Abstract
Brain cannabinoid CB1 receptors contribute to alcohol-related behaviors in experimental animals, but their potential role in humans with alcohol dependence is poorly understood. We measured CB1 receptors in alcohol dependent patients in early and protracted abstinence, and in comparison with control subjects without alcohol use disorders, using positron emission tomography (PET) and [18F]FMPEP-d2, a radioligand for CB1 receptors. We scanned 18 male inpatients with alcohol dependence twice, within 3–7 days of admission from ongoing drinking, and after 2–4 weeks of supervised abstinence. Imaging data were compared with those from 19 age-matched healthy male control subjects. Data were also analyzed for potential influence of a common functional variation (rs2023239) in the CB1 receptor gene (CNR1) that may moderate CB1 receptor density. On the first scan, CB1 receptor binding was 20–30% lower in patients with alcohol dependence than in control subjects in all brain regions and was negatively correlated with years of alcohol abuse. After 2–4 weeks of abstinence, CB1 receptor binding remained similarly reduced in these patients. Irrespective of diagnostic status, C allele carriers at rs2023239 had higher CB1 receptor binding compared to non-carriers. Alcohol dependence is associated with a widespread reduction of cannabinoid CB1 receptor binding in the human brain and this reduction persists at least 2–4 weeks into abstinence. The correlation of reduced binding with years of alcohol abuse suggests an involvement of CB1 receptors in alcohol dependence in humans.
Keywords: alcohol dependence, alcoholism, cannabinoid CB1 receptor, positron emission tomography, brain imaging, withdrawal, endocannabinoid
Introduction
Animal studies suggest that the brain cannabinoid system contributes to alcohol-related behaviors1–3. This system consists of endogenous cannabinoids such as anandamide and 2-arachidonoylglycerol, enzymes responsible for their synthesis and catabolism, and cannabinoid receptors of the CB1 and CB2 subtypes. The CB1 receptor is widely distributed in the human brain, with highest densities in basal ganglia, hippocampus, cingulate cortex, and the molecular layer of cerebellum4,5. Most CB1 receptors are pre-synaptic, and inhibit release of other neurotransmitters, such as γ-amino butyric acid and glutamate6,7.
Animal studies suggest that reinforcing properties of alcohol are in part mediated through the endocannabinoid system. Stimulation of CB1 receptors increases alcohol intake across a variety of rodent models8, whereas the opposite is observed with genetic9–11 or pharmacological8,12–14 CB1 receptor blockade. Chronic alcohol exposure increases the concentration of endogenous cannabinoids in most brain regions3 and decreases the density of CB1 receptors3,15–17, a change that is reversible upon abstinence16,17. Accordingly, a post-mortem study recently found decreased CB1 receptor density in the ventral striatum of patients with alcohol dependence18. Whether cannabinoid CB1 receptors are decreased in vivo in human subjects with alcohol dependence is unknown.
Functional genetic variation that moderates endocannabinoid signaling in response to alcohol might moderate susceptibility for excessive alcohol consumption. Accordingly, a common single nucleotide polymorphism (SNP), rs2023239, in the gene encoding CB1 receptors (CNR1) tags a haplotype associated with substance use disorders including alcohol dependence19. A recent study of patients with alcohol dependence linked the rs2023239 C allele with greater subjective reward from alcohol, greater midbrain and prefrontal cortex activation in response to alcohol cues, and higher density of CB1 receptors in post-mortem samples of prefrontal cortex20. Whether this SNP also moderates CB1 receptor density in vivo is unknown.
Here, we evaluated CB1 receptor binding in patients with alcohol dependence and controls without alcohol use disorders. We used positron emission tomography (PET) and a recently developed inverse agonist radioligand for CB1 receptors, [18F]FMPEP-d221,22, with high affinity and selectivity in human brain21. Patients were scanned twice: within 3–7 days of admission from ongoing alcohol use, and after 2–4 weeks of abstinence on a monitored unit. Based on animal experiments8, we hypothesized that CB1 receptor binding would be decreased in patients with alcohol dependence immediately after ongoing alcohol use. Based on human post-mortem data20, we predicted that the C allele of rs2023239 would be associated with higher CB1 receptor binding. Finally, we asked whether a hypothesized decrease in CB1 receptor binding in patients with alcohol dependence recovers after abstinence.
Subjects and methods
The NIH CNS Institutional Review Board approved the protocol and the consent forms. Written informed consent was obtained from all subjects.
Subjects
Detailed eligibility criteria can be found at clinicaltrials.gov (NCT00816439).
Patients (N=18) were males with alcohol dependence according to DSM-IV23, established by structured diagnostic interviews (SCID)24, and alcohol consumption within the last month (Table 1). Subjects did not have other substance use disorders except nicotine dependence, as determined by the SCID. Urine samples on admission were negative for cannabinoids, opiates, amphetamines, cocaine metabolites, and benzodiazepines. Patients were admitted to an inpatient unit at the NIH Clinical Center, and assessed for severity of alcohol dependence (Alcohol Dependence Scale, ADS25), alcohol use in the previous 90 days (Time-Line Follow Back, TLFB26), alcohol craving (Penn Alcohol Craving Scale, PACS27) and symptoms of depression and anxiety (Comprehensive Psychopathology Rating Scale, CPRS28). Withdrawal intensity was assessed with the Clinical Institute Withdrawal Assessment Scale (CIWA-Ar29). Ten (56%) patients received oxazepam to relieve withdrawal symptoms. The cumulative oxazepam dose (mean: 273 mg, range: 30–540 mg) was used to control for possible medication effects. Thirteen of the patients (72%) smoked cigarettes. Throughout the study, all subjects participated in a standard behavioral inpatient alcohol rehabilitation program, but did not receive any prescription medications other than benzodiazepines as described above and vitamin B1 (thiamine) supplementation according to clinical guidelines.
Table 1.
Demographic, clinical, and radiochemical information of the study participants.
| Alcohol | Control | P value | |
|---|---|---|---|
|
|
|||
| Number of subjects | 18 | 19 | |
| Age (years) | 44 ± 10 | 40 ± 8 | 0.251 |
| Body mass index (kg/m2) | 27 ± 4 | 28 ± 5 | 0.738 |
| Tobacco smokers/non-smokers (N) | 11/7 | 2/17 | 0.001 |
| Amount of alcohol use (drinks/day, TLFB) | 18 ± 6 | <1 | <0.001 |
| Alcohol Dependence Severity (ADS) | 20 ± 6 | <1 | <0.001 |
| Baseline craving (PACS) | 12 ± 8 | 1 ± 2 | <0.001 |
| Baseline peak withdrawal (CIWA) | 9 ± 5 | N.A. | N.A. |
| Injected activity of [18F]FMPEP-d2 (MBq) | 187 ± 10 | 181 ± 12 | 0.103 |
| Injected amount of [18F]FMPEP-d2 (nmol) | 1.7 ± 0.9 | 1.9 ± 0.8 | 0.547 |
| Fraction of free [18F]FMPEP-d2 in plasma (%) | 0.38 ± 0.1 | 0.39 ± 0.2 | 0.886 |
Values are number, mean ± standard deviation, or range. N.A., not applicable.
Healthy male controls (N=19) were free of somatic and psychiatric illness as confirmed by history, physical examination, structured diagnostic interviews (SCID, full version) electrocardiogram, and blood and urine tests. Control subjects did not have current or lifetime history of alcohol use or other substance use disorders, and had urine samples negative for cannabinoids, opiates, amphetamines, cocaine metabolites, and benzodiazepines. Control subjects completed assessments of alcohol use and severity of alcohol dependence as described for the patients. None of the controls were total abstainers, but 7 out of 19 (37%) reported no alcohol use in the past 90 days. Two control subjects (11%) smoked cigarettes (Table 1.).
Genotyping
Genotyping for CNR1 rs202039 was done largely as described previously20 and in detail in Supplementary Methods online.
Positron emission tomography and measurement of parent radioligand in arterial plasma
[18F]FMPEP-d2 was prepared as described previously22 and in detail in our Investigational New Drug Application 105,198, submitted to the U.S. Food and Drug Administration (available at http://pdsp.med.unc.edu/snidd/). The radioligand was obtained in high radiochemical purity (>99%) and had a specific radioactivity of 110 ± 43 MBq/nmol at time of injection.
After intravenous injection of [18F]FMPEP-d2 (Table 1), images were acquired for 120 min using an Advance camera (GE Healthcare) as previously described21. Arterial blood samples were drawn as previously described30. Plasma time-activity curve was corrected for the fraction of unchanged radioligand by radio-high-performance liquid chromatography separation31, and the plasma free fraction was measured using ultrafiltration32.
PET images were analyzed by applying a template of volumes of interest33 as implemented in PMOD, version 3.0 (PMOD Technologies Ltd)34, in the standard stereotactic space35 as previously described30. Distribution volume (VT) was estimated according to the two-tissue compartmental model36 with concentration of parent radioligand in plasma as input function, using PMOD, as previously described21. To assess the potential confound of brain atrophy, PET images were corrected for partial volume effects using the Rousset method implemented in PVElab software37. Statistical parametric mapping of VT values at voxel level was done using SPM8 as previously described30.
Statistical analysis of VT data
Data were analyzed using SPSS Statistics 17.0 for Windows (Release 17.0.0, copyright SPSS Inc., 1993–2007). VT values were normally distributed except for 4 out of 18 brain regions in the patient group (Shapiro-Wilk test). Therefore, results were confirmed with non-parametric Mann-Whitney U tests. Variance was homogeneous across groups (Levene’s test). To test whether CB1 receptors were decreased in patients with alcohol dependence at baseline, we first applied a mixed model two-way analysis of variance (ANOVA), with group status (alcohol dependence vs. control) as between-subject factor and brain region as within-subject factor. Body mass index (BMI) entered the model as a covariate since it affects VT30. To assess the contribution of the CNR1 rs2023239 SNP, this factor was then entered into the model. Correlations with clinical variables were assessed with Pearson’s correlation coefficient. To test whether CB1 receptors increased after abstinence, we applied a two-way ANOVA with time point (early vs. protracted abstinence) and brain region as within-subject factors. Potential effects of genotype were also evaluated with time × genotype interaction. P-values smaller than 0.05 were considered statistically significant.
Results
Overall, VT of [18F]FMPEP-d2 was lower in patients with alcohol dependence than in control subjects (main effect of group: F[1, 34]=10.1, p=0.003). A decrease was seen in all brain regions examined, but its magnitude varied significantly between regions (group × region interaction: F[3, 103]=4.21, p=0.007; Fig. 1); decrease in BMI-adjusted VT ranged from 21% in hippocampus to 35% in white matter. This finding was confirmed by an independent statistical parametric mapping analysis of voxel-wise VT values (Fig. 2) and a non-parametric Mann-Whitney U test (all p<0.019). The main effect of diagnostic status was also significant when analyzed without correcting for BMI (F[1, 35]=9.36, p=0.004). Among patients with alcohol dependence, years of alcohol use correlated negatively with VT in midbrain (R2=0.28, p=0.023), posterior cingulate cortex (R2=0.37, p=0.008) (Supplementary Fig. 1), and putamen (R2=0.26, p=0.030); patients who had been drinking longer had lower VT than those who had been drinking for a shorter period. This analysis was not confounded by age, since age did not correlate with VT in control subjects30. Whole brain VT did not correlate with alcohol dependence severity (ADS) (R2=0.02, p=0.56), measures of craving (R2=0.08, p=0.26), average drinks per day (R2=0.01, p=0.97), peak withdrawal score (R2=0.16, p=0.10), benzodiazepine dose received (R2=0.05, p=0.38) or ratings of depression (R2=0.01, p=0.92) or anxiety (R2=0.01, p=0.90).
Figure 1.
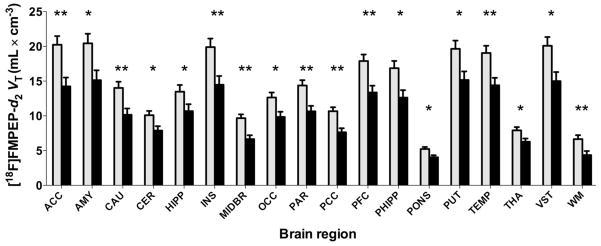
At baseline (within 3–7 days of ongoing alcohol use), of VT [18F]FMPEP-d2 is lower in patients with alcohol dependence (black bars, n=18) than in control subjects (gray bars, n=19). Values are estimated marginal means from the repeated measures analysis variance and adjusted to an average BMI of 27.3 kg/m2. Error bars are standard error of the mean. ACC, anterior cingulate cortex; AMY, amygdala; CAU, caudate nucleus; CER, cerebellum; HIPP, hippocampus; INS, insula; MIDBR, midbrain; OCC, occipital cortex; PAR, parietal cortex; PCC, posterior cingulate cortex; PFC, prefrontal cortex; PHIPP, parahippocampal gyrus; PUT, putamen; TEMP, lateral temporal cortex; THA, thalamus; VST, ventral striatum; WM, white matter; *p<0.05; **p<0.005, two-tailed t-test.
Figure 2.
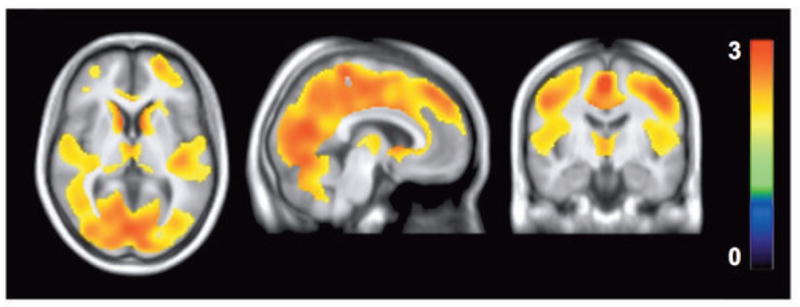
Statistical parametric mapping (SPM) analysis shows lower VT in patients with alcohol dependence (n=18) than in control subjects (n=19) at baseline as a large single cluster. This cluster comprised of 81,026 voxels and had a maximum t value of 3.2 at [0, −34, 60] and a cluster-level family-wise error (FWE) corrected p value of 0.038. Color bar represents t value in each voxel within the significant cluster.
Among control subjects and patients, 28% and 39% carried the rs2023239 C allele, respectively (χ2=0.50; p=0.48). When we included genotype as a factor in the analysis, the main effect of group remained (F[1, 31]=7.72, p=0.009), and a main genotype effect was found (F[1, 31]=4.44, p=0.043). There was no significant group × genotype (F[1, 31]=1.47, p=0.24) or group × genotype × region (F[3, 96]=2.00, p=0.12) interaction, suggesting that genotype affected CB1 binding similarly in both groups. Carriers of the C allele had 31% higher VT than non-carriers among patients with alcohol dependence and 19% among control subjects (Fig. 3). There were no statistically significant differences between carriers and non-carriers of the C allele in any of the demographic, or in the clinical variables among patients with alcohol dependence.
Figure 3.
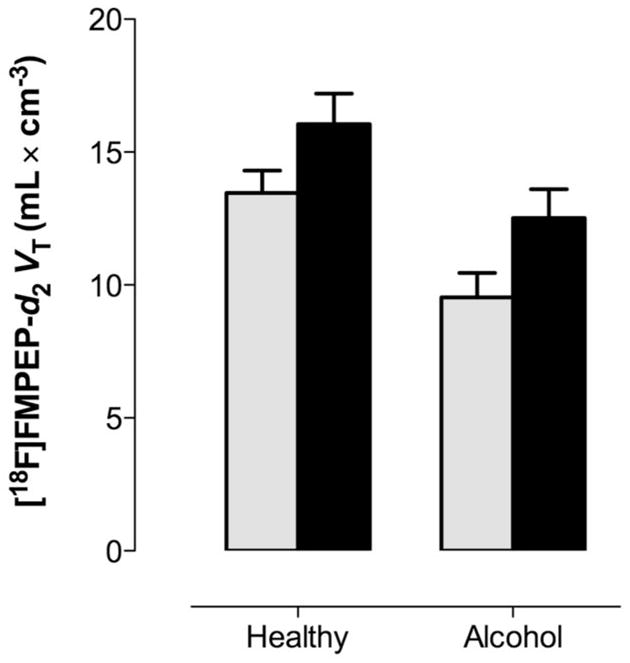
Variation in rs2023239 in the CNR1 gene regulates whole brain VT of [18F]FMPEP-d2: VT carriers of the C allele (black bars) have higher than non-carriers (gray bars), irrespective of diagnostic status.
More patients with alcohol dependence (61%) than control subjects (11%) smoked cigarettes. However, smoking had no effect on VT among patients with alcohol dependence (main effect: F[1, 33]=0.01, p=0.93; smoking × region interaction: F[3, 99]=0.34, p=0.80). Furthermore, lower VT in patients with alcohol dependence than in control subjects remained significant even after smoking was included in the statistical model (main effect of group: F[1, 33]=7.32, p=0.011). Therefore, cigarette smoking was unlikely to significantly confound the main finding.
To control for a potential confound of brain atrophy in the patient group, we corrected the PET data for partial volume effects. After correction, group difference in VT remained significant (main effect of group: F[1, 34]=8.42, p=0.006; group × region interaction: F[3, 99]=3.79, p=0.011).
Finally, to determine whether decreased VT in patients with alcohol dependence is reversible, we repeated PET measurements after 19 ± 4 days of abstinence (range 14–28 days). After protracted abstinence, VT did not significantly change in any brain region (main effect of time: F[1, 17]=1.18, p=0.29; time × region interaction: F[3, 49]=0.71, p=0.55), and thus, remained significantly decreased (Fig. 4.). The average value of whole brain VT increased by only +5%. Change after abstinence remained not significant after controlling for rs2023239 genotype (main effect of time: F[1, 16]=0.83, p=0.38; time × genotype interaction: F[1, 16]=0.34, p=0.57). Change in whole brain VT correlated positively with baseline Alcohol Dependence Score (R2=0.27, p=0.027) (Supplementary Figure 2.), but not with other clinical variables at baseline, with change in clinical variables after abstinence, or with interval between two PET scans. Fraction of free radioligand in arterial plasma did not change after abstinence (p=0.75).
Figure 4.
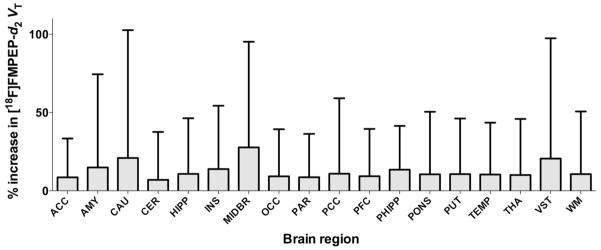
VT of [18F]FMPEP-d2 did not change after abstinence in patients with alcohol dependence. Error bars are standard deviation of percent change. Abbreviations of brain regions are same as those in Figure 1.
Discussion
When evaluated within one week of admission from ongoing alcohol use, patients with alcohol dependence had 20–30% lower VT of [18F]FMPEP-d2 in all brain regions compared to control subjects without substance use or other psychiatric disorders, consistent with a wide-spread downregulation of CB1 receptors. This downregulation was likely related to long-term alcohol exposure, because it was more pronounced in patients who had used alcohol longer than in those who had done so for a shorter time, while no effect of age per se was observed in controls. VT of [18F]FMPEP-d2 remained similarly lowered in the alcohol dependent patients after 2–4 weeks of abstinence. This lack of reversibility is in contrast with the reversible downregulation of cortical CB1 receptors recently found in subjects who chronically abuse cannabis30, and suggests a more persistent neuroadaptive change in CB1 receptors in alcohol dependence. We also demonstrated a significant effect of genetic variation at rs2023239, a CNR1 SNP previously associated with risk for substance use disorders including alcohol dependence19, with brain responses to alcohol associated cues in functional imaging, and with cortical CB1 receptor density on post mortem analysis20. Consistent with the post mortem binding data, carriers of the C allele in our study had higher VT of [18F]FMPEP-d2 than non-carriers in vivo, providing additional support for the validity of our data.
Decreased VT of [18F]FMPEP-d2 in patients with alcohol dependence was unlikely caused by methodological confounds. First, potential alterations in peripheral distribution or metabolism of the radioligand in patients with alcohol dependence did not affect our results, because the outcome measure VT corrects for these effects. Second, only free radioligand (not bound to plasma proteins) is able to enter the brain, while the fraction of free radioligand was similar between the groups (Table 1). Third, about 90% of [18F]FMPEP-d2 VT in monkey brain represents specific binding to CB1 receptors21; therefore, changes in non-specific binding (e.g., to brain lipids) would be unlikely to confound the main finding. Fourth, brain atrophy in patients with alcohol dependence may have “diluted” the PET signal via partial volume effects. However, the group differences remained similar even after correction for partial volume effect based on structural images. Fifth, cigarette smoking was more prevalent among patients with alcohol dependence than among control subjects. Yet, the main finding persisted after controlling for smoking in the statistical model, and VT was not different between smoking and non-smoking patients. This is in agreement with our recent observation that cigarette smoking did not influence [18F]FMPEP-d2 VT in people who chronically abuse cannabis30. Sixth, a lack of correlation between withdrawal intensity, benzodiazepine dose, and VT of [18F]FMPEP-d2, as well as the persistence of the findings 2–4 weeks after admission makes a confound by these factors unlikely.
Finally, we considered whether differential displacement of the radioligand [18F]FMPEP-d2 by endogenous cannabinoids in patients compared to control subjects could confound our results30. Because some PET radioligands can be displaced by endogenous neurotransmitters, we have previously tested the effects of cannabinoid agonists on the binding of [11C]MePPEP, a closely related analog, in rat brain38. In that study, we used high doses of cannabinoid agonists (anandamide, methanandamide, or CP 55,940) or an inhibitor of fatty acid amide hydrolyse (FAAH), the main enzyme that breaks down endocannabinoids. Although rimonabant, an inverse CB1 agonist, potently displaced [11C]MePPEP, none of the direct agonists did so. Importantly, potentiation of endogenous cannabinoid transmission by FAAH was also without effect. These findings suggests that [11C]MePPEP, a close structural analog of [18F]FMPEP-d2 used in the present study, is not displaced by direct CB1 agonists, including the endogenous ligand anandamide38. Based on these observations, we believe it unlikely that differential displacement of our PET ligand by endocannabinoids accounts for the differences observed between patients and controls in the present study. Nevertheless, additional studies would be required to directly determine whether [18F]FMPEP-d2 can be displaced by endogenous (and exogenous) CB1 receptor agonists in humans.
Animal studies utilizing genetic inactivation as well as pharmacological blockade of CB1 receptors have consistently shown that reinforcement of alcohol consumption is in part mediated by endocannabinoids acting at this receptor3,8. Accordingly, chronic alcohol exposure has been shown to result in elevated tissue levels of endocannabinoids3, while more recent microdialysis experiments have directly shown that self-administration of alcohol is associated with increased central levels of extracellular endocannabinoids, and that this increase is correlated with the amount of alcohol consumed39. Consistent with increased endocannabinoid activity resulting from alcohol consumption, most animal studies have found downregulation of CB1 receptors in several brain regions after chronic alcohol exposure15–17 (although see Ref.40 for a negative finding). One post mortem human study also found decreased CB1 receptor protein in the ventral striatum of alcoholics18. Our present finding that long-term alcohol exposure in alcohol dependence is associated with downregulation of CB1 receptors in vivo thus represents a direct translation of the animal data. Our findings diverge from the animal studies in that those found evidence of reversibility after 1 day16 and 40 days17. A likely reason for this divergence is the difference in duration of alcohol exposure, which was 3–60 days in the animal studies and 5–50 years in our patients.
Our finding of downregulated CB1 receptors in alcohol dependence is consistent with a conceptualization of this condition as an allostatic process41,42. According to this framework, alcohol is initially consumed for its pleasurable, positively reinforcing properties, but neuroadaptions to continued heavy alcohol use gradually reduce these alcohol actions. Because of the established role of CB1 receptors in positive reinforcement from alcohol, their downregulation with prolonged heavy alcohol use is a plausible candidate mechanism that might contribute to this process. While the positively reinforcing effects of alcohol are gradually lost with time, brain stress systems become overactive, and alcohol use becomes pursued in large part to ameliorate negative emotionality and promote stress coping42. Animal studies have shown that CB1 receptors have a critical role in modulating stress responses through actions in basolateral amygdala and hippocampus43,44. Downregulation of CB1 receptors in these structures in later stages of alcohol dependence can therefore be expected to contribute to enhanced negative emotionality and impaired stress coping. This in turn would provide an incentive for resumption of alcohol intake to restore endocannabinoid signaling and its ability to promote stress coping to normal levels. In this context, we note that the CB1 receptor inverse agonist rimonabant was not effective when evaluated in chronic alcohol dependence45,46. Several factors may have contributed to these negative results, but one possibility is that a blockade by rimonabant of positively reinforcing properties of alcohol was in part offset by an increased incentive for consumption in order to restore affective homeostasis. If this interpretation is correct, augmenting rather than inhibiting endogenous cannabinoid function (e.g., by inhibiting their metabolism47) might be of interest for treatment of alcohol dependence. First, augmented endocannabinoid function might restore affective homeostasis in the absence of alcohol, thereby reducing or eliminating the incentive to consume alcohol for its negatively reinforcing properties. Secondly, and more generally, CB1 receptor stimulation reduces glutamate release, and is therefore neuroprotective7. Chronic alcohol dependence is characterized by a hyperglutamatergic state48 and CB1 receptor stimulation protects against glutamatergic excitotoxic cell death during alcohol withdrawal49.
In a direct in vivo correlate of post mortem ligand binding data20, we found higher CB1 ligand binding in carriers of the C allele at the rs2023239 CNR1 SNP, irrespective of diagnostic group. Genetic association data suggest a small but significant contribution of this allele to the risk for substance use disorders, including alcohol dependence19. This risk increase may be mechanistically accounted for by the observations that carriers of the C allele exhibit greater brain responses to alcohol associated cues and greater alcohol-induced reward, presumably in part via higher CB1 receptor density20. As indicated above, rewarding, or positively reinforcing properties of alcohol are likely most important in early stages of alcohol dependence, and enhanced reward from alcohol due to a gain-of-function mutation at the CNR1 locus would be expected to increase alcoholism risk. In later stages of alcohol dependence, alcohol reward becomes less important, stress systems are activated, and restored CB1 receptor function may be beneficial by counteracting negative emotionality and glutamate excitotoxicity.
In conclusion, we found that CB1 receptor binding is decreased in patients with alcohol dependence and that this downregulation persists several weeks into abstinence. We also found that the C allele of the rs2023239 locus is associated with higher CB1 receptor binding in vivo, similar to what has been found on post mortem analysis. Our findings suggest that CB1 receptor plays a different role in early vs. late phases of alcohol dependence. A potential implication of our findings is that enhanced, rather than blocked CB1 signaling may be beneficial in late stage, treatment seeking alcohol dependent patients.
Supplementary Material
Correlation between duration of alcohol use and VT of [18F]FMPEP-d2 in posterior cingulate cortex in patients with alcohol dependence. Curved dashed lines are bounds for the 95% confidence interval of the regression line.
Alcohol Dependence Scores at baseline correlated positively with change in VT of [18F]FMPEP-d2 after abstinence in the insular cortex: patients with higher scores had higher increase in VT than those with lower scores. Insular cortex is representative of all brain regions.
Acknowledgments
We thank Kimberly Jenko, Kacey Anderson, and David Clark for assisting in the measurements of radioligand in plasma; Maria D. Ferraris Araneta, Gerald Hodges, William C. Kreisl, and Barbara Scepura as well as Chris Geyer and the NIAAA nursing staff for subject recruitment and care; the NIH PET Department for imaging; and PMOD Technologies for providing its image analysis and modeling software. This research was supported by the Intramural Programs of NIMH and NIAAA. Jussi Hirvonen was supported by personal grants from The Academy of Finland; The Finnish Cultural Foundation; The Finnish Foundation for Alcohol Studies; The Finnish Medical Foundation; The Instrumentarium Foundation; The Jalmari and Rauha Ahokas Foundation; The Paulo Foundation; The Research Foundation of Orion Corporation; and The Yrjö Jahnsson Foundation.
This research was supported by the Intramural Programs of NIMH (project # Z01-MH-002852-04) and NIDA (project # Z01-DA000413-13). Jussi Hirvonen was supported by personal grants from The Academy of Finland; The Finnish Cultural Foundation; The Finnish Foundation for Alcohol Studies; The Finnish Medical Foundation; The Instrumentarium Foundation; The Jalmari and Rauha Ahokas Foundation; The Paulo Foundation; The Research Foundation of Orion Corporation; and The Yrjö Jahnsson Foundation.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Supplementary information is available at Molecular Psychiatry’s website.
ClinicalTrials.gov Identifier: NCT00816439
References
- 1.Maccioni P, Colombo G, Carai MA. Blockade of the cannabinoid CB1 receptor and alcohol dependence: preclinical evidence and preliminary clinical data. CNS Neurol Disord Drug Targets. 2010;9:55–59. doi: 10.2174/187152710790966623. [DOI] [PubMed] [Google Scholar]
- 2.Mechoulam R, Parker L. Cannabis and alcohol - a close friendship. Trends in Pharmacological Sciences. 2003;24:266–268. doi: 10.1016/S0165-6147(03)00107-X. [DOI] [PubMed] [Google Scholar]
- 3.Basavarajappa BS. The endocannabinoid signaling system: a potential target for next-generation therapeutics for alcoholism. Mini Rev Med Chem. 2007;7:769–779. doi: 10.2174/138955707781387920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid Receptor Localization in Brain. Proceedings of the National Academy of Sciences. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass M, Dragunow M, Faull RL. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience. 1997;77:299–318. doi: 10.1016/s0306-4522(96)00428-9. [DOI] [PubMed] [Google Scholar]
- 6.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 7.Wilson RI, Nicoll RA. Endocannabinoid Signaling in the Brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- 8.Colombo G, Serra S, Vacca G, Carai M, Gessa G. Endocannabinoid system and alcohol addiction: Pharmacological studies. Pharmacology Biochemistry and Behavior. 2005;81:369–380. doi: 10.1016/j.pbb.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Hungund BL, Szakall I, Adam A, Basavarajappa BS, Vadasz C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Liu J, Harvey-White J, Zimmer A, Kunos G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proceedings of the National Academy of Sciences. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Racz I, Bilkei-Gorzo A, Toth ZE, Michel K, Palkovits M, Zimmer A. A critical role for the cannabinoid CB1 receptors in alcohol dependence and stress-stimulated ethanol drinking. J Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20:1109–1114. [PubMed] [Google Scholar]
- 13.Arnone M, Maruani J, Chaperon F, Thiebot MH, Poncelet M, Soubrie P, et al. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology (Berl) 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- 14.Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, et al. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR–141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 15.Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 16.Vinod K, Yalamanchili R, Xie S, Cooper T, Hungund B. Effect of chronic ethanol exposure and its withdrawal on the endocannabinoid system. Neurochemistry International. 2006;49:619–625. doi: 10.1016/j.neuint.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 17.Mitrirattanakul S, López-Valdés HE, Liang J, Matsuka Y, Mackie K, Faull KF, et al. Bidirectional Alterations of Hippocampal Cannabinoid 1 Receptors and Their Endogenous Ligands in a Rat Model of Alcohol Withdrawal and Dependence. Alcoholism: Clinical and Experimental Research. 2007;31:855–867. doi: 10.1111/j.1530-0277.2007.00366.x. [DOI] [PubMed] [Google Scholar]
- 18.Vinod KY, Kassir SA, Hungund BL, Cooper TB, Mann JJ, Arango V. Selective alterations of the CB1 receptors and the fatty acid amide hydrolase in the ventral striatum of alcoholics and suicides. Journal of Psychiatric Research. 2010;44:591–597. doi: 10.1016/j.jpsychires.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang PW, Ishiguro H, Ohtsuki T, Hess J, Carillo F, Walther D, et al. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Molecular Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison KE, Haughey H, Niculescu M, Schacht J, Kaiser A, Stitzel J, et al. The incentive salience of alcohol: translating the effects of genetic variant in CNR1. Arch Gen Psychiatry. 2008;65:841–850. doi: 10.1001/archpsyc.65.7.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry GE, Hirvonen J, Liow JS, Zoghbi SS, Gladding R, Tauscher JT, et al. Imaging and quantitation of cannabinoid CB1 receptors in human and monkey brains using (18)F-labeled inverse agonist radioligands. J Nucl Med. 2010;51:112–120. doi: 10.2967/jnumed.109.067074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donohue SR, Krushinski JH, Pike VW, Chernet E, Phebus L, Chesterfield AK, et al. Synthesis, ex vivo evaluation, and radiolabeling of potent 1,5-diphenylpyrrolidin-2-one cannabinoid subtype-1 receptor ligands as candidates for in vivo imaging. J Med Chem. 2008;51:5833–5842. doi: 10.1021/jm800416m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diagnostic and statistical manual of mental disorders (4th ed., text revision) American Psychiatric Association; Washington, D.C: 2000. [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Clinician Version (SCID-CV) American Psychiatric Press, Inc; Washington, D.C: 1996. [Google Scholar]
- 25.Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- 26.Sobell LC, Sobell MB. Timeline Follow-back: A technique for assessing self-reported ethanol consumption. In: Allen J, Litten R, editors. Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Humana Press; Totowa, N.J: 1992. pp. 41–72. [Google Scholar]
- 27.Flannery BA, Volpicelli JR, Pettinati HM. Psychometric properties of the Penn Alcohol Craving Scale. Alcoholism, clinical and experimental research. 1999;23:1289–1295. [PubMed] [Google Scholar]
- 28.Svanborg P. State and trait measures in the affective disorders. Karolinska Institute, Department of Clinical Neuroscience, Psychiatry Institute; Stockholm: 1999. [Google Scholar]
- 29.Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- 30.Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, Morse C, et al. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Molecular Psychiatry. 2011 doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gandelman MS, Baldwin RM, Zoghbi SS, Zea-Ponce Y, Innis RB. Evaluation of ultrafiltration for the free-fraction determination of single photon emission computed tomography (SPECT) radiotracers: beta-CIT, IBF, and iomazenil. J Pharm Sci. 1994;83:1014–1019. doi: 10.1002/jps.2600830718. [DOI] [PubMed] [Google Scholar]
- 32.Zoghbi SS, Shetty HU, Ichise M, Fujita M, Imaizumi M, Liow JS, et al. PET imaging of the dopamine transporter with 18F-FECNT: a polar radiometabolite confounds brain radioligand measurements. J Nucl Med. 2006;47:520–527. [PubMed] [Google Scholar]
- 33.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 34.Burger C, Mikolajczyk K, Grodzki M, Rudnicki P, Szabatin M, Buck A. Java tools for quantitative post-processing of brain PET data. Journal of Nuclear Medicine. 1998;39:277p–278p. [Google Scholar]
- 35.Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith C, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- 36.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 37.Quarantelli M, Berkouk K, Prinster A, Landeau B, Svarer C, Balkay L, et al. Integrated software for the analysis of brain PET/SPECT studies with partial-volume-effect correction. Journal of Nuclear Medicine. 2004;45:192–201. [PubMed] [Google Scholar]
- 38.Terry G, Liow J, Chernet E, Zoghbi S, Phebus L, Felder C, et al. Positron emission tomography imaging using an inverse agonist radioligand to assess cannabinoid CB1 receptors in rodents. Neuroimage. 2008;41:690–698. doi: 10.1016/j.neuroimage.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caille S, Alvarez-Jaimes L, Polis I, Stouffer DG, Parsons LH. Specific Alterations of Extracellular Endocannabinoid Levels in the Nucleus Accumbens by Ethanol, Heroin, and Cocaine Self-Administration. Journal of Neuroscience. 2007;27:3695–3702. doi: 10.1523/JNEUROSCI.4403-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend. 2002;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- 41.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 42.Koob GF. A Role for Brain Stress Systems in Addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hill MN, Patel S, Campolongo P, Tasker JG, Wotjak CT, Bains JS. Functional interactions between stress and the endocannabinoid system: from synaptic signaling to behavioral output. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14980–14986. doi: 10.1523/JNEUROSCI.4283-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hill MN, McEwen BS. Involvement of the endocannabinoid system in the neurobehavioural effects of stress and glucocorticoids. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:791–797. doi: 10.1016/j.pnpbp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Soyka M, Koller G, Schmidt P, Lesch OM, Leweke M, Fehr C, et al. Cannabinoid Receptor 1 Blocker Rimonabant (SR 141716) for Treatment of Alcohol Dependence. Journal of Clinical Psychopharmacology. 2008;28:317–324. doi: 10.1097/JCP.0b013e318172b8bc. [DOI] [PubMed] [Google Scholar]
- 46.George DT, Herion DW, Jones CL, Phillips MJ, Hersh J, Hill D, et al. Rimonabant (SR141716) has no effect on alcohol self-administration or endocrine measures in nontreatment-seeking heavy alcohol drinkers. Psychopharmacology. 2009;208:37–44. doi: 10.1007/s00213-009-1704-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cippitelli A, Cannella N, Braconi S, Duranti A, Tontini A, Bilbao A, et al. Increase of brain endocannabinoid anandamide levels by FAAH inhibition and alcohol abuse behaviours in the rat. Psychopharmacology. 2008;198:449–460. doi: 10.1007/s00213-008-1104-0. [DOI] [PubMed] [Google Scholar]
- 48.Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, et al. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Archives of general psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubio M, Villain H, Docagne F, Roussel BD, Ramos JA, Vivien D, et al. Pharmacological activation/inhibition of the cannabinoid system affects alcohol withdrawal-induced neuronal hypersensitivity to excitotoxic insults. PloS one. 2011;6:e23690. doi: 10.1371/journal.pone.0023690. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlation between duration of alcohol use and VT of [18F]FMPEP-d2 in posterior cingulate cortex in patients with alcohol dependence. Curved dashed lines are bounds for the 95% confidence interval of the regression line.
Alcohol Dependence Scores at baseline correlated positively with change in VT of [18F]FMPEP-d2 after abstinence in the insular cortex: patients with higher scores had higher increase in VT than those with lower scores. Insular cortex is representative of all brain regions.


