Abstract
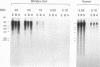
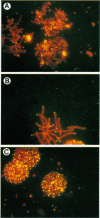
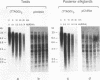
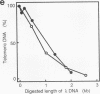
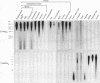
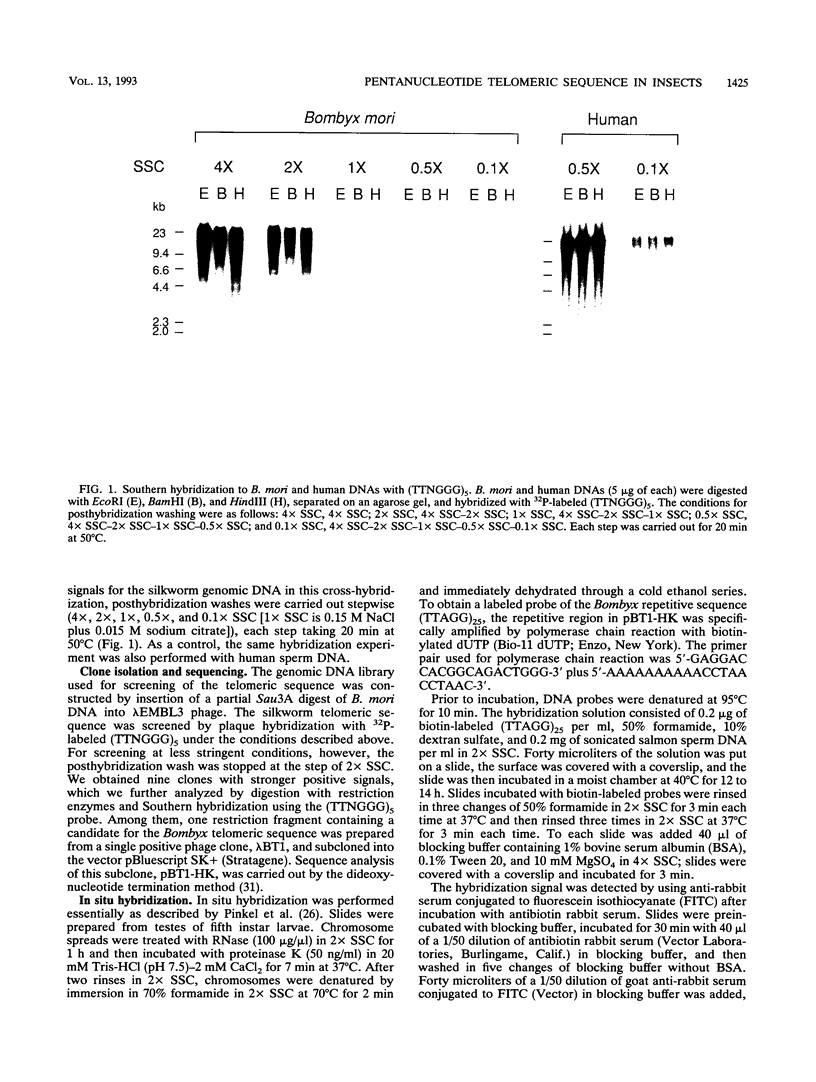
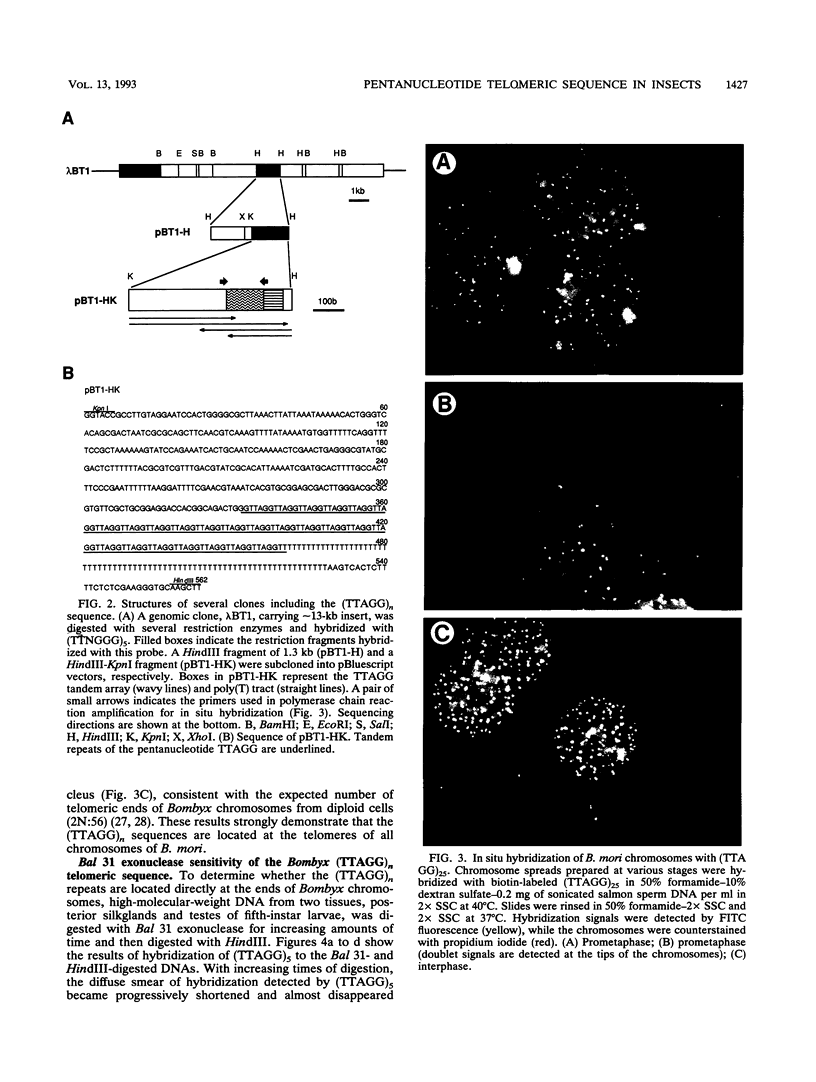
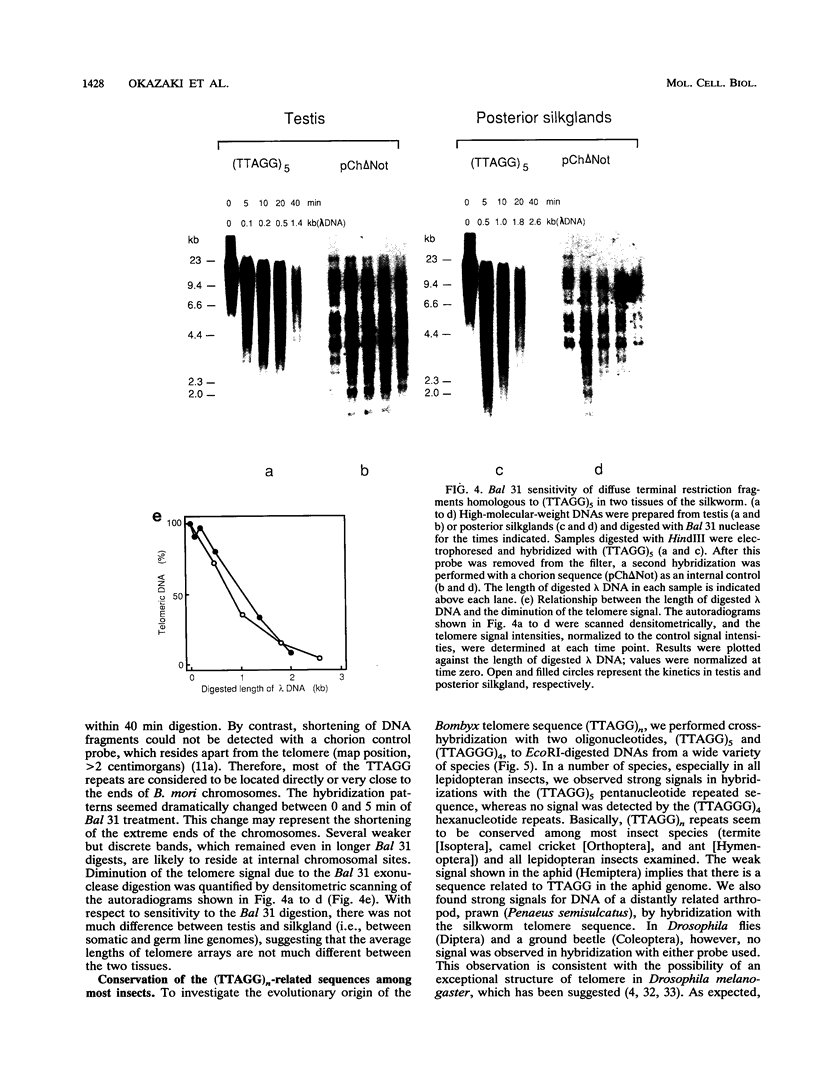
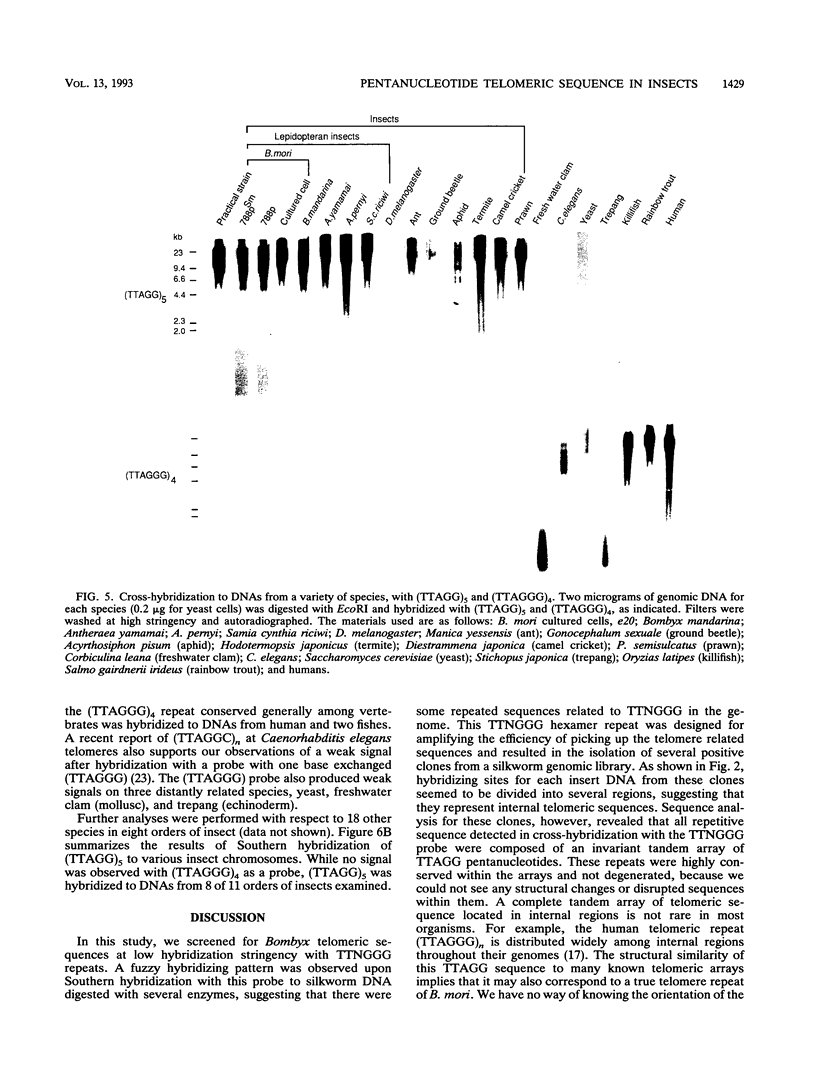
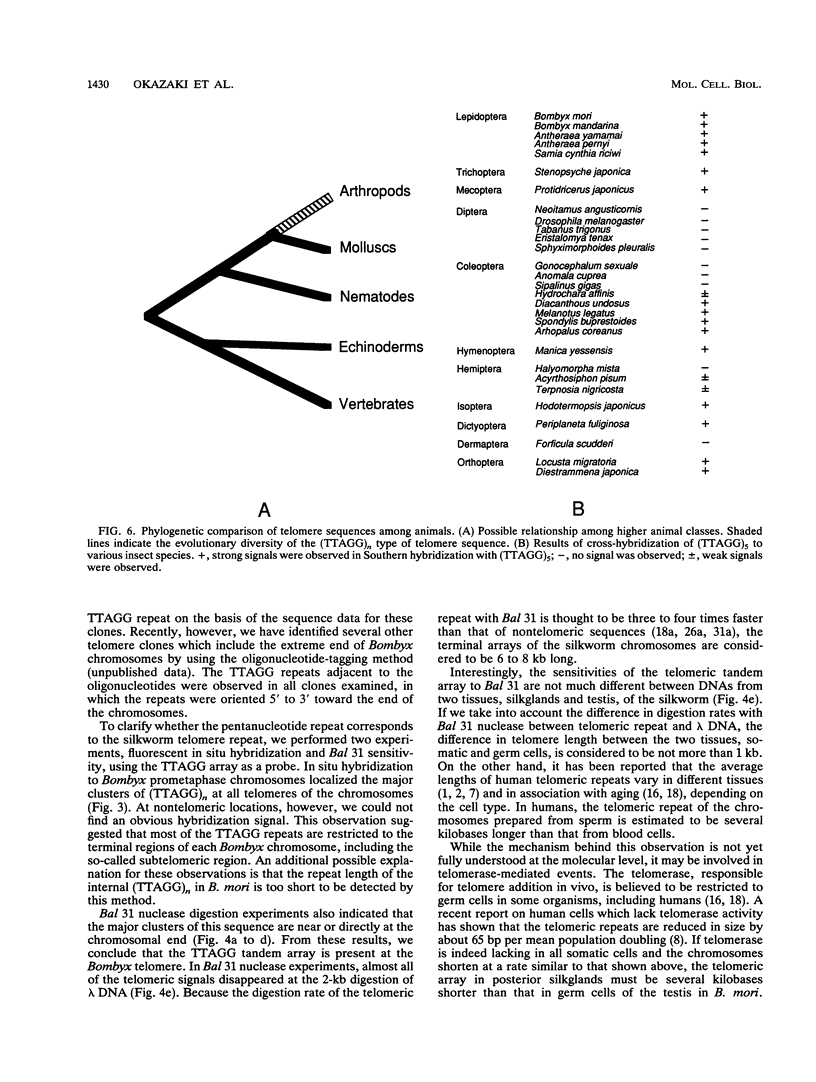
A pentanucleotide repetitive sequence, (TTAGG)n, has been isolated from a silkworm genomic library, using cross-hybridization with a (TTNGGG)5 sequence, which is conserved among most eukaryotic telomeres. Both fluorescent in situ hybridization and Bal 31 exonuclease experiments revealed major clusters of (TTAGG)n at the telomeres of all Bombyx chromosomes. To determine the evolutionary origin of this sequence, two types of telomeric sequence, (TTAGG)5 and a hexanucleotide repetitive sequence, (TTAGGG)4, which is conserved mainly among vertebrate and several invertebrate telomeres so far examined, were hybridized to DNAs from a wide variety of eukaryotic species under highly stringent hybridization conditions. The (TTAGGG)5 oligonucleotide hybridized to genomic DNAs from vertebrates and several nonvertebrate species, as has been reported so far, but not to any DNAs from insects. On the other hand, the Bombyx type of telomere sequence, (TTAGG)n, hybridized to DNAs from 8 of 11 orders of insect species tested but not to vertebrate DNAs, suggesting that this TTAGG repetitive sequence is conserved widely among insects.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allshire R. C., Dempster M., Hastie N. D. Human telomeres contain at least three types of G-rich repeat distributed non-randomly. Nucleic Acids Res. 1989 Jun 26;17(12):4611–4627. doi: 10.1093/nar/17.12.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allshire R. C., Gosden J. R., Cross S. H., Cranston G., Rout D., Sugawara N., Szostak J. W., Fantes P. A., Hastie N. D. Telomeric repeat from T. thermophila cross hybridizes with human telomeres. Nature. 1988 Apr 14;332(6165):656–659. doi: 10.1038/332656a0. [DOI] [PubMed] [Google Scholar]
- Biessmann H., Carter S. B., Mason J. M. Chromosome ends in Drosophila without telomeric DNA sequences. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1758–1761. doi: 10.1073/pnas.87.5.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biessmann H., Mason J. M., Ferry K., d'Hulst M., Valgeirsdottir K., Traverse K. L., Pardue M. L. Addition of telomere-associated HeT DNA sequences "heals" broken chromosome ends in Drosophila. Cell. 1990 May 18;61(4):663–673. doi: 10.1016/0092-8674(90)90478-w. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Dobson M. J., MacKinnon P. Telomere cloning and mammalian chromosome analysis. J Cell Sci. 1990 Apr;95(Pt 4):521–526. doi: 10.1242/jcs.95.4.521. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Smith B. A. Variability at the telomeres of the human X/Y pseudoautosomal region. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):213–219. doi: 10.1101/sqb.1986.051.01.026. [DOI] [PubMed] [Google Scholar]
- Counter C. M., Avilion A. A., LeFeuvre C. E., Stewart N. G., Greider C. W., Harley C. B., Bacchetti S. Telomere shortening associated with chromosome instability is arrested in immortal cells which express telomerase activity. EMBO J. 1992 May;11(5):1921–1929. doi: 10.1002/j.1460-2075.1992.tb05245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara H., Ishikawa H. Structure of the Bombyx mori rDNA: initiation site for its transcription. Nucleic Acids Res. 1987 Feb 11;15(3):1245–1258. doi: 10.1093/nar/15.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage L. P. Polyploidization of the silk gland of Bombyx mori. J Mol Biol. 1974 Jun 15;86(1):97–108. doi: 10.1016/s0022-2836(74)80010-0. [DOI] [PubMed] [Google Scholar]
- Goldsmith M. R., Kafatos F. C. Developmentally regulated genes in silkmoths. Annu Rev Genet. 1984;18:443–487. doi: 10.1146/annurev.ge.18.120184.002303. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989 Jan 26;337(6205):331–337. doi: 10.1038/337331a0. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985 Dec;43(2 Pt 1):405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- Greider C. W., Blackburn E. H. The telomere terminal transferase of Tetrahymena is a ribonucleoprotein enzyme with two kinds of primer specificity. Cell. 1987 Dec 24;51(6):887–898. doi: 10.1016/0092-8674(87)90576-9. [DOI] [PubMed] [Google Scholar]
- Harley C. B., Futcher A. B., Greider C. W. Telomeres shorten during ageing of human fibroblasts. Nature. 1990 May 31;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Allshire R. C. Human telomeres: fusion and interstitial sites. Trends Genet. 1989 Oct;5(10):326–331. doi: 10.1016/0168-9525(89)90137-6. [DOI] [PubMed] [Google Scholar]
- Hastie N. D., Dempster M., Dunlop M. G., Thompson A. M., Green D. K., Allshire R. C. Telomere reduction in human colorectal carcinoma and with ageing. Nature. 1990 Aug 30;346(6287):866–868. doi: 10.1038/346866a0. [DOI] [PubMed] [Google Scholar]
- Katinka M. D., Bourgain F. M. Interstitial telomeres are hotspots for illegitimate recombination with DNA molecules injected into the macronucleus of Paramecium primaurelia. EMBO J. 1992 Feb;11(2):725–732. doi: 10.1002/j.1460-2075.1992.tb05105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyne J., Baker R. J., Hobart H. H., Hsu T. C., Ryder O. A., Ward O. G., Wiley J. E., Wurster-Hill D. H., Yates T. L., Moyzis R. K. Distribution of non-telomeric sites of the (TTAGGG)n telomeric sequence in vertebrate chromosomes. Chromosoma. 1990 Apr;99(1):3–10. doi: 10.1007/BF01737283. [DOI] [PubMed] [Google Scholar]
- Meyne J., Ratliff R. L., Moyzis R. K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin G. B. The human telomere terminal transferase enzyme is a ribonucleoprotein that synthesizes TTAGGG repeats. Cell. 1989 Nov 3;59(3):521–529. doi: 10.1016/0092-8674(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Murakami A., Imai H. T. Cytological evidence for holocentric chromosomes of the silkworms, Bombyx mori and B. mandarina, (Bombycidae, Lepidoptera). Chromosoma. 1974;47(2):167–178. doi: 10.1007/BF00331804. [DOI] [PubMed] [Google Scholar]
- Müller F., Wicky C., Spicher A., Tobler H. New telomere formation after developmentally regulated chromosomal breakage during the process of chromatin diminution in Ascaris lumbricoides. Cell. 1991 Nov 15;67(4):815–822. doi: 10.1016/0092-8674(91)90076-b. [DOI] [PubMed] [Google Scholar]
- Pimpinelli S., Goday C. Unusual kinetochores and chromatin diminution in Parascaris. Trends Genet. 1989 Sep;5(9):310–315. doi: 10.1016/0168-9525(89)90114-5. [DOI] [PubMed] [Google Scholar]
- Pinkel D., Gray J. W., Trask B., van den Engh G., Fuscoe J., van Dekken H. Cytogenetic analysis by in situ hybridization with fluorescently labeled nucleic acid probes. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):151–157. doi: 10.1101/sqb.1986.051.01.018. [DOI] [PubMed] [Google Scholar]
- Raibaud A., Gaillard C., Longacre S., Hibner U., Buck G., Bernardi G., Eisen H. Genomic environment of variant surface antigen genes of Trypanosoma equiperdum. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4306–4310. doi: 10.1073/pnas.80.14.4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S. W. The meotic prophase in Bombyx mori females analyzed by three dimensional reconstructions of synaptonemal complexes. Chromosoma. 1976 Feb 23;54(3):245–293. doi: 10.1007/BF00293453. [DOI] [PubMed] [Google Scholar]
- Rasmussen S. W. The transformation of the Synaptonemal Complex into the 'elimination chromatin' in Bombyx mori oocytes. Chromosoma. 1977 Apr 19;60(3):205–221. doi: 10.1007/BF00329771. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. Isolation of a telomeric DNA sequence from Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1041–1046. doi: 10.1101/sqb.1978.042.01.104. [DOI] [PubMed] [Google Scholar]
- Saiga H., Edström J. E. Long tandem arrays of complex repeat units in Chironomus telomeres. EMBO J. 1985 Mar;4(3):799–804. doi: 10.1002/j.1460-2075.1985.tb03700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shampay J., Szostak J. W., Blackburn E. H. DNA sequences of telomeres maintained in yeast. Nature. 1984 Jul 12;310(5973):154–157. doi: 10.1038/310154a0. [DOI] [PubMed] [Google Scholar]
- Traverse K. L., Pardue M. L. Studies of He-T DNA sequences in the pericentric regions of Drosophila chromosomes. Chromosoma. 1989 Jan;97(4):261–271. doi: 10.1007/BF00371965. [DOI] [PubMed] [Google Scholar]
- Valgeirsdóttir K., Traverse K. L., Pardue M. L. HeT DNA: a family of mosaic repeated sequences specific for heterochromatin in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7998–8002. doi: 10.1073/pnas.87.20.7998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B. S., Pession A., Traverse K. L., French C., Pardue M. L. Telomere regions in Drosophila share complex DNA sequences with pericentric heterochromatin. Cell. 1983 Aug;34(1):85–94. doi: 10.1016/0092-8674(83)90138-1. [DOI] [PubMed] [Google Scholar]
- Zahler A. M., Prescott D. M. Telomere terminal transferase activity in the hypotrichous ciliate Oxytricha nova and a model for replication of the ends of linear DNA molecules. Nucleic Acids Res. 1988 Jul 25;16(14B):6953–6972. doi: 10.1093/nar/16.14.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A. Structure and function of telomeres. Annu Rev Genet. 1989;23:579–604. doi: 10.1146/annurev.ge.23.120189.003051. [DOI] [PubMed] [Google Scholar]