Abstract
Objectives
Although candidaemia is a well-known complication of hospital stay and has a crude mortality of ∼40%, few data are available for episodes diagnosed within 10 days after hospital admission. In this paper, we compared the risk factors for mortality according to the onset of candidaemia.
Methods
This was a retrospective study of hospitalized patients with early-onset candidaemia (EOC; ≤10 days) or late-onset candidaemia (LOC; >10 days) to identify any distinct clinical characteristics and risk factors for 30 day mortality in two Italian academic centres.
Results
A total of 779 patients were included in the study: 183 EOC and 596 LOC. Mortality was significantly lower in EOC (71/183, 38.8% versus 283/596, 47.5%, P = 0.03). In EOC, multivariate analysis showed that inadequate initial antifungal therapy (IIAT) (P = 0.005, OR 3.02, 95% CI 1.40–6.51), Candida albicans aetiology (P = 0.02, OR 2.17, 95% CI 1.11–4.26) and older age (P < 0.001, OR 1.05, 95% CI 1.02–1.07) were independent risk factors for mortality. In LOC, liver disease (P = 0.003, OR 2.46, 95% CI 1.36–4.43), IIAT (P = 0.002, OR 2.01, 95% CI 1.28–3.15) and older age (P < 0.001, OR 1.03, 95% CI 1.02–1.04) were independently associated with a fatal outcome, while treatment with caspofungin was associated with survival (P < 0.001, OR 0.42, 95% CI 0.26–0.67).
Conclusions
EOC has different clinical characteristics and risk factors for mortality compared with LOC. Although EOC mortality is significantly lower, the rate of inappropriate antifungal treatment is higher. Treatment with caspofungin is significantly associated with survival in patients with LOC. Efforts are needed to improve the diagnosis and treatment of EOC.
Keywords: caspofungin, risk factors, mortality, healthcare-associated infections, early-onset candidaemia
Introduction
Candida species represent the fourth most common cause of bloodstream infection (BSI) and are the leading cause of invasive fungal infection among hospitalized patients.1,2 In a recent study conducted in the USA,3 the overall, crude 12 week mortality rate was 35.2% and candidaemia in adult patients has been associated with a 14.5% increase in mortality with a mean 10 day increase in length of stay.4 The epidemiological trend of candidaemias has changed in recent years, due to the increased frequency of infections caused by Candida spp. other than Candida albicans and increased diagnosis in medical wards.5–7 The risk factors and comorbidities for candidaemia have been widely investigated and most infections are observed a median of 3–4 weeks after hospital admission.8,9
There are several reports of candidaemia ≤48 h after admission and these cases are usually described as healthcare associated (HCA).5,10–14 HCA infections are caused by pathogens previously limited to hospital settings and now are recognized as a distinct entity in terms of epidemiology, microbiology and outcome.15 There are few studies on the clinical characteristics and outcomes of early-onset candidaemias (EOCs) with variable time definitions, ranging from 2 to 14 days after hospital admission;10,13,14,16 EOC was compared with late-onset candidaemia (LOC) in only one study.14 Moreover, in a cohort of critically ill patients with candidaemia onset within 14 days of hospital admission, higher mortality and hospital costs were significantly associated with inadequate initial antifungal therapy (IIAT).16
In this retrospective, 4 year study from two large Italian hospitals, we compared the clinical characteristics, risk factors for mortality and impact of appropriate antifungal treatment in patients with EOC and LOC.
Methods
Study design
This study was conducted in two Italian academic centres: the 1200 bed San Giovanni Battista University Hospital in Turin and the 1400 bed Agostino Gemelli University Hospital in Rome, which offer a full range of clinical services. The local institutional review committees approved the study and informed consent was waived because of the observational nature of the study. The computerized databases of the hospitals' microbiology laboratories were used to identify adult patients hospitalized from 1 January 2004 to 31 December 2008 and diagnosed with candidaemia, defined as the presence of at least one positive blood culture for Candida spp. Each patient was included in the study only once, at the time of the initial positive blood culture.
The primary objective of this retrospective cohort study was to describe the clinical characteristics and compare the risk factors for death at 30 days after the diagnosis of candidaemia in two groups of patients, divided according to the time of onset of the disease after hospital admission: ≤10 days for EOC and >10 days for LOC. Patients were included only if complete data series could be collected from medical records.
Data collection
Data regarding demographic characteristics and clinical risk factors were collected from patients' paper and electronic medical records.
The variables analysed included age, sex, type of admission (medical versus surgical versus intensive care), time at risk (defined as the number of hospital days from admission to the date of the first positive blood culture) and severity of illness, as calculated by the APACHE III score within 24 h after candidaemia onset.17
The following comorbidities were documented: chronic liver disease, malignancy (solid organ or haematological malignancy), diabetes, chronic obstructive pulmonary disease (COPD), congestive heart failure, chronic renal failure (indicated by previous creatinine level >2.0 mg/dL or use of dialysis), HIV infection, neutropenia (defined as absolute neutrophil count <500/mm3), prior organ transplantation, prior haematopoietic stem cell transplantation, total parenteral nutrition or use of immunosuppressive agents, including corticosteroids, in the 30 days before BSI onset, abdominopelvic surgery or other surgery in the 30 days before BSI onset, invasive procedures (i.e. central venous and/or urinary catheterization, endoscopy, nasogastric tube insertion and mechanical ventilation) during the 72 h before BSI onset, exposure to antibiotics or antifungal therapy in the 30 days preceding candidaemia and the Charlson comorbidity score.18
Candidaemia was considered central venous catheter (CVC) related when the same Candida species was isolated from simultaneous quantitative blood cultures obtained through the catheter and the peripheral vein with a growth ratio ≥5:1 cfu, or when the semi-quantitative culture of the removed CVC tip yielded >15 cfu of the same Candida species isolated from peripheral blood cultures.14
IIAT was defined as a lack of antifungal treatment within 24 h of drawing the first positive blood culture or as the administration of antifungal treatment to which there was resistance in vitro.19 Appropriate definitive treatment was considered as the administration of at least one in vitro-active antifungal for ≥72 h.
Amongst EOC, episodes occurring ≤48 h after hospital admission were defined as either community acquired (CA) or HCA with the absence or presence, respectively, of one or more of the following characteristics: hospital admission during the last 6 months, parenteral antibiotic therapy during the last 4 weeks, dialysis or residence in long-term chronic care facilities. All the other episodes were hospital acquired (HA).
Microorganism identification
Blood specimens were processed by an automated blood culture system (BACTEC 9240) and viable yeasts were subcultured on Sabouraud dextrose agar. Species identification was based on germ tube production, distinctive colour and morphology on Candida ID2 agar, together with sugar assimilation profiles obtained using the API ID 32C system. All isolates were stored at −80°C in 10% (v/v) glycerol. Candida spp. were identified to the species level by phenotyping methods.
Statistical analysis
Continuous variables were compared by Student's t-test if normally distributed and the Mann–Whitney U-test if non-normally distributed. Categorical variables were evaluated using χ2 or the two-tailed Fisher's exact test. ORs and 95% CIs were calculated to evaluate the strength of any association. Values for continuous and categorical variables are expressed as the mean ± SD and median (IQR) or percentage of the group from which they are derived, respectively. Two-tailed tests were used to determine statistically significant association with P < 0.05. Multivariate analysis to identify independent risk factors for mortality was performed using a logistic regression model that incorporated all variables with a P value of <0.20 in the univariate test. Calibration was assessed using the Hosmer–Lemeshow test for goodness of fit, which evaluates expected and observed probabilities in population deciles.
The Mann–Whitney test was used to investigate differences in the distribution of time to discharge, grouped by explanatory variables. The relationship between the time to discharge and factors was analysed using multiple linear regression analysis; in this case, since the histogram of time to discharge showed a strongly skewed distribution, the data were transformed using a log scale.
All statistical analyses were performed using the Stata IC 11 program (Stata Corporation, USA).
Results
Characteristics of patients and isolates
A total of 779 patients with candidaemia were observed during the study period (434 in Turin and 345 in Rome): 183 were classified as EOC and 596 as LOC. Candidaemia occurred after a median (IQR) of 6 (2–8) and 27 (18–42) days after admission in the EOC and LOC groups, respectively. In the EOC group, 21/183 (11.5%) and 9/183 (4.9%) of candidaemias were HCA and CA, respectively. The main characteristics of the patients are detailed in Table 1.
Table 1.
Characteristics of the 779 patients with BSI caused by Candida spp. according to the time at risk
| Variable | No. (%) of patients |
P value | |
|---|---|---|---|
| EOC (≤10 days) (n = 183) | LOC (>10 days) (n = 596) | ||
| Demographic information | |||
| male | 109 (59.6) | 334 (56.0) | 0.40 |
| age (years), median (IQR) | 67 (53–77) | 69 (59–77) | 0.03 |
| Ward | |||
| medicine | 94 (51.4) | 249 (41.8) | 0.02 |
| surgery | 39 (21.3) | 209 (35.1) | <0.001 |
| ICU | 50 (27.3) | 138 (23.2) | 0.24 |
| Epidemiological category | |||
| CA | 9 (4.9) | — | — |
| HCA | 21 (11.5) | — | — |
| HA | 153 (83.6) | — | — |
| Time at risk (days), median (IQR) | 6 (2–8) | 27 (18–42) | <0.001 |
| Time to discharge (days), median (IQR) | 14 (7–26) | 22 (12–39) | 0.002 |
| Clinical presentation | |||
| previous bacterial infections | 93 (50.8) | 367 (61.6) | 0.009 |
| CVC | 126 (68.9) | 470 (78.9) | 0.005 |
| urinary catheter | 129 (70.5) | 463 (77.7) | 0.04 |
| total parenteral nutrition | 92 (50.3) | 351 (58.9) | 0.03 |
| corticosteroid therapy | 47 (25.7) | 171 (28.7) | 0.42 |
| surgery | 70 (38.3) | 361 (60.6) | <0.001 |
| neutrophil count <500/mm3 | 10 (5.5) | 31 (5.2) | 0.89 |
| previous antibiotic therapy | 148 (80.9) | 570 (95.6) | <0.001 |
| Comorbidity | |||
| diabetes mellitus | 43 (23.5) | 135 (22.7) | 0.81 |
| COPD | 57 (31.2) | 224 (37.6) | 0.11 |
| chronic renal failure | 69 (37.7) | 198 (33.2) | 0.26 |
| liver disease | 14 (7.7) | 60 (10.1) | 0.32 |
| solid tumour | 81 (44.3) | 247 (41.4) | 0.49 |
| haematological malignancy | 13 (7.1) | 54 (9.1) | 0.40 |
| HIV | 2 (1.1) | 4 (0.7) | 0.56 |
| Charlson comorbidity score, median (IQR) | 3 (2–5) | 3 (2–4) | 0.42 |
| APACHE III score, median (IQR) | 12 (8–17) | 14 (9–19) | 0.02 |
| Species isolated monofungal | |||
| C. albicans | 98 (53.6) | 349 (58.6) | 0.23 |
| C. glabrata | 24 (13.1) | 62 (10.4) | 0.23 |
| C. tropicalis | 18 (9.8) | 34 (5.7) | 0.05 |
| C. parapsilosis | 33 (18.0) | 98 (16.4) | 0.62 |
| Candida krusei | 2 (1.1) | 1 (0.2) | 0.08 |
| Candida guilliermondii | 2 (1.1) | 1 (0.2) | 0.08 |
| other species | 3 (1.6) | 10 (1.7) | 0.97 |
| polyfungal | 3 (1.6) | 24 (4.0) | 0.12 |
| Definitive antifungal therapy | |||
| fluconazole | 112 (61.2) | 420 (70.5) | 0.02 |
| caspofungin | 33 (18.0) | 108 (18.1) | 0.97 |
| amphotericin B | 29 (15.9) | 84 (14.1) | 0.55 |
| voriconazole | 6 (3.3) | 22 (3.7) | 0.79 |
| IIAT | 44 (24.0) | 109 (18.3) | 0.009 |
| Outcome | |||
| death | 71 (38.8) | 283 (47.5) | 0.03 |
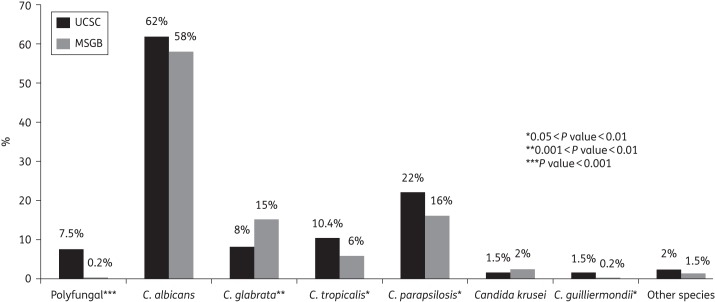
C. albicans was most commonly isolated (447/779, 57.4%), whereas the majority of the other Candida species isolated included Candida parapsilosis (131/779, 16.8%), Candida glabrata (86/779, 11.0%) and Candida tropicalis (52/779, 6.7%). Twenty-seven out of 779 patients (3.5%) had polyfungal candidaemia. The overall rate of fluconazole resistance was 6.6% (51/779): it was 3.3% (6/183) in EOC and 7.6% (45/596) in LOC (P = 0.04). In Figure 1, the differences between the two centres in the species of Candida isolated are shown.
Figure 1.
Distribution of yeast species isolated at the Agostino Gemelli University Hospital (UCSC) and San Giovanni Battista University Hospital (MSGB). Polyfungal: 27 blood culture samples yielded two different Candida spp., including C. albicans/C. parapsilosis (n = 9), C. albicans/C. glabrata (n = 6), C. albicans/C. tropicalis (n = 5), C. albicans/Candida guilliermondii (n = 2), C. glabrata/C. tropicalis (n = 2), C. glabrata/C. parapsilosis (n = 1), C. parapsilosis/C. tropicalis (n = 1) and C. parapsilosis/C. guilliermondii (n = 1). Other species include Candida lusitaniae, Candida lambica, Candida famata and Blastoschizomyces capitatus.
Risk factors associated with death in patients with EOC
Univariate analysis of the factors significantly associated with mortality amongst 183 patients with EOC (non-survivors = 71 and survivors = 112) is presented in Table 2. Multivariate analysis showed that IIAT (P = 0.005, OR 3.02, 95% CI 1.40–6.51), BSI caused by C. albicans (P = 0.02, OR 2.17, 95% CI 1.11–4.26) and older age (P < 0.001, OR 1.05, 95% CI 1.02–1.07) were independent risk factors for 30 day mortality in patients with EOC (Table 2). The results of Hosmer–Lemeshow χ2 testing (χ2 = 111.82, P = 0.64) were indicative of good calibration.
Table 2.
Univariate and multivariate analyses of factors associated with death among patients with EOC (time at risk, ≤10 days)
| Variable | No. (%) of patients |
|||
|---|---|---|---|---|
| Non-survivors (n = 71) | Survivors (n = 112) | P value | OR (95% CI) | |
| Univariate analysis | ||||
| Demographic information | ||||
| male | 39 (54.9) | 70 (62.5) | 0.31 | 0.73 (0.38–1.40) |
| age (years), median (IQR) | 72 (63–80) | 62 (47–71) | <0.001 | — |
| hospital (Rome) | 38 (53.5) | 50 (44.6) | 0.24 | 1.42 (0.75–2.71) |
| Ward | ||||
| medicine | 39 (54.9) | 55 (49.1) | 0.44 | 1.26 (0.67–2.40) |
| surgery | 10 (14.1) | 29 (25.9) | 0.06 | 0.47 (0.19–1.09) |
| ICU | 22 (31.0) | 28 (25) | 0.38 | 1.35 (0.66–2.74) |
| Epidemiological category | ||||
| CA | 3 (4.2) | 6 (5.4) | 0.73 | 0.78 (0.12–3.80) |
| HCA | 7 (9.9) | 14 (12.5) | 0.58 | 0.77 (0.25–2.17) |
| HA | 61 (85.9) | 92 (82.1) | 0.50 | 1.33 (0.55–3.39) |
| Clinical presentation | ||||
| CVC | 48 (67.6) | 78 (69.6) | 0.77 | 0.91 (0.46–1.82) |
| corticosteroid therapy | 23 (32.4) | 24 (21.4) | 0.09 | 1.75 (0.85–3.63) |
| neutrophil count <500/mm3 | 5 (7.0) | 5 (4.5) | 0.45 | 1.62 (0.36–7.31) |
| previous antibiotic therapy | 62 (87.3) | 86 (76.8) | 0.08 | 2.08 (0.87–5.39) |
| previous bacterial infection | 37 (52.1) | 56 (50.0) | 0.78 | 1.09 (0.58–2.06) |
| Comorbidity | ||||
| diabetes mellitus | 22 (31.0) | 21 (18.8) | 0.06 | 1.95 (0.92–4.12) |
| COPD | 31 (43.7) | 26 (23.2) | 0.004 | 2.56 (1.28–5.12) |
| chronic renal failure | 31 (43.7) | 38 (33.9) | 0.19 | 1.51 (0.78–2.90) |
| liver disease | 7 (9.9) | 7 (6.3) | 0.37 | 1.64 (0.47–5.75) |
| solid tumour | 28 (39.4) | 53 (47.3) | 0.30 | 0.72 (0.38–1.38) |
| haematological malignancy | 4 (5.6) | 9 (8.0) | 0.54 | 0.68 (0.15–2.57) |
| HIV | 0 | 2 (1.8) | 0.52 | — |
| Charlson comorbidity score, median (IQR) | 3 (2–5) | 2 (2–5) | 0.02 | — |
| APACHE III score, median (IQR) | 14 (9–19) | 11 (7–17) | 0.07 | — |
| Species isolated monofungal | ||||
| C. albicans | 47 (66.2) | 51 (45.5) | 0.006 | 2.34 (1.21–4.56) |
| C. glabrata | 8 (11.3) | 16 (14.2) | 0.55 | 0.76 (0.26–2.02) |
| C. tropicalis | 7 (9.9) | 11 (9.8) | 0.99 | 1.01 (0.31–3.01) |
| C. parapsilosis | 5 (7.0) | 28 (25) | 0.002 | 0.23 (0.06–0.64) |
| Candida krusei | 1 (1.4) | 1 (0.9) | 0.74 | 1.58 (0.02–125.57) |
| Candida guilliermondii | 0 | 2 (1.8) | 0.25 | — |
| other species | 2 (2.8) | 1 (0.9) | 0.31 | 3.22 (0.16–191.47) |
| polyfungal | 1 (1.4) | 2 (1.8) | 0.84 | 0.79 (0.01–15.38) |
| Definitive antifungal therapy | ||||
| fluconazole | 39 (54.9) | 73 (65.2) | 0.17 | 0.65 (0.34–1.25) |
| caspofungin | 15 (21.1) | 18 (16.1) | 0.39 | 1.40 (0.60–3.20) |
| amphotericin B | 13 (18.3) | 16 (14.3) | 0.47 | 1.34 (0.55–3.22) |
| voriconazole | 0 | 6 (5.4) | 0.05 | — |
| IIAT | 28 (39.4) | 16 (14.3) | <0.001 | 3.91 (1.81–8.52) |
| Multivariate analysis | ||||
| IIAT | — | — | 0.005 | 3.02 (1.40–6.51) |
| C. albicans BSI | — | — | 0.02 | 2.17 (1.11–4.26) |
| Age | — | — | <0.001 | 1.05 (1.02–1.07) |
Risk factors associated with death in patients with LOC
Univariate analysis for 596 patients with LOC (non-survivors = 283 and survivors = 313) is presented in Table 3. Multivariate analysis for mortality among patients with LOC showed that liver disease (P = 0.003, OR 2.46, 95% CI 1.36–4.43), IIAT (P = 0.002, OR 2.01, 95% CI 1.28–3.15) and older age (P < 0.001, OR 1.03, 95% CI 1.02–1.04) were significantly associated with a fatal outcome, while definitive antifungal treatment with caspofungin was significantly associated with survival (P < 0.001, OR 0.42, 95% CI 0.26–0.67) (Table 3). The results of Hosmer–Lemeshow χ2 testing (χ2 = 187.15; P = 0.60) were indicative of good calibration.
Table 3.
Univariate and multivariate analyses of factors associated with death among patients with LOC (time at risk, >10 days)
| Variable | No. (%) of patients |
P value | OR (95% CI) | |
|---|---|---|---|---|
| Non-survivors (n = 283) | Survivors (n = 313) | |||
| Univariate analysis | ||||
| Demographic information | ||||
| male | 157 (55.5) | 177 (56.6) | 0.79 | 0.96 (0.68–1.34) |
| age (years), median (IQR) | 73 (63–79) | 66 (54–75) | <0.001 | — |
| hospital (Rome) | 119 (42.1) | 138 (44.1) | 0.62 | 0.92 (0.66–1.29) |
| Ward | ||||
| medicine | 126 (44.5) | 123 (39.3) | 0.20 | 1.24 (0.88–1.74) |
| surgery | 79 (27.9) | 130 (41.5) | <0.001 | 0.55 (0.38–0.78) |
| ICU | 78 (27.6) | 60 (19.2) | 0.02 | 1.60 (1.07–2.40) |
| Clinical presentation | ||||
| CVC | 224 (79.2) | 246 (78.6) | 0.87 | 1.03 (0.68–1.56) |
| corticosteroid therapy | 84 (29.7) | 87 (27.8) | 0.61 | 1.10 (0.76–1.59) |
| neutrophil count <500/mm3 | 15 (5.3) | 16 (5.1) | 0.92 | 1.04 (0.47–2.28) |
| previous antibiotic therapy | 274 (96.8) | 296 (94.6) | 0.12 | 1.97 (0.79–5.35) |
| previous bacterial infection | 184 (65.0) | 183 (58.4) | 0.10 | 1.32 (0.93–1.87) |
| Comorbidity | ||||
| diabetes mellitus | 83 (29.3) | 52 (16.6) | <0.001 | 2.08 (1.38–3.15) |
| COPD | 124 (43.8) | 100 (32.0) | 0.003 | 1.66 (0.17–2.35) |
| chronic renal failure | 112 (39.6) | 86 (27.5) | 0.002 | 1.73 (1.21–2.48) |
| liver disease | 37 (13.1) | 23 (7.4) | 0.02 | 1.90 (1.06–3.44) |
| solid tumour | 105 (37.1) | 142 (45.4) | 0.04 | 0.71 (0.50–0.99) |
| haematological malignancy | 25 (8.8) | 29 (9.3) | 0.85 | 0.95 (0.52–1.73) |
| HIV | 1 (0.4) | 3 (1.0) | 0.37 | 0.37 (0.01–4.60) |
| Charlson comorbidity score, median (IQR) | 3 (2–5) | 3 (2–4) | <0.001 | — |
| APACHE III score, median (IQR) | 16 (11–21) | 12 (8–16) | <0.001 | — |
| Species isolated monofungal | ||||
| C. albicans | 164 (58.0) | 185 (59.1) | 0.77 | 0.95 (0.67–1.34) |
| C. glabrata | 33 (11.7) | 29 (9.3) | 0.33 | 1.29 (0.73–2.27) |
| C. tropicalis | 16 (5.7) | 18 (5.8) | 0.95 | 0.98 (0.45–2.08) |
| C. parapsilosis | 44 (15.6) | 54 (17.3) | 0.57 | 0.88 (0.55–1.39) |
| Candida krusei | 6 (2.1) | 6 (1.9) | 0.89 | 0.92 (0.21–3.66) |
| Candida guilliermondii | 0 | 1 (0.3) | 0.34 | — |
| other species | 3 (1.1) | 7 (2.2) | 0.26 | 0.46 (0.07–2.27) |
| polyfungal | 15 (5.3) | 9 (2.9) | 0.13 | 1.89 (0.76–4.98) |
| Definitive antifungal therapy | ||||
| fluconazole | 197 (69.6) | 223 (71.3) | 0.66 | 0.92 (0.64–1.34) |
| caspofungin | 31 (11.0) | 77 (24.6) | <0.001 | 0.38 (0.23–0.60) |
| amphotericin B | 29 (10.3) | 55 (17.6) | 0.01 | 0.54 (0.32–0.89) |
| voriconazole | 8 (2.8) | 14 (4.5) | 0.29 | 0.62 (0.22–1.61) |
| IIAT | 71 (25.1) | 38 (12.1) | <0.001 | 2.42 (1.54–3.84) |
| Multivariate analysis | ||||
| Liver disease | — | — | 0.003 | 2.46 (1.36–4.43) |
| Age | — | — | <0.001 | 1.03 (1.02–1.04) |
| IIAT | — | — | 0.002 | 2.01 (1.28–3.15) |
| Definitive therapy with caspofungin | — | — | <0.001 | 0.42 (0.26–0.67) |
Simple and multiple linear regression analysis of time to discharge
The univariate analysis of predictors of time to discharge from the onset of candidaemia, calculated only in patients who survived, is shown in Table 4.
Table 4.
Univariate analysis of predictors of time to discharge between surviving patients
| P value | No. of surviving patients | Time to discharge, median (IQR) | |
|---|---|---|---|
| Sex | 0.16 | ||
| male | 247 | 25 (16–43) | |
| female | 178 | 25 (15–39) | |
| Ward | 0.02 | ||
| medicine | 178 | 22 (15–39) | |
| surgery | 159 | 25 (16–41) | |
| ICU | 88 | 26 (16.5–49.5) | |
| Time to onset of infection (LOC versus EOC) | 0.36 | ||
| EOC (time at risk, ≤10 days) | 112 | 25.5 (14.5–40) | |
| LOC (time at risk, >10 days) | 313 | 25 (16–41) | |
| Comorbidity | |||
| Diabetes mellitus | 0.54 | ||
| yes | 73 | 25 (17–41) | |
| no | 352 | 25 (15–40.5) | |
| COPD | 0.24 | ||
| yes | 126 | 25 (15–46) | |
| no | 299 | 25 (15–39) | |
| Chronic renal failure | 0.09 | ||
| yes | 124 | 28 (16–44.5) | |
| no | 301 | 24 (15–39) | |
| Liver disease | 0.25 | ||
| yes | 30 | 28.5 (16–48) | |
| no | 395 | 25 (15–40) | |
| Solid tumour | 0.51 | ||
| yes | 195 | 25 (15–44) | |
| no | 230 | 25 (15–39) | |
| Haematological malignancy | 0.93 | ||
| yes | 38 | 26 (15–43) | |
| no | 387 | 25 (15–41) | |
| HIV | 0.17 | ||
| yes | 5 | 10 (10–30) | |
| no | 420 | 25 (15.5–41) | |
| Isolated pathogen | |||
| C. albicans | 0.14 | ||
| yes | 244 | 25 (16–43.5) | |
| no | 181 | 23 (15–39) | |
| C. glabrata | 0.55 | ||
| yes | 48 | 26.5 (16–42.5) | |
| no | 377 | 25 (15–40) | |
| C. tropicalis | 0.51 | ||
| yes | 32 | 28 (19–40) | |
| no | 393 | 25 (15–41) | |
| C. parapsilosis | 0.058 | ||
| yes | 89 | 20 (14–38) | |
| no | 336 | 26 (16–41) | |
| Candida krusei | 0.87 | ||
| yes | 7 | 28 (15–38) | |
| no | 418 | 25 (15–41) | |
| Candida guilliermondii | 0.02 | ||
| yes | 3 | 12 (7–13) | |
| no | 422 | 25 (16–41) | |
| other species | 0.43 | ||
| yes | 5 | 26 (13–31) | |
| no | 420 | 25 (15–41) | |
| Polyfungal | 0.30 | ||
| yes | 11 | 25 (20–53) | |
| no | 414 | 25 (15–40) | |
| IIAT | <0.001 | ||
| yes | 54 | 39.5 (22–50) | |
| no | 371 | 24 (15–39) |
At multiple regression analysis, IIAT (P < 0.04) and intensive care unit (ICU) stay (P < 0.001) were independent factors for a longer time to discharge, which was significantly shorter for patients with C. parapsilosis BSI (P = 0.03) (Table 5).
Table 5.
Multiple linear regression model: predictors of time to discharge among surviving patients
| Variablea | Coeff. | 95% CI | P value |
|---|---|---|---|
| Ward: ICU | 0.17 | 0.01–0.33 | 0.04 |
| C. parapsilosis | −0.17 | −0.33 to −0.01 | 0.03 |
| Inappropriate empirical therapy | 0.38 | 0.19–0.58 | <0.001 |
| Intercept | 3.16 | 3.07–3.25 | <0.001 |
aDependent variable was log transformed prior to analysis.
Discussion
Efforts have been made to highlight strategies for the early recognition and treatment of candidaemia in patients at risk, but few data have so far reported the risk factors and characteristics according to the onset after hospital admission. In this study, for the first time, we defined as EOC those episodes occurring ≤10 days and as LOC those occurring >10 days after admission. We agree that there is no scientific agreement on the definition of EOC and most previous studies identified EOC as occurring within 48 h of hospital admission.10,13,14 However, in our cohort, there was a significant difference in mortality by comparing episodes of candidaemia within or after 10 days from the admission and accordingly we aimed to investigate the clinical characteristics and prognostic factors (focusing also on the impact of different definitive antifungal regimens) of EOC and LOC.
The main results of our study are that the crude mortality rate was significantly lower in EOC compared with LOC patients, that IIAT was significantly associated with mortality in both EOC and LOC, although it was more frequent in EOC, and that caspofungin was significantly associated with survival in LOC. Our data are in line with an Australian surveillance study conducted by Chen et al.,14 who found that 30 day mortality was significantly lower for patients with EOC (i.e. ≤48 h after admission) compared with patients with candidaemia diagnosed ≥48 h after admission (11% versus 31%); of note, our findings are similar, despite the fact that in our study the definition of EOC was extended to cases of candidaemia with onset ≤10 days. Chen et al.14 hypothesized that this finding could be related to the more severe clinical condition of patients with candidaemia onset at ≥48 h after admission, and this could be true also in our cohort, as evidenced by the significant differences in the mean APACHE III score between the EOC and LOC cases.
The impact of IIAT on mortality in patients with candidaemia has been previously demonstrated in several studies6,8,19,20 and also in EOC (i.e. from ≤48 h to ≤14 days).13,16 In line with previous reports, in our cohort, IIAT was an independent predictor of mortality in both EOC and LOC. Moreover, IIAT was significantly more frequent in EOC compared with LOC, thus confirming the hypothesis that the low index of suspicion for candidaemia is very important in EOC. By extending the definition of HCA infections to beyond 48 h but within 10 days after admission, as many as one-third of EOC are still classifiable as HCA candidaemias (31.1%; 57/183), compared with only 11.4% of those with the classic definition. In fact, the pathogenesis of candidaemia most likely reflects a multistep process where comorbidities, host factors and intestinal colonization contribute to the invasion of the bloodstream by Candida spp.9 Nowadays, the pathogenesis may well begin outside of the hospital because of medical comorbidities and HCA attributes and candidaemia may also present within 10 days after hospital admission.
Other studies have reported significantly lower mortality in patients treated with echinocandins, mostly caspofungin, compared with other drugs.21–23 By comparing patients' outcome with candidaemia onset, we demonstrated for the first time the efficacy of definitive treatment with echinocandins (mostly caspofungin) also in patients with LOC.
Amongst patients who survived, the time to discharge was significantly longer in ICU patients and in those with IIAT, whereas it was significantly shorter in candidaemia caused by C. parapsilosis. The impact of IIAT on the post-candidaemia length of stay has previously been reported by others.16,24 The excess of hospitalization for ICU patients after candidaemia could be due to the higher severity of disease. The shorter time to discharge observed in infections caused by C. parapsilosis could be related to the high association with medical devices and their subsequent removal at diagnosis of candidaemia, as widely recommended.25
Our study has at least two main limitations that have to be acknowledged. First, the retrospective nature may underestimate the role of certain factors and, consequently, our conclusions do need to be confirmed in larger clinical trials. Second, it was conducted in only two centres and, therefore, although there were no significant differences in mortality between the two hospitals, unknown risk factors for mortality might have been unequally distributed between the different groups.
In conclusion, we found that EOC has different characteristics and risk factors for mortality from LOC. Early infections are at higher risk of being inappropriately treated than late infections, but IIAT was an important predictor of mortality in both groups. Definitive treatment with caspofungin was significantly associated with survival in patients with LOC. Strong efforts should be made to improve the diagnosis and adequate treatment of patients with EOC.
Funding
The Clinic of Infectious Diseases of Turin, Amedeo di Savoia Hospital, has received a grant from MSD Europe to study EOC (NCT trial number 01406093). No grant or reimbursement has ever been received by the Catholic University of Rome.
Transparency declarations
F. G. D. R., S. D. G., M. S., R. C., G. D. P. and M. T. have been speakers or consultants for Gilead Sciences, MSD and Pfizer. All other authors: none to declare.
References
- 1.Becksague CM, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal-infections in the United States, 1980–1990. J Infect Dis. 1993;167:1247–51. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 2.Edmond MB, Wallace SE, McClish DK, et al. Nosocomial bloodstream infections in United States hospitals: a three-year analysis. Clin Infect Dis. 1999;29:239–44. doi: 10.1086/520192. [DOI] [PubMed] [Google Scholar]
- 3.Horn DL, Neofytos D, Anaissie EJ, et al. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis. 2009;48:1695–703. doi: 10.1086/599039. [DOI] [PubMed] [Google Scholar]
- 4.Zaoutis TE, Argon J, Chu J, et al. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin Infect Dis. 2005;41:1232–9. doi: 10.1086/496922. [DOI] [PubMed] [Google Scholar]
- 5.Sofair AN, Lyon GM, Huie-White S, et al. Epidemiology of community-onset candidemia in Connecticut and Maryland. Clin Infect Dis. 2006;43:32–9. doi: 10.1086/504807. [DOI] [PubMed] [Google Scholar]
- 6.Tumbarello M, Sanguinetti M, Trecarichi EM, et al. Fungaemia caused by Candida glabrata with reduced susceptibility to fluconazole due to altered gene expression: risk factors, antifungal treatment and outcome. J Antimicrob Chemother. 2008;62:1379–85. doi: 10.1093/jac/dkn381. [DOI] [PubMed] [Google Scholar]
- 7.Bassetti M, Taramasso L, Nicco E, et al. Epidemiology, species distribution, antifungal susceptibility and outcome of nosocomial candidemia in a tertiary care hospital in Italy. PLoS ONE. 2011;6:e24198. doi: 10.1371/journal.pone.0024198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassetti M, Trecarichi EM, Righi E, et al. Incidence, risk factors, and predictors of outcome of candidemia. Survey in 2 Italian university hospitals. Diagn Microbiol Infect Dis. 2007;58:325–31. doi: 10.1016/j.diagmicrobio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Leon C, Ruiz-Santana S, Saavedra P, et al. Usefulness of the ‘Candida score’ for discriminating between Candida colonization and invasive candidiasis in non-neutropenic critically ill patients: a prospective multicenter study. Crit Care Med. 2009;37:1624–33. doi: 10.1097/CCM.0b013e31819daa14. [DOI] [PubMed] [Google Scholar]
- 10.Shorr AF, Gupta V, Sun XW, et al. Burden of early-onset candidemia: analysis of culture-positive bloodstream infections from a large US database. Crit Care Med. 2009;37:2519–26. doi: 10.1097/CCM.0b013e3181a0f95d. [DOI] [PubMed] [Google Scholar]
- 11.Hajjeh RA, Sofair AN, Harrison LH, et al. Incidence of bloodstream infections due to Candida species and in vitro susceptibilities of isolates collected from 1998 to 2000 in a population-based active surveillance program. J Clin Microbiol. 2004;42:1519–27. doi: 10.1128/JCM.42.4.1519-1527.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulia J, Aryal S, Saadlla H, et al. Healthcare-associated candidemia: a distinct entity? J Hosp Med. 2010;5:298–301. doi: 10.1002/jhm.652. [DOI] [PubMed] [Google Scholar]
- 13.Kung HC, Wang JL, Chang SC, et al. Community-onset candidemia at a university hospital, 1995–2005. J Microbiol Immunol Infect. 2007;40:355–63. [PubMed] [Google Scholar]
- 14.Chen S, Slavin M, Nguyen Q, et al. Australian Candidemia Study. Active surveillance for candidemia, Australia. Emerg Infect Dis. 2006;12:1508–16. doi: 10.3201/eid1210.060389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman ND, Kaye KS, Stout JE, et al. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Int Med. 2002;137:791–7. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 16.Zilberberg MD, Kollef MH, Arnold H, et al. Inappropriate empiric antifungal therapy for candidemia in the ICU and hospital resource utilization: a retrospective cohort study. BMC Infect Dis. 2010;10:150. doi: 10.1186/1471-2334-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knaus WA, Wagner DP, Draper EA, et al. The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest. 1991;100:1619–36. doi: 10.1378/chest.100.6.1619. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Tumbarello M, Posteraro B, Trecarichi EM, et al. Biofilm production by Candida species and inadequate antifungal therapy as predictors of mortality for patients with candidemia. J Clin Microbiol. 2007;45:1843–50. doi: 10.1128/JCM.00131-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garey KW, Rege M, Pai MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: a multi-institutional study. Clin Infect Dis. 2006;43:25–31. doi: 10.1086/504810. [DOI] [PubMed] [Google Scholar]
- 21.Ortega M, Marco F, Soriano A, et al. Candida spp. bloodstream infection: influence of antifungal treatment on outcome. J Antimicrob Chemother. 2010;65:562–8. doi: 10.1093/jac/dkp495. [DOI] [PubMed] [Google Scholar]
- 22.Reboli AC, Shorr AF, Rotstein C, et al. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: a multivariate analysis of factors associated with improved outcome. BMC Infect Dis. 2011;11:261. doi: 10.1186/1471-2334-11-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tumbarello M, Fiori B, Trecarichi EM, et al. Risk factors and outcomes of candidemia caused by biofilm-forming isolates in a tertiary care hospital. PLoS ONE. 2012;7:e33705. doi: 10.1371/journal.pone.0033705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold HM, Micek ST, Shorr AF, et al. Hospital resource utilization and costs of inappropriate treatment of candidemia. Pharmacotherapy. 2010;30:361–8. doi: 10.1592/phco.30.4.361. [DOI] [PubMed] [Google Scholar]
- 25.Silva S, Negri M, Henriques M, et al. Candida glabrata, Candida parapsilosis and Candida tropicalis: biology, epidemiology, pathogenicity and antifungal resistance. FEMS Microbiol Rev. 2012;36:288–305. doi: 10.1111/j.1574-6976.2011.00278.x. [DOI] [PubMed] [Google Scholar]