Abstract
Chronic kidney disease (CKD) is associated with increased coronary artery disease (CAD) and coronary artery calcification. We hypothesized that the osteogenic factor, bone morphogenetic protein-4 (sBMP-4), is elevated in subjects with both CKD and CAD. Serum was collected from 79 subjects undergoing diagnostic angiography and stratified according to CAD and CKD status. Subjects with both CAD and CKD had significantly elevated sBMP-4 compared to those with only one or no disease. sBMP-4 continued to be associated with the presence of both diseases after adjustment for other risk factors. To determine if sBMP-4 is associated with coronary artery calcification, we compared coronary artery calcium scores (CAC) to sBMP-4 in 22 subjects. A positive correlation between CAC and sBMP-4 was seen. In conclusion, sBMP-4 is elevated in patients with both CAD and CKD and positively correlates with CAC, suggesting a role for sBMP-4 in the increased CAD seen in CKD patients.
Keywords: Bone morphogenetic protein, chronic kidney disease, coronary artery disease, vascular calcification
Introduction
Patients with chronic kidney disease (CKD) are more likely to die from cardiovascular disease than progress to kidney failure [1]. The prevalence and incidence of CKD are rising in the United States driving increased medical costs and diminishing patient outcomes [2]. CKD is associated both with increased risk of coronary artery disease (CAD) and also an increased severity of CAD [3]. While this patient population exhibits higher levels of traditional risk factors for CAD, these risk factors alone do not account for the increased risk observed in these patients and tools such as the Framingham Risk Equation often under predict the risk of CAD in the CKD population [4]. Thus, additional pro-atherosclerotic factors, such as increased oxidative stress, inflammation, and vascular calcification, may play a role in CAD in the presence of CKD.
Coronary artery calcification is increased in patients in the early stages of CKD and progresses as renal function declines [5–7]. Vascular calcification is an active cell mediated process regulated by factors that stimulate and factors that inhibit osteogenesis. Among the known inhibitory factors, osteoprotegrin (OPG) is elevated in the plasma of patients with severe (Stage IV and V) CKD and was an independent predictor of coronary artery calcification and mortality [8]. Thus, circulating osteogenic factors are associated with the progression of CAD in the CKD population.
Bone morphogenetic proteins (BMPs) are osteoinductive growth factors that play a key role in cell differentiation, proliferation, migration, development, and apoptosis [9, 10]. Vascular endothelial cells and smooth muscle cells (VSMCs) both express BMP receptors and secrete BMPs. BMP-2, −4, and −6 have been shown to be present in regions of vascular calcification [11–14]. BMP-4 has been linked to the receptor-activator of nuclear factor-κB ligand (RANKL) mediated calcification in VSMCs [15]. Additional reports suggest roles for BMP-4 in endothelial cell dysfunction, inflammation, and hypertension [16–18].
Here we report that serum BMP-4 concentration (sBMP-4) is elevated uniquely in patients with the combination of both CAD and CKD. We also report in a small study that sBMP-4 directly correlates with coronary calcium scores. These findings further support a role for BMP-4 in the increased vascular calcification and increased CAD seen in CKD patients.
Methods
Subjects
This report includes data from two cross-sectional, observational studies performed at Ochsner Clinic Foundation. Both protocols were approved by the Institutional Review Board of Ochsner Clinic Foundation (Protocol # 2004.224.A and 2006.092.A), and each participant signed the informed consent. Criteria for inclusion in the study were a planned catheterization and ability to consent. sBMP-4 was measured in a total of 79 patients seen in the Cardiac Catheterization Laboratory at Ochsner Clinic Foundation from 2005 to 2006. Blood was drawn from the arterial access site prior to the procedure. Relevant medical histories were obtained and compiled into a database. CKD was defined using the National Kidney Foundation Disease Outcomes Quality Initiative guidelines as an eGFR < 60 ml/min/1.73 m2 as calculated by the Modified Diet and Renal Disease equation for greater than 3 months [19]. CAD was defined as a >50% narrowing of one or more coronary arteries as determined angiographically. For those subjects that underwent a catheterization procedure that did not include a coronary angiogram, a prior angiogram was obtained from the medical records.
In a separate study, sBMP-4 levels and coronary artery calcium score were measured in a total of 22 volunteers identified from patients seen in the Vascular Medicine Program at Ochsner Clinic Foundation. The blood sample was obtained via venipuncture prior to the procedure. Serum coronary artery calcium score was obtained from 2 sequential cardiac computed tomography (CT) -scans using a Philips Mx-8000 16 detector CT scanner coupled with the Extended Brilliance coronary calcium scoring software (Philips Healthcare USA, Andover, Massachusetts).
Serum BMP-4 and sCRP Measurement
Serum BMP-4 was measured using the BMP-4 Duoset enzyme linked immunoassay kit (R & D Systems, Minneapolis, MN) according to manufacturer's instructions. Samples were assayed in duplicate. The interassay coefficient of variance was < 14% and the detection limit of the assay was 15.6 pg/mL. Specificity of the kit for the full length BMP-4 was confirmed using western blotting (Supplementary Figure 1). sCRP was measured by the Ochsner Pathology and Laboratory Medicine Department using the high sensitivity CRP Vario assay (Abbott Laboratories, Abbott Park, Illinois).
Statistical Analyses
Results are presented as mean ± standard error of the mean. Chi-squares analysis was used to determine significant differences in categorical variables. One-way ANOVA coupled with Tukey's HSD test was used to determine significant differences in continuous variables. Student's t-test was used to make pairwise comparisons of continuous variables. For calculation of the ROC curves, sensitivity was calculated as the percentage of correct positive response and specificity as the percent of correct negative response for a given threshold. Stepwise multivariate logisitic regression was performed using SAS statistical software package (SAS Institute, Inc. Cary, NC).
Results
Subject Characteristics
A total of 79 subjects (Table 1), ranging from 23 to 84 years of age, were stratified into four groups (No CAD or CKD, CAD only, CKD only, and CKD and CAD) according to their medical histories. The indications for catheterization of these subjects were angina or dyspnea (n = 52), peripheral artery disease assessment (n = 15), assessment prior to surgery (transplant, n = 8; and valve, n = 2), patent foramen closure (n = 1), and alcohol septal ablation (n = 1). No significant difference in sBMP-4 was observed across the indications (p = 0.51). Increased hypertension was observed in the CKD groups and both CAD groups exhibited increases in the number of subjects on statin therapy. The group with neither CAD nor CKD had a significantly lower mean age than both CAD groups (Table 2). There was no significance difference in the percentage of subjects in the individual stages of CKD across the groups, with the majority being in stage III (n = 6 (66%) for CKD alone, n = 17 (92%) for CKD with CAD).
Table 1.
Study population characteristics
| Characteristic | Normal |
Chronic Kidney Disease |
p value | ||
|---|---|---|---|---|---|
| No CAD | CAD | No CAD | CAD | ||
| Total | 14 | 32 | 9 | 24 | |
| Male | 10 (71%) | 18 (56%) | 5 (56%) | 16 (67%) | 0.72 |
| African-American | 6 (43%) | 6 (19%) | 4 (44%) | 4 (17%) | 0.13 |
| Smoker | 8 (57%) | 22 (69%) | 3 (33%) | 18 (75%) | 0.14 |
| Diabetes | 5 (36%) | 7 (22%) | 3 (33%) | 11 (46%) | 0.30 |
| Hypertension | 10 (71%) | 20 (63%) | 9 (100%) | 22 (92%) | 0.02 |
| Statins | 4 (29%) | 13 (41%) | 3 (33%) | 16 (67%) | <0.01 |
| Osteoporosis | 2 (14%) | 3 (9%) | 2 (22%) | 6 (25%) | 0.44 |
Table 2.
Demographics of study population Bold indicates a significant difference.
| Normal |
Chronic Kidney Disease |
p value | |||
|---|---|---|---|---|---|
| No CAD | CAD | No CAD | CAD | ||
| Age (years) | 55.7 ± 2.6 | 65.6 ± 1.9 | 57.9 ± 5.4 | 67.0 ± 2.2 | 0.01 |
| BMI (kg/m2) | 33.4 ± 2.2 | 28.9 ± 0.9 | 32.0 ± 3.4 | 28.8 ± 1.0 | 0.07 |
| Lipid Panel | |||||
| HDL (mg/dL) | 39.8 ± 2.0 | 48.1 ± 3.5 | 47.3 ± 4.1 | 44.9 ± 3.2 | 0.51 |
| LDL (mg/dL) | 110.4 ± 10.5 | 101.0 ± 8.1 | 90.9 ± 7.6 | 88.9 ± 8.2 | 0.43 |
| Total (mg/dL) | 173.5 ± 10.0 | 171.9 ± 9.8 | 165.3 ± 8.5 | 161.0 ± 9.2 | 0.81 |
| TriG (mg/dL) | 116.4 ± 17.6 | 113.9 ± 7.7 | 135.8 ± 16.9 | 131.0 ± 12.8 | 0.55 |
| Kidney Function | |||||
| sCrea (mg/dL) | 1.0 ± 0.0 | 1.1 ± 0.0 | 4.5 ± 1.6 | 2.1 ± 0.4 | <0.001 |
| eGFR (mL/min) | 82.5 ± 4.9 | 68.1 ± 2.2 | 38.1 ± 8.1 | 41.5 ± 2.8 | <0.001 |
BMI Body mass index, HDL High density lipoprotein, LDL Low density lipoprotein, Total Total Cholesterol, TriG Triglycerides, sCrea Serum creatinine, eGFR Estimated glomerular filtration rate
Elevation of sBMP-4 in Patients with both CAD and CKD
Elevated levels of sBMP-4 were observed in patients with hypertension, CKD, CAD, or those currently on statin therapy (Table 3). When stratified into the four groups, sBMP-4 concentrations were significantly elevated only in the samples from patients with both CAD and CKD (Figure 1a). As figures 1b and 1c confirms, the increase in sBMP-4 was not related to renal function or age. The increases in sBMP-4 seen in those patients on statin therapy or with a history of hypertension were not seen when the analysis was limited to those patients with either no disease, CKD only, or CAD only, suggesting the increase was associated with the increased number of patients in the CKD and CAD group on statin therapy or with hypertension (Supplementary Table 1).
Table 3.
sBMP-4 concentration according to demographics
| Group | Mean | N | p-value |
|---|---|---|---|
| Gender | |||
| Male | 81.66 ± 11.95 | 30 | 0.79 |
| Female | 85.53 ± 7.87 | 49 | |
| African American | |||
| Yes | 85.59 ± 7.84 | 59 | 0.68 |
| No | 79.53 ± 12.49 | 20 | |
| Smoker | |||
| Yes | 80.49 ± 12.19 | 28 | 0.70 |
| No | 86.02 ± 7.86 | 51 | |
| Diabetes Mellitus | |||
| No | 84.56 ± 9.17 | 53 | 0.90 |
| Yes | 83.04 ± 7.67 | 26 | |
| Hypertension | |||
| No | 62.79 ± 7.46 | 18 | 0.02 |
| Yes | 90.34 ± 8.14 | 61 | |
| Chronic Kidney Disease | |||
| No | 64.88 ± 5.37 | 46 | <0.01 |
| Yes | 110.80 ± 12.69 | 33 | |
| Coronary Artery Disease | |||
| No | 69.46 ± 6.79 | 23 | 0.07 |
| Yes | 90.06 ± 8.83 | 56 | |
| Osteoporosis | |||
| No | 82.95 ± 7.52 | 66 | 0.71 |
| Yes | 89.72 ± 13.13 | 13 | |
| Statin Use | |||
| No | 65.92 ± 5.61 | 35 | 0.01 |
| Yes | 98.49 ± 10.59 | 44 |
Fig. 1.
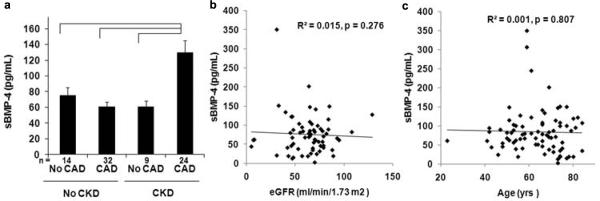
a sBMP-4 is increased uniquely in subjects with the combination of CAD and CKD. Mean sBMP-4 concentration in subjects without CAD or CKD, CAD alone, CKD alone or CAD and CKD are shown. Brackets indicate p < 0.05. b sBMP-4 does not correlate with eGFR. An X–Y scatter plot of sBMP-4 versus eGFR is shown. Line indicates linear regression fit to the data (R2 = 0.015, p = 0.276). c sBMP-4 does not correlate with age. An X–Y scatter plot of sBMP-4 versus age is shown. Line indicates linear regression fit to the data (R2 = 0.001, p = 0.807)
Association of sBMP-4 with CAD and CKD is independent of additional risk factors
As sBMP-4 was elevated under multiple conditions, stepwise logistic regression was performed to determine if the association of sBMP-4 with CAD and CKD remained when other risk factors are included in our analysis. When adjusted for age, gender, body mass index, low density lipoprotein, high density lipoprotein, total cholesterol, triglycerides, the presence of Diabetes Mellitus, statin therapy, and hypertension, sBMP-4 did not correlate with the presence of hypertension, or CAD alone. In contrast, sBMP-4 was significantly associated with the presence of the combination of CAD and CKD when adjusted for these factors (p < 0.001).
Characterization of sBMP-4 levels associated with the presence of CAD and CKD
Using receiver operating characteristic (ROC) curves, we identified 91 pg/mL as the threshold value associated with the presence of both CAD and CKD with a favorable c-statistic of 0.83 (95% CI 0.727 to 0.932, p < 0.001; Figure 2A). Because renal function is routinely assessed in the adult population, we also examined sBMP-4 thresholds in those patients with known CKD. In this case the threshold value was determined to be 81 pg/mL with a c-statistic of 0.89 (95% CI 0.775 to 0.999, p < 0.001; Figure 2B). The sensitivity, specificity, likelihood ratios, and accuracy of these two thresholds are given in Table 4. We also compared the association of CAD with serum levels of C-reactive protein (sCRP), a commonly used clinical test for the prediction of CAD, in a subset of our subjects with CKD (n=17) and found a decreased c-statistic for sCRP (0.60 95% CI 0.285 to 0.908, p = 0.57) suggesting a stronger association of sBMP-4 with CAD in CKD patients.
Fig. 2.
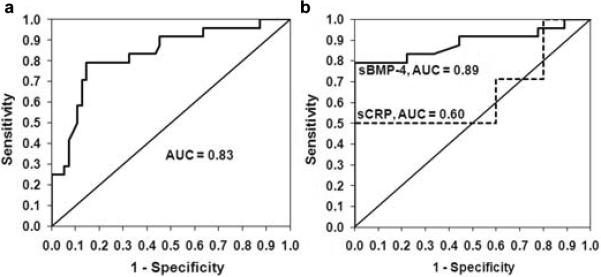
a Receiver Operating Characteristic curves for sBMP-4 predicting the presence of CAD and CKD in all subjects. b Receiver Operating Characteristic curve for sBMP-4 predicting the presence of CAD in subjects with CKD. Dashed lines indicate ROC curve for sCRP in predicting CAD in CKD patients. AUC, Area under the Curve (c-statistic).
Table 4.
Diagnostic accuracy of sBMP-4 concentration as a predictor of CAD in CKD patients
| eGFR (ml/mm/1.73m2) | Threshold (pg/mL) | Sensitivity | Specificity | LR + | LR − | Accuracy, % |
|---|---|---|---|---|---|---|
| All | 94 | 0.85 | 0.80 | 4.34 | 0.19 | 82% |
| <60 | 88 | 0.85 | 0.89 | 7.65 | 0.17 | 86% |
LR + Likelihood ratio positive, LR − Likelihood ratio negative
Correlation of sBMP-4 with coronary artery calcification
sBMP-4 has both pro-osteogenic or pro-atherosclerotic effects on the vasculature. As a first step in determining if the association of sBMP-4 with CAD in CKD patients was due to its osteogenic effects, we compared coronary artery calcium scores and sBMP-4 concentrations in a set of 22 patients. As shown in figure 3, there was a positive correlation between coronary artery calcium score and sBMP-4 measurements (R2 = 0.48, p < 0.001). These data, while not ruling out additional pro-atherosclerotic effects, support a role for the osteogenic effects of sBMP-4 in the increased CAD seen in CKD patients.
Fig. 3.
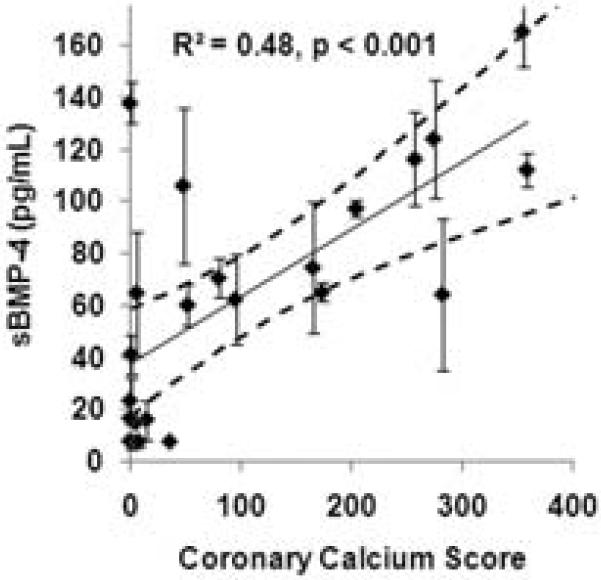
sBMP-4 positively correlates with coronary artery calcium score. An X–Y scatter plot of sBMP-4 versus coronary artery calcium score is shown. The 95% confidence interval is presented by the dashed lines.
Discussion
The increased risk of CAD in patients with CKD coupled with the fact that traditional risk factors are insufficient in predicting disease in this population suggest that additional unique factors play a role in the progression of CAD in CKD patients [4]. Calcification is common in the coronary arteries of dialysis patients, including adolescents and young adults [20, 21]. In patients with known coronary artery disease, those with CKD have been shown to have 2- to 5- fold greater coronary artery calcification, as measured by electron-beam CT scan [22]. Similarly, comparisons of coronary atherosclerotic lesions from age/gender-matched autopsy specimens showed a similar lesion size, but with greater calcification in those patients with end-stage renal disease [23]. These data suggest that the atherosclerosis in CKD patients may progress at a similar rate as normal patients, but with greater calcification [24].
RANKL and its decoy receptor, osteoprotegrin (OPG), form a signaling axis that regulates osteogenesis and vascular calcification. Serum levels of both OPG and RANKL are elevated in hemodialysis patients and have been investigated as potential biomarkers of CAD in patients with stage 4 and 5 CKD [25–27]. Elevated serum OPG levels correlate with increased coronary artery calcification and mortality [8, 25, 26]. In stage 4 CKD and hemodialysis patients, elevated plasma OPG is an independent risk factor for mortality and a indicator of coronary artery calcification [8]. Low serum levels of RANKL correlate with survival in these patient groups [8, 26].
Given recent findings that link BMP-4 to RANKL mediated vascular calcification [15], we measured sBMP-4 in subjects being seen in the catheterization laboratory to determine if it is elevated in patients with both CAD and CKD. Our data demonstrate that sBMP-4 is elevated uniquely in those patients with CKD and CAD. After adjustment for additional risk factors, elevated sBMP-4 continues to be significantly associated with the combination of diseases. Importantly, the majority of CKD patients in our study are in stage 3, suggesting sBMP-4 may be part of the progression of atherosclerosis that occurs in earlier stages of CKD.
We characterized the sBMP-4 levels associated with the presence of CAD and CKD under conditions where renal function is not known and then if stage 3 or higher CKD has been confirmed using ROC curves. In both cases favorable c-statistics were obtained. We also examined whether the association of sBMP-4 with CAD in CKD patients was stronger than that of sCRP. The more favorable c-statistic for sBMP-4 suggests sBMP-4 may be more closely linked to CAD progression in CKD patients than general markers of inflammation associated with CAD. Finally, we found, in a small study, that sBMP-4 correlated with coronary artery calcium scores suggesting a role for sBMP-4 in the increased vascular calcification seen in CKD patients. Future studies combining measurement of sBMP-4 with serum concentrations of other mediators of vascular calcification, including RANKL, osteoprotegrin, and vitamin D, are required to obtain a comprehensive understanding of the pathogenesis of CAD in the CKD population.
This is a small pilot cross-sectional, observational study with limitations that should be noted. First, the subjects were selected from patients referred for diagnostic angiography. Thus, the subjects are not representative of the general population, but instead representative of those undergoing cardiac and vascular assessment. It is possible that if performed in the general population additional factors not represented in this population may produce false positives. Despite these limitations, these data do form a basis for future studies examining the relationship between sBMP-4 and CAD in CKD patients.
In summary, sBMP-4 is elevated in patients with both CKD and CAD and correlates with an increase in coronary artery calcium scores. Stepwise logistic regression suggests that the association of elevated sBMP-4 levels with the combination of these two diseases is independent of additional risk factors. Further studies are therefore warranted examining the role of sBMP-4 in the progression of CAD in CKD patients.
Supplementary Material
Acknowledgements
This study was supported by Award Number P20RR018766 from the National Center for Research Resources of the National Institutes of Health.
References
- 1.Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension. 1989;13:I80–93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics--2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21–181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 3.Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am J Nephrol. 2008;28:354–60. doi: 10.1159/000111829. [DOI] [PubMed] [Google Scholar]
- 4.Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS, et al. The Framingham predictive instrument in chronic kidney disease. J Am Coll Cardiol. 2007;50:217–24. doi: 10.1016/j.jacc.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Kestenbaum BR, Adeney KL, de Boer IH, Ix JH, Shlipak MG, Siscovick DS. Incidence and progression of coronary calcification in chronic kidney disease: the Multi-Ethnic Study of Atherosclerosis. Kidney Int. 2009;76:991–8. doi: 10.1038/ki.2009.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakano T, Ninomiya T, Sumiyoshi S, Fujii H, Doi Y, Hirakata H, et al. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: the Hisayama study. Am J Kidney Dis. 2010;55:21–30. doi: 10.1053/j.ajkd.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 7.Russo D, Palmiero G, De Blasio AP, Balletta MM, Andreucci VE. Coronary artery calcification in patients with CRF not undergoing dialysis. Am J Kidney Dis. 2004;44:1024–30. doi: 10.1053/j.ajkd.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 8.Mesquita M, Demulder A, Damry N, Melot C, Wittersheim E, Willems D, et al. Plasma osteoprotegerin is an independent risk factor for mortality and an early biomarker of coronary vascular calcification in chronic kidney disease. Clin Chem Lab Med. 2009;47:339–46. doi: 10.1515/CCLM.2009.075. [DOI] [PubMed] [Google Scholar]
- 9.Kiyono M, Shibuya M. Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression. Mol Cell Biol. 2003;23:4627–36. doi: 10.1128/MCB.23.13.4627-4636.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graff JM. Embryonic patterning: to BMP or not to BMP, that is the question. Cell. 1997;89:171–4. doi: 10.1016/s0092-8674(00)80196-8. [DOI] [PubMed] [Google Scholar]
- 11.Dhore CR, Cleutjens JP, Lutgens E, Cleutjens KB, Geusens PP, Kitslaar PJ, et al. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1998–2003. doi: 10.1161/hq1201.100229. [DOI] [PubMed] [Google Scholar]
- 12.Schluesener HJ, Meyermann R. Immunolocalization of BMP-6, a novel TGF-beta-related cytokine, in normal and atherosclerotic smooth muscle cells. Atherosclerosis. 1995;113:153–6. doi: 10.1016/0021-9150(94)05438-o. [DOI] [PubMed] [Google Scholar]
- 13.Bostrom K, Watson KE, Horn S, Wortham C, Herman IM, Demer LL. Bone morphogenetic protein expression in human atherosclerotic lesions. J Clin Invest. 1993;91:1800–9. doi: 10.1172/JCI116391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, et al. Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res. 2010;107:485–94. doi: 10.1161/CIRCRESAHA.110.219071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–8. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 16.Sorescu GP, Sykes M, Weiss D, Platt MO, Saha A, Hwang J, et al. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress stimulates an inflammatory response. J Biol Chem. 2003;278:31128–35. doi: 10.1074/jbc.M300703200. [DOI] [PubMed] [Google Scholar]
- 17.Miriyala S, Gongora Nieto MC, Mingone C, Smith D, Dikalov S, Harrison DG, et al. Bone morphogenic protein-4 induces hypertension in mice: role of noggin, vascular NADPH oxidases, and impaired vasorelaxation. Circulation. 2006;113:2818–25. doi: 10.1161/CIRCULATIONAHA.106.611822. [DOI] [PubMed] [Google Scholar]
- 18.Wong WT, Tian XY, Chen Y, Leung FP, Liu L, Lee HK, et al. Bone morphogenic protein-4 impairs endothelial function through oxidative stress-dependent cyclooxygenase-2 upregulation: implications on hypertension. Circ Res. 2010;107:984–91. doi: 10.1161/CIRCRESAHA.110.222794. [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 20.Oh J, Wunsch R, Turzer M, Bahner M, Raggi P, Querfeld U, et al. Advanced coronary and carotid arteriopathy in young adults with childhood-onset chronic renal failure. Circulation. 2002;106:100–5. doi: 10.1161/01.cir.0000020222.63035.c0. [DOI] [PubMed] [Google Scholar]
- 21.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, et al. Coronaryartery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med. 2000;342:1478–83. doi: 10.1056/NEJM200005183422003. [DOI] [PubMed] [Google Scholar]
- 22.Braun J, Oldendorf M, Moshage W, Heidler R, Zeitler E, Luft FC. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996;27:394–401. doi: 10.1016/s0272-6386(96)90363-7. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218–23. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 24.Moe SM, Chen NX. Pathophysiology of vascular calcification in chronic kidney disease. Circ Res. 2004;95:560–7. doi: 10.1161/01.RES.0000141775.67189.98. [DOI] [PubMed] [Google Scholar]
- 25.Nitta K, Akiba T, Uchida K, Kawashima A, Yumura W, Kabaya T, et al. The progression of vascular calcification and serum osteoprotegerin levels in patients on long-term hemodialysis. Am J Kidney Dis. 2003;42:303–9. doi: 10.1016/s0272-6386(03)00655-3. [DOI] [PubMed] [Google Scholar]
- 26.Morena M, Terrier N, Jaussent I, Leray-Moragues H, Chalabi L, Rivory JP, et al. Plasma osteoprotegerin is associated with mortality in hemodialysis patients. J Am Soc Nephrol. 2006;17:262–70. doi: 10.1681/ASN.2005030260. [DOI] [PubMed] [Google Scholar]
- 27.Gonnelli S, Montagnani A, Caffarelli C, Cadirni A, Campagna MS, Franci MB, et al. Osteoprotegerin (OPG) and receptor activator of NF-kB ligand (RANK-L) serum levels in patients on chronic hemodialysis. J Endocrinol Invest. 2005;28:534–9. doi: 10.1007/BF03347242. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


