Abstract
We report a magneto-nanosensor biochip for fungal detection. The chip is made of arrays of giant magnetoresistive (GMR) spin-valve sensors, and is able to detect protein biomarkers at low concentrations in solutions. As a demonstration, a standard curve for fungal pathogen Asp f 1 was obtained by measuring signals from various concentrations of Asp f 1 spiked in PBS solutions, indicating a detection limit of ~100 pg/ml. Five positive and negative Asp f 1 solution samples were discriminated correctly in blind experiments. Our data suggest that the magneto-nanosensor biochips are very promising as sensitive diagnostic devices for fungal pathogens. Given the generality of the detection scheme used in the magneto-nanosensor, we anticipate that the platform will be very useful for the detection of many types of biomarkers.
Index Terms: magneto-nanosensor, biochip, immunoassay, magnetic nanoparticles
I. Introduction
Sensitive and rapid detection of protein biomarkers is expected to play an important role in molecular diagnosis of numerous diseases as well as personalized medical treatment of patients [1]–[3]. In spite of recent advances in various detection methods, development of diagnostic tools suitable for ultra-sensitive and multiplex detection of protein biomarkers remains in its infancy stage. Magneto-nanosensor biochip, capable of multiplexed detection of up to 64 protein biomarkers, has emerged as a practical contender as a biomedical diagnostic platform [4]–[6]. Compared with non-magnetic technologies, this platform offers several advantages such as large linear dynamic range, facile integration with electronics, and enhanced signal-to-noise ratio due to ultra-sensitive GMR effect and negligible magnetic background signals from mostly non-magnetic biological species [5]. In this study, we demonstrate the feasibility of using the magneto-nanosensor biochips for the detection of Asp f 1, a major allergen of fungal pathogen Aspergillus Fumigatus, which can cause serious fungal infections in humans and animals [7].
II. Materials and Methods
All experiments were performed in ambient condition except the surface cleaning steps (cleaning with acetone, methanol, isopropanol, and oxygen plasma) which must be performed in a fume hood.
A. Materials
Asp f 1 enzyme-linked immunosorbent assay (ELISA) kit (Indoor biotechnologies, EL-AF1), EZ-Link micro sulfo-NHS-biotinylation kit (Thermo scientific, 21925), poly(allylamine hydrochloride) (Polyscience), poly(ethylene-alt-maleic anhydride) (Aldrich), Phosphate buffered saline (PBS) (Invitrogen), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (EDC) (Thermo scientific), N-hydroxysuccinimide (NHS) (Aldrich), 1% bovine serum albumin (BSA) (Aldrich), biotinylated-bovine serum albumin (biotin-BSA) (Pierce), Tween 20 (Aldrich), and streptavidin-coated MicroBeads (Miltenyi, 130-048-101) were used as received without further purification. The magneto-nanosensor biochips were fabricated according to the previously reported method [8].
B. Biotinylation of detection antibody
EZ-Link micro sulfo-NHS-biotinylation kit was used for the biotinylation of the detection antibody. First, 100 μl of detection antibody solution was diluted with 100 μl of PBS. Separately, 1 mg of sulfo-NHS-biotin was dissolved in 110 μl of PBS, resulting in a solution of 20 mM concentration. The prepared two solutions were mixed together and incubated at room temperature for 45 minutes. Excess biotins were removed using a desalt spin column included in the kit. Prior to the removal of the excess biotins, a desalt spin column was buffer exchanged by centrifuging at 1,000 × g (e.g. 2,500 rpm if 15 cm distant from center) for 2 minutes and centrifuging 3 times more with an addition of 1 ml of PBS for each additional step. After that, the premixed and incubated solution of the sulfo-NHS-biotin and the detection antibody was added to the buffer exchanged desalt spin column. A purified solution of biotinylated-detection antibody was obtained after the final centrifugation at 1,000 × g for 2 minutes.
C. Sensor surface preparation
The sensor chip surface was cleaned with acetone, methanol, and isopropanol. After subsequent cleaning with oxygen plasma, the sensor chip surface was coated with 1% solution of poly(allylamine hydrochloride) for 5 minutes. The chip was washed with deionized water, and then annealed at 120°C for 1 hour. Following the coating with 2% poly(ethylene-alt-maleic anhydride) solution and washing with deionized water, a 1:1 mixture solution of 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride and N-hydroxysuccinimide was added to activate the carboxyl groups on the biochip surface. The chip was incubated for 1 hour before washing and deposition of 0.05% Asp f 1 capture antibody, 0.1% BSA, and 0.1% biotin-BSA PBS solutions. The deposition was done using a robotic spotter to ensure a drop of 1.5 nl volume completely covers each of the designated sensors. Some sensors were covered by epoxy to prevent them from participating in binding reactions and to serve as electrical signal reference sensors. The prepared chip was stored in a humidity chamber at 4°C before use.
D. Biochip immunoassay protocol
The sensor chip was taken out of the humidity chamber and washed with a washing buffer (0.1% BSA and 0.05% Tween 20 in PBS). The surface was further blocked for 1 hour using a 1% BSA solution. The surface was washed again. Following the incubation with an analyte solution of Asp f 1 for 2 hours and washing, an Asp f 1 detection antibody solution with a concentration of 10 μg/ml was added. The chip was incubated for 1 hour and washed. Finally, the chip was placed in a biochip reader station with electric signal read-out capability which was implemented according to Hall et al. [9]. Signal acquisition was done in real-time with the addition of streptavidin-coated magnetic nanoparticles. The obtained signal was fitted to a double exponential curve to estimate a final value.
III. Results and Discussion
The magneto-nanosensor biochip immunoassay relies on GMR spin-valve sensors to quantify the number of magnetic nanoparticle labels selectively bound to the sensor surface. We used 10 mm × 12 mm-sized prototype biochips with an array of 64 individually addressable sensors (each sensor is made of 48 spin-valve strips with a size of 90 μm × 0.75 μm) covered by ultrathin and biochemically-stable silicon oxide passivation [10]. Fig. 1 illustrates the detection scheme of the magneto-nanosensor biochip immunoassay. Capture antibodies are immobilized on the sensor surface covalently (Fig. 1a) to capture Asp f 1 allergens. After Asp f 1 allergens are captured by the capture antibodies (Fig. 1b), detection antibodies are added, which bind to the allergens in the form of a sandwich structure (Fig. 1c). The detection antibodies are biotinylated in advance (Section II.B), and thus they can be subsequently labeled by ~50 nm-diameter streptavidin-coated magnetic nanoparticles (Fig. 1d). Finally, the magnetic nanoparticles generate a stray magnetic field in real time and perturb the oscillating external magnetic field which is applied during the measurement. As a result of the GMR effect, the resistance of the spin-valve is changed as the magnetic nanoparticles bind to the sensor surface, which leads to the specific biological signal we measure [9].
Fig. 1.
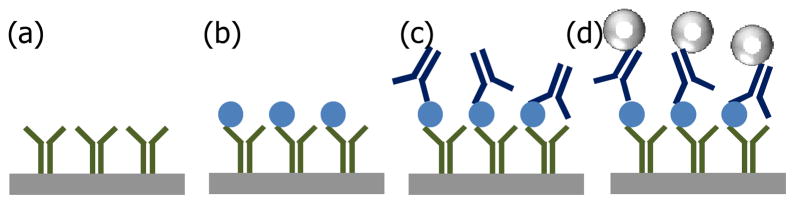
A schematic of magneto-nanosensor biochip immunoassay: (a) Capture antibodies are immobilized on the sensor surface. (b) Target antigens are captured. (c) Sandwich structures are formed by the addition of biotinylated-detection antibodies. (d) Magnetic nanoparticles bound to the detection antibodies generate stray magnetic field, and the sensor resistance is changed as a result of the GMR effect.
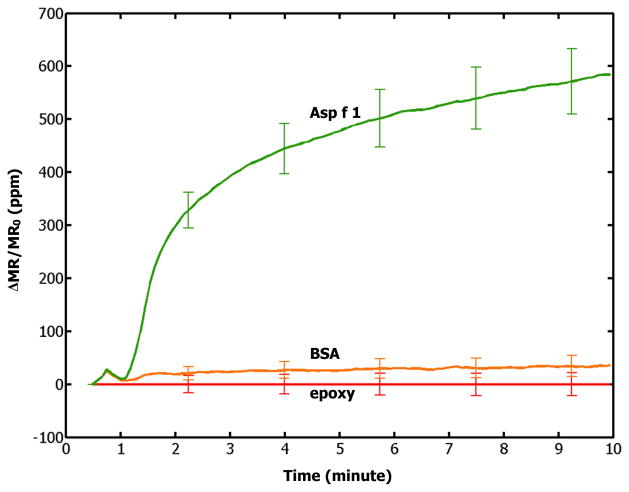
The measured signals are related to Asp f 1 concentrations in a manner similar to the Langmuir absorption behavior. A representative binding curve is shown in Fig. 2, where the Asp f 1 analyte concentration was 10 ng/ml. Whereas the Asp f 1 allergen (green) showed signals around 600 ppm after 10 minute of measurement, BSA-coated negative control sensors (orange) produced very small signals because they were designed not to bind with Asp f 1 allergens or streptavidin-coated magnetic nanoparticles. These small signals measured for the BSA-coated negative control sensors are mostly originated from the insignificant but observable non-specific binding of the Asp f 1 allergens or streptavidin-coated magnetic nanoparticles. The closeness between the negative control signals and the electrical reference signals (red) indicates that the observed large signals from the Asp f 1 capture antibody-coated sensors indeed come from the specific binding of the Asp f 1 allergens to the Asp f 1 capture antibodies.
Fig. 2.
A binding curve from the assay of 10 ng/ml Asp f 1 in PBS solution: Signals from Asp f 1 capture antibody-coated (green), BSA-coated (orange), and epoxy-covered (red) sensors are shown. Error bars indicate ±1 standard deviations.
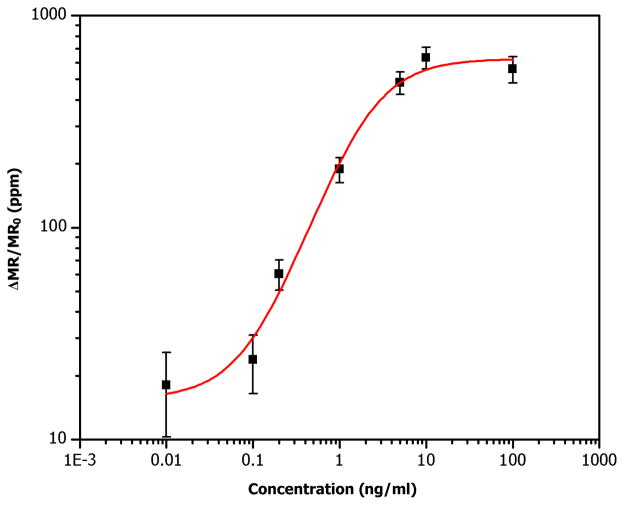
The Asp f 1 standard curve was obtained by measuring signals of spiked solutions with various Asp f 1 concentrations and subtracting the signals from BSA-coated negative control sensors (Fig. 3). A total of seven chips were measured for 10 minutes each. The subtraction of the BSA signal was done to remove the effect of the non-specific bindings even though it was very small. The salient features of our results are: We are able to get measurable signals from the Asp f 1 solutions of concentrations down to ~0.1 ng/ml. This detection limit is smaller than that of commercially available ELISA kit (Indoor biotechnologies, EL-AF1) which claims a detection limit of ~0.3 ng/ml. The highest signal we measured is ~600 ppm for 10 ng/ml standard solution of Asp f 1, and the signals begin to saturate at higher concentrations. The linear dynamic range of the standard curve is about two orders of magnitude (from ~0.1 to ~10 ng/ml), which is somehow much smaller than the 4~6 logs reported previously [6]. We believe this is largely limited by the availability of high affinity reagents rather than by the magnetic sensors or nanoparticles at this point.
Fig. 3.
Asp f 1 standard curve measured with magneto-nanosensor biochips. Each square represents an average of redundant sensors from a biochip. Error bars indicate ±1 standard deviations. Red line is a trend line.
In separate experiments, five Asp f 1 solutions with their concentrations undisclosed to the experimenter were measured. In other words, experiments were “blinded” so that the experimenter was not aware of the real concentrations of the Asp f 1 in the solutions, and the dilution ratios were suggested to test the positive and negative responses. As can be seen in Table I, positive and negative response samples could be determined correctly using the measurement results, indicating the proper working of the magneto-nanosensor biochips.
TABLE I.
Asp f 1 SOLUTION BLIND TEST
| Sample # | Concentration (mg/ml) | Dilution ratio used | Measured signal (ppm) | Remarks |
|---|---|---|---|---|
| A | 0 | 10× | < 20 | Negative |
| B | 0 | 10× | < 20 | Negative |
| C | 1 | 1,000× | ~ 630 | Positive |
| D | 1 | 10× | ~ 600 | Positive |
| E | 0 | 1,000× | < 20 | Negative |
IV. Conclusion
It was demonstrated that the magneto-nanosensor biochip immunoassay could be used for the quantification of Aspergillus fumigatus allergen Asp f 1 with high sensitivity. The magneto-nanosensor biochip has a detection limit lower than that of the commercial ELISA kit, and is expected to be very useful for the ultra-sensitive detection of several allergic disorders and infections caused by the fungal pathogens. Taken together with previous reports [4]-[6], this platform technology holds great potentials for biomedical diagnosis of many diseases since the detection scheme used in our study is applicable to numerous types of protein biomarkers.
Acknowledgments
This work was supported by the National Institute of Health under Grant 5R21AI085566-02 and Stanford BioX program. The authors would like to thank Dr. Karl V. Clemons for helpful discussion and provision of experimental samples.
References
- 1.Wulfkuhle Julia D, Liotta Lance A, Petricoin Emanuel F. Proteomic applications for the early detection of cancer. Nat Rev Cancer. 2003;3:267–275. doi: 10.1038/nrc1043. [DOI] [PubMed] [Google Scholar]
- 2.Duff Michael J, Crown John. A personalized approach to cancer treatment: how biomarkers can help. Clin Chem. 2008;54(11):1770–1779. doi: 10.1373/clinchem.2008.110056. [DOI] [PubMed] [Google Scholar]
- 3.Pepe Margaret Sullivan, Etzioni Ruth, Feng Ziding, Potter John D, Thompson Mary Lou, Thornquist Mark, Winget Marcy, Yasui Yutaka. Phase of biomarker development for early detection of cancer. J Natl Cancer I. 2001;93(14):1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 4.Osterfeld Sebastian J, Yu Heng, Gaster Richard S, Caramuta Stefano, Xu Liang, Han Shu-Jen, Hall Drew A, Wilson Robert J, Sun Shouheng, White Robert L, Davis Ronald W, Pourmand Nader, Wang Shan X. Multiplex protein assays based on real-time magnetic nanotag sensing. Proc Natl Acad Sci USA. 2008;105(52):20637–20640. doi: 10.1073/pnas.0810822105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Shan X, Li Guanxiong. Advances in giant magnetoresistance biosensors with magnetic nanoparticle tags: review and outlook. IEEE Trans Magn. 2008;44(7):1687–1702. [Google Scholar]
- 6.Gaster Richard S, Hall Drew A, Nielsen Carsten H, Osterfeld Sebastian J, Yu Heng, Mach Kathleen E, Wilson Robert J, Murmann Boris, Liao Joseph C, Gambhir Sanjiv S, Wang Shan X. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat Med. 2009;15:1327–1332. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latgé Jean-Paul. Aspergillus fumigatus and Aspergillosis. Clin Microbiol Rev. 1999;12(2):310–350. doi: 10.1128/cmr.12.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Liang, Yu Heng, Han Shu-Jen, Osterfeld Sebastian J, White Robert L, Pourmand Nader, Wang Shan X. Giant magnetoresistive sensors for DNA microarray. IEEE Trans Magn. 2008;44(11):3989–3991. doi: 10.1109/TMAG.2008.2002795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall DA, Gaster RS, Lin T, Osterfeld SJ, Han S, Murmann B, Wang SX. GMR biosensor arrays: a system perspective. Biosens Bioelectron. 2010;25(9):2051–2057. doi: 10.1016/j.bios.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Liang, Yu Heng, Akhras Michael S, Han Shu-Jen, Osterfeld Sebastian J, White Robert L, Pourmand Nader, Wang Shan X. Giant magnetoresistive biochip for DNA detection and HPV genotyping. Biosens Bioelectron. 2008;24:99–103. doi: 10.1016/j.bios.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]