Abstract
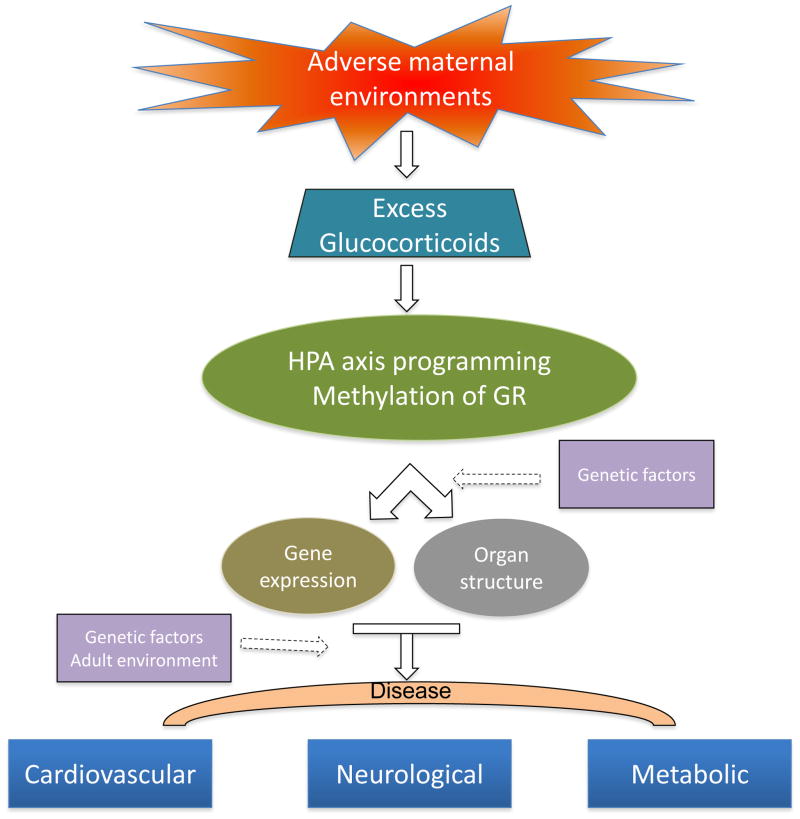
Adverse environments during the fetal and neonatal development period may permanently program physiology and metabolism, and lead to increased risk of diseases in later life. Programming of the hypothalamic-pituitary-adrenal (HPA) axis is one of the key mechanisms that contribute to altered metabolism and response to stress. Programming of the HPA axis often involves epigenetic modification of the glucocorticoid receptor (GR) gene promoter, which influences tissue-specific GR expression patterns and response to stimuli. This review summarizes the current state of research on the HPA axis and programming of health and disease in the adult, focusing on the epigenetic regulation of GR gene expression patterns in response to fetal and neonatal stress. Aberrant GR gene expression patterns in the developing brain may have a significant negative impact on protection of the immature brain against hypoxic-ischemic encephalopathy in the critical period of development during and immediately after birth.
Keywords: Hypothalamic-pituitary-adrenal axis, glucocorticoids, programming, neurological dysfunction, hypertension, metabolic disease, epigenetic, glucocorticoid receptor
1. Introduction
Survival of an organism depends on its ability to maintain the equilibrium with changing environmental conditions. Although environmental conditions are considered to influence life after birth, recent epidemiological studies have demonstrated the importance of environment in utero on health and disease of individuals in later life. There is compelling evidence that early life events play a causal role in developmental programming of adult disease. During the period of rapid growth and development, the fetus is highly susceptible to alterations in the maternal environment. The concept of “programming” has been developed to explain the process by which an organism adapts to environmental events through generating stable alterations in the phenotype. This usually occurs at early period of development. Exposure to an adverse environment may disturb the process of cell proliferation and differentiation, alter fetal growth and have long-lasting effects on the individual’s health. The concept of “fetal programming of adult disease” was first proposed several decades ago, and it has now been widely accepted [13,146].
Stress is an internal response to stimuli or pressures that challenge or disrupt the homeostasis of organism in a changing environment. Environmental signals that influence the process of fetal development cause fetal stress. Stress responses are mediated mainly by the sympatho-adrenomedullary and the hypothalamic-pituitary-adrenal (HPA) systems. The sympatho-adrenomedullary system provides a rapid response to acute stress through changes in cardiovascular, respiratory, renal, and endocrine systems, while the HPA system provides prolonged response to stress via the effects of glucocorticoids. These two systems interact with each other to promote homeostasis and facilitate the adaption to stress. The HPA axis is of vital importance in mediating the process of fetal programming. Although these stress response actions may provide short-term benefit and facilitate the survival, the resultant alteration of gene expression patterns may bring about modified responses in the adulthood and set the stage for the development of disease in later life. In this review we discuss major stress factors, their effects on programming of the HPA axis, the subsequent effects on disease development, and the molecular mechanisms that may be involved.
2. The HPA axis and perinatal stress
2.1 The HPA axis
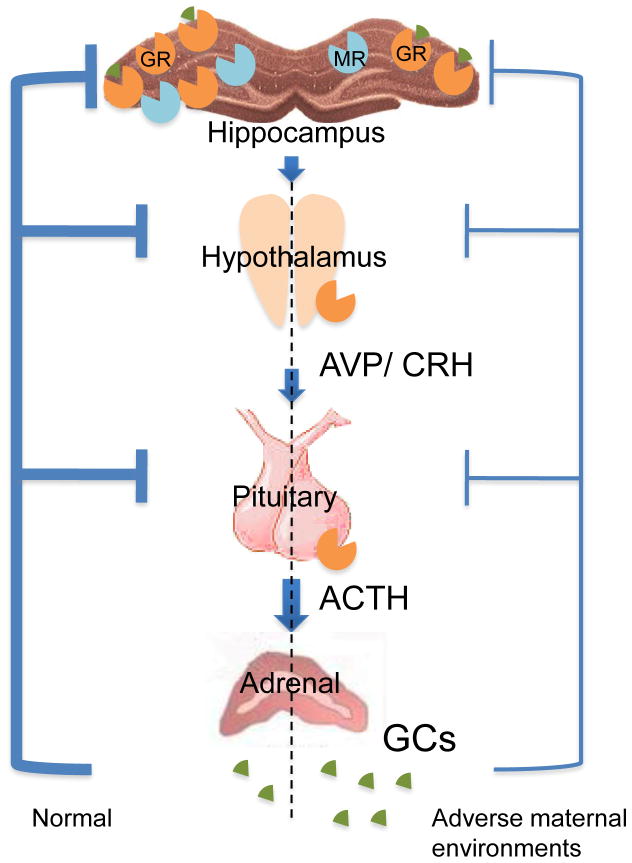
The HPA axis consists of a complex set of interactions between the hypothalamus, pituitary gland and adrenal cortex, which mediates the stress response through the actions of glucocorticoids. Main anatomical components of the HPA axis and glucocorticoid feedback loop are illustrated in Figure 1. The hippocampus, hypothalamic paraventricular nucleus (PVN), anterior pituitary, and adrenal cortex are integrated into a tightly regulated, interdependent system that maintains internal homeostasis. In response to stress, the PVN releases corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP), which enter hypophyseal portal circulation and stimulate the synthesis and secretion of adrenocorticotropin (ACTH) into the peripheral circulation. ACTH is produced from cleavage of the precursor molecule proopiomelanocortin in anterior pituitary corticotrope cells. Once released, ACTH initiates the production and secretion of glucocorticoid (cortisol in humans, sheep, and guinea pigs, and corticosterone in rodents) from the adrenal cortex. During stress, glucocorticoids redirect the metabolism to meet energy demands for stress response, and restrain defense reactions to stress in order to protect the body from further damage.
Figure 1. The HPA components and feedback regulation of HPA activity.
The AVP (arginine vasopressin) and CRH (corticotrophin-releasing hormone) produced from PVN of hypothalamus stimulate the synthesis and secretion of ACTH in anterior pituitary. The ACTH then promotes the production and release of GCs (glucocorticoids) from adrenal cortex. The HPA activity is controlled by negative feedback regulation of GCs at pituitary, PVN and limbic system level through effects of GR (glucocorticoid receptor) and MR (mineralocorticoid receptor). Adverse maternal environments can result in attenuated negative feedback regulation of HPA activity through decreased expression of GR in the hippocampus.
At the molecular level, these stress response actions are achieved mainly by the regulation of gene expressions. Glucocorticoids exert their effects through two different types of receptors, the type I mineralocorticoid receptor (MR) and the type II glucocorticoid receptor (GR). Both MR and GR are members of the nuclear hormone superfamily of ligand-activated transcription factors. The unligated GR is located in the cytoplasm as a multi-protein complex containing several chaperones including heat-shock proteins and kinases. Binding of glucocorticoids to the GR results in dissociation of chaperones from the protein complex, and the activated glucocorticoid/GR complex is translocated into the nucleus where it binds to the glucocorticoid response elements (GREs) in the promoter region of target genes and modulates gene expressions [3]. Due to potential damaging effects of extended glucocorticoid exposure, the HPA axis activity is modulated by a feedback regulation of glucocorticoids and ACTH at the levels of the hippocampus, PVN and pituitary gland [213]. In addition to the classic genomic effects, glucocorticoids can also mediate rapid cellular responses through non-specific interaction with cellular membranes, especially the plasma and mitochondrial membranes. It has been shown that at high concentrations glucocorticoids dissolve into membranes and can affect physicochemical membrane properties, as well as the activities of membrane-associated proteins [35]. This results in inhibition of calcium and sodium cycling across the plasma membranes of immune cells, and contributes to the clinically observed immunosupression associated with high-dose glucocorticoid therapy. Non-genomic effects may also be mediated by the binding of glucocorticoids to the GR located in the cytoplasm or on the membrane [87,276]. As noted above, binding of glucocorticoids to the cytoplastic GR may result in the release of signaling molecules such as the Src from the multi-protein complex, and the Src is considered to mediate rapid glucocorticoid effects [53]. Recent studies show that rapid non-genomic effects of glucocorticoids are also mediated by a membrane-linked GR in T cells. The Src family members Lac and Fyn are expressed in T cells and involved in T cell receptor-mediated signal transduction. Glucocorticoid treatment rapidly inhibits the activities of Lac and Fyn through a GR-dependent pathway [166,167]. In the hypothalamus, glucocorticoid-mediated non-genomic effects participate in rapid glucocorticoid feedback inhibition of hypothalamic hormone secretion [72]. The molecular mechanisms of genomic and non-genomic GR actions have been reviewed in detail by Stahn et al. [262,263]. Genomic effects of glucocorticoids normally take place within hours or days in the nanomolar range, while non-genomic effects of glucocorticoids may occur within seconds or minutes at significantly higher concentrations. Both genomic and non-genomic effects of glucocorticoids have been described at cellular levels, the level of whole organ function and in vivo physiological responses.
Molecular and biochemical studies have demonstrated the expression of both MR and GR in brain tissues. In the rat brain, the GR is expressed in virtually all cell types, while the expression of MR is more restricted and is distributed mainly in the PVN and in certain subfields of the hippocampus. The MR binds to glucocorticoids with high affinity, approximately 10-fold higher than that of GR [234]. Glucocorticoids may thus predominantly bind to and occupy the MR at basal concentrations, which may play an important role in the activity of HPA system, such as in maintaining the basal activity (e.g. circadian fluctuations) and controlling the sensitivity of stress response. In conditions where glucocorticoid concentrations rise markedly such as after stress, the GR is activated, together with MR, which modulates appropriate stress responses by controlling and terminating stress-induced HPA reactions through the feedback regulation. Under such circumstances, the GR mobilizes energy resources required for stress response and facilitates the recovery from the stress insult [54]. In other words, the activation of MR by glucocorticoids results in initiation of the stress response, while GR-mediated effects lessen and limit the response and promote the adaptation.
The impact of fetal glucocorticoid exposure depends on the expression of the genes encoding GR and MR in the developing brain at the time of exposure. The developmental expression patterns of GR and MR are highly species specific. In fetal rat brains, both GR and MR are expressed at low levels throughout gestation, but increase rapidly after birth. GR mRNA expression is first seen on embryonic days (E) 12.5 in the neuroepithelium of fetal rats, and its expression levels increase from that time on [73]. Kitraki et al. studied GR gene expression in the rat brain from E12 to E17 by in situ hybridization, and found that GR mRNA was first detected in E13 embryos [141]. In another study, GR expression was evaluated at both the mRNA and protein levels in E15 to E22 rat brains. A moderate to strong GR mRNA signal was observed in multiple regions of fetal rat brains from E15, and a weak to moderate nuclear GR protein signal developed in nerve cells 1 or 2 days after the appearance of GR mRNA signal [46]. The expression of MR was present in the fetal rat brain later in the gestation, and was limited to certain regions. The MR mRNA expression was demonstrated in fetal rat brains from E15.5, and maintained at a low level until E22.5 [73]. In species that give birth to mature newborns such as guinea pigs, sheep or non-human primates, GR and MR expression patterns are more complicated. For example, GR mRNA was found widely distributed in guinea pig brains, with the highest levels of expression in the cortex, hippocampus, amygdala, and PVN, while MR mRNA was limited to the limbic structures [170]. In the hippocampus, MR expression decreases while GR expression increases with progression of the gestation, indicating that there is differential developmental regulation of these two receptors [170]. Moreover, GR mRNA levels undergo dynamic and site-specific changes in the brain of fetal guinea pigs. The hippocampal GR mRNA levels increase with progression of the gestation and reach the highest levels near the term, while PVN GR mRNA levels are highest on gestational days 40–45, and dramatically decrease in the last 25 days of gestation [170]. A similar reduction in the GR expression pattern is found in the PVN of fetal lamb brains [4]. In the common marmoset brain, the expression of MR and GR is higher in the hippocampus than in the cortex, striatum and cerebellum [224]. In the hippocampus, the expression of MR is site-specific, with highest level in the dentate gyrus (DG), lower levels in the subiculum and presubiculum, and very low levels in the lateral nucleus of amygdala. The distribution of GR mRNA in the brain is more widespread than that of MR, and shows significant differences between areas within one tissue. For example in the hippocampus GR mRNA is expressed higher in DG than in the CA1-CA4. MR mRNA expression is higher in infants than in adults, and intermediate values are found in neonates and juveniles, while the hippocampal GR mRNA expression occurs in infants and juveniles but not in neonates or adults. A study in the fetal rhesus monkey showed that pulmonary GR mRNA levels increased significantly with the progression of gestation [318]. In the developing human hippocampus, both MR and GR are expressed between 24 and 34 weeks of the gestation, and GR mRNA expression levels are lower than those of MR mRNA. Moreover, the expression levels of MR and GR mRNA do not show large changes over time [199]. This dynamic regulation of MR and GR expression levels may be a reason for the distinctly different reactions to common stimuli applied at different times of gestation in the programming process.
The HPA axis is highly susceptible to programming during the development, and glucocorticoids act as the primary mediators of HPA programming [136,172]. Glucocorticoid-induced plasticity of the neural circuitry regulating the HPA axis may constitute a means through which exposure to stress plays a role in a spectrum of HPA abnormalities, including aberrant HPA circadian rhythms, abnormal HPA response to stress, and basal HPA dysregulation [156]. In view of the regulatory effects of HPA hormones on the gene expression profile, exposure of glucocorticoids-responsive organs such as the brain, liver or pancreas to excess glucocorticoids during the critical period of the development may result in a permanent alteration of physiology and later health.
2.2 Perinatal stress of malnutrition, hypoxia and glucocorticoid exposure
Growth and development in utero are a complex and dynamic process, and require the interaction of maternal and fetal components in order to sustain the survival and optimal growth throughout the gestation. If adverse environmental factors such as shortage of nutrition are encountered during pregnancy, it is logical for a “stress” signal to be transmitted to the developing fetus, which results in changes in the structure and function of fetal tissues, the activity of fetal HPA axis and behaviors that promote the survival after birth. Prenatal stress may lead to low birth weight that is an important marker of intrauterine growth restriction (IUGR) in the fetus. Perinatal stress includes altered nutrients (for example maternal hypoxia or malnutrition), exposure to harmful chemical agents (such as cocaine, nicotine, or alcohol), physiological or psychological stress, perturbed endocrine signaling (e.g. excessive glucocorticoid exposure) between the mother and the developing fetus, as well as postnatal manipulation (e.g. postnatal handling of the newborn animals). Although fetal or neonatal response to such disturbances enhances the maintenance of critical tissue function and the capacity for survival of the organism, this adaptive response may contribute to irreversible changes in tissue structure or function, and significantly increase subsequent susceptibility to disease, especially when the adult environment does not match the environment that originally induced the programming process and developmental plasticity [104]. Two major hypotheses that have been proposed to explain the association of perinatal stress and postnatal health are nutritional programming [13] and hormonal programming through overexposure to glucocorticoids [256]. In addition, fetal development and programming may be influenced by exposure to many other adverse factors during the perinatal period. For example, chronic hypoxia during pregnancy plays an important role in IUGR-independent of maternal malnutrition [129,186,231]. Conditions such as increased maternal stress or fetal exposure to adverse substances may also result in low birth weight and cause programming that leads to heightened vulnerability to disease in adult life [31,59,260].
2.2.1 Perinatal malnutrition
Since the original studies by Barker et al. [12], compelling evidence from other large epidemiological studies has demonstrated a relationship between prenatal malnutrition and negative consequences on the health of offspring in later life. It is increasingly clear that prenatal malnutrition is associated with increased risk of cardiovascular disease, metabolic syndromes or neuroendocrine deficits [249,273]. In the “thrift phenotype” proposed by Hales and Barker, insufficient prenatal nutrition may bring about the adaption that facilitates fetal survival and postnatal growth under continued nutritional deprivation. However, when plentiful postnatal nutrition is provided after birth, these adaptations that were beneficial under the original conditions may increase the risk of development of type 2 diabetes and other features of metabolic syndrome [116]. Prenatal undernutrition followed by subsequent rapid catch-up growth in neonates supported by sufficient nutrition or overnutrition, lead to disease in the adulthood. There has been extensive investigation of inadequate prenatal nutrition followed by discordant postnatal nutrition. However, rapid postnatal growth is itself associated with increased risk of obesity and metabolic disease in the adulthood [286]. Rapid weight gain during infancy has been associated with adiposity, high blood pressure, high fasting triglyceride levels and other risk factors, which are independent of birth weight [286]. The fetal and infant period from pregnancy to 24 months of age is a crucial window of opportunity for reduction of malnutrition and its long-term adverse effects.
Adequate nutrients throughout the gestation are important for the optimal fetal growth and development. Insufficient nutrition at any stage of pregnancy is associated with adverse outcomes in pregnancy or infancy, and leads to increased risk of adult disease. Due to conditions such as severe morning sickness (hyperemesis gravidarum), adequate maternal nutrition may be a challenge, especially in early pregnancy. In the earliest stages of pregnancy, concentrations of nutrients influence embryonic and trophoblast growth even before the implantation, and the one-cell embryo is particularly sensitive [95]. During middle and late gestation, severe malnutrition may cause restricted development of both the fetus and placenta. Nutritional deprivation in early development affects the differentiation of hypothalamic centers regulating energy metabolism, and increased food availability in subsequent life thus leads to the accumulation of excess fat. Individuals with insufficient prenatal nutrition are prone to obesity [228,265], and have increased risk of both hypertension and coronary heart disease [242,264].
In addition to general caloric restriction, deficiency of one or more essential nutrients during the perinatal period may also increase risk of disease in later life. A low-protein diet programs hypertension, insulin resistance and endothelial dysfunction in rats [145]. Maternal and/or neonatal deficiency of iron is associated with increased blood pressure and adverse metabolic responses [157], as well as behavioral consequences [168], which most likely result from abnormal brain development [15]. Zinc deficiency during fetal or postnatal growth leads to increased arterial blood pressure and the impairment of renal function in adulthood [280]. The increasing knowledge of the vital roles played by essential nutrients such as iron and zinc in the fetal development and in the onset of diseases in adulthood is of importance and may enable the development of early interventions to control the specific nutrition deficiency in the current nutritional programs.
Although many previous studies have discussed the prevalence of insufficient nutrition, overnutrition is also a major factor in developmental programming of disease as has been demonstrated in more recent studies. Maternal obesity and prenatal overnutrition are associated with the onset of metabolic syndrome and cardiovascular disease in offspring. As a consequence of increasing consumption of fat-rich diets, more and more people in the world are overweight. In the United States, over 70% of the adult population was reported to be overweight or obese in 2010 [90]. Moreover, the number of obese women of childbearing age tripled between 1960 and 2000 [89]. In several epidemiological studies, maternal BMI and gestational diabetes have been related to offspring obesity [26,143]. In animal models, a high fat maternal diet during pregnancy and lactation produces a phenotype in offspring that closely resembles the human metabolic syndrome, including obesity, dyslipidaemia, hyperglycemia and insulin resistance [7,112]. In addition, impaired vascular function and elevated blood pressure that are associated with cardiovascular diseases are also observed in these animal models [102,139]. Evidence from both epidemiological studies and animal models suggests that overnutrition in maternal/fetal and/or neonatal period may lead to obesity and increased risk of metabolic diseases in offspring. It is thus important to include women during their reproductive period and children during their infancy and early childhood for the efforts to decrease obesity and related metabolic diseases.
Animal model studies of malnutrition demonstrate compromised development of the pancreas, adipose tissue, brain, kidney and liver, and these changes are involved in programming of metabolic physiology and disease [32,41,70,196]. Circulating concentrations of factors like insulin and leptin are generally altered by exposure to malnutrition, and these alterations are associated with long-lasting consequences on the circuitry that regulates the metabolism and energy balance. Changes in pancreatic structure and function such as lower β-cell mass and insufficient insulin secretion, are observed in both fetal and postnatal life in animals exposed to maternal malnutrition [41,43,180]. In rat dams fed a low-protein diet, the β-cell mass, proliferation and function are decreased, and expression levels of transcription factors that regulate pancreatic development and function are also altered, which are associated with changes in programmed metabolism in later life [237]. A high-fat diet has been shown to cause β-cell failure, insulin resistance and eventually type-2 diabetes [41]. During pregnancy and lactation, reduction of maternal nutrition in the baboon programs offspring metabolic responses, increases insulin resistance and β-cell responsiveness, and results in emergence of an overall phenotype that predisposes to type-2 diabetes in later life [44]. Leptin is usually produced by adipocytes and its major function is to regulate food intake and energy expenditure. During human pregnancy, the placenta is also an important source of leptin, and leptin receptors are also expressed in the placenta, suggesting that leptin may act through a paracrine or autocrine mechanism in the regulation of placental growth and fetal development [173]. In rodents leptin comes mainly from the maternal circulation as the rodent placenta does not produce endocrinologically significant amounts of leptin [233]. Leptin levels in human intrauterine growth restriction (IUGR) infants are lower than in infants of normal weight [128]. In rat offspring with hypoxia-induced IUGR, the energy intake and physical activity are decreased compared to controls. When fed a high-fat diet, plasma leptin levels of the IUGR offspring are increased, accompanied by changes in lipid metabolism and insulin resistance [245]. The dexamethasone treatment reduces placental expression of leptin receptor mRNA and protein, whereas metyrapone, an inhibitor of glucocorticoid synthesis, stimulates placental expression of leptin receptors. Moreover, the treatment of dexamethasone and carbenoxolone (an inhibitor of 11β-hydroxysteroid dehydrogenase 2) also markedly reduces fetal plasma leptin concentrations [261]. Taken together, evidence from these studies suggests that leptin is a mediator of the effects of developmental programming by prenatal stress. Understanding of the role of leptin may provide novel strategies for therapeutic interventions. In pregnant rats, a low-protein diet results in an increased fetal to placental weight ratio, and continuous infusion of leptin in the third trimester in low-protein diet dams reduces the fetal to placenta weight ratio to that of the control group, suggesting that the elevated leptin levels in the low-protein dams prevent placental insufficiency [268]. In newborn rats, blockage of leptin with a recombinant antagonist leads to long-term leptin resistance and susceptibility to diet-induced obesity [9]. Moreover, neonatal leptin treatment in rat pups reverses the development programming induced by severe maternal under-nutrition and restores the normal programmed phenotype in adult rats [290].
2.2.2 Maternal hypoxia
Oxygen is a major nutrient for the developing fetus, and maternal hypoxia is a common form of stress during fetal development. The fetus may experience hypoxia under different conditions. For pregnant women who live at high altitude, fetal hypoxia is a common condition [185] that may lead to low birth weight [103,197]. Many women continue to smoke throughout pregnancy despite its well-known harmful effects, and tobacco smoking can result in prolonged hypoxemia in the fetus. The placenta serves as a critical component of the maternal-fetal interaction. Insufficient placental function or decreased maternal pO2 resulting from anemia, preeclampsia, or heart and lung disease may also lead to fetal hypoxia [193].
In response to a short-term episode of hypoxia, the fetus may undergo a redistribution of blood flow away from peripheral circulation to vital organs, such as the heart, adrenals and brain [235]. Sustained redistribution of blood flow to essential organ circulation ensures fetal survival, but this adaptation may give rise to many unfavorable health results in later life. Maternal hypoxia may reprogram expression patterns of vital genes, alter the structure or function of multiple organs, and increase susceptibility to disease in later life. Animal model studies show that maternal hypoxia may result in IUGR and increase susceptibility to diet-induced metabolic syndrome [187,244]. In adult rat offspring that have been exposed to maternal hypoxia before birth, the expression of hepatic AKT and the muscle glucose transporter GLUT4 that are early markers of insulin resistance, is changed by hypoxia independent of undernutrition [38]. The developing brain is highly sensitive to reduced oxygen and undergoes important changes in response to hypoxia, which lead to brain injury and impaired function [79,113,230]. The cardiovascular system is particularly sensitive to hypoxia [317]. Maternal hypoxia causes aberrant expression of important genes in the heart, and results in alteration of heart structure or function [77,278]. Recent studies in rats demonstrated that maternal hypoxia in the last week of gestation caused changes in matrix metalloproteinase expression patterns in fetal and neonatal hearts, leading to collagen deposition in the heart and a decrease in ventricular wall thickness [281]. Fetal hypoxia-induced down-regulation of PKCε and up-regulation of the angiotensin II type 2 receptor (AT2R) in the developing heart have been shown to increase susceptibility to cardiac ischemic injury in offspring [214,314,313]. Vascular and endothelial dysfunction has also been associated with prenatal hypoxia [188,308]. As fetal hypoxia is capable of inducing dysfunction in multiple organs, there is increased risk of cardiovascular disease, metabolic syndrome or neurological dysfunction in offspring with exposure to maternal hypoxia.
2.2.3 Exposure to excessive glucocorticoids
Glucocorticoids are steroid hormones produced predominantly by the adrenal gland. Glucocorticoids have broad but nuanced effects in maintaining the homeostasis and facilitating the short-term survival and recovery from challenges under stress [251]. Glucocorticoids play important roles in the fetal development by accelerating the maturation of tissues and organs, notably the lung [236,294]. It is for this reason that the synthetic glucocorticoids (e.g. dexamethasone and betamethasone) have been widely used in the treatment of pregnant women at risk of preterm delivery, which have effectively improved outcomes for premature babies over the last 30 years [236]. However, evidence from epidemiological studies and animal models has shown an association of prenatal exposure to high circulating concentrations of glucocorticoids and increased risk of cardiovascular disease, metabolic disorders and neuroendocrine dysfunction in later life [50,117,256], in which programming of the HPA axis is commonly involved.
Exposure to excess glucocorticoids is common in pregnancy. The fetus is exposed to elevated levels of endogenous glucocorticoids in conditions where the levels of endogenous glucocorticoids are elevated in the mother who is under stress or when the placental 11β-hydroxysteroid dehydrogenase 2 (11β-HSD2) barrier decreases. In normal pregnancy, glucocorticoid concentrations in the maternal circulation are markedly higher than those in the fetal circulation that is very low. This results mostly from the synthesis of 11β-HSD2 by the placenta, which acts as a maternal-fetal ‘barrier’ to prevent inappropriate action on glucocorticoid sensitive tissues during the fetal development [17]. 11β-HSD2 is expressed by the placental syncytiotrophoblasts that are the site of fetal-maternal exchange. Placental 11β-HSD2 oxidizes most (~90%) of the maternal cortisol or corticosteron into inactive 11-keto derivates (cortisone or 11-dehydrocorticosterone), and the remaining 10~20% of the maternal glucocorticoids enter the fetal circulation [17]. During human pregnancy, the expression and/or activity of 11β-HSD2 steadily increases as gestation progresses, and then drops precipitously near term (38–40 weeks) so that maternal glucocorticoids are available to the fetus to promote the maturation of fetal organs, including the lung and brain [191,254]. In humans, mutation of the 11β-HSD2 gene is rarely reported, but is a deleterious mutation that leads to very low birth weight [57]. As maternal glucocorticoid levels are markedly higher than those of the fetus, modest alterations in placental 11β-HSD2 expression and activity can have a profound impact on fetal glucocorticoid exposure. An association between restricted fetal growth and low levels of placental 11β-HSD2 (decreased expression or activity) has been found in humans in some studies [177,267], although other studies have failed to confirm this association [238]. Maternal physiological stress results in secretion of catecholamines, which may down-regulate human placental 11β-HSD2 mRNA levels through the alpha adrenergic pathway during early gestation and term pregnancy [252]. Evidence from animal studies further demonstrates the relation of placental 11β-HSD2 levels, fetal exposure to glucocorticoids and the compromised development. Maternal undernutrition or low protein diet during pregnancy selectively attenuates the expression of placental 11β-HSD2, leading to overexposure of the fetus to endogenous glucocorticoids and subsequent low birth weight [20,30], which play an important role in programming of adult hypertension [84,152]. Inhibition of 11β-HSD2 with carbenoxolone in pregnant rats leads to fetal overexposure to glucocorticoids with associated increased risk of hypertension, hyperglycemia and impaired stress response in offspring [163,162,303]. These effects of carbenoxolone further highlight the role of maternal glucocorticoids in programming, since these effects are apparently independent of changes in other maternal factors such as blood pressure or obesity.
Fetuses may also suffer exposure to excess synthetic glucocorticoids in conditions in which antenatal glucocorticoids are administered, such as in the treatment for asthma or congenital adrenal hyperplasia. About 10% of pregnant women are at risk for preterm delivery in the United States, and need to be treated with synthetic glucocorticoids in order to improve newborn survival [236]. Unlike the endogenous glucocorticoids that are inactivated by 11β-HSD2 in the placenta, synthetic glucocorticoids readily cross the placenta. Although dexamethasone is a substrate for 11β-HSD2, the product 11-ketodexamethasone can effectively bind to and activate glucocorticoid receptors [229]. It is in part for this reason that dexamethasone is extensively used in investigating programming by glucocorticoids. In rat models, the treatment with dexamethasone for the final week of gestation results in elevated basal and stress-induced plasma corticosterone levels in adult male offspring, indicating that HPA function is altered [153,226,257].
Another potent mechanism that serves to protect the fetus from maternal glucocorticoids is the multidrug resistance P-glycoprotein (P-gp)-mediated efflux of glucocorticoids from syncytiotrophoblasts [134]. P-gp is an important drug transporter that is expressed in many tissues including the placenta. P-gp in the placenta is specifically expressed on the microvillus membrane that faces the maternal blood supply, of the syncytiotrophoblast [277]. P-gp protects the fetus by extruding exogenous chemical agents and regulating transfer of endogenous compounds. The expression of P-gp in the placenta is high in early gestation and dramatically decreases near term [271], and this decrease in late gestation may be an important factor in allowing increased fetal exposure to endogenous glucocorticoids. Both dexamethasone and betamethasone are substrates of P-gp, and these compounds also have regulatory effects on the expression of placental P-gp [319]. Dexamethasone has been shown to increase the expression of P-gp in a placental cell line [216]. Given that the expression of P-gp is developmentally regulated, outcomes of maternal exposure to synthetic glucocorticoids may result at least partly from P-gp-induced alteration of endogenous factors that may be important for the fetal development.
Maternal health may directly affect the fetal environment. In addition to prenatal malnutrition, hypoxia or excess glucocorticoids, maternal conditions such as tobacco smoking, alcohol or abuse of cocaine, and subclinical maternal infection or inflammation may also potentially alter the fetal environment, triggering programming of the HPA axis and subsequent disease in offspring. It is important to note that these stressors are not mutually exclusive. Alterations in maternal nutrition also affect fetal glucocorticoid exposure [24,201]. Conversely, prenatal stress or maternal administration of glucocorticoids influences maternal feeding behavior that may lead to alteration of nutrition. Preeclampsia and hypoxia are associated with decreased 11β-HSD2 in the placenta, which leads to fetal glucocorticoid overexposure [2]. Alcohol has also been reported to decrease placental 11β-HSD2 in female offspring [307]. The actions of glucocorticoids are potent and can modulate metabolic processes to achieve appropriate stress response, and excess glucocorticoids may thus represent a common pathway by which adverse environmental signals are transferred from the mother to the fetus, triggering changes in fetal and offspring development and permanently affecting physiological function and disease development in later life. During this process, programming of the HPA axis plays a pivotal role.
2.3 Effect of perinatal stress on the HPA axis
Maternal stress has a profound effect on programming of the HPA axis [36,274], and glucocorticoids are primary mediators in programming of HPA function. In human pregnancies complicated by IUGR, fetal cortisol levels are significantly elevated at term [106], and elevated cortisol both reduces birth weight and influences the developing HPA axis and emotional behavior [140]. In rats and non-human primates, high glucocorticoid exposure during the fetal development leads to permanent elevation of basal glucocorticoid levels in offspring [68,153]. Prenatal stress and excess maternal or fetal corticosterone cause downregulation of fetal GR and MR receptors and impair the feedback regulation of the HPA axis in both infancy and adulthood [301]. The pivotal role of glucocorticoids in programming has been demonstrated by maternal adrenalectomy. In studies of adrenalectomized pregnant rats with basal corticosterone replacement, neither prenatal stress nor food-restriction showed any effect on HPA function in offspring [10,152], suggesting that maternal glucocorticoids mediate the stress-induced programming of HPA function in offspring. Programming of adult disease by maternal stress or glucocorticoid overexposure is associated with a ‘resetting’ of the HPA axis sensitivity, and this often involves perturbations in the development of glucocorticoid responsive organs, including the brain, kidney, adipose tissue and pancreas.
2.3.1 Human studies
Since Barker and colleagues reported the association of low birth weight with higher risk of coronary heart disease, diabetes, and hypertension in later life in multiple populations [11,13,12], programming of adult disease by adverse early life environments has been extensively studied in humans. Despite its tragic effect on the general health of the population, the Dutch Winter famine of 1944–1945 has provided an epidemiological study model of maternal undernutrition and long-term disease, demonstrating that prenatal exposure to famine results in increased risk of obesity, coronary heart disease, glucose intolerance, stress sensitivity and breast cancer [240]. The professional perinatal medical care records and official rationing records make this cohort the equivalent of an experiment intentionally set up for investigation of the effects of prenatal undernutrition in humans. During the famine, the daily rations of some mothers fell below 1000 calories per day, resulting in low infant birth weight. A recent study of people with prenatal exposure to the Biafran famine that occurred during the Nigerian civil war of 1967–1970 also demonstrates increased cardiovascular disease and Type 2 diabetes risk in later life [126]. A large body of recent human data indicates that early life events are capable of programming the HPA axis by permanently altering the set point of the HPA axis in utero, resulting in increased HPA activity and cortisol levels in adulthood, which predispose to cardiovascular disease and metabolic syndromes [5,243,302]. Investigations from a variety of populations demonstrate the association of low birth weight and elevated fasting plasma cortisol concentrations [154,221]. Increased responsiveness to dexamethasone suppression and corticotropin releasing hormone or ACTH stimulation has also been shown in people born with small stature [132,133,211,293], although in other studies investigators failed to find HPA activity alteration at the adrenal level in adults with prenatal exposure to the Dutch famine [65,64]. Other adverse prenatal factors also show effects on programming of human HPA function. Cocaine-exposed infants had a high amplitude trajectory of cortisol reactivity compared to non-cocaine-exposed infants [85]. Increased umbilical ACTH has been observed in infants with prenatal exposure to nicotine, which may link maternal smoking to HPA programming [176]. Given the widespread use of synthetic glucocorticoids to treat women at risk of preterm delivery, the direct effects of HPA axis programming by glucocorticoids have also been studied in humans. The use of synthetic glucocorticoids was endorsed by the US National Institutes of Health and was widely adopted elsewhere in 1994. Although the short-term benefits in neonatal outcomes are clear, the long-term effects of antenatal synthetic glucocorticoid exposure in humans have only recently begun to be reported, and at the present little is known specifically about the potential alterations in HPA function. In a follow-up study of late gestation synthetic gluocorticoid therapy in 30-year olds, early markers of insulin resistance were associated with prenatal betamethasone exposure, but neither patent adverse neurological or physiological effects, nor cardiovascular risk factors were identified in this group [55,56]. Further follow up studies should give more information about the association between synthetic glucocorticoids and HPA function in humans.
Due to the significant improvement in neonatal intensive care techniques, the survival rate of preterm infants has increased markedly, including survival of early preterm infants born before 28 weeks. Thus, the investigation of the potential role of synthetic glucocorticoids in developmental programming of health and disease has become increasingly important. However, although human studies of HPA function programming are of greater clinical importance as compared to animal models, difficulty in obtaining samples and using experimental intervention for detailed analysis of mechanisms limits human studies, and animal models are thus of particular importance for further investigation.
2.3.2 Animal studies
Animal models are valuable for providing a platform to direct clinical investigation and for identification of relevant mechanisms. Investigation with animal models may provide observations that are simplified but are still clinically relevant, although some effects observed in animals may not necessarily occur in humans. Rodents have comprised nearly 70% of the animals used for fetal programming studies, and other animal species commonly used for such investigation include mice, guinea pigs, sheep and monkeys [292].Rodents have strong similarity to humans at the cellular and molecular levels due to possession of similar genes (99% of mouse genes have analogues in humans) and biochemical pathways. Experimental techniques in animal studies of fetal programming include uterine artery ligation, maternal malnutrition (caloric restriction, alteration of one or more essential nutrients, such as low protein diet, high fat diet, iron restriction or zinc deficiency), glucocorticoid overexposure, maternal hypoxia and maternal administration of substances (e.g. nicotine, cocaine or alcohol). Many of these techniques, including malnutrition, hypoxia, and glucocorticoid overexposure are capable of programming HPA function in different animal models [24,201,246]. Pregnant rats fed with a low protein diet during late gestation show an increase in the HPA axis activity in newborn offspring as indicated by elevated levels of basal plasma ACTH and corticosterone [152]. Exposure to betamethasone on day 80 to 81 of gestation in sheep increases the proportion of angiotensin II type 1 receptor (AT1R), but decreases the proportion of AT2R in the kidney of adult sheep, and this may be partly responsible for the sustained blood pressure elevation in offspring [114]. In sheep, modest prenatal undernutrition leads to elevated postnatal activity of the HPA axis, as indicated by greater cortisol response after a CRH plus AVP challenge [118].
It is clear that the effects of glucocorticoid exposure on programming of subsequent physiology or pathology differ in offspring according to differences in the time point and duration of the exposure. In sheep, although the HPA axis shows vulnerability to inappropriate levels of glucocorticoids during long periods of fetal life, the early part of gestation appears to be the time of greatest vulnerability to maternal stress [226]. In pregnant rats treated with oral glucocorticoids, the β- and α-cell mass was reduced in the fetus by mechanisms that differed according to the stage of fetal development. In fetuses exposed to excessive glucocorticoids in the last week of gestation only, β-cell mass was reduced 18% and this reduction resulted mainly from impairment of β-cell commitment. However, in fetuses exposed to glucocorticoids throughout gestation, islet vascularization and decreased β-cell proliferation were involved, resulting in a 62% reduction of the β-cell mass [82]. It is probable that this phenomenon is related to the developmentally regulated expression of GR and MR in fetal organs.
It is important to acknowledge that there are differences in the development among animal species. Although the embryonic development of rats or mice shares many similarities with that of humans, there are still important differences. For example, brain development in the 22-day gestational period in rats is equivalent to that found in the first two trimesters of human gestation, and the first two weeks of postnatal development of the rat is equivalent to the third trimester of human development [14,83]. Thus, caution is required in generalizing animal data to human studies. For studies with endpoints related to data collection, different time points in gestation are another factor that must be taken into consideration, as specific time windows may vary between species. Studies in animals with short gestations may greatly overestimate some effects. These differences limit the validity of generalizing studies in animal models to humans.
3. The HPA axis and programming of neurological dysfunction
3.1 HPA and brain development
The development of the nervous system is characterized by proliferation of neurons, which requires a relatively long period of time during gestation. The brain is therefore a principal target for programming influences. At gestational age (GA) 3 weeks, the neural plate forms along the dorsal side of the embryo, and gives rise to the majority of neurons and glial cells that will function in the mature brain. The neurons that are generated move to different parts of the brain and self-organize into different structures [194]. Between GA 8 and 16 weeks, cortical plates form secondary to cell accumulation in the outer cerebral wall, and these later develop into cerebral cortex. By GA 24 weeks, synapses extend from axons and dendrites, and organization of neural circuits is gradually established by synaptic communication between neurons, which conduct sensory and motor signals and produce behavior [155]. The prenatal period is considered to be an important time for determination of neurological changes, and the nervous system is particularly vulnerable to various influences. As the end product of HPA axis activation, glucocorticoids are important for normal development and maturation of the brain, as they act to bring about initiation of terminal maturation, remodeling of axons and dendrites, and modulate the process of neural survival and process of programming cell death [182]. Dexamethasone has been shown to inhibit proliferation and accelerate maturation [1]. During the fetal development, the central nervous system’s exposure to free glucocorticoids is fairly low, due to the inactivation of glucocorticoids by placental 11β-HSD2, binding of circulating glucocorticoids to GBP, and extrusion of glucocorticoids by P-gp on the blood-brain-barrier. Approaching term (38–40 weeks), maternal glucocorticoids continue to increase while the expression of placental 11β-HSD2 and P-gp drops precipitously, which allows a glucocorticoid surge in the fetus that accelerates tissue maturation. Birth is a very stressful process, and the glucocorticoid surge near term has a profound effect on preparing the fetus for life outside of the uterus, especially in protecting the vulnerable brain from perinatal hypoxic-ischemic brain damage, known as hypoxic-ischemic encephalopathy (HIE) during the process of birth and in newborns. HIE is one of the major brain injuries in neonates, which may lead to acute mortality or neurological disability in survivors [42]. Prenatal stress may increase the vulnerability of the newborn brain to hypoxic-ischemic brain damage, and glucocorticoids and GR are involved in this process [158]. Indeed, pre-treatment of newborn rats with dexamethasone reduces hypoxic-ischemic induced brain injury, and this effect can be inhibited by a GR blocker [88], indicating that the protective role is mediated by the GR. Programming of the HPA axis caused by prenatal stress and changes in glucocorticoid receptors may reduce the protective effect of the glucocorticoid surge and GR activation.
Prenatal exposure to excess glucocorticoids has widespread effects on neuronal structure and synapse formation and may result in permanent changes in brain structure and function [130,171]. In a recent study, excess glucocorticoids were shown to retard the radial migration of post-mitotic neurons during the development of cerebral cortex [93]. Caldesmon is an actin regulatory protein that negatively controls the function of myosin II and leads to changes in cell shape and migration. Excess glucocorticoid exposure results in up-regulation of caldesmon and impairs the radial migration of neurons during cortical development. In murine neural stem cells (NSCs), dexamethasone significantly increases the sensitivity to oxidative stress-induced apoptosis and alters the phenotype of NCSs, as well as causes changes in the expression of mitochondrial respiratory chain associated genes. These effects can be blocked by the GR antagonist mifepristone, suggesting the involvement of GR [192]. Adverse maternal environments can lead to elevations of both glucocorticoids and oxidative stress in the developing fetus, which is associated with loss of neurons in the programmed brain. In rat fetuses, repetitive stress caused robust decreases in actively proliferating neural cells, reduced neurogenesis and increased cell death in females [169]. A prospective study showed that maternal stress and anxiety during pregnancy influenced brain morphology [33]. Cognitive dysfunction in offspring from mothers with maternal stress may be caused by abnormal development of fetal brain morphology. For example, reduced gray matter volume in the fetal brain may render the developing individuals more vulnerable to neurodevelopmental and psychiatric disorders, as well as to cognitive and intellectual impairment. Studies in experimental animals have explored abnormal nervous system development caused by glucocorticoids in a precise manner. Prenatal glucocorticoid administration delays the maturation of neurons, myelination, glia, and vasculature [124,123,227]. During the development, exposure of the fetal brain to high levels of stress-triggered or exogenous glucocorticoids is associated with abnormal behaviors in experimental animals and an increased risk of psychiatric disorders in humans [93]. Many studies indicate that the trajectory of brain development may be affected by the prenatal environment, such as the gestation period, maternal stress and so on [34,62], although direct evidence linking HPA to brain morphology dysfunction is still lacking.
3.2 HPA and programming of neurological dysfunction
Compelling evidence from human studies shows that programming of HPA function by early life events is associated with various kinds of neuroendocrine dysfunction, including schizophrenia, anxiety, depression, impaired stress response and abnormal behavior in offspring. Behavioral and mood disturbance in the infant and toddler period are associated with prenatal exposure to stress and stress hormones, accompanied by alterations of the HPA axis [60,59]. O’Connor and colleagues measured diurnal cortisol levels in 10 year old children with exposure to prenatal anxiety, and found that prenatal anxiety significantly elevated the cortisol level, suggesting that prenatal anxiety has lasting effects on HPA axis function in humans [204]. Antenatal exposure to maternal anxiety at 12–22 weeks of pregnancy was consistently associated with a high, flattened daytime cortisol profile, together with depressive symptoms in adult offspring [288]. These studies thus suggest the involvement of the HPA axis linking maternal anxiety and depressive symptoms in offspring. Sandman and colleagues found that elevated maternal cortisol levels at 30–32 weeks of gestation were significantly associated with increased maternal reporting of infant anxiety and depression [61]. Elevated stress, depression and anxiety during the prenatal period may slow down infant cognitive and neuromotor development [61,125], and this dysfunction persists into adolescence [179]. Additional studies from Sandman et al. reported that the psychosocial stress caused by maternal cortisol during the prenatal period was time-dependent [58]. Early exposure to elevated cortisol concentrations in gestation was associated with lower mental developmental scores at 12 months. However, late elevation of maternal cortisol in gestation was associated with higher mental developmental scores at 12 months. In contrast to the strong preclinical evidence demonstrating the relation of prenatal stress and depression and/or anxiety in adulthood, there is only relatively weak evidence of this relation in clinical reports. This may in part be related to the challenges inherent in clinical trials and studies involving pregnant women with collection of follow-up data in offspring. In patients who are diagnosed with depression and/or anxiety, the etiology is often thought to be related to genetic susceptibility or environmental inducers, such as career stress, losing a loved one or other life difficulties. The perinatal environment of an individual is barely taken into account. In addition, a deficiency of detailed perinatal records regarding maternal stress is a potential challenge to investigation of the prenatal environmental and its association with adult depression and/or anxiety. Moreover, maternal sensitivity and responsibility in the care of human infants can moderate an altered stress response that has been programmed by exposure to maternal stress [110,109], and this may further obscure the association of adult depression and anxiety to prenatal stress in clinical investigations.
Studies in experimental animals present further supporting evidence for understanding HPA function and programming of neurological dysfunction. In rats, elevated prenatal glucocorticoid levels are associated with growth retardation, sexually dimorphic alterations in HPA regulation of offspring, and programming of other physiological systems [306]. Maternal stress leads to increased anxiety and depression-like behaviors that result from dysregulation of the HPA axis in monkey and rodent offspring. The offspring have higher levels of CRH, reduced hippocampal GR expression and less endogenous opioid and GABA/BDZ (benzodiazepine) inhibitory activity [299]. Excess CRH and cortisol that pass through the placenta may impair fetal habituation to stimuli and difficulties with temperament in infants. Gestational stress also increases CRH activity in the amygdala and depression-like behavior in rats and non-human primates [301]. In addition to prenatal stress, antenatal glucocorticoid administration in animals leads to programming of neurological disorder in adulthood, which is accompanied by alterations in the HPA axis [310]. Single or repeated maternal betamethasone injections reduced the brain weight at 3.5 years postnatal age in sheep [190]. These studies indicate that prenatal glucocorticoid exposure changes the development of HPA axis, leading to persistent deficits of neurological function in offspring. Moreover, the effect of glucocorticoids on postnatal HPA responses may vary according to the time of steroid injections in gestation, and whether they are administered directly into the fetal versus maternal compartments [190,259]. In adult rat offspring, both paradigms reduce exploratory behavior in an open field, elevate plus-maze and impair behavioral responses and learning in a forced swim test. This behavioral disorder results from the increase of CRH in the amygdala and persistent reduction of hippocampal corticosteroid receptors, accompanied by the attenuation of HPA axis feedback sensitivity [304]. The hippocampus is one of the control centers of the HPA axis, and it is especially sensitive to glucocorticoids. Prenatal exposure to dexamethasone in fetal rhesus macaques decreases the number of pyramidal neurons in the hippocampal CA regions and decreases the number of granular neurons in the dentate gyrus, which is associated with degeneration of neuronal perikarya and dendrites [287].
It is noteworthy that previous studies have shown that decreased maternal glucocorticoids may also program some of the same physiological functions in the developing fetus in a biphasic manner. In maternal adrenalectomy offspring, some of the consequences are similar to those observed in offspring exposed to excess maternal glucocorticoids in utero, such as lower birth weight and higher basal plasma corticosterone levels as compared with controls. Wilcoxon and colleagues [306] observed increased depression-like behavior in adult offspring from maternal adrenalectomy animals, which is similar to those in offspring that have been prenatally stressed or exposed to elevated glucocorticoids [80,300]. Further studies are necessary to determine the mechanisms by which decreased maternal glucocorticoids developmentally program the HPA axis leading to depression-like behavior in offspring. It is possible that a common mechanism may underlie the effects of too low or too high maternal glucocorticoids on adult HPA function and programming of behavior in offspring.
4. The HPA axis and programming of cardiovascular disease
Programming of the HPA axis predicts cardiovascular disease in adulthood, and hypertension is one of the most significant risk factors for cardiovascular disease. The effect of antenatal glucocorticoids on blood pressure in offspring has been well documented. Direct infusion of exogenous cortisol into ovine fetuses increases fetal blood pressure [275], and maternal antenatal betamethasone treatment also elevates blood pressure at birth in animals [19]. Epidemiologic studies suggest that hypertension and cardiovascular disease may be programmed by prenatal factors including undernutrition and glucocorticoid overexposure through the action of elevated glucocorticoids [13,12]. Exposure to excess glucocorticoids typically leads to low birth weight in the newborn baby, and the association of low birth weight with hypertension is further supported by recent studies [63,127,147].
Animal studies provide further evidence and possible mechanisms for programming of hypertension and other cardiovascular disease by elevated HPA activity in the fetus. In rats, prenatal glucocorticoid overexposure is associated with reduced birth weight and a persistent elevation of arterial blood pressure in adult offspring [18,309]. In addition, glucocorticoids mediate the effects of maternal protein deprivation on offspring hypertension [96,115]. Inhibition of maternal glucocorticoid synthesis in pregnant rats prevents increased blood pressure in the offspring of low protein diet fed rat dams [96]. Other studies also show that although combined application of maternal undernutrition and glucocorticoids result in an additive reduction of fetal skeletal muscle mass, they may bring about this effect through a mechanism that differs from that operates when these factors act separately [75,105,195].
Blood pressure control involves many systems including the central nervous system, heart, vasculature and kidney. Insults in a key period of the fetal development may disturb the gene expression and function of these vital organs, and thus lead to impaired control of blood pressure [21,27,74,159]. Prenatal hypoxia can cause down-regulation of GR and GR-related programming of the AT2R gene, which leads to increased AT2R expression in the heart and contributes to increased cardiac vulnerability to adult ischemic injury in rat offspring [314]. Glucose transport is the rate-limiting step in cardiac glucose metabolism, and GLUT1 is a major mediator of basal cardiac glucose uptake. Maternal dexamethasone treatment in rats selectively up-regulates cardiac GLUT1 protein expression and activation of Akt, together with modest activation of the anti-apoptotic proteins PKCε and PKCα in the heart of male offspring, which may produce adverse consequences including hypertension [144]. A study using a sheep model demonstrates that prenatal cortisol treatment results in significantly higher mean arterial pressure and leads to up-regulation of angiotensinogen, AT1R, MR, and GR mRNA in the hippocampus in fetuses at 130 days of gestation, but this up-regulation of MR and GR mRNA is not found in animals at 2 months of age [76], indicating the programming effect of cortisol in the sheep model may be time dependent, and the HPA axis may not be the mechanism whereby prenatal exposure to dexamethasone programmed hypertension in sheep offspring. However this hypothesis needs further investigation. In addition to altered gene expression patterns and subsequent physiologic changes in the brain and heart, various studies point to a central role for the kidney in the etiology of programmed hypertension [316]. Nephron deficit is a consistent feature of hypertension that is programmed by fetal glucocorticoid exposure. Administration of dexamethasone by 2 daily intraperitoneal injections in pregnant rats results in reduced numbers of glomeruli and hypertension in offspring, but these consequences have a relationship with the timing of dexamethasone administration and sex of the offspring [210]. Fetal glucocorticoid exposure can disturb the development of the rennin-angiotensin system and thus participate in programming of hypertension. Prenatal glucocorticoid exposure up-regulates adult plasma renin and angiotensinogen levels, as well as renal expression of AT2R [207,258]. Moreover, increased GR and reduced 11β-HSD2 expression are found in the kidney of rats and sheep treated with a low-protein diet [20,305], which may increase renal glucocorticoid sensitivity and thus contribute to hypertension by direct effect or through the regulation of an associated gene such as Na/KATPase [311]. The timing of glucocorticoids exposure seems to be species specific for programming of hypertension, as exposure to glucocorticoids during the final week of pregnancy is sufficient to produce permanent adult hypertension in rats. However in sheep, these effects are seen in offspring exposed to glucocorticoids at early gestational age [97].
5. The HPA axis and programming of metabolic disease
The HPA axis plays a pivotal role in the regulation of metabolic function, and this effect constitutes an important component in stress response. Many studies suggest that the HPA activity plays a key role in perinatal programming of metabolic disease [291]. In humans, low birth weight may predict insulin resistance and diabetes in adult life. Overexposure of the fetus to maternal glucocorticoids may explain the link between low birth weight and subsequent glucose intolerance. People exposed to the Dutch Winter Hunger famine were found to have elevated glucose levels or glucose intolerance as adults [67,131], which may be due to insulin secretion deficit [66]. Those exposed to famine in early gestation had lipid profiles that were more atherogenic [241]. Increased HPA activity resulting from adverse early life events has been shown to result in permanent changes in systems that regulate metabolic processes including glucose homeostasis and adipose function. For example, prenatal glucocorticoid exposure in rats causes upregulation of hepatic GR expression, which increases both the expression and activity of the gluconeogenic enzyme phosphoenolypyruvate carboyxkinase (PEPCK), and programs insulin resistance and impairs glucose tolerance in adult life [48,202]. Application of glucocorticoids in the last week of gestation or throughout gestation leads to reduced beta-cell mass accompanied by decreased secretion of insulin in the fetal pancreas [82], which may be associated with hyperglycemia in adult offspring [202]. Suppression of 11β-HSD2 throughout pregnancy in rats reduces birth weight and causes hyperglycemia in adult offspring [248]. Different from 11β-HSD2 that unidirectionally inactivates glucocorticoids, 11β-HSD1 is a bidirectional enzyme that acts predominantly as an oxidoreductase to regenerate active glucocorticoids from their inactive 11-keto derivatives. Tissue glucocorticoid levels are therefore determined by both concentrations of circulating glucocorticoids and the local 11β-HSD1 activity. 11β-HSD1 is expressed in multiple tissues including the brain, adipose, liver, pancreas and placenta, and plays a critical role in regulating glucocorticoid action. In offspring of the common marmoset monkey, the mRNA expression and activity of 11β-HSD1 are permanently increased in the liver, pancreas, and subcutaneous adipose tissue following prenatal dexamethasone treatment, which may amplify glucocorticoid concentrations in local tissues involving in programming of the metabolic syndrome [203].
In addition to glucose metabolism, fat metabolism in offspring is also affected by HPA programming. In rats exposed to prenatal dexamethasone, high-fat feeding results in adipose accumulation in the liver, which may be mediated by alterations of associated gene transcription, as dexamethasone can direct depot-specific changes in the expression of genes involved in fatty acid esterification and triglyceride synthesis and storage [81]. Another study reveals that prenatal dexamethasone exposure markedly increases GR mRNA specifically in visceral adipose tissue, while fatty acid uptake is attenuated [47]. Visceral fat has a higher level of GR expression, and this depot is therefore especially sensitive to glucocorticoids [219]. In humans, lipid accumulation in visceral fat is related to other features of the metabolic syndrome [23]. These metabolic regulating mechanisms that are modified by glucocorticoids may partly explain the onset of later metabolic disease.
6. Prenatal stress and programming of immune function
Although glucocorticoids play a fundamental role in maintaining immune function homeostasis, little attention has been paid to their role in immune system programming in humans. An individual’s response to common antigens or other pathogenic factors is highly specific, suggesting a possibility of programming of the immune system by factors in the developmental environment, such as prenatal stress [181].
Evidence from recent studies shows that psychosocial stress and low levels of social support increase the levels of serum pro-inflammatory cytokines in pregnant women [51,52], and this increase in circulating maternal cytokine levels may be associated with a higher risk of allergy for the infant in later life [222]. Data from animal studies provides further evidence of alteration of immune function by prenatal stress. In rats exposed to maternal stress during the last week of gestation, both cellular and humoral immune responses are reduced in male juveniles, but enhanced in both male and female adult offspring [142]. When pregnant rats are treated with stress caused by noise and light three days a week throughout gestation, immune function is suppressed in 2-month old offspring [138]. In pregnant mice submitted to foot-shock once daily from gestation day 15 to 19, there is decreased macrophage spreading and phagocytosis, and increased growth of Ehrlich tumors in vivo in 2-month old male offspring, indicating that the immune response is suppressed [212]. In rhesus monkeys, blood cells from juvenile offspring exposed to maternal stress released reduced levels of tumor necrosis factor α and interlukin 6 upon lipopolysaccharide stimulation in vitro [49]. Prenatal stress was associated with a suppressive effect on immune function in pig offspring in a recent study. Thymus weights were significantly reduced in prenatally stressed piglets on the first and 35th days of life, and morbidity and mortality during the suckling period were significantly increased in prenatally stressed litters due to high frequencies of disease [282]. A decrease in total lymphocytes as well as in CD4+ and CD8+ lymphocytes was observed in rats exposed to prenatal stress, indicating that thymic function was altered [107,165].
Prenatal stress may induce the release of stress hormones such as glucocorticoids that play important roles in the development of fetal immune organs and immune cells. In transgenic mice with impaired glucocorticoid function, the role of glucocorticoids in the ontogeny of the immune system has been demonstrated [247]. The partial blockade of T-cell differentiation during ontogeny and profound alterations of the stromal cell compartment suggest that glucocorticoids play a key role in coordinating the physiological dialogue between developing thymocytes and their microenvironment.
7. Programming of the HPA axis
Since GR mediates the major effects of glucocorticoids in the stress response, alteration in GR levels plays a pivotal role in response to stress and programming of the HPA axis. In transgenic mice a reduction of GR levels by only 30–50% results in significant neuroendocrine, metabolic and immunological disorders [220,289], as well as in impaired HPA and blood pressure adaption [183]. Specific knock-out of GR in the forebrain impairs the negative feedback regulation of the HPA axis [8]. Studies with transgenic mice demonstrate the association of GR-overexpression with increased resistance to stress [232]. In the rat, prenatal stress or glucocorticoid overexposure instantly decreases the expression of GR in the hippocampus, accompanied by the elevation of basal glucocorticoids and altered response to stress [174,304]. Thus, programming effects on the HPA axis often involve the alteration of GR gene expression in regions of the brain that regulate stress response.
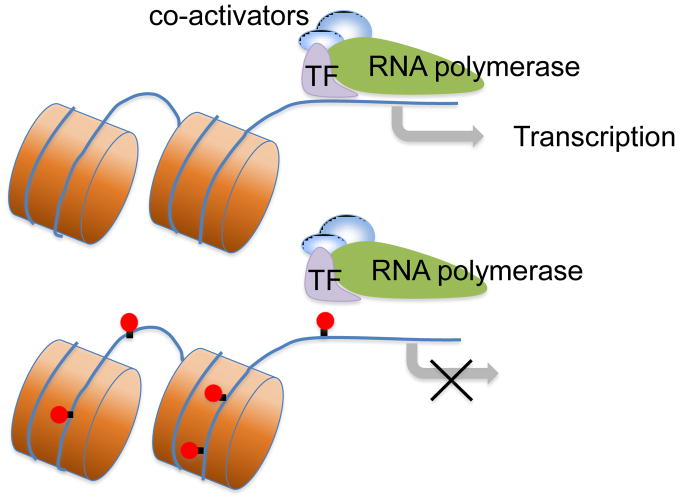
Epigenetic regulation often involves alteration of chromatin structure through histone modifications (e.g. acetylation or phosphorylation) or DNA methylation. Acetylation neutralizes the positive charge on the DNA binding histone, and “opens” the structure of the chromatin to facilitate transcription-factor binding to DNA, and consequently promotes expression of the corresponding gene. Epigenetic modification of methylation at 5′-cytosine of CpG dinucleotides is associated with long-term gene silencing either by inhibition of transcription factor binding or chromatin inactivation (Figure 2). Thus, hypomethylation of CpG dinucleotides is associated with an active chromatin structure and increased transcriptional activity [178]. Maternal stress including fetal hypoxia, maternal malnutrition and glucocorticoid overexposure has been shown to alter epigenetic modification of specific genes related to programming of the HPA axis [101,255].
Figure 2. DNA methylation inhibits gene transcription.
Transcription factors (TF) bind to corresponding binding sites on the GR (glucocorticoid receptor) promoter, and promote GR transcription. Methylation (red solid circles) modification of GR gene inhibits the transcription factor binding to the GR promoter, and leads to long-term silence of GR.
7.1 Glucocorticoid receptor gene
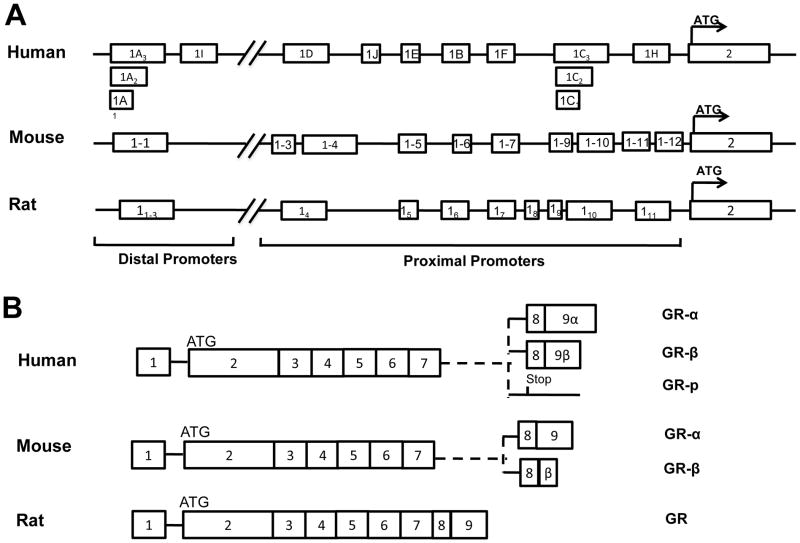
The GR gene has been extensively studied in human, mouse and rat [25,223,283]. The human GR gene spans more than 80 kb and contains 8 coding exons (exon2-9) that encode three isoforms of protein GRα, GRβ and GRp through specific splicing of exon9 [121]. Similarly, the mouse and rat GR genes span regions of more than 80 kb and 110 kb, respectively. Although the rodent GR gene is thought to encode only one type of GR protein that is equivalent to human GRα, a recent study showed a GRβ protein in mice [119]. All isoforms of the GR protein are encoded by mRNAs spliced from a common exon2, and the mRNA sequence is conserved with more than 80% similarity in humans and rodents [285]. The ATG translation start site is located in exon2, and an in-frame stop codon (TAA for mouse and rat, TGA for human) is located three codons upstream of the ATG. As a result the alternate first exons of the GR gene are untranslated and are not encoded in the protein sequence of the GR, although they have active roles in regulating tissue-specific GR expression levels. Both the human and rodent GR genes contain multiple alternate first exons, each preceded with its own promoters. In the mouse GR gene, an additional five novel exon1s have recently been cloned and these exon1s are all designated by numerical descriptors based on their distance from the translation start site, instead of by the old style alphabetical description [25]. Most of these exon1s (human Exon 1-B to 1-H, rat exon14 to 111 and mouse exon1.3 to 1.7, 1.9 to 1.12) are located in the proximal promoter region. Sequences for proximal exons range from approximately −1.1 to −4.5 kb in the mouse, −1.8 kb to −4.5 kb in humans, and −1.7 to −4.2kb in rats as measured from the translation start site. In contrast, the human Exon1-A, 1-I, rat exon11 to 13 and mouse exon1.1 are located in a distal promoter region approximately 27–30 kb upstream [98]. As previously demonstrated, high sequence homology and structural similarity of the alternate exon1s are found among rat, mouse and human GR genes [283]. The structures of human, mouse and rat GR genes are shown in Figure 3.
Figure 3. Structure of the human, mouse and rat GR genes and the potential mRNA transcripts.
(A) scheme of the human, mouse and rat GR first exons. (B) potential mRNA transcripts of GR. Human GRα is encoded by exon2-8 and exon9α, GRβ by exon2-8 and exon9β, and GR-p by exon2-7. Mouse GRα is encoded by exon2-8 and exon9, while mouse GRβ is encoded by exon2-8 and a proximal portion of intron 8. Rats have only one GR isoform that is equivalent to human GRα.
7.2 Alternative exon1s and promoter usage for tissue-specific GR expression
GR expression is regulated at both the transcriptional and post-transcriptional level. Although some studies indicate that post-transcriptional mechanisms such as mRNA stability have effect on GR expression [78], the major mechanism for control of GR expression is transcriptional [218,269,285]. Each of the alternate first exon1s of the GR gene has its own promoter, except in humans where exon1-A and 1-C have three splicing variants that share the same promoter region. The promoters of exon1s show specific activity in different tissues and this may govern the tissue specific expression of the GR. In humans, although in general not all exon1s are expressed in all tissues, the hippocampus seems to show the expression of all exon1s, and exon1-D is uniquely expressed in this region. Exon1-B and 1-C are broadly expressed in many tissues except the liver, subcutaneous adipose and heart muscle, while exon1-E and 1-F are predominantly expressed in the immune system [283]. In the rat, exon16 and exon110 are broadly expressed in many tissues as are their human homologues exon1-B and 1-C, and these represent 77–87% of total GR transcripts. The remaining exon1s show tissue specific transcription. Expression of exon11 has been observed in the thymus, but not in the liver or hippocampus. Exons 15, 17, and 111 have all been identified in the hippocampus, and 17 is uniquely expressed in this region as is its human homologue exon1-D [175]. In the mouse, exon1.6 and 1.11 are abundantly expressed in most tissues, although in some tissues exon1.1 (e.g. pituitary) and 1.12 (e.g. hippocampus and liver) are also major transcripts. Transcripts 1.4c, 1.5 and 1.10 show moderate expression (lower than 5%), and exons 1.7, 1.9 and 1.3 show the lowest levels of expression (lower than 1%) [25].
Specific promoter activity of the alternate exon1s is associated not only with tissue specific expression, but also with specific transcriptional response to stimuli. There is extensive evidence that the perinatal environment can program the HPA function through influencing the first exon usage of the GR gene in a tissue specific manner. In rats, the GR protein is dynamically expressed at different postnatal periods in the hippocampus and is involved in the regulation of neonatal HPA axis hyporesponsiveness as well as HPA function [94]. Neonatal handling significantly increases GR mRNA by 40–50% in all hippocampal subfields, which is accompanied by a 2–3 folds elevation of exon17-containing GR mRNA transcripts [205].
Similarly, the adult offspring of a mother who shows high level licking and grooming behavior (High-LG) show increased GR mRNA expression in the hippocampus and reduced HPA axis responses to restraint stress as compared with the offspring of a low-LG mother [164]. Cross-fostering studies show that offspring of a low-LG mother reared by a high-LG mother resemble the normal offspring of a high-LG mother [91]. These studies demonstrate the causal effects of maternal care levels on the tissue specific regulation of GR expression and programming of the HPA response to stress.
7.3 Epigenetic regulation of GR
Accumulating evidence indicates that environmental events affect GR gene expression mainly through epigenetic mechanisms. In a recent study the methylation status of CpG dinucleotides in all of the human exon1 promoters located in the CpG island of the GR gene was investigated, and the results showed that the human GR promoter region was extensively methylated, while levels of methylation and methylation sites were highly diverse in individuals. Most of the multiple transcription factor binding sites in the GR promoter contain methylatable CpG, suggesting that there is an active operation of epigenetic mechanisms throughout the CpG island of the GR gene [284]. The offspring of rats fed with a protein restricted diet during pregnancy show tissue-specific alterations in the expression of GR [20], and the increased expression of GR in the liver is associated with protein restriction induced hypomethylation of the GR exon110 promoter and this hypomethylation of exon110 promoter can be prevented by supplementation of folic acid [160]. Moreover, decreased methylation of the GR exon110 promoter and increased expression of hepatic GR are associated with decreased expression of DNA methyltransferase-1 [161]. Taken together, these findings show that balanced nutrition intake and the capacity for metabolism of methyl groups during pregnancy are important for the induction or prevention of tissue-specific GR promoter methylation and gene expression in offspring. A variety of transcription factor binding sites have been defined on the promoter of GR, and there are corresponding transcription factors that influence the expression of GR including YY1 (Ying Yang 1) [28], Sp1 [200], NGFI-A [198], GRE (glucocorticoid response element) [99] and others. Among these, methylation of the NGFI-A binding site in the rat promoter 17 and human promoter 1F has been extensively studied in relation to perinatal programming of subsequent disease.
It has been demonstrated that the promoter of the GR exon17 NGFI-A binding site contains two CpG dinucleotides that are potential targets for epigenetic modification [295]. The 5 CpG dinucleotide is commonly methylated in the offspring of low-LG mothers, while it is rarely methylated in the offspring of high-LG mothers. The hypomethylation of CpG dinucleotides in high-LG offspring is associated with increased NGFI-A binding, increased hippocampal GR expression and enhanced HPA feedback regulation. These changes in methylation at the NGFI-A binding site, together with some other alterations may be reversed by cross-fostering, indicating that there is a direct association between maternal care level and alterations of DNA methylation of the exon17 GR promoter. In addition, infusion of a methyl donor L-methinoine into the brain of high-LG offspring results in increased methylation in the NGFI-A binding site and decreased NGFI-A binding, which is accompanied by reduced GR expression and increased HPA response [296]. Moreover, pharmacological inhibition of histone deacetylation by trichostatin A (TSA) results in increased demethylation of the exon17 GR promoter and increased binding of NGFI-A in the adult hippocampus of low-LG offspring, and this inhibition reverses the effects of low-LG on stress response [298]. These studies demonstrate the direct effects of postnatal manipulation on programming of HPA function through epigenetic modification of the GR promoter. More importantly, these studies raise the possibility that although epigenetic programming of GR and HPA function is established early and persists throughout life, it may be modified in post-mitotic neurons that potentially provides an opportunity to correct the consequences of programming caused by adverse early life events. In the human hippocampus, the GR exon 1-F promoter is generally not methylated at the NGFI-A binding site as it is in the rat homologue exon 17 [189,284], yet prenatal exposure to maternal depression and/or anxiety results in a significant increase in GR promoter methylation and increased stress response in the adult [208]. A recent study demonstrated the alteration of gene-specific DNA methylation patterns by maternal depression with decreasing methylation of the SLC6A4 gene [71]. The SLC6A4 gene encodes an integral membrane protein serotonin transporter (5-HTT) that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons, and thus terminates the effect of serotonin. A repeat length polymorphism in the promoter of this gene has been shown to affect the uptake of serotonin and increase risk for depression in humans [39,266]. It is of note that heightened serotonin signaling causes an increase in the expression of key transcription factors, notably NGFI-A that binds to the GR exon17 promoter and increases GR expression in the hippocampus [297]. Thus, decreased methylation of the SLC6A4 gene through exposure to maternal depression leads to decreased NGFI-A binding to the GR exon17 promoter through attenuated serotonin signaling. Indeed, the 5-HTT genotype interacts with prenatal stress and shows marked influence on hippocampal GR expression and HPA function [16]. These findings underscore the importance of individual genetic background as a determinant of the consequences of epigenetic programming, at least in the case of GR, and this may in part explain the highly individualized epigenetic modification and reaction resulting from a given common stress.
7.4 Regulation of GR by glucocorticoids
In most tissues and cultured cell lines glucocorticoids down-regulate the expression of GR [45, 250, 253, 272]. Both GR protein and mRNA decreased more than 50% after 24 hours of dexamethasone treatment in cultured cell lines or in rat liver [78,120,209]. However, up-regulation of GR expression by glucocorticoids has also been found in some cell lines, mostly T-lymphocytes [6,86,111]. The glucocorticoid-induced reduction of GR transcription may be associated with repression of GR promoter activity caused by the glucocorticoid treatment [108]. The promoter 1A of the human GR gene has been associated with GR expression in many cancer cell lines, and dexamethasone treatment significantly increases 1A activity in Jurkat T cells that express GR under co-transfection [29]. Dexamethasone-induced GR up-regulation in CEM-C7 T-lymphoblast cells and down-regulation in IM-9 B B-lymphoma cells also involve the regulation of the promoter 1A [217]. Several GREs have been located within the GR promoter and may mediate the autoregulation of GR expression [100,99,98]. The promoter 1A contains a GRE adjacent to overlapping binding sites for c-Myb and c-Ets family proteins. In the presence of c-Myb, GRs bind to the promoter and increase its activity, while the interaction of GR with c-Ets family members results in down-regulated transcription of the GR [100,98]. Similar molecular mechanisms are involved in the autoregulation of GR exon1B and 1C transcription, and the glucocorticoid response unit resembles that of the 1A promoter [99]. Binding of GR and c-Myb to the GR promoter is responsible for auto-up-regulation, while an overlapping Ets-binding site (overlap with either GR or c-Myb) may blunt the auto-up-regulation and perhaps even reverse it with resulting auto-down-regulation. The mode of regulation depends both on the promoter elements and the availability of transcription factors in the cell lineage. These data may partly explain the regulation of GR by glucocorticoids in different tissue or cells, although some studies have suggested that the reduction of GR gene expression by glucocorticoids may be independent of the GR promoter. In view of the fact that glucocorticoids may be common mediators for HPA programming by early life events, the regulation of GR by glucocorticoids may play an important role in the programming process.
7.5 Involvement of oxidative stress in perinatal programming
Reactive oxygen species (ROS) are free radicals generated from the reduction of oxygen to a variety of intermediates including superoxide anion, hydrogen peroxide, hydroxyl radical and nitric oxide. ROS participate in redox signaling and mediate a wide variety of cellular functions under physiological conditions, while excess ROS production may cause oxidative stress that result in damage to cell structure and function. Oxidative stress has been observed in a variety of physiologic and pathophysiologic conditions including smoking, diabetes, hypertension and hypoxia, which are not uncommon in pregnant women. These conditions may result in increased oxidative stress either in the mother or in the developing fetus, which may be associated with programming of later disease in offspring [69,149,270,279]. Pregnant women diagnosed with IUGR show elevated values of indices of oxidative stress in the serum, indicating occurrence of oxidative stress [22,137]. In infants with IUGR, antioxidant enzyme activities are significantly decreased while the level of lipid peroxidation is significantly higher [122]. Children with low birth weight also show higher levels of lipid peroxidation and higher systolic blood pressure at 8–13 year of age as compared to age-matched children of normal birth weight [92,184]. Animal studies provide further evidence that the oxidative stress is involved in programming of disease by perinatal stress. Exposure of neonatal rats to hyperoxia resulted in increased blood pressure and vascular dysfunction in adulthood associated with enhanced vascular production of ROS [315]. In rat offspring with maternal exposure to a low-protein diet, antenatal administration of antioxidant in the dam prevents adult hypertension and vascular dysfunction [37]. In a model in which sheep were exposed to synthetic glucocorticoids in early gestation, the basal superoxide production diminished in fetal coronary arteries, and increased in 4-month-old lamb coronary arteries, suggesting programming of coronary function by ROS [239].
Increased ROS are important mediators of epigenetic modification to DNA or chromatin [40]. The influence of ROS on epigenetic alterations has been extensively studied in cancer [320]. In several cancer cell lines, ROS induces methylation and suppresses expression of important tumor suppress genes [135,225]. Cellular oxidative stress induced by hydrogen peroxide may cause formation and relocalization of a protein complex including DNA methyltransferase 1, and the function of this complex may explain cancer-specific aberrant DNA methylation and transcriptional silencing [206]. Additionally, perinatal stress is capable of producing ROS in the developing fetus, and thus may alter the epigenetic modification of important genes and lead to programming of disease in later life. Studies in the rat model demonstrate that maternal hypoxia induces increased ROS generation in the fetal heart, which increases methylation of Sp1 binding sites on the promoter of PKCε gene [215]. Oxidative stress-mediated methylation of the PKCε promoter is also found in fetal hearts in a rat model of maternal nicotine treatment [148]. In rat embryonic cardiomyocyte cell line H9c2, prolonged exposure to norepinephrine leads to increased methylation of the PKCε gene promoter through NADPH oxidase 1 dependent ROS production [312]. Taken together, these studies suggest that elevated oxidative stress in adverse intrauterine environments plays an important role in epigenetic modification of genes affecting susceptibility to subsequent disease. As a critical mediator of the programming process, the GR is susceptible to epigenetic modification and it is plausible that oxidative stress is involved in the epigenetic modification of the GR gene. Indeed, hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of GR expression [151,150]. Hypoxia or over-expression of HIF1α causes up-regulation of GR at the transcriptional level. Five putative HIF-1 binding HRE sites have been observed in the GR promoter, but the function of these sites needs further experimental evaluation.
8. Conclusion and perspectives
Accumulating evidence from epidemiologic and animal studies clearly suggests that perinatal stress sets the stage for subsequent disease and have great influence on health. It is an independent risk factor that is distinctly different from factors including genetic susceptibility or lifestyle in later life. Developmental programming of the HPA axis may be one of the common mechanisms involved because prenatal stress including malnutrition, hypoxia and glucocorticoid exposure may lead to ‘resetting’ of HPA activity and sensitivity, which is central to metabolic regulation (Figure 4). Altered metabolic features persist into adulthood and increase an individual’s susceptibility to disease. Epigenetic modification of the GR promoter is likely to play a central role in mediating these programming processes. Thus, although the administration of synthetic glucocorticoids in obstetric and neonatal practice is of obvious benefit for short-term pregnancy and neonatal outcomes, the potential undesired adverse long-term effects of these agents are too important to be ignored.
Figure 4. Programming of the HPA axis and disease.
Adverse maternal environments lead to increased exposure of the fetus to excess glucocorticoids and reset of the fetal HPA activity. Epigenetic regulation of GR (glucocorticoid receptor) plays a center role in this process. Programming of the HPA axis alters the gene expression patterns and/or permanently changes the organ structure, leading to various diseases in adulthood.
Highlights.
Adverse perinatal environments affect developmental programming of health and disease.
Reprogramming of the HPA axis is a mechanism that is commonly involved.
Epigenetic modification of GR gene expression patterns plays a central role in reprogramming of the HPA axis.
Acknowledgments
This work was supported in part by National Institutes of Health Grants HL067745 (LZ), HL082779 (LZ), HL083966 (LZ), HL089012 (LZ), HL110125 (LZ), and HD031226 (LZ). We greatly appreciate Dr. Michael A. McNutt for his valuable suggestions in editing the manuscript. We apologize to those authors whose excellent studies covered by the scope of this review were unable to be cited due to space restriction.
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aden P, Paulsen RE, Maehlen J, Loberg EM, Goverud IL, Liestol K, Lomo J. Glucocorticoids dexamethasone and hydrocortisone inhibit proliferation and accelerate maturation of chicken cerebellar granule neurons. Brain Res. 2011;1418:32–41. doi: 10.1016/j.brainres.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 2.Alfaidy N, Gupta S, DeMarco C, Caniggia I, Challis JR. Oxygen regulation of placental 11 beta-hydroxysteroid dehydrogenase 2: physiological and pathological implications. J Clin Endocrinol Metab. 2002;87:4797–805. doi: 10.1210/jc.2002-020310. [DOI] [PubMed] [Google Scholar]
- 3.Almawi WY, Melemedjian OK. Molecular mechanisms of glucocorticoid antiproliferative effects: antagonism of transcription factor activity by glucocorticoid receptor. J Leukoc Biol. 2002;71:9–15. [PubMed] [Google Scholar]
- 4.Andrews MH, Matthews SG. Regulation of glucocorticoid receptor mRNA and heat shock protein 70 mRNA in the developing sheep brain. Brain Res. 2000;878:174–82. doi: 10.1016/s0006-8993(00)02735-9. [DOI] [PubMed] [Google Scholar]
- 5.Andrews RC, Herlihy O, Livingstone DE, Andrew R, Walker BR. Abnormal cortisol metabolism and tissue sensitivity to cortisol in patients with glucose intolerance. J Clin Endocrinol Metab. 2002;87:5587–93. doi: 10.1210/jc.2002-020048. [DOI] [PubMed] [Google Scholar]
- 6.Antakly T, Thompson EB, O’Donnell D. Demonstration of the intracellular localization and up-regulation of glucocorticoid receptor by in situ hybridization and immunocytochemistry. Cancer Res. 1989;49:2230s–2234s. [PubMed] [Google Scholar]
- 7.Armitage JA, Poston L, Taylor PD. Developmental origins of obesity and the metabolic syndrome: the role of maternal obesity. Front Horm Res. 2008;36:73–84. doi: 10.1159/000115355. [DOI] [PubMed] [Google Scholar]
- 8.Arnett MG, Kolber BJ, Boyle MP, Muglia LJ. Behavioral insights from mouse models of forebrain--and amygdala-specific glucocorticoid receptor genetic disruption. Mol Cell Endocrinol. 2011;336:2–5. doi: 10.1016/j.mce.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Attig L, Solomon G, Ferezou J, Abdennebi-Najar L, Taouis M, Gertler A, Djiane J. Early postnatal leptin blockage leads to a long-term leptin resistance and susceptibility to diet-induced obesity in rats. Int J Obes (Lond) 2008;32:1153–60. doi: 10.1038/ijo.2008.39. [DOI] [PubMed] [Google Scholar]
- 10.Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–9. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 12.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–80. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 13.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 14.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicology. 1993;14:83–144. [PubMed] [Google Scholar]
- 15.Beard JL. Why iron deficiency is important in infant development. J Nutr. 2008;138:2534–6. doi: 10.1093/jn/138.12.2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belay H, Burton CL, Lovic V, Meaney MJ, Sokolowski M, Fleming AS. Early adversity and serotonin transporter genotype interact with hippocampal glucocorticoid receptor mRNA expression, corticosterone, and behavior in adult male rats. Behav Neurosci. 2011;125:150–60. doi: 10.1037/a0022891. [DOI] [PubMed] [Google Scholar]
- 17.Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46:161–6. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- 18.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–41. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 19.Berry LM, Polk DH, Ikegami M, Jobe AH, Padbury JF, Ervin MG. Preterm newborn lamb renal and cardiovascular responses after fetal or maternal antenatal betamethasone. Am J Physiol. 1997;272:R1972–9. doi: 10.1152/ajpregu.1997.272.6.R1972. [DOI] [PubMed] [Google Scholar]
- 20.Bertram C, Trowern AR, Copin N, Jackson AA, Whorwood CB. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11beta-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology. 2001;142:2841–53. doi: 10.1210/endo.142.7.8238. [DOI] [PubMed] [Google Scholar]
- 21.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol. 2011;26:1529–33. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 22.Biri A, Bozkurt N, Turp A, Kavutcu M, Himmetoglu O, Durak I. Role of oxidative stress in intrauterine growth restriction. Gynecol Obstet Invest. 2007;64:187–92. doi: 10.1159/000106488. [DOI] [PubMed] [Google Scholar]
- 23.Bjorntorp P. Metabolic implications of body fat distribution. Diabetes Care. 1991;14:1132–43. doi: 10.2337/diacare.14.12.1132. [DOI] [PubMed] [Google Scholar]
- 24.Bloomfield FH, Oliver MH, Hawkins P, Holloway AC, Campbell M, Gluckman PD, Harding JE, Challis JR. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology. 2004;145:4278–85. doi: 10.1210/en.2004-0424. [DOI] [PubMed] [Google Scholar]
- 25.Bockmuhl Y, Murgatroyd CA, Kuczynska A, Adcock IM, Almeida OF, Spengler D. Differential regulation and function of 5′-untranslated GR-exon 1 transcripts. Mol Endocrinol. 2011;25:1100–10. doi: 10.1210/me.2010-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005;115:e290–6. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 27.Brawley L, Poston L, Hanson MA. Mechanisms underlying the programming of small artery dysfunction: review of the model using low protein diet in pregnancy in the rat. Arch Physiol Biochem. 2003;111:23–35. doi: 10.1076/apab.111.1.23.15138. [DOI] [PubMed] [Google Scholar]
- 28.Breslin MB, Vedeckis WV. The human glucocorticoid receptor promoter upstream sequences contain binding sites for the ubiquitous transcription factor, Yin Yang 1. J Steroid Biochem Mol Biol. 1998;67:369–81. doi: 10.1016/s0960-0760(98)00138-1. [DOI] [PubMed] [Google Scholar]
- 29.Breslin MB, Geng CD, Vedeckis WV. Multiple promoters exist in the human GR gene, one of which is activated by glucocorticoids. Mol Endocrinol. 2001;15:1381–95. doi: 10.1210/mend.15.8.0696. [DOI] [PubMed] [Google Scholar]
- 30.Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–7. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 31.Bruin JE, Gerstein HC, Holloway AC. Long-term consequences of fetal and neonatal nicotine exposure: a critical review. Toxicol Sci. 2010;116:364–74. doi: 10.1093/toxsci/kfq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgueno AL, Cabrerizo R, Gonzales Mansilla N, Sookoian S, Pirola CJ. Maternal high-fat intake during pregnancy programs metabolic-syndrome-related phenotypes through liver mitochondrial DNA copy number and transcriptional activity of liver PPARGC1A. J Nutr Biochem. 2012 doi: 10.1016/j.jnutbio.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 33.Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35:141–53. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–5. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buttgereit F, Scheffold A. Rapid glucocorticoid effects on immune cells. Steroids. 2002;67:529–34. doi: 10.1016/s0039-128x(01)00171-4. [DOI] [PubMed] [Google Scholar]
- 36.Cadet R, Pradier P, Dalle M, Delost P. Effects of prenatal maternal stress on the pituitary adrenocortical reactivity in guinea-pig pups. J Dev Physiol. 1986;8:467–75. [PubMed] [Google Scholar]
- 37.Cambonie G, Comte B, Yzydorczyk C, Ntimbane T, Germain N, Le NL, Pladys P, Gauthier C, Lahaie I, Abran D, Lavoie JC, Nuyt AM. Antenatal antioxidant prevents adult hypertension, vascular dysfunction, and microvascular rarefaction associated with in utero exposure to a low-protein diet. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1236–45. doi: 10.1152/ajpregu.00227.2006. [DOI] [PubMed] [Google Scholar]
- 38.Camm EJ, Martin-Gronert MS, Wright NL, Hansell JA, Ozanne SE, Giussani DA. Prenatal hypoxia independent of undernutrition promotes molecular markers of insulin resistance in adult offspring. FASEB J. 2011;25:420–7. doi: 10.1096/fj.10-158188. [DOI] [PubMed] [Google Scholar]
- 39.Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 40.Cerda S, Weitzman SA. Influence of oxygen radical injury on DNA methylation. Mutat Res. 1997;386:141–52. doi: 10.1016/s1383-5742(96)00050-6. [DOI] [PubMed] [Google Scholar]
- 41.Cerf ME. High fat programming of beta-cell failure. Adv Exp Med Biol. 2010;654:77–89. doi: 10.1007/978-90-481-3271-3_5. [DOI] [PubMed] [Google Scholar]
- 42.Chen W, Ostrowski RP, Obenaus A, Zhang JH. Prodeath or prosurvival: two facets of hypoxia inducible factor-1 in perinatal brain injury. Exp Neurol. 2009;216:7–15. doi: 10.1016/j.expneurol.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cherif H, Reusens B, Dahri S, Remacle C. A protein-restricted diet during pregnancy alters in vitro insulin secretion from islets of fetal Wistar rats. J Nutr. 2001;131:1555–9. doi: 10.1093/jn/131.5.1555. [DOI] [PubMed] [Google Scholar]
- 44.Choi J, Li C, McDonald TJ, Comuzzie A, Mattern V, Nathanielsz PW. Emergence of insulin resistance in juvenile baboon offspring of mothers exposed to moderate maternal nutrient reduction. Am J Physiol Regul Integr Comp Physiol. 2011;301:R757–62. doi: 10.1152/ajpregu.00051.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cidlowski JA, Cidlowski NB. Regulation of glucocorticoid receptors by glucocorticoids in cultured HeLa S3 cells. Endocrinology. 1981;109:1975–82. doi: 10.1210/endo-109-6-1975. [DOI] [PubMed] [Google Scholar]
- 46.Cintra A, Solfrini V, Bunnemann B, Okret S, Bortolotti F, Gustafsson JA, Fuxe K. Prenatal development of glucocorticoid receptor gene expression and immunoreactivity in the rat brain and pituitary gland: a combined in situ hybridization and immunocytochemical analysis. Neuroendocrinology. 1993;57:1133–47. doi: 10.1159/000126480. [DOI] [PubMed] [Google Scholar]
- 47.Cleasby ME, Kelly PA, Walker BR, Seckl JR. Programming of rat muscle and fat metabolism by in utero overexposure to glucocorticoids. Endocrinology. 2003;144:999–1007. doi: 10.1210/en.2002-220559. [DOI] [PubMed] [Google Scholar]
- 48.Cleasby ME, Livingstone DE, Nyirenda MJ, Seckl JR, Walker BR. Is programming of glucocorticoid receptor expression by prenatal dexamethasone in the rat secondary to metabolic derangement in adulthood? Eur J Endocrinol. 2003;148:129–38. doi: 10.1530/eje.0.1480129. [DOI] [PubMed] [Google Scholar]
- 49.Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87:675–81. doi: 10.1210/jcem.87.2.8233. [DOI] [PubMed] [Google Scholar]
- 50.Cottrell EC, Seckl JR. Prenatal stress, glucocorticoids and the programming of adult disease. Front Behav Neurosci. 2009;3:19. doi: 10.3389/neuro.08.019.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coussons-Read ME, Okun ML, Nettles CD. Psychosocial stress increases inflammatory markers and alters cytokine production across pregnancy. Brain Behav Immun. 2007;21:343–50. doi: 10.1016/j.bbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 52.Coussons-Read ME, Okun ML, Schmitt MP, Giese S. Prenatal stress alters cytokine levels in a manner that may endanger human pregnancy. Psychosom Med. 2005;67:625–31. doi: 10.1097/01.psy.0000170331.74960.ad. [DOI] [PubMed] [Google Scholar]
- 53.Croxtall JD, Choudhury Q, Flower RJ. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol. 2000;130:289–98. doi: 10.1038/sj.bjp.0703272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dallman MF, Akana SF, Levin N, Walker CD, Bradbury MJ, Suemaru S, Scribner KS. Corticosteroids and the control of function in the hypothalamo-pituitary-adrenal (HPA) axis. Ann N Y Acad Sci. 1994;746:22–31. doi: 10.1111/j.1749-6632.1994.tb39206.x. discussion 31–2, 64–7. [DOI] [PubMed] [Google Scholar]
- 55.Dalziel SR, Lim VK, Lambert A, McCarthy D, Parag V, Rodgers A, Harding JE. Antenatal exposure to betamethasone: psychological functioning and health related quality of life 31 years after inclusion in randomised controlled trial. BMJ. 2005;331:665. doi: 10.1136/bmj.38576.494363.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dalziel SR, Walker NK, Parag V, Mantell C, Rea HH, Rodgers A, Harding JE. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–62. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 57.Dave-Sharma S, Wilson RC, Harbison MD, Newfield R, Azar MR, Krozowski ZS, Funder JW, Shackleton CH, Bradlow HL, Wei JQ, Hertecant J, Moran A, Neiberger RE, Balfe JW, Fattah A, Daneman D, Akkurt HI, De Santis C, New MI. Examination of genotype and phenotype relationships in 14 patients with apparent mineralocorticoid excess. J Clin Endocrinol Metab. 1998;83:2244–54. doi: 10.1210/jcem.83.7.4986. [DOI] [PubMed] [Google Scholar]
- 58.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–29. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davis EP, Glynn LM, Dunkel Schetter C, Hobel C, Chicz-Demet A, Sandman CA. Corticotropin-releasing hormone during pregnancy is associated with infant temperament. Dev Neurosci. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- 61.Davis EP, Glynn LM, Schetter CD, Hobel C, Chicz-Demet A, Sandman CA. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–46. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 62.Davis EP, Buss C, Muftuler LT, Head K, Hasso A, Wing DA, Hobel C, Sandman CA. Children’s Brain Development Benefits from Longer Gestation. Front Psychol. 2011;2:1. doi: 10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226–34. doi: 10.1161/HYPERTENSIONAHA.111.181784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Rooij SR, Painter RC, Phillips DI, Osmond C, Tanck MW, Bossuyt PM, Roseboom TJ. Cortisol responses to psychological stress in adults after prenatal exposure to the Dutch famine. Psychoneuroendocrinology. 2006;31:1257–65. doi: 10.1016/j.psyneuen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 65.de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Bossuyt PM, Bleker OP, Roseboom TJ. Hypothalamic-pituitary-adrenal axis activity in adults who were prenatally exposed to the Dutch famine. Eur J Endocrinol. 2006;155:153–60. doi: 10.1530/eje.1.02193. [DOI] [PubMed] [Google Scholar]
- 66.de Rooij SR, Painter RC, Phillips DI, Osmond C, Michels RP, Godsland IF, Bossuyt PM, Bleker OP, Roseboom TJ. Impaired insulin secretion after prenatal exposure to the Dutch famine. Diabetes Care. 2006;29:1897–901. doi: 10.2337/dc06-0460. [DOI] [PubMed] [Google Scholar]
- 67.de Rooij SR, Painter RC, Roseboom TJ, Phillips DI, Osmond C, Barker DJ, Tanck MW, Michels RP, Bossuyt PM, Bleker OP. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia. 2006;49:637–43. doi: 10.1007/s00125-005-0136-9. [DOI] [PubMed] [Google Scholar]
- 68.de Vries A, Holmes MC, Heijnis A, Seier JV, Heerden J, Louw J, Wolfe-Coote S, Meaney MJ, Levitt NS, Seckl JR. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117:1058–67. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dennery PA. Oxidative stress in development: nature or nurture? Free Radic Biol Med. 2010;49:1147–51. doi: 10.1016/j.freeradbiomed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 70.Desai M, Ross MG. Fetal programming of adipose tissue: effects of intrauterine growth restriction and maternal obesity/high-fat diet. Semin Reprod Med. 2011;29:237–45. doi: 10.1055/s-0031-1275517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One. 2010;5:e12201. doi: 10.1371/journal.pone.0012201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J Neurosci. 2003;23:4850–7. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diaz R, Brown RW, Seckl JR. Distinct ontogeny of glucocorticoid and mineralocorticoid receptor and 11beta-hydroxysteroid dehydrogenase types I and II mRNAs in the fetal rat brain suggest a complex control of glucocorticoid actions. J Neurosci. 1998;18:2570–80. doi: 10.1523/JNEUROSCI.18-07-02570.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dodic M, Moritz K, Koukoulas I, Wintour EM. Programmed hypertension: kidney, brain or both? Trends Endocrinol Metab. 2002;13:403–8. doi: 10.1016/s1043-2760(02)00693-8. [DOI] [PubMed] [Google Scholar]
- 75.Dodic M, Abouantoun T, O’Connor A, Wintour EM, Moritz KM. Programming effects of short prenatal exposure to dexamethasone in sheep. Hypertension. 2002;40:729–34. doi: 10.1161/01.hyp.0000036455.62159.7e. [DOI] [PubMed] [Google Scholar]
- 76.Dodic M, Hantzis V, Duncan J, Rees S, Koukoulas I, Johnson K, Wintour EM, Moritz K. Programming effects of short prenatal exposure to cortisol. FASEB J. 2002;16:1017–26. doi: 10.1096/fj.01-1045com. [DOI] [PubMed] [Google Scholar]
- 77.Dong Y, Thompson LP. Differential expression of endothelial nitric oxide synthase in coronary and cardiac tissue in hypoxic fetal guinea pig hearts. J Soc Gynecol Investig. 2006;13:483–90. doi: 10.1016/j.jsgi.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 78.Dong Y, Poellinger L, Gustafsson JA, Okret S. Regulation of glucocorticoid receptor expression: evidence for transcriptional and posttranslational mechanisms. Mol Endocrinol. 1988;2:1256–64. doi: 10.1210/mend-2-12-1256. [DOI] [PubMed] [Google Scholar]
- 79.Dong Y, Yu Z, Sun Y, Zhou H, Stites J, Newell K, Weiner CP. Chronic fetal hypoxia produces selective brain injury associated with altered nitric oxide synthases. Am J Obstet Gynecol. 2011;204:254, e16–28. doi: 10.1016/j.ajog.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drago F, Di Leo F, Giardina L. Prenatal stress induces body weight deficit and behavioural alterations in rats: the effect of diazepam. Eur Neuropsychopharmacol. 1999;9:239–45. doi: 10.1016/s0924-977x(98)00032-7. [DOI] [PubMed] [Google Scholar]
- 81.Drake AJ, Raubenheimer PJ, Kerrigan D, McInnes KJ, Seckl JR, Walker BR. Prenatal dexamethasone programs expression of genes in liver and adipose tissue and increased hepatic lipid accumulation but not obesity on a high-fat diet. Endocrinology. 2010;151:1581–7. doi: 10.1210/en.2009-1088. [DOI] [PubMed] [Google Scholar]
- 82.Dumortier O, Theys N, Ahn MT, Remacle C, Reusens B. Impairment of rat fetal beta-cell development by maternal exposure to dexamethasone during different time-windows. PLoS One. 2011;6:e25576. doi: 10.1371/journal.pone.0025576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dwyer JB, Broide RS, Leslie FM. Nicotine and brain development. Birth Defects Res C Embryo Today. 2008;84:30–44. doi: 10.1002/bdrc.20118. [DOI] [PubMed] [Google Scholar]
- 84.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–7. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 85.Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Dev. 2009;80:528–43. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eisen LP, Elsasser MS, Harmon JM. Positive regulation of the glucocorticoid receptor in human T-cells sensitive to the cytolytic effects of glucocorticoids. J Biol Chem. 1988;263:12044–8. [PubMed] [Google Scholar]
- 87.Evanson NK, Tasker JG, Hill MN, Hillard CJ, Herman JP. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology. 2010;151:4811–9. doi: 10.1210/en.2010-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feng Y, Rhodes PG, Bhatt AJ. Dexamethasone pre-treatment protects brain against hypoxic-ischemic injury partially through up-regulation of vascular endothelial growth factor A in neonatal rats. Neuroscience. 2011;179:223–32. doi: 10.1016/j.neuroscience.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 89.Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–7. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- 90.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 91.Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–8. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- 92.Franco MC, Kawamoto EM, Gorjao R, Rastelli VM, Curi R, Scavone C, Sawaya AL, Fortes ZB, Sesso R. Biomarkers of oxidative stress and antioxidant status in children born small for gestational age: evidence of lipid peroxidation. Pediatr Res. 2007;62:204–8. doi: 10.1203/PDR.0b013e3180986d04. [DOI] [PubMed] [Google Scholar]
- 93.Fukumoto K, Morita T, Mayanagi T, Tanokashira D, Yoshida T, Sakai A, Sobue K. Detrimental effects of glucocorticoids on neuronal migration during brain development. Mol Psychiatry. 2009;14:1119–31. doi: 10.1038/mp.2009.60. [DOI] [PubMed] [Google Scholar]
- 94.Galeeva A, Ordyan N, Pivina S, Pelto-Huikko M. Expression of glucocorticoid receptors in the hippocampal region of the rat brain during postnatal development. J Chem Neuroanat. 2006;31:216–25. doi: 10.1016/j.jchemneu.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 95.Gardner DK, Pool TB, Lane M. Embryo nutrition and energy metabolism and its relationship to embryo growth, differentiation, and viability. Semin Reprod Med. 2000;18:205–18. doi: 10.1055/s-2000-12559. [DOI] [PubMed] [Google Scholar]
- 96.Gardner DS, Jackson AA, Langley-Evans SC. Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension. 1997;30:1525–30. doi: 10.1161/01.hyp.30.6.1525. [DOI] [PubMed] [Google Scholar]
- 97.Gatford KL, Wintour EM, De Blasio MJ, Owens JA, Dodic M. Differential timing for programming of glucose homoeostasis, sensitivity to insulin and blood pressure by in utero exposure to dexamethasone in sheep. Clin Sci (Lond) 2000;98:553–60. doi: 10.1042/cs0980553. [DOI] [PubMed] [Google Scholar]
- 98.Geng CD, Vedeckis WV. Steroid-responsive sequences in the human glucocorticoid receptor gene 1A promoter. Mol Endocrinol. 2004;18:912–24. doi: 10.1210/me.2003-0157. [DOI] [PubMed] [Google Scholar]
- 99.Geng CD, Schwartz JR, Vedeckis WV. A conserved molecular mechanism is responsible for the auto-up-regulation of glucocorticoid receptor gene promoters. Mol Endocrinol. 2008;22:2624–42. doi: 10.1210/me.2008-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Geng CD, Pedersen KB, Nunez BS, Vedeckis WV. Human glucocorticoid receptor alpha transcript splice variants with exon 2 deletions: evidence for tissue- and cell type-specific functions. Biochemistry. 2005;44:7395–405. doi: 10.1021/bi047485e. [DOI] [PubMed] [Google Scholar]
- 101.Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol. 2010;54:507–23. doi: 10.1387/ijdb.082770cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghosh P, Bitsanis D, Ghebremeskel K, Crawford MA, Poston L. Abnormal aortic fatty acid composition and small artery function in offspring of rats fed a high fat diet in pregnancy. J Physiol. 2001;533:815–22. doi: 10.1111/j.1469-7793.2001.00815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res. 2001;49:490–4. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- 104.Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004;15:183–7. doi: 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 105.Gokulakrishnan G, Estrada IJ, Sosa HA, Fiorotto ML. In utero Glucocorticoid Exposure Reduces Fetal Skeletal Muscle Mass in Rats Independent of Effects on Maternal Nutrition. Am J Physiol Regul Integr Comp Physiol. 2012 doi: 10.1152/ajpregu.00466.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goland RS, Jozak S, Warren WB, Conwell IM, Stark RI, Tropper PJ. Elevated levels of umbilical cord plasma corticotropin-releasing hormone in growth-retarded fetuses. J Clin Endocrinol Metab. 1993;77:1174–9. doi: 10.1210/jcem.77.5.8077309. [DOI] [PubMed] [Google Scholar]
- 107.Gotz AA, Stefanski V. Psychosocial maternal stress during pregnancy affects serum corticosterone, blood immune parameters and anxiety behaviour in adult male rat offspring. Physiol Behav. 2007;90:108–15. doi: 10.1016/j.physbeh.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 108.Govindan MV, Pothier F, Leclerc S, Palaniswami R, Xie B. Human glucocorticoid receptor gene promotor-homologous down regulation. J Steroid Biochem Mol Biol. 1991;40:317–23. doi: 10.1016/0960-0760(91)90197-d. [DOI] [PubMed] [Google Scholar]
- 109.Grant KA, McMahon C, Reilly N, Austin MP. Maternal sensitivity moderates the impact of prenatal anxiety disorder on infant responses to the still-face procedure. Infant Behav Dev. 2010;33:453–62. doi: 10.1016/j.infbeh.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Grant KA, McMahon C, Austin MP, Reilly N, Leader L, Ali S. Maternal prenatal anxiety, postnatal caregiving and infants’ cortisol responses to the still-face procedure. Dev Psychobiol. 2009;51:625–37. doi: 10.1002/dev.20397. [DOI] [PubMed] [Google Scholar]
- 111.Gruol DJ, Rajah FM, Bourgeois S. Cyclic AMP-dependent protein kinase modulation of the glucocorticoid-induced cytolytic response in murine T-lymphoma cells. Mol Endocrinol. 1989;3:2119–27. doi: 10.1210/mend-3-12-2119. [DOI] [PubMed] [Google Scholar]
- 112.Guo F, Jen KL. High-fat feeding during pregnancy and lactation affects offspring metabolism in rats. Physiol Behav. 1995;57:681–6. doi: 10.1016/0031-9384(94)00342-4. [DOI] [PubMed] [Google Scholar]
- 113.Guo R, Hou W, Dong Y, Yu Z, Stites J, Weiner CP. Brain injury caused by chronic fetal hypoxemia is mediated by inflammatory cascade activation. Reprod Sci. 2010;17:540–8. doi: 10.1177/1933719110364061. [DOI] [PubMed] [Google Scholar]
- 114.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension. 2011;57:620–6. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Habib S, Gattineni J, Twombley K, Baum M. Evidence that prenatal programming of hypertension by dietary protein deprivation is mediated by fetal glucocorticoid exposure. Am J Hypertens. 2011;24:96–101. doi: 10.1038/ajh.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 117.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–89. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 118.Hawkins P, Steyn C, McGarrigle HH, Calder NA, Saito T, Stratford LL, Noakes DE, Hansona MA. Cardiovascular and hypothalamic-pituitary-adrenal axis development in late gestation fetal sheep and young lambs following modest maternal nutrient restriction in early gestation. Reprod Fertil Dev. 2000;12:443–56. doi: 10.1071/rd99071. [DOI] [PubMed] [Google Scholar]
- 119.Hinds TD, Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, Sanchez ER. Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Mol Endocrinol. 2010;24:1715–27. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hoeck W, Rusconi S, Groner B. Down-regulation and phosphorylation of glucocorticoid receptors in cultured cells. Investigations with a monospecific antiserum against a bacterially expressed receptor fragment. J Biol Chem. 1989;264:14396–402. [PubMed] [Google Scholar]
- 121.Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–41. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hracsko Z, Orvos H, Novak Z, Pal A, Varga IS. Evaluation of oxidative stress markers in neonates with intra-uterine growth retardation. Redox Rep. 2008;13:11–6. doi: 10.1179/135100008X259097. [DOI] [PubMed] [Google Scholar]
- 123.Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Int J Dev Neurosci. 2001;19:415–25. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 124.Huang WL, Harper CG, Evans SF, Newnham JP, Dunlop SA. Repeated prenatal corticosteroid administration delays astrocyte and capillary tight junction maturation in fetal sheep. Int J Dev Neurosci. 2001;19:487–93. doi: 10.1016/s0736-5748(01)00035-1. [DOI] [PubMed] [Google Scholar]
- 125.Huizink AC, Robles de Medina PG, Mulder EJ, Visser GH, Buitelaar JK. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–8. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- 126.Hult M, Tornhammar P, Ueda P, Chima C, Bonamy AK, Ozumba B, Norman M. Hypertension, diabetes and overweight: looming legacies of the Biafran famine. PLoS One. 2010;5:e13582. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J Hypertens. 2000;18:815–31. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 128.Jaquet D, Leger J, Levy-Marchal C, Oury JF, Czernichow P. Ontogeny of leptin in human fetuses and newborns: effect of intrauterine growth retardation on serum leptin concentrations. J Clin Endocrinol Metab. 1998;83:1243–6. doi: 10.1210/jcem.83.4.4731. [DOI] [PubMed] [Google Scholar]
- 129.Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health. 1997;87:1003–7. doi: 10.2105/ajph.87.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Joels M, Karst H, Krugers HJ, Lucassen PJ. Chronic stress: implications for neuronal morphology, function and neurogenesis. Front Neuroendocrinol. 2007;28:72–96. doi: 10.1016/j.yfrne.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Kahn HS. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 2001;357:1798–9. doi: 10.1016/S0140-6736(00)04911-4. [DOI] [PubMed] [Google Scholar]
- 132.Kajantie E, Eriksson J, Barker DJ, Forsen T, Osmond C, Wood PJ, Andersson S, Dunkel L, Phillips DI. Birthsize, gestational age and adrenal function in adult life: studies of dexamethasone suppression and ACTH1–24 stimulation. Eur J Endocrinol. 2003;149:569–75. doi: 10.1530/eje.0.1490569. [DOI] [PubMed] [Google Scholar]
- 133.Kajantie E, Feldt K, Raikkonen K, Phillips DI, Osmond C, Heinonen K, Pesonen AK, Andersson S, Barker DJ, Eriksson JG. Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. J Clin Endocrinol Metab. 2007;92:4094–100. doi: 10.1210/jc.2007-1539. [DOI] [PubMed] [Google Scholar]
- 134.Kalabis GM, Kostaki A, Andrews MH, Petropoulos S, Gibb W, Matthews SG. Multidrug resistance phosphoglycoprotein (ABCB1) in the mouse placenta: fetal protection. Biol Reprod. 2005;73:591–7. doi: 10.1095/biolreprod.105.042242. [DOI] [PubMed] [Google Scholar]
- 135.Kang KA, Zhang R, Kim GY, Bae SC, Hyun JW. Epigenetic changes induced by oxidative stress in colorectal cancer cells: methylation of tumor suppressor RUNX3. Tumour Biol. 2012;33:403–12. doi: 10.1007/s13277-012-0322-6. [DOI] [PubMed] [Google Scholar]
- 136.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Karowicz-Bilinska A, Kedziora-Kornatowska K, Bartosz G. Indices of oxidative stress in pregnancy with fetal growth restriction. Free Radic Res. 2007;41:870–3. doi: 10.1080/10715760701291647. [DOI] [PubMed] [Google Scholar]
- 138.Kay G, Tarcic N, Poltyrev T, Weinstock M. Prenatal stress depresses immune function in rats. Physiol Behav. 1998;63:397–402. doi: 10.1016/s0031-9384(97)00456-3. [DOI] [PubMed] [Google Scholar]
- 139.Khan IY, Dekou V, Douglas G, Jensen R, Hanson MA, Poston L, Taylor PD. A high-fat diet during rat pregnancy or suckling induces cardiovascular dysfunction in adult offspring. Am J Physiol Regul Integr Comp Physiol. 2005;288:R127–33. doi: 10.1152/ajpregu.00354.2004. [DOI] [PubMed] [Google Scholar]
- 140.Khashan AS, McNamee R, Abel KM, Pedersen MG, Webb RT, Kenny LC, Mortensen PB, Baker PN. Reduced infant birthweight consequent upon maternal exposure to severe life events. Psychosom Med. 2008;70:688–94. doi: 10.1097/PSY.0b013e318177940d. [DOI] [PubMed] [Google Scholar]
- 141.Kitraki E, Alexis MN, Papalopoulou M, Stylianopoulou F. Glucocorticoid receptor gene expression in the embryonic rat brain. Neuroendocrinology. 1996;63:305–17. doi: 10.1159/000126971. [DOI] [PubMed] [Google Scholar]
- 142.Klein SL, Rager DR. Prenatal stress alters immune function in the offspring of rats. Dev Psychobiol. 1995;28:321–36. doi: 10.1002/dev.420280603. [DOI] [PubMed] [Google Scholar]
- 143.Laitinen J, Power C, Jarvelin MR. Family social class, maternal body mass index, childhood body mass index, and age at menarche as predictors of adult obesity. Am J Clin Nutr. 2001;74:287–94. doi: 10.1093/ajcn/74.3.287. [DOI] [PubMed] [Google Scholar]
- 144.Langdown ML, Holness MJ, Sugden MC. Early growth retardation induced by excessive exposure to glucocorticoids in utero selectively increases cardiac GLUT1 protein expression and Akt/protein kinase B activity in adulthood. J Endocrinol. 2001;169:11–22. doi: 10.1677/joe.0.1690011. [DOI] [PubMed] [Google Scholar]
- 145.Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–22. doi: 10.1042/cs0860217. discussion 121. [DOI] [PubMed] [Google Scholar]
- 146.Langley-Evans SC. Developmental programming of health and disease. Proc Nutr Soc. 2006;65:97–105. doi: 10.1079/pns2005478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Law CM, Shiell AW, Newsome CA, Syddall HE, Shinebourne EA, Fayers PM, Martyn CN, de Swiet M. Fetal, infant, and childhood growth and adult blood pressure: a longitudinal study from birth to 22 years of age. Circulation. 2002;105:1088–92. doi: 10.1161/hc0902.104677. [DOI] [PubMed] [Google Scholar]
- 148.Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes PKCepsilon gene repression by promoter methylation in rat hearts. Cardiovasc Res. 2011;89:89–97. doi: 10.1093/cvr/cvq270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Leduc L, Levy E, Bouity-Voubou M, Delvin E. Fetal programming of atherosclerosis: possible role of the mitochondria. Eur J Obstet Gynecol Reprod Biol. 2010;149:127–30. doi: 10.1016/j.ejogrb.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 150.Leonard MO, Godson C, Brady HR, Taylor CT. Potentiation of glucocorticoid activity in hypoxia through induction of the glucocorticoid receptor. J Immunol. 2005;174:2250–7. doi: 10.4049/jimmunol.174.4.2250. [DOI] [PubMed] [Google Scholar]
- 151.Leonard MO, Cottell DC, Godson C, Brady HR, Taylor CT. The role of HIF-1 alpha in transcriptional regulation of the proximal tubular epithelial cell response to hypoxia. J Biol Chem. 2003;278:40296–304. doi: 10.1074/jbc.M302560200. [DOI] [PubMed] [Google Scholar]
- 152.Lesage J, Blondeau B, Grino M, Breant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- 153.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–8. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 154.Levitt NS, Lambert EV, Woods D, Hales CN, Andrew R, Seckl JR. Impaired glucose tolerance and elevated blood pressure in low birth weight, nonobese, young south african adults: early programming of cortisol axis. J Clin Endocrinol Metab. 2000;85:4611–8. doi: 10.1210/jcem.85.12.7039. [DOI] [PubMed] [Google Scholar]
- 155.Levitt P. Structural and functional maturation of the developing primate brain. J Pediatr. 2003;143:S35–45. doi: 10.1067/s0022-3476(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 156.Levy BH, Tasker JG. Synaptic regulation of the hypothalamic-pituitary-adrenal axis and its modulation by glucocorticoids and stress. Front Cell Neurosci. 2012;6:24. doi: 10.3389/fncel.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lewis RM, Petry CJ, Ozanne SE, Hales CN. Effects of maternal iron restriction in the rat on blood pressure, glucose tolerance, and serum lipids in the 3-month-old offspring. Metabolism. 2001;50:562–7. doi: 10.1053/meta.2001.22516. [DOI] [PubMed] [Google Scholar]
- 158.Li Y, Gonzalez P, Zhang L. Fetal stress and programming of hypoxic/ischemic-sensitive phenotype in the neonatal brain: Mechanisms and possible interventions. Prog Neurobiol. 2012;98:145–65. doi: 10.1016/j.pneurobio.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ligi I, Grandvuillemin I, Andres V, Dignat-George F, Simeoni U. Low birth weight infants and the developmental programming of hypertension: a focus on vascular factors. Semin Perinatol. 2010;34:188–92. doi: 10.1053/j.semperi.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 160.Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J Nutr. 2005;135:1382–6. doi: 10.1093/jn/135.6.1382. [DOI] [PubMed] [Google Scholar]
- 161.Lillycrop KA, Slater-Jefferies JL, Hanson MA, Godfrey KM, Jackson AA, Burdge GC. Induction of altered epigenetic regulation of the hepatic glucocorticoid receptor in the offspring of rats fed a protein-restricted diet during pregnancy suggests that reduced DNA methyltransferase-1 expression is involved in impaired DNA methylation and changes in histone modifications. Br J Nutr. 2007;97:1064–73. doi: 10.1017/S000711450769196X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lindsay RS, Lindsay RM, Waddell BJ, Seckl JR. Prenatal glucocorticoid exposure leads to offspring hyperglycaemia in the rat: studies with the 11 beta-hydroxysteroid dehydrogenase inhibitor carbenoxolone. Diabetologia. 1996;39:1299–305. doi: 10.1007/s001250050573. [DOI] [PubMed] [Google Scholar]
- 163.Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–4. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 164.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 165.Llorente E, Brito ML, Machado P, Gonzalez MC. Effect of prenatal stress on the hormonal response to acute and chronic stress and on immune parameters in the offspring. J Physiol Biochem. 2002;58:143–9. doi: 10.1007/BF03179851. [DOI] [PubMed] [Google Scholar]
- 166.Lowenberg M, Tuynman J, Bilderbeek J, Gaber T, Buttgereit F, van Deventer S, Peppelenbosch M, Hommes D. Rapid immunosuppressive effects of glucocorticoids mediated through Lck and Fyn. Blood. 2005;106:1703–10. doi: 10.1182/blood-2004-12-4790. [DOI] [PubMed] [Google Scholar]
- 167.Lowenberg M, Verhaar AP, Bilderbeek J, Marle J, Buttgereit F, Peppelenbosch MP, van Deventer SJ, Hommes DW. Glucocorticoids cause rapid dissociation of a T-cell-receptor-associated protein complex containing LCK and FYN. EMBO Rep. 2006;7:1023–9. doi: 10.1038/sj.embor.7400775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. discussion S72–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mandyam CD, Crawford EF, Eisch AJ, Rivier CL, Richardson HN. Stress experienced in utero reduces sexual dichotomies in neurogenesis, microenvironment, and cell death in the adult rat hippocampus. Dev Neurobiol. 2008;68:575–89. doi: 10.1002/dneu.20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Matthews SG. Dynamic changes in glucocorticoid and mineralocorticoid receptor mRNA in the developing guinea pig brain. Brain Res Dev Brain Res. 1998;107:123–32. doi: 10.1016/s0165-3806(98)00008-x. [DOI] [PubMed] [Google Scholar]
- 171.Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47:291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 172.Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002;13:373–80. doi: 10.1016/s1043-2760(02)00690-2. [DOI] [PubMed] [Google Scholar]
- 173.Maymo JL, Perez AP, Gambino Y, Calvo JC, Sanchez-Margalet V, Varone CL. Review: Leptin gene expression in the placenta--regulation of a key hormone in trophoblast proliferation and survival. Placenta. 2011;32(Suppl 2):S146–53. doi: 10.1016/j.placenta.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 174.McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55–61. doi: 10.1016/0165-3806(94)00153-q. [DOI] [PubMed] [Google Scholar]
- 175.McCormick JA, Lyons V, Jacobson MD, Noble J, Diorio J, Nyirenda M, Weaver S, Ester W, Yau JL, Meaney MJ, Seckl JR, Chapman KE. 5′-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: differential regulation of variant transcripts by early-life events. Mol Endocrinol. 2000;14:506–17. doi: 10.1210/mend.14.4.0438. [DOI] [PubMed] [Google Scholar]
- 176.McDonald SD, Walker M, Perkins SL, Beyene J, Murphy K, Gibb W, Ohlsson A. The effect of tobacco exposure on the fetal hypothalamic-pituitary-adrenal axis. BJOG. 2006;113:1289–95. doi: 10.1111/j.1471-0528.2006.01089.x. [DOI] [PubMed] [Google Scholar]
- 177.McTernan CL, Draper N, Nicholson H, Chalder SM, Driver P, Hewison M, Kilby MD, Stewart PM. Reduced placental 11beta-hydroxysteroid dehydrogenase type 2 mRNA levels in human pregnancies complicated by intrauterine growth restriction: an analysis of possible mechanisms. J Clin Endocrinol Metab. 2001;86:4979–83. doi: 10.1210/jcem.86.10.7893. [DOI] [PubMed] [Google Scholar]
- 178.Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med. 2007;13:269–77. doi: 10.1016/j.molmed.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 179.Mennes M, Stiers P, Lagae L, Van den Bergh B. Long-term cognitive sequelae of antenatal maternal anxiety: involvement of the orbitofrontal cortex. Neurosci Biobehav Rev. 2006;30:1078–86. doi: 10.1016/j.neubiorev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 180.Merezak S, Reusens B, Renard A, Goosse K, Kalbe L, Ahn MT, Tamarit-Rodriguez J, Remacle C. Effect of maternal low-protein diet and taurine on the vulnerability of adult Wistar rat islets to cytokines. Diabetologia. 2004;47:669–75. doi: 10.1007/s00125-004-1357-z. [DOI] [PubMed] [Google Scholar]
- 181.Merlot E, Couret D, Otten W. Prenatal stress, fetal imprinting and immunity. Brain Behav Immun. 2008;22:42–51. doi: 10.1016/j.bbi.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 182.Meyer JS. Early adrenalectomy stimulates subsequent growth and development of the rat brain. Exp Neurol. 1983;82:432–46. doi: 10.1016/0014-4886(83)90415-6. [DOI] [PubMed] [Google Scholar]
- 183.Michailidou Z, Carter RN, Marshall E, Sutherland HG, Brownstein DG, Owen E, Cockett K, Kelly V, Ramage L, Al-Dujaili EA, Ross M, Maraki I, Newton K, Holmes MC, Seckl JR, Morton NM, Kenyon CJ, Chapman KE. Glucocorticoid receptor haploinsufficiency causes hypertension and attenuates hypothalamic-pituitary-adrenal axis and blood pressure adaptions to high-fat diet. FASEB J. 2008;22:3896–907. doi: 10.1096/fj.08-111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mohn A, Chiavaroli V, Cerruto M, Blasetti A, Giannini C, Bucciarelli T, Chiarelli F. Increased oxidative stress in prepubertal children born small for gestational age. J Clin Endocrinol Metab. 2007;92:1372–8. doi: 10.1210/jc.2006-1344. [DOI] [PubMed] [Google Scholar]
- 185.Moore LG. Human genetic adaptation to high altitude. High Alt Med Biol. 2001;2:257–79. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- 186.Moore LG. Fetal growth restriction and maternal oxygen transport during high altitude pregnancy. High Alt Med Biol. 2003;4:141–56. doi: 10.1089/152702903322022767. [DOI] [PubMed] [Google Scholar]
- 187.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35:730–43. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 188.Morton JS, Rueda-Clausen CF, Davidge ST. Flow-mediated vasodilation is impaired in adult rat offspring exposed to prenatal hypoxia. J Appl Physiol. 2011;110:1073–82. doi: 10.1152/japplphysiol.01174.2010. [DOI] [PubMed] [Google Scholar]
- 189.Moser D, Molitor A, Kumsta R, Tatschner T, Riederer P, Meyer J. The glucocorticoid receptor gene exon 1-F promoter is not methylated at the NGFI-A binding site in human hippocampus. World J Biol Psychiatry. 2007;8:262–8. doi: 10.1080/15622970701429862. [DOI] [PubMed] [Google Scholar]
- 190.Moss TJ, Doherty DA, Nitsos I, Sloboda DM, Harding R, Newnham JP. Effects into adulthood of single or repeated antenatal corticosteroids in sheep. Am J Obstet Gynecol. 2005;192:146–52. doi: 10.1016/j.ajog.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 191.Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–44. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 192.Mutsaers HA, Tofighi R. Dexamethasone Enhances Oxidative Stress-Induced Cell Death in Murine Neural Stem Cells. Neurotox Res. 2012 doi: 10.1007/s12640-012-9308-9. [DOI] [PubMed] [Google Scholar]
- 193.Myatt L. Placental adaptive responses and fetal programming. J Physiol. 2006;572:25–30. doi: 10.1113/jphysiol.2006.104968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–50. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 195.Newnham JP, Moss TJ. Antenatal glucocorticoids and growth: single versus multiple doses in animal and human studies. Semin Neonatol. 2001;6:285–92. doi: 10.1053/siny.2001.0064. [DOI] [PubMed] [Google Scholar]
- 196.Niculescu MD, Lupu DS. High fat diet-induced maternal obesity alters fetal hippocampal development. Int J Dev Neurosci. 2009;27:627–33. doi: 10.1016/j.ijdevneu.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Niermeyer S, Andrade Mollinedo P, Huicho L. Child health and living at high altitude. Arch Dis Child. 2009;94:806–11. doi: 10.1136/adc.2008.141838. [DOI] [PubMed] [Google Scholar]
- 198.Nobukuni Y, Smith CL, Hager GL, Detera-Wadleigh SD. Characterization of the human glucocorticoid receptor promoter. Biochemistry. 1995;34:8207–14. doi: 10.1021/bi00025a028. [DOI] [PubMed] [Google Scholar]
- 199.Noorlander CW, De Graan PN, Middeldorp J, Van Beers JJ, Visser GH. Ontogeny of hippocampal corticosteroid receptors: effects of antenatal glucocorticoids in human and mouse. J Comp Neurol. 2006;499:924–32. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- 200.Nunez BS, Vedeckis WV. Characterization of promoter 1B in the human glucocorticoid receptor gene. Mol Cell Endocrinol. 2002;189:191–9. doi: 10.1016/s0303-7207(01)00676-1. [DOI] [PubMed] [Google Scholar]
- 201.Nunez H, Ruiz S, Soto-Moyano R, Navarrete M, Valladares L, White A, Perez H. Fetal undernutrition induces overexpression of CRH mRNA and CRH protein in hypothalamus and increases CRH and corticosterone in plasma during postnatal life in the rat. Neurosci Lett. 2008;448:115–9. doi: 10.1016/j.neulet.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 202.Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J Clin Invest. 1998;101:2174–81. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Nyirenda MJ, Carter R, Tang JI, de Vries A, Schlumbohm C, Hillier SG, Streit F, Oellerich M, Armstrong VW, Fuchs E, Seckl JR. Prenatal programming of metabolic syndrome in the common marmoset is associated with increased expression of 11beta-hydroxysteroid dehydrogenase type 1. Diabetes. 2009;58:2873–9. doi: 10.2337/db09-0873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biol Psychiatry. 2005;58:211–7. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 205.O’Donnell D, Larocque S, Seckl JR, Meaney MJ. Postnatal handling alters glucocorticoid, but not mineralocorticoid messenger RNA expression in the hippocampus of adult rats. Brain Res Mol Brain Res. 1994;26:242–8. doi: 10.1016/0169-328x(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 206.O’Hagan HM, Wang W, Sen S, Destefano Shields C, Lee SS, Zhang YW, Clements EG, Cai Y, Van Neste L, Easwaran H, Casero RA, Sears CL, Baylin SB. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell. 2011;20:606–19. doi: 10.1016/j.ccr.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.O’Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. Am J Physiol Endocrinol Metab. 2004;287:E863–70. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- 208.Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics. 2008;3:97–106. doi: 10.4161/epi.3.2.6034. [DOI] [PubMed] [Google Scholar]
- 209.Okret S, Poellinger L, Dong Y, Gustafsson JA. Down-regulation of glucocorticoid receptor mRNA by glucocorticoid hormones and recognition by the receptor of a specific binding sequence within a receptor cDNA clone. Proc Natl Acad Sci U S A. 1986;83:5899–903. doi: 10.1073/pnas.83.16.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–34. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Osterholm EA, Hostinar CE, Gunnar MR. Alterations in stress responses of the hypothalamic-pituitary-adrenal axis in small for gestational age infants. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 212.Palermo Neto J, Massoco CO, Favare RC. Effects of maternal stress on anxiety levels, macrophage activity, and Ehrlich tumor growth. Neurotoxicol Teratol. 2001;23:497–507. doi: 10.1016/s0892-0362(01)00164-7. [DOI] [PubMed] [Google Scholar]
- 213.Papadimitriou A, Priftis KN. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation. 2009;16:265–71. doi: 10.1159/000216184. [DOI] [PubMed] [Google Scholar]
- 214.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKC{epsilon} gene repression in rat hearts. Circ Res. 2010;107:365–73. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Patterson AJ, Xiao D, Xiong F, Dixon B, Zhang L. Hypoxia-derived oxidative stress mediates epigenetic repression of PKCepsilon gene in foetal rat hearts. Cardiovasc Res. 2012;93:302–10. doi: 10.1093/cvr/cvr322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pavek P, Cerveny L, Svecova L, Brysch M, Libra A, Vrzal R, Nachtigal P, Staud F, Ulrichova J, Fendrich Z, Dvorak Z. Examination of Glucocorticoid receptor alpha-mediated transcriptional regulation of P-glycoprotein, CYP3A4, and CYP2C9 genes in placental trophoblast cell lines. Placenta. 2007;28:1004–11. doi: 10.1016/j.placenta.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 217.Pedersen KB, Vedeckis WV. Quantification and glucocorticoid regulation of glucocorticoid receptor transcripts in two human leukemic cell lines. Biochemistry. 2003;42:10978–90. doi: 10.1021/bi034651u. [DOI] [PubMed] [Google Scholar]
- 218.Pedersen KB, Geng CD, Vedeckis WV. Three mechanisms are involved in glucocorticoid receptor autoregulation in a human T-lymphoblast cell line. Biochemistry. 2004;43:10851–8. doi: 10.1021/bi049458u. [DOI] [PubMed] [Google Scholar]
- 219.Pedersen SB, Jonler M, Richelsen B. Characterization of regional and gender differences in glucocorticoid receptors and lipoprotein lipase activity in human adipose tissue. J Clin Endocrinol Metab. 1994;78:1354–9. doi: 10.1210/jcem.78.6.8200937. [DOI] [PubMed] [Google Scholar]
- 220.Pepin MC, Pothier F, Barden N. Impaired type II glucocorticoid-receptor function in mice bearing antisense RNA transgene. Nature. 1992;355:725–8. doi: 10.1038/355725a0. [DOI] [PubMed] [Google Scholar]
- 221.Phillips DI, Walker BR, Reynolds RM, Flanagan DE, Wood PJ, Osmond C, Barker DJ, Whorwood CB. Low birth weight predicts elevated plasma cortisol concentrations in adults from 3 populations. Hypertension. 2000;35:1301–6. doi: 10.1161/01.hyp.35.6.1301. [DOI] [PubMed] [Google Scholar]
- 222.Prescott SL, Taylor A, Roper J, Wahdan A, Noakes P, Thornton C, Dunstan J, Upham JW. Maternal reactivity to fetal alloantigens is related to newborn immune responses and subsequent allergic disease. Clin Exp Allergy. 2005;35:417–25. doi: 10.1111/j.1365-2222.2005.02171.x. [DOI] [PubMed] [Google Scholar]
- 223.Presul E, Schmidt S, Kofler R, Helmberg A. Identification, tissue expression, and glucocorticoid responsiveness of alternative first exons of the human glucocorticoid receptor. J Mol Endocrinol. 2007;38:79–90. doi: 10.1677/jme.1.02183. [DOI] [PubMed] [Google Scholar]
- 224.Pryce CR, Feldon J, Fuchs E, Knuesel I, Oertle T, Sengstag C, Spengler M, Weber E, Weston A, Jongen-Relo A. Postnatal ontogeny of hippocampal expression of the mineralocorticoid and glucocorticoid receptors in the common marmoset monkey. Eur J Neurosci. 2005;21:1521–35. doi: 10.1111/j.1460-9568.2005.04003.x. [DOI] [PubMed] [Google Scholar]
- 225.Quan X, Lim SO, Jung G. Reactive oxygen species downregulate catalase expression via methylation of a CpG island in the Oct-1 promoter. FEBS Lett. 2011;585:3436–41. doi: 10.1016/j.febslet.2011.09.035. [DOI] [PubMed] [Google Scholar]
- 226.Rakers F, Frauendorf V, Rupprecht S, Schiffner R, Bischoff S, Kiehntopf M, Reinhold P, Witte OW, Schubert H, Schwab M. Effects of early and late-gestational maternal stress and synthetic glucocorticoid on development of the fetal hypothalamus-pituitary-adrenal axis in sheep. Stress. 2012 doi: 10.3109/10253890.2012.686541. [DOI] [PubMed] [Google Scholar]
- 227.Raschke C, Schmidt S, Schwab M, Jirikowski G. Effects of betamethasone treatment on central myelination in fetal sheep: an electron microscopical study. Anat Histol Embryol. 2008;37:95–100. doi: 10.1111/j.1439-0264.2007.00807.x. [DOI] [PubMed] [Google Scholar]
- 228.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349–53. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 229.Rebuffat AG, Tam S, Nawrocki AR, Baker ME, Frey BM, Frey FJ, Odermatt A. The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol Cell Endocrinol. 2004;214:27–37. doi: 10.1016/j.mce.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 230.Rees S, Harding R, Walker D. The biological basis of injury and neuroprotection in the fetal and neonatal brain. Int J Dev Neurosci. 2011;29:551–63. doi: 10.1016/j.ijdevneu.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Regnault TR, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and mechanisms of fetal hypoxia in severe fetal growth restriction. Placenta. 2007;28:714–23. doi: 10.1016/j.placenta.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 232.Reichardt HM, Umland T, Bauer A, Kretz O, Schutz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol. 2000;20:9009–17. doi: 10.1128/mcb.20.23.9009-9017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Reitman ML, Bi S, Marcus-Samuels B, Gavrilova O. Leptin and its role in pregnancy and fetal development--an overview. Biochem Soc Trans. 2001;29:68–72. doi: 10.1042/0300-5127:0290068. [DOI] [PubMed] [Google Scholar]
- 234.Reul JM, de Kloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–11. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 235.Richardson BS, Bocking AD. Metabolic and circulatory adaptations to chronic hypoxia in the fetus. Comp Biochem Physiol A Mol Integr Physiol. 1998;119:717–23. doi: 10.1016/s1095-6433(98)01010-1. [DOI] [PubMed] [Google Scholar]
- 236.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 237.Rodriguez-Trejo A, Ortiz-Lopez MG, Zambrano E, de Granados-Silvestre ML, Mendez C, Blondeau B, Breant B, Nathanielsz PW, Menjivar M. Developmental programming of neonatal pancreatic beta-cells by a maternal low-protein diet in rats involves a switch from proliferation to differentiation. Am J Physiol Endocrinol Metab. 2012;302:E1431–9. doi: 10.1152/ajpendo.00619.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rogerson FM, Kayes KM, White PC. Variation in placental type 2 11beta-hydroxysteroid dehydrogenase activity is not related to birth weight or placental weight. Mol Cell Endocrinol. 1997;128:103–9. doi: 10.1016/s0303-7207(97)04027-6. [DOI] [PubMed] [Google Scholar]
- 239.Roghair RD, Miller FJ, Jr, Scholz TD, Lamb FS, Segar JL. Endothelial superoxide production is altered in sheep programmed by early gestation dexamethasone exposure. Neonatology. 2008;93:19–27. doi: 10.1159/000105521. [DOI] [PubMed] [Google Scholar]
- 240.Roseboom TJ, Painter RC, van Abeelen AF, Veenendaal MV, de Rooij SR. Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas. 2011;70:141–5. doi: 10.1016/j.maturitas.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 241.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72:1101–6. doi: 10.1093/ajcn/72.5.1101. [DOI] [PubMed] [Google Scholar]
- 242.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, van Montfrans GA, Michels RP, Bleker OP. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–8. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rosmond R, Bjorntorp P. The hypothalamic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J Intern Med. 2000;247:188–97. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- 244.Rueda-Clausen CF, Morton JS, Lopaschuk GD, Davidge ST. Long-term effects of intrauterine growth restriction on cardiac metabolism and susceptibility to ischaemia/reperfusion. Cardiovasc Res. 2011;90:285–94. doi: 10.1093/cvr/cvq363. [DOI] [PubMed] [Google Scholar]
- 245.Rueda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JR, Davidge ST. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 2011;60:507–16. doi: 10.2337/db10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rybnikova E, Glushchenko T, Churilova A, Pivina S, Samoilov M. Expression of glucocorticoid and mineralocorticoid receptors in hippocampus of rats exposed to various modes of hypobaric hypoxia: Putative role in hypoxic preconditioning. Brain Res. 2011;1381:66–77. doi: 10.1016/j.brainres.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 247.Sacedon R, Vicente A, Varas A, Morale MC, Barden N, Marchetti B, Zapata AG. Partial blockade of T-cell differentiation during ontogeny and marked alterations of the thymic microenvironment in transgenic mice with impaired glucocorticoid receptor function. J Neuroimmunol. 1999;98:157–67. doi: 10.1016/s0165-5728(99)00091-0. [DOI] [PubMed] [Google Scholar]
- 248.Saegusa H, Nakagawa Y, Liu YJ, Ohzeki T. Influence of placental 11beta-hydroxysteroid dehydrogenase (11beta-HSD) inhibition on glucose metabolism and 11beta-HSD regulation in adult offspring of rats. Metabolism. 1999;48:1584–8. doi: 10.1016/s0026-0495(99)90249-4. [DOI] [PubMed] [Google Scholar]
- 249.Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Sapolsky RM, Krey LC, McEwen BS. Stress down-regulates corticosterone receptors in a site-specific manner in the brain. Endocrinology. 1984;114:287–92. doi: 10.1210/endo-114-1-287. [DOI] [PubMed] [Google Scholar]
- 251.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 252.Sarkar S, Tsai SW, Nguyen TT, Plevyak M, Padbury JF, Rubin LP. Inhibition of placental 11beta-hydroxysteroid dehydrogenase type 2 by catecholamines via alpha-adrenergic signaling. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1966–74. doi: 10.1152/ajpregu.2001.281.6.R1966. [DOI] [PubMed] [Google Scholar]
- 253.Schlechte JA, Ginsberg BH, Sherman BM. Regulation of the glucocorticoid receptor in human lymphocytes. J Steroid Biochem. 1982;16:69–74. doi: 10.1016/0022-4731(82)90145-5. [DOI] [PubMed] [Google Scholar]
- 254.Schoof E, Girstl M, Frobenius W, Kirschbaum M, Repp R, Knerr I, Rascher W, Dotsch J. Course of placental 11beta-hydroxysteroid dehydrogenase type 2 and 15-hydroxyprostaglandin dehydrogenase mRNA expression during human gestation. Eur J Endocrinol. 2001;145:187–92. doi: 10.1530/eje.0.1450187. [DOI] [PubMed] [Google Scholar]
- 255.Seckl JR. Prenatal glucocorticoids and long-term programming. Eur J Endocrinol. 2004;151(Suppl 3):U49–62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- 256.Seckl JR, Meaney MJ. Glucocorticoid programming. Ann N Y Acad Sci. 2004;1032:63–84. doi: 10.1196/annals.1314.006. [DOI] [PubMed] [Google Scholar]
- 257.Shoener JA, Baig R, Page KC. Prenatal exposure to dexamethasone alters hippocampal drive on hypothalamic-pituitary-adrenal axis activity in adult male rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1366–73. doi: 10.1152/ajpregu.00757.2004. [DOI] [PubMed] [Google Scholar]
- 258.Singh RR, Cullen-McEwen LA, Kett MM, Boon WM, Dowling J, Bertram JF, Moritz KM. Prenatal corticosterone exposure results in altered AT1/AT2, nephron deficit and hypertension in the rat offspring. J Physiol. 2007;579:503–13. doi: 10.1113/jphysiol.2006.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- 260.Slotkin TA. Fetal nicotine or cocaine exposure: which one is worse? J Pharmacol Exp Ther. 1998;285:931–45. [PubMed] [Google Scholar]
- 261.Smith JT, Waddell BJ. Leptin receptor expression in the rat placenta: changes in ob-ra, ob-rb, and ob-re with gestational age and suppression by glucocorticoids. Biol Reprod. 2002;67:1204–10. doi: 10.1095/biolreprod67.4.1204. [DOI] [PubMed] [Google Scholar]
- 262.Stahn C, Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nat Clin Pract Rheumatol. 2008;4:525–33. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 263.Stahn C, Lowenberg M, Hommes DW, Buttgereit F. Molecular mechanisms of glucocorticoid action and selective glucocorticoid receptor agonists. Mol Cell Endocrinol. 2007;275:71–8. doi: 10.1016/j.mce.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 264.Stein AD, Zybert PA, van der Pal-de Bruin K, Lumey LH. Exposure to famine during gestation, size at birth, and blood pressure at age 59 y: evidence from the Dutch Famine. Eur J Epidemiol. 2006;21:759–65. doi: 10.1007/s10654-006-9065-2. [DOI] [PubMed] [Google Scholar]
- 265.Stein AD, Kahn HS, Rundle A, Zybert PA, van der Pal-de Bruin K, Lumey LH. Anthropometric measures in middle age after exposure to famine during gestation: evidence from the Dutch famine. Am J Clin Nutr. 2007;85:869–76. doi: 10.1093/ajcn/85.3.869. [DOI] [PubMed] [Google Scholar]
- 266.Stein MB, Schork NJ, Gelernter J. Gene-by-environment (serotonin transporter and childhood maltreatment) interaction for anxiety sensitivity, an intermediate phenotype for anxiety disorders. Neuropsychopharmacology. 2008;33:312–9. doi: 10.1038/sj.npp.1301422. [DOI] [PubMed] [Google Scholar]
- 267.Stewart PM, Rogerson FM, Mason JI. Type 2 11 beta-hydroxysteroid dehydrogenase messenger ribonucleic acid and activity in human placenta and fetal membranes: its relationship to birth weight and putative role in fetal adrenal steroidogenesis. J Clin Endocrinol Metab. 1995;80:885–90. doi: 10.1210/jcem.80.3.7883847. [DOI] [PubMed] [Google Scholar]
- 268.Stocker CJ, Arch JR, Cawthorne MA. Fetal origins of insulin resistance and obesity. Proc Nutr Soc. 2005;64:143–51. doi: 10.1079/pns2005417. [DOI] [PubMed] [Google Scholar]
- 269.Strahle U, Schmidt A, Kelsey G, Stewart AF, Cole TJ, Schmid W, Schutz G. At least three promoters direct expression of the mouse glucocorticoid receptor gene. Proc Natl Acad Sci U S A. 1992;89:6731–5. doi: 10.1073/pnas.89.15.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Strakovsky RS, Pan YX. In Utero Oxidative Stress Epigenetically Programs Antioxidant Defense Capacity and Adulthood Diseases. Antioxid Redox Signal. 2012 doi: 10.1089/ars.2011.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Sun M, Kingdom J, Baczyk D, Lye SJ, Matthews SG, Gibb W. Expression of the multidrug resistance P-glycoprotein, (ABCB1 glycoprotein) in the human placenta decreases with advancing gestation. Placenta. 2006;27:602–9. doi: 10.1016/j.placenta.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 272.Svec F, Rudis M. Glucocorticoids regulate the glucocorticoid receptor in the AtT-20 cell. J Biol Chem. 1981;256:5984–7. [PubMed] [Google Scholar]
- 273.Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol. 2009;5:604–10. doi: 10.1038/nrendo.2009.195. [DOI] [PubMed] [Google Scholar]
- 274.Takahashi LK, Turner JG, Kalin NH. Prolonged stress-induced elevation in plasma corticosterone during pregnancy in the rat: implications for prenatal stress studies. Psychoneuroendocrinology. 1998;23:571–81. doi: 10.1016/s0306-4530(98)00024-9. [DOI] [PubMed] [Google Scholar]
- 275.Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Effect of cortisol on blood pressure and vascular reactivity in the ovine fetus. Exp Physiol. 1992;77:709–17. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- 276.Tasker JG, Herman JP. Mechanisms of rapid glucocorticoid feedback inhibition of the hypothalamic-pituitary-adrenal axis. Stress. 2011;14:398–406. doi: 10.3109/10253890.2011.586446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Thiebaut F, Tsuruo T, Hamada H, Gottesman MM, Pastan I, Willingham MC. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc Natl Acad Sci U S A. 1987;84:7735–8. doi: 10.1073/pnas.84.21.7735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Thompson LP, Dong Y. Chronic hypoxia decreases endothelial nitric oxide synthase protein expression in fetal guinea pig hearts. J Soc Gynecol Investig. 2005;12:388–95. doi: 10.1016/j.jsgi.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 279.Thompson LP, Al-Hasan Y. Impact of Oxidative Stress in Fetal Programming. J Pregnancy. 2012;2012:582748. doi: 10.1155/2012/582748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tomat AL, de Costa ML, Arranz CT. Zinc restriction during different periods of life: influence in renal and cardiovascular diseases. Nutrition. 2011;27:392–8. doi: 10.1016/j.nut.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 281.Tong W, Xue Q, Li Y, Zhang L. Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats. Am J Physiol Heart Circ Physiol. 2011;301:H2113–21. doi: 10.1152/ajpheart.00356.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Tuchscherer M, Kanitz E, Otten W, Tuchscherer A. Effects of prenatal stress on cellular and humoral immune responses in neonatal pigs. Vet Immunol Immunopathol. 2002;86:195–203. doi: 10.1016/s0165-2427(02)00035-1. [DOI] [PubMed] [Google Scholar]
- 283.Turner JD, Muller CP. Structure of the glucocorticoid receptor (NR3C1) gene 5′ untranslated region: identification, and tissue distribution of multiple new human exon 1. J Mol Endocrinol. 2005;35:283–92. doi: 10.1677/jme.1.01822. [DOI] [PubMed] [Google Scholar]
- 284.Turner JD, Pelascini LP, Macedo JA, Muller CP. Highly individual methylation patterns of alternative glucocorticoid receptor promoters suggest individualized epigenetic regulatory mechanisms. Nucleic Acids Res. 2008;36:7207–18. doi: 10.1093/nar/gkn897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Turner JD, Schote AB, Macedo JA, Pelascini LP, Muller CP. Tissue specific glucocorticoid receptor expression, a role for alternative first exon usage? Biochem Pharmacol. 2006;72:1529–37. doi: 10.1016/j.bcp.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 286.Tzoulaki I, Sovio U, Pillas D, Hartikainen AL, Pouta A, Laitinen J, Tammelin TH, Jarvelin MR, Elliott P. Relation of immediate postnatal growth with obesity and related metabolic risk factors in adulthood: the northern Finland birth cohort 1966 study. Am J Epidemiol. 2010;171:989–98. doi: 10.1093/aje/kwq027. [DOI] [PubMed] [Google Scholar]
- 287.Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res. 1990;53:157–67. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- 288.Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–45. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 289.Vanderbilt JN, Miesfeld R, Maler BA, Yamamoto KR. Intracellular receptor concentration limits glucocorticoid-dependent enhancer activity. Mol Endocrinol. 1987;1:68–74. doi: 10.1210/mend-1-1-68. [DOI] [PubMed] [Google Scholar]
- 290.Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146:4211–6. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- 291.Vieau D. Perinatal nutritional programming of health and metabolic adult disease. World J Diabetes. 2011;2:133–6. doi: 10.4239/wjd.v2.i9.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Vuguin PM. Animal models for small for gestational age and fetal programming of adult disease. Horm Res. 2007;68:113–23. doi: 10.1159/000100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Ward AM, Syddall HE, Wood PJ, Chrousos GP, Phillips DI. Fetal programming of the hypothalamic-pituitary-adrenal (HPA) axis: low birth weight and central HPA regulation. J Clin Endocrinol Metab. 2004;89:1227–33. doi: 10.1210/jc.2003-030978. [DOI] [PubMed] [Google Scholar]
- 294.Ward RM. Pharmacologic enhancement of fetal lung maturation. Clin Perinatol. 1994;21:523–42. [PubMed] [Google Scholar]
- 295.Weaver IC, Diorio J, Seckl JR, Szyf M, Meaney MJ. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann N Y Acad Sci. 2004;1024:182–212. doi: 10.1196/annals.1321.099. [DOI] [PubMed] [Google Scholar]
- 296.Weaver IC, Champagne FA, Brown SE, Dymov S, Sharma S, Meaney MJ, Szyf M. Reversal of maternal programming of stress responses in adult offspring through methyl supplementation: altering epigenetic marking later in life. J Neurosci. 2005;25:11045–54. doi: 10.1523/JNEUROSCI.3652-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Weaver IC, D’Alessio AC, Brown SE, Hellstrom IC, Dymov S, Sharma S, Szyf M, Meaney MJ. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: altering epigenetic marks by immediate-early genes. J Neurosci. 2007;27:1756–68. doi: 10.1523/JNEUROSCI.4164-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–54. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- 299.Weinstock M. Does prenatal stress impair coping and regulation of hypothalamic-pituitary-adrenal axis? Neurosci Biobehav Rev. 1997;21:1–10. doi: 10.1016/s0149-7634(96)00014-0. [DOI] [PubMed] [Google Scholar]
- 300.Weinstock M. Can the behaviour abnormalities induced by gestational stress in rats be prevented or reversed? Stress. 2002;5:167–76. doi: 10.1080/1025389021000010503. [DOI] [PubMed] [Google Scholar]
- 301.Weinstock M. The potential influence of maternal stress hormones on development and mental health of the offspring. Brain Behav Immun. 2005;19:296–308. doi: 10.1016/j.bbi.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 302.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–28. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 303.Welberg LA, Seckl JR, Holmes MC. Inhibition of 11beta-hydroxysteroid dehydrogenase, the foeto-placental barrier to maternal glucocorticoids, permanently programs amygdala GR mRNA expression and anxiety-like behaviour in the offspring. Eur J Neurosci. 2000;12:1047–54. doi: 10.1046/j.1460-9568.2000.00958.x. [DOI] [PubMed] [Google Scholar]
- 304.Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–9. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- 305.Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11beta-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin ii receptor in neonatal sheep. Endocrinology. 2001;142:2854–64. doi: 10.1210/endo.142.7.8264. [DOI] [PubMed] [Google Scholar]
- 306.Wilcoxon JS, Redei EE. Maternal glucocorticoid deficit affects hypothalamic-pituitary-adrenal function and behavior of rat offspring. Horm Behav. 2007;51:321–7. doi: 10.1016/j.yhbeh.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wilcoxon JS, Schwartz J, Aird F, Redei EE. Sexually dimorphic effects of maternal alcohol intake and adrenalectomy on left ventricular hypertrophy in rat offspring. Am J Physiol Endocrinol Metab. 2003;285:E31–9. doi: 10.1152/ajpendo.00552.2002. [DOI] [PubMed] [Google Scholar]
- 308.Williams SJ, Hemmings DG, Mitchell JM, McMillen IC, Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol. 2005;565:125–35. doi: 10.1113/jphysiol.2005.084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289:R955–62. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 310.Wyrwoll CS, Holmes MC. Prenatal Excess Glucocorticoid Exposure and Adult Affective Disorders: A Role for Serotonergic and Catecholamine Pathways. Neuroendocrinology. 2011 doi: 10.1159/000331345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension. 2007;50:579–84. doi: 10.1161/HYPERTENSIONAHA.107.091603. [DOI] [PubMed] [Google Scholar]
- 312.Xiong F, Xiao D, Zhang L. Norepinephrine causes epigenetic repression of PKC{varepsilon} gene in rodent hearts by activating Nox1-dependent reactive oxygen species production. FASEB J. 2012 doi: 10.1096/fj.11-199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther. 2009;330:624–32. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res. 2011;89:300–8. doi: 10.1093/cvr/cvq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Yzydorczyk C, Comte B, Cambonie G, Lavoie JC, Germain N, Ting Shun Y, Wolff J, Deschepper C, Touyz RM, Lelievre-Pegorier M, Nuyt AM. Neonatal oxygen exposure in rats leads to cardiovascular and renal alterations in adulthood. Hypertension. 2008;52:889–95. doi: 10.1161/HYPERTENSIONAHA.108.116251. [DOI] [PubMed] [Google Scholar]
- 316.Zandi-Nejad K, Luyckx VA, Brenner BM. Adult hypertension and kidney disease: the role of fetal programming. Hypertension. 2006;47:502–8. doi: 10.1161/01.HYP.0000198544.09909.1a. [DOI] [PubMed] [Google Scholar]
- 317.Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Investig. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 318.Zhang Q, Wu WX, Hong Ma X, Nathanielsz PW, Brenna JT. Pulmonary phospholipid saturation increases with glucocorticoid receptor mRNAs in late gestation but not due to labor in the fetal rhesus monkey. Prostaglandins Leukot Essent Fatty Acids. 1997;57:311–21. doi: 10.1016/s0952-3278(97)90550-0. [DOI] [PubMed] [Google Scholar]
- 319.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802–32. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]
- 320.Ziech D, Franco R, Pappa A, Panayiotidis MI. Reactive oxygen species (ROS)--induced genetic and epigenetic alterations in human carcinogenesis. Mutat Res. 2011;711:167–73. doi: 10.1016/j.mrfmmm.2011.02.015. [DOI] [PubMed] [Google Scholar]