Abstract
Structural neuroimaging studies of the amygdala and hippocampus in bipolar disorder have been largely inconsistent. This may be due in part to differences in the proportion of subjects taking lithium or experiencing an acute mood state, as both factors have recently been shown to influence gray matter structure. To avoid these problems, we evaluated euthymic subjects not currently taking lithium. Thirty-two subjects with bipolar type I disorder and 32 healthy subjects were scanned using magnetic resonance imaging. Subcortical regions were manually traced, and converted to three-dimensional meshes to evaluate the main effect of bipolar illness on radial distance. Statistical analyses found no evidence for a main effect of bipolar illness in either region, although exploratory analyses found a significant age by diagnosis interaction in the right amygdala, as well as positive associations between radial distance of the left amygdala and both prior hospitalizations for mania and current medication status. These findings suggest that, when not treated with lithium or in an acute mood state, patients with bipolar disorder exhibit no structural abnormalities of the amygdala or hippocampus. Future studies, nevertheless, that further elucidate the impact of age, course of illness, and medication on amygdala structure in bipolar disorder are warranted.
Keywords: mood disorder, magnetic resonance imaging, hippocampus, amygdala, lithium, bipolar disorder
1. Introduction
Lesion and behavioral studies have implicated the involvement of the amygdala (Flor-Henry 1969; Bear and Fedio 1977; Shukla et al. 1987; Starkstein et al. 1988; George et al. 1998; Murphy et al. 1999; Yurgelun-Todd et al. 2000; Lembke and Ketter 2002) and hippocampus (Flor-Henry 1969; Bear and Fedio 1977; Shukla et al. 1987; Starkstein et al. 1988; Altshuler et al. 2005; Malhi et al. 2007) in the pathophysiology of bipolar disorder, but findings from structural neuroimaging studies of these brain regions have been largely inconsistent. Many MRI studies, including our own, have found abnormally large amygdala volumes in adults with bipolar disorder (Altshuler et al. 1998; Strakowski et al. 1999; Brambilla et al. 2003; Frangou 2005), but other studies have reported normal (Killgore et al. 2009; Brown et al. 2011) or smaller (Blumberg et al. 2003; Rosso et al. 2007; Savitz et al. 2010) amygdala volumes in adult patients. MRI studies of the hippocampus have also been inconsistent, with volume reductions (Noga et al. 2001; Strasser et al. 2005; Bearden et al. 2008; Chepenik et al. 2009) or no difference (Altshuler et al. 1998; Strakowski et al. 1999; Altshuler et al. 2000; Brambilla et al. 2003) reported in bipolar adults compared to healthy controls.
It is not clear why these discrepancies exist, but possibilities include methodological differences (i.e., differences in MRI acquisition and analysis procedures), age or biological factors affecting brain structure (e.g., genetics). Additionally, differences in findings may relate to variations in clinical characteristics of the patient samples. Many prior reports, for example, have sampled from bipolar participants receiving lithium treatment, a mood stabilizing medication known to significantly increase subcortical gray matter volume (Yucel et al. 2007; Bearden et al. 2008; Foland et al. 2008; Usher et al. 2009; Hallahan et al. 2011). Prior studies have also included patients in various states of mania, depression or euthymia, both within and across studies. This factor (mood state) may also significantly confound results; two recent reports found an effect of acute depression on cortical structure (Brooks et al. 2009; Nery et al. 2009). Findings of amygdala structure in bipolar disorder from our laboratory (Foland-Ross et al. in press), as well as a study of the hippocampus in major depression (Bearden et al. 2009) have similarly found effects of depressed mood on subcortical gray matter. Additional studies, therefore, that control for the possible effects of lithium status and mood state are needed to elucidate structural differences in the medial temporal brain region between adults with bipolar disorder and healthy controls.
Here we attempted to control for the above issues through examining whether the anatomical structure of the amygdala and hippocampus were altered in a sample of bipolar I subjects who were in a euthymic mood state and were currently not taking lithium. We employed a specialized three-dimensional (3D) surface mesh modeling approach that visualizes the spatial profile of neuropathological abnormalities, allowing for a refined neuroanatomic localization of regionally specific alterations in bipolar patients (Thompson et al. 2004). Given that the prior studies that have found subcortical enlargement in one or both of these structures have primarily sampled from bipolar patients treated with lithium (Altshuler et al. 1998; Altshuler et al. 2000; Brambilla et al. 2003), and evidence that volumetric reductions in these regions are present in bipolar populations not treated with this medication (Blumberg et al. 2003; Rosso et al. 2007; Savitz et al. 2010), we hypothesized that lithium-free euthymic bipolar subjects in the current study would show regional volumetric reductions in the amygdala and hippocampus relative to healthy subjects. We additionally explored possible associations between 3D morphology of medial temporal lobe structures and clinical and demographic variables.
2. Methods
2.1 Subjects
The Institutional Review Boards at UCLA and the VA Greater Los Angeles Healthcare System approved the study. Each subject provided written informed consent. Subjects with bipolar I disorder were recruited through the outpatient UCLA Mood Disorders Clinic, and the outpatient Bipolar Disorders Clinic of the Veterans Affairs Greater Los Angeles Health Care System. Control subjects were recruited by advertisement in local newspapers and campus flyers. Control and patient populations were evaluated using the Structured Clinical Interview for DSMIV (SCID) to confirm diagnosis or absence thereof. Exclusion criteria for all subjects included left-handedness, hypertension, neurological illness, metal implants and a history of skull fracture or head trauma with loss of consciousness >5 minutes. Bipolar subjects who were experiencing an acute mood state, had other active Axis I co-morbidities or were currently receiving treatment with lithium were excluded. Euthymia was defined as not currently meeting criteria for a manic, hypomanic or depressive mood episode, according to the SCID, a Young Mania Rating Scale (YMRS; Young et al. 1978) score of ≤7, and a 21-item Hamilton Depression Rating Scale (HAMD; Hamilton 1960) score of ≤7 on the day of scanning. Current lithium use was defined as patients who were taking lithium medication at the time of scanning or had done so in the previous month. Information on lifetime exposure to lithium, as well as prior course of illness and current medication use was additionally obtained by self-report, by reference to medical records when available, and by corroboration of family members or significant others when subjects allowed this. Other exclusionary criteria for healthy controls included current or past psychiatric diagnosis (including history of substance abuse) or current medication use.
In total, 32 subjects with bipolar type I disorder (20f; 37.9±11.9 years), currently euthymic, and 32 healthy control subjects (19f; 38.3±12.9 years) were included in the study. Demographic and clinical characteristics, including current medication information for all subjects are presented in Table 1.
Table 1.
Subject demographics
| Demographic variable | Subjects with bipolar disorder (N=32) | Healthy controls (N=32) | Group difference |
|---|---|---|---|
| Age (Mean ± SD) | 37.9±11.9 | 38.3±12.9 | p=0.91 t=0.12 |
| N Female (%) | 20 (60.6) | 19 (59.4) | p=1.00 |
| Education levela | 3.0±0.6 | 3.2±0.6 | p=0.35 |
| Race | p=0.21 | ||
| N Caucasian (%) | 23 (71.9) | 23 (71.9) | - |
| N Asian (%) | 2 (6.3) | 6 (18.8) | - |
| N African American (%) | 6 (18.8) | 3 (9.4) | - |
| N Other (%) | 1 (3.1) | 0 (0) | - |
| HAMDb | 4.5±2.3 | - | - |
| YMRSc | 1.8±2.2 | - | - |
| Duration of Illness (years) | 19.1±12.4 | - | - |
| Age at Onset | 17.1±6.5 | - | - |
| Prior Manias | 8.8±11.4 | - | - |
| Prior depressions | 10.6±18.8 | - | - |
| Prior hospitalizations | 2.5±2.7 | - | - |
| N with a history of psychosis (%) | 16 (48.5) | - | - |
| Months Euthymic | 17.9±22.3 | - | - |
| Patient medications | |||
| N Unmedicated (%) | 10 (31) | - | - |
| N Lithium (%)* | 0 (0) | - | - |
| N Anticonvulsantsd (%) | 14 (44) | - | - |
| N Antipsychoticse (%) | 15 (47) | - | - |
| N Antidepressantsf (%) | 4 (13) | - | - |
Educational level for each subject was rated on a four-point scale (1, grade 8 or less; 2, grade 9–12; 3, 1–4 years of college or university; 5, five or more years of college or university);
Hamilton Depression Rating Scale;
Young Mania Rating Scale;
includes treatment with Divalproex sodium (N=5), Lamotrigine (N=10), Oxcarbazepine (N=1) or Carbamazepine (N=2);
includes treatment with Aripiprazole (N=8), Risperidone (N=1), Ziprasidone (N=1), Olanzapine (N=3), Quetiapine (N=4);
includes treatment with Paroxetine (N=1), Fluoxetine (N=2), Citalopram (N=1), Escitalopram (N=1),Venlafaxine (N=1),Bupropion (N=2),Trazodone (N=1);
includes treatment with Temazepam (N=1). Months euthymic indicated time euthymic prior to scanning. All p values indicate 2-tailed significance levels.
Although no patients in the current study were taking lithium at the time of scanning, (25%) were documented as having lifetime exposure to this medication.
2.2 Image acquisition
Each subject was positioned in a supine orientation with their head stabilized by foam pads inside a radio frequency (RF) head coil. Sound-insulating earplugs reduced discomfort associated with scanner noise. A sagittal high-resolution 3D MP-RAGE T1-weighted image volume was obtained using a 1.5 Tesla Siemens Sonata MRI scanner (Milwaukee, WI) at the UCLA Ahmanson-Lovelace Brain Mapping Center (FOV: 256 mm; 160 slices; isotropic voxel size 1 mm3; TR=1900 ms; TE=4.38 ms; flip angle: 15 degrees; averages=4; total scan time=8.14 min).
2.3 Analysis of demographic variables
Statistical analysis of demographic variables was performed using the R statistical software package (http://www.r-project.org). Group differences in categorical and continuous demographic variables were computed using 2-tailed Fisher’s exact and independent t-tests, respectively. A two-tailed α level of p<0.05 was used as the threshold for statistical significance for these and all other analyses.
2.4 Image preprocessing
Image preprocessing steps consisted of correction of artifactual intensity non-uniformities due to magnetic field inhomogeneities (Zijdenbos and Dawant 1994), adjustment for head position and transformation of imaging data into a common stereotaxic coordinate system without scaling using a three-translation and three-rotation rigid-body transformation (Woods et al. 1998) and reslicing in the coronal plane. These procedures corrected for differences in head position and orientation and allowed images to be displayed in a coronal plane perpendicular to the longitudinal axis of the hippocampus (Bartzokis et al. 1993). Additionally, all images were processed to remove non-brain tissue using automated algorithms (Shattuck and Leahy 2002) to allow for the calculation of total brain volume (TBV).
2.5 Manual delineation of subcortical regions of interest
The amygdala was manually traced using a previously defined protocol (Bartzokis et al. 1993; Altshuler et al. 2000). A trained image analyst (C.P.) who was blind to diagnostic, demographic and clinical variables performed the tracings of this structure. Hippocampi were traced bilaterally according to a predefined protocol (Altshuler 2000) by a separate trained image analyst (R.V.), who was also blind to all demographic and clinical variables. To assess intra-rater reliability, ten different randomly selected brains were re-traced by each investigator and intraclass correlation coefficients (ICCs) were computed. Results from intra-rater testing revealed excellent reliability, with ICCs of 0.83 and 0.86 for the left and right amygdala, respectively, and 0.89 and 0.92 for the left and right hippocampus, respectively.
2.6 Subcortical mesh mapping
For the 3D morphometric analysis of the amygdala and hippocampus, surface meshes were constructed for each subject using a surface-based anatomical modeling approach that has been detailed previously (Thompson et al. 1996). This method converts manual tracings to mesh surfaces through first digitizing points along the amygdalar and hippocampal tissue boundaries (identified by tracings), then resamples these points to make structural boundaries spatially uniform by stretching a regular parametric grid over each surface (Figure 1). A medial core, or curve threading down the longitudinal center of each region, was then computed separately for the amygdala and hippocampus. The resulting distance from the subcortical structure’s medial core to each surface point of that structure determine radial distance, the dependent variable in our analysis of subcortical structure; smaller radial distances reflect local areas of small volume, whereas larger radial distances reflect local areas of large volume. Because the same surface grid was initially imposed on each subject, statistical comparisons could be made at homologous subcortical surface points to index diagnosis-related (between-group) differences in radial distance, as described below. Probability values from statistical comparisons, mapped at each surface point, generate a three-dimensional representation of structural differences between groups.
Figure 1.
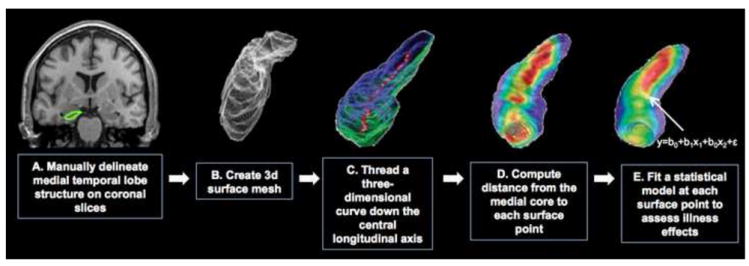
Schematic of the mesh mapping method. The surface-based mesh mapping method relies on (A) manually tracing the subcortical structure (B) computing a three-dimensional parametric mesh model of the structure, (C) estimating the distance between the central core of the structure to each surface point (i.e., the radial distance), and recording radial distance estimates at each surface point to create subject and group average color-coded maps of the radial distance (D). These maps are then assessed statistically by fitting a statistical model separately at each surface point to provide group differences in radial distance, or associations with clinical and demographic variables (E).
2.7 Analysis of diagnostic effects
A general linear model (GLM) was fit at each location along the amygdala and hippocampus surface mesh to identify surface points having a significant association between diagnosis and radial distance. This model included age, gender and TBV as covariates. Because mesh-mapping methods assess point-wise, highly localized effects of structural differences, we, like prior studies that have used these procedures (Narr et al. 2004; Thompson et al. 2004; Bearden et al. 2008) did not include hemisphere as a repeated measure. Results from the surface-based GLM analyses were mapped onto the average surface mesh as uncorrected, color-coded pvalues to provide a visual representation of illness effects.
Because multiple comparisons were made across many surface points, permutation testing was used, as previously described (Thompson et al. 2004) to assess regional significance. This procedure measures the distribution of features in the statistical maps that would be observed by accident if group assignment were random, and provides an overall p value for the observed effects that is corrected for multiple comparisons.
2.8 Exploratory analyses of associations between subcortical structure and clinical and demographic variables
To explore possible associations between prior course of illness and subcortical radial size, individual linear regression models were fit for the clinical variables listed in Table 1. For prior course of illness variables that were highly collinear with age (such as illness duration, r=0.82, p<0.0001), gender and TBV, but not age, were included as covariates; all other models controlled for all three variables.
Associations between radial distance and current medications were explored through direct group comparisons between patients who were (N=22) or were not (N=10) treated at the time of scanning. Follow-up multiple linear regression models were used to further explore whether there were significant associations between radial distance and specific medication type (anticonvulsants, N=14; antipsychotics, N=15; and antidepressants, N=4). Additionally, although all subjects in our sample were lithium free at the time of scanning, and although there is not evidence to suggest a permanent effect of this medication on neural structure, we none-the-less conducted additional analyses comparing radial distance between patients who had (N=8) and who had not (N=20) taken lithium in the past to determine whether prior exposure was itself a significant confounder, as well as compared radial distance between lithium-naïve patients and healthy controls.
Finally, since at least one previous study found an abnormal relationship between age and subcortical structure in bipolar adolescents (Chen et al. 2004), we also explored the effects of this factor on radial distance of the amygdala and hippocampus. In this exploratory analysis, we refit the primary main effects models adding the interaction of diagnosis and age. This revised model was run at each surface point, controlling for gender and TBV. Additionally, the interaction of diagnosis and gender was explored.
3. Results
3.1 Subject demographics
Bipolar subjects did not differ significantly from healthy controls in age, gender, educational level or race (Table 1). On the day of the scan, bipolar patients’ average HAMD and YMRS scores were 4.5+/-2.3 and 1.8+/- 2.2, respectively.
3.2 Mesh mapping results: effect of bipolar illness
Statistical analyses of the radial distance of the hippocampus and amygdala revealed no significant differences between groups for either region (corrected p’s for the left and right amygdala: 0.475 and 0.489, respectively; corrected p’s for the left and right hippocampus: 0.804 and 0.375, respectively).
3.3 Mesh mapping results: associations with clinical and demographic variables
Results from our exploratory analysis of medication effects revealed no significant differences between medicated and unmedicated bipolar subjects and radial size in the hippocampus. A significant difference in radial distance, however, was observed between medicated (N=22) and unmedicated (N=10) subjects in the left amygdala (Figure 2a), with radial size increases observed in medicated compared to unmedicated bipolar subjects (corrected p values: left=0.047; right=0.827). Visual comparisons of these maps with surface based representations of amygdala subnuclei (provided by Yang et al. 2009) suggest that medication associations were present in the central nucleus, and midline portions of the lateral and basolateral nuclei. Follow-up multiple linear regression analyses indicated that no particular medication type (anticonvulsants, N=14; antipsychotics, N=15; and antidepressants, N=4) drove this group difference. Furthermore, follow-up pairwise comparisons revealed no significant differences in radial size between medicated bipolar subjects and healthy subjects (corrected p’s>0.2) or between unmedicated bipolar subjects and healthy subjects (corrected p’s>0.498). Moreover, no group differences in radial size were observed in the amygdala or hippocampus between patients who had (N=8) and who had not (N=20) had prior exposure to lithium medication, or between lithium-naïve patients and healthy controls (all p’s>0.4).
Figure 2.
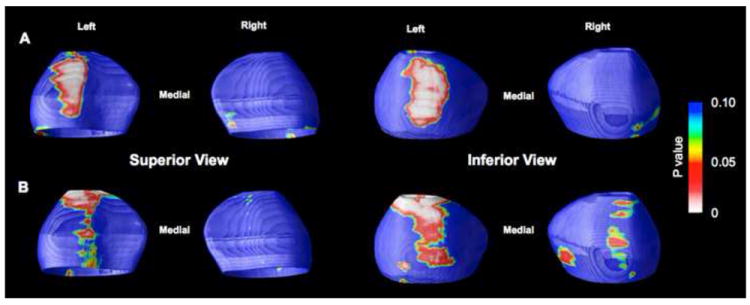
Statistical maps showing regional positive associations between amygdala surface structure and current medication use (A) and prior hospitalizations for mania (B). Probability maps show thresholded, uncorrected p values in color. See the Results section of the text for corrected p values.
Exploratory analyses examining the effect of prior course of illness revealed significant positive correlations between radial distance in the left amygdala and prior number of hospitalizations for mania (corrected p values: left=0.046; right=0.682; Figure 2b). Visual comparisons of these maps with surface based representations of amygdala subnuclei (provided by Yang et al. 2009) show that these associations were again localized to the central nucleus, and midline portions of the lateral and basolateral nuclei. All other prior course of illness variables showed no significant associations with radial distance of the amygdala or hippocampus (all corrected p’s<0.2).
Finally, a diagnosis by age interaction (controlling for gender and TBV) was found for the right amygdala that was localized to a gray matter subregion roughly corresponding to the basolateral nucleus (corrected p’s: left=0.172; right=0.046; Figure 3). Follow-up within-group analyses, controlling for gender and TBV, showed that radial size of this region decreased with age in healthy controls (corrected p’s: left=0.075, right=0.013), but not in bipolar patients (corrected p’s: left=0.897, right=0.451). No interaction effects were found for the left or right hippocampus (corrected p’s: left=0.600, right=0.388). Moreover, no diagnosis by gender interaction effects were found for either structure (corrected p’s=n.s.).
Figure 3.
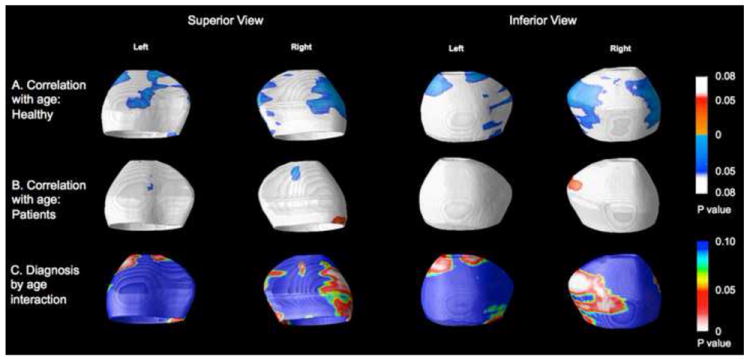
Associations between amygdala radial size and age in healthy controls (A) and bipolar subjects (B). The significance of these correlations is plotted as uncorrected color-coded p values, with cool colors indicating negative correlations and hot colors, positive correlations. Red areas in (C) indicate areas in areas of significant age by diagnosis interactions (i.e. areas in which age-related changes in radial size differ between groups).
Given that our initial exploratory analyses found a significant positive association between radial distance of the amygdala and current use of medication, prior hospitalizations for mania and age, we refit our statistical model to include all of these variables, while controlling for gender and TBV. This joint model allowed us to determine whether these variables had unique contributions to radial distance, or had overlapping effects. Results from this analysis showed that the effects of these individual factors on radial distance were no longer significant in the joint model (corrected p’s for prior hospitalizations for mania: left=0.076, right=0.505; corrected p’s for current medication treatment: left=0.139, right=0.554), suggesting that associations between amygdala structure and age may be related to exposure to medications that induce gray matter hypertrophy, the occurrence of manic episodes, or both.
4. Discussion
Using surface based mesh-mapping methods, our study found no significant differences in the regional morphology of either the amygdala or hippocampus between lithium-free euthymic bipolar subjects and healthy controls. These data add to the existing literature examining structural differences in the amygdala and hippocampus in this disorder and suggest that when the possible confounding effects of lithium treatment and mood are controlled for, no structural differences are evident between bipolar patients and healthy control subjects. Although contrary to our a priori hypotheses, these findings regarding the main effect of diagnosis on amygdala structure agree with at least two recent studies of bipolar disorder (Killgore et al. 2009; Brown et al. 2011). Likewise, the lack of a between-group difference in the hippocampus is consistent with prior neuroimaging reports of subjects with bipolar disorder both taking or not taking lithium (Strakowski et al. 1999; Hauser et al. 2000; Brambilla et al. 2003; Chen et al. 2004).
A secondary aim of the current study was to explore potential associations between regional morphology of the amygdala and hippocampus and demographic and course of illness variables. Several interesting findings resulted from these secondary analyses. First, an age by diagnosis interaction was observed in the basolateral portion of the right amygdala that was driven by an inverse association between age and radial distance in healthy controls, but not bipolar subjects. Such a pattern of age-related reduction in amygdala volume in healthy subjects is consistent with prior investigations (Allen et al. 2005; Walhovd et al. 2005). Moreover, the age by diagnosis interaction that we observed in this study of bipolar adults is consistent with at least one study examining age-related changes in amygdala volume in young adults with bipolar disorder (Chen et al. 2004). These data add support to the suggestion that altered age-related trajectories of this region may explain why smaller amygdala size is more consistently found in studies of bipolar children and adolescents (Blumberg et al. 2003; DelBello et al. 2004; Blumberg et al. 2005; Chang et al. 2005) and in bipolar individuals experiencing a first-episode of mania or depression (Rosso et al. 2007), whereas amygdala enlargement is more consistently found in studies of chronically ill bipolar adults (that is, those with a greater number of prior episodes or longer duration of illness; Altshuler et al. 1998; Strakowski et al. 1999; Altshuler et al. 2000; Brambilla et al. 2003; Frangou 2005).
The factors underlying the apparent abnormalities in age-related changes in amygdala structure in bipolar patients are unknown. However, given that medication and the number of prior hospitalizations for mania were also found to be associated with increases in radial distance in separate exploratory analyses, and given that the effects of these individual factors on radial distance were no longer significant in the joint model, the apparent absence of an age related structural decline in this region of patients could be related to exposure to medications that induce gray matter hypertrophy, the occurrence of severe manic episodes, or both. Future studies, therefore, that attempt to tease apart the unique impact of each of these variables on regional amygdala morphology, while controlling for lithium and mood state in bipolar disorder are needed.
The positive correlations that we observed between radial distance of the left amygdala and number of prior of hospitalizations for mania is consistent with a prior study by our group, in which we found a positive correlation between left amygdala volume and prior number of manic episodes (Altshuler et al. 2000). While it is possible that this finding may be reflective of a more severe form of bipolar illness, another possibility is that an episode of mania itself may have enduring hypertrophic effects on gray matter. Indeed, no studies, to our knowledge have explicitly controlled for mood state when investigating subcortical gray matter abnormalities in bipolar disorder, despite recent evidence showing a significant effect of mood state on brain structure (Brooks et al. 2009; Nery et al. 2009; Foland-Ross et al. in press). Sampling from an entirely euthymic bipolar sample, therefore, represents a unique strength of the current investigation. Future studies that address the impact of mood state on brain structure in bipolar disorder using a longitudinal approach would be helpful in delineating the specific impact of mania on brain structure, as well as in determining whether mood-state related anomalies in structure are transient or enduring.
Finally, in addition to the associations that we observed between amygdala structure, prior hospitalizations for mania, and age, we also observed a relationship between current medication status (treated versus untreated) and radial size of the left amygdala. The significant enlargement that was present in this region in medicated versus unmedicated bipolar patients is in agreement with a recent study by Savitz (2010), who reported reduced total amygdala volume in 18 unmedicated, compared to 17 medicated bipolar subjects. This prior finding, in concert with our own, may suggest that neuroplastic changes in this region could be moderated by any number of mood stabilizing medications. Unlike Savitz, however, we did not observe a significant reduction in overall amygdala size in unmedicated bipolar patients versus healthy controls. As the number of unmedicated subjects in the current study was small (N=10), however, we had limited power to examine this issue. This lower sample size may also have diminished our power to detect a significant association between amygdala volume and specific medication type (antipsychotics, antidepressants or anticonvulsants). Further investigation on the impact of medication type in larger bipolar samples is needed.
It is interesting that the effects of medication and mania history were found in the left, but not right hemisphere in our patient sample. This apparent laterality is consistent with prior work from our group, showing increased left amygdala activation in manic bipolar patients during the visual processing of emotional stimuli (Altshuler et al. 2005), and increased volume or the left amygdala in patients reporting a higher number of manic episodes (Altshuler et al. 2000). The meaning of this apparent lateralization, and its causes, are not currently well understood. However, prior work suggests that the left amygdala, which is typically smaller than the right (Pedraza et al. 2004), is more critically involved in the explicit, conscious, and verbal processing of affective material (Gazzaniga 1995; Morris et al. 1998). In this context, it would be interesting for future studies of bipolar disorder to examine possible associations between structural characteristics of the left amygdala, and performance on various left-lateralized amygdaladependent behavioral tasks and activation paradigms. Along these lines, given that an interaction of diagnosis and age was found in the right, but not left hemisphere, future studies should examine whether anomalies in age-related declines in the radial distance of this structure are also related to abnormal changes in right amygdala-based functions and behaviors (e.g., the subconscious processing of emotional material; Gazzaniga 1995; Morris et al. 1998).
Our findings should be considered in light of several limitations. First, the number of subjects in our patient sample that were unmedicated was small, limiting our power to detect areas that were significantly impacted by current medication status. Additionally, a small number of subjects in our patient sample (N=8) had reported taking lithium in the years prior to scanning, and previous exposure to this medication could have affected our results. Yet, to our knowledge, there is no evidence to suggest that the effects of lithium on neural structure are enduring. In fact, in our sample, comparisons between patients who had and had not previously taken this medication indicated that prior lithium use itself was not a significant confounder. We may have been underpowered to thoroughly address this issue however; future studies that follow subjects longitudinally both before, during and after treatment with lithium, are needed to assess whether gray matter hypertrophy caused by this medication is reversible upon medication withdrawal. Second, it is important to note that a majority of the subjects within our patient sample (69%) were receiving treatment with one or more psychotropic medications at the time of scanning. As such, it remains possible, particularly given our findings of a hypertropic effect of current medication status on structure in the right amygdala, that medication may have masked any diagnosis-specific alterations in amygdalar and hippocampal gray matter morphology. Indeed, this limitation represents a major issue for studies of chronically ill patients, as recruitment of large numbers of unmedicated individuals with bipolar disorder remains difficult (Phillips et al. 2008). It will be critical, therefore, for future investigations to continue to try to elucidate the effects of medication on brain structure. Third, while a logical next step in our analysis could involve the use of model selection to identify the best group of predictors, the point-wise analysis tools currently available for the analysis of subcortical mesh maps do not easily allow for this. Future methodological development and/or use of normalized measures of illness history in studies that examine subcortical structure using the mesh mapping approach would therefore be of interest. Fourth, the manual tracing method, which we utilized in order to derive surface meshes, is susceptible to measurement error. We attempted to minimize these errors, however, through training our analysts (C.P, R.V.) using well-established and specific tracing protocols and through blinding these individuals to diagnostic, demographic and clinical variables. Fifth, while we have highlighted the involvement of mood state, medication and neuroimaging analysis approach as possible factors contributing to prior inconsistencies in the neuroimaging literature of bipolar disorder, many other factors not considered here (e.g. genes, age, chronicity, comorbidity) may have contributed to variability in prior findings. Future studies that take these other factors into account are needed. Finally, caution should be exercised in interpreting our findings regarding associations between radial distance and clinical variables. Illness history was assessed retrospectively, and may be influenced by errors in subject recall and/or reporting bias. Further, multiple analyses between subcortical structure and clinical variables were conducted, and the possibility of Type I error cannot be excluded. Because we did not have specific hypotheses regarding which clinical variables might be associated with subcortical structure, these findings were considered exploratory and require replication in future studies to ensure their validity.
In conclusion, using mesh-based structural neuroimaging analysis methods, we found no evidence for a structural abnormality of the amygdala or hippocampus between lithium-free euthymic bipolar adults and healthy control subjects. Exploratory analyses nevertheless revealed, in bipolar subjects, a lack of the age-related structural decline that was observed in the basloateral portion of the right amygdala of healthy subjects. Additionally, enlargement in dorsal and ventral midline subregions of the left amygdala was found in patients having a higher number of prior hospitalizations for mania and in patients who were receiving (non-lithium) medication. Taken together, these findings suggest that when bipolar patients are not treated with lithium, and are in a euthymic mood state, there is no evidence of a structural abnormality in amygdala or hippocampus, but other clinical variables may nevertheless impact gray matter structure in the amygdala. Future studies that replicate these findings would help to establish the validity of these observations.
Acknowledgments
This work was supported by grants from the National Institute of Mental Health (MH078556 to LCFR, and MH075944 and MH01848 to LLA). Additional support for algorithm development was provided by the NIA, NIBIB, and the National Center for Research Resources (AG016570, EB01651, RR019771 to PT). For their generous support, the authors also thank the National Association for Research on Schizophrenia and Affective Disorders (NARSAD), Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family and Northstar Fund. The project was also supported by Grants RR12169, RR13642 and RR00865 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH).
Footnotes
Financial Disclosures: Dr. Altshuler has served on advisory boards or speaker’s bureaus for Forest Laboratories, Merck, and Sepracor. The other authors report no financial relationships with commercial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, Wilkins J, Gerner R, Mintz J. An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biological Psychiatry. 2000;48:147–162. doi: 10.1016/s0006-3223(00)00836-2. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J. Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: an MRI study demonstrating neuroanatomic specificity. Archives of General Psychiatry. 1998;55:663–664. doi: 10.1001/archpsyc.55.7.663. [DOI] [PubMed] [Google Scholar]
- Altshuler LL, Bookheimer SY, Proenza MA, Townsend J, Sabb F, Firestine A, Bartzokis G, Mintz J, Mazziotta J, Cohen MS. Increased amygdala activation during mania: a functional magnetic resonance imaging study. American Journal of Psychiatry. 2005;162:1211–1213. doi: 10.1176/appi.ajp.162.6.1211. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Mintz J, Marx P, Osborn D, Gutkind D, Chiang F, Phelan CK, Marder SR. Reliability of in vivo volume measures of hippocampus and other brain structures using MRI. Magnetic Resonance Imaging. 1993;11:993–1006. doi: 10.1016/0730-725x(93)90218-3. [DOI] [PubMed] [Google Scholar]
- Bear DM, Fedio P. Quantitative analysis of interictal behavior in temporal lobe epilepsy. Archives of Neurology. 1977;34:454–467. doi: 10.1001/archneur.1977.00500200014003. [DOI] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Avedissian C, Klunder AD, Nicoletti M, Dierschke N, Brambilla P, Soares JC. Altered hippocampal morphology in unmedicated patients with major depressive illness. ASN Neuro. 2009;1:265–273. doi: 10.1042/AN20090026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MA, Nicoletti M, Dierschke N, Hayashi KM, Klunder AD, Glahn DC, Brambilla P, Sassi RB, Mallinger AG, Soares JC. Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology. 2008;33:1229–1238. doi: 10.1038/sj.npp.1301507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Fredericks C, Wang F, Kalmar JH, Spencer L, Papademetris X, Pittman B, Martin A, Peterson BS, Fulbright RK, Krystal JH. Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disorders. 2005;7:570–576. doi: 10.1111/j.1399-5618.2005.00264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Archives of General Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, Kupfer DJ, Keshavan MS, Soares JC. MRI investigation of temporal lobe structures in bipolar patients. Journal of Psychiatric Research. 2003;37:287–295. doi: 10.1016/s0022-3956(03)00024-4. [DOI] [PubMed] [Google Scholar]
- Brooks JO, Bonner JC, Rosen AC, Wang PW, Hoblyn JC, Hill SJ, Ketter TA. Dorsolateral and dorsomedial prefrontal gray matter density changes associated with bipolar depression. Psychiatry Research. 2009;172:200–204. doi: 10.1016/j.pscychresns.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GG, Lee JS, Strigo IA, Caligiuri MP, Meloy MJ, Lohr J. Voxel-based morphometry of patients with schizophrenia or bipolar I disorder: a matched control study. Psychiatric Research. 2011;194:149–156. doi: 10.1016/j.pscychresns.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K, Karchemskiy A, Barnea-Goraly N, Garrett A, Simeonova DI, Reiss A. Reduced amygdalar gray matter volume in familial pediatric bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:565–573. doi: 10.1097/01.chi.0000159948.75136.0d. [DOI] [PubMed] [Google Scholar]
- Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, Brambilla P, Keshavan MS, Ryan ND, Birmaher B, Soares JC. Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biological Psychiatry. 2004;56:399–405. doi: 10.1016/j.biopsych.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Chepenik LG, Fredericks C, Papademetris X, Spencer L, Lacadie C, Wang F, Pittman B, Duncan JS, Staib LH, Duman RS, Gelernter J, Blumberg HP. Effects of the brain-derived neurotrophic growth factor val66met variation on hippocampus morphology in bipolar disorder. Neuropsychopharmacology. 2009;34:944–951. doi: 10.1038/npp.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM. Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disorders. 2004;6:43–52. doi: 10.1046/j.1399-5618.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- Flor-Henry P. Schizophrenia-like reactions and affective psychoses associated with temporal lobe epilepsy: etiological factors. American Journal of Psychiatry. 1969;126:400–404. doi: 10.1176/ajp.126.3.400. [DOI] [PubMed] [Google Scholar]
- Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, Narr KL, Asuncion DM, Toga AW, Thompson PM. Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport. 2008;19:221–224. doi: 10.1097/WNR.0b013e3282f48108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foland-Ross LC, Brooks JO, Mintz J, Bartzokis G, Townsend J, Thompson PM, Altshuler LL. Mood-state effects on amygdala volume in bipolar disorder. Journal of Affective Disorders. doi: 10.1016/j.jad.2012.03.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S. The Maudsley bipolar disorder project. Epilepsia. 2005;46:19–25. doi: 10.1111/j.1528-1167.2005.463005.x. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. The Cognitive Neurosciences. MIT Press; Cambridge, MA: 1995. [Google Scholar]
- George MS, Huggins T, McDermut W, Parekh PI, Rubinow D, Post RM. Abnormal facial emotion recognition in depression: serial testing in an ultra-rapid cycling patient. Behavior Modification. 1998;22:192–204. doi: 10.1177/01454455980222007. [DOI] [PubMed] [Google Scholar]
- Hallahan B, Newell J, Soares JC, Brambilla P, Strakowski SM, Fleck DE, Kieseppä T, Altshuler LL, Fornito A, Malhi GS, McIntosh AM, Yurgelun-Todd DA, Labar KS, Sharma V, MacQueen GM, Murray RM, McDonald C. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biological Psychiatry. 2011;69:326–335. doi: 10.1016/j.biopsych.2010.08.029. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry. 1960;12:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser P, Matochik J, Altshuler LL, Denicoff KD, Conrad A, Li X, Post RM. MRI-based measurements of temporal lobe and ventricular structures in patients with bipolar I and bipolar II disorders. Journal of Affective Disorders. 2000;60:25–32. doi: 10.1016/s0165-0327(99)00154-8. [DOI] [PubMed] [Google Scholar]
- Killgore WD, Rosso IM, Gruber SA, Yurgelun-Todd DA. Amygdala volume and verbal memory performance in schizophrenia and bipolar disorder. Cognitive and Behavioral Neurology. 2009;22:28–37. doi: 10.1097/WNN.0b013e318192cc67. [DOI] [PubMed] [Google Scholar]
- Lembke A, Ketter TA. Impaired recognition of facial emotion in mania. American Journal of Psychiatry. 2002;159:302–304. doi: 10.1176/appi.ajp.159.2.302. [DOI] [PubMed] [Google Scholar]
- Malhi GS, Lagopoulos J, Sachdev PS, Ivanovski B, Shnier R, Ketter TA. Is a lack of disgust something to fear? A functional magnetic resonance imaging facial emotion recognition study in euthymic bipolar disorder patients. Bipolar Disorders. 2007;9:345–357. doi: 10.1111/j.1399-5618.2007.00485.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, Ohman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature Biotechnology. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykel ES. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Narr KL, Thompson PM, Szeszko P, Robinson D, Jang S, Woods RP, Kim S, Hayashi KM, Asunction D, Toga AW, Bilder RM. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage. 2004;21:1563–1575. doi: 10.1016/j.neuroimage.2003.11.011. [DOI] [PubMed] [Google Scholar]
- Nery FG, Chen HH, Hatch JP, Nicoletti MA, Brambilla P, Sassi RB, Mallinger AG, Keshavan MS, Soares JC. Orbitofrontal cortex gray matter volumes in bipolar disorder patients: a region-of-interest MRI study. Bipolar Disorders. 2009;11:145–153. doi: 10.1111/j.1399-5618.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- Noga JT, Vladar K, Torrey EF. A volumetric magnetic resonance imaging study of monozygotic twins discordant for bipolar disorder. Psychiatry Research. 2001;106:25–34. doi: 10.1016/s0925-4927(00)00084-6. [DOI] [PubMed] [Google Scholar]
- Pedraza O, Bowers D, Gilmore R. Asymmetry of the hippocampus and amygdala in MRI volumetric measurements of normal adults. Journal of International Neuropsychology Society. 2004;10:664–678. doi: 10.1017/S1355617704105080. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. American Journal of Psychiatry. 2008;165:313–320. doi: 10.1176/appi.ajp.2007.07071066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso IM, Killgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA. Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biological Psychiatry. 2007;61:743–749. doi: 10.1016/j.biopsych.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Savitz J, Nugent AC, Bogers W, Liu A, Sills R, Luckenbaugh DA, Bain EE, Price JL, Zarate C, Manji HK, Cannon DM, Marrett S, Charney DS, Drevets WC. Amygdala volume in depressed patients with bipolar disorder assessed using high resolution 3T MRI: The impact of medication. Neuroimage. 2010;49:2966–2976. doi: 10.1016/j.neuroimage.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck D, Leahy R. Brainsuite: An automated cortical surface identification tool. Medical Image Analysis. 2002;6:129–142. doi: 10.1016/s1361-8415(02)00054-3. [DOI] [PubMed] [Google Scholar]
- Shukla S, Cook BL, Mukherjee S, Godwin C, Miller MG. Mania following head trauma. American Journal of Psychiatry. 1987;144:93–96. doi: 10.1176/ajp.144.1.93. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Boston JD, Robinson RG. Mechanisms of mania after brain injury: 12 case reports and review of the literature. Journal of Nervous and Mental Disease. 1988;176:87–100. doi: 10.1097/00005053-198802000-00004. [DOI] [PubMed] [Google Scholar]
- Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER. Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Archives of General Psychiatry. 1999;56:254–260. doi: 10.1001/archpsyc.56.3.254. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biological Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Thompson P, Hayashi K, Sowell E, Gogtay N, Giedd J, Rapoport J, de Zubicaray G, Janke A, Rose S, Semple J, Doddrell D, Wang Y, van Erp T, Cannon T, Toga A. Mapping cortical change in Alzheimer’s disease, brain development, and schizophrenia. Neuroimage. 2004;23:S2–18. doi: 10.1016/j.neuroimage.2004.07.071. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, De Zubicaray GI, Janke AL, Rose SE, Semple J, Hong MS, Herman DH, Gravano D, Doddrell DM, Toga AW. Mapping hippocampal and ventricular change in Alzheimer disease. Neuroimage. 2004;22:1754–1766. doi: 10.1016/j.neuroimage.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. Neuroimage. 1996;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Usher J, Menzel P, Schneider-Axmann T, Kemmer C, Reith W, Falkai P, Gruber O, Scherk H. Increased right amygdala volume in lithium-treated patients with bipolar I disorder. Acta Psychiatrica Scandinavica. 2009;121:119–124. doi: 10.1111/j.1600-0447.2009.01428.x. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Woods R, Grafton S, Holmes C, Cherry S, Mazziotta J. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- Yang Y, Raine A, Narr KL, Colletii P, Toga A. Localization of deformations within the amygdala in individuals with psychopathy. Archives of General Psychiatry. 2009;66:986–994. doi: 10.1001/archgenpsychiatry.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R, Biggs J, Ziegler V, Meyer D. A rating scale for mania: Reliability, validity and sensitivity. British Journal of Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Yucel K, McKinnon MC, Taylor VH, Macdonald K, Alda M, Young LT, MacQueen GM. Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: a longitudinal MRI study. Psychopharmacology. 2007;195:357–367. doi: 10.1007/s00213-007-0906-9. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd DA, Gruber SA, Kanayama G, Killgore WD, Baird AA, Young AD. fMRI during affect discrimination in bipolar affective disorder. Bipolar Disorders. 2000;2:237–248. doi: 10.1034/j.1399-5618.2000.20304.x. [DOI] [PubMed] [Google Scholar]
- Zijdenbos A, Dawant B. Brain segmentation and white matter lesion detection in mr images. Critical Reviews in Biomedical Engineering. 1994;22:401–465. [PubMed] [Google Scholar]


