Abstract
Objective
Several mechanisms of disease have been implicated in the pathophysiology of SGA including an anti-angiogenic state, failure of physiologic transformation of spiral arteries, and an exaggerated intravascular pro-inflammatory response. Adiponectin, an insulin-sensitizing, anti-atherogenic, anti-inflammatory and angiogenic adipokine circulates in oligomeric complexes including low-molecular-weight (LMW) trimers, medium-molecular-weight (MMW) hexamers and high-molecular-weight (HMW) isoforms. Adiponectin plays a role in a wide range of biological activities including those that have been implicated in the pathophysiology SGA. Thus, the aim of this study was to determine if third trimester adiponectin concentrations differed between women with normal weight infants and those with an SGA neonate.
Study design
This cross-sectional study included women with: 1) a normal pregnancy (n=234); and 2) an SGA neonate (n=78). The study population was further stratified by first trimester BMI (normal weight <25 kg/m2 vs. overweight/obese ≥25 kg/m2). Maternal serum adiponectin multimers (total, HMW, MMW and LMW) concentrations were determined by ELISA. Non-parametric statistics were used for analyses.
Results
1) The median maternal serum concentrations of total, HMW and MMW adiponectin were significantly lower in patients with an SGA neonate than in those with normal pregnancies; 2) patients with an SGA neonate had a significantly lower median HMW/total adiponectin ratio and higher median MMW/total adiponectin and LMW/total adiponectin ratios than those with a normal pregnancy; 3) among patients with an SGA neonate, neither maternal serum concentrations of adiponectin multimers, nor their relative distribution differ between normal weight and overweight/obese patients.
Conclusion
1) Pregnancies complicated by an SGA neonate are characterized by a alterations in the maternal serum adiponectin multimers concentrations and their relative abundance; 2) in contrast to normal pregnancies, those complicated by an SGA neonate are not associated with low circulating adiponectin multimers in overweight/obese individuals suggesting altered regulation of this adipokine in the presence of an SGA neonate; 3) collectively, the findings reported herein suggest that maternal adipose tissue may play a role, in the pathogenesis of SGA.
Keywords: Adipokines, Pregnancy, High-molecular-weight (HMW) adiponectin, Medium-molecular-weight (MMW) adiponectin, Low-molecular-weight (LMW) adiponectin, BMI, overweight, obesity, fetal growth, SGA, pregnancy, Adipose tissue
Introduction
Small-for-gestational-age (SGA) neonate, one of the “great obstetrical syndromes”[109] [110], is usually defined as a birthweight below the 10th percentile for gestational age at birth according to the birth weight distribution of a particular population.[124] In accordance with its syndromic nature, several mechanisms of disease have been implicated in the pathophysiology of this complication including: endothelial cell dysfunction, [17] an anti-angiogenic state, [21, 22, 34, 42, 111, 118, 139] failure of physiologic transformation of the spiral arteries, [18, 39] and an increased maternal intravascular pro-inflammatory response.[52, 66, 120, 125, 132] Interestingly, maternal overweight/obesity, has a protective effect for the development of SGA fetuses.[7, 19, 27, 33, 123, 145]
Adipose tissue, once considered a passive depot for energy storage, is now recognized as a potent endocrine organ.[134] Adipocytes, and other cellular components of adipose tissue, have a high capacity to produce and secrete adipokines. Recently, adipokines have been implicated in the metabolic adaptation of normal gestation, [20, 36, 73, 75, 76, 78, 80, 82, 84, 91, 92, 117, 130] as well as in complications of pregnancy such as preeclampsia, [29, 31, 46, 47, 54, 70, 85, 88, 90, 107, 120, 140] gestational diabetes mellitus (GDM), [10, 23, 43, 56, 59, 60, 63, 68, 77, 108, 133, 149, 150] intra-amniotic infection/inflammation, [65, 83, 141] and abnormal fetal growth.[24, 35, 50, 53, 62, 67, 69, 79, 81, 95, 105, 136, 143]
Much attention has been focused on the biological actions of adiponectin, the adipokine[48, 71, 86, 119] that circulates at highest concentrations in humans.[5] Unlike other adipokines, circulating concentrations of this adiponectin are lower in obese than in non-obese subjects, [8, 48] suggesting a negative feedback on its production or secretion by the adipose tissue. In addition to its insulin sensitizing, [12, 28, 72, 151] anti-atherogenic, [26, 38, 64, 94, 98] anti-inflammatory, [25, 122, 127] and angiogenic[16, 100, 126] properties, adiponectin has been consistently shown to have protective effects on vasculature.[74, 93, 96–98, 101] Of note, several of the abovementioned conditions in which adiponectin has been implicated (e.g. angiogenesis, hypertension, atherosclerosis and inflammation) are know risk factors for the development of an SGA neonate.
Adiponectin circulates in human plasma in distinct forms: 1) low-molecular-weight (LMW) trimers; 2) medium-molecular-weight (MMW) hexamers; and 3) high-molecular-weight (HMW) oligomers (12 to 18 subunits).[9, 103, 104, 137, 138, 144] A growing body of evidence suggests that the physiological activity of adiponectin is determined not only by the absolute concentrations of its multimeric complexes but, to a large extent, by the relative distribution of these isoforms.[61, 103, 104, 135, 138, 144] Thus, a comprehensive evaluation of its isoforms is essential to elucidate its role.
To date, no study has evaluated maternal circulating concentrations of adiponectin multimers in patients with an SGA neonate. Thus, the aim of this study was to determine whether there are changes in adiponectin multimers in patients with an SGA neonate.
Materials and methods
Study groups and inclusion criteria
A cross-sectional study was conducted including patients in the following groups: 1) women with a normal pregnancy (n=234); and 2) patients with an SGA neonate (n=78). The study population was further stratified by first trimester body mass index (BMI: normal weight 18.5–24.9 kg/m2 vs. overweight/obese ≥25 kg/m2).
Samples and data were retrieved from our bank of biological samples and clinical databases. Many of these samples have previously been employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Written informed consent was obtained from all participants after approval by the Institutional Review Boards of the Sotero del Rio Hospital (Chile), Wayne State University (Detroit, Michigan, USA) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD; Bethesda, Maryland, USA).
Clinical Definitions
The inclusion criteria for women with a normal pregnancy were: singleton gestation, no prior diabetes mellitus, no maternal or fetal complications during pregnancy, normal plasma glucose concentrations in the first trimester, normal oral glucose challenge test, [1] and delivery at term of a healthy neonate with a birthweight above the 10th percentile for gestational age.[6, 41] The diagnosis of SGA was based on ultrasonographic estimated fetal weight and confirmed by a birth weight below the 10th percentile for gestational age.[6, 41]
The BMI was calculated according to the formula: weight (kg)/height (m2). Normal weight women were defined as those with a BMI of 18.5–24.9 kg/m2 according to the definition of the World Health Organization (WHO)[2] Pregnant women were classified by their first trimester BMI into two groups: normal weight and overweight/obese (BMI ≥25 kg/m2).
Sample collection and quantitative determination of maternal serum adiponectin multimers
Maternal blood samples were collected with a vacutainer into tubes. Samples were centrifuged and the sera were stored at −80°C until analysis. Sensitive enzyme-linked immunoassays were used to determine the concentrations of adiponectin multimeric forms in maternal serum. Immunoassays kits were purchased from ALPCO Diagnostics (47-ADPHU-E01, Salem, NH, USA). The assays were run according to the manufacturer’s recommendations. Maternal serum plasma samples that treated with SDS-containing acid buffer to convert multimeric adiponectin to a dimmer form were assayed to determine total adiponectin concentrations. To detect HMW adiponectin, serum samples were pretreated with a specific protease that selectively digested MMW and LMW adiponectin and than treated with the SDS-buffer that also stopped the digestion reaction. We were also able to determine the combined HMW and MMW adiponectin concentrations by pretreating the samples with a protease that specifically digested LMW adiponectin. Briefly, recombinant adiponectin (standards) and SDS-buffer-treated or protease-pretreated maternal serum samples were incubated in duplicate wells of the micro titer plates, which had been pre-coated with a monoclonal antibody specific for adiponectin. During this incubation any adiponectin present in the standards and SDS-buffer-treated or protease-pretreated maternal serum samples was bound by the immobilized antibodies. After repeated washing and aspiration to remove all unbound substances, an enzyme-linked polyclonal antibody specific for adiponectin was added to the wells. Unbound materials were removed with repeated washing and a substrate solution was added to the wells and color developed in proportion to the amount of adiponectin bound in the initial step. The color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA, USA). The concentration of adiponectin in SDS-buffer-treated or protease-pretreated maternal serum samples was determined by interpolation from individual standard curves composed of human adiponectin. Total, HMW, and HMW-MMW adiponectin concentrations were derived directly from the assay plates. MMW adiponectin concentrations were obtained by subtracting HMW adiponectin value from the combined HMW-MMW value. Finally, the LMW adiponectin value was computed by subtracting HMW and MMW adiponectin values from the total adiponectin values. The calculated inter- and intra-assay coefficients of variation for adiponectin multimer immunoassays in our laboratory were 2.2% and 4.2%, respectively. The sensitivity was calculated to be 0.04 ng/ml.
Statistical analysis
Normality of the data was tested using the Kolmogorov-Smirnov test. Since serum multimeric adiponectin isoforms concentrations were not normally distributed, Mann-Whitney U tests were used for comparisons of continuous variables. Comparison of proportions was performed with Chi-square tests. Multiple linear regression analysis was performed to determine which factors were significantly and independently associated with maternal serum adiponectin isoforms concentrations as well as their relative distribution. Due to skewed distribution of the data, logarithmic (log) transformation was employed in the latter analysis. The following parameters were included in the model: maternal age, maternal first trimester BMI, gestational age at blood sampling, gestational age at delivery, and the presence of SGA. A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 14 (SPSS Inc., Chicago, IL, USA).
Results
The demographic and clinical characteristics of women with a normal pregnancy and those with an SGA neonate are displayed in Table 1. Women with a normal pregnancy had a higher median maternal age. There were no significant differences in parity, gestational age at blood sampling, gestational age at delivery and first trimester BMI between patients with a normal pregnancy and those with an SGA neonate (Table 1). Table 2 displays the demographic and clinical characteristics of the study population according to BMI. Among women with a normal pregnancy, those with normal weight were younger than overweight/obese patients. There were no significant differences in parity, gestational age at blood sampling, gestational age at delivery and neonatal birthweight between normal weight and overweight/obese women in both groups (Table 2).
Table 1.
Clinical and demographic characteristics of the study population
| Normal Pregnancy (n=234) | SGA (n=78) | p | |
|---|---|---|---|
| Maternal age (years) | 28.0 (22.0–32.0) | 24.0 (19.0–31.8) | 0.004 |
| Parity | 1 (0–2) | 2 (1–3) | NS |
| First trimester BMI (kg/m2) | 24.9 (22.8–27.3) | 25.9 (22.6–30.9) | NS |
| Gestational age at blood sampling (weeks) | 32.7 (28.7–39.3) | 34.5 (27.8–38.5) | NS |
| Gestational age at delivery (weeks) | 39.9 (39.0–40.4) | 38.5 (37.2–39.5) | NS |
| Birth weight (g) | 3470 (3220–3700) | 2452 (2140–2651) | <0.001 |
Values are expressed as median (interquartile range); SGA: Small for Gestational Age; NS: Not significant; BMI: Body Mass Index
Table 2.
Clinical and demographic characteristics of the study population according to body mass index
| Normal Pregnancy | p | SGA | p | |||
|---|---|---|---|---|---|---|
| Normal Weight (n=118) | Overweight/Obese (n=116) | Normal Weight (n=35) | Overweight/Obese (n=43) | |||
| Maternal age (years) | 26.5 (21.0–31.0) | 29.0 (24.0–34.0) | <0.01 | 21.0 (18.2–29.7) | 24.0 (20.0–32.0) | NS |
| Parity | 1 (0–2) | 1 (1–2) | NS | 2 (1–3) | 2 (1–3) | NS |
| First trimester BMI (kg/m2) | 22.7 (21.4–23.8) | 27.3 (25.9–30.3) | <0.01 | 22.1 (20.2–23.5) | 30.6 (28.6–32.2) | <0.01 |
| Gestational age at blood sampling (weeks) | 33.7 (31.2–40.0) | 31.5 (27.3–33.8) | NS | 33.8 (28.0–37.5) | 35.0 (27.0–38.6) | NS |
| Gestational age at delivery (weeks) | 40.0 (39.1–40.2) | 39.8 (38.8–40.5) | NS | 36.0 (32.7–39.0) | 38.6 (37.3–40.0) | NS |
| Birth weight (grams) | 3420 (3205–3690) | 3505 (3245–3735) | NS | 2422 (1813–2643) | 2455 (2245–2650) | NS |
Values are expressed as median (interquartile range); SGA: Small for Gestational Age; NS: Not significant; BMI: Body Mass Index
Adiponectin multimers concentrations and their relative distribution in SGA vs. normal pregnancy
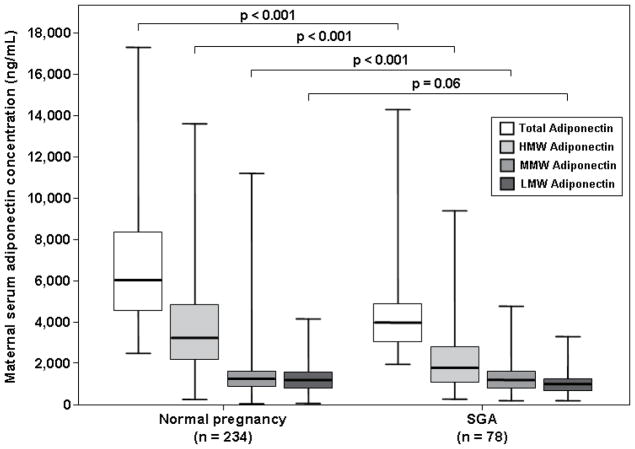
The median maternal serum concentration of total adiponectin was lower in patients with an SGA neonate than that of women with a normal pregnancy (median: 3,961 ng/mL, interquartile range [IQR] 3,075–4,958 vs. 6,070 ng/mL, IQR 4,640–8,240; p<0.001, Figure 1). Similarly, patients with an SGA neonate had lower median serum concentrations of HMW (1,575 ng/mL, IQR 973–2,600 vs. 3,312 ng/mL, IQR 2,236–4,773; p<0.001, Figure 1) and MMW adiponectin (1,062 ng/mL, IQR 775–1,361 vs. 1,355 ng/mL, IQR 913–1,804; p<0.001, Figure 1) than women with a normal pregnancy. The median maternal serum concentration of LMW adiponectin did not differ significantly between patients with an SGA neonate and women with a normal pregnancy (1,120 ng/mL, IQR 811–1,493 vs. 1,272 ng/mL, IQR 870–1,722; respectively; p=0.06), Figure 1).
Figure 1. Comparison of the median serum total, HMW, MMW and LMW adiponectin concentrations between women with normal pregnancies and those with an SGA neonate.
The median maternal serum concentrations of total, HMW and MMW adiponectin were lower in patients with an SGA neonate than that of those with a normal pregnancy. The median maternal serum concentration of LMW adiponectin did not differ significantly between patients with SGA and those with a normal pregnancy.
The median maternal HMW/total adiponectin ratio was lower in patients with an SGA neonate than in those with a normal pregnancy (0.42, IQR 0.33–0.54 vs. 0.55, IQR 0.47–0.62; p<0.001, Figure 2). In contrast, patients with an SGA neonate had higher median MMW/total adiponectin (0.27, IQR 0.20–0.35 vs. 0.22, IQR 0.16–0.28; p<0.001, Figure 2) and LMW/total adiponectin ratios (0.28, IQR 0.21–0.37 vs. 0.21, IQR 0.15–0.28; p=0.001, Figure 2) than women with a normal pregnancy.
Figure 2. Comparison of HMW/Total adiponectin MMW/Total adiponectin and LMW/Total adiponectin ratio between women with normal pregnancies and those with an SGA neonate.
The median maternal HMW/Total adiponectin ratio was lower in patients with an SGA neonate than that of those with a normal pregnancy. In contrast, patients with an SGA neonate had higher median MMW/Total adiponectin and LMW/Total adiponectin ratios than that of those with a normal pregnancy.
Adiponectin multimers concentrations and their relative distribution in normal weight pregnant women: normal pregnancy vs. SGA
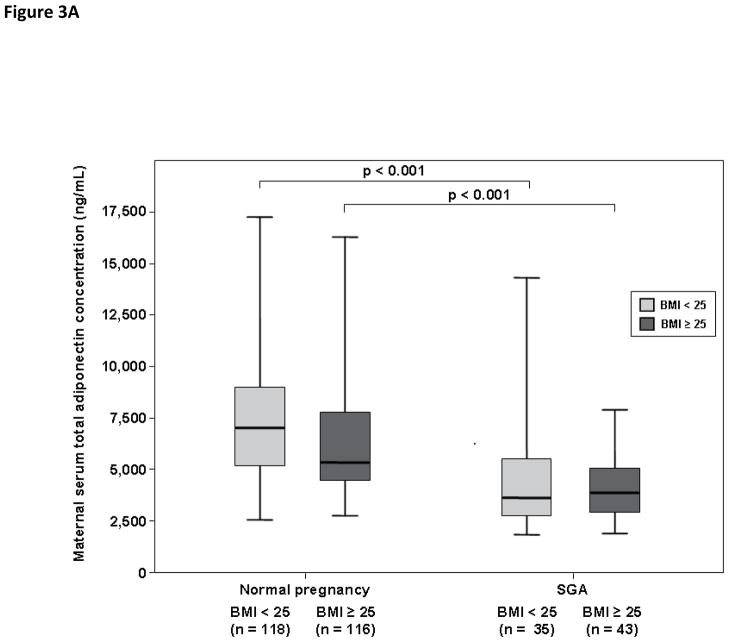
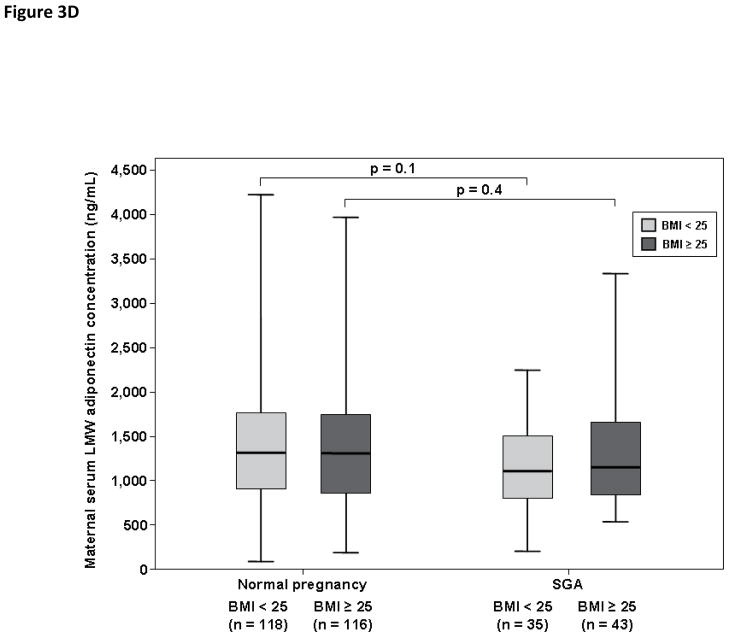
Among pregnant women with a normal weight, patients with an SGA neonate had lower median serum concentrations of total adiponectin (3,993 ng/mL, IQR 2,869–5,515 vs. 6,859 ng/mL, IQR 4,982–8,695; p<0.001, Figure 3A), HMW adiponectin (1,717 ng/mL, IQR 878–2,609 vs. 3,910 ng/mL, IQR 2,467–5,134; p<0.001, Figure 3B) and MMW adiponectin (1,025 ng/mL, IQR 775–1,376 vs. 1,411 ng/mL, IQR 961–1,911; p=0.006, Figure 3C) than women with a normal pregnancy. There was no significant difference in the median maternal serum concentrations of LMW adiponectin between patients with an SGA neonate and those with a normal pregnancy (1,145 ng/mL, IQR 788–1,490 vs. 1,295 ng/mL, IQR 915–1,731; respectively; p=0.1, Figure 3D).
Figure 3. Maternal serum total adiponectin (A), HMW adiponectin (B), MMW adiponectin (C) and LMW adiponectin (D) concentrations in women with normal pregnancies and patients with an SGA neonate according to first trimester BMI (normal weight vs. overweight/obese).
Among patients with an SGA neonate, normal weigh pregnant women and those with overweight/obesity did not differ in the median serum concentrations of total, HMW, MMW, and LMW adiponectin. Among patients with a normal weight, the median serum concentrations of total (A), HMW (B), and MMW (C) adiponectin were higher in pregnant women with a normal pregnancy than that of those with an SGA neonate. Similar findings were found among patients with overweight/obesity. The median maternal serum concentration of LMW did not differ significantly between pregnant women with a normal pregnancy and those with an SGA neonate regardless of maternal first trimester BMI category (D).
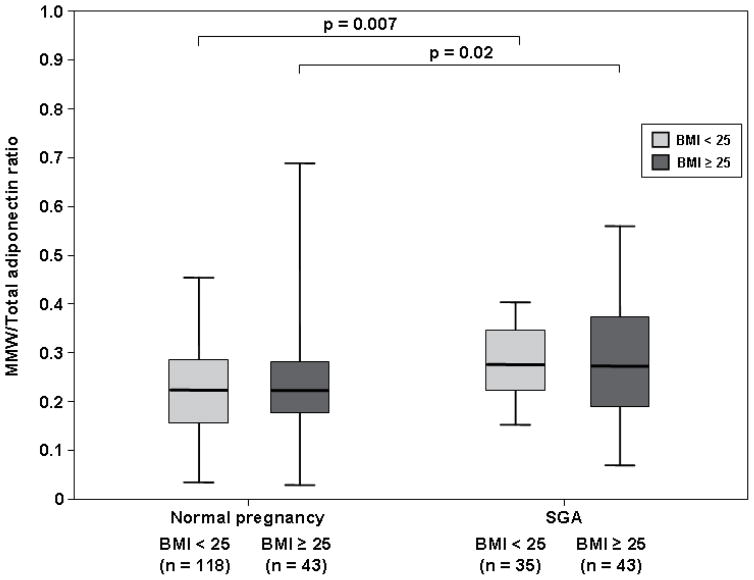
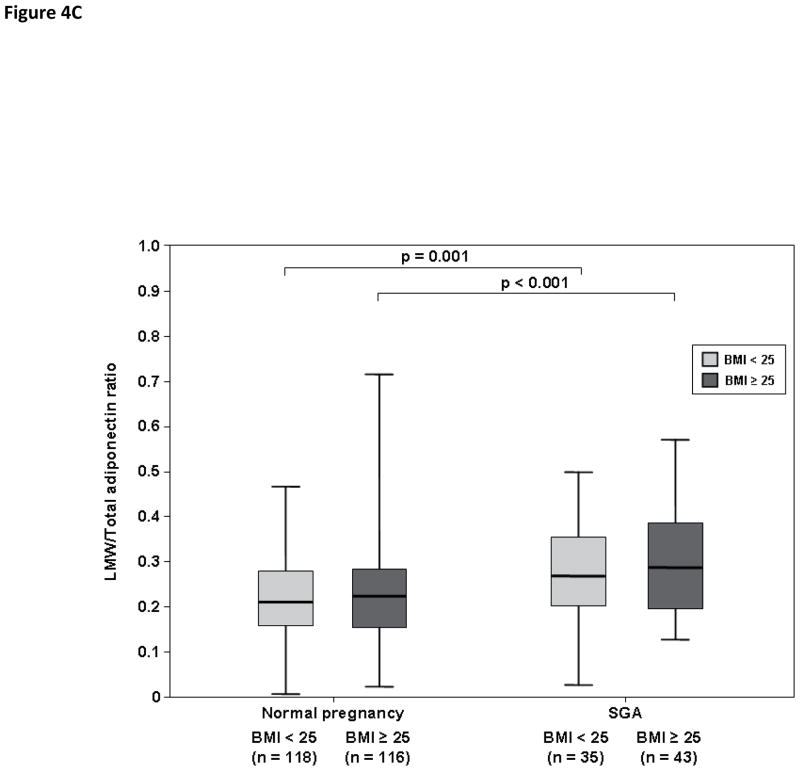
Among normal weight women, patients with an SGA neonate had a lower median HMW/total adiponectin ratio than those with a normal pregnancy (0.45, IQR 0.33–0.55 vs. 0.56, IQR 0.49–0.63; p<0.001, Figure 4A). In contrast, the median MMW/total adiponectin (0.26, IQR 0.22–0.33 vs. 0.23, IQR 0.15–0.29; p=0.007, Figure 4B) and LMW/total adiponectin ratios (0.27, IQR 0.21–0.36 vs. 0.21, IQR 0.15–0.27; p=0.001, Figure 4C) were higher in patients with an SGA neonate than in those with a normal pregnancy.
Figure 4. Comparison of HMW/Total adiponectin (A) MMW/Total adiponectin (B) and LMW/Total adiponectin (C) ratios between women with a normal pregnancy and those with an SGA neonate according to first trimester BMI (normal weight vs. overweight/obese).
Among pregnant women with an SGA neonate, normal weight patients and those with overweight/obesity had comparable median HMW/Total adiponectin, MMW/Total adiponectin and LMW/Total adiponectin ratios. Both normal weight and overweight/obese women with a normal pregnancy had a higher HMW/Total adiponectin ratio than that of those with an SGA neonate (A). In contrast, MMW/Total adiponectin (B) and LMW/Total adiponectin ratios (C) were lower in pregnant women with a normal pregnancy than that of those with an SGA neonate, regardless of first trimester maternal BMI category.
Adiponectin multimers concentrations and their relative distribution in overweight/obese women: normal pregnancy vs. SGA
Among pregnant women with overweight/obesity, patients with an SGA neonate had lower median serum concentrations of total adiponectin (3,950 ng/mL, IQR 3,086–4,939 vs. 5,434 ng/mL, IQR 4,481–7,732; p<0.001, Figure 3A), HMW adiponectin (1,526 ng/mL, IQR 1,112–2,624 vs. 3,019 ng/mL, IQR 2,133–4,405; p<0.001, Figure 3B) and MMW adiponectin (1,080 ng/mL, IQR 695–1,387 vs. 1,344 ng/mL, IQR 898–1,722; p=0.01, Figure 3C) than women with a normal pregnancy. There was no significant difference in the median maternal serum concentrations of LMW adiponectin (1,130 ng/mL, IQR 824–1,622 vs. 1,265 ng/mL, IQR 826–1,719; respectively; p=0.4, Figure 3D) between patients with an SGA neonate and those with a normal pregnancy.
Overweight/obese women with an SGA neonate had a lower HMW/total adiponectin ratio than those with a normal pregnancy (0.42, IQR 0.32–0.55 vs. 0.54, IQR 0.46–0.62; p<0.001, Figure 4A). In contrast, MMW/total adiponectin (0.27, IQR 0.19–0.36 vs. 0.22, IQR 0.18–0.28; p=0.02, Figure 4B) and LMW/total adiponectin (0.28, IQR 0.20–0.39 vs. 0.22, IQR 0.15–0.28; p<0.001, Figure 4C) ratios were higher in overweight/obese pregnant women with an SGA neonate than in those with a normal pregnancy.
Adiponectin multimers concentrations and their relative distribution in women with an SGA neonate: normal weight vs. overweight/obesity
Among patients with an SGA neonate, normal weight pregnant women and those with overweight/obesity did not differ significantly in the median serum concentrations of total adiponectin (p=0.98, Figure 3A), HMW adiponectin (p=0.7, Figure 3B), MMW adiponectin (p=0.9, Figure 3C) and LMW adiponectin (p=0.7, Figure 3D). Similarly, patients with an SGA neonate with normal weight and those with overweight/obesity had comparable median HMW/total adiponectin (p=0.4, Figure 4A), MMW/total adiponectin (p=0.8, Figure 4B), and LMW/total adiponectin (p=0.8, Figure 4C) ratios.
Linear regression analysis was used to examine the contribution of the presence of SGA on the serum concentration of adiponectin isoforms, while adjusting for maternal age, maternal first trimester BMI, gestational age at blood sampling, and gestational age at delivery. The final regression model suggested that the presence of SGA and first trimester BMI were independently associated with low maternal serum concentrations of total adiponectin (p<0.001 and p=0.01, respectively), and HMW adiponectin (p<0.001 and p=0.002, respectively). In addition, only the presence of SGA was independently associated with low maternal serum concentrations of MMW adiponectin (p=0.04), and HMW/total adiponectin ratio (p<0.001) as well as with high MMW/total adiponectin (p=0.001) and LMW/total adiponectin ratios (p<0.001).
Discussion
Principal findings of the study
1) The median maternal serum concentrations of total, HMW and MMW adiponectin were significantly lower in patients with an SGA neonate than that of those with normal pregnancies; 2) patients with an SGA neonate had a significantly lower median HMW/total adiponectin ratio and higher median MMW/total adiponectin and LMW/total adiponectin ratios than those with a normal pregnancy; 3) among patients with an SGA neonate, neither maternal serum concentrations of adiponectin multimers, nor their relative distribution differed between normal weight and overweight/obese patients; and 4) the presence of SGA was independently associated with a low maternal serum total, HMW, and MMW adiponectin concentration, as well as with low HMW/total adiponectin ratio and high MMW/total adiponectin and LMW/total adiponectin ratios.
What is the rationale to assess maternal circulating adiponectin in patients with SGA fetuses?
The rationale to determine the association between maternal circulating adiponectin concentrations and fetal growth hinges on the following findings: 1) Adiponectin is an important mediator of several biological processes that have been implicated in the pathophysiology of SGA. Adiponectin has insulin sensitizing, [12, 28] anti-atherogenic, [26, 38, 64, 94] anti-inflammatory, [25, 51, 114] and angiogenic [14, 16, 100] properties, as well as vasculature protective effects.[74, 93, 96–98, 101] This unique and diverse combination of biological activities made adiponectin an attractive candidate to account for both physiologic adaptations and pathologic states. Indeed, SGA has been associated with an anti-angiogenic state, [15, 21, 22, 30, 34, 40, 42, 111, 118, 139, 142, 146] an exaggerated intravascular pro-inflammatory response, [40, 52, 66, 115, 120, 125, 132] and hypertension;[128, 129] and 2) a growing body of evidence supports a role for adiponectin in complications of pregnancy, including those closely associated with SGA, such as preeclampsia. Indeed, preeclampsia is associated with altered maternal adiponectin concentrations[29, 32, 46, 47, 54, 70, 88, 90, 107, 131] and low circulating maternal adiponectin concentrations have been reported in GDM;[10, 59, 108, 133, 150] and overweight pregnant patients.[91]
Modifications of maternal serum adiponectin multimers – functional implications
A solid body of evidence suggests that the biological effects of adiponectin are determined not only by the absolute concentrations of adiponectin multimeric complexes, but, to a large extent, by the relative distribution of its isoforms.[9, 13, 61, 103, 104, 135, 138, 144] Moreover, each isoform has different biological effects. This view is supported by the following findings: 1) in vitro, HMW and MMW adiponectin have pro-inflammatory effects such as induction of IL-6 secretion by human monocytes and activation of nuclear factor (NF)-κB;[4, 45, 112, 138] 2) LMW adiponectin inhibits the release of IL-6, [89, 121] and increases the secretion of IL-10, [89] an anti-inflammatory cytokine; 3) administration of HMW adiponectin multimers to adiponectin knock-out mice results in a dose-dependent reduction in serum glucose concentrations.[104] This effect could not be reproduced by the administration of LMW adiponectin; and 4) plasma HMW/total adiponectin ratio has a better correlation with insulin resistance indices than total adiponectin concentrations. Collectively, these data suggest a structure-function relationship of adiponectin multimers. Importantly, the post-translational modifications resulting in the various adiponectin isoforms, are performed exclusively by adipocytes.[147, 148] Indeed, none of the multimeric forms interchange with each other after their secretion.[103] Thus, the dysregulation of adiponectin multimers can be viewed as an index for adipose tissue dysfunction.
Pregnancy complicated by an SGA neonate – a state of adipose tissue dysregulation?
The present report is the first study explicitly designed to compare maternal serum concentrations of adiponectin multimers between normal pregnant women and those who delivered an SGA neonate. The findings that the median maternal serum concentrations of total, HMW and MMW adiponectin are lower in patients with SGA neonates than in those with a normal pregnancy are novel. The lower total adiponectin concentrations in patients with SGA neonates is the consequence of a significant lowering in maternal serum HMW adiponectin concentrations, as evidenced by the low HMW/total adiponectin ratio, yet high MMW/total and LMW/total adiponectin ratios. The shift from the HMW adiponectin isoform to MMW and LMW adiponectin multimers may have functional implications since the metabolic effects of adiponectin are primarily mediated by HMW adiponectin.
The finding concerning low total adiponectin in patients with SGA fetuses is in agreement with that of Kyriakakou et al[67] who reported lower maternal serum concentrations of total adiponectin in 20 patients when compared to SGA than in 20 controls. Consistent with the latter report, Ong et al.[95] reported a negative correlation between neonatal birth weight and maternal total adiponectin, HMW adiponectin and HMW/total adiponectin ratio in 58 women with normal pregnancies and AGA neonates. The results of the present study extend the previous studies by demonstrating that the low maternal serum total adiponectin concentration is the result of a selective lowering in HMW adiponectin. Moreover, we were able to show that low maternal serum adiponectin concentrations can be detected in patients with SGA in a wide range of gestational age, and that are a feature of normal weight as well as overweight/obese patients. Our findings are in contrast with those of Verhaeghe et al.[143] Fasshauer et al.[35] and Savvidou et al.[118] who reported similar maternal circulating total adiponectin concentrations in patients with SGA neonates and those with a normal pregnancy. Differences in study design, definition of SGA, and clinical characteristics of the study population can explain the disparity among studies. In particular, our study included a large group of mothers with SGA fetuses, at a wider range of gestational ages and a relatively higher rate of overweight/obese patients.
Why do patients with SGA fetuses have low serum concentrations of total and HMW adiponectin concentrations?
The alteration in maternal serum adiponectin multimers reported herein favors insulin resistance. In such observational study it is difficult to unravel a cause and effect relationship between circulating maternal adiponectin isoforms concentrations and the delivery of an SGA neonate. However, it is tempting to postulate that these changes are aimed at meeting the metabolic demands of the small fetus. Indeed, it has been proposed that the insulin-sensitizing properties of adiponectin are mediated specifically by the HMW isoforms.[11, 37, 44, 55, 61, 87, 104]
An alternative explanation can be that the low adiponectin concentrations are not an adaptive metabolic response to a primary insult that resulted in an SGA fetus, but rather a consequence of this primary insult. Pregnancies complicated by an SGA neonate are associated with an anti-angiogenic state and an intravascular pro-inflammatory response. Since adiponectin has anti-inflammatory and angiogenic properties, it is conceivable that its low concentration is a reflection of these underlying mechanisms. Indeed, low circulating adiponectin concentrations have been reported in other conditions associated with pro-inflammatory state such as overweight/obesity[3, 8, 48, 58, 91] and systemic lupus erythematosus, [113, 116] diseases characterized by impairment in vasculature integrity such as atherosclerosis[99, 106] and hypertension, [51, 57, 101] and those with anti-angiogenic states such as preeclampsia.[29, 31, 32, 49, 102]
Adiponectin multimers in normal and overweight/obese patients with SGA fetuses – evidence for altered regulation of adiponectin multimers
In contrast to normal pregnancy, [8, 48, 75, 91] overweight/obese patients with SGA neonates did not have lower serum concentrations of total adiponectin or its multimers compared to normal weight patients with SGA. Similarly, overweight/obese and normal weight patients with SGA did not differ in the relative distribution of adiponectin isoforms. These findings suggest a genuine association between the presence of SGA and alterations in maternal circulating adiponectin, regardless of the presence of obesity. This is further supported by the fact that the presence of SGA was independently associated with a low maternal serum concentration of HMW adiponectin, as well as with a low HMW/total adiponectin ratio. Collectively, these findings suggest altered regulation of maternal serum adiponectin multimeric complexes in the presence of SGA.
In conclusion, the present study is the first to determine maternal serum adiponectin multimers concentrations in patients with SGA neonates. The findings reported herein suggest that maternal adipose tissue plays an important and hitherto unrecognized role in the pathogenesis of SGA. These observations lend support to the hypothesis that adipokines play an important role not only in the physiologic adaptation of human gestation but also in complications of pregnancy. The implicit promise of adipokines research during normal and abnormal pregnancy is that lifestyle or pharmacologic interventions may be helpful in the prevention and/or treatment of pregnancy complications such as the delivery of an SGA neonate.
Acknowledgments
This research was supported by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.ACOG Practice Bulletin. Clinical management guidelines for obstetrician-gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994). Gestational diabetes. Obstet Gynecol. 2001;98:525–538. [PubMed] [Google Scholar]
- 2.Diet, nutrition and the prevention of chronic diseases. World Health Organ Tech Rep Ser. 2003;916:i–149. backcover. [PubMed] [Google Scholar]
- 3.Abbasi F, Chu JW, Lamendola C, McLaughlin T, Hayden J, Reaven GM, et al. Discrimination between obesity and insulin resistance in the relationship with adiponectin. Diabetes. 2004;53:585–590. doi: 10.2337/diabetes.53.3.585. [DOI] [PubMed] [Google Scholar]
- 4.Abke S, Neumeier M, Weigert J, Wehrwein G, Eggenhofer E, Schaffler A, et al. Adiponectin-induced secretion of interleukin-6 (IL-6), monocyte chemotactic protein-1 (MCP-1, CCL2) and interleukin-8 (IL-8, CXCL8) is impaired in monocytes from patients with type I diabetes. Cardiovasc Diabetol. 2006;5:1–8. doi: 10.1186/1475-2840-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahima RS, Flier JS. Adipose tissue as an endocrine organ. Trends Endocrinol Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- 6.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 7.Andreasen KR, Andersen ML, Schantz AL. Obesity and pregnancy. Acta Obstet Gynecol Scand. 2004;83:1022–1029. doi: 10.1111/j.0001-6349.2004.00624.x. [DOI] [PubMed] [Google Scholar]
- 8.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 9.Aso Y, Yamamoto R, Wakabayashi S, Uchida T, Takayanagi K, Takebayashi K, et al. Comparison of serum high-molecular weight (HMW) adiponectin with total adiponectin concentrations in type 2 diabetic patients with coronary artery disease using a novel enzyme-linked immunosorbent assay to detect HMW adiponectin. Diabetes. 2006;55:1954–1960. doi: 10.2337/db05-1525. [DOI] [PubMed] [Google Scholar]
- 10.Ategbo JM, Grissa O, Yessoufou A, Hichami A, Dramane KL, Moutairou K, et al. Modulation of adipokines and cytokines in gestational diabetes and macrosomia. J Clin Endocrinol Metab. 2006;91:4137–4143. doi: 10.1210/jc.2006-0980. [DOI] [PubMed] [Google Scholar]
- 11.Basu R, Pajvani UB, Rizza RA, Scherer PE. Selective downregulation of the high molecular weight form of adiponectin in hyperinsulinemia and in type 2 diabetes: differential regulation from nondiabetic subjects. Diabetes. 2007;56:2174–2177. doi: 10.2337/db07-0185. [DOI] [PubMed] [Google Scholar]
- 12.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 13.Bobbert T, Rochlitz H, Wegewitz U, Akpulat S, Mai K, Weickert MO, et al. Changes of adiponectin oligomer composition by moderate weight reduction. Diabetes. 2005;54:2712–2719. doi: 10.2337/diabetes.54.9.2712. [DOI] [PubMed] [Google Scholar]
- 14.Bora PS, Kaliappan S, Lyzogubov VV, Tytarenko RG, Thotakura S, Viswanathan T, et al. Expression of adiponectin in choroidal tissue and inhibition of laser induced choroidal neovascularization by adiponectin. FEBS Lett. 2007;581:1977–1982. doi: 10.1016/j.febslet.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 15.Boutsikou T, Malamitsi-Puchner A, Economou E, Boutsikou M, Puchner KP, Hassiakos D. Soluble vascular endothelial growth factor receptor-1 in intrauterine growth restricted fetuses and neonates. Early Hum Dev. 2006;82:235–239. doi: 10.1016/j.earlhumdev.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 16.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci US A. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bretelle F, Sabatier F, Blann A, D’Ercole C, Boutiere B, Mutin M, et al. Maternal endothelial soluble cell adhesion molecules with isolated small for gestational age fetuses: comparison with pre-eclampsia. BJOG. 2001;108:1277–1282. doi: 10.1111/j.1471-0528.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 18.Brosens I, Dixon HG, Robertson WB. Fetal growth retardation and the arteries of the placental bed. Br J Obstet Gynaecol. 1977;84:656–663. doi: 10.1111/j.1471-0528.1977.tb12676.x. [DOI] [PubMed] [Google Scholar]
- 19.Catalano PM. Management of obesity in pregnancy. Obstet Gynecol. 2007;109:419–433. doi: 10.1097/01.AOG.0000253311.44696.85. [DOI] [PubMed] [Google Scholar]
- 20.Catalano PM, Hoegh M, Minium J, Huston-Presley L, Bernard S, Kalhan S, et al. Adiponectin in human pregnancy: implications for regulation of glucose and lipid metabolism. Diabetologia. 2006;49:1677–1685. doi: 10.1007/s00125-006-0264-x. [DOI] [PubMed] [Google Scholar]
- 21.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, et al. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaiworapongsa T, Romero R, Gotsch F, Espinoza J, Nien JK, Goncalves L, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan TF, Chen YL, Lee CH, Chou FH, Wu LC, Jong SB, et al. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J Soc Gynecol Investig. 2006;13:364–367. doi: 10.1016/j.jsgi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Chan TF, Yuan SS, Chen HS, Guu CF, Wu LC, Yeh YT, et al. Correlations between umbilical and maternal serum adiponectin levels and neonatal birthweights. Acta Obstet Gynecol Scand. 2004;83:165–169. doi: 10.1111/j.0001-6349.2004.0298.x. [DOI] [PubMed] [Google Scholar]
- 25.Chandran M, Phillips SA, Ciaraldi T, Henry RR. Adiponectin: more than just another fat cell hormone? Diabetes Care. 2003;26:2442–2450. doi: 10.2337/diacare.26.8.2442. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Montagnani M, Funahashi T, Shimomura I, Quon MJ. Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J Biol Chem. 2003;278:45021–45026. doi: 10.1074/jbc.M307878200. [DOI] [PubMed] [Google Scholar]
- 27.Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:147–152. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- 28.Combs TP, Berg AH, Obici S, Scherer PE, Rossetti L. Endogenous glucose production is inhibited by the adipose-derived protein Acrp30. J Clin Invest. 2001;108:1875–1881. doi: 10.1172/JCI14120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cortelazzi D, Corbetta S, Ronzoni S, Pelle F, Marconi A, Cozzi V, et al. Maternal and foetal resistin and adiponectin concentrations in normal and complicated pregnancies. Clin Endocrinol (Oxf) 2007;66:447–453. doi: 10.1111/j.1365-2265.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 30.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–207. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 31.D’Anna R, Baviera G, Corrado F, Giordano D, De VA, Nicocia G, et al. Adiponectin and insulin resistance in early- and late-onset pre-eclampsia. BJOG. 2006;113:1264–1269. doi: 10.1111/j.1471-0528.2006.01078.x. [DOI] [PubMed] [Google Scholar]
- 32.D’Anna R, Baviera G, Corrado F, Giordano D, Di Benedetto A, Jasonni VM. Plasma adiponectin concentration in early pregnancy and subsequent risk of hypertensive disorders. Obstet Gynecol. 2005;106:340–344. doi: 10.1097/01.AOG.0000168441.79050.03. [DOI] [PubMed] [Google Scholar]
- 33.Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal-weight women: effects of gestational weight change. Obstet Gynecol. 1996;87:389–394. doi: 10.1016/0029-7844(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 34.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern Fetal Neonatal Med. 2008;21:279–287. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fasshauer M, Bluher M, Stumvoll M, Tonessen P, Faber R, Stepan H. Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental function. Clin Endocrinol (Oxf) 2007;66:434–439. doi: 10.1111/j.1365-2265.2007.02751.x. [DOI] [PubMed] [Google Scholar]
- 36.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, et al. Adiponectin gene expression and secretion is inhibited by interleukin-6 in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2003;301:1045–1050. doi: 10.1016/s0006-291x(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 37.Fisher FF, Trujillo ME, Hanif W, Barnett AH, McTernan PG, Scherer PE, et al. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia. 2005;48:1084–1087. doi: 10.1007/s00125-005-1758-7. [DOI] [PubMed] [Google Scholar]
- 38.Funahashi T, Nakamura T, Shimomura I, Maeda K, Kuriyama H, Takahashi M, et al. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern Med. 1999;38:202–206. doi: 10.2169/internalmedicine.38.202. [DOI] [PubMed] [Google Scholar]
- 39.Gerretsen G, Huisjes HJ, Elema JD. Morphological changes of the spiral arteries in the placental bed in relation to pre-eclampsia and fetal growth retardation. Br J Obstet Gynaecol. 1981;88:876–881. doi: 10.1111/j.1471-0528.1981.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 40.Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–2175. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, et al. A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000. Rev Med Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 42.Gotsch F, Romero R, Kusanovic JP, Chaiworapongsa T, Dombrowski M, Erez O, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haider DG, Handisurya A, Storka A, Vojtassakova E, Luger A, Pacini G, et al. Visfatin response to glucose is reduced in women with gestational diabetes mellitus. Diabetes Care. 2007;30:1889–1891. doi: 10.2337/dc07-0013. [DOI] [PubMed] [Google Scholar]
- 44.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, et al. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 45.Haugen F, Drevon CA. Activation of nuclear factor-kappaB by high molecular weight and globular adiponectin. Endocrinology. 2007;148:5478–5486. doi: 10.1210/en.2007-0370. [DOI] [PubMed] [Google Scholar]
- 46.Haugen F, Ranheim T, Harsem NK, Lips E, Staff AC, Drevon CA. Increased plasma levels of adipokines in preeclampsia: relationship to placenta and adipose tissue gene expression. Am J Physiol Endocrinol Metab. 2006;290:E326–E333. doi: 10.1152/ajpendo.00020.2005. [DOI] [PubMed] [Google Scholar]
- 47.Hendler I, Blackwell SC, Mehta SH, Whitty JE, Russell E, Sorokin Y, et al. The levels of leptin, adiponectin, and resistin in normal weight, overweight, and obese pregnant women with and without preeclampsia. Am J Obstet Gynecol. 2005;193:979–983. doi: 10.1016/j.ajog.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 48.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 49.Ichida K, Moriyama T, Morita H, Kondo T, Yoshida S, Ohara N, et al. Plasma adiponectin concentrations and placental adiponectin expression in pre-eclamptic women. Gynecol Endocrinol. 2007;23:238–243. doi: 10.1080/09513590701297740. [DOI] [PubMed] [Google Scholar]
- 50.Inami I, Okada T, Fujita H, Makimoto M, Hosono S, Minato M, et al. Impact of serum adiponectin concentration on birth size and early postnatal growth. Pediatr Res. 2007;61:604–606. doi: 10.1203/pdr.0b013e3180459f8a. [DOI] [PubMed] [Google Scholar]
- 51.Iwashima Y, Katsuya T, Ishikawa K, Ouchi N, Ohishi M, Sugimoto K, et al. Hypoadiponectinemia is an independent risk factor for hypertension. Hypertension. 2004;43:1318–1323. doi: 10.1161/01.HYP.0000129281.03801.4b. [DOI] [PubMed] [Google Scholar]
- 52.Johnston TA, Greer IA, Dawes J, Calder AA. Neutrophil activation in small for gestational age pregnancies. Br J Obstet Gynaecol. 1991;98:105–106. doi: 10.1111/j.1471-0528.1991.tb10321.x. [DOI] [PubMed] [Google Scholar]
- 53.Kajantie E, Hytinantti T, Hovi P, Andersson S. Cord plasma adiponectin: a 20-fold rise between 24 weeks gestation and term. J Clin Endocrinol Metab. 2004;89:4031–4036. doi: 10.1210/jc.2004-0018. [DOI] [PubMed] [Google Scholar]
- 54.Kajantie E, Kaaja R, Ylikorkala O, Andersson S, Laivuori H. Adiponectin concentrations in maternal serum: elevated in preeclampsia but unrelated to insulin sensitivity. J Soc Gynecol Investig. 2005;12:433–439. doi: 10.1016/j.jsgi.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 55.Katsuki A, Suematsu M, Gabazza EC, Murashima S, Nakatani K, Togashi K, et al. Decreased high-molecular weight adiponectin-to-total adiponectin ratio in sera is associated with insulin resistance in Japanese metabolically obese, normal-weight men with normal glucose tolerance. Diabetes Care. 2006;29:2327–2328. doi: 10.2337/dc06-1239. [DOI] [PubMed] [Google Scholar]
- 56.Kautzky-Willer A, Pacini G, Tura A, Bieglmayer C, Schneider B, Ludvik B, et al. Increased plasma leptin in gestational diabetes. Diabetologia. 2001;44:164–172. doi: 10.1007/s001250051595. [DOI] [PubMed] [Google Scholar]
- 57.Kazumi T, Kawaguchi A, Sakai K, Hirano T, Yoshino G. Young men with high-normal blood pressure have lower serum adiponectin, smaller LDL size, and higher elevated heart rate than those with optimal blood pressure. Diabetes Care. 2002;25:971–976. doi: 10.2337/diacare.25.6.971. [DOI] [PubMed] [Google Scholar]
- 58.Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–1785. doi: 10.2337/diabetes.52.7.1779. [DOI] [PubMed] [Google Scholar]
- 59.Kinalski M, Telejko B, Kuzmicki M, Kretowski A, Kinalska I. Tumor necrosis factor alpha system and plasma adiponectin concentration in women with gestational diabetes. Horm Metab Res. 2005;37:450–454. doi: 10.1055/s-2005-870238. [DOI] [PubMed] [Google Scholar]
- 60.Kirwan JP, Hauguel-De MS, Lepercq J, Challier JC, Huston-Presley L, Friedman JE, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–2213. doi: 10.2337/diabetes.51.7.2207. [DOI] [PubMed] [Google Scholar]
- 61.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, et al. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kotani Y, Yokota I, Kitamura S, Matsuda J, Naito E, Kuroda Y. Plasma adiponectin levels in newborns are higher than those in adults and positively correlated with birth weight. Clin Endocrinol (Oxf) 2004;61:418–423. doi: 10.1111/j.1365-2265.2004.02041.x. [DOI] [PubMed] [Google Scholar]
- 63.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin Sci (Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 64.Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, et al. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863–25866. doi: 10.1074/jbc.C200251200. [DOI] [PubMed] [Google Scholar]
- 65.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in Amniotic Fluid and its Association with Intra-amniotic Infection and Inflammation. The Journal of Maternal-Fetal and Neonatal Medicine. 2008;21:902–916. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kusanovic JP, Romero R, Hassan SS, Gotsch F, Edwin S, Chaiworapongsa T, et al. Maternal serum soluble CD30 is increased in normal pregnancy, but decreased in preeclampsia and small for gestational age pregnancies. J Matern Fetal Neonatal Med. 2007;20:867–878. doi: 10.1080/14767050701482993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kyriakakou M, Malamitsi-Puchner A, Militsi H, Boutsikou T, Margeli A, Hassiakos D, et al. Leptin and adiponectin concentrations in intrauterine growth restricted and appropriate for gestational age fetuses, neonates, and their mothers. Eur J Endocrinol. 2008;158:343–348. doi: 10.1530/EJE-07-0692. [DOI] [PubMed] [Google Scholar]
- 68.Lewandowski KC, Stojanovic N, Press M, Tuck SM, Szosland K, Bienkiewicz M, et al. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia. 2007;50:1033–1037. doi: 10.1007/s00125-007-0610-7. [DOI] [PubMed] [Google Scholar]
- 69.Lopez-Bermejo A, Fernandez-Real JM, Garrido E, Rovira R, Brichs R, Genaro P, et al. Maternal soluble tumour necrosis factor receptor type 2 (sTNFR2) and adiponectin are both related to blood pressure during gestation and infant’s birthweight. Clin Endocrinol (Oxf) 2004;61:544–552. doi: 10.1111/j.1365-2265.2004.02120.x. [DOI] [PubMed] [Google Scholar]
- 70.Lu D, Yang X, Wu Y, Wang H, Huang H, Dong M. Serum adiponectin, leptin and soluble leptin receptor in pre-eclampsia. Int J Gynaecol Obstet. 2006 doi: 10.1016/j.ijgo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- 72.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 73.Malamitsi-Puchner A, Briana DD, Boutsikou M, Kouskouni E, Hassiakos D, Gourgiotis D. Perinatal circulating visfatin levels in intrauterine growth restriction. Pediatrics. 2007;119:e1314–e1318. doi: 10.1542/peds.2006-2589. [DOI] [PubMed] [Google Scholar]
- 74.Matsuda M, Shimomura I, Sata M, Arita Y, Nishida M, Maeda N, et al. Role of adiponectin in preventing vascular stenosis. The missing link of adipo-vascular axis. J Biol Chem. 2002;277:37487–37491. doi: 10.1074/jbc.M206083200. [DOI] [PubMed] [Google Scholar]
- 75.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez OE, Gotsch F, et al. Maternal Serum Adiponectin Multimers in Preeclampsia. The Journal of Perinatal Medicine. 2009;34:349–363. doi: 10.1515/JPM.2009.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than GN, et al. Maternal visfatin concentration in normal pregnancy. Journal Of Perinatal Medicine. 2009;37:206–217. doi: 10.1515/JPM.2009.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mazaki-Tovi S, Romero R, Kusanovic JP, Vaisbuch E, Erez O, Than GN, et al. Visfatin in human pregnancy: maternal gestational diabetes vis-a-vis neonatal birthweight. Journal Of Perinatal Medicine. 2008 doi: 10.1515/JPM.2009.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Efraty Y, Schiff E, et al. Determining the source of fetal adiponectin. J Reprod Med. 2007;52:774–778. [PubMed] [Google Scholar]
- 79.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Schiff E, Sivan E. Cord blood adiponectin in large-for-gestational age newborns. Am J Obstet Gynecol. 2005;193:1238–1242. doi: 10.1016/j.ajog.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 80.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Wiser A, Schiff E, et al. Maternal serum adiponectin levels during human pregnancy. J Perinatol. 2007;27:77–81. doi: 10.1038/sj.jp.7211639. [DOI] [PubMed] [Google Scholar]
- 81.Mazaki-Tovi S, Kanety H, Pariente C, Hemi R, Yinon Y, Wiser A, et al. Adiponectin and leptin concentrations in dichorionic twins with discordant and concordant growth. J Clin Endocrinol Metab. 2009;44:892–898. doi: 10.1210/jc.2008-2118. [DOI] [PubMed] [Google Scholar]
- 82.Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr Diab Rep. 2005;5:278–281. doi: 10.1007/s11892-005-0023-2. [DOI] [PubMed] [Google Scholar]
- 83.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008 doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Vaisbuch E, Gotsch F, et al. Adiponectin multimers in maternal plasma. J Matern Fetal Neonatal Med. 2008;21:796–815. doi: 10.1080/14767050802266881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McCarthy JF, Misra DN, Roberts JM. Maternal plasma leptin is increased in preeclampsia and positively correlates with fetal cord concentration. Am J Obstet Gynecol. 1999;180:731–736. doi: 10.1016/s0002-9378(99)70280-2. [DOI] [PubMed] [Google Scholar]
- 86.Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem (Tokyo) 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- 87.Nakashima R, Kamei N, Yamane K, Nakanishi S, Nakashima A, Kohno N. Decreased total and high molecular weight adiponectin are independent risk factors for the development of type 2 diabetes in Japanese-Americans. J Clin Endocrinol Metab. 2006;91:3873–3877. doi: 10.1210/jc.2006-1158. [DOI] [PubMed] [Google Scholar]
- 88.Naruse K, Yamasaki M, Umekage H, Sado T, Sakamoto Y, Morikawa H. Peripheral blood concentrations of adiponectin, an adipocyte-specific plasma protein, in normal pregnancy and preeclampsia. J Reprod Immunol. 2005;65:65–75. doi: 10.1016/j.jri.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 89.Neumeier M, Weigert J, Schaffler A, Wehrwein G, Muller-Ladner U, Scholmerich J, et al. Different effects of adiponectin isoforms in human monocytic cells. J Leukoc Biol. 2006;79:803–808. doi: 10.1189/jlb.0905521. [DOI] [PubMed] [Google Scholar]
- 90.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Adiponectin in severe preeclampsia. J Perinat Med. 2007;35:503–512. doi: 10.1515/JPM.2007.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nien JK, Mazaki-Tovi S, Romero R, Erez O, Kusanovic JP, Gotsch F, et al. Plasma adiponectin concentrations in non-pregnant, normal and overweight pregnant women. J Perinat Med. 2007;35:522–531. doi: 10.1515/JPM.2007.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nien JK, Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, et al. Resistin: a hormone which induces insulin resistance is increased in normal pregnancy. J Perinat Med. 2007;35:513–521. doi: 10.1515/JPM.2007.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okamoto Y, Arita Y, Nishida M, Muraguchi M, Ouchi N, Takahashi M, et al. An adipocyte-derived plasma protein, adiponectin, adheres to injured vascular walls. Horm Metab Res. 2000;32:47–50. doi: 10.1055/s-2007-978586. [DOI] [PubMed] [Google Scholar]
- 94.Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- 95.Ong GK, Hamilton JK, Sermer M, Connelly PW, Maguire G, Zinman B, et al. Maternal serum adiponectin and infant birthweight: the role of adiponectin isoform distribution. Clin Endocrinol (Oxf) 2007;67:108–114. doi: 10.1111/j.1365-2265.2007.02846.x. [DOI] [PubMed] [Google Scholar]
- 96.Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- 97.Ouchi N, Kihara S, Arita Y, Nishida M, Matsuyama A, Okamoto Y, et al. Adipocyte-derived plasma protein, adiponectin, suppresses lipid accumulation and class A scavenger receptor expression in human monocyte-derived macrophages. Circulation. 2001;103:1057–1063. doi: 10.1161/01.cir.103.8.1057. [DOI] [PubMed] [Google Scholar]
- 98.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, et al. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 99.Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- 100.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, et al. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–234. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 102.Ouyang Y, Chen H, Chen H. Reduced plasma adiponectin and elevated leptin in pre-eclampsia. Int J Gynaecol Obstet. 2007;98:110–114. doi: 10.1016/j.ijgo.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 103.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 104.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, et al. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 105.Pardo IM, Geloneze B, Tambascia MA, Barros-Filho AA. Hyperadiponectinemia in newborns: relationship with leptin levels and birth weight. Obes Res. 2004;12:521–524. doi: 10.1038/oby.2004.59. [DOI] [PubMed] [Google Scholar]
- 106.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 107.Ramsay JE, Jamieson N, Greer IA, Sattar N. Paradoxical elevation in adiponectin concentrations in women with preeclampsia. Hypertension. 2003;42:891–894. doi: 10.1161/01.HYP.0000095981.92542.F6. [DOI] [PubMed] [Google Scholar]
- 108.Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83:341–347. doi: 10.1111/j.0001-6349.2004.00413.x. [DOI] [PubMed] [Google Scholar]
- 109.Romero R. The child is the father of the man. Prenat Neonat Med. 1996:8–11. [Google Scholar]
- 110.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann NY Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 111.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rovin BH, Song H. Chemokine induction by the adipocyte-derived cytokine adiponectin. Clin Immunol. 2006;120:99–105. doi: 10.1016/j.clim.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 113.Rovin BH, Song H, Hebert LA, Nadasdy T, Nadasdy G, Birmingham DJ, et al. Plasma, urine, and renal expression of adiponectin in human systemic lupus erythematosus. Kidney Int. 2005;68:1825–1833. doi: 10.1111/j.1523-1755.2005.00601.x. [DOI] [PubMed] [Google Scholar]
- 114.Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, et al. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975–981. doi: 10.1253/circj.68.975. [DOI] [PubMed] [Google Scholar]
- 115.Sabatier F, Bretelle F, D’Ercole C, Boubli L, Sampol J, gnat-George F. Neutrophil activation in preeclampsia and isolated intrauterine growth restriction. Am J Obstet Gynecol. 2000;183:1558–1563. doi: 10.1067/mob.2000.108082. [DOI] [PubMed] [Google Scholar]
- 116.Sada KE, Yamasaki Y, Maruyama M, Sugiyama H, Yamamura M, Maeshima Y, et al. Altered levels of adipocytokines in association with insulin resistance in patients with systemic lupus erythematosus. J Rheumatol. 2006;33:1545–1552. [PubMed] [Google Scholar]
- 117.Sagawa N, Yura S, Itoh H, Mise H, Kakui K, Korita D, et al. Role of leptin in pregnancy--a review. Placenta. 2002;23 (Suppl A):S80–S86. doi: 10.1053/plac.2002.0814. [DOI] [PubMed] [Google Scholar]
- 118.Savvidou MD, Sotiriadis A, Kaihura C, Nicolaides KH, Sattar N. Circulating levels of adiponectin and leptin at 23–25 weeks of pregnancy in women with impaired placentation and in those with established fetal growth restriction. Clin Sci (Lond) 2008;115:219–224. doi: 10.1042/CS20070409. [DOI] [PubMed] [Google Scholar]
- 119.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 120.Schiff E, Friedman SA, Baumann P, Sibai BM, Romero R. Tumor necrosis factor-alpha in pregnancies associated with preeclampsia or small-for-gestational-age newborns. Am J Obstet Gynecol. 1994;170:1224–1229. doi: 10.1016/s0002-9378(94)70130-x. [DOI] [PubMed] [Google Scholar]
- 121.Schober F, Neumeier M, Weigert J, Wurm S, Wanninger J, Schaffler A, et al. Low molecular weight adiponectin negatively correlates with the waist circumference and monocytic IL-6 release. Biochem Biophys Res Commun. 2007;361:968–973. doi: 10.1016/j.bbrc.2007.07.106. [DOI] [PubMed] [Google Scholar]
- 122.Schulze MB, Rimm EB, Shai I, Rifai N, Hu FB. Relationship between adiponectin and glycemic control, blood lipids, and inflammatory markers in men with type 2 diabetes. Diabetes Care. 2004;27:1680–1687. doi: 10.2337/diacare.27.7.1680. [DOI] [PubMed] [Google Scholar]
- 123.Sebire NJ, Jolly M, Harris JP, Wadsworth J, Joffe M, Beard RW, et al. Maternal obesity and pregnancy outcome: a study of 287,213 pregnancies in London. Int J Obes Relat Metab Disord. 2001;25:1175–1182. doi: 10.1038/sj.ijo.0801670. [DOI] [PubMed] [Google Scholar]
- 124.Seeds JW. Impaired fetal growth: definition and clinical diagnosis. Obstet Gynecol. 1984;64:303–310. [PubMed] [Google Scholar]
- 125.Selvaggi L, Lucivero G, Iannone A, dell’Osso A, Loverro G, Antonaci S, et al. Analysis of mononuclear cell subsets in pregnancies with intrauterine growth retardation. Evidence of chronic B-lymphocyte activation. J Perinat Med. 1983;11:213–217. doi: 10.1515/jpme.1983.11.4.213. [DOI] [PubMed] [Google Scholar]
- 126.Shibata R, Ouchi N, Kihara S, Sato K, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis in response to tissue ischemia through stimulation of amp-activated protein kinase signaling. J Biol Chem. 2004;279:28670–28674. doi: 10.1074/jbc.M402558200. [DOI] [PubMed] [Google Scholar]
- 127.Shimabukuro M, Higa N, Asahi T, Oshiro Y, Takasu N, Tagawa T, et al. Hypoadiponectinemia is closely linked to endothelial dysfunction in man. J Clin Endocrinol Metab. 2003;88:3236–3240. doi: 10.1210/jc.2002-021883. [DOI] [PubMed] [Google Scholar]
- 128.Sibai BM, Ewell M, Levine RJ, Klebanoff MA, Esterlitz J, Catalano PM, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–1010. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 129.Sibai BM, Gordon T, Thom E, Caritis SN, Klebanoff M, McNellis D, et al. Risk factors for preeclampsia in healthy nulliparous women: a prospective multicenter study. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. Am J Obstet Gynecol. 1995;172:642–648. doi: 10.1016/0002-9378(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 130.Sivan E, Mazaki-Tovi S, Pariente C, Efraty Y, Schiff E, Hemi R, et al. Adiponectin in human cord blood: relation to fetal birth weight and gender. J Clin Endocrinol Metab. 2003;88:5656–5660. doi: 10.1210/jc.2003-031174. [DOI] [PubMed] [Google Scholar]
- 131.Suwaki N, Masuyama H, Nakatsukasa H, Masumoto A, Sumida Y, Takamoto N, et al. Hypoadiponectinemia and circulating angiogenic factors in overweight patients complicated with pre-eclampsia. Am J Obstet Gynecol. 2006;195:1687–1692. doi: 10.1016/j.ajog.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 132.Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med. 2008;21:429–442. doi: 10.1080/14767050802041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thyfault JP, Hedberg EM, Anchan RM, Thorne OP, Isler CM, Newton ER, et al. Gestational diabetes is associated with depressed adiponectin levels. J Soc Gynecol Investig. 2005;12:41–45. doi: 10.1016/j.jsgi.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 134.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 135.Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, et al. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53:1621–1629. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 136.Tsai PJ, Yu CH, Hsu SP, Lee YH, Chiou CH, Hsu YW, et al. Cord plasma concentrations of adiponectin and leptin in healthy term neonates: positive correlation with birthweight and neonatal adiposity. Clin Endocrinol (Oxf) 2004;61:88–93. doi: 10.1111/j.1365-2265.2004.02057.x. [DOI] [PubMed] [Google Scholar]
- 137.Tsao TS, Murrey HE, Hug C, Lee DH, Lodish HF. Oligomerization state-dependent activation of NF-kappa B signaling pathway by adipocyte complement-related protein of 30 kDa (Acrp30) J Biol Chem. 2002;277:29359–29362. doi: 10.1074/jbc.C200312200. [DOI] [PubMed] [Google Scholar]
- 138.Tsao TS, Tomas E, Murrey HE, Hug C, Lee DH, Ruderman NB, et al. Role of disulfide bonds in Acrp30/adiponectin structure and signaling specificity. Different oligomers activate different signal transduction pathways. J Biol Chem. 2003;278:50810–50817. doi: 10.1074/jbc.M309469200. [DOI] [PubMed] [Google Scholar]
- 139.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences. J Clin Endocrinol Metab. 2003;88:5555–5563. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 140.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–30822. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 141.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than GN, Kim SK, et al. Retinol Binding Protein 4: An Adipokine Associated with Intra-amniotic Infection/Inflammation. J Matern Fetal Neonatal Med. 2009 doi: 10.3109/14767050902994739. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 143.Verhaeghe J, van BR, Van HE. Maternal body size and birth weight: can insulin or adipokines do better? Metabolism. 2006;55:339–344. doi: 10.1016/j.metabol.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 144.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, et al. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 145.Waller DK, Dawson TE. Relationship between maternal obesity and adverse pregnancy outcomes. Nestle Nutr Workshop Ser Pediatr Program. 2005;55:197–207. doi: 10.1159/000082603. [DOI] [PubMed] [Google Scholar]
- 146.Wallner W, Sengenberger R, Strick R, Strissel PL, Meurer B, Beckmann MW, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin Sci (Lond) 2007;112:51–57. doi: 10.1042/CS20060161. [DOI] [PubMed] [Google Scholar]
- 147.Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem. 2006;281:16391–16400. doi: 10.1074/jbc.M513907200. [DOI] [PubMed] [Google Scholar]
- 148.Wang Y, Xu A, Knight C, Xu LY, Cooper GJ. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin. Potential role in the modulation of its insulin-sensitizing activity. J Biol Chem. 2002;277:19521–19529. doi: 10.1074/jbc.M200601200. [DOI] [PubMed] [Google Scholar]
- 149.Williams MA, Qiu C, Muy-Rivera M, Vadachkoria S, Song T, Luthy DA. Plasma adiponectin concentrations in early pregnancy and subsequent risk of gestational diabetes mellitus. J Clin Endocrinol Metab. 2004;89:2306–2311. doi: 10.1210/jc.2003-031201. [DOI] [PubMed] [Google Scholar]
- 150.Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knofler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191:2120–2124. doi: 10.1016/j.ajog.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 151.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, et al. The fat derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]