Abstract
Manganese (Mn) is an essential metal for biological systems, however occupational or clinical exposure to high levels of Mn can produce a neurological disorder called manganism. Oxidative stress and neuroinflammation play major roles in the Mn-induced neurodegeneration leading to dysfunction of the basal ganglia. We investigated the toxic effects of MnCl2in an immortalized rat brain endothelial cell line (RBE4) and the protective effects of the radical scavenging aminosalicylic acids, 5-aminosalicylic acid (5-ASA) and 4-aminosalicylic acid (4-PAS). Mn cytotoxicity was determined with 3-[4,5-dimethylth-iazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) reduction and lactate dehydrogenase (LDH) activity. A significant decrease in MTT reduction concomitant with increased LDH release was noted in RBE4 cells exposed for 24 h to MnCl2 (600 and 800 μM)(p<0.0001). Our results establish that compared to 4-PAS, 5-ASA has greater efficacy in protecting RBE4 cells from Mn-induced neurotoxicity after pre-exposure to MnCl2 800 μM (p<0.0001).
Index Entries: manganese, neurotoxicity, neuroprotection, RBE4 cells, blood, brain barrier, endothelium
Introduction
Mn is an essential nutrient and it plays a fundamental role in cellular homeostasis. However, excessive Mn exposure causes neurotoxicity (Cotzias et al. 1968). Clinical signs, such as Parkinsonism, characterized by an extrapyramidal movement disorder, commonly appear after a primarily psychiatric presentation (locura manganica) (Mergler et al. 1999, Roels et al. 1985). Symptoms associated with manganism have been treated with limited efficacy with psychiatric and anti-parkinsonian drugs (Herrero Hernandez et al. 2006, Koller, Lyons and Truly 2004, Blanc 1990). In contrast to Parkinson’s disease, Mn predominantly targets the globus pallidus (Herrero Hernandez et al. 2006). Chelation serves to alleviate the acute symptoms, but there is little evidence to support that such therapy has chronic consequences (e.g. Parkinsonism) (Rosenstock and Cullen 1994). Treatment of workers with occupational Mn-induced parkinsonism with ethylene diamine tetra-acetic acid (EDTA) and anti-parkinsonian drugs has led to improvement in clinical outcome (Herrero Hernandez et al. 2006).
Herein, we utilized an established blood–brain barrier (BBB) model of immortalized rat brain endothelium 4 (RBE4) cells to evaluate Mn-induced cytotoxicity. The RBE4 cell line preserves features of the in vivo brain endothelium(Demeuse et al. 2002), expressing a number of BBB transporters (P-glycoprotein) and endothelial markers (VIII-related antigen)(Durieu-Trautmann et al. 1993). RBE4cells were derived from rat brain microvascular endothelial cells immortalized with the plasmid pE1A-neo, containing the E1A region of adenovirus 2 and a neomycin-resistance gene (Bressler et al. 2004, Roux et al. 1994).
Given the limited success in treating chronic states of Mn-induced disease with anti-parkinsonian drugs(Sadek, Rauch and Schulz 2003, Herrero Hernandez et al. 2006, Koller et al. 2004), an increasing and immediate need exists for efficacious therapy against Mn-induced neurological impairment. We evaluated the efficacy of two carboxylic acids, 4-aminosalicylic acid (4-PAS) and 5-aminosalicylic acid (5-ASA), analyzing two biochemical indicators of cell toxicity, lactate dehydrogenase (LDH) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), following RBE4 cells treatment with MnCl2 for 24 hours (h).
Previous studies in rats demonstrated that treatment with 4-PAS markedly reduced brain Mn levels in animals sub-chronically and sub-acutely exposed to this metal (Zheng et al. 2009, Santos et al. 2012a). However, 4-PAS is used as a second line anti-tuberculosis (TB) agent. Given (1) the indiscriminate use of antibiotics (Fitzwater et al. 2012) that threatens their clinical efficacy,(2) the documented efficacy of 5-ASA as an antioxidant and anti-inflammatory agent (Pearson, Jourd’heuil and Meddings 1996, Goncalves, Almeida and Dinis 1998), and (3) the role of reactive oxygen species (ROS) and neuroinflammation in the etiology of Mn-induced neurotoxicity (Milatovic and Aschner 2009, Zhang et al. 2001), the present study was designed to assess the efficacy of 5-ASA (vs. 4-PAS) in mitigating the cytotoxicity of this metal in RBE4 cells.
Materials and Methods
Chemicals
Manganese chloride tetrahydrate (MnCl2.4H2O), 4-PAS, 5-ASA and penicillin/streptomycin solution were obtained from Sigma Aldrich. Heat- inactivated fetal bovine serum (FBS), trypsin, nutrient mixture Ham F10, Geneticin G-418 and minimal essential medium (MEM) were purchased from Gibco. Basic fibroblastic growth factor (bFGF) were purchased from Life Technologies - Invitrogen.
Cell Culture
RBE4 cultures were cultured in 44.5% minimum essential medium (MEM), 44.5 %Ham F10 with glycine, 10% fetal bovine serum (FBS), and 1% of a penicillin/streptomycin solution and kept at 37 °C with 5% CO2. For subculturing, the cells were dissociated with 0.25% trypsin, split 1:3, and subcultured in flasks coated with collagen with 75 cm2 of growth area. The cells reached confluence at a density of 1,48 × 105cells/ml.
Cells Treatment
The RBE4 cells in 96-well culture plates were treated with MnCl2.4H2O at 400, 600 and 800 μM and combinations of MnCl2.4H2O and the anti- oxidants 4-PAS (Mn + 4-PAS 1 mM; Mn + 4-PAS 2 mM) and 5-ASA (Mn + 4- PAS 1 mM; Mn + 4-PAS 2 mM). Cells were treated with manganese chloride for 24 hours followed by the anti-oxidants 4-PAS or 5-ASA for 45 minutes. Mn (Marreilha dos Santos et al. 2008), 4-PAS and 5-ASA concentrations were based on previous experiments in the same cell line.
LDH Toxicity Assay
RBE4 cells were grown for 24 h, in 96-well culture plates, at 37°C, until confluency at a density of 1.48 × 105cells/ml. Forty-five minutes after 4-PAS or 5-ASA or medium (control) treatment, cultures were processed in accordance with the LDH-based colorimetric assay (Legrand et al. 1992). The LDH released into the supernatant medium was analyzed according to the manufacture’s protocol (Promega) and quantified with an ELISA plate reader (Zenyth 3100) at 490 nm.
MTT Toxicity Assay
The MTT assay was performed according to the manufacturer’s protocol (Sigma). After treatments (see above) and at the end of the respective incubation periods, 50 μl of MTT solution was added to each well, followed by 2 h incubation of the plates at 37°C in a CO2 incubator. The reaction was terminated by the addition of 50 μl of MTT solubilization solution, and the results were quantified by measuring the absorbance at 570 nm. Treated samples are reported as percentage of viability in comparison to controls.
Statistical Analysis
All results are expressed as means +standard error of the mean (SEM) of 3 or more individual assays. Multiple comparisons were performed by utilizing one-way analysis of variance (ANOVA), followed by Bonferroni test for comparison of treatments with the controls. Results were analyzed by GraphPad Prism 4 (GraphPad software, San Diego, California); P values less than 0.05 were considered to be significant.
Results
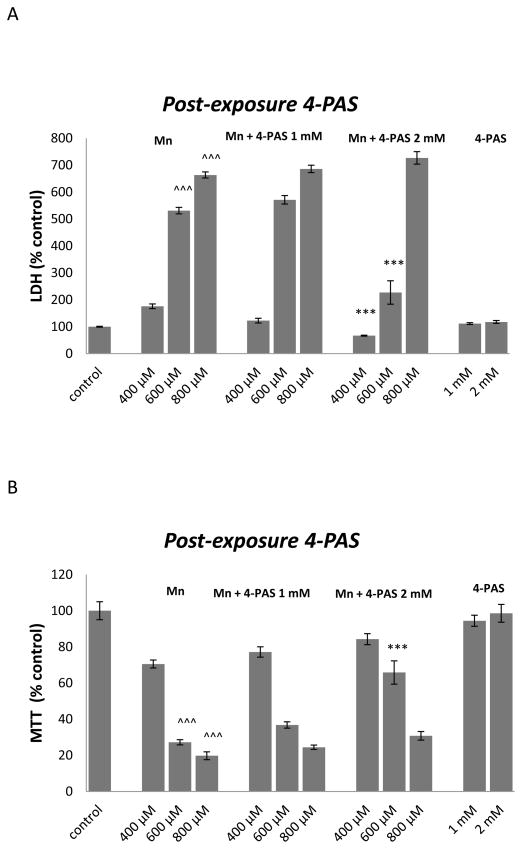
4-PAS (2 mM) was effective in protecting RBE4 cells treated for 24 h with 600 μM of MnCl2, as evidenced by significant (p<0.0001) increase in MTT levels (figure 1B) and decrease in LDH release (figure 1A).
Figure 1.
The effect of 4-PAS on MTT reduction (A) and membrane integrity (B) in RBE4 cells cultured in 96-well culture plates treated for 24 h with 400, 600 or 800 μM MnCl2 or medium alone. After 24 h RBE4 cells were treated for 45 minutes with medium or 1 or 2 mM 4-PAS in DMSO. Values of MTT (% control) (A) and LDH (% control) (B) represent mean ± SEM (n=12). ^^^ p<0.0001 significantly different from control group, *** p<0.0001,** p<0.001 significantly different from RBE4 cells exposed to the respective Mn 400, 600 and 800 μM group by one-way ANOVA followed by Bonferroni’s multiple comparison tests.
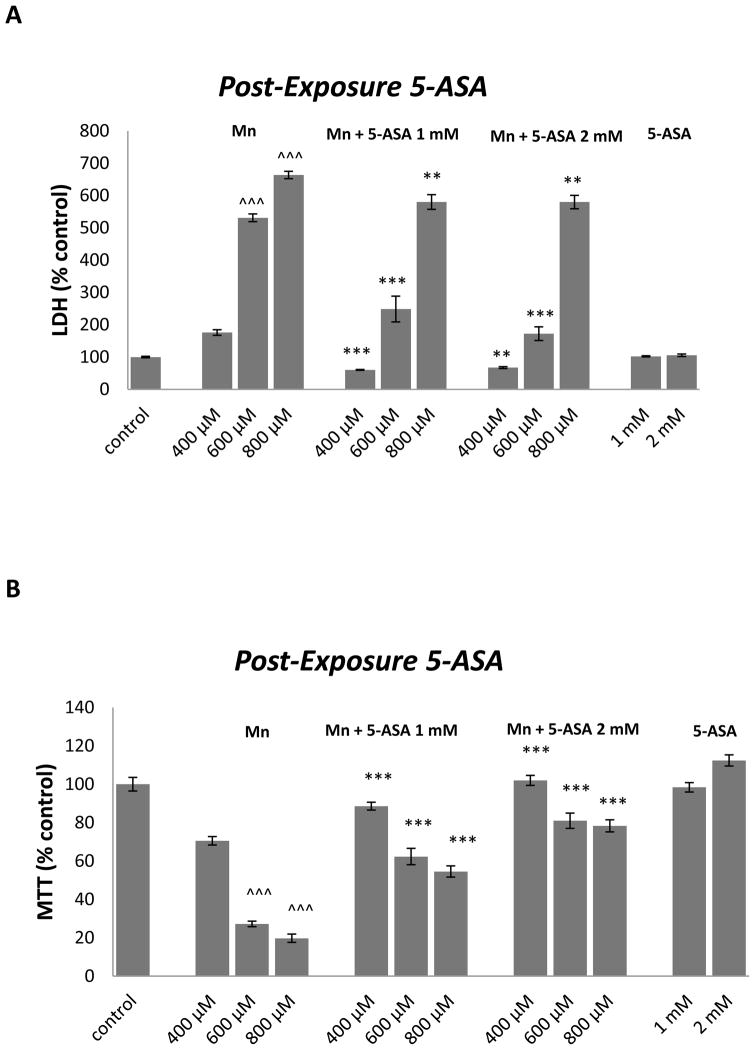
Figure 2A shows that 1 and 2 mM 5-ASA significantly attenuated LDH release in RBE4 cells exposed for 24 h to400 (p<0.0001 and p<0.001), 600 (p<0.0001 and p<0.0001) or 800 μM (p<0.001 and p<0.001) MnCl2. 5-ASA (1 and 2 mM) also significantly increased the conversion of MTT to formazan in RBE4 cells treated for 24h with MnCl2within the tested concentration range (p<0.0001) (figure 2B).
Figure 2.
The effect of 5-ASA on MTT reduction (A) and membrane integrity (B) in RBE4 cells cultured in 96-well culture plates treated for 24 h with 400, 600 or 800 μM MnCl2 or medium alone. After 24 h RBE4 cells were treated for 45 minutes with medium, or 1 or 2 mM 5-ASA in HCl. Values of MTT (% control) (A) and LDH (% control) (B) represent mean ± SEM (n=12). ^^^ p<0.0001 significantly different from control group, *** p<0.0001, ** p<0.001 significantly different from RBE4 cells exposed to the respective Mn 400, 600 and 800 μM group by one-way ANOVA followed by Bonferroni’s multiple comparison tests.
Discussion
4-PAS and 5-ASA were assessed for their ability to attenuate Mn-induced cytotoxicity in RBE4 cells. The present study confirms that in cells exposed to MnCl2 alone, the increase in LDH release is concordant with the decrease in MTT levels, corroborating previous reports (Marreilha dos Santos et al. 2008, dos Santos et al. 2010). 5-ASA significantly decreased Mn cytotoxicity in RBE4 cells exposed to MnCl2 for 24 h.
Several recent reports have suggested that inflammation and oxidative stress contribute to Mn-induced neurodegeneration (Zhao et al. 2009, Milatovic et al. 2009, Santos et al. 2012b). The aminosalicylic acids used 4-PAS and 5-ASA provide an anti-oxidative protection through the deactivation of excited oxygen species (Yppolito et al. 2002).
For the first time, our results show that 5-ASA has greater efficacy in protecting RBE4 cells from Mn-induced cytotoxicity in comparison to 4-PAS after pre-exposure to MnCl2 800 μM (p<0.0001).
The efficacy of 4-PAS has been ascribed to its role in reducing Mn concentrations in body fluids and brain, acting as a Mn-chelating agent (Zheng et al. 2009). A recent report in rats co-exposed to MnCl2and 4-PAS showed that 4-PAS was also effective in attenuating MnCl2-induced neurotoxicity (Santos et al. 2012a).
5-ASA is clinically used as an anti-inflammatory agent against intestinal mucosa and joint synovial inflammation (Svartz 1988), where oxidative stress has been implicated (Miles and Grisham 1994). Brain cells are especially susceptible to oxidative stress. 5-ASA showed protection against the oxidation in gerbil cortical synaptosomes system caused by azo-bis(isobutyronitrile) (AIBN) and 2,2′-azobis (amidino propane) dihydrochloride (AAPH) (Kanski, Lauderback and Butterfield 2001).
Recent studies also showed that 5-ASA inhibits nuclear factor-kB (NF-kB) activation preventing the expression of genes encoding proinflammatory cytokines, chemokines, adhesion molecules and inflammatory mediators (Barnes and Karin 1997). In PC12 cells, Mn induced NFκ-B (Ramesh, Ghosh and Gunasekar 2002) expression, lending support to the role of oxidative stress in its neurotoxicity. Pretreatment with either vitamin E or an NFκ-B inhibitor protected against Mn toxicity in mesencephalic cells (Prabhakaran et al. 2008). Notably, NFκ-B is not a target of 4-PAS. Induction of Mn-superoxide dismutase (Mn-SOD) activity by 5-ASA may also contribute to the therapeutic mechanism of 5-ASA (Valentine 2001). Notably, MnSOD is not a target of 4-PAS.
Although 5-ASA protects RBE4 cells from the deleterious effects of Mn,, it doesn’t cross the BBB(UKPAR 2009). These findings suggest that new strategies to deliver it to the brain should be developed in order to prevent Mn-induced degeneration or other neurotoxicities associated with oxidative stress. Furthermore, we suggest the use of in vivo and in vitro models to study the effectiveness of other NFκ-B inhibitors and/or enhancers of Mn-SOD activity that readily cross the BBB. These studies should profitable advance strategies to control and mitigate the adverse effects of Mn in the brain.
Acknowledgments
I am thankful to Prof. Eunsook Lee from Meharry Medical College and Dr. Yingchun Yu from Vanderbilt University. The study was supported by FCT (Foundation for Science and Technology of Portugal; SFRH/BD/64128/ 2009) and National Institute of Environmental Health Sciences (NIEHS) R01 ES 10563 to MA.
References
- Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–71. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Blanc P. Poisoning and Drug Overdose. Norwalk, California: Appleton & Lange; 1990. [Google Scholar]
- Bressler JP, Olivi L, Cheong JH, Kim Y, Bannona D. Divalent metal transporter 1 in lead and cadmium transport. Ann N Y Acad Sci. 2004;1012:142–52. doi: 10.1196/annals.1306.011. [DOI] [PubMed] [Google Scholar]
- Cotzias GC, Horiuchi K, Fuenzalida S, Mena I. Chronic manganese poisoning. Clearance of tissue manganese concentrations with persistance of the neurological picture. Neurology. 1968;18:376–82. doi: 10.1212/wnl.18.4.376. [DOI] [PubMed] [Google Scholar]
- Demeuse P, Kerkhofs A, Struys-Ponsar C, Knoops B, Remacle C, van den Bosch de Aguilar P. Compartmentalized coculture of rat brain endothelial cells and astrocytes: a syngenic model to study the blood-brain barrier. J Neurosci Methods. 2002;121:21–31. doi: 10.1016/s0165-0270(02)00225-x. [DOI] [PubMed] [Google Scholar]
- dos Santos AP, Milatovic D, Au C, Yin Z, Batoreu MC, Aschner M. Rat brain endothelial cells are a target of manganese toxicity. Brain Res. 2010;1326:152–61. doi: 10.1016/j.brainres.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieu-Trautmann O, Federici C, Creminon C, Foignant-Chaverot N, Roux F, Claire M, Strosberg AD, Couraud PO. Nitric oxide and endothelin secretion by brain microvessel endothelial cells: regulation by cyclic nucleotides. J Cell Physiol. 1993;155:104–11. doi: 10.1002/jcp.1041550114. [DOI] [PubMed] [Google Scholar]
- Fitzwater SP, Sechler GA, Jave O, Coronel J, Mendoza A, Gilman RH, Friedland JS, Moore DA. Second-line anti-TB drug concentrations for susceptibility testing in the MODS assay. Eur Respir J. 2012 doi: 10.1183/09031936.00059812. [DOI] [PubMed] [Google Scholar]
- Goncalves E, Almeida LM, Dinis TC. Antioxidant activity of 5-aminosalicylic acid against peroxidation of phosphatidylcholine liposomes in the presence of alpha-tocopherol: a synergistic interaction? Free Radic Res. 1998;29:53–66. doi: 10.1080/10715769800300071. [DOI] [PubMed] [Google Scholar]
- Herrero Hernandez E, Discalzi G, Valentini C, Venturi F, Chio A, Carmellino C, Rossi L, Sacchetti A, Pira E. Follow-up of patients affected by manganese-induced Parkinsonism after treatment with CaNa2EDTA. Neurotoxicology. 2006;27:333–9. doi: 10.1016/j.neuro.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Kanski J, Lauderback C, Butterfield DA. 5-Aminosalicylic acid protection against oxidative damage to synaptosomal membranes by alkoxyl radicals in vitro. Neurochem Res. 2001;26:23–9. doi: 10.1023/a:1007620330168. [DOI] [PubMed] [Google Scholar]
- Koller WC, Lyons KE, Truly W. Effect of levodopa treatment for parkinsonism in welders: A double-blind study. Neurology. 2004;62:730–3. doi: 10.1212/01.wnl.0000113726.34734.15. [DOI] [PubMed] [Google Scholar]
- Legrand C, Bour JM, Jacob C, Capiaumont J, Martial A, Marc A, Wudtke M, Kretzmer G, Demangel C, Duval D, et al. Lactate dehydrogenase (LDH) activity of the cultured eukaryotic cells as marker of the number of dead cells in the medium [corrected] J Biotechnol. 1992;25:231–43. doi: 10.1016/0168-1656(92)90158-6. [DOI] [PubMed] [Google Scholar]
- Marreilha dos Santos AP, Santos D, Au C, Milatovic D, Aschner M, Batoreu MC. Antioxidants prevent the cytotoxicity of manganese in RBE4 cells. Brain Res. 2008;1236:200–5. doi: 10.1016/j.brainres.2008.07.125. [DOI] [PubMed] [Google Scholar]
- Mergler D, Baldwin M, Belanger S, Larribe F, Beuter A, Bowler R, Panisset M, Edwards R, de Geoffroy A, Sassine MP, Hudnell K. Manganese neurotoxicity, a continuum of dysfunction: results from a community based study. Neurotoxicology. 1999;20:327–42. [PubMed] [Google Scholar]
- Milatovic D, Aschner M. Measurement of isoprostanes as markers of oxidative stress in neuronal tissue. Curr Protoc Toxicol. 2009;Chapter 12(Unit 12):14. doi: 10.1002/0471140856.tx1214s39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milatovic D, Zaja-Milatovic S, Gupta RC, Yu Y, Aschner M. Oxidative damage and neurodegeneration in manganese-induced neurotoxicity. Toxicol Appl Pharmacol. 2009;240:219–25. doi: 10.1016/j.taap.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles AM, Grisham MB. Antioxidant properties of aminosalicylates. Methods Enzymol. 1994;234:555–72. doi: 10.1016/0076-6879(94)34128-1. [DOI] [PubMed] [Google Scholar]
- Pearson DC, Jourd’heuil D, Meddings JB. The anti-oxidant properties of 5-aminosalicylic acid. Free Radic Biol Med. 1996;21:367–73. doi: 10.1016/0891-5849(96)00031-7. [DOI] [PubMed] [Google Scholar]
- Prabhakaran K, Ghosh D, Chapman GD, Gunasekar PG. Molecular mechanism of manganese exposure-induced dopaminergic toxicity. Brain Res Bull. 2008;76:361–7. doi: 10.1016/j.brainresbull.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Ramesh GT, Ghosh D, Gunasekar PG. Activation of early signaling transcription factor, NF-kappaB following low-level manganese exposure. Toxicol Lett. 2002;136:151–8. doi: 10.1016/s0378-4274(02)00332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roels H, Sarhan MJ, Hanotiau I, de Fays M, Genet P, Bernard A, Buchet JP, Lauwerys R. Preclinical toxic effects of manganese in workers from a Mn salts and oxides producing plant. Sci Total Environ. 1985;42:201–6. doi: 10.1016/0048-9697(85)90022-1. [DOI] [PubMed] [Google Scholar]
- Rosenstock L, Cullen M. Metals and related compounds. In: Rosenstock L, Cullen M, editors. Textbook of Clinical and Occupational and Environmental Medicine. Philadelphia: W.B. Saunders Company; 1994. [Google Scholar]
- Roux F, Durieu-Trautmann O, Chaverot N, Claire M, Mailly P, Bourre JM, Strosberg AD, Couraud PO. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994;159:101–13. doi: 10.1002/jcp.1041590114. [DOI] [PubMed] [Google Scholar]
- Sadek AH, Rauch R, Schulz PE. Parkinsonism due to manganism in a welder. Int J Toxicol. 2003;22:393–401. doi: 10.1177/109158180302200511. [DOI] [PubMed] [Google Scholar]
- Santos AP, Lucas RL, Andrade V, Mateus ML, Milatovic D, Aschner M, Batoreu MC. Protective effects of ebselen (Ebs) and para-aminosalicylic acid (PAS) against manganese (Mn)-induced neurotoxicity. Toxicol Appl Pharmacol. 2012a;258:394–402. doi: 10.1016/j.taap.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Santos D, Milatovic D, Andrade V, Batoreu MC, Aschner M, Marreilha dos Santos AP. The inhibitory effect of manganese on acetylcholinesterase activity enhances oxidative stress and neuroinflammation in the rat brain. Toxicology. 2012b;292:90–8. doi: 10.1016/j.tox.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svartz N. Sulfasalazine: II. Some notes on the discovery and development of salazopyrin. Am J Gastroenterol. 1988;83:497–503. [PubMed] [Google Scholar]
- UKPAR; M. a. H. P. R. Agency, editor. Pentasa slow release tablets 1 g. Vol. 19. United Kingdom: Ferring Pharmaceuticals Limited; 2009. [Google Scholar]
- Valentine JF. Mesalamine induces manganese superoxide dismutase in rat intestinal epithelial cell lines and in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1044–50. doi: 10.1152/ajpgi.2001.281.4.G1044. [DOI] [PubMed] [Google Scholar]
- Yppolito R, Pappano N, Debattista N, Miskoski S, Bertolotti SG, Garcia NA. On the antioxidant properties of therapeutic drugs: quenching of singlet molecular oxygen by aminosalicylic acids. Redox Rep. 2002;7:229–33. doi: 10.1179/135100002125000613. [DOI] [PubMed] [Google Scholar]
- Zhang D, He X, Huang S, Li Y. Effect of manganese exposure on brain development in postnatal mice. Wei Sheng Yan Jiu. 2001;30:260–2. [PubMed] [Google Scholar]
- Zhao F, Cai T, Liu M, Zheng G, Luo W, Chen J. Manganese induces dopaminergic neurodegeneration via microglial activation in a rat model of manganism. Toxicol Sci. 2009;107:156–64. doi: 10.1093/toxsci/kfn213. [DOI] [PubMed] [Google Scholar]
- Zheng W, Jiang YM, Zhang Y, Jiang W, Wang X, Cowan DM. Chelation therapy of manganese intoxication with para-aminosalicylic acid (PAS) in Sprague-Dawley rats. Neurotoxicology. 2009;30:240–8. doi: 10.1016/j.neuro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]