Abstract
Background
Chronic inflammation contributes to the development of pathological disorders including insulin resistance and atherosclerosis. Identification of anti-inflammatory natural products can prevent the inflammatory diseases.
Methods
Anti-inflammatory effects of blue-green algae (BGA), i.e., Nostoc commune var. Sphaeroides Kützing (NO) and Spirulina Platensis (SP), were compared in RAW 264.7 and mouse bone marrow-derived macrophages (BMM) as well as splenocytes from apolipoprotein E knockout (apoE−/−) mice fed BGA.
Results
When macrophages pretreated with 100 μg/ml NO lipid extract (NOE) or SP lipid extract (SPE) were activated by lipopolysaccharide (LPS), expression and secretion of pro-inflammatory cytokines, such as tumor necrosis factor α (TNFα), interleukin 1β (IL-1β), and IL-6, were significantly repressed. NOE and SPE also significantly repressed the expression of TNFα and IL-1β in BMM. LPS-induced secretion of IL-6 was lower in splenocytes from apoE−/− fed an atherogenic diet containing 5% NO or SP for 12 weeks. In RAW 264.7 macrophages, NOE and SPE markedly decreased nuclear translocation of NF-κB. The degree of repression of pro-inflammatory gene expression by algal extracts was much stronger than that of SN50, an inhibitor of NF-κB nuclear translocation. Trichostatin A, a pan histone deacetylase inhibitor, increased basal expression of IL-1β and attenuated the repression of the gene expression by SPE. SPE significantly down-regulated mRNA abundance of 11 HDAC isoforms, consequently increasing acetylated histone 3 levels.
Conclusion
NOE and SPE repress pro-inflammatory cytokine expression and secretion in macrophages and splenocytes via inhibition of NF-κB pathway. Histone acetylation state is likely involved in the inhibition.
General significance
This study underscores natural products can exert anti-inflammatory effects by epigenetic modifications such as histone acetylation.
Keywords: blue-green algae, anti-inflammation, RAW 264.7 macrophages, NF-κB, cytokine array, histone deacetylation
1. INTRODUCTION
Chronic inflammation is an underlying cause of several pathological disorders including insulin resistance, dementia, rheumatoid arthritis, atherosclerosis, and cancer [1, 2]. Non-steroidal anti-inflammatory drugs (NSAID) are generally used to treat acute and chronic inflammatory conditions. However, due to their adverse side-effects and increased cardiovascular disease (CVD) risk associated with chronic use of several NSAID [3], there is a critical need to identify natural products with anti-inflammatory properties. Several anti-inflammatory bioactive compounds that have been extensively studied thus far include curcumin, resveratrol, anthocyanin, and green tea polyphenols [4–8].
Nuclear factor κ B (NF-κB) is a major transcriptional factors responsible for pro-inflammatory cytokine production, such as tumor necrosis factor α (TNFα), interleukins (ILs), inducible nitric oxide synthase and cyclooxygenase-2, under inflammatory conditions [9, 10]. NF-κB exists as homo- or heterodimers consisting of five subunits of Rel family, i.e., p50, p52, p65, c-Rel and RelB [11]. In an unstimulated state, NF-κB is present in the cytoplasm bound with inhibitor of κB α (IκBα), which masks the nuclear localization sequence of p65 [12] and NF-κB activation largely depends on IκB kinase β (IKKβ). Extracellular inflammatory signals including lipopolysaccharide (LPS), pro-inflammatory cytokines, reactive oxygen species, advanced glycation end products, and oxidized low-density lipoprotein trigger the phosphorylation of IKKβ, which in turn phosphorylates IκBα for ubiquitination and degradation by proteasome [13–18]. This event liberates NF-κB to enter the nucleus for the induction of pro-inflammatory gene expression [19–24].
Blue-green algae (BGA), also known as cyanobacteria, are one of the most primitive forms of photosynthetic prokaryotes. They have been consumed as food or medicine for centuries and human consumption of BGA was recorded in the 14th century during the Aztec civilization [25]. They are recognized for their protective effects against viral and bacterial infections, cancer, allergy, diabetes, inflammation and hyperlipidemia [26–29]. At present, Spirulina platensis (SP) is the most commonly consumed and commercialized BGA species. We have previously reported that lipid extract of Nostoc commune var. sphaeroides Kützing (NO), another BGA species, inhibited NF-κB DNA binding activity and consequently repressed the pro-inflammatory gene expression in RAW 264.7 macrophages [30]. In this study, we compared anti-inflammatory effects of two BGA species using several model systems: RAW 264.7 macrophages, bone marrow-derived macrophages (BMM), and splenocytes isolated from BGA-fed apolipoprotein E knockout (apoE−/−) mice. We found that in all of three systems, NO and SP either as lipid extract or as whole algae repressed pro-inflammatory gene expression and production.
2. METHODS AND MATERIALS
2.1. Preparation of BGA lipid extraction
BGA powder was kindly provided by Algaen Corporation (Winston Salam, NC) for NO and by Earthrise Nutritionals (Irvine, CA) for SP. Lipid extracts of BGA into chloroform/methanol (1:2) were prepared as previously described [30, 31]. Lipid extracts were stored under N2 gas at −20°C for short term or at −80°C for long term. The lipid extracts were dried down under N2 to remove solvents and then dissolved in cell medium by sonication.
2.2. Bone marrow isolation and macrophage differentiation
BMM were isolated from C57BL/6J mice (Jackson Laboratory, Bar harbor, ME). Briefly, mouse legs were removed from the hip joint and cleaned, after which the femur and tibia were cut at the tip and subsequently bone marrow was collected by centrifugation. The bone marrow cells were differentiated into macrophages in low-glucose DMEM containing 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, 30 U/mL penicillin, 300 μg/mL streptomycin, 20% FBS, and 30% L929 cell conditioned media. L929 cells were generously provided by Dr. John Parks (Wake Forest University School of Medicine, Winston-Salem, NC). BMM were incubated in a humidified chamber at 37°C with 5% CO2 and cell culture medium was changed every 3 days for 7 days until they became confluent.
2.3. Macrophage cell culture and treatment
RAW 264.7 macrophages (ATCC, Manasas, VA) and BMM were incubated in RPMI-1640 containing 10% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin, 1 x vitamins and 2 mmol/L L-glutamine in a 37°C humidified chamber with a supply of 5% CO2. Macrophages were incubated with 0–100 μg/mL of NO lipid extract (NOE) or SP lipid extract (SPE) for 12 h and subsequently activated by LPS (Sigma-Aldrich, St. Louis, MO) at 100 ng/ml concentration for additional 18 h. Cells and medium were collected for pro-inflammatory cytokine expression and secretion.
SN50, a cell permeable inhibitor specific for NF-κB nuclear translocation, was purchased from Enzo Life Science (Plymouth meeting, PA) and dissolved in sterile water. RAW 264.7 macrophages were incubated with 50 μg/mL of NOE or SPE for 11 h followed by 1 h incubation with 50 μg/mL of SN50. Subsequently, the cells were treated with LPS (100 ng/mL) in the presence of algal lipid extract and SN50 for additional 3 h. For the experiment with trichostatin A (TSA), a pan histone deacetylase (HDAC) inhibitor, RAW 264.7 macrophages were treated with algal lipid extract (100 μg/ml) and 50 nmol/L of TSA for 12 h prior to LPS stimulation for 7 h. All cell culture supplies were purchased from Thermo Scientific Hyclone (Logan, UT), unless stated otherwise.
For all cell culture experiments, cells without exposure to any algal extract were considered as control. Algal extracts were incorporated into cell culture medium by sonication and therefore no solvent was used as a vehicle.
2.4. Splenocyte isolation and culture
Spleens were harvested from male apoE−/− mice (Jackson Laboratory) fed a high fat/high cholesterol (15% fat, 0.2% cholesterol by wt) containing 5% NO or SP by wt for 12 wk from 8 wk of age. After being anesthetized with ketamine HCl (50 mg/kg)/xylazine (10 mg/kg) and subsequently euthanized by cardiac puncture and cervical dislocation, spleen of each mouse was excised aseptically and ground in RPMI-1640 medium containing 10% FBS, 100 U/mL penicillin and 100 μg/ml streptomycin. After removal of red blood cells by a pre-warmed RBC lysis buffer (eBioScience, San Diego, CA), the cells were resuspended in the medium and centrifuged at 2,000 rpm for 5 min. Cell pellet was resuspended in PBS and filtered through 40 μm strainer (BD Biosciences, San Jose, CA). After washing with PBS, cells were resuspended in RPMI-1640 complete media and plated at a density of 1 x 106/0.5 mL for experiments. Cells were incubated with LPS (500 ng/mL) for 20 h and IL-6 concentrations in the media were measure by ELISA (eBioScience) according to the manufacturer’s instruction. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Connecticut.
2.5. Quantitative realtime PCR (qRT-PCR)
Total RNA was extracted using TRIzol reagent (Life technology, Carlsbad, CA) and cDNA synthesis was performed as previously described [30, 31]. qRT-PCR was conducted using Bio-Rad CFX96 Real-Time system (Bio-Rad, Hercules CA). Primers were designed according to GenBank database and the sequences were previously published [30].
2.6. Cytokine array
RAW 264.7 macrophages were pretreated with 100 μg/mL of NOE or SPE for 12 h, after which they were incubated with LPS for 18 h. Conditioned medium was collected and centrifuged at 12,000 x g for 5 min to remove any cell debris or dead cells. Secretion of cytokines was assessed by RayBio Mouse Cytokine Antibody Array (RayBiotech, Norcross, GA) according to the manufacturer’s protocol. Signals were captured using ChemiDoc XRS+ system (Bio-Rad, Hercules, CA).
2.7. NF-κB translocation by Western blot analysis
RAW 264.7 macrophages incubated with 100 μg/ml of NOE or SPE for 12 h were activated by LPS for 1 or 2 h. Nuclear and cytoplasmic fractions of the cells were separated by using Cayman nuclear extraction kit (Ann Arbor, MI) and Western blot analysis was conduct as we previously described [30, 31]. Polyclonal anti-p65 and anti-GAPDH antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibody against TATA binding protein was obtained from Abcam (Cambridge, MA) and used as a loading control for nuclear fraction. Protein expression were detected using Westpico horseradish peroxidase chemiluminescence (Pierce, Rockford, IL) and imaged using a Chemidoc XRS+ system (Bio-Rad) and Image Lab software (Bio-Rad).
2.8. Acetylation of histone 3 (H3)
After incubation with 100 μg/mL of SPE for 12 h and LPS for additional 18 h, RAW 264.7 macrophages were washed twice with ice-cold PBS and cells were collected by centrifugation at 12,000 x g for 5 min. Cells were then resuspended in Triton X extraction buffer containing 0.5% Trition X-100 (v/v), 0.02% NaN3 (w/v) in PBS and 1 X protease inhibitor cocktail (Calbiochem, San Diego, CA) for 10 min on ice. Subsequently, the cell lysates were centrifuged for 10 min at 4°C to pellet nuclei and the pellets were then acid extracted in 0.2 N HCl overnight at 4 C. After centrifugation at 12,000 x g for 10 min, the supernatants were collected for Western blot analysis as described above. Antibodies against total H3 and acetyl-H3K9 were purchased from Cell Signaling Technology (Danvers, MA).
2.9. Statistical analysis
One-way analysis of variance (ANOVA) and Newman-Keuls pairwise comparisons to detect significant difference between groups were performed using GraphPad InStat 5 (GraphPad Software, La Jolla, CA). P values less than 0.05 were considered significant. Values were expressed as mean ± SEM.
3. RESULTS
3.1. Pro-inflammatory cytokine expression and secretion were repressed by NOE and SPE in macrophages
We examined the effects of NOE and SPE on pro-inflammatory cytokine expression. In RAW 264.7 macrophages, both algal lipid extracts significantly repressed LPS-induced TNFα, IL-1β and IL-6 mRNA expression compared with control and their repressive effects showed a similar potency (Figure 1). Significant reductions in the gene expression were observed in a dose-dependent manner as low as 25 μg/mL (data not shown).
Figure 1. Repression of pro-inflammatory expression by NOE and SPE in RAW 264.7 macrophages.
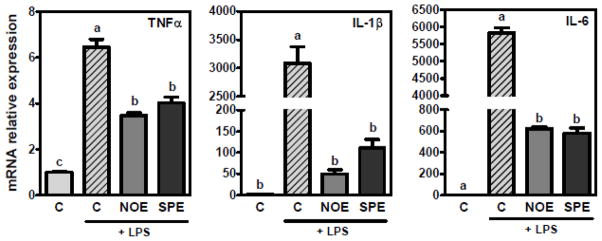
Cells were pre-treated with 100 μg/mL of NOE or SPE for 12 h followed by co-incubation of 100 ng/mL LPS and algal extracts for additional 18 h. qRT-PCR analysis was conducted to measure mRNA levels of TNFα, IL-1β and IL-6. Values are expressed as mean ± SEM, P < 0.05, n = 15–18.
Furthermore, NOE and SPE markedly decreased the secretion of TNFα, IL-6, granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor, and monocyte chemoattractant protein 1 (Figure 2).
Figure 2. Inhibition of pro-inflammatory cytokine secretion by NOE and SPE in RAW 264.7 macrophages.
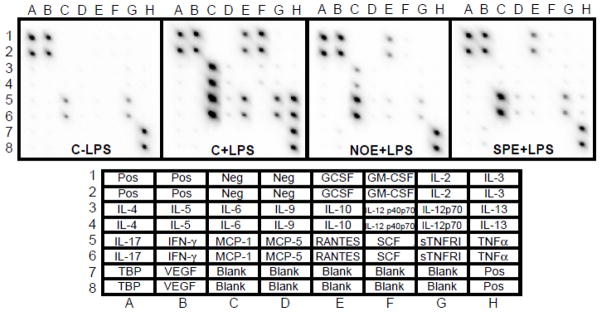
Cells were incubated with 50 μg/mL of NOE or SPE for 12 h and subsequently activated by 100 ng/mL LPS for 18 h. Bottom chart indicates location of cytokines in the array. GCSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; IL, interleukins; IFNγ, interferon gamma; MCP-1, monocyte chemoattractant protein 1; RANTES (CCL5), chemokine C-C motive ligand 5; SCF, stem cell factor; sTNFR1, soluble tumor necrosis factor receptor 1; TNFα, tumor necrosis factor alpha; TBP, TNF binding protein; VEGF, vascular endothelial growth factor.
Consistent with our observations in RAW 264.7 macrophages, BMM treated with NOE and SPE showed significantly lower expression of TNFα and IL-1β compared with control BMM (Figure 3).
Figure 3. Inhibitory effects of TNFα and IL-1β expression by NOE and SPE in BMM.
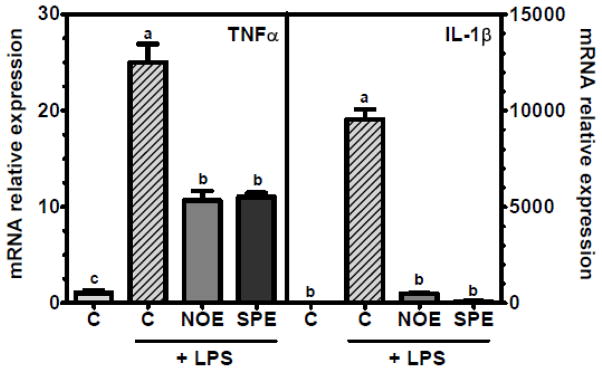
Bone marrow derived macrophages isolated from C57BL/6J mice were treated with 100 μg/mL of NOE or SPE for 12 h, after which they were co-incubated with 100 ng/mL for 18 h. Values are expressed as mean ± SEM, P < 0.05, n = 3.
3.2. IL-6 secretion was decreased from splenocytes of mice fed NO and SP supplements
Fresh splenocytes were isolated from apoE−/− mice fed an atherogenic diet containing 5% of NO or SP for 12 wk. When the splenocytes were challenged by LPS for 20 h, cells from NO or SP-fed mice showed significantly lower IL-6 levels in cell medium than control mice (Figure 4).
Figure 4. Decreased IL-6 secretion in spelnocytes from apoE−/− mice fed NO or SP.
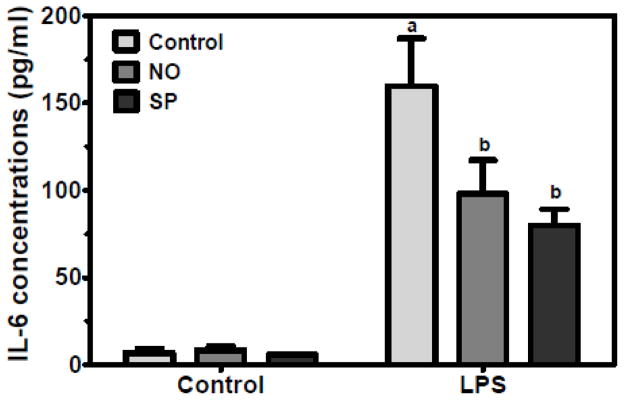
Splenocytes were isolated from apoE−/− mice fed an atherogenic diet supplemented with 5% NO or SP for 12 weeks. The cells were treated with and without 500 ng/mL of LPS for 20 h. Values are expressed as mean ± SEM, P < 0.05, n = 5.
3.3. Algal lipid extracts inhibited NF-κB nuclear translocation in macrophages
Translocation of NF-κB p50/p65 dimer to the nucleus is one of the major steps for its activation to increase transcription of pro-inflammatory cytokines. We previously reported that NOE significantly decreased NF-κB p65 DNA binding activity [30]. To address if the decrease in DNA binding activity is, at least in part, due to the inhibition of p65 nuclear translocation and to compare a potency of the repression between NOE and SPE, RAW 264.7 macrophages pre-treated with algal lipid extracts were stimulated by LPS for 1 or 2 h. At 1 h LPS stimulation, control cells showed markedly increased p65 in the nucleus with p65 being non-detectible in the cytoplasmic fraction (Figure 5). In contrast, macrophages pretreated with NOE and SPE had less p65 translocation to the nucleus and detectible amount of p65 was present in the cytoplasm. The similar trend was shown at 2 h LSP stimulation.
Figure 5. Attenuation of nuclear translocation of NF-κB p65 by NOE and SPE in RAW 264.7 macrophage.
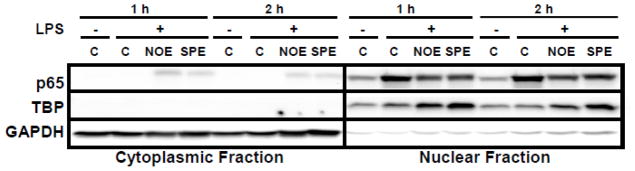
Cells were pre-treated with 50 μg/mL of NOE or SPE for 12 h and subsequently they were activated by 100 ng/mL LPS for 1 or 2 h. Cytoplasmic and nuclear fractions were isolated and Western blot analysis for p65 was conducted. TATA binding protein (TBP) and GAPDH were used as a nuclear and cytoplasmic marker, respectively.
3.4. Inhibition of NF-κB nuclear translocation could not solely account for the repression of pro-inflammatory cytokine expression by NOE and SPE in macrophages
Although translocation of NF-κB to the nucleus is a major regulatory step for its transcriptional activity, other posttranslational modifications in NF-κB have been recently recognized as important regulatory mechanisms for NF-κB activity [32, 33]. To evaluate contribution of nuclear translocation of p65 to the repressive effect of NOE and SPE on TNFα and IL-1β expression, we used SN50, an inhibitor specific for NF-κB translocation to the nucleus. SN50 binds to the nuclear localization sequence (NLS) of p65 and therefore p65 cannot be recognized by karyopherin α, resulting in cytoplasmic sequestration. With the treatment of SN50, LPS-induced nuclear translocation of p65 was blocked (data not shown). Although SN50 significantly lowered mRNA levels of TNFα and IL-1β compared with control, repression of the gene expression was markedly stronger in NOE or SPE-treated RAW 264.7 macrophages than SN50-treated cells (Figure 6A). Interestingly, TSA significantly increased basal expression of IL-1β and TNFα and furthermore the repressive effect of SPE on the gene expression was attenuated by TSA (Figure 6B). NOE also elicited the similar results (data not shown).
Figure 6. Alternative mechanisms for anti-inflammatory effects of NOE and SPE in RAW 264.7 macrophages.
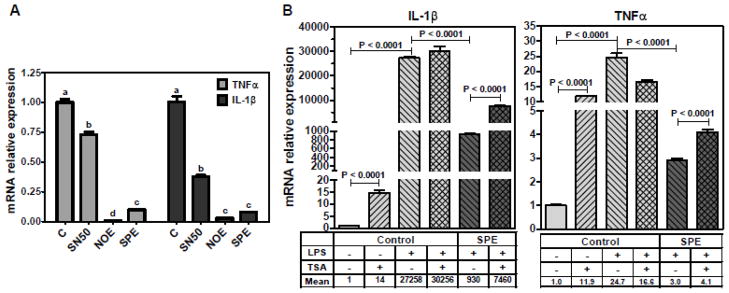
A. Effect of SN50 on pro-inflammatory gene expression. Cells were incubated with NOE or SPE (50 μg/mL) for 12 h. Subsequently, they were incubated with 50 μg/mL of SN50, a NF-κB translocation inhibitor, for 1 h, after which they were activated with 100 ng/mL LPS for 3 h. qRT-PCR analysis was conducted to measure mRNA levels of TNFα and IL-1β. Value are expressed as mean ± SEM, P < 0.05, n = 3. B. RAW 264.7 macrophages were treated with NOE or SPE (100 μg/ml) and 50 nmol/L of TSA for 12 h and then they were activated by LPS (100 ng/mL) for 7 h. qRT-PCR analysis was conducted to measure mRNA levels of TNFα and IL-1β. Value are expressed as mean ± SEM, P < 0.05, n = 3.
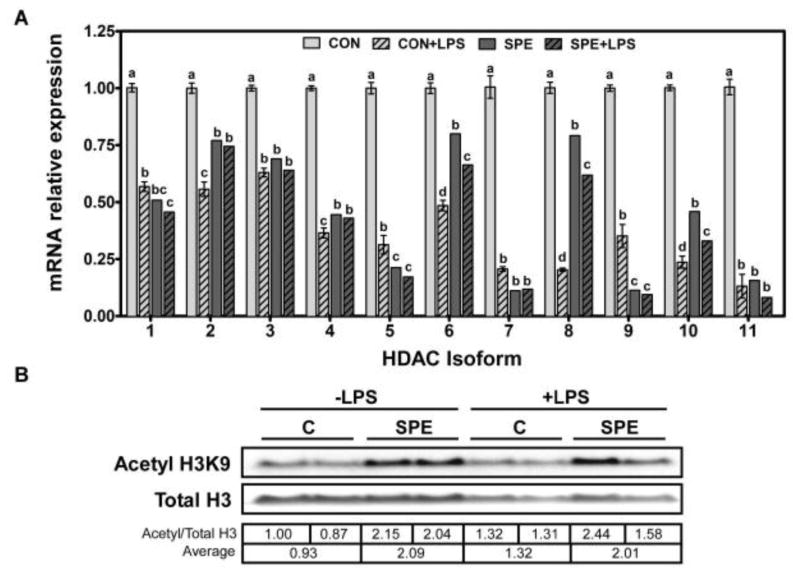
3.5. Down regulation of HDAC expression and subsequent increases in acetylated histone 3 by SPE in macrophages
Inhibition of HDAC by TSA attenuated the repression of pro-inflammatory gene expression by SPE in LPS-stimulated macrophages, suggesting HDAC may play a role in the anti-inflammatory function of the algal extract. Therefore, we next evaluated if SPE can alter the expression of 11 HDAC isoforms in RAW 264.7 macrophages. mRNA abundance of all HDAC isoforms was significantly decreased by SPE in both unstimulated and LPS-stimulated macrophages (Figure 7A). HDAC expression was also significantly lowered by LPS. Consistent with significant decreases in HDAC mRNA levels by SPE in both unstimulated and LPS-stimulated macrophages, SPE increased the level of acetylated H3 by ~2-fold (Figure 7B). Interestingly, although LPS repressed HDAC mRNA levels to a similar extent to SPE, there was only ~40% increase in acetylated H3 in LPS-stimulated cells compared with controls.
Figure 7. Regulation of HDAC expression and levels of acetyl H3 by SPE in RAW 264.7 macrophages.
A. RAW 264.7 macrophages were pre-treated with 100 μg/mL of SPE for 12 h and subsequently with 100 ng/mL LPS and algal extracts for additional 18 h. qRT-PCR analysis was conducted to detect HDAC isoform mRNA abundance. Values are expressed as mean ± SEM, P < 0.05, n = 6. B. Cells were treated with SPE and LPS as described above and Western blot analysis was conducted to detect total and acetyl H3 levels. One representative blot of 2 separate experiments is shown. Densitometry analysis was conducted for the ratios of acetyl H3K9 to total H3. A ratio of each band and an averaged value of each treatment are listed in the table.
4. DISCUSSION
Chronic low-grade inflammation is causally linked to the pathogenesis of several metabolic diseases, such as insulin resistance, metabolic syndrome, type 2 diabetes, CVD and non-alcoholic fatty liver disease. NSAID are commonly used to treat acute and chronic inflammatory conditions, but not without their adverse effects [3]. Therefore, identification of anti-inflammatory natural products with minimal side-effects, yet effective in blocking pathways leading to chronic inflammation is critically needed. We previously reported that NOE exerts ant-inflammatory effects by inhibiting NF-κB DNA binding activity in macrophages [30]. In the present study, we further demonstrated that SPE inhibits pro-inflammatory mediator expression and secretion to a similar extent to NOE in macrophage cell line as well as primary mouse macrophages. Furthermore, the anti-inflammatory was confirmed in splenocytes isolated from NO or SP-fed apoE−/− mice, demonstrating the protection against inflammation by the two BGA species is likely to occur in vivo.
Humans have a long history of BGA consumption as food or medicine for hundreds of years, yet scientific understanding of their health-promoting properties only began ~ 30 years ago. Currently, SP is the most commonly consumed and commercialized BGA species [25]. NO is another edible BGA species with several purported health benefits. However, there has been a concern on safety of BGA for human consumption. Certain BGA species produce toxins, such as microcystins, nodularins, saxitoxins, anatoxins, and β-methylamino-L-alanine and therefore naturally harvested BGA products may contain the toxins due to contamination with toxin-producing algae species during harvest [34–37]. The two BGA species used in the present study are cultivated in the controlled environment to minimize any toxin contamination. We previously demonstrated that SP and NO are free of microcystins, the major algal toxin, and long-term supplementation of the two BGA did not show any adverse side-effects in mice [37]. Furthermore, in 2011, The Dietary Supplements Information Expert Committee assigned a Class A safety rating for SP and permitted the admission of quality monographs for SP in the United States Pharmacopeia and National Formulary [38]. As safety is prerequisite to investigation on health-promoting properties of any natural products, we believe the two BGA species may be safe natural products for human consumption to gain health benefits if their functions are scientifically proven.
Studies have demonstrated that BGA consumption promotes immunity and protects against inflammatory diseases such as colitis, arthritis, and allergic rhinitis in animals and humans [39–42]. In particular, Rasool et al. [42] showed that Spirulina fusiformis supplementation minimized inflammatory response to near normal condition in mice injected with inflammatory inducer. We previously showed that NOE repressed the expression and secretion of pro-inflammatory cytokine such as TNFα and IL-1β in RAW 264.7 macrophages [30]. Consistent with our previous findings, NOE significantly decreased mRNA levels of the pro-inflammatory cytokines not only in RAW 264.7 macrophages but also in BMM. SPE also elicited the same anti-inflammatory effect with similar potency to NOE. Phycocyanin (PC) and γ-linoleic acid are the most well-known anti-inflammatory components in BGA. In particular, C-PC, a natural blue pigment (~14% of SP dry weight) [40, 43, 44], has shown to have a strong inhibitory property against inflammation in macrophages [45, 46] and in animal models [47, 48]. We found that in NOE, unsaturated fatty acids, which account for ~15% of the extract mass, are partly responsible for the repression of pro-inflammatory cytokine production but other compounds are required to achieve full degree of repression in RAW 264.7 macrophages [30]. Studies on identification of anti-inflammatory compounds in NOE and SPE are currently underway. As both algal extracts elicits marked repression of inflammation, the identified compounds may be used as anti-inflammatory drugs alternative to NSAID.
In addition to the inhibitory role of NOE and SPE in the production of pro-inflammatory mediators shown in vitro using RAW 264.7 macrophages and BMM, we sought to evaluate if the BGA supplementation can accumulate enough amount of putative anti-inflammatory compounds in other immune-modulating cells in mice to inhibit inflammation. It has been shown that spleen is a monocyte reservoir and plays an important role not only in monocyte production but also in monocyte/macrophage clearance during inflammation [49, 50]. Therefore, we isolated splenocytes from apoE−/− mice, a common mouse model for atherosclerosis, that were fed an atherogenic diet supplemented with 5% of NO or SP for 12 wk. When the splenocytes from mice fed a NO or SP-containing diet were stimulated by LPS, IL-6 secretion was significantly lower than cells from control mice. This result suggests that NO and SP supplementation can be beneficial to inhibit inflammation in vivo.
Mounting evidence suggests that activation of NF-κB pathway is a major underlying event for the development of inflammatory diseases and its inhibition can decrease the disease progression [51–53]. Epidemiological and clinical studies have demonstrated the effect of polyphenol-rich foods, such as green tea and extra virgin olive oils, on the inhibition of NF-κB activation [54]. We previously demonstrated that NOE repressed pro-inflammatory cytokine production, at least in part, by inhibiting NF-κB p65 DNA binding activity in RAW 264.7 macrophages [30]. In the present study, both NOE and SPE inhibited nuclear translocation of NF-κB p65 in LPS-stimulated macrophages. However, of interest is that blockage of p65 translocation to the nucleus by a chemical inhibitor was significantly less effective in repressing TNFα and IL-1β expression than NOE and SPE. This suggests that mechanisms other than NF-κB nuclear translocation may exist for the algal extract to elicit the drastic reduction in the expression of pro-inflammatory cytokine expression.
Although NF-κB nuclear translocation is the best-known mechanism for its activation [12], several lines of evidence indicate that posttranslational modifications in p65 subunit, such as phosphorylation and acetylation, impact its activity by altering DNA binding, interaction with inhibitor of κB α (IκBα) in the nucleus, and association with chromatin structure modifiers such as histone acetyltransferases (HAT) and HDAC [32, 33]. It is our particular interest that acetylation of p65 at Lys218, 221 and 310 by p300 and cAMP response element binding (CREB) binding protein (CBP) regulates its transcriptional activity by increasing p65 DNA binding and by inhibiting its association with nuclear IκBα for the prevention of NF-κB relocation to the cytoplasm [55]. In contrast, deacetylation at Lys310 by corepressor complexes inhibits p65 activity [56, 57]. In the present study, we observed that basal IL-1β and TNFα mRNA levels were significantly induced by TSA, a pan HDAC inhibitor, in unstimulated macrophages. Bailey and Ghosh [58] suggested that NF-κB in association with a corepressor complex actively represses pro-inflammatory gene expression in unstimulated macrophages and the corepressor complex is composed of nuclear receptor corepressor, HDAC3 and others. Therefore, the induction of IL-1β and TNFα expression by TSA in the present study is well in line with their findings that active transcriptional repression of the pro-inflammatory genes occurs when macrophages are not stimulated by inflammatory insults. Furthermore, we observed that repression of the pro-inflammatory gene expression by NOE and SPE was significantly ameliorated by TSA in LPS-activated RAW 264.7 macrophages. The data suggest that HDAC may be involved in basal transrepression of IL-1β and TNFα when there are no extracellular inflammatory insults, while it mediates the repressive effect of the algal extracts on the expression of the pro-inflammatory genes in the activated macrophages. We found that the mRNA expression of 11 HDAC isoforms was significantly repressed by SPE both in unstimulated and activated macrophages. Among 11 HDAC isoforms, HDAC 1, 2, 3, 4, 5 and 9 are major isoforms in RAW 264.7 macrophages (data not shown). High levels of acetyl H3 observed in the SPE-treated cells are likely due to the down-regulation of HDAC expression although we cannot rule out possible alterations in HAT by SPE. LPS also repressed HDAC mRNA levels to a similar extent to SPE. Although a degree of repression of HDAC mRNA by SPE and LPS was similar, acetyl H3 levels were markedly higher in SPE-treated cells than LPS-activated cells. It can be speculated that LPS and SPE may differentially regulate HDAC expression at the posttranscriptional levels and/or HAT activity may also be altered by the treatments to alter H3 acetylation. Conundrum is that both unstimulated cells and SPE treatment in LPS-treated cells showed low pro-inflammatory gene expression but their acetyl H3 levels were very different. It seems important to understand the effect of SPE and LPS on the expression/activity of each HDAC isoform rather than overall changes in H3 acetylation state for gaining mechanistic insights into critical roles of HDAC in inflammation. Future study is warranted to identify specific HDAC isoforms that are involved in the regulation of the inflammatory gene expression and to determine how their activity is regulated such as cellular localization, posttranslational modification and association with other regulatory proteins. The information can be used to develop effective therapeutic targets to inhibit inflammation.
In conclusion, we found that NO and SP either as a whole or a lipid extract have an anti-inflammatory effect using several model systems, i.e., RAW 264.7 macrophages, BMM and mouse splenocytes. Repression of pro-inflammatory mediator expression by the BGA is mediated partly by inhibiting NF-κB nuclear translocation. In addition, our results also support that HDAC and modulations in chromatin structure are likely involved in the anti-inflammatory function of NO and SP. Dysregulation of pro-inflammatory mediator production from macrophages is a major contributor to the development of pathological conditions related to inflammation such as insulin resistance and atherosclerosis. Therefore, the two BGA species tested in the present study may be used as a natural product to prevent inflammatory diseases.
Highlights.
Anti-inflammatory effects of edible blue-green algae in macrophages
Inhibition of NF-κB pathway for the anti-inflammatory effects in macrophages
Repression of IL-6 secretion from splenocytes of mice fed blue-green algae
Role of histone deacetylases in the anti-inflammatory role of blue-green algae
Acknowledgments
This work was supported by National Institute Health grant R21AT005152 and fund from College of Agriculture and Natural Resources at the University of Connecticut to J. Lee.
Abbreviations
- apoE−/−
apolipoprotein E knock out
- BGA
blue-green algae
- BMM
bone marrow-derived macrophages
- CBP
CREB-binding protein
- CREB
cAMP-response element-binding protein
- C-PC
C-phycocyanin
- CVD
cardiovascular disease
- HAT
histone acetyltransferase
- HDAC
histone deacetylases
- IκBα
inhibitor of kappa B α
- IL
interleukins
- LPS
lipopolysaccharide
- NLS
nuclear localization sequence
- NO
Nostoc commune var. sphaeroides Kützing
- NOE
NO lipid extract
- SP
Spirulina platensis
- SPE
SP lipid extract
- TNFα
tumor necrosis factor α
- TSA
trichostatin A
Footnotes
C. S. Ku, Y. Park, B. Kim, T.X. Pham and M. Shin conducted experiments; C. S. Ku, and Y. Park contributed to manuscript preparation; I. Kang contributed to data analysis; and J. Lee designed the study, analyzed data and largely contributed to manuscript preparation. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30. doi: 10.1016/j.arr.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Prete A, Allavena P, Santoro G, Fumarulo R, Corsi MM, Mantovani A. Molecular pathways in cancer-related inflammation. Biochem Med (Zagreb) 2011;21:264–275. doi: 10.11613/bm.2011.036. [DOI] [PubMed] [Google Scholar]
- 3.Jugdutt BI. Cyclooxygenase inhibition and adverse remodeling during healing after myocardial infarction. Circulation. 2007;115:288–291. doi: 10.1161/CIRCULATIONAHA.106.675306. [DOI] [PubMed] [Google Scholar]
- 4.Lubbad A, Oriowo MA, Khan I. Curcumin attenuates inflammation through inhibition of TLR-4 receptor in experimental colitis. Mol Cell Biochem. 2009;322:127–135. doi: 10.1007/s11010-008-9949-4. [DOI] [PubMed] [Google Scholar]
- 5.Tsai SH, Lin-Shiau SY, Lin JK. Suppression of nitric oxide synthase and the down-regulation of the activation of NFkappaB in macrophages by resveratrol. Br J Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terra X, Valls J, Vitrac X, Merrillon JM, Arola L, Ardevol A, Blade C, Fernandez-Larrea J, Pujadas G, Salvado J, Blay M. Grape-seed procyanidins act as antiinflammatory agents in endotoxin-stimulated RAW 264.7 macrophages by inhibiting NFkB signaling pathway. J Agric Food Chem. 2007;55:4357–4365. doi: 10.1021/jf0633185. [DOI] [PubMed] [Google Scholar]
- 7.Kumar B, Gupta SK, Nag TC, Srivastava S, Saxena R. Green tea prevents hyperglycemia-induced retinal oxidative stress and inflammation in streptozotocin-induced diabetic rats. Ophthalmic Res. 2012;47:103–108. doi: 10.1159/000330051. [DOI] [PubMed] [Google Scholar]
- 8.Recio MC, Andujar I, Rios JL. Anti-inflammatory agents from plants: progress and potential. Curr Med Chem. 2012;19:2088–2103. doi: 10.2174/092986712800229069. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, Jung KJ, Kim JW, Yu BP, Chung HY. Alteration of soluble adhesion molecules during aging and their modulation by calorie restriction. FASEB J. 2004;18:320–322. doi: 10.1096/fj.03-0849fje. [DOI] [PubMed] [Google Scholar]
- 10.Chung HY, Sung B, Jung KJ, Zou Y, Yu BP. The molecular inflammatory process in aging. Antioxid Redox Signal. 2006;8:572–581. doi: 10.1089/ars.2006.8.572. [DOI] [PubMed] [Google Scholar]
- 11.Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 12.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 13.Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM, Buckbinder L, Greene WC. NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol. 2005;25:7966–7975. doi: 10.1128/MCB.25.18.7966-7975.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen ZJ, Bhoj V, Seth RB. Ubiquitin TAK1 and IKK: is there a connection? Cell Death Differ. 2006;13:687–692. doi: 10.1038/sj.cdd.4401869. [DOI] [PubMed] [Google Scholar]
- 15.Krappmann D, Scheidereit C. A pervasive role of ubiquitin conjugation in activation and termination of IkappaB kinase pathways. EMBO Rep. 2005;6:321–326. doi: 10.1038/sj.embor.7400380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pamukcu B, Lip GY, Shantsila E. The nuclear factor--kappa B pathway in atherosclerosis: a potential therapeutic target for atherothrombotic vascular disease. Thromb Res. 2011;128:117–123. doi: 10.1016/j.thromres.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Yan SF, Ramasamy R, Naka Y, Schmidt AM. Glycation, inflammation, and RAGE: a scaffold for the macrovascular complications of diabetes and beyond. Circ Res. 2003;93:1159–1169. doi: 10.1161/01.RES.0000103862.26506.3D. [DOI] [PubMed] [Google Scholar]
- 18.Foncea R, Carvajal C, Almarza C, Leighton F. Endothelial cell oxidative stress and signal transduction. Biol Res. 2000;33:89–96. doi: 10.4067/s0716-97602000000200008. [DOI] [PubMed] [Google Scholar]
- 19.DiDonato J, Mercurio F, Rosette C, Wu-Li J, Suyang H, Ghosh S, Karin M. Mapping of the inducible IkappaB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang CC, Chen J, Thomas MA, Cheng D, Del Priore VA, Newton RS, Pape ME, Chang TY. Regulation and immunolocalization of acyl-coenzyme A: cholesterol acyltransferase in mammalian cells as studied with specific antibodies. J Biol Chem. 1995;270:29532–29540. doi: 10.1074/jbc.270.49.29532. [DOI] [PubMed] [Google Scholar]
- 21.Brown K, Gerstberger S, Carlson L, Franzoso G, Siebenlist U. Control of I kappa B-alpha proteolysis by site-specific, signal-induced phosphorylation. Science. 1995;267:1485–1488. doi: 10.1126/science.7878466. [DOI] [PubMed] [Google Scholar]
- 22.DiDonato JA, Mercurio F, Karin M. Phosphorylation of I kappa B alpha precedes but is not sufficient for its dissociation from NF-kappa B. Mol Cell Biol. 1995;15:1302–1311. doi: 10.1128/mcb.15.3.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alkalay I, Yaron A, Hatzubai A, Jung S, Avraham A, Gerlitz O, Pashut-Lavon I, Ben Neriah Y. In vivo stimulation of I kappa B phosphorylation is not sufficient to activate NF-kappa B. Mol Cell Biol. 1995;15:1294–1301. doi: 10.1128/mcb.15.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karin M. The beginning of the end: IkappaB kinase (IKK) and NF-kappaB activation. J Biol Chem. 1999;274:27339–27342. doi: 10.1074/jbc.274.39.27339. [DOI] [PubMed] [Google Scholar]
- 25.Ciferri O. Spirulina, the edible microorganism. Microbiol Rev. 1983;47:551–578. doi: 10.1128/mr.47.4.551-578.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samuels R, Mani UV, Iyer UM, Nayak US. Hypocholesterolemic effect of spirulina in patients with hyperlipidemic nephrotic syndrome. J Med Food. 2002;5:91–96. doi: 10.1089/109662002760178177. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Hernandez A, Ble-Castillo JL, Juarez-Oropeza MA, Diaz-Zagoya JC. Spirulina maxima prevents fatty liver formation in CD-1 male and female mice with experimental diabetes. Life Sci. 2001;69:1029–1037. doi: 10.1016/s0024-3205(01)01185-7. [DOI] [PubMed] [Google Scholar]
- 28.Kushak RI, Drapeau C, Van Cott EM. Favorable effects of blue-green algae Aphanizomenon flos-aquae on rat plasma lipids. Journal of the American Nutraceutical Association. 2000;2:59–65. [Google Scholar]
- 29.Hori K, Ishibashi G, Okita T. Hypocholesterolemic effect of blue-green alga, ishikurage (Nostoc commune) in rats fed atherogenic diet. Plant Foods Hum Nutr. 1994;45:63–70. doi: 10.1007/BF01091230. [DOI] [PubMed] [Google Scholar]
- 30.Park YK, Rasmussen HE, Ehler SJ, Blobaum KR, Lu F, Schlegel VL, Carr TP, Lee JY. Repression of proinflammatory gene expression by lipid extract of Nostoc commune var sphaeroides Kutzing, a blue-green alga, via inhibition of nuclear factor-kappa B in RAW 264.7 macrophages. Nutr Res. 2008;28:83–92. doi: 10.1016/j.nutres.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 31.Rasmussen HE, Blobaum KR, Park YK, Ehlers SJ, Lu F, Lee JY. Lipid extract of Nostoc commune var. sphaeroides Kutzing, a blue-green alga, inhibits the activation of sterol regulatory element binding proteins in HepG2 cells. J Nutr. 2008;138:476–481. doi: 10.1093/jn/138.3.476. [DOI] [PubMed] [Google Scholar]
- 32.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor kappa B pathway. Oncogene. 2006;25:6717–6730. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 33.Neumann M, Naumann M. Beyond IkappaBs: alternative regulation of NF-kappaB activity. FASEB J. 2007;21:2642–2654. doi: 10.1096/fj.06-7615rev. [DOI] [PubMed] [Google Scholar]
- 34.Carmichael WW. The toxins of cyanobacteria. Scientific America. 1994;270:78–86. doi: 10.1038/scientificamerican0194-78. [DOI] [PubMed] [Google Scholar]
- 35.Cox PA, Banack SA, Murch SJ, Rasmussen U, Tien G, Bidigare RR, Metcalf JS, Morrison LF, Codd GA, Bergman B. Diverse taxa of cyanobacteria produce beta-N-methylamino-L-alanine, a neurotoxic amino acid. Proc Natl Acad Sci U S A. 2005;102:5074–5078. doi: 10.1073/pnas.0501526102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson HE, King SR, Banack SA, Webster C, Callanaupa WJ, Cox PA. Cyanobacteria (Nostoc commune) used as a dietary item in the Peruvian highlands produce the neurotoxic amino acid BMAA. J Ethnopharmacol. 2008;118:159–165. doi: 10.1016/j.jep.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Yang Y, Park Y, Cassada DA, Snow DD, Rogers DG, Lee J. In vitro and in vivo safety assessment of edible blue-green algae, Nostoc commune var. sphaeroides Kutzing and Spirulina plantensis. Food Chem Toxicol. 2011;49:1560–1564. doi: 10.1016/j.fct.2011.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klivenyi P, Andreassen OA, Ferrante RJ, Dedeoglu A, Mueller G, Lancelot E, Bogdanov M, Andersen JK, Jiang D, Beal MF. Mice deficient in cellular glutathione peroxidase show increased vulnerability to malonate, 3-nitropropionic acid, and 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. J Neurosci. 2000;20:1–7. doi: 10.1523/JNEUROSCI.20-01-00001.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Selmi C, Leung PS, Fischer L, German B, Yang CY, Kenny TP, Cysewski GR, Gershwin ME. The effects of Spirulina on anemia and immune function in senior citizens. Cell Mol Immunol. 2011;8:248–254. doi: 10.1038/cmi.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao TK, Van de Water J, Gershwin ME. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. J Med Food. 2005;8:27–30. doi: 10.1089/jmf.2005.8.27. [DOI] [PubMed] [Google Scholar]
- 41.Coskun ZK, Kerem M, Gurbuz N, Omeroglu S, Pasaoglu H, Demirtas C, Lortlar N, Salman B, Pasaoglu OT, Turgut HB. The study of biochemical and histopathological effects of spirulina in rats with TNBS-induced colitis. Bratisl Lek Listy. 2011;112:235–243. [PubMed] [Google Scholar]
- 42.Rasool M, Sabina EP, Lavanya B. Anti-inflammatory effect of Spirulina fusiformis on adjuvant-induced arthritis in mice. Biol Pharm Bull. 2006;29:2483–2487. doi: 10.1248/bpb.29.2483. [DOI] [PubMed] [Google Scholar]
- 43.Deng R, Chow TJ. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther. 2010;28:e33–45. doi: 10.1111/j.1755-5922.2010.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Romay C, Gonzalez R, Ledon N, Remirez D, Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4:207–216. doi: 10.2174/1389203033487216. [DOI] [PubMed] [Google Scholar]
- 45.Cherng SC, Cheng SN, Tarn A, Chou TC. Anti-inflammatory activity of c-phycocyanin in lipopolysaccharide-stimulated RAW 264.7 macrophages. Life Sci. 2007;81:1431–1435. doi: 10.1016/j.lfs.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 46.Remirez D, Fernandez V, Tapia G, Gonzalez R, Videla LA. Influence of C-phycocyanin on hepatocellular parameters related to liver oxidative stress and Kupffer cell functioning. Inflamm Res. 2002;51:351–356. doi: 10.1007/pl00000314. [DOI] [PubMed] [Google Scholar]
- 47.Romay AJ, Remirez D, González R, Ledon N, García I. Antioxidant and anti-inflammatory properties of C-phycocyanin from blue-green algae. Inflamm Res. 1998;47:36–41. doi: 10.1007/s000110050256. [DOI] [PubMed] [Google Scholar]
- 48.Gonzalez R, Rodriguez S, Romay C, Ancheta O, Gonzalez A, Armesto J, Remirez D, Merino N. Anti-inflammatory activity of phycocyanin extract in acetic acid-induced colitis in rats. Pharmacol Res Commun. 1999;39:55–59. [PubMed] [Google Scholar]
- 49.Swirski FK, Wildgruber M, Ueno T, Figueiredo JL, Panizzi P, Iwamoto Y, Zhang E, Stone JR, Rodriguez E, Chen JW, Pittet MJ, Weissleder R, Nahrendorf M. Myeloperoxidase-rich Ly-6C+ myeloid cells infiltrate allografts and contribute to an imaging signature of organ rejection in mice. J Clin Invest. 2010;120:2627–2634. doi: 10.1172/JCI42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med. 2012;209:123–137. doi: 10.1084/jem.20111009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rial NS, Choi K, Nguyen T, Snyder B, Slepian MJ. Nuclear factor kappa B (NF-kappaB): a novel cause for diabetes, coronary artery disease and cancer initiation and promotion? Med Hypotheses. 2012;78:29–32. doi: 10.1016/j.mehy.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Madonna R, De Caterina R. Relevance of new drug discovery to reduce NF-κB activation in cardiovascular disease. Vascular Pharmacology. 2012;57:41–47. doi: 10.1016/j.vph.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 54.Santangelo C, Vari R, Scazzocchio B, Di Benedetto R, Filesi C, Masella R. Polyphenols, intracellular signalling and inflammation. Ann Ist Super Sanita. 2007;43:394–405. [PubMed] [Google Scholar]
- 55.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J. 2002;21:6539–6548. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoberg JE, Yeung F, Mayo MW. SMRT derepression by the IkappaB kinase alpha: a prerequisite to NF-kappaB transcription and survival. Mol Cell. 2004;16:245–255. doi: 10.1016/j.molcel.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 57.Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 2004;23:2369–2380. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bailey ST, Ghosh S. ‘PPAR’ting ways with inflammation. Nat Immunol. 2005;6:966–967. doi: 10.1038/ni1005-966. [DOI] [PubMed] [Google Scholar]