Abstract
OBJECTIVE
When exposed to heat stress, increases in cutaneous blood flow and sweating in well-healed grafted skin are severely attenuated, which could impair whole-body heat loss if skin grafts cover a large portion of total body surface area (TBSA). It is unknown to what extent whole-body heat loss is impaired when skin grafts cover a significant (e.g., >50%) proportion of TBSA.
METHODS
We examined whole-body heat exchange during and following 60-min of cycling exercise in the heat (35°C, 25% RH) at a fixed rate of metabolic heat production (~400W) in a female (age = 36 years; mass = 78.2 kg) with well-healed (17+ years) skin grafts covering 75% of TBSA. Her responses were compared to 2 non-injured control subjects. Whole-body evaporative and dry heat exchange were measured by direct calorimetry.
RESULTS
While exercising in the same ambient conditions and at the same rate of heat production, relative evaporative heat loss of non-grafted skin in the grafted subject (i.e., evaporative heat loss per m2) was nearly twice that of the control subjects. However total rate of evaporative heat loss reached only 59% of the amount required for heat balance in the skin-grafted subject compared to 92±3% in controls. Thus, the increase in core temperature was two-fold greater for the grafted (1.22°C) versus control (0.61±0.19°C) individuals.
CONCLUSION
This case study demonstrates that a large area of grafted skin greatly diminishes maximum evaporative heat loss during exercise in the heat, making a compensable environment for control subjects uncompensable for skin-grafted individuals.
Keywords: calorimeter, hyperthermia, thermoregulation, burn
INTRODUCTION
Every year in the US approximately 9,000 individuals experience burns that cover 20% or more of their total body surface area (TBSA).1 Many severe burns require the removal of the dermal layer and a subsequent skin graft. Although split-thickness skin grafts help repair the damaged area, the blood vessels and sweat glands necessary for temperature regulation remain damaged.2, 3 When an individual is exposed to a heat stress, cutaneous vasodilation and sweating in well-healed grafted skin is severely impaired compared to adjacent non-damaged skin.4, 5 Likewise, grafted skin does not vasodilate or sweat appropriately upon exogenous administration of local vasodilators and sudorific drugs (i.e., sodium nitroprusside and acetylcholine), suggesting post-synaptic impairments4, 6 which are not resolved 4 to 8 years post-surgery.4 These impairments in grafted skin become barriers to whole-body heat dissipation, especially when grafted skin represents a significant proportion of TBSA.7–9 During physical activity, increases in metabolic heat production that are not properly compensated for by adequate heat loss responses (e.g., skin vasodilation and sweating) can lead to dangerous increases in core body temperature. Therefore, the ability of individuals with split-thickness grafts to safely participate in physical activity may be limited by their capacity to dissipate heat, especially when physical activity is performed in the heat.
A change in core body temperature is typically used to assess one’s ability to adequately dissipate heat. For example, differences in core body temperature between population groups are thought to be indicative of inverse responses to skin vasodilation and sweating (i.e., greater increases in core body temperature due to attenuated increases in skin blood flow and sweating). Some studies suggest that core temperature responses to physical activity in the heat may be exacerbated in individuals with ≤55% of TBSA of grafted skin.7, 8, 10 However, core temperature alone does not provide an accurate representation of one’s ability to dissipate heat. In some cases, greater increases in core temperature are not paralleled by a reduced capacity to dissipate heat.11 Whole-body calorimetry is a gold standard method to directly determine whole-body evaporative heat loss from sweat production and dry heat exchange with the surrounding environment. The evaluation of whole-body heat exchange during physical activity in the heat in individuals with grafted skin covering a significant portion of TBSA (i.e., >55%) has not been performed.
Therefore, the purpose of this case report was to compare the rates of whole-body evaporative and dry heat exchange, as well as the change in body heat content, in an individual with well-healed split-thickness skin grafts covering 75% of her body relative to control, non-injured individuals during and following exercise in the heat performed at a fixed rate of metabolic heat production.
CASE DESCRIPTION
Prior to testing, subjects signed an informed consent form approved by the University of Ottawa Health Sciences and Science Research Ethics Board. A recreationally active female with well-healed split-thickness skin grafts (17+ years since last surgery) covering 75% of her TBSA participated in this investigation (age = 36 years; mass = 78.2 kg; height = 170 cm). Her responses were compared to two female control subjects with similar physical characteristics (Table 1). None of the subjects were taking medications at the time of testing.
Table 1.
Participant characteristics.
| Age (yrs) |
Height (cm) |
Mass (kg) |
AD (m2) |
VO2max (l·min−1) |
VO2max (mLO2·kg−1·min−1) |
|
|---|---|---|---|---|---|---|
| Control #1 | 34 | 165 | 76.0 | 1.83 | 3.38 | 44.5 |
| Control #2 | 37 | 170 | 73.5 | 1.85 | 3.53 | 48.0 |
| Mean ± SD for Controls | 36 ± 2 | 168 ± 4 | 74.8 ± 1.8 | 1.84 ± .01 | 3.45 ± 0.11 | 46.3 ± 2.47 |
| Grafted Subject | 36 | 170 | 78.2 | 1.90 | 3.35 | 43.3 |
AD, body surface area.
Prior to experimental testing, subjects underwent incremental cycle ergometer testing to determine maximal oxygen uptake (VO2max). From measured height and mass, body surface area was calculated according to the formula of DuBois and DuBois.12 Rule of Nine’s was used to calculate skin area covered by split-thickness grafts.13 Experimental testing occurred in a whole-body direct calorimeter (see below for description) regulated to an air temperature of 35°C, a relative humidity of 25%, and an air mass flow of ~5.65 kg of air·min−1. Subjects rested for 30 min in the calorimeter followed by 60 min of upright seated cycling at a fixed rate of metabolic heat production equal to 400 W. Preliminary testing indicated that these test conditions would be achievable by all subjects without core temperature rising to dangerous levels (i.e. >39.5°C). Following exercise, subjects remained seated resting in the calorimeter for 45 min.
Core body temperature was measured with an ingestible temperature pill (HQ, Palmetto, FL, USA) swallowed upon arrival at the laboratory (~60 min prior to testing). Mean skin temperature was calculated from skin temperature at 4 points (upper arm, upper back, upper leg and calf) using 0.3 mm diameter T-type (copper/constantan) thermocouples (Concept Engineering, Old Saybrook, CT, USA). Heart rate (HR) was measured using a Polar Advantage interface and Polar Precision Performance software (Polar Electro OY, Kempele, Finland). Rating of Perceived Exertion (RPE) was measured every 15 min during exercise using Borg’s 6–20 scale.14 Thermal Comfort (‘0 = Neutral’ to ‘7 = Extremely Hot’) was recorded at baseline resting, every 15 min during exercise and at the end of the 45 min recovery period.
Temperature data were collected using a HP Agilent data acquisition module (model 3497A) at a sampling rate of 15 s. Data were simultaneously displayed and recorded in spreadsheet format on a personal computer (IBM ThinkCentre M50) with LabVIEW software (Version 7.0, National Instruments, TX, USA).
Whole-Body Direct Calorimetry
Heat exchange between the human body and surrounding environment is determined by the heat balance equation:15
S = M ± W ± (R+C) − E
Where: S is rate of body heat storage, M is metabolic rate, W is rate of external work, R+C is rate of dry exchange, and E is rate of evaporative heat loss (all units in Watts).
The modified Snellen direct air calorimeter was used for the direct measurement of whole-body dry and evaporative heat exchange. A full description of the technical aspects of measurements is provided elsewhere,16
Rate of evaporative heat loss (E) was calculated from the calorimetry data every minute using the following equation:
E = [(Massflow · (Humidityout − Humidityin) · 2426] / 60
where mass flow is the rate of air mass flow (kg air s−1); and 2426 is the latent heat of vaporization of sweat at 30°C (J g sweat−1).17
Rate of dry heat exchange (R+C) from radiation (R), and conduction/convection (C) was calculated from the calorimetry data every minute using the following equation:
R+C = [(Massflow · (Temperatureout-Temperaturein) · 1005] / 60
where mass flow is the rate of flow of air mass ((kg air)·s−1); (Temperatureout – Temperaturein) is the calorimeter outflow-inflow difference in air temperature (°C), and 1005 is the specific heat of air (J · (kg air)−1 · °C).
Total heat loss was calculated by adding together dry and evaporative heat exchange, and total heat loss per area of non-grafted skin was calculated by dividing total heat loss by TBSA of non-grafted skin for each subject. A 6 l fluted mixing box housed within the calorimeter was utilized for the concurrent measurement of metabolic energy expenditure (M). Expired gas was analyzed for oxygen (error of ±0.01%) and carbon dioxide concentrations (error of ±0.02%) using calibrated electrochemical gas analyzers (AMETEK model S-3A/1 and CD 3A, Applied Electrochemistry, Pittsburgh, PA, USA). Expired air was recycled back into the calorimeter chamber in order to account for respiratory dry and evaporative heat loss. Rate of metabolic energy expenditure was calculated from minute-average values for oxygen consumption and the respiratory exchange ratio.18 The calorimetry data were then used to calculate change in body heat content (ΔHb) using the following equation:
ΔHb @ time (t) = (M − [HE + HD] −W) dt
where: M is metabolic rate; HE is rate of evaporative heat loss; HD is rate of dry heat exchange; and W is rate of external work.
To quantify the extent to which evaporative heat loss is impaired by skin grafts, differences between groups in evaporative heat loss were compared in relation to the required evaporation for heat balance (Ereq),19 which was calculated as: Ereq = (M−W) − (R+C), where M is metabolic rate, W is rate of external work, and R+C is rate of dry heat exchange.
RESULTS
Pre-exercise
Core temperature at the end of the 15 min pre-exercise rest period was 37.57°C for the grafted subject versus 37.28 ± 0.10°C for the control subjects. Mean skin temperature was 34.36°C for the grafted participant versus control 34.67 ± 0.37°C for control subjects (Figure 1). Thermal comfort after 15 min of seated rest in the heat prior to exercise was 1 ± 0 for the controls and 3 for the grafted subject. Heart rate in controls was 71 ± 1 bpm compared to 80 bpm in the skin-grafted subject prior to exercise.
Figure 1.

Mean skin temperature (left panel) and change in core temperature from rest (right panel), during 15 min of baseline rest, 60 min of exercise and 45 min resting recovery.
Exercise response
During exercise, the control subjects exercised at an external workload of 81 ± 1 W, while the grafted subject exercised at 50 W. By design, rate of metabolic heat production was similar between the skin-grafted individual (419 W) and matched controls (394 ± 3 W). Since ambient temperature was close to mean skin temperature, rate of dry heat exchange was minimal during baseline rest. During exercise, rate of dry heat exchange became slightly negative (representing a dry heat gain from the environment) for the control subjects. In contrast, rate of dry heat exchange became progressively more positive (representing a greater rate of dry heat loss to the environment) during exercise for the grafted subject (Figure 2). Evaporative heat loss represented the main avenue of heat exchange during exercise, accounting for more than 90% of total heat loss in all subjects. However, due to the differences in dry heat exchange, the required evaporation needed for heat balance at the end of exercise was 398 W in the skin-grafted subject relative to 439 ± 35 W for the control subjects (Figure 3).
Figure 2.
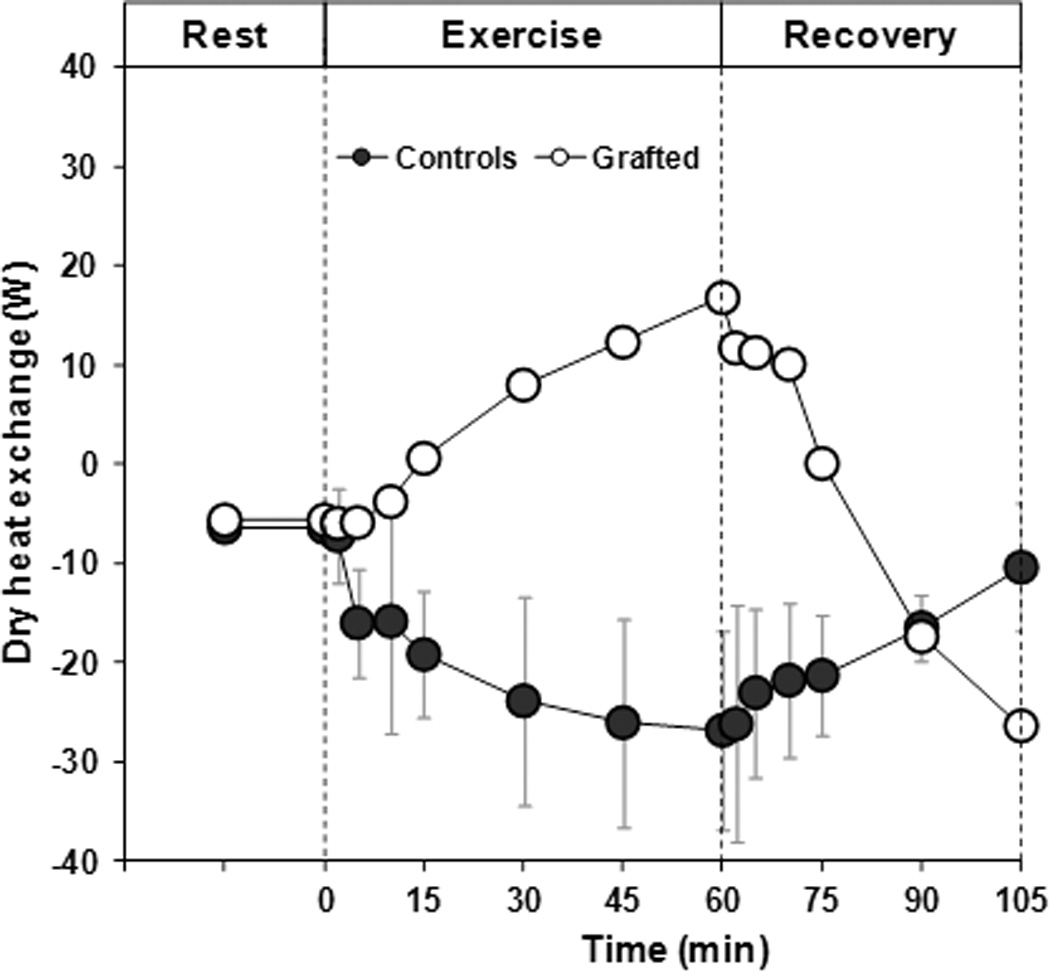
Rate of dry heat exchange during 15 min of baseline rest, 60 min of exercise and a 45 min resting recovery. Note that a positive value represents a dry heat loss from the body to the environment, while a negative value represents a dry heat gain from the environment to the body.
Figure 3.

Required evaporation for heat balance (Ereq) and evaporative heat loss (He) in control (left panel) and skin grafted (right panel) subjects during 15 min of baseline rest, 60 min of exercise and 45 min resting recovery.
Despite a lower required evaporation for heat balance, rate of evaporative heat loss in the skin-grafted subject reached only 59% of the value required for heat balance, compared to 92 ± 5% in controls (Figure 3). This difference was due to a rate of evaporative heat loss in the skin-grafted subject which was 47% lower than control subjects. When evaporative heat loss was expressed relative to area of non-grafted skin, it was nearly twice as much in the skin-grafted subject compared to control subjects (167%; Figure 4). The lower rate of evaporative heat loss in the skin-grafted subject resulted in a change in body heat content during exercise of +663 kJ, representing a 3-fold greater change than control subjects (+224 ± 58 kJ). The greater change in body heat content was paralleled by greater increases in end-exercise mean skin (Figure 1) and core body temperature (grafted subjects: 37.57 to 38.79°C; control subjects: 37.28 ± 0.10 to 37.90 ± 0.30°C; Figure 1). Thermal sensation at end-exercise averaged 3 ± 1 and 5 ± 1 for the control and grafted subjects, respectively.
Figure 4.
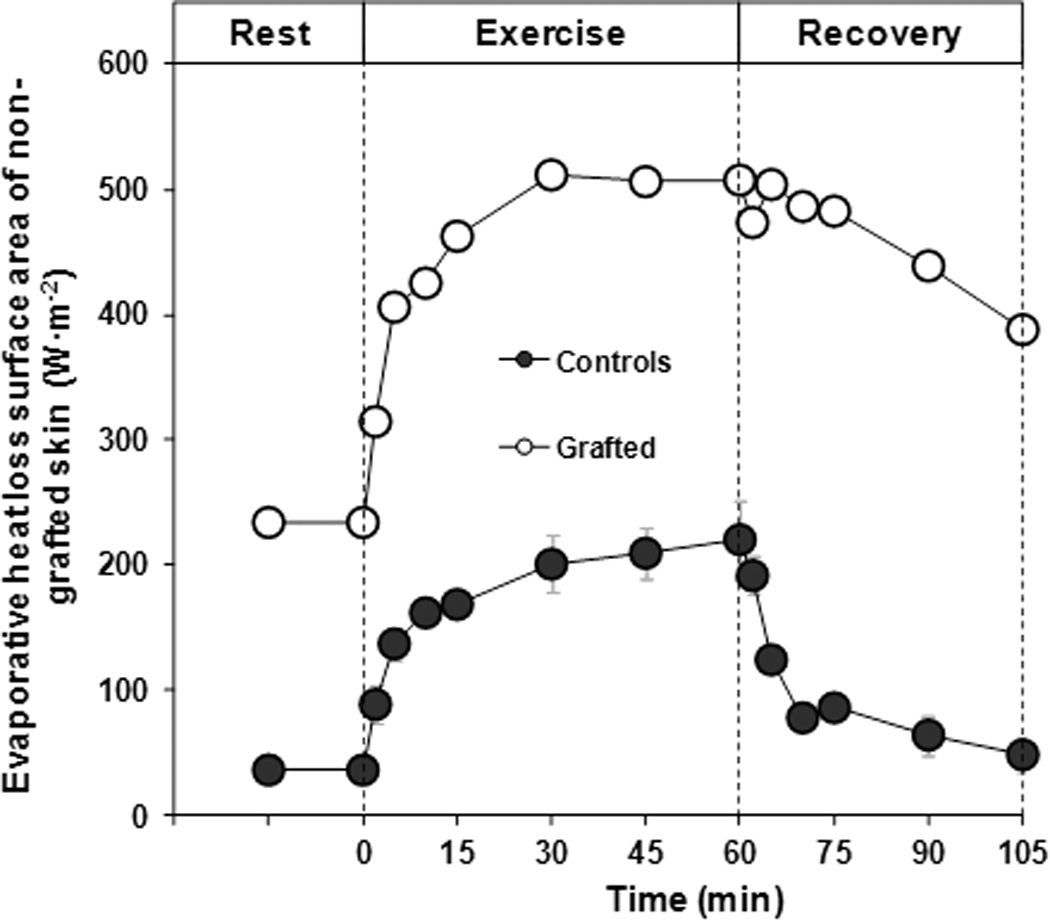
Evaporative heat loss per area of non-grafted skin during 15 min of baseline rest, 60 min of exercise and 45 min resting recovery.
The rating of perceived exertion during exercise was comparable between controls (12 ± 0) and the grafted subject (13 ± 1). This was paralleled by a similar HR response during exercise between controls (122 ± 11 bpm) and the skin-grafted subject (121 ± 6 bpm).
Post-exercise response
Interestingly, rate of evaporative heat loss for the grafted subject remained elevated near end-exercise values during the recovery period. This is in sharp contrast to the control subjects in which rate of evaporative heat loss returned to near baseline values within 30 min post-exercise (Figure 3). As such, changes in body heat content post-exercise were greater for the grafted (−169 kJ) versus control (−79 ± 41 kJ) subjects. Nonetheless, combining exercise and post-exercise changes, net body heat content for the control subjects (+145 ± 17 kJ) was still more than 3-fold less than the grafted subject (+494 kJ). Consequently, the grafted subject still had a greater absolute and change in core temperature throughout the experimental trial compared to the control subjects (Figure 1). Heart rate during 45 min of recovery was lower in control subjects (88 ± 11 bpm) compared to the skin-grafted subject (106 ± 12 bpm).
DISCUSSION
The purpose of this case report was to examine whole-body evaporative and dry heat exchange during exercise in the heat of an individual with skin grafts covering the majority of her body (75% of TBSA). We show that this individual exhibits a reduced capacity to dissipate heat when exercise is performed in warm ambient conditions at a moderate fixed rate of heat production, and therefore a similar requirement for heat loss, relative to controls with no grafted skin. Specifically, we show that in this setting 1) whole-body evaporative heat loss is severely impaired when significant portions of the TBSA are grafted, and 2) relative evaporative heat loss of non-grafted skin in the grafted subject was nearly twice that of the control subjects. Thus, despite normal skin blood flow and sweating responses in non-grafted skin, skin grafts which cover the majority of the body and have impaired or absence of vasomotor and sudomotor function5 limit the body’s ability to dissipate heat (see below).
Unique to this study is the direct assessment of whole-body evaporative heat loss from sweat production in relation to the evaporation needed to achieve heat balance. Heat balance occurs when whole-body heat loss is equal to the combination of metabolic heat production and dry heat exchange; resulting in a steady-state core temperature. Contrary to previous studies that only examined differences in core temperature,7, 8, 10, 20 we were able to directly examine the extent to which skin grafts impair whole-body heat loss when a significant portion of TBSA is grafted. In the current study, control participants and the skin graft individual exercised at the same rate of metabolic heat production in the same environmental conditions. This ensured that the required evaporation for heat balance was similar between groups (Figure 3). In the control subjects, whole-body sweat production was sufficient to attain evaporative heat loss values that nearly balanced the combination of metabolic heat production and dry heat exchange. In contrast, it is clearly evident that whole-body sweat production was not sufficient to attain heat balance in the skin graft individual, as the level of evaporative heat loss achieved was markedly below the required evaporation for heat balance. The extent to which skin grafts impair whole-body evaporation is best exemplified by the fact that the control subjects attained ~93% of the value required to attain heat balance, compared to only 59% in the skin-grafted subject.
The relationship between evaporative heat loss and the required evaporation needed for heat balance is physiologically important because it dictates whether any combination of environmental conditions and metabolic heat production will be compensable or not. A compensable heat stress situation is exemplified by an increase in core temperature that eventually attains a plateau when heat production is balanced by heat dissipation. In contrast, an uncompensable condition occurs when heat production exceeds the body’s ability to dissipate heat, and core temperature continuously increases. Although the combination of environmental conditions and metabolic heat in the present study were compensable for the control subjects, they represented an uncompensable heat stress in the skin-grafted individual. This is evidenced by the rapid, continuous increase in core temperature (Figure 1B). Prior investigations examining core temperature responses in individuals with skin grafts while exercising in the heat have mixed findings.7, 8, 10, 20, 21 Generally, individuals with a greater proportion of TBSA grafted have greater impairments in thermoregulation (i.e., greater increases in core temperature versus controls). However this finding is not consistent. For example, Austin et. al.21 observed that individuals with as little as 35% and as much as 90% TBSA grafted had similar impairments in thermoregulation compared to controls. The purpose of the current study was not to identify the percentage TBSA of grafted skin necessary to impaired thermoregulation. It is possible that mixed findings between and within prior studies is likely due to varying levels of metabolic heat production generated by the subjects.7, 8, 10, 20, 21 By clamping metabolic heat production, we were able to accurately quantify thermoregulatory response between graft and non-grafted subjects. Identifying impairments in thermoregulation and the resulting greater increase in core temperature is important because it implies that individuals with 75% TBSA grafted may be at a greater risk for heat illnesses such as heat exhaustion and heat stroke.
It is interesting to note that the skin-grafted subject maintained relatively greater levels of evaporative heat loss during the recovery period, which resulted in an approximately 2-fold greater loss of body heat content. This may be caused by a compensatory response to partially compensate for the greater heat gain during exercise, whereby the greater end-exercise and post-exercise core temperature creates a drive to maintain sweat production at elevated levels for a prolonged period. Nonetheless, the elevated levels of evaporative heat loss were not sufficient to offset the greater heat gain during exercise, and the skin-grafted subject ended the recovery period with a total change in body heat content that was approximately 3-fold greater than controls.
The primary explanation for the attenuated heat loss response in the grafted individual is due to impaired evaporative heat exchange (Figure 3B). The grafted individual had evaporative heat exchange values that were 47% lower than control subjects. This is likely due to the fact that grafted skin has little or no increases in sweating when exposed to heat stress.4, 5 These impairments are post-synaptic in nature4, 6 and are not resolved 4 to 8 years post-surgery.4 From the present investigation, it is clear that these local deficiencies carry over to marked reductions in the body’s overall capacity to dissipate heat. Although grafted skin has a compromised vasomotor and sudomotor response during heat stress,4, 5 it is largely unknown if the non-injured skin of an individual with well-healed skin grafts demonstrates a compensatory or adaptive response (i.e., has an elevated capacity to dissipate heat relative to individuals without grafted skin). In order to provide insight into this question, we divided evaporative heat loss by body surface area of non-grafted/non-injured skin for both groups. This provides an estimate of the evaporation (i.e. sweating) that occurs per meter squared of non-grafted skin (i.e., relative evaporative heat loss for non-injured skin). Relative evaporative heat loss of non-grafted skin in the grafted subject was nearly twice that of the control subjects (Figure 4). One possible explanation for this response is that the greater increase in core temperature, relative to controls, resulted in a greater thermoregulatory drive for sweating. Additionally, it is possible that the 25% of non-grafted skin of the grafted subject had an adaptive or compensatory increase in skin blood flow and sweating, such as that which occurs during heat acclimation,22 compared to the non-injured skin of the controls. It is also possible that given the extended period of time since this individual completed her surgeries (17+ years), her grafted skin may have “healed” and was able to at least partially contribute to whole body heat loss. Davis et al.4 showed that impairments in skin blood flow and sweating of grafted skin in individuals 2–3 years post-surgery were similar to individuals 4–8 years post-surgery.4 Thus it is unlikely that the duration since the grafted subject had surgery played a role in the grafted skin’s ability, or lack thereof, to dissipate heat.
It should be recognized that although grafted skin has an impaired ability to dissipate heat,4 some individuals show small increases in skin blood flow, and perhaps sweating, in their grafted skin when exposed to heat stress.4 Thus, our estimation of evaporative heat loss per area of non-grafted skin may be slightly over-estimated, but it is unlikely to explain the large differences observed between grafted and non-grafted subjects (i.e., 167% greater relative heat loss; Figure 4). It is important to point out that although the non-grafted skin in this grafted individual had a greater ability to dissipate heat, the individual was still unable to achieve heat balance due to the significant portion of body surface area covered by skin grafts. Thus whole-body heat loss remained attenuated as evidenced by the greater change in body heat content and elevations in core temperature.
In summary, when metabolic heat production was matched between subjects, evaporative heat loss was greatly compromised in an individual with 75% TBSA of split-thickness grafts, which led to a continuous increase in core temperature relative to control subjects. Despite the increased capacity of the non-grafted skin to lose heat via evaporation in the grafted subject, whole-body heat loss was severely compromised exemplified by the fact that the skin-grafted subject only attained 59% of the value required for heat balance compared to 92% in the control subjects. It can be concluded that the combination of exercise intensity and environmental conditions which was compensable for the control subjects, represented an uncompensable heat stress for the grafted subject. This suggests that individuals with significant portions of their body covered with skin grafts are at a greater risk for heat illnesses such as heat exhaustion and heat stroke, particularly during exercise in hot environments.
ACKNOWLEDGMENTS
We thank the subjects for their participation.
Source of Funding: This work was supported by the National Institutes of General Medical Sciences Grant GM068865, the Natural Sciences and Engineering Research Council (RGPIN-298159-2009), and Leaders Opportunity Fund from the Canada Foundation for Innovation (22529). G.P. Kenny is supported by a University of Ottawa Research Chair in Environmental Physiology.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
Conflicts of Interest: The authors have no conflict of interest to declare.
REFERENCES
- 1.Anonymous . American Burn Association National Burn Repository (2006 Report) Chicago, IL: American Burn Association; 2007. [Google Scholar]
- 2.Ablove RH, Howell RM. The physiology and technique of skin grafting. Hand Clin. 1997;13:163–173. [PubMed] [Google Scholar]
- 3.Ponten B. Grafted skin. Acta chirurgica Scandinavica. 1960;(Suppl 257):1–78. [PubMed] [Google Scholar]
- 4.Davis SL, Shibasaki M, Low DA, et al. Sustained impairments in cutaneous vasodilation and sweating in grafted skin following long-term recovery. J Burn Care Res. 2009;30:675–685. doi: 10.1097/BCR.0b013e3181abfd43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis SL, Shibasaki M, Low DA, et al. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res. 2007;28:427–434. doi: 10.1097/BCR.0B013E318053D312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis SL, Shibasaki M, Low DA, et al. Skin grafting impairs postsynaptic cutaneous vasodilator and sweating responses. J Burn Care Res. 2007;28:435–441. doi: 10.1097/BCR.0B013E318053d32E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roskind JL, Petrofsky J, Lind AR, Paletta FX. Quantitation of thermoregulatory impairment in patients with healed burns. Ann Plas Surg. 1978;1:172–176. doi: 10.1097/00000637-197803000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Shapiro Y, Epstein Y, Ben-Simchon C, Tsur H. Thermoregulatory responses of patients with extensive healed burns. J Appl Physiol. 1982;53:1019–1022. doi: 10.1152/jappl.1982.53.4.1019. [DOI] [PubMed] [Google Scholar]
- 9.Wingo JE, Low DA, Keller DM, et al. Heat acclimation of an adult female with a large surface area of grafted skin. J Burn Care Res. 2008;29:848–851. doi: 10.1097/BCR.0b013e3181848b5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Simchon C, Tsur H, Keren G, Epstein Y, Shapiro Y. Heat tolerance in patients with extensive healed burns. Plast Reconstr Surg. 1981;67:499–504. doi: 10.1097/00006534-198104000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Gagnon D, Dorman LE, Jay O, Hardcastle S, Kenny GP. Core temperature differences between males and females during intermittent exercise: Physical considerations. Eur J Appl Physiol. 2009;105:453–461. doi: 10.1007/s00421-008-0923-3. [DOI] [PubMed] [Google Scholar]
- 12.DuBois D, Dubois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Med. 1916;17:863–871. [Google Scholar]
- 13.Sakson JA. Simplified chart for estimating burn areas. Am J Surg. 1959;98:693–694. doi: 10.1016/0002-9610(59)90492-1. [DOI] [PubMed] [Google Scholar]
- 14.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- 15.Bligh J, Johnson KG. Glossary of terms for thermal physiology. J Appl Physiol. 1973;35:941–961. doi: 10.1152/jappl.1973.35.6.941. [DOI] [PubMed] [Google Scholar]
- 16.Reardon FD, Leppik KE, Wegmann R, Webb P, Ducharme MB, Kenny GP. The snellen human calorimeter revisited, re-engineered and upgraded: Design and performance characteristics. Med Biol Eng Comput. 2006;44:721–728. doi: 10.1007/s11517-006-0086-5. [DOI] [PubMed] [Google Scholar]
- 17.Wenger CB. Heat of evaporation of sweat: Thermodynamic considerations. J Appl Physiol. 1972;32:456–459. doi: 10.1152/jappl.1972.32.4.456. [DOI] [PubMed] [Google Scholar]
- 18.Nishi Y. Measurement of thermal balance in man. In: Cena K, Clark J, editors. Bioengineering, Thermal Physiology and Comfort. New York: Elsevier; 1981. pp. 29–39. [Google Scholar]
- 19.Gagge AP, Gonzales RR. Handbook of Physiology: Environmental Physiology. Bethesda, MD: American Physiological Society; 1996. Mechanisms of heat exchange; pp. 45–84. [Google Scholar]
- 20.McGibbon B, Beaumont WV, Strand J, Paletta FX. Thermal regulation in patients after the healing of large deep burns. Plastic and reconstructive surgery. 1973;52:164–170. doi: 10.1097/00006534-197308000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Austin KG, Hansbrough JF, Dore C, Noordenbos J, Buono MJ. Thermoregulation in burn patients during exercise. J Burn Care Rehabil. 2003;24:9–14. doi: 10.1097/00004630-200301000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Wenger CB. Human heat acclimatization. In: Pandolf KB, Sawka MN, Gonzalez RR, editors. Human Performance Physiology and Environmental Medicine at Terrestrial Extremes. 1st ed. Traverse City: Benchmark Press; 1988. pp. 153–197. [Google Scholar]


