Abstract
Adenosine monophosphate-activated kinase (AMPK) plays a central role in regulating energy homeostasis in eukaryotic cells. AMPK also regulates lipid synthesis by inhibiting acetyl-CoA carboxylase (ACC) and regulates mTOR signaling by activating TSC2. Due to its important roles in cell metabolism, AMPK is an attractive target for metabolic diseases, such as type II diabetes and obesity. AMPK activators, such as metformin, that are used for diabetes treatment are also effective anticancer agents. However, the efficacies of many known AMPK activators are relatively low. For example, metformin activates AMPK at millimolar levels. In this study, we identified a novel family of AMPK activators, namely fluorinated N,N’-diarylureas, that activate AMPK at 1–3µM concentrations. These novel agents strongly inhibit the proliferation of colon cancer cells. We studied the potential mechanisms of these agents, performed a structure-activity relationship (SAR) study and identified several fluorinated N,N’- diarylureas as potent AMPK activators.
Adenosine monophosphate-activated kinase (AMPK) plays a dominant role in cell maintenance and cell cycle progression as a central part of the mechanism for regulating energy homeostasis1–4. Neoplastic tissues make effective use of this system to sustain unregulated growth, and the development of “small molecule” AMPK activators, which interdict this activation process either directly or indirectly, represents a potential target for cancer therapeutics as well as the treatment of diabetes and obesity1,2,4. Among these diverse AMPK activators are nucleosides such 5-amino-1-β-Dribofuranosyl- imidazole-4-carboxamide (AICAR), its phosphorylated analog ZMP, drugs such as metformin, and a number of other drug candidates3–5.
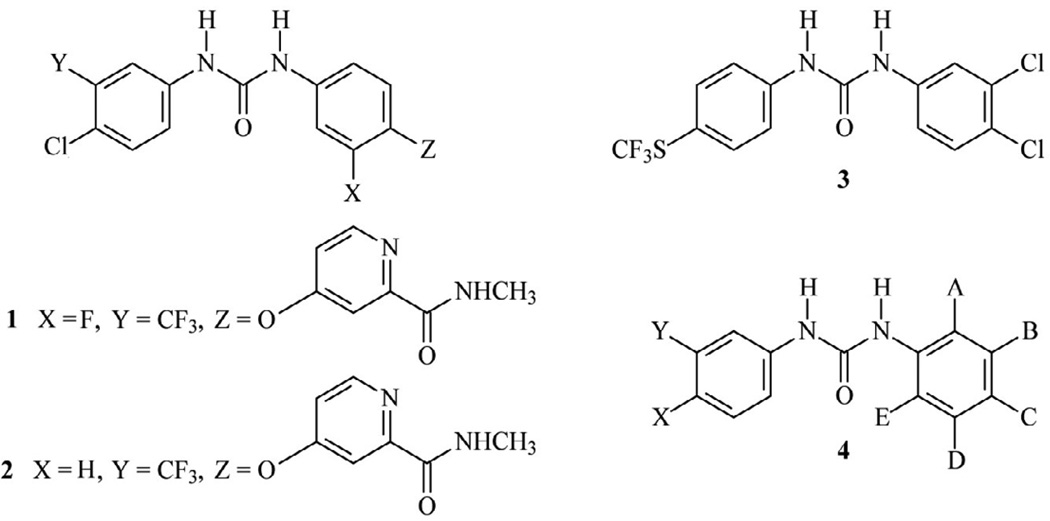
Our search for activators of AMPK began with a high-throughput screen that identified several N,N’-diarylureas that structurally resembled the multikinase inhibitors, regorafenib (1) and sorafenib (2) (Fig. 1). Regorafenib (1) and sorafenib (2) are approved for the treatment of colon cancer, renal cancer and advanced liver cancer6,7. The leading compound from our screening efforts was 1-(3,5-dichlorophenyl)-3-(4-(trifluoromethylthio)phenyl)urea (3), hereinafter designated as urea-3,4-diCl (3) (Fig. 1). We sought to develop more potent analogs than urea-3,4-diCl (3) that would promote AMPK activation and prove suitable for cancer treatment. We also wanted to delineate whether this lead compound possessed activity different from those reported for other N,N’-diarylureas used for cancer treatment8. As a first step, we explored structure-activity studies within this series with a focus exclusively on the potential for these compounds to serve as antineoplastic agents, and we report here that only fluorinated or chlorinated N,N’-diarylureas having general structure (4) (Fig. 1) possessed the desired activity and surprisingly, behaved differently from several other N,N’-diarylureas, including the multikinase inhibitor, sorafenib (2).
Figure 1. N,N-Diarylurea structures: regorafenib (1), sorafenib (2), urea-3,4-diCl (3), and N,N’-diarylurea analogs (4).
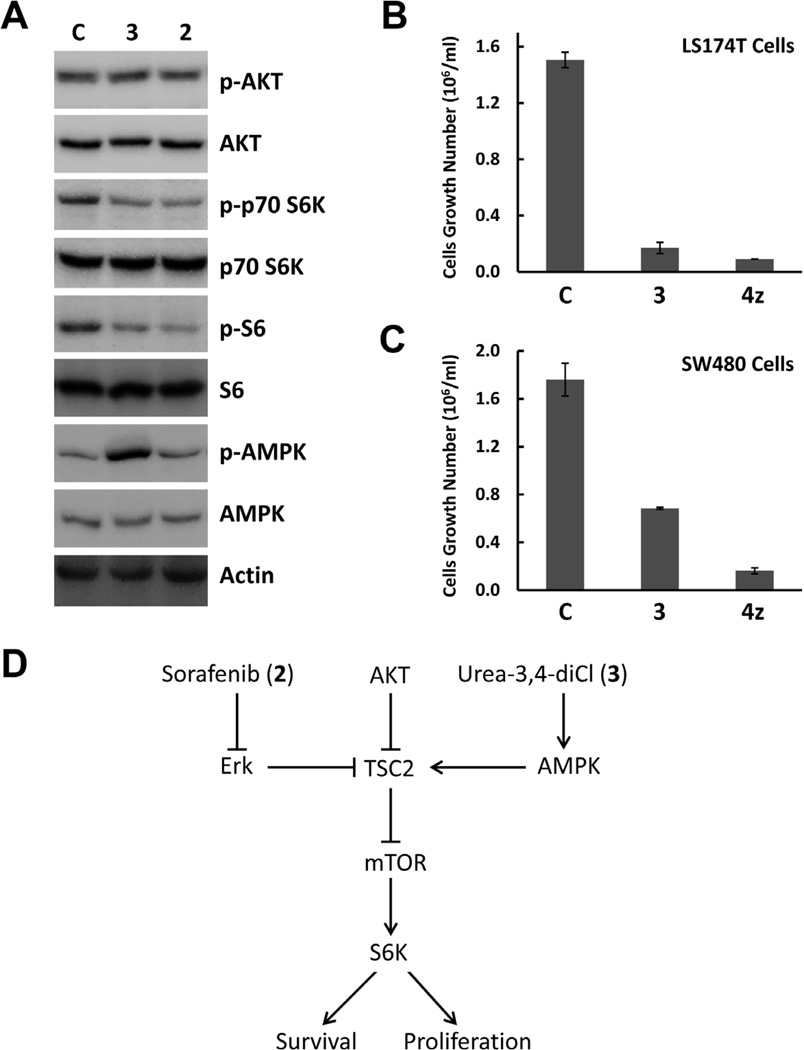
Since sorafenib (2) is a multi-kinase inhibitor7, we began by treating LS174T colon cancer cells with urea-3,4-diCl (3) and analyzed its effect on several key substrates of cancer-related kinases. We found that the phosphorylation of p70S6K was depressed (Fig. 2A), suggesting that urea-3,4-diCl (3) inhibited p70S6K activity. As a result, the phosphorylation of S6, a substrate of p70S6K3, was decreased (Fig. 2A). Sorafenib (2) has similar effects on p70S6K and S6 (Fig. 2A). Since AKT regulates mTOR signaling and p70S6K activity,3 we analyzed AKT activation using anti-Phospho-AKT (Ser473). Surprisingly, neither urea-3,4-diCl (3) nor sorafenib (2) had significantly effects on AKT activation at 3µM.
Figure 2. N,N-Diarylureas inhibit mTOR and the proliferation of colon cancer cells.
A. Effects of urea-3,4-diCl (3) and sorafenib (2) on activities of AKT, AMPK and p70S6K.
B and C. Effects of N,N-diarylureas (3) and (4z) on colon cancer cell growth.
D. N,N-Diarylureas inhibit cancer growth by distinct mechanisms.
To test if urea-3,4-diCl (3) inhibits mTOR signaling by activating AMPK, we treated LS174T cells with urea-3,4-diCl (3) at 3µM and analyzed AMPK activation by Western blotting with anti-Phospho-AMPKα (Thr172). The cellular levels of AMPK were analyzed as a control. We found that urea-3,4-diCl (3) had no effect on overall AMPK levels, but at 1–3µM concentrations, urea-3,4-diCl (3) significantly increased the amount of phosphorylated AMPK (Fig. 2A), suggesting that urea-3,4-diCl (3) was a promising AMPK activator. Interestingly, although sorafenib (2) has a similar structure to urea-3,4-diCl (3), sorafenib had no effect on AMPK phosphorylation at similar concentrations (Fig. 2A). Since AMPK activation and mTOR inhibition contribute to cancer inhibition3, we tested urea-3,4-diCl (3) and its analog, urea 4z (Table 1) using cell proliferation assays (Fig. 2B and 2C). We found that urea-3,4-diCl (3) and urea 4z significantly inhibited the growth of multiple human cancer cell lines, including colon cancer cell lines LS174T and SW480 (Fig 2B and 2C). Metformin, which is a known activator of AMPK used for diabetes treatment and cancer prevention, activates AMPK at millimolar levels5. These data suggested that N,N’-diarylureas, such as urea-3,4-diCl (3) and its analogs (4), represent a novel family of AMPK activators that warranted further study.
Table 1. Halogenated N,N-Diarylureas (4) with substitutents X or Y in the “first” aryl ring and substituents A, B, C, D, and E in the “second aryl” ring and their activities in AMPK activation.
Ratio is the activity of each compound in AMPK activation compared with the activity of compound 3.
| Compound | X | Y | A | B | C | D | E | Ratio |
|---|---|---|---|---|---|---|---|---|
| 3 | CF3S | H | Cl | Cl | ||||
| 4a | CF3O | H | CF3 | 2.37 | ||||
| 4b | CF3O | H | Cl | SCF3 | 2.60 | |||
| 4c | CF3O | H | F | F | 1.44 | |||
| 4d | CF3O | H | Cl | Cl | 0.82 | |||
| 4e | CF3O | H | F | F | F | 0.99 | ||
| 4f | CF3O | H | Cl | Cl | Cl | 0.96 | ||
| 4g | CF3O | H | F | F | F | F | 0.80 | |
| 4h | H | CF3S | CF3 | 2.16 | ||||
| 4i | H | CF3S | F | F | 1.83 | |||
| 4j | H | CF3S | Cl | Cl | 1.94 | |||
| 4k | CF3 | H | F | F | F | 4.00 | ||
| 4l | CF3S | H | Cl | 0.71 | ||||
| 4m | CF3S | H | F | 0.74 | ||||
| 4n | CF3S | H | CF3 | 1.19 | ||||
| 4o | CF3S | H | CN | 1.05 | ||||
| 4p | CF3S | H | Cl | Cl | 1.59 | |||
| 4q | CF3S | H | F | F | 1.11 | |||
| 4r | CF3S | H | F | F | 1.14 | |||
| 4s | CF3S | H | Cl | F | 1.05 | |||
| 4t | CF3S | H | F | Cl | 0.72 | |||
| 4u | CF3S | H | CF3 | Cl | 1.64 | |||
| 4v | CF3S | H | CF3 | F | 1.52 | |||
| 4w | CF3S | H | CN | F | 0.96 | |||
| 4x | CF3S | H | Cl | SCF3 | 0.93 | |||
| 4y | CF3S | H | F | F | F | 1.21 | ||
| 4z | CF3S | H | F | F | F | 2.02 | ||
| 4aa | CF3S | H | Cl | Cl | Cl | 2.32 | ||
| 4bb | CF3S | H | F | F | F | F | 2.48 | |
| 4cc | CF3S | H | F | F | CO2H | F | F | 0.86 |
The addition of suitably functionalized anilines to aryl isocyanates secured an array of functionalized ureas possessing a spectrum of electron-withdrawing and donating groups. Among the several hundred compounds surveyed in this study, only the halogenated N,N’-diarylureas possessed activity comparable to or better than that of the lead compound, urea-3,4-diCl (3) in AMPK activation (Table 1). Specifically, halogenated N,N’-diarylureas were potent AMPK activators if they possessed one aryl ring bearing a 4’-trifluoromethoxy group (4’-CF3O), a 3’-trifluoromethylthio group (3’-CF3S), a 4’-trifluoromethyl group (4’-CF3), or preferably, a 4’-trifluoromethylthio group (4’-CF3S) and if they possessed a second aryl ring bearing various chlorine or fluorine substituents. With respect to the first aryl ring, substitutions with groups other than the four that are listed above led to inactive compounds (data not shown). With respect to the second aryl ring bearing the two chlorine groups in urea-3,4-diCl (3), the introduction of heteroaryl or polycyclic aryl rings; replacement of the chlorine substituents with electron-withdrawing cyano or carboxylate groups (with few exceptions); introduction of bromine or iodine as the halogen substituents; and modifications, such as N-alkylation, of the urea functional group in urea-3,4-diCl (3) also led to inactive compounds (data not shown).
Replacing the 4’-trifluoromethylthio group in urea-3,4-diCl (3) with a 4’- trifluoromethoxy group (4’-CF3O), a 3’-trifluoromethylthio group (3’-CF3S), or a 4’- trifluoromethyl group (4’-CF3) led to the active compounds summarized in Table 1. To facilitate comparisons, we report the AMPK activation ratios of various N,N’-diarylureas (4) (Fig. 1) relative to the original lead compound, urea-3,4-diCl (3). In the series in which the first aryl ring bears a 4-trifluoromethoxy group (4’-CF3O) in place of the 4’-trifluoromethylthio group found in urea-3,4-diCl (3), the N,N’-diarylureas (4) with either a 4-trifluoromethyl group (4a) or a 3-chloro-4-trifluoromethylthio group (4b) in the second aryl ring possessed activity approximately twice that of urea-3,4-diCl (3) (Table 1). Unlike other series, the inclusion of additional halogen substituents, as found in N,N’-diarylureas 4c-4g, only produced compounds with activity comparable to urea-3,4-diCl (3), and the inclusion of non-halogen substituents led to inactive compounds. In the series in which the first aryl ring bears a 3’-trifluoromethylthio group (3’-CF3S) in place of the 4’-trifluoromethylthio group in urea-3,4-diCl (3), the N,N’-diarylureas (4) with either a 4-trifluoromethyl substituent (4h), 3,5-fluoro substituents (4i), or 3,5-dichloro substituents (4j) possessed activity approximately twice that of the lead compound, urea-3,4-diCl (3). In the series in which the first aryl ring bears a 4’-trifluoromethyl group (4’- CF3) in place of the 4’-trifluoromethylthio group in urea-3,4-diCl (3), only one N,N’-diarylurea with 3,4,5-trifluoro substituents, namely (4k), possessed activity four times that of the lead compound, urea-3,4-diCl (3). Unfortunately, other modifications of this platform failed to produce active compounds. In summary, in three of the four modifications of the first aryl ring in urea-3,4-diCl (3), only a limited number of compounds emerged as equipotent or slightly more potent analogs of the lead compound, despite explorations designed to uncover activity in N,N’-diarylureas (4) in which the second aryl ring would have different electron-withdrawing groups than halogens or combinations of electron-withdrawing and donating groups.
These findings led us to focus on modifications of the urea-3,4-diCl (3) in which the first aryl ring bearing the 4’-trifluoromethylthio group (4’-CF3S) was retained and the second aryl ring was altered. These N,N’-diaryl ureas (4) (Table 1) provided an interesting series of active compounds and the following structure-activity relationships. Eliminating one of the chlorines in the urea-3,4-diCl (3) reduced activity as seen in various monosubstituted analogs (4l-4o). Only modest improvement in activity was seen by relocating the 3,4-dichloro substituents in urea-3,4-diCl (3) to other positions such as the 3,5-dichloro urea (4p); substituting other halogens for the chloro substituents in urea- 3,4-diCl (3) as in the 3,4-difluoro urea (4q) and the 3,5-difluoro urea (4r); replacing one of the two chlorines in urea-3,4-diCl (3) with a fluorine as in the 3-chloro-4-fluoro urea (4s) and the 3-chloro-2-fluoro urea (4t); or replacing one of the chloro substituents in urea-3,4-diCl (3) with a trifluromethyl group as in the 3-trifluoromethyl-4-chloro urea (4u) or 3-trifluoromethyl-4-fluoro urea (4v). The introduction of cyano groups in the 3- cyano-4-fluoro urea (4w) or additional trifluoromethylthio groups as in the 4-trifluoromethylthio-3-chloro urea (4x) produced compounds only comparable in activity to the lead compound. Finally, the inclusion of additional halogens beyond the two chlorines in urea-3,4-diCl (3) produced compounds either comparable to or slightly more active than 3 as in the 2,4,5-trifluoro urea (4y), 3,4,5-trifluoro urea (4z), 3,4,5-trichloro urea (4aa), or the 2,3,4,5-tetrafluoro urea (4bb). In conclusion, halogenated N,N’- diarylureas activate AMPK at concentrations in the 1–3 µM range, well below that of other known AMPK activators, such as metformin.
Supplementary Material
Acknowledgments
We thank Center for Clinical and Translational Science at University of Kentucky for CCTS Pilot Award. CL was supported by R01 DK071976 from the NIH. DSW was supported by NIH Grant Number 2P20 RR020171 from the National Center for Research Resources. JT was a summer research student supported the REU program under NSF DBI-1004931 (to Trevor Creamer, PI). This study was supported in part by NIH Grant Number P20GM103486 from the National Institute of General Medical Sciences, its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the NIGMS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Luo Z, Saha AK, Xiang X, Ruderman NB. Trends Pharmacol Sci. 2005;26:69. doi: 10.1016/j.tips.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Luo Z, Zang M, Guo W. Future Oncol. 2010;6:457. doi: 10.2217/fon.09.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shackelford DB, Shaw RJ. Nat Rev Cancer. 2009;9:563. doi: 10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou G, Sebhat IK, Zhang BB. Acta Physiol (Oxf) 2009;196:175. doi: 10.1111/j.1748-1716.2009.01967.x. [DOI] [PubMed] [Google Scholar]
- 5.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. J Clin Invest. 2001;108:1167. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carr BI, Cavallini A, Lippolis C, D'Alessandro R, Messa C, Refolo MG, Tafaro A. J Cell Physiol. 2013;228:292. doi: 10.1002/jcp.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilhelm S, Carter C, Lynch M, Lowinger T, Dumas J, Smith RA, Schwartz B, Simantov R, Kelley S. Nat Rev Drug Discov. 2006;5:835. doi: 10.1038/nrd2130. [DOI] [PubMed] [Google Scholar]
- 8.Denoyelle S, Chen T, Chen L, Wang Y, Klosi E, Halperin JA, Aktas BH, Chorev M. Bioorg Med Chem Lett. 2012;22:402. doi: 10.1016/j.bmcl.2011.10.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W, Sviripa V, Kril LM, Chen X, Yu T, Shi J, Rychahou P, Evers BM, Watt DS, Liu C. J Med Chem. 2011;54:1288. doi: 10.1021/jm101248v. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.