Abstract
Background & Aims
Tumor suppressor proteins are inactivated by many different mechanisms, including nuclear exclusion by chromosome region maintenance (CRM)-1. Increased tumor levels of CRM-1 have been correlated with poor prognosis of patients with pancreatic cancer, making it a therapeutic target. Selective inhibitors of nuclear export (SINEs) bind to CRM-1 to irreversibly inhibit its ability to export proteins; we investigated a new class of SINEs in pancreatic cancer cells.
Methods
We studied the effects of SINE analogs in a panel of pancreatic cancer cell lines and non-transformed human pancreatic ductal epithelial (HPDE) cells using proliferation, apoptosis, immunoblot, co-immunoprecipitation, small inhibitor (Si)-RNA, and fluorescence microscopy analyses. The effects of the SINEs were also investigated in mice with subcutaneous and orthotopic tumors.
Results
SINEs (KPT-185, KPT-127, KPT-205 and KPT-227) inhibited proliferation and promoted apoptosis of pancreatic cancer cells, but did not affect HPDE cells. The nuclei of cells incubated with KPT-185 accumulated tumor suppressor proteins (p27, FOXO, p73, and PAR-4) and inhibited interactions between CRM-1 and these proteins. Mutations in the region of CRM-1 that binds to SINEs (Cys-528), or siRNA knockdown of PAR-4, prevented the ability of KPT-185 to block proliferation and induce apoptosis of pancreatic cancer cells. Oral administration of KPT-330 to mice reduced growth of subcutaneous and orthotopic xenograft tumors without major toxicity. Analysis of tumor remnants showed that KPT-330 disrupted the interaction between CRM-1 and PAR-4, activated PAR-4 signaling, and reduced proliferation of tumor cells.
Conclusion
We identified SINEs that inhibit CRM-1 and promote nuclear accumulation of tumor suppressor proteins in pancreatic cancer cells. Oral administration of the drug to mice reduces growth of xenograft tumors.
Keywords: EXPORTIN, translocation, nucleus, small molecule inhibitor
Introduction
Treatment of pancreatic cancer is an unmet clinical problem with annual deaths exceeding breast and prostate cancers, largely due to the lack of effective drug therapies 1. Such dismal statistics indicates that newer targets and drugs need to be urgently identified. CRM-1 (chromosome region maintenance 1; also referred to as exportin1 or Xpo1) is one of the major, non-redundant receptor for the export of proteins out of the nucleus. It is a member of the importin β superfamily of nuclear transport receptors, recognizing proteins bearing a leucine-rich nuclear export sequence (NES) 2. Among the various targets of CRM-1 are tumor suppressor proteins (TSPs) such as p53, p27, p21, FOXO and prostate apoptosis response-4 (PAR-4) 3. Nuclear exclusion of these and other TSPs by CRM-1 renders cancer cells resistant to apoptosis 4. A significant mechanism of action of the commonly used chemotherapeutic drugs such gemcitabine, 5FU and platinum based compounds is by activation of various TSPs through their nuclear retention. However, elevated CRM-1 expression levels in cancer cells result in mislocalization of important TSPs via a constant nuclear export, resulting in the attenuation of their tumor suppressor function, and contributing to treatment failure. In support of its role in cancer maintenance, elevated CRM-1 expression has been found to be correlated with poor overall survival in multiple tumors including pancreatic cancer 5. Therefore, targeted inhibition of CRM-1 is an attractive therapeutic strategy for forcing the TSPs to be retained in the nucleus in order to allow them to function properly and induce cancer specific apoptosis.
Earlier approaches to target CRM-1 led to the development of the natural product CRM-1 inhibitor Leptomycin B (LMB) 6, 7. However, a single phase I trial of parenterally administered LMB showed marked gastrointestinal toxicity and constitutional intolerance thereby limiting its clinical use 8. Semi-synthetic modification of LMB markedly improved pharmacokinetic (PK) properties, and improved the therapeutic window of these parenterally administered LMB-derivatives in animals 9. Nevertheless, their clinical potential has not been explored. Together, these data suggest that newer CRM-1 inhibitors with high specificity, cancer cell selectivity and low toxicity are needed.
Using high throughput in silico structure-based design, we have developed novel, small molecule highly selective inhibitors of nuclear export (SINEs) that covalently bind to the CRM-1 cargo-binding groove and irreversibly inhibit the protein’s export function. SINEs have broad specificity against different tumors types with IC50s in the submicromolar range sparing normal cells (Supplementary Figure 1) and have recently entered Phase I clinical trials for both solid tumors (NCT01607905) and advanced hematological malignancies (NCT01607892).
As documented earlier that PAR-4 is a cancer cell-selective pro-apoptotic protein that functions intra-cellularly in the cytoplasmic and nuclear compartments as a TSP 10. Under external stress conditions in most cancer cells, ectopic PAR-4 readily translocates to the nucleus to induce apoptosis 11. In contrast, in normal cells, ectopic PAR-4 is predominantly localized to the cytoplasm and does not induce apoptosis unless a second apoptotic insult occurs. Consistent with these observations, PAR-4 has been found to be down-regulated in many cancers, such as renal cell carcinoma 12, neuroblastoma 13, endometrial cancer 14, breast cancer 15 and pancreatic cancer 16. We were the first to show that activation and nuclear localization of PAR-4 can lead to significant apoptosis in pancreatic cancer cells 17, 18. Based on these investigations, we proposed PAR-4 activation as a potential therapeutic strategy in pancreatic cancer 19. As the TSP PAR-4 contains a canonical leucine rich NES, it may be a cargo for CRM-1 mediated nuclear export, and therefore an ideal candidate to investigate using our newly developed SINEs.
In this report, we investigated the anti-cancer potential of SINEs against pancreatic cancer cell lines and in three xenograft models (one subcutaneous and two orthotopic) and evaluated the role of different TSPs in general, and more specifically, the role of PAR-4 in these effects. The data presented here demonstrates that SINEs activity is mediated through nuclear localization of multiple tumor suppressor proteins particularly PAR-4. Our findings support the development of SINEs for the clinical treatment of pancreatic cancer.
Materials and Methods
Cell lines and culture condition
AsPC-1, BxPC-3 and HPAC cells were obtained from ATCC. Colo-357 and HPDE cells were a gift from Dr. Paul Chiao, MD Anderson Cancer Center, Texas. Pancreatic cancer cells were grown in DMEM media with 5% penicillin and streptomycin supplemented with Fetal Bovine Serum (Sigma) and 5% Glutamine (Invitrogen). HPDE was cultured in keratinocyte serum-free (KSF) medium supplemented by epidermal growth factor and bovine pituitary extract (Life Technologies). These cell lines have been tested and authenticated in our Core facility.
Cell growth inhibition 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (MTT)
Pancreatic cancer cells and normal HPDE cells were seeded at a density of 3×103 cells per well in 96-well micro-titer culture plates. After overnight incubation, medium was removed and replaced with fresh medium containing KPT SINEs at indicated concentrations (0–200 nM). In another set of experiments, pancreatic cancer cells were first exposed to Calyculin (0–15 nM) for 5–30 minutes followed by KPT-SINEs (0–150 nM for 24–72 hrs). Upon completion of incubation, MTT assay was performed according to published methods 17
Quantification of apoptosis by Histone DNA ELISA and Annexin V FITC Assay
Cell Apoptosis was detected using Annexin V FITC Biovision Danvers MA) and Histone DNA ELISA Detection Kit (Roche, Life Sciences) according to the manufacturer's protocol 18.
Western blot analysis
Preparation of cellular lysates, protein concentration determination and SDS-PAGE analysis has been previously described 20.
Immunofluorescence and PAR-4 cellular staining
For protein localization experiments, cells were grown on glass chamber slides and exposed to KPT-185 in the absence or presence of different siRNAs (control siRNA and PAR-4 specific siRNA) at indicated concentrations for 24 hrs. In another set of experiments, cells on cultured slides were exposed to short term treatment with Calyculin A (0–15 nM) for 5–30 minutes followed by KPT-185 treatment for 24 hrs. At the end of the treatment, immunofluorescence was performed according to our previously published methods 20.
siRNA and transfections
Cells were transfected with either control siRNA or PAR-4 siRNA using LipofectAMINE 2000 (Invitrogen) according to published methods 21. After the siRNA treatment period cells were further treated with KPT-185 in 96 well plates for MTT assay, and 6 well plates for Annexin V FITC assays, respectively. Transfection efficiency was evaluated by fluorescence microscopy as published previously 20.
Site directed mutations in CRM-1
CRM-1 cys528 mutants (that carry aberrant KPT-SINE recognizable NES recognizing sequence) were developed by transiently transfecting AsPC-1 and BxPC-3 cells with wild-type and mutant Ser-528 CRM-1. The stable cell lines were cloned by antibiotics ampicillin and confirmed by immunofluorescence and western blot analysis according to published methods 22.
Development of Subcutaneous and Orthotopic Animal Xenografts and Pre-clinical Efficacy Trial
Four-week-old female ICR-SCID mice (Taconic Laboratory) were adapted to animal housing and xenografts were developed as described earlier 23. To test the efficacy of KPT-330, bilateral fragments of the MiaPaCa-2 xenograft were implanted s.c. into naive, similarly adapted mice. One week later, MiaPaCa-2 developed into palpable tumors (~50 mg); tumor-bearing animals were randomly assigned to different cohorts and treated with either diluents (control group), 15 or 20 mg/kg (maximum tolerated dose; MTD) orally, for 3 weeks All mice were followed for measurement of s.c. tumors and observed for changes in body weight and any side effects. Orthotopic models were developed as described earlier 24. Briefly, 1x106 AsPC-1 or MiaPaCa-2 cells were injected in the pancreas of mice (16 animals per cell line). Twelve days after inoculation, mice were randomly divided and received KPT-330 at 20mg/kg orally; QD X5 for 3 weeks. All in vivo studies were conducted under Animal Investigation Committee-approved protocol. At the end of the treatment period, tumors were excised and minced in proteins isolation buffer using our well established methods 23. The membranes were probed for PAR-4, Phospho-PAR-4, Bax, PARP, Caspase-9 and β-actin. The expression of PAR-4 was detected using histologic sections of tumor xenografts according to published methods 21.
Results
Rational Design of KPT SINEs as Potent and Specific Small-molecule Inhibitors
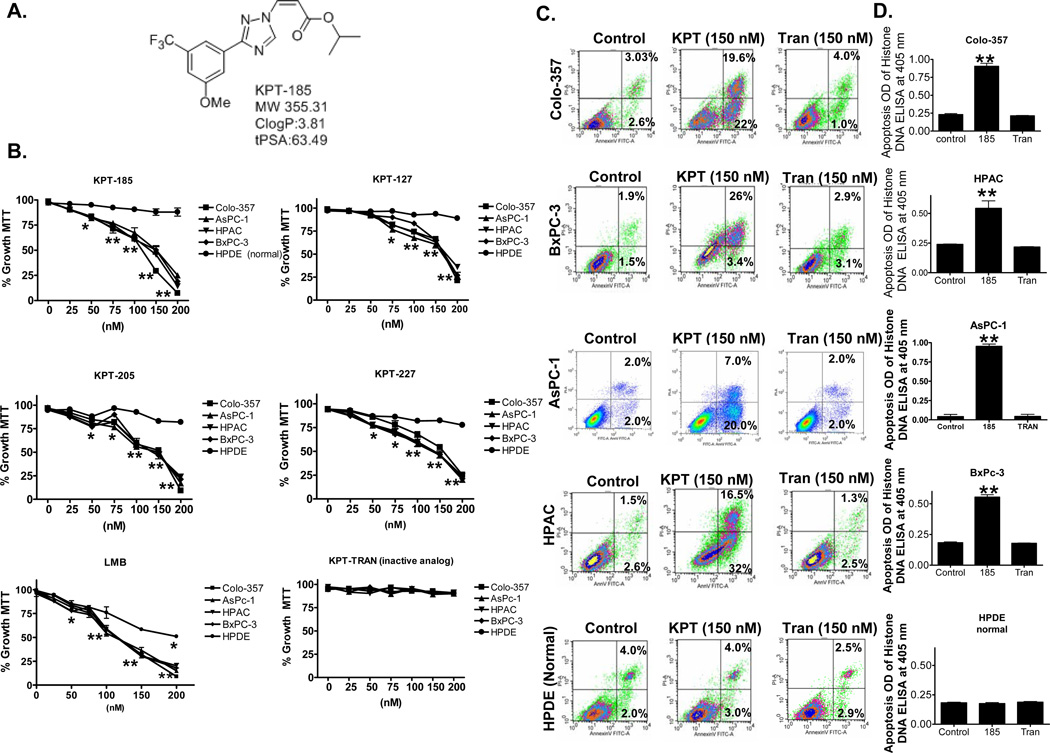
Employing a structure-based approach using the published crystal structure of CRM-1, we have designed various SINEs (Figure 1A Structure of most potent analog KPT-185) as a new class of small molecule, drug like inhibitors of CRM-1. KPT-185 binds to CRM-1 with high affinity that consequently restricts its ability to mediate the nuclear export of TSPs and other proteins 4. Four analogs KPT-185, KPT-127, KPT-205 and KPT-227 (structures published earlier 4) were investigated for their growth inhibitory and apoptotic potential against a panel of pancreatic cancer cells lines (Colo-357, AsPC-1, HPAC and BxPC-3) and a normal human pancreatic ductal epithelial (HPDE) cell line using MTT assay. As can be seen from results presented in Figure 1B, exposure of different pancreatic cancer cell lines to sub-micromolar concentrations of SINEs for 72 hrs resulted in statistically significant growth inhibition (IC50 ~150 nM). Most significantly, at the concentrations tested, the SINEs showed no effect on growth of normal pancreatic ductal epithelial (HPDE) cells. Additionally, at comparative doses, KPT-Trans (the trans analog of KPT-185 with >10X reduced CRM-1 inhibition potency) did not induce statistically significant growth inhibition.
Figure 1. KPT SINEs induce cancer cell selective growth inhibition and apoptosis.
[A] Structure of KPT-185. [B] Cell growth inhibition curves using MTT assay 20 at 72 hrs of KPT-SINE treatment. All points represent triplicate experiments with 8 replicates per concentration. * p <0.05 and ** p<0.01. [C] Pancreatic cancer and HPDE cells (50,000) were exposed to indicated concentrations of KPT SINEs or inactive analog KPT-TRAN for 72 hrs. Apoptosis was analyzed using Annexin V FITC and using Histone DNA ELISA 20 [D]. Results are representative of three independent experiments. * p <0.05 and ** p<0.01.
KPT SINEs Induce Apoptosis in Pancreatic Cancer Cells
Because CRM-1 is the non-redundant exporter of most TSPs, we investigated whether its targeted inhibition could reactivate TSP function and lead to apoptosis in the tested cancer cell lines by using Annexin V FITC assay. As expected and in line with the growth inhibition results, active SINEs at 150 nM (not KPT-Trans analog) induced apoptosis in three different pancreatic cancer cell lines while sparing normal HPDE cells (early and late apoptotic events for cancer cell lines Colo-357-19.6% and 22%; for HPAC-16.5% and 32%; BxPC-3-26.4% and 3.4% and normal cell line HPDE 4.06% and 3.0%, respectively Figure 1C). We also tested apoptosis induction using Histone/DNA ELISA assay and comparable apoptosis was observed (Figure 1D). Together, these results provide unequivocal proof of the potential of SINEs as new class of anti-cancer drugs.
KPT-SINE Activity is Mediated through Nuclear localization of Various TSPs
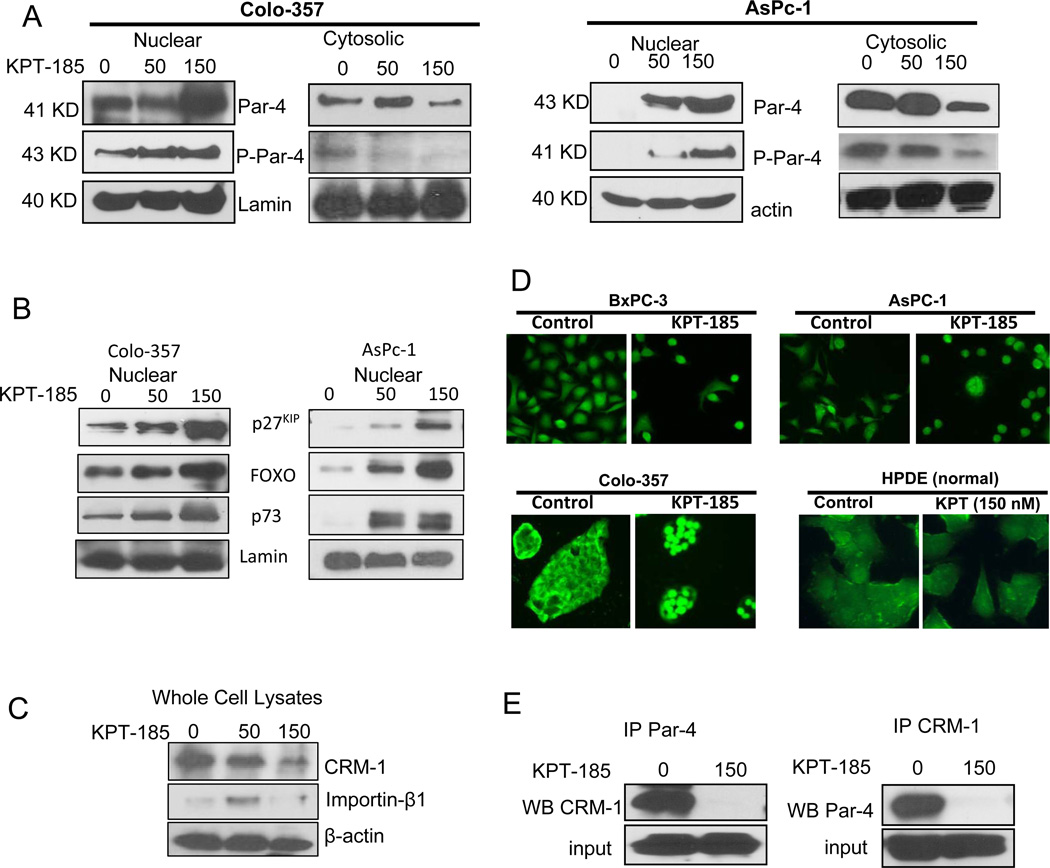
In order to verify whether CRM-1 inhibition by KPT-SINEs leads to nuclear accumulation of different TSPs, western blot assays on KPT-185 treated nuclear cell lysates were performed. We have earlier established PAR-4 as one of the central players in apoptosis induction in pancreatic cancer cells 17, 18. As PAR-4 carries a NES and could therefore undergo targeted nuclear export by CRM-1, we first investigated whether SINE treatment leads to PAR-4 nuclear accumulation. As can be seen in Figure 2A, exposure of Colo-357 and AsPC-1 cells to KPT-185 resulted in progressive nuclear accumulation of PAR-4. Further, the nuclear accumulation of phospho-PAR-4 (p-PAR-4) showed similar enhancement in nuclear fraction following SINE induced CRM-1 inhibition. On the other hand, cytosolic fraction of PAR-4 and p-PAR-4 were reduced (Figure 2A, right panels). KPT-185 exposure did not result in any significant activation of PAR-4 in normal HPDE cells (data not shown). We also evaluated three other TSPs (FOXO, p73 and p27) that were found to be localized in the nucleus upon KPT-185 treatment (Figure 2B). Further, CRM-1 expression was found to decrease and importin expression was also lost at high KPT-185 concentration (Figure 2C). Nuclear PAR-4 was also evaluated using immunofluorescence assay (Figure 2D). DMSO control Colo-357, BxPC-3 and AsPC-1 cells show minimal PAR-4 nuclear staining. However, exposure to 150 nM KPT-185 for 24 hrs resulted in nuclear accumulation of PAR-4. As PAR-4 is found in the cytoplasm of untreated cells, these data indicate that nuclear import of PAR-4 is constantly occurred and is unaffected by SINE treatment, suggesting that SINE treatment leads only to nuclear export blockade and nuclear import proceeds unimpeded, leading to marked nuclear accumulation of PAR-4. We did not observe any significant accumulation of PAR-4 in normal HPDE cells (hollow nucleus and low intensity in both treated and untreated cells, Figure 2D far right panel). Direct evidence showing that KPT-185 can disrupt CRM-1-PAR-4 interaction came from the results of co-immunoprecipitation. Lysates from DMSO control and KPT-185 treated cells were pulled down with CRM-1 antibody and probed with PAR-4 using western blotting and vice versa. As can be seen from the results of Figure 2E (left and right panels), KPT-185 treatment resulted in reduced association between PAR-4 and CRM-1 as little protein precipitated in treated samples.
Figure 2. KPT-185 induces nuclear localization of different TSPs including PAR-4 in cancer selective manner.
[A] Nuclear lysates (50 μg) from KPT-185 treated Colo-357 and AsPC-1 cells were resolved using western blotting. The membranes were probed with TSP PAR-4 antibody (Santa Cruz, CA) and p-PAR-4 (Cell Signaling Danvers, MA). [B] Western blot showing nuclear accumulation of other important TSPs. [C] Whole cell lysates from KPT-185 treated Colo-357 cells (KPT-185 0-150 nM for 24 hrs) were resolved for CRM-1 and importin β expression. [D] Immunofluorescence assay demonstrating PAR-4 nuclear localization by KPT-185 in Colo-357 and AsPC-1 cells and not in HPDE cells. Images are representative of three independent experiments [E] Co-immunoprecipitation assay demonstrating disruption of CRM-1-PAR-4 interaction. Blots are representative of two independent experiments.
Cys528 in CRM-1 is a Specific Target Site for KPT-SINE
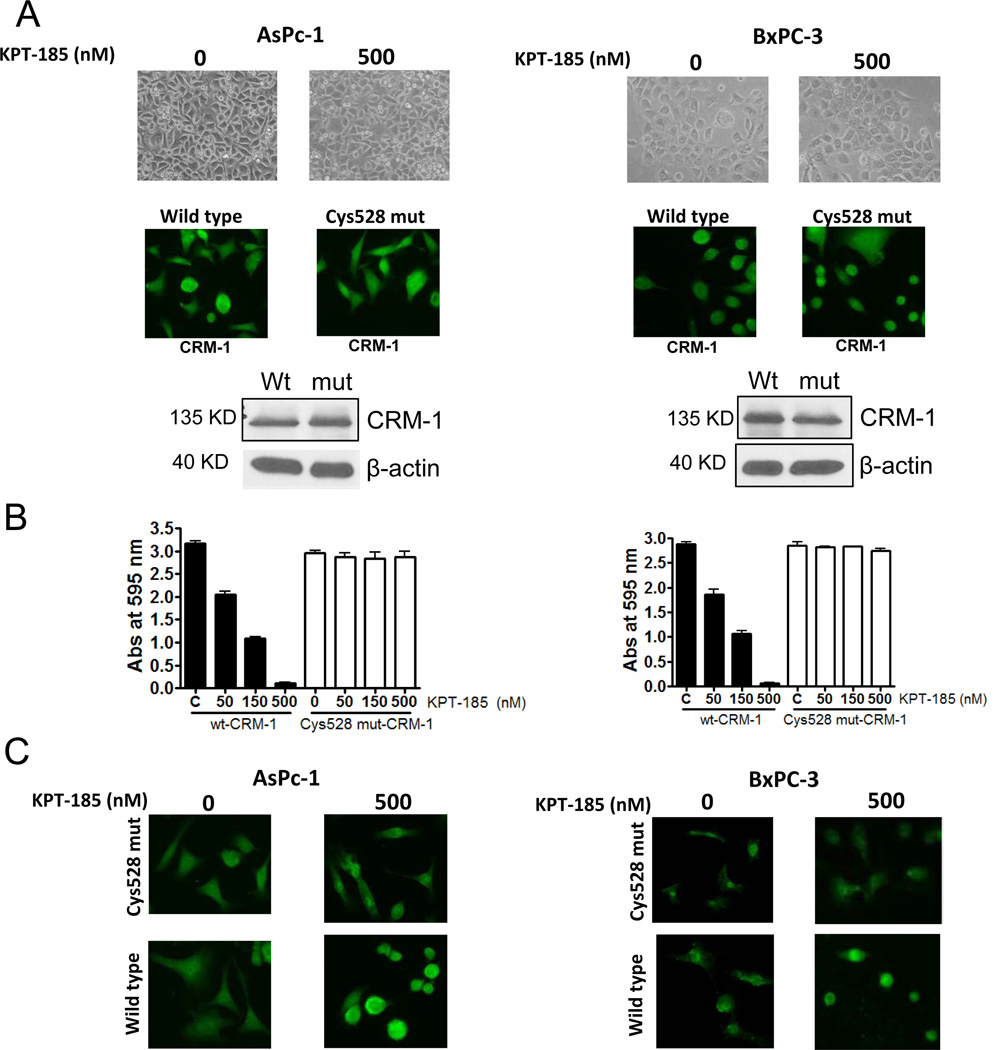
Earlier it was shown that the potent inhibitor of CRM-1 LMB very selectively bound to Cys-528 of human CRM-1 and yeast cells carrying a mutation at the corresponding site are LMB-resistant 25. Building on these existing results, we transiently transfected AsPC-1 and BxPC-3 cells with cys528 CRM-1 mutant protein and tested the effect of KPT-185. Transfection with wt- or mut- cys528 CRM-1 did not alter cell morphology or growth pattern in the two cells (Figure 3A top panels). Additionally, we did not observe loss of CRM-1 expression in both cell lines (Figure 3A; middle and lower panels showing immunofluorescence and western blot with CRM-1 antibody). In line with our previous observation, KPT-185 treatment resulted in a statistically significant growth inhibition at 150 and 500 nM concentrations (Figure 3B; solid bars). However, in mut-528 cys expressing AsPC-1 or BxPC-3 cells, exposure to KPT-185 did not induce growth inhibition even at doses up to 500 nM (Figure 3B; white bars). We further evaluated whether KPT-185 loses its ability to retain PAR-4 in the nucleus of Cys528 mutants. In agreement with our proposed hypothesis, Cys528 mutants did not show PAR-4 nuclear expression even at KPT-185 doses as high as 500 nM while wt- cells showed PAR-4 nuclear expression after drug treatment.
Figure 3. KPT-SINE is ineffective in cells expressing Cys528 mutant CRM-1.
[A] (Top panels) showing photomicrographs of growth patterns of AsPC-1and BxPC-3 cells. (Middle panels are immunofluorescence images demonstrating CRM-1 expression in both cell lines. (Lower panel) Western blot analyses of whole cell lysates for CRM-1 expression in both cell lines. β-actin was used as loading control. [B] Growth inhibition assay in wt- and Cys528 mutant cells. 8000 cells were seeded in 96 well plates and exposed to KPT-185 at indicated concentrations for 72 hrs. Growth inhibition was assessed using MTT assay. [C] Immunofluorescence images of PAR-4 stained AsPC-1 and BxPC-3 cells exposed to indicated concentrations of KPT-185 for 24 hrs.
Role of PAR-4-PKA Axis in KPT-185 Cancer Selective Activity
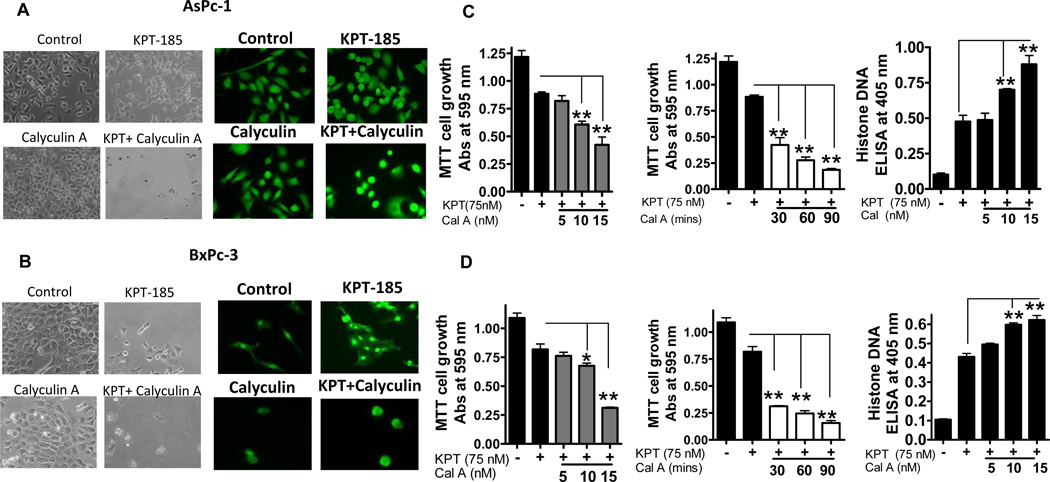
PAR-4 cancer cell selectivity has been attributed to its propensity to be phosphorylated by protein kinase A (PKA), a protein found to be over-expressed in cancer cells but not in normal cells 26. To further validate the role of PKA-PAR-4 axis, we performed both dose and time kinetics experiments using Calyculin A (a chemical activator of PKA). Figure 4 A and B (left and right panels) are photomicrographs and immunofluorescence images of AsPC-1 and BxPC-3 showing enhanced cell growth inhibition and greater PAR-4 nuclear localization in cells that were pre-exposed to Calyculin A. Figure 4C and D show time and dose kinetics MTT and apoptosis assays after KPT-185 treatment in the presence of Calyculin A. Our results clearly show that 30 minute pre-exposure of pancreatic cancer cells to increasing doses of Calyculin A (5-15 nM) followed by KPT-185 (sub IC50 doses of 75 nM for 72 hrs) resulted in statistically significant enhancement in growth inhibition (p=0.025). A time course assessment in which fixed dose of Calyculin A (15 nM) at different time points (30, 60 and 90 min) in the presence of KPT-185 (75 nM for 72 hrs) showed similar trend. Apoptosis was also enhanced under similar treatment conditions in both cells lines (Figure 4 C and D; far right panels). Collectively, these results provide definitive proof in support of a PKA mediated PAR-4 phosphorylation/activation by KPT-SINE drugs and are consistent with cancer cell selective mechanism of action of SINE suggesting the involvement of PAR-4.
Figure 4. PKA-PAR-4 axis in KPT-SINE action.
[A and B] Photomicrographs and immunofluorescence images of AsPC-1 and BxPC cells exposed to vehicle control; KPT-185 75 nM; Calyculin A (15 nM for 30 minutes) and Calyculin A (15 nM for 30 minutes followed by KPT-185 75 nM for 72 hrs. [C and D] AsPC-1and BxPC-3 cells were exposed to indicated concentrations of KPT-185 and Calyculin A and growth inhibition and apoptosis was assessed using MTT and Histone DNA ELISA assays.
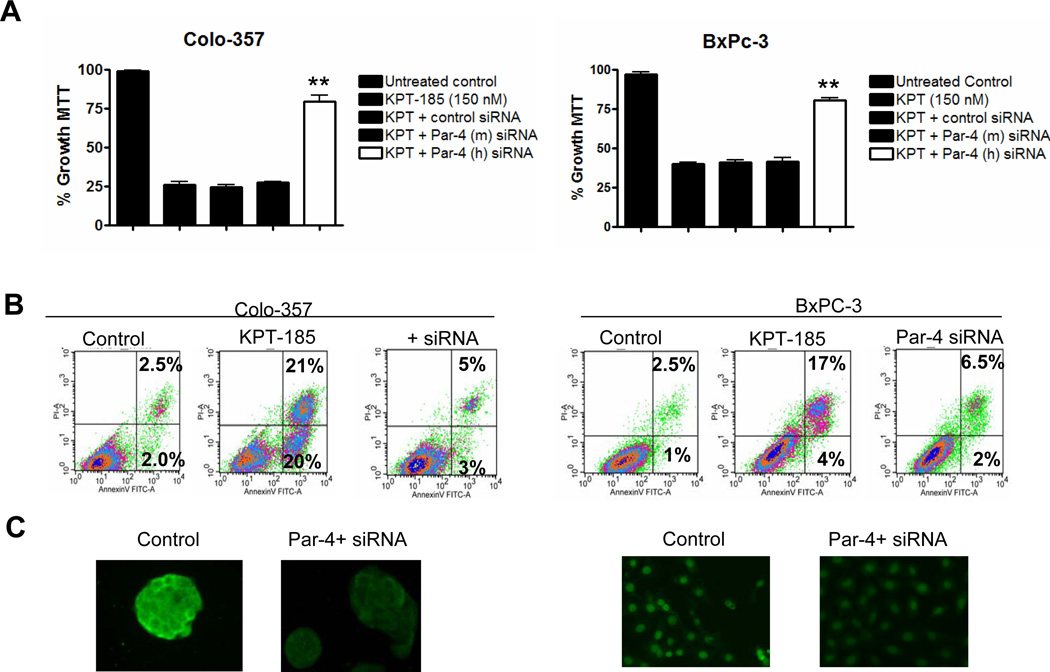
PAR-4 Knockdown by RNA Interference Abrogates SINE Cytotoxicity in pancreatic cancer Cells
In order to determine whether KPT-185 induced apoptosis involves PAR-4, we investigated the effect of PAR-4 siRNA on the activity of the SINE. As can be seen in Figure 5A, siRNA knockdown of PAR-4 markedly reduced growth inhibition by KPT-185 (MTT assay). As a control, mouse specific PAR-4 siRNA [siRNA PAR-4 (m)], that does not target human PAR-4, did not suppress SINE mediated growth inhibition. Furthermore, apoptosis induction by SINE was abrogated by PAR-4 siRNA treatment [reduction from 41% to 8% (early and late apoptotic events combined)] (Figure 5 B). The suppression of PAR-4 by siRNA was validated using fluorescent microscopy which is shown in Figure 5C. As another control, siRNA silencing was also used to investigate the role of p53, a major TSP that is typically mutated or lost in pancreatic cancer. Consistent with the fact that p53 is mutated in the tested pancreatic cancer cell lines; siRNA against p53 showed no effect on KPT-185 mediated growth inhibition or apoptosis (Supplementary Figure 2). These data indicate that PAR-4 is a potentially functional TSP and that CRM-1 inhibition can restore nuclear localization of PAR-4 in pancreatic cancer lines. Furthermore, our data show clearly that SINE can kill cells lacking functional p53, and are therefore likely to be relevant to pancreatic cancer and other cancer types.
Figure 5. siRNA against PAR-4 abrogates KPT SINE activity.
[A] Colo-357 and BxPC-3 cells were exposed to 150 nM of KPT-185 in the absence or presence of control siRNA, PAR-4 mouse specific siRNA and PAR-4 human specific siRNA. Growth inhibition was evaluated using MTT assay. [B] Apoptosis by KPT-185 was evaluated under similar siRNA silencing conditions. Results are representative of three independent experiments. [C] siRNA silencing of PAR-4 was evaluated using confocal microscopy.
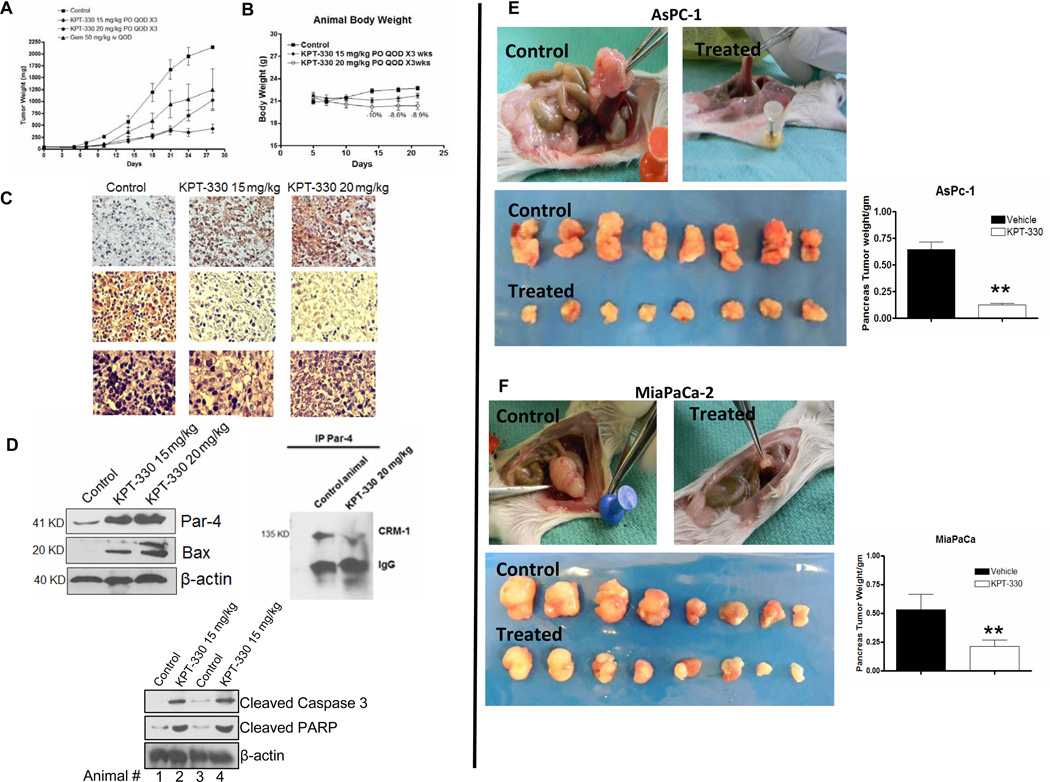
Oral-KPT SINE shows In Vivo Antitumor Activity in Subcutaneous and Orthotopic Models
We next evaluated the anti-tumor activity of KPT-330 (the clinical candidate analog of KPT-185 which has suitable PK in animals). Oral administration of KPT-330 resulted in statistically significant tumor growth inhibition compared to control or gemcitabine treatment (50 mg/kg i.v. QOD Figure 6A). At 15 mg/kg or 20 mg/kg daily×5 each week (for 3 weeks) did not show significant body weight loss or toxicity (Figure 6B). The maximum body weight loss was 10% (more than 15% is considered toxic). In order to demonstrate SINE activity in vivo, tumors were excised at the end of the treatment period for molecular analyses. In line with our in vitro results, immunohistochemical examination of tumor sections revealed substantial enhancement of PAR-4 in KPT-330 treated mice, and this was coupled with marked suppression of Ki67 (an index of proliferation; Figure 6C Left Panel). Additionally, western blot expression analyses of tumor tissue lysates showed similar dose-dependent effects with emergence of PAR-4 and pro-apoptotic Bax (Figure 6D) along with other major apoptotic cmarkers such as cleaved caspase-3 and PARP. These in vivo data indicate that KPT-330 mediates CRM-1 inhibition in the tumor and induces changes similar to those shown in vitro. Taken together, these results fortify our hypothesis that the anti-cancer activity of SINEs is due, in part, to the activation of intracellular PAR-4 signaling mediated apoptosis.
Figure 6. Pre-clinical Anti-tumor Efficacy Trial of clinical grade KPT-330.
[A] Efficacy trial of KPT-330. Note KPT-330 has similar anti-tumor effects compared to chemotherapeutic drug gemcitabine. [B] KPT-330 treated ICR SCID animal’s body weight loss evaluation. [C] Tumor tissue histology showing enhancement of PAR-4 in treated group compared to vehicle control and reduction of proliferation marker ki-67 and H&E. [D] (Top left panel) western blot analysis on tumor tissue lysates showing enhancement in PAR-4 and its downstream pro-apoptotic target Bax. (right panel) Immunoprecipitation assay showing disruption of PAR-4-CRM-1 interaction in tumors by KPT-330 and (Lower panel) tumor tissue lysate’s western blot showing enhancement of pro-apoptotic cleaved caspase-3 and PARP cleavage. [E and F] Preclinical efficacy trials of KPT-330 in AsPC-1 and MiaPaCa-2 orthotopic models. Photographs of gross examination of mice Pancreas/tumors in control (vehicle treated) and KPT-330 treated (similar to subcutaneous model). Right panels shows data-plot comparing pancreas/tumor weight of control vs KPT-185 treated mice (p<0.01).
We further investigated the oral anti-tumor efficacy of KPT-330 in two additional orthotopic models (AsPC-1 and MiaPaCa-2). As can be seen from the results of Figure 6, control (vehicle treated) animals had large tumors growing at the pancreas site in both models (E and F; Top lower left panels). However, in the KPT-330 treated animals, the size of orthotopic tumors was drastically reduced (6E Top and lower right panels). Figures 6E and F show the pancreas/tumors from 8 different mice (upper line-untreated and lower line treated with KPT-330). Figure 6E and F (top and lower panels) are graphical representation of the pancreas/tumor weight of the two models. Taken together, our pre-clinical efficacy trial in multiple animal models provide very strong evidence in support of KPT-SINEs against pancreatic cancer.
Discussion
There is an urgent need for newer and effective drugs for pancreatic cancer and in this paper; we demonstrate the anti-cancer potential of a new class of orally active nuclear export protein inhibitors for this deadly cancer. Our results highlight a pancreatic cancer cell selective mechanism of SINE CRM-1 antagonists that involves nuclear retention of various tumor suppressor proteins (TSPs). Our preclinical animal data forms a solid foundation that could accelerate the clinical translation of SINEs for the treatment of pancreatic cancer.
A common and highly efficient mechanism controlling gene expression involves alterations in the subcellular localization of transcriptional regulators. Many transcription factors and co-factors possess nuclear localization sequences (NLSs) and nuclear export sequences (NESs) that co-ordinate entry into and exit from the nucleus, respectively 27. Nuclear export of proteins and RNAs are mediated by seven different exportins. Proteins containing canonical hydrophobic leucine-rich NESs, as well as some mRNAs and microRNAs (miRNAs), are exported by Exportin 1 (XPO1), more commonly called CRM-1 28, 29. Chemotherapy and other targeted therapies work, at least in part, by forcing nuclear accumulation of TSPs that initiate cascade of pathways, resulting in cell death. However, CRM-1 overexpression that is common to pancreatic cancer 5 results in constitutive nuclear export of TSPs, thereby suppressing the activation of their anti-tumor functions. This, in turn, translates to low efficacy of many anti-cancer agents 4. Therefore, targeted inhibition of CRM-1 becomes an attractive anti-cancer strategy.
Apart from Leptomycin B (LMB) and its derivatives, there have not been any serious attempts to develop targeted inhibitors against CRM-1 9. LMB is a Streptomyces metabolite that covalently attaches to the sulfhydryl group of cysteine at position 529 in CRM-1 and thereby prohibits CRM-1 from associating with NES-containing cargo 25. Although, the inhibition of CRM-1 by LMB is a highly efficient process, the poor pharmacological properties have contributed to its extreme toxicities – severe diarrhea and profound fatigue 8. There is certainly a drive in the pharmaceutical research arena to develop novel targeted small molecule CRM-1 inhibitors with desirable PK parameters and lesser toxicity. To our knowledge, our SINEs are the most advanced compounds, which have shown high specificity towards CRM-1 and showed PK properties including oral bioavailability that are superior to LMB, and showed substantially reduced toxicity.
Most normal cells treated with CRM-1 inhibitors undergo cell cycle arrest, but apoptosis was very uncommon (unpublished observations). In fact, SINE treatment of the ‘normal’ pancreatic ductal epithelial line evaluated here showed minimal effects on proliferation (cell cycle) at drug doses that killed pancreatic cancer cells. Along these lines, our data provide a strong rationale supporting SINE mediated localization of various TSPs such as FOXO, p27, p73 and PAR-4 (Figure 2) in pancreatic cancer but not normal cells.
Pancreatic tumors are generally resistant to chemotherapy, harbor complex genomic alterations and lack clear druggable targets 30. Studies from our laboratory and those of others have demonstrated that down-regulation of the TSP PAR-4 is directly correlated to poor overall survival in pancreatic cancer 16. This led to our proposed hypothesis that PAR-4 as a legitimate therapeutic target in pancreatic cancer 17. We further hypothesize that PAR-4 is a TSP that carries a NES, and therefore likely to be exported from the nucleus by CRM-1. CRM-1 mediated nuclear export of PAR-4, like many TSPs, leads to its functional inactivation as a tumor suppressor. Gurumurthy and group have established that PAR-4 activation and the selective killing of cancer cells is due in part to its phosphorylation by a cancer specific protein PKA 18. Low PKA expression and negligible PAR-4 phosphorylation in normal cells highlights a therapeutic window for cancer selective killing by our SINE CRM-1 inhibitors (Summary Diagram in Supplemental Figure 3). Supporting these observations, our results show that SINE induced PAR-4 expression, its nuclear localization and phosphorylation, specifically in pancreatic cancer. Additional validation came from experiments in which we chemically induced the expression of PAR-4 using Calyculin A, which resulted in enhanced cell growth inhibition and apoptosis by KPT-SINE. Furthermore, PAR-4 siRNA mediated silencing abrogates the CRM-1 inhibitor efficacy, and this directly documented the involvement of PAR-4. We also showed that the anti-tumor efficacy of KPT-330 in vivo was mediated through the activation of PAR-4 signaling in the tested tumor models (Figure 6 C and D).
Oral administration of KPT-330 at its MTD resulted in a statistically significant tumor growth inhibition in subcutaneous and two orthotopic pancreatic cancer xenograft models (greater than the efficacy of gemcitabine in subcutaneous model). Molecular analysis of treated tumors revealed substantial enhancement of PAR-4, Bax and other major apoptotic markers such as cleaved caspase-3 and PARP along with marked suppression of proliferation index as assessed by Ki67 immunostaining.
In conclusion, we have rationally designed small molecule, orally bioavailable, drug-like SINEs that irreversibly block CRM-1, and performed critical in vitro and in vivo evaluations to demonstrate their therapeutic potential in multiple pancreatic cancer xenograft models. In particular, we have provided strong evidence in support of a SINE mechanism of action that involves nuclear retention of different TSPs especially PAR-4. Based on these strong pre-clinical studies, we are progressing oral SINEs in Phase I clinical trials for the treatment of pancreatic cancer.
Acknowledgments
Funding Source: National Cancer Institute, NIH grants R01CA109389 and R21CA169848 to R.M. Mohammad are acknowledged. We are thankful to Karyopharm Therapeutics for partially funding this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Asfar S. Azmi: Study concept and design, acquisition of data; analysis and interpretation of data; drafting of the manuscript and statistical analysis
Amro Aboukameel: acquisition of data, analysis and interpretation of data
Bin Bao: acquisition of data, analysis and interpretation of data
Fazlul H Sarkar: Critical revision of the manuscript for important intellectual content
Philip a. Philip: Critical revision of the manuscript for important intellectual content
Michael Kauffman: Technical, or material support; study supervision, critical revision of the manuscript for important intellectual content
Sharon Shacham: Technical, or material support, study supervision, critical revision of the manuscript for important intellectual content
Ramzi M. Mohammad: Study concept and design, analysis and interpretation of data
Conflict Statement: Sharon Shacham and Michael Kauffman own equity in Karyopharm Therapeutics and this study was partially funded by Karyopharm Therapeutics. Asfar S. Azmi, Amro Aboukameel, Bin Bao, Philip A. Philip, Fazlul Sarkar and Ramzi M Mohammad have no potential conflict of interest.
Reference List
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Hutten S, Kehlenbach RH. CRM1-mediated nuclear export: to the pore and beyond. Trends Cell Biol. 2007;17:193–201. doi: 10.1016/j.tcb.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 4.Turner JG, Dawson J, Sullivan DM. Nuclear export of proteins and drug resistance in cancer. Biochem Pharmacol. 2012;83:1021–1032. doi: 10.1016/j.bcp.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang WY, Yue L, Qiu WS, Wang LW, Zhou XH, Sun YJ. Prognostic value of CRM1 in pancreas cancer. Clin Invest Med. 2009;32:E315. [PubMed] [Google Scholar]
- 6.Kau TR, Way JC, Silver PA. Nuclear transport and cancer: from mechanism to intervention. Nat Rev Cancer. 2004;4:106–117. doi: 10.1038/nrc1274. [DOI] [PubMed] [Google Scholar]
- 7.Kau TR, Silver PA. Nuclear transport as a target for cell growth. Drug Discov Today. 2003;8:78–85. doi: 10.1016/s1359-6446(02)02562-x. [DOI] [PubMed] [Google Scholar]
- 8.Newlands ES, Rustin GJ, Brampton MH. Phase I trial of elactocin. Br J Cancer. 1996;74:648–649. doi: 10.1038/bjc.1996.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutka SC, Yang WQ, Dong SD, Ward SL, Craig DA, Timmermans PB, Murli S. Identification of nuclear export inhibitors with potent anticancer activity in vivo. Cancer Res. 2009;69:510–517. doi: 10.1158/0008-5472.CAN-08-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sells SF, Han SS, Muthukkumar S, Maddiwar N, Johnstone R, Boghaert E, Gillis D, Liu G, Nair P, Monnig S, Collini P, Mattson MP, Sukhatme VP, Zimmer SG, Wood DP, Jr, McRoberts JW, Shi Y, Rangnekar VM. Expression and function of the leucine zipper protein Par-4 in apoptosis. Mol Cell Biol. 1997;17:3823–3832. doi: 10.1128/mcb.17.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty M, Qiu SG, Vasudevan KM, Rangnekar VM. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 2001;61:7255–7263. [PubMed] [Google Scholar]
- 12.Cook J, Krishnan S, Ananth S, Sells SF, Shi Y, Walther MM, Linehan WM, Sukhatme VP, Weinstein MH, Rangnekar VM. Decreased expression of the proapoptotic protein Par-4 in renal cell carcinoma. Oncogene. 1999;18:1205–1208. doi: 10.1038/sj.onc.1202416. [DOI] [PubMed] [Google Scholar]
- 13.Kogel D, Reimertz C, Mech P, Poppe M, Fruhwald MC, Engemann H, Scheidtmann KH, Prehn JH. Dlk/ZIP kinase-induced apoptosis in human medulloblastoma cells: requirement of the mitochondrial apoptosis pathway. Br J Cancer. 2001;85:1801–1808. doi: 10.1054/bjoc.2001.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Bueno G, Fernandez-Marcos PJ, Collado M, Tendero MJ, Rodriguez-Pinilla SM, Garcia-Cao I, Hardisson D, az-Meco MT, Moscat J, Serrano M, Palacios J. Inactivation of the candidate tumor suppressor par-4 in endometrial cancer. Cancer Res. 2007;67:1927–1934. doi: 10.1158/0008-5472.CAN-06-2687. [DOI] [PubMed] [Google Scholar]
- 15.Zapata-Benavides P, Mendez-Vazquez JL, Gonzalez-Rocha TR, Zamora-Avila DE, Franco-Molina MA, Garza-Garza R, Rodriguez-Padilla C. Expression of prostate apoptosis response (Par-4) is associated with progesterone receptor in breast cancer. Arch Med Res. 2009;40:595–599. doi: 10.1016/j.arcmed.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed MM, Sheldon D, Fruitwala MA, Venkatasubbarao K, Lee EY, Gupta S, Wood C, Mohiuddin M, Strodel WE. Downregulation of PAR-4, a pro-apoptotic gene, in pancreatic tumors harboring K-ras mutation. Int J Cancer. 2008;122:63–70. doi: 10.1002/ijc.23019. [DOI] [PubMed] [Google Scholar]
- 17.Azmi AS, Ahmad A, Banerjee S, Rangnekar VM, Mohammad RM, Sarkar FH. Chemoprevention of pancreatic cancer: characterization of Par-4 and its modulation by 3,3' diindolylmethane (DIM) Pharm Res. 2008;25:2117–2124. doi: 10.1007/s11095-008-9581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azmi AS, Wang Z, Burikhanov R, Rangnekar VM, Wang G, Chen J, Wang S, Sarkar FH, Mohammad RM. Critical role of prostate apoptosis response-4 in determining the sensitivity of pancreatic cancer cells to small-molecule inhibitor-induced apoptosis. Mol Cancer Ther. 2008;7:2884–2893. doi: 10.1158/1535-7163.MCT-08-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azmi AS, Philip PA, Zafar SF, Sarkar FH, Mohammad RM. PAR-4 as a possible new target for pancreatic cancer therapy. Expert Opin Ther Targets. 2010;14:611–620. doi: 10.1517/14728222.2010.487066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Azmi AS, Philip PA, Beck FW, Wang Z, Banerjee S, Wang S, Yang D, Sarkar FH, Mohammad RM. MI-219-zinc combination: a new paradigm in MDM2 inhibitor-based therapy. Oncogene. 2011;30:117–126. doi: 10.1038/onc.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Azmi AS, Wang Z, Burikhanov R, Rangnekar VM, Wang G, Chen J, Wang S, Sarkar FH, Mohammad RM. Critical role of prostate apoptosis response-4 in determining the sensitivity of pancreatic cancer cells to small-molecule inhibitor-induced apoptosis. Mol Cancer Ther. 2008;7:2884–2893. doi: 10.1158/1535-7163.MCT-08-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Azmi AS, Aboukameel A, Banerjee S, Wang Z, Mohammad M, Wu J, Wang S, Yang D, Philip PA, Sarkar FH, Mohammad RM. MDM2 inhibitor MI-319 in combination with cisplatin is an effective treatment for pancreatic cancer independent of p53 function. Eur J Cancer. 2010;46:1122–1131. doi: 10.1016/j.ejca.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banerjee S, Zhang Y, Ali S, Bhuiyan M, Wang Z, Chiao PJ, Philip PA, Abbruzzese J, Sarkar FH. Molecular evidence for increased antitumor activity of gemcitabine by genistein in vitro and in vivo using an orthotopic model of pancreatic cancer. Cancer Res. 2005;65:9064–9072. doi: 10.1158/0008-5472.CAN-05-1330. [DOI] [PubMed] [Google Scholar]
- 25.Kudo N, Taoka H, Toda T, Yoshida M, Horinouchi S. A novel nuclear export signal sensitive to oxidative stress in the fission yeast transcription factor Pap1. J Biol Chem. 1999;274:15151–15158. doi: 10.1074/jbc.274.21.15151. [DOI] [PubMed] [Google Scholar]
- 26.Gurumurthy S, Goswami A, Vasudevan KM, Rangnekar VM. Phosphorylation of Par-4 by protein kinase A is critical for apoptosis. Mol Cell Biol. 2005;25:1146–1161. doi: 10.1128/MCB.25.3.1146-1161.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ullman KS, Powers MA, Forbes DJ. Nuclear export receptors: from importin to exportin. Cell. 1997;90:967–970. doi: 10.1016/s0092-8674(00)80361-x. [DOI] [PubMed] [Google Scholar]
- 28.Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 29.Fornerod M, Ohno M. Exportin-mediated nuclear export of proteins and ribonucleoproteins. Results Probl Cell Differ. 2002;35:67–91. doi: 10.1007/978-3-540-44603-3_4. [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Li Y, Ahmad A, Banerjee S, Azmi AS, Kong D, Sarkar FH. Pancreatic cancer: understanding and overcoming chemoresistance. Nat Rev Gastroenterol Hepatol. 2011;8:27–33. doi: 10.1038/nrgastro.2010.188. [DOI] [PubMed] [Google Scholar]