Abstract
Accumulated evidence suggests that an altered ambulatory blood pressure (BP) profile, particularly elevated nighttime BP, reflects target organ injury and is a better predictor of further cardiorenal risk than the clinic BP or daytime BP in hypertensive patients complicated by chronic kidney disease (CKD). In this study, we examined the beneficial effects of olmesartan, an angiotensin II type 1 receptor blocker (ARB), on ambulatory BP profiles and renal function in hypertensive CKD patients. Forty-six patients were randomly assigned to the olmesartan add-on group (n=23) or the non-ARB group (n=23). At baseline and after the 16-week treatment period, ambulatory BP monitoring was performed and renal function parameter measurements were collected. Although the baseline clinic BP levels and the after-treatment/baseline (A/B) ratios of clinic BP levels were similar in the olmesartan add-on and non-ARB groups, the A/B ratios of ambulatory 24-h and nighttime BP levels in the olmesartan add-on group were significantly lower. Furthermore, the A/B ratios of urinary protein, albumin and type IV collagen excretion in the olmesartan add-on group were significantly lower than those in the non-ARB group (urinary protein excretion, 0.72±0.41 vs. 1.45±1.48, P=0.030; urinary albumin excretion, 0.73±0.37 vs. 1.50±1.37, P 0.005; urinary type IV collagen excretion, 0.87±0.42 vs. 1.48±0.87, P=0.014) despite comparable A/B ratios for the estimated glomerular filtration rate in the two groups. These results indicate that in hypertensive patients with CKD, olmesartan add-on therapy improves the ambulatory BP profile via a preferential reduction in nighttime BP with concomitant renal injury inhibition.
Keywords: ambulatory blood pressure, angiotensin receptor blocker, estimated glomerular filtration rate, proteinuria
INTRODUCTION
Clinical trials have shown that strict blood pressure (BP) control is critical for preventing target organ damage and cardiovascular mortality in hypertensive patients.1,2 Hypertensive patients with chronic kidney disease (CKD) are increasing in number, and cardiovascular complications are the most common cause of death in these patients. Thus, it is of considerable importance to identify therapeutic targets in cases of hypertension accompanied by the complication of CKD. Recent studies have indicated that the ambulatory and the clinic BP profiles are important for proper estimation of BP control. In particular, ambulatory BP monitoring has allowed accurate diagnosis of hypertension3,4 and determination of the circadian BP rhythm under different pathophysiological conditions, including CKD, and thus may have prognosis predictability superior to clinic BP measurement.5,6 The circadian BP pattern in hypertensive patients with CKD has been found to exhibit a blunted nocturnal BP decrease, which is associated with autonomic neuropathy and nephropathy.7,8 Conversely, the loss of nocturnal BP dipping is considered a risk factor for nephropathy progression and is of prognostic value with respect to target organ damage and cardiovascular morbidity in these CKD patients.6,9–12
Activation of the renin–angiotensin system has been demonstrated to be involved in CKD pathogenesis and its cardiovascular complications through the generation of angiotensin II (Ang II), a key regulator of cardiovascular homeostasis. The Ang II type 1 receptor (AT1R) is responsible for most Ang II-mediated pathophysiological effects, and inhibition of the renin–angiotensin system by angiotensin-converting enzyme inhibitors (ACEIs) and AT1R-specific blockers (ARBs) has been shown to exert various protective effects against CKD progression and cardiovascular complications, at least partially through a reduction in urinary protein/albumin excretion.13–16
Furthermore, recent clinical study results and a meta-analysis of several large-scale cohort studies indicated that preservation of the estimated glomerular filtration rate (eGFR), as well as reduction in proteinuria/albuminuria are important for the suppression of CKD progression and cardiovascular complications in hypertensive CKD patients.17–19 Of the clinically available ARBs, olmesartan has been reported to exert a long-lasting BP-lowering effect via its characteristic `double-chain domain' structure20 and is expected to efficiently improve the altered ambulatory BP profile of hypertensive CKD patients.21 Previous studies have shown that nocturnal hypertension is closely related to an increase in urinary protein excretion and renal function deterioration in CKD patients. We hypothesized that olmesartan may effectively lower nocturnal BP levels with concomitant renal protective effects such as improvements in proteinuria and markers of renal injury. Therefore, in this study, we examined the therapeutic effects of olmesartan add-on therapy on the ambulatory BP profile and renal function of hypertensive patients with CKD.
METHODS
Study design
This was a randomized open-label parallel-group controlled study; it was conducted at the outpatient clinic of the Department of Internal Medicine, Yokohama City University Hospital (Yokohama, Japan). The study consisted of a 2-week run-in period and a 16-week active treatment period. This study was approved by the Ethics Committees of Yokohama City University Hospital, and written informed consent was obtained from every participant.
Study participants
Inclusion criteria were an age of 20 years or older, a history of mild-to-moderate hypertension (clinic systolic BP ≥130 mm Hg and/or diastolic BP ≥80 mm Hg or receiving antihypertensives) and CKD. When patients were already being treated for hypertension, anti-hypertensive drugs other than ARBs were continued during the run-in period, and then, if the hypertension was still uncontrolled (BP ≥130/80 mm Hg), the patients were considered for recruitment into the study. CKD was diagnosed by the presence of albuminuria (urinary albumin excretion rate (UACR) ≥30 mg per g creatinine), proteinuria (urinary protein excretion rate (UPCR) ≥0.15 g per g creatinine), or eGFR <60 ml min per 1.73 m2 for a period of more than 3 months. We calculated the eGFR using a revised equation for the Japanese population: eGFR (ml min−1 per 1.73 m2)=194 × serum creatinine−1.094 × age−0.287 × 0.739 (if female).22 Exclusion criteria included patients who were on dialysis, women who were nursing or pregnant, and patients with clinically significant heart disease, stroke, renal artery stenosis, hepatic dysfunction or known hypersensitivity to any ingredient in the study medications.
Study treatment
After the run-in period and the discontinuation of any previous ARB treatment, eligible patients were randomized to the olmesartan add-on group or the non-ARB group. Patients in the olmesartan add-on group were initially given 10 mg of olmesartan once daily in the morning; the dose of olmesartan was titrated up to 40 mg daily, as needed, during the 16-week active treatment period. Patients in the non-ARB group were given either an increased dose of their existing treatment or an additional conventional treatment other than ARB to achieve the BP goal (BP <130/80 mm Hg).
Clinic BP measurement and 24-h ambulatory BP monitoring
Clinic BP was measured at the trough of the medication cycle (24±2 h post dose) using a calibrated standard mercury sphygmomanometer and the recommended cuff sizes in a sitting position.23 Two measurements were taken at 1- to 2-min intervals, and their average was used to calculate the clinic BP.
The ambulatory BP and heart rate were monitored every 30 min with a fully automated device (TM-2425, A&D, Tokyo, Japan), as described previously.24–30 Ambulatory BP monitoring was repeated in patients who had <20% of values missing, <30% error rate for the total readings, or values missing for more than 2 consecutive hours. The following readings were considered technical artifacts and were omitted: systolic BP <250 mm Hg or >70 mm Hg, diastolic BP <130 mm Hg or >30 mm Hg, pulse pressure <160 mm Hg or >20 mm Hg, systolic differences <60 mm Hg or diastolic differences <30 mm Hg compared with the immediately preceding or subsequent values. The patients were instructed to fill out a diary to record the time of sleeping, rising and other daytime activities. Therefore, the terms `daytime' and `nighttime' in the present study reflect the average period during which the subjects were awake/upright and asleep/supine, respectively. We defined the morning BP as the average BP during the initial 2 h after awakening, as described previously.31–33
Laboratory measurements
Blood and urine sampling was performed between 0800 and 1000 hours after an overnight fast. After the patients had spent 30 min at quiet rest in a recumbent position, blood samples were collected to determine laboratory parameters using routine methods in the Department of Clinical Chemistry, Yokohama City University Hospital. The urinary concentrations of type IV collagen and 8-hydroxydeoxyguanosine (8-OHdG) were determined using an enzyme immunoassay kit (SRL laboratory, Tokyo, Japan) and an ELISA kit (Japan Institute for Control of Aging, Shizuoka, Japan), respectively.34 Urinary angiotensinogen (AGT) levels were measured using a sandwich ELISA, as described previously.35,36 Urinary type IV collagen, 8-OHdG and AGT concentrations were normalized to urinary creatinine concentration (Table 1).
Table 1.
Patient baseline characteristics
| Variable | Olmesartan, n=22 | Non-ARB, n=23 | P-value |
|---|---|---|---|
| Age (years); mean (s.d.) | 64.7±10.4 | 67.0±7.9 | 0.413 |
| Male (%) | 86 | 87 | 1.000 |
| Waist circumference (cm); mean (s.d.) | 88.3±7.7 | 90.1±10.1 | 0.892 |
| Diabetes (%) | 41 | 51 | 0.554 |
| CKD stage (n) | 0.167 | ||
| Stage 1 | 0 | 0 | |
| Stage 2 | 6 | 1 | |
| Stage 3 | 10 | 16 | |
| Stage 4 | 3 | 4 | |
| Stage 5 | 3 | 2 | |
| Serum creatinine (mg dl−1); mean (s.d.) | 1.73±1.04 | 1.73±1.10 | 0.742 |
| eGFR (ml min−1 per 1.73 m2); mean (s.d.) | 43.0±22.2 | 40.5±17.9 | 0.751 |
| UPCR (g per g Cr); mean (s.d.) | 2.12±2.58 | 1.58±2.23 | 0.517 |
| UACR (mg per g Cr); mean (s.d.) | 1605.3±1889.7 | 1182.0±1614.9 | 0.388 |
| hs-CRP (mg dl−1); mean (s.d.) | 0.14±0.17 | 0.07±0.05 | 0.175 |
| Urinary AGT (μg per g Cr); mean (s.d.) | 514.8±843.3 | 454.3±811.3 | 0.835 |
| Urinary 8-OHdG (ng per mg Cr); mean (s.d.) | 3.60±3.53 | 2.65±2.74 | 0.455 |
| Urinary type IV collagen (μg per g Cr); mean (s.d.) | 12.70±12.72 | 8.78±8.85 | 0.301 |
Abbreviations: ARB, angiotensin II type 1 receptor blocker; AGT, angiotensinogen; CDK, chronic kidney disease; eGFR, estimated glomerular filtration rate; hs-CRP, high-sensitivity c-reactive protein; 8-OHdG, 8-hydroxydeoxyguanosine; UACR, urinary albumin-to-creatinine ratio; UPCR, urinary protein-to-creatinine ratio.
Statistical analysis
The quantitative data are expressed as the mean±s.d. To examine the effects of anti-hypertensive treatment, the values of the variables after the 16-week active treatment period were normalized to those of their respective variables at baseline and were expressed as after-treatment/baseline (A/B) ratios. For the statistical analysis of the difference between the olmesartan add-on group and non-ARB group, the Mann-Whitney U-test was performed for continuous variables, and the χ2 test was performed for qualitative variables using SPSS (Statistical Package for the Social Sciences) software (Version 16.0, Chicago, IL, USA). A P-value <0.05 was considered statistically significant.
RESULTS
Patient characteristics
Forty-six hypertensive patients with CKD were enrolled between October 2007 and March 2010. The causes of CKD were hypertensive nephrosclerosis (n = 17), diabetic nephropathy (n = 14), chronic glomerulonephritis (n = 6), polycystic kidney disease (n = 1), other renal diseases (n = 4) or of unknown cause (n = 4). Patients were randomly assigned to the olmesartan add-on group (n = 23) or the non-ARB group (n = 23). One patient in the olmesartan add-on group was discontinued from the study; therefore, a total of 45 patients completed the study. This patient was unavailable for follow-up due to a transfer to another hospital. Table 1 shows the demographics of the study participants, including the number of participants in each CKD stage and the baseline characteristics of the participants.
The olmesartan add-on therapy was well tolerated without any significant adverse events, and the average additional dose of olmesartan was 15.9±8.9 mg per day after a 16-week treatment period. The additional treatments in both groups were primarily calcium channel blockers, diuretics, ACEIs and statins; more patients in the non-ARB group were treated with ACEIs than the olmesartan add-on group during the study period (Table 2). Although ~50% of patients in both groups had diabetes, there were no significant differences in the use of oral hypoglycemic agents and insulin.
Table 2.
Medications used during the study
| Medication | Olmesartan, n=22 | Non-ARB, n=23 | P-value |
|---|---|---|---|
| Calcium channel blockers (%) | 77 | 96 | 0.096 |
| Diuretics (%) | 36 | 57 | 0.175 |
| ACEI (%) | 14 | 57 | 0.003 |
| Statins (%) | 23 | 35 | 0.372 |
| Oral hypoglycemic agents (%) | 32 | 17 | 0.187 |
| Insulin (%) | 18 | 4 | 0.260 |
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II type 1 receptor blocker.
Effects of olmesartan add-on therapy on the BP profile
At baseline, the mean clinical BP values were similar in the olmesartan add-on and non-ARB groups. After the 16-week active treatment period, the A/B ratios of the two groups were comparable (Table 3). With respect to the ambulatory BP profile, the baseline daytime systolic/diastolic BP levels were comparable in the olmesartan add-on and non-ARB groups, while the baseline 24-h and nighttime systolic/diastolic BP levels were significantly higher in the olmesartan add-on group (Table 4). However, compared with the non-ARB group, the 24 h diastolic BP and nighttime systolic/diastolic BP level A/B ratios in the olmesartan add-on group were significantly lower after the 16-week active treatment period (Table 4; 24-h diastolic BP, 0.92±0.07 vs. 0.98±0.07, P = 0.017; nighttime systolic BP, 0.91±0.10 vs. 1.00±0.08, P = 0.001; nighttime diastolic BP 0.90±0.11 vs. 0.98±0.10, P = 0.024), and the 24-h systolic BP A/B ratio tended to be decreased in the olmesartan add-on group (Table 4; 24-h systolic BP, 0.93±0.07 vs. 0.99±0.08, P = 0.053). However, the daytime systolic/diastolic BP A/B ratios were comparable in the olmesartan add-on group and the non-ARB group (Table 4; daytime systolic BP, 0.94±0.07 vs. 0.98±0.09, P = 0.261; daytime diastolic BP, 0.93±0.07 vs. 0.97±0.07, P = 0.105). In addition, although the differences did not reach statistical significance, there was a trend toward a decrease in morning systolic/diastolic BP A/B ratios in the olmesartan add-on group compared with the non-ARB group (Table 4).
Table 3.
Effects of anti-hypertensive therapy on clinic BP
| Variable | Olmesartan, n=22 | Non-ARB, n=23 | P-value |
|---|---|---|---|
| Clinic BP | |||
| Systolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 144.1±13.7 | 144.1±17.8 | 0.955 |
| A/B ratio; mean (s.d.) | 0.99±0.12 | 0.96±0.11 | 0.714 |
| Diastolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 79.7±19.0 | 84.6±11.5 | 0.474 |
| A/B ratio; mean (s.d.) | 1.36±1.61 | 0.95±0.12 | 0.182 |
Abbreviations: A/B ratio, after-treatment/baseline ratio; ARB, angiotensin II type 1 receptor blocker; BP, blood pressure.
Table 4.
Effects of anti-hypertensive therapy on the ambulatory BP profile
| Variable | Olmesartan, n=22 | Non-ARB, n=23 | P-value |
|---|---|---|---|
| 24 h | |||
| Systolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 142.6±14.4 | 135.0±11.3 | 0.036 |
| A/B ratio; mean (s.d.) | 0.93±0.07 | 0.99±0.08 | 0.053 |
| Diastolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 83.6±10.4 | 78.2±7.5 | 0.045 |
| A/B ratio; mean (s.d.) | 0.92±0.07 | 0.98±0.07 | 0.017 |
| Daytime | |||
| Systolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 146.6±14.8 | 140.5±11.5 | 0.180 |
| A/B ratio; mean (s.d.) | 0.94±0.07 | 0.98±0.09 | 0.261 |
| Diastolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 86.3±10.8 | 81.5±8.4 | 0.104 |
| A/B ratio; mean (s.d.) | 0.93±0.07 | 0.97±0.07 | 0.105 |
| Nighttime | |||
| Systolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 133.4±15.9 | 123.9±13.9 | 0.031 |
| A/B ratio; mean (s.d.) | 0.91±0.10 | 1.00±0.08 | 0.001 |
| Diastolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 77.8±10.7 | 71.8±7.8 | 0.013 |
| A/B ratio; mean (s.d.) | 0.90±0.11 | 0.98±0.10 | 0.024 |
| Morning | |||
| Systolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 144.6±16.8 | 138.9±16.9 | 0.107 |
| A/B ratio; mean (s.d.) | 0.95±0.11 | 1.02±0.15 | 0.095 |
| Diastolic BP | |||
| Baseline (mm Hg); mean (s.d.) | 84.1±12.2 | 80.9±10.5 | 0.340 |
| A/B ratio; mean (s.d.) | 0.95±0.12 | 1.03±0.13 | 0.068 |
Abbreviations: BP, blood pressure; A/B ratio, after-treatment/baseline ratio.
Effects of olmesartan add-on therapy on markers of renal function
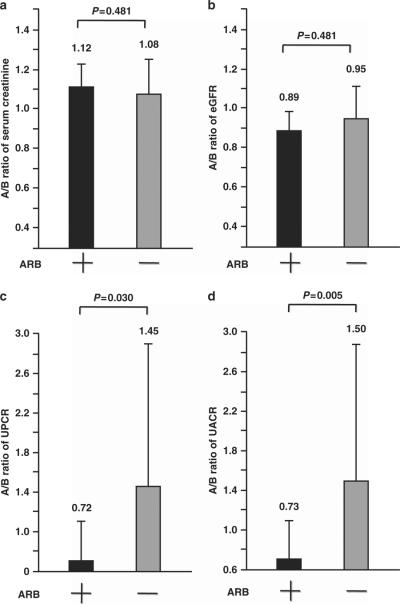
At baseline, serum creatinine, eGFR, UPCR and UACR were similar in the olmesartan add-on and non-ARB groups (Table 1). After the 16-week active treatment period, serum creatinine and eGFR A/B ratios were comparable in the two groups (Figure 1). In contrast, the UPCR and UACR A/B ratios were significantly lower in the olmesartan add-on group than in the non-ARB group (Figure 1; UPCR, 0.72±0.41 vs. 1.45±1.48, P = 0.030; UACR, 0.73±0.37 vs. 1.50±1.37, P = 0.005) after the 16-week active treatment period. Overall, the results of correlation analysis showed that there were significant positive associations between the A/B ratios of nighttime systolic BP and UPCR (R = 0.440, P = 0.004), and between the A/B ratios of nighttime systolic BP and UACR (R = 0.472, P = 0.002) in all patient groups.
Figure 1.
Effects of olmesartan add-on therapy on the after-treatment/baseline (A/B) ratios of (a) serum creatinine, (b) eGFR, (c) UPCR and (d) UACR. Forty-six hypertensive patients with CKD were randomly assigned to the olmesartan add-on group (ARB +) or the non-ARB group (ARB −). At baseline and after the 16-week treatment period, measurements were collected. To compare the effects of anti-hypertensive treatment in each group, the values of the variables after the 16-week active treatment period were normalized to the respective variables at baseline and were expressed as the A/B ratio. The values of the A/B ratios are expressed as the mean ± s.d.
Effects of olmesartan add-on therapy on markers of inflammation, the renal renin–angiotensin system, oxidative stress and fibrosis
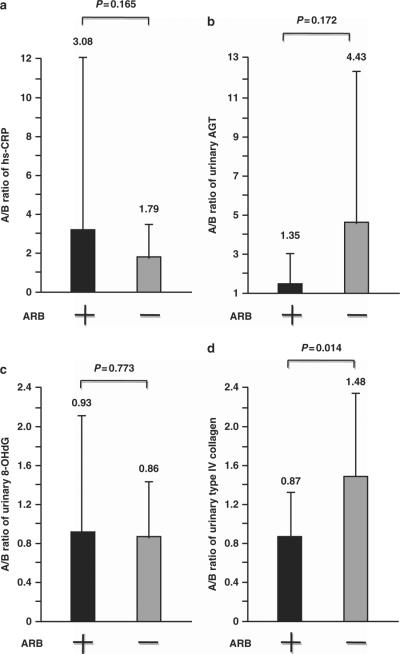
At baseline, high-sensitivity C-reactive protein (hs-CRP), urinary AGT, 8-OHdG and type IV collagen levels were similar in the olmesartan add-on and non-ARB groups (Table 1). The hs-CRP and urinary 8-OHdG A/B ratios after the 16-week active treatment period were comparable in both groups (Figure 2). The urinary AGT A/B ratio after the 16-week active treatment period was lower in the olmesartan add-on group than in the non-ARB group, but the difference did not reach statistical significance (Figure 2; urinary AGT, 1.35±1.59 vs. 4.43±7.39, P = 0.172). In contrast, the urinary type IV collagen A/B ratio following the 16-week treatment was significantly suppressed in the olmesartan add-on group compared with the non-ARB group (Figure 2; urinary type IV collagen, 0.87±0.42 vs. 1.48±0.87, P = 0.014). The correlation analysis demonstrated that there was a significant positive relationship between the A/B ratios of nighttime systolic BP and urinary type IV collagen (R = 0.451, P = 0.003), and further revealed a trend toward a positive association between the A/B ratios of nighttime systolic BP and urinary AGT (R = 0.332, P = 0.051).
Figure 2.
Effects of olmesartan add-on therapy on the after-treatment/baseline (A/B) ratios of (a) hs-CRP, (b) urinary AGT, (c) urinary 8-OHdG and (d) urinary type IV collagen. Forty-six hypertensive patients with CKD were randomly assigned to the olmesartan add-on group (ARB +) or the non-ARB group (ARB −). Measurements were collected at baseline and after the 16-week treatment period. To compare the effects of anti-hypertensive treatment in each group, the values of the variables after the 16-week active treatment period were normalized to those of the respective variables at baseline and are expressed as A/B ratios. The values of the A/B ratios are expressed as the mean ± s.d.
Comparison of renal function parameters between patients with and without ACEI treatment in the non-ARB group
As shown in Table 2, there was a significant difference in the percentage of patients in the olmesartan add-on group and non-ARB group that were prescribed ACEIs (14% vs. 57%, P = 0.003). Previous studies have shown that ACEIs exert renoprotective effects in CKD patients, and thus, ACEIs could also affect the renal function parameters measured in the present study.37 Therefore, we compared the renal function parameters between patients in the non-ARB group that were either treated with ACEIs (ACEI + patients) or not treated with ACEIs (ACEI − patients). In the non-ARB group, the UPCR and UACR in the ACEI − patients both showed an increasing trend after the 16-week active treatment period, and the UPCR and UACR A/B ratios after treatment in the ACEI − patients were higher than those in the ACEI + patients (Table 5; UPCR, 2.27±2.08 vs. 0.89±0.37, P = 0.060; UACR, 2.27±1.80 vs. 0.92±0.43, P = 0.015). These differences were not observed between ACEI + patients and ACEI − patients when the olmesartan add-on group and all of the subjects were analyzed (data not shown). These results support the renoprotective effects of ACEIs in hypertensive CKD patients.
Table 5.
Comparison of UPCR, UACR and urinary type IV collagen between ACEI+ patients and ACEI− patients in the non-ARB group
| Variable | ACEI+, n=13 | ACEI−, n=10 | P-value |
|---|---|---|---|
| UPCR | |||
| Baseline (g per g Cr); mean (s.d.) | 1.97±2.18 | 1.09±2.30 | 0.343 |
| A/B ratio; mean (s.d.) | 0.89±0.37 | 2.27±2.08 | 0.060 |
| UACR | |||
| Baseline (mg per g Cr); mean (s.d.) | 1511±1507 | 788±1730 | 0.123 |
| A/B ratio; mean (s.d.) | 0.92±0.43 | 2.27±1.80 | 0.015 |
| Urinary type IV collagen | |||
| Baseline (μg per g Cr); mean (s.d.) | 11.05±10.33 | 5.83±5.66 | 0.148 |
| A/B ratio; mean (s.d.) | 1.28±0.84 | 1.76±0.87 | 0.186 |
Abbreviations: A/B ratio, after-treatment/baseline ratio; ACEI, angiotensin-converting enzyme inhibitor; UACR, urinary albumin-to-creatinine ratio; UPCR, urinary protein-to-creatinine ratio.
DISCUSSION
Accumulated evidence suggests that elevated nighttime BP levels reflect target organ injury and are a better predictor of further cardiovascular and renal risk than daytime or 24-h BP levels in hypertensive patients with diabetes and CKD.6,38 In addition, efficient reduction in nighttime BP by methods such as bedtime dosing, as determined by ambulatory BP monitoring, has been shown to reduce cardiovascular risk in cases of resistant hypertension and hypertension complicated by diabetes or CKD.39–41 The results of the present study showed that olmesartan add-on therapy exerted a significantly better BP-lowering effect than non-ARB therapy, particularly during the nighttime period; similar daytime BP lowering was observed in the olmesartan add-on group and non-ARB group.
The results of this study show that both the olmesartan add-on therapy and non-ARB anti-hypertensive therapy were well tolerated in hypertensive patients with CKD. Although the baseline clinic BP levels were similar in the olmesartan add-on and non-ARB groups, the baseline 24-h and nighttime BP levels from the ambulatory BP recording were significantly higher in the olmesartan add-on group than the non-ARB group (Table 4). Thus, to strictly compare the effects of the two treatment regimens after the 16-week active treatment period, the values of the variables, including the ambulatory BP parameters, were normalized to those of the respective variables at baseline and were expressed as A/B ratios.
In this study, although more patients were treated with ACEIs in the non-ARB group than in the olmesartan add-on group, the olmesartan add-on therapy significantly decreased urinary protein, albumin and type IV collagen excretion. Thus, these data indicate that the benefit of olmesartan add-on therapy for patients with hypertension and CKD results from inhibition of proteinuria/albuminuria and pathological renal injury, and these beneficial effects of olmesartan are exerted, at least in part, through a preferential reduction in nocturnal BP, which is an important potential therapeutic target for cardiorenal protection in hypertension with CKD.
The role of the renin–angiotensin system in promoting hypertension- and CKD-related organ damage is well established. Reducing proteinuria/albuminuria is critically important for the regression of CKD,42 and the evidence indicates that ARBs and ACEIs reduce cardiovascular and renal risks primarily by reducing proteinuria/albuminuria.13,16,43 In a recent, large-scale ORIENT clinical trial, olmesartan treatment significantly decreased clinic BP and proteinuria compared with the control anti-hypertensive treatment that included ACEIs, and there was no renal dysfunction acceleration in type 2 diabetic patients with overt nephropathy.44 The present study included non-diabetic glomerulopathy patients as well as diabetic nephropathy patients. The results of the present study demonstrate that a preferential decrease in nighttime BP levels was accompanied by decreases in urinary protein and albumin excretion and urinary type IV collagen excretion, an important marker of renal injury in diabetic nephropathy and non-diabetic renal disease,45,46 without any decrease in eGFR resulting from the olmesartan add-on therapy. The results of the ACCOMPLISH study indicated that not only a reduction of albuminuria but also long-term preservation of eGFR are important for the suppression of CKD progression and cardiovascular complications.17,19 Therefore, it is likely that olmesartan effectively inhibits renal deterioration through improvement in the circadian BP rhythm and maintenance of eGFR, in addition to its general BP-lowering effect in hypertensive patients with CKD.
In contrast, proteinuria/albuminuria showed an increasing trend after the treatment period in the non-ARB group. We previously showed that urinary protein excretion correlated positively with nighttime BP in hypertensive CKD patients with diabetic nephropathy or non-diabetic glomerulopathy,25 and a recent study reported that nocturnal BP reduction is critically important for the inhibition of CKD progression.41 Therefore, the lack of a decrease in nighttime BP may have caused the increasing trend in proteinuria/albuminuria in the non-ARB group after treatment. In addition, proteinuria/albuminuria in the ACEI – patients in the non-ARB group showed an increasing trend after the 16-week active treatment period, and urinary protein/albumin excretion after treatment was higher in the ACEI – patients than in the ACEI + patients (Table 5) thereby also supporting the renoprotective effects of ACEIs in hypertensive CKD patients.
What might the underlying mechanism be that is responsible for the efficient nocturnal BP-lowering effects of olmesartan? In comparison with other ARBs, olmesartan is reported to exert a long-lasting BP-lowering effect via its characteristic `double-chain domain' structure20 and to efficiently improve the altered ambulatory BP profile in hypertensive patients, even if olmesartan is only administered once in the morning.21 In addition, sodium retention due to a reduced GFR and impaired renal sodium excretion capacity are characteristic pathophysiological states in hypertensive patients with CKD. Recent animal studies reported that activation of AT1R in the kidney mediates chronic hypertensive effects; by stimulation of the intrarenal renin-angiotensin system, sodium reabsorption from the renal tubules is promoted.47,48 Furthermore, previous clinical studies showed that the urinary AGT level closely reflects renal renin–angiotensin system activity in hypertension and CKD.36,49–51 In this regard, a recent study suggested that inhibition of renal sodium reabsorption may be an important mechanism involved in the olmesartan-mediated improvement in the circadian BP rhythm via a preferential lowering of nocturnal BP levels in CKD patients.52 Although the decrease in urinary AGT excretion by the olmesartan add-on therapy did not reach statistical significance in the current study, there was also a trend toward a positive relationship between nocturnal BP and urinary AGT. Therefore, olmesartan-mediated preferential reduction in nocturnal BP may be effected via the prevention of renal tubular sodium reabsorption through the inhibition of renal tubule AT1R.
The limitations of the present study include the background difference in ambulatory BP levels between the olmesartan add-on and non-ARB groups, although the clinic BP levels in these groups at baseline were comparable. To examine the effects of olmesartan add-on therapy on ambulatory BP levels, the values of the variables after the 16-week active treatment period were normalized to the respective variables at baseline and were expressed as A/B ratios. Furthermore, the sample size is relatively small, which limits our ability to determine significance.
In summary, the results of this study indicate that olmesartan add-on therapy improves the ambulatory BP profile by preferential reduction in nighttime BP and may afford protective renal effects in patients with hypertension and CKD. Further studies are needed to examine the mechanistic basis of the olmesartan-mediated therapeutic effects on ambulatory BP profiles and renal function.
ACKNOWLEDGEMENTS
This work was supported by a Health and Labor Sciences Research grant and by grants from the Japanese Ministry of Education, Science, Sports and Culture, the Salt Science Research Foundation (No. 1134), the Kidney Foundation, Japan (JKFB11-25) and the Novartis Foundation for Gerontological Research (2012). We thank Dr Boru for editing the English of this manuscript.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristiansson K, Lederballe-Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Wedel H. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- 2.Casas JP, Chua W, Loukogeorgakis S, Vallance P, Smeeth L, Hingorani AD, MacAllister RJ. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet. 2005;366:2026–2033. doi: 10.1016/S0140-6736(05)67814-2. [DOI] [PubMed] [Google Scholar]
- 3.Hodgkinson J, Mant J, Martin U, Guo B, Hobbs FD, Deeks JJ, Heneghan C, Roberts N, McManus RJ. Relative effectiveness of clinic and home blood pressure monitoring compared with ambulatory blood pressure monitoring in diagnosis of hypertension: systematic review. BMJ. 2011;342:d3621. doi: 10.1136/bmj.d3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lovibond K, Jowett S, Barton P, Caulfield M, Heneghan C, Hobbs FD, Hodgkinson J, Mant J, Martin U, Williams B, Wonderling D, McManus RJ. Cost-effectiveness of options for the diagnosis of high blood pressure in primary care: a modelling study. Lancet. 2011;378:1219–1230. doi: 10.1016/S0140-6736(11)61184-7. [DOI] [PubMed] [Google Scholar]
- 5.Tamura K, Kanaoka T, Ohsawa M, Haku S, Azushima K, Maeda A, Dejima T, Wakui H, Ozawa M, Shigenaga A, Toya Y, Umemura S. Emerging concept of anti-hypertensive therapy based on ambulatory blood pressure profile in chronic kidney disease. Am J Cardiovasc Dis. 2011;1:236–243. [PMC free article] [PubMed] [Google Scholar]
- 6.Minutolo R, Agarwal R, Borrelli S, Chiodini P, Bellizzi V, Nappi F, Cianciaruso B, Zamboli P, Conte G, Gabbai FB, De Nicola L. Prognostic role of ambulatory blood pressure measurement in patients with nondialysis chronic kidney disease. Arch Int Med. 2011;171:1090–1098. doi: 10.1001/archinternmed.2011.230. [DOI] [PubMed] [Google Scholar]
- 7.Spallone V, Bernardi L, Ricordi L, Solda P, Maiello MR, Calciati A, Gambardella S, Fratino P, Menzinger G. Relationship between the circadian rhythms of blood pressure and sympathovagal balance in diabetic autonomic neuropathy. Diabetes. 1993;42:1745–1752. doi: 10.2337/diab.42.12.1745. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Munemura M, Usami T, Nakao N, Takeuchi O, Kamiya Y, Yoshida A, Kimura G. Nocturnal blood pressure is elevated with natriuresis and proteinuria as renal function deteriorates in nephropathy. Kid Int. 2004;65:621–625. doi: 10.1111/j.1523-1755.2004.00419.x. [DOI] [PubMed] [Google Scholar]
- 9.Nakano S, Fukuda M, Hotta F, Ito T, Ishii T, Kitazawa M, Nishizawa M, Kigoshi T, Uchida K. Reversed circadian blood pressure rhythm is associated with occurrences of both fatal and nonfatal vascular events in NIDDM subjects. Diabetes. 1998;47:1501–1506. doi: 10.2337/diabetes.47.9.1501. [DOI] [PubMed] [Google Scholar]
- 10.Sturrock ND, George E, Pound N, Stevenson J, Peck GM, Sowter H. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000;17:360–364. doi: 10.1046/j.1464-5491.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- 11.Astrup AS, Nielsen FS, Rossing P, Ali S, Kastrup J, Smidt UM, Parving HH. Predictors of mortality in patients with type 2 diabetes with or without diabetic nephropathy: a follow-up study. J Hypertens. 2007;25:2479–2485. doi: 10.1097/HJH.0b013e3282f06428. [DOI] [PubMed] [Google Scholar]
- 12.Palmas W, Pickering TG, Teresi J, Schwartz JE, Moran A, Weinstock RS, Shea S. Ambulatory blood pressure monitoring and all-cause mortality in elderly people with diabetes mellitus. Hypertension. 2009;53:120–127. doi: 10.1161/HYPERTENSIONAHA.108.118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galle J. Reduction of proteinuria with angiotensin receptor blockers. Nat Clin Pract Cardiovasc Med. 2008;5:S36–S43. doi: 10.1038/ncpcardio0806. [DOI] [PubMed] [Google Scholar]
- 14.Ruggenenti P, Remuzzi G. Proteinuria: Is the ontarget renal substudy actually off target? Nat Rev Nephrol. 2009;5:436–437. doi: 10.1038/nrneph.2009.109. [DOI] [PubMed] [Google Scholar]
- 15.Ito S. Usefulness of ras inhibition depends on baseline albuminuria. Nat Rev Nephrol. 2010;6:10–11. doi: 10.1038/nrneph.2009.203. [DOI] [PubMed] [Google Scholar]
- 16.Holtkamp FA, de Zeeuw D, de Graeff PA, Laverman GD, Berl T, Remuzzi G, Packham D, Lewis JB, Parving HH, Lambers Heerspink HJ. Albuminuria and blood pressure, independent targets for cardioprotective therapy in patients with diabetes and nephropathy: a post hoc analysis of the combined RENAAL and IDNT trials. Eur Heart J. 2011;32:1493–1499. doi: 10.1093/eurheartj/ehr017. [DOI] [PubMed] [Google Scholar]
- 17.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, Hester A, Gupte J, Gatlin M, Velazquez EJ. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med. 2008;359:2417–2428. doi: 10.1056/NEJMoa0806182. [DOI] [PubMed] [Google Scholar]
- 18.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bakris GL, Sarafidis PA, Weir MR, Dahlof B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V, Chiang YT, Weber MA. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375:1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 20.Kiya Y, Miura S, Fujino M, Imaizumi S, Karnik SS, Saku K. Clinical and pharmacotherapeutic relevance of the double-chain domain of the angiotensin II type 1 receptor blocker olmesartan. Clin Exp Hypertens. 2010;32:129–136. doi: 10.3109/10641960903254430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smolensky MH, Hermida RC, Portaluppi F. Comparison of the efficacy of morning versus evening administration of olmesartan in uncomplicated essential hypertension. Chronobiol Int. 2007;24:171–181. doi: 10.1080/07420520600969277. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–992. doi: 10.1053/j.ajkd.2008.12.034. [DOI] [PubMed] [Google Scholar]
- 23.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, Jones DH, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans: an aha scientific statement from the council on high blood pressure research professional and public education subcommittee. J Clin Hypertens. 2005;7:102–109. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Tsurumi Y, Sakai M, Tanaka Y, Okano Y, Yamauchi J, Ishigami T, Kihara M, Hirawa N, Toya Y, Yabana M, Tokita Y, Ohnishi T, Umemura S. A possible relationship of nocturnal blood pressure variability with coronary artery disease in diabetic nephropathy. Clin Exp Hypertens. 2007;29:31–42. doi: 10.1080/10641960601096760. [DOI] [PubMed] [Google Scholar]
- 25.Tamura K, Yamauchi J, Tsurumi-Ikeya Y, Sakai M, Ozawa M, Shigenaga A, Azuma K, Okano Y, Ishigami T, Toya Y, Yabana M, Tokita Y, Ohnishi T, Umemura S. Ambulatory blood pressure and heart rate in hypertensives with renal failure: comparison between diabetic nephropathy and non-diabetic glomerulopathy. Clin Exp Hypertens. 2008;30:33–43. doi: 10.1080/10641960701813890. [DOI] [PubMed] [Google Scholar]
- 26.Mitsuhashi H, Tamura K, Yamauchi J, Ozawa M, Yanagi M, Dejima T, Wakui H, Masuda S, Azuma K, Kanaoka T, Ohsawa M, Maeda A, Tsurumi-Ikeya Y, Okano Y, Ishigami T, Toya Y, Tokita Y, Ohnishi T, Umemura S. Effect of losartan on ambulatory short-term blood pressure variability and cardiovascular remodeling in hypertensive patients on hemodialysis. Atherosclerosis. 2009;207:186–190. doi: 10.1016/j.atherosclerosis.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 27.Masuda S, Tamura K, Wakui H, Kanaoka T, Ohsawa M, Maeda A, Dejima T, Yanagi M, Azuma K, Umemura S. Effects of angiotensin II type 1 receptor blocker on ambulatory blood pressure variability in hypertensive patients with overt diabetic nephropathy. Hypertens Res. 2009;32:950–955. doi: 10.1038/hr.2009.131. [DOI] [PubMed] [Google Scholar]
- 28.Ozawa M, Tamura K, Okano Y, Matsushita K, Yanagi M, Tsurumi-Ikeya Y, Oshikawa J, Hashimoto T, Masuda S, Wakui H, Shigenaga A, Azuma K, Ishigami T, Toya Y, Ishikawa T, Umemura S. Identification of an increased short-term blood pressure variability on ambulatory blood pressure monitoring as a coronary risk factor in diabetic hypertensives. Clin Exp Hypertens. 2009;31:259–270. doi: 10.1080/10641960902822518. [DOI] [PubMed] [Google Scholar]
- 29.Ozawa M, Tamura K, Okano Y, Matsushita K, Ikeya Y, Masuda S, Wakui H, Dejima T, Shigenaga A, Azuma K, Ishigami T, Toya Y, Ishikawa T, Umemura S. Blood pressure variability as well as blood pressure level is important for left ventricular hypertrophy and brachial-ankle pulse wave velocity in hypertensives. Clin Exp Hypertens. 2009;31:669–679. doi: 10.3109/10641960903407033. [DOI] [PubMed] [Google Scholar]
- 30.Kanaoka T, Tamura K, Moriya T, Tanaka K, Konno Y, Kondoh S, Toyoda M, Umezono T, Fujikawa T, Ohsawa M, Dejima T, Maeda A, Wakui H, Haku S, Yanagi M, Mitsuhashi H, Ozawa M, Okano Y, Ogawa N, Yamakawa T, Mizushima S, Suzuki D, Umemura S. Effects of multiple factorial intervention on ambulatory BP profile and renal function in hypertensive type 2 diabetic patients with overt nephropathy - a pilot study. Clin Exp Hypertens. 2011;33:255–263. doi: 10.3109/10641963.2011.583971. [DOI] [PubMed] [Google Scholar]
- 31.Kario K, Pickering TG, Umeda Y, Hoshide S, Hoshide Y, Morinari M, Murata M, Kuroda T, Schwartz JE, Shimada K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: a prospective study. Circulation. 2003;107:1401–1406. doi: 10.1161/01.cir.0000056521.67546.aa. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Thijs L, Hansen TW, Kikuya M, Boggia J, Richart T, Metoki H, Ohkubo T, Torp-Pedersen C, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Sandoya E, Kawecka-Jaszcz K, Ibsen H, Imai Y, Wang J, Staessen JA. Prognostic value of the morning blood pressure surge in 5645 subjects from 8 populations. Hypertension. 2010;55:1040–1048. doi: 10.1161/HYPERTENSIONAHA.109.137273. [DOI] [PubMed] [Google Scholar]
- 33.Verdecchia P, Angeli F, Mazzotta G, Garofoli M, Ramundo E, Gentile G, Ambrosio G, Reboldi G. Day-night dip and early-morning surge in blood pressure in hypertension: prognostic implications. Hypertension. 2012;60:34–42. doi: 10.1161/HYPERTENSIONAHA.112.191858. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa S, Mori T, Nako K, Kato T, Takeuchi K, Ito S. Angiotensin II type 1 receptor blockers reduce urinary oxidative stress markers in hypertensive diabetic nephropathy. Hypertension. 2006;47:699–705. doi: 10.1161/01.HYP.0000203826.15076.4b. [DOI] [PubMed] [Google Scholar]
- 35.Katsurada A, Hagiwara Y, Miyashita K, Satou R, Miyata K, Ohashi N, Navar LG, Kobori H. Novel sandwich ELISA for human angiotensinogen. Am J Physiol - Ren Physiol. 2007;293:F956–F960. doi: 10.1152/ajprenal.00090.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konishi Y, Nishiyama A, Morikawa T, Kitabayashi C, Shibata M, Hamada M, Kishida M, Hitomi H, Kiyomoto H, Miyashita T, Mori N, Urushihara M, Kobori H, Imanishi M. Relationship between urinary angiotensinogen and salt sensitivity of blood pressure in patients with IgA nephropathy. Hypertension. 2011;58:205–211. doi: 10.1161/HYPERTENSIONAHA.110.166843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mann JF, Gerstein HC, Yi QL, Lonn EM, Hoogwerf BJ, Rashkow A, Yusuf S. Development of renal disease in people at high cardiovascular risk: results of the HOPE randomized study. J Am Soc Nephrol. 2003;14:641–647. doi: 10.1097/01.asn.0000051594.21922.99. [DOI] [PubMed] [Google Scholar]
- 38.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Sleep-time blood pressure as a therapeutic target for cardiovascular risk reduction in type 2 diabetes. Am J Hypertens. 2012;25:325–334. doi: 10.1038/ajh.2011.231. [DOI] [PubMed] [Google Scholar]
- 39.Ayala DE, Hermida RC, Portaluppi F, Smolensky MH. Bedtime hypertension treatment increases ambulatory blood pressure control and reduces cardiovascular risk in resistant hypertension. Hypertension. 2011;58:e26. doi: 10.1161/HYPERTENSIONAHA.111.178665. author reply e27. [DOI] [PubMed] [Google Scholar]
- 40.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Influence of time of day of blood pressure-lowering treatment on cardiovascular risk in hypertensive patients with type 2 diabetes. Diabetes Care. 2011;34:1270–1276. doi: 10.2337/dc11-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hermida RC, Ayala DE, Mojon A, Fernandez JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22:2313–2321. doi: 10.1681/ASN.2011040361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Remuzzi G, Benigni A, Remuzzi A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J Clin Invest. 2006;116:288–296. doi: 10.1172/JCI27699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haller H, Ito S, Izzo JL, Jr, Januszewicz A, Katayama S, Menne J, Mimran A, Rabelink TJ, Ritz E, Ruilope LM, Rump LC, Viberti G. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 44.Imai E, Chan JC, Ito S, Yamasaki T, Kobayashi F, Haneda M, Makino H. Effects of olmesartan on renal and cardiovascular outcomes in type 2 diabetes with overt nephropathy: a multicentre, randomised, placebo-controlled study. Diabetologia. 2011;54:2978–2986. doi: 10.1007/s00125-011-2325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Banu N, Hara H, Okamura M, Egusa G, Yamakido M. Urinary excretion of type IV collagen and laminin in the evaluation of nephropathy in NIDDM: comparison with urinary albumin and markers of tubular dysfunction and/or damage. Diabetes Res Clin Pract. 1995;29:57–67. doi: 10.1016/0168-8227(95)01119-x. [DOI] [PubMed] [Google Scholar]
- 46.Furumatsu Y, Nagasawa Y, Shoji T, Yamamoto R, Iio K, Matsui I, Takabatake Y, Kaimori JY, Iwatani H, Kaneko T, Tsubakihara Y, Imai E, Isaka Y, Rakugi H. Urinary type IV collagen in nondiabetic kidney disease. Nephron Clin Pract. 2011;117:c160–c166. doi: 10.1159/000319794. [DOI] [PubMed] [Google Scholar]
- 47.Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1a angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469–475. doi: 10.1016/j.cmet.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crowley SD, Zhang J, Herrera M, Griffiths R, Ruiz P, Coffman TM. Role of AT receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2011;301:F1124–F1130. doi: 10.1152/ajprenal.00305.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobori H, Ohashi N, Katsurada A, Miyata K, Satou R, Saito T, Yamamoto T. Urinary angiotensinogen as a potential biomarker of severity of chronic kidney diseases. J Am Soc Hypertens. 2008;2:349–354. doi: 10.1016/j.jash.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG. Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension. 2009;53:344–350. doi: 10.1161/HYPERTENSIONAHA.108.123802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishiyama A, Konishi Y, Ohashi N, Morikawa T, Urushihara M, Maeda I, Hamada M, Kishida M, Hitomi H, Shirahashi N, Kobori H, Imanishi M. Urinary angiotensinogen reflects the activity of intrarenal renin-angiotensin system in patients with IgA nephropathy. Nephrol Dial Transplant. 2011;26:170–177. doi: 10.1093/ndt/gfq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukuda M, Wakamatsu-Yamanaka T, Mizuno M, Miura T, Tomonari T, Kato Y, Ichikawa T, Miyagi S, Shirasawa Y, Ito A, Yoshida A, Kimura G. Angiotensin receptor blockers shift the circadian rhythm of blood pressure by suppressing tubular sodium reabsorption. Am J Physiol Renal Physiol. 2011;301:F953–F957. doi: 10.1152/ajprenal.00167.2011. [DOI] [PubMed] [Google Scholar]