Abstract
Objectives
This study explores the relationship of lymph node ratio (LNR) and radiotherapy to overall survival (OS) for patients with resected pancreatic cancer. The impact of adjuvant radiotherapy, number of lymph nodes (LN) resected, positive LN resected, and disease extension was also evaluated.
Methods
The SEER database from 1998 – 2006 was reviewed, and 3,314 patients with non-metastatic carcinoma of the pancreas, surgical resection, examination of the regional LN, and a survival of > 2 months were identified. Of these, 1,597 patients received radiotherapy. Cox proportional hazards regression models and the logrank test were used to determine if specific variables were related to OS.
Results
Median OS for patients having surgery alone was 14 months (1-yr survival 58.1%, 2-yr survival 33.6%) and for patients having adjuvant radiotherapy was 19 months (1-yr survival 73.5%, 2-yr survival 41.4%), p-value < 0.001. For patients with LNR of 0%, OS was better compared to patients with any LN involvement, regardless of treatment group. Multivariable analysis found OS significantly related to LNR, total LN resected, positive resected LN, year of diagnosis, and regional extent of disease in patients without adjuvant radiotherapy. In patients that received adjuvant radiotherapy, OS significance was only persistent for LNR, the total LN resected, and positive resected LN.
Conclusions
A higher LNR was indicative of worse OS in all patients. A strong association with improvement in OS was seen in patients having received adjuvant radiotherapy.
Keywords: pancreatic cancer, lymph node, adjuvant radiotherapy, SEER database
Introduction
Pancreatic cancer remains largely fatal despite aggressive trials of surgical resection and/or multimodality adjuvant therapies. Surgical resection is considered the best potentially curative treatment but can be offered to only 15%–20% of the diagnosed population.1 Despite surgical resection, local failure rates may be as high as 50%–80%.2
The role of adjuvant radiotherapy (RT) after surgical resection in pancreatic cancer remains controversial due to lack of proven survival benefit. In particular, the ESPAC trial suggested a detrimental effect of adjuvant chemoradiotherapy; however, the validity of these findings has been questioned due to what was considered by some to be poor trial design.3–5 A non-statistically significant increase in 2-year OS was seen in the EORTC trial with the addition of concurrent RT and 5-FU in the subset of patients with pancreatic cancers.6 In addition, retrospective single institutional reviews have suggested a benefit to adjuvant RT and chemotherapy.7,8 This benefit was evident in the above studies despite more adverse prognostic factors within the adjuvantly treated population. Adjuvant therapy (RT and chemotherapy combined) was found to be beneficial in the presence of extensive disease, high histological grade, and involved regional lymph nodes (LN).7
Surgical series have addressed the benefit of a standard versus extended LN resection and, separately, the impact of the lymph node ratio (LNR), the ratio of involved nodes to nodes resected, in respect to patient survival.9–11 The afore mentioned studies detected a possible advantage in an extended LN dissection and in patients with a decreased LNR. LN status, however, was not evaluated in the setting of adjuvant RT.
Our study explores the relationship of the LNR on outcomes in patients receiving adjuvant RT compared to those not receiving adjuvant RT. The primary goal is to assess the impact of adjuvant RT in pancreatic cancer in relationship to the LNR. The secondary goals include separately assessing the impact of adjuvant RT, total number of LN resected, absolute number of positive resected LN, and the extension of disease in pancreatic cancer on clinical outcomes.
Materials and Methods
The Surveillance Epidemiology and End Results (SEER) Registry Data provides incidence and survival data as well as patient, tumor, and treatment variables from specific geographic areas representing 28% of the US population.12 The SEER 17 Limited Use database was queried for patients with adenocarcinoma of the pancreas from 1998 – 2006. The search was limited to patients having undergone surgical resection and pathological examination of the specimen and regional LN. Patients were further limited to those with only local or local/regional disease (no distant metastatic sites) and a survival time of ≥2 months. Patients who survived <2 months were excluded from the dataset to prevent a bias for overall survival (OS) in the RT arm.13 From the patients selected, the search was further limited to patients that had known radiation status. Chemotherapy use in this disease site is not captured in the SEER database.
Data collected for each patient included patient demographics (region of the SEER registry, race/ethnicity, sex, age at presentation, year of diagnosis), tumor characteristics (T-stage, extension of disease, number of excised LN, number of positive LN) and treatment modality (status of adjuvant radiotherapy, type of radiation, sequence of radiation). Lymph node ratio (LNR) is defined as the number of positive lymph nodes divided by the total number of lymph nodes excised and is reported as a percentage (i.e. percent of positive lymph nodes). T-stage was only reported for years 2004 and later. Variations in the American Joint Committee on Cancer (AJCC) Staging Manuals with new editions limited the subgrouping in the collected patient time period to T1/T2 versus T3/T4.14 Each patient’s extent of local-regional disease was extracted directly from the SEER database for patients after 2000. For patients from 1998–2000, local-regional extent of disease was derived from extent of the primary tumor and LN status. All patients’ extent of disease were normalized to the “Derived SS2000” definition currently in use by the SEER database regardless of the patient’s year of diagnosis.15
The primary outcome is overall survival (OS), defined as the time from surgery until death, or the last date known to be alive. Cox proportional hazards regression models were used to determine if specific variables were related to OS. The proportional hazards (PH) assumption was tested and assumed to be appropriate if the p-value was greater than 0.05. When the assumption was violated (p≤0.05), graphical approaches were used to determine the extent of the violation. For continuous variables and multivariable models, the graphical approach involved plotting the scaled residuals against time to see if the smoothing-spline fit has a slope near zero. For binary variables (e.g. RT), the log of the empirically estimated hazard rate per group was estimated over time per group. These estimates were then combined to generate a hazard ratio over time. A plot of the empirical hazard ratio versus time allowed us to assess the severity of the violation of the PH assumption. Those variables that violated the assumption (yet did not have crossing hazards) were compared using logrank tests instead of Cox regression. Based on the above described approaches, we determined that the PH assumption was not met for RT. As a result, separate Cox models were fit for patients who received RT and for those patients who did not.
Multivariable analyses represent the measures of association between risk factors and OS adjusting for other risk factors. That is, the results are determined based on a multivariable Cox regression model with multiple predictors included in the model simultaneously. Based on the univariate results, risk factors are included in the multiple regression model. Due to the violation of proportional hazards for OS as a function of RT, separate models are estimated in the patients who did and did not receive RT. Extent of disease is collapsed into local vs. regional. Number of positive lymph nodes, age at diagnosis, and year of diagnosis are treated as continuous variables (HRs represent a 1 unit increase). LNR is treated as categorical, as defined above with 0 as the reference category. Number of LN excised is also categorical with ≥12 LN resected is compared to a reference of <12 LN.
Results
Patient Characteristics
Patient characteristics are displayed in Table 1. A total of 3,314 patients were identified: 1,597 treated with adjuvant RT and 1,717 without adjuvant RT. Of the patients having received RT, 1,561 patients were treated with external beam RT (EBRT), 27 with RT not otherwise specified (NOS), 6 with a combination of EBRT and implants/radioisotopes, and 3 patients with radioisotopes. The sequence was RT after surgical excision in 1,486 patients, prior to surgery in 74 patients, intra-operatively in 13 patients, RT before and after surgery in 7 patients, intra-operatively plus an additional form of delivery in 8 patients, and 9 patients unknown. Patients were categorized by LNR as delineated in the Hopkins surgical review.11 N0 disease was classified as 0%. Positive nodal disease was further stratified as: 1%–19%, 20%–40%, and >40%. Of the 3,314 patients in the study, 39% had LNR of 0, 28% had LNR between 1 and 19%, 16% had LNR between 20% and 40%, and 17% had LNR>40%. Patients that received radiotherapy tended to be younger, but also tended to have more locally advanced T-stage and LN involvement compared to patients that did not receive radiotherapy.
Table 1.
Patient Characteristics.
| Characteristic | No Adjuvant RT (Total=1,717) N(%) |
Adjuvant RT (Total =1,597) N(%) |
|---|---|---|
| Sex | ||
| Male | 816 (48) | 845 (53) |
| Female | 901 (52) | 752 (47) |
| Race | ||
| Caucasian | 1,454 (85) | 1,366 (86) |
| Black | 159 (9) | 139 (9) |
| Other | 96 (6) | 90 (6) |
| Unknown | 8 (0.5) | 2 (0.1) |
| Median age at diagnosis (interquartile range) | 69 (60–76) | 64 (55–71) |
| Year of Diagnosis | ||
| 1998 | 75 (4) | 97 (6) |
| 1999 | 84 (5) | 88 (6) |
| 2000 | 182 (11) | 206 (13) |
| 2001 | 211 (12) | 210 (13) |
| 2002 | 200 (12) | 199 (12) |
| 2003 | 202 (12) | 193 (12) |
| 2004 | 241 (14) | 220 (14) |
| 2005 | 286 (17) | 210 (13) |
| 2006 | 236 (14) | 174 (11) |
| Derived AJCC T-stage (6th edition)* | ||
| T1–T2 | 184 (24) | 112 (19) |
| T3–T4 | 569 (75) | 484 (80) |
| Extension of Disease | ||
| Local disease no LN | 284 (17) | 170 (11) |
| Local disease with LN | 159 (9) | 167 (10) |
| Regional disease no LN | 454 (26) | 371 (23) |
| Regional disease with LN | 808 (47) | 883 (55) |
| Unknown | 12 (1) | 6 (0.4) |
| Median number of excised LN (interquartile range) | 9 (5–14) | 10 (6–15) |
| Median number of positive LN (interquartile range) | 1 (0–3) | 1 (0–3) |
| Median LNR (interquartile range) | 8% (0–29) | 13% (0–29) |
Abbreviations: RT, radiotherapy; LN, lymph node; LNR, lymph node ratio (number of positive nodes/total number of nodes excised).
T-stage available only for 2004–2006.
Overall Survival
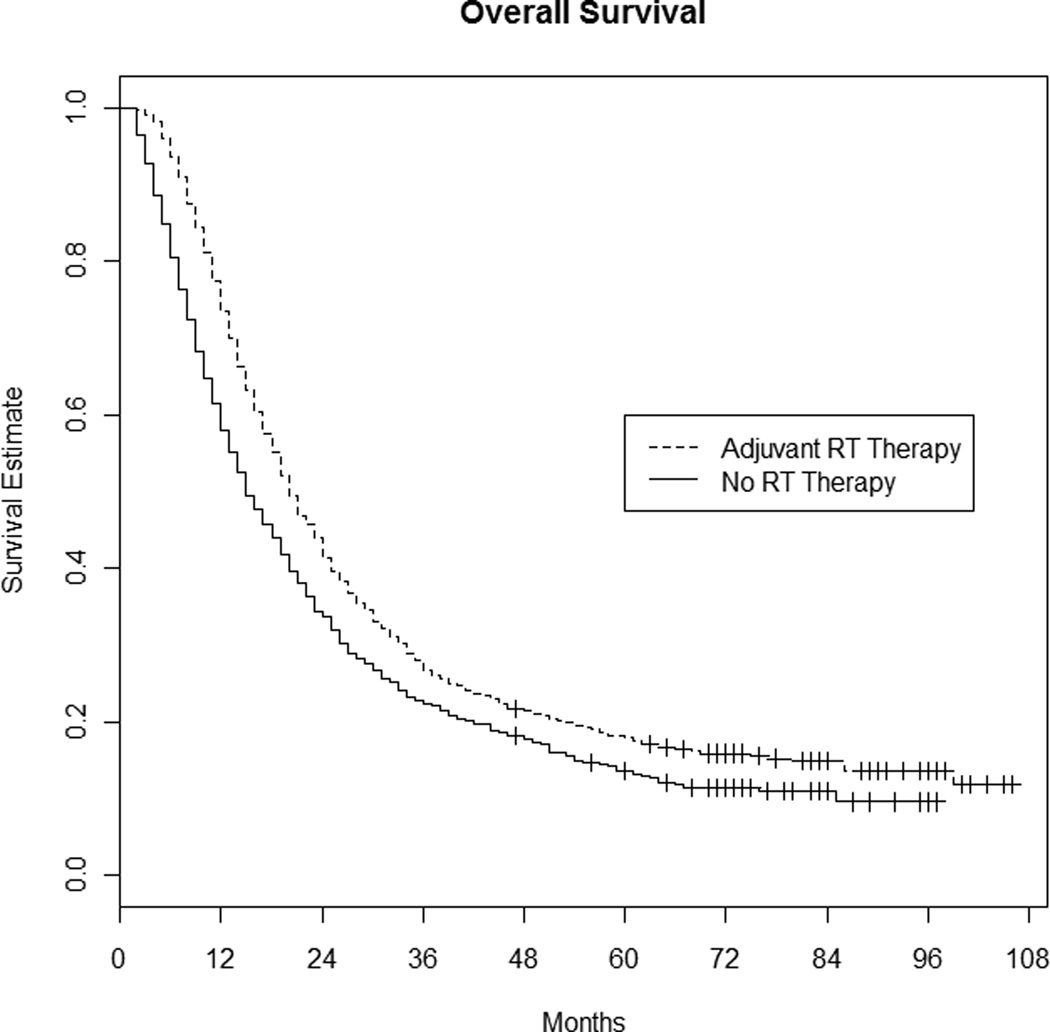
At the time of analysis 2,221 patients had died. The median survival time for all patients was 17 months. Median survival for patients having surgery alone was 14 months (1-year survival 58.1%, 2-year survival 33.6%), while median survival for patients having adjuvant RT was 19 months (1-year survival 73.5%, 2-year survival 41.4%), p-value < 0.001 (see Figure 1).
Figure 1. Overall survival: Radiation therapy versus no radiation therapy.
Univariate Analysis
Univariate analyses are based on Cox regression models in which only one predictor is included in the model and, as a result, represent unadjusted hazard ratios. Univariate analysis showed statistically significant positive associations between time to death and stage (T3/T4 vs. T1/T2: HR=1.57; 95% CI: 1.26–1.95; p<0.001), number of positive lymph nodes (HR=1.06 per lymph node; 95% CI: 1.05–1.08; p<0.001) and extension of disease (HR=1.42; 95% CI: 1.28–1.57; p<0.001) compared to local only disease. Significant negative associations were found with earlier year of diagnosis (HR=0.95 per each year; 95%CI: 0.93–0.98; p<0.001), and number of LN resected (HR= per LN; 95% CI: 0.99–1.00; p=0.005). The number of LN resected was also analyzed at a discrete cut-point of <12 versus ≥12 resected, as previously tested in the surgical literature,11 and this was found to be significant in favor of a more complete resection (HR=0.87; 95% CI: 0.79–0.94; p=0.001). LNR divided into the discrete groupings defined above was also found to be significantly related to OS (p<0.001). Age was found to be significantly related to OS (p<0.001) with older patients having a higher risk of death. Factors not found to be statistically significant for OS included: race (p=0.6), region of the country (p=0.7), and sex of patient (p=0.2).
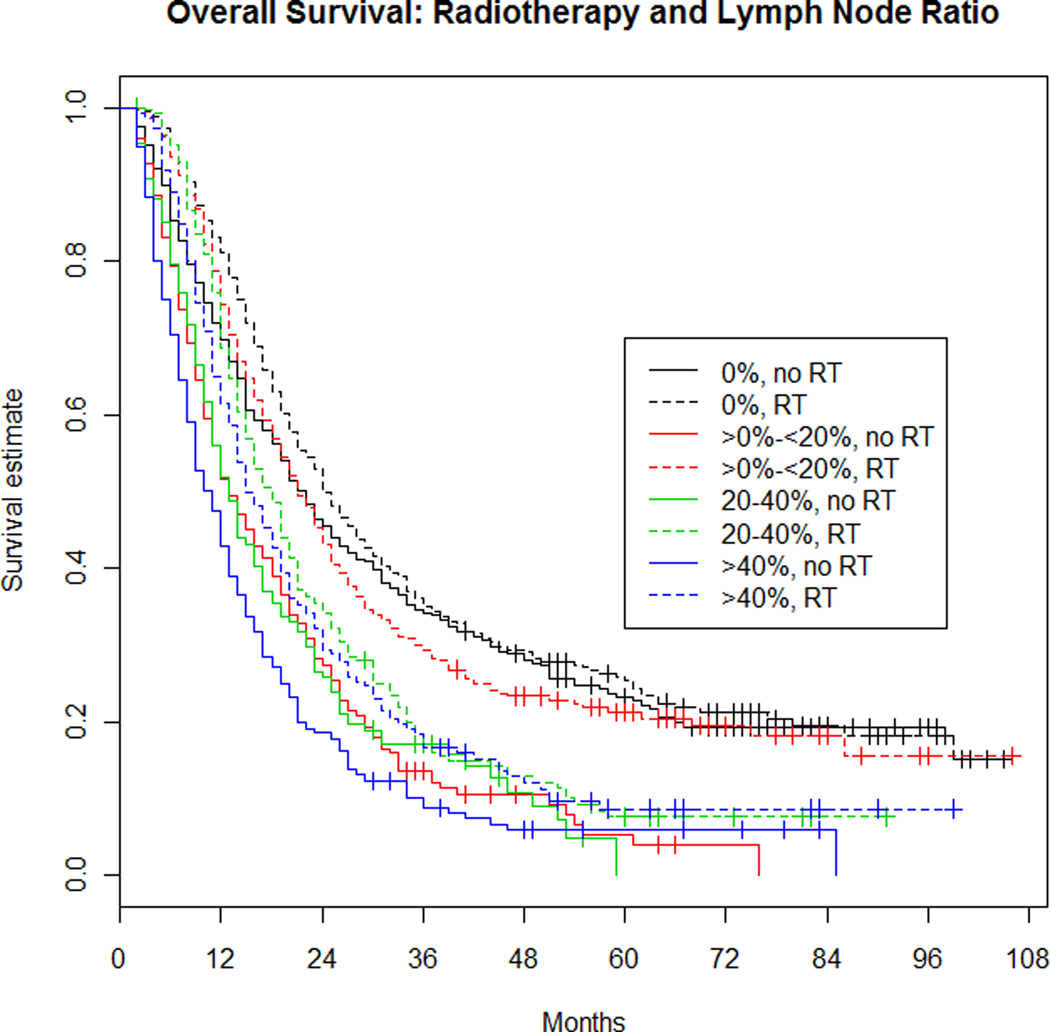
The relationship between RT and LNR was examined by estimating the effect of LNR on OS separately within RT patients and patients not receiving RT (see Figure 2). Patients with a ratio of 0 were used as the reference group. For patients receiving RT, as the ratio of involved LN increased, the risk of death also increased. Patients with 1–19% involvement had an 18% increased risk of death (HR=1.18; 95% CI: 1.01–1.38; p=0.03), patients with 20–40% involvement had a 60% increased risk of death (HR=1.60; 95% CI: 1.35–1.90; p<0.001), and patients with >40% involvement had a 78% increased risk of death (HR=1.78; 95% CI: 1.49–2.12; p<0.001). For patients not receiving RT, as the ratio of involved LN increased, the risk of death also increased. Patients with 1–19% involvement had a 75% increased risk of death (HR=1.75; 95% CI: 1.51–2.03; p<0.001), patients with 20–40% involvement had a 76% increased risk of death (HR=1.76; 95% CI: 1.47–2.10; p<0.001), and the risk of death in patients with >40% involvement was 2.25 times higher than those with no involvement (HR=2.25; 95% CI: 1.92–2.64; p<0.001).
Figure 2. Overall survival: Radiotherapy and lymph node ratio.
LNR within the RT groups (colored, dotted lines) were compared to the 0% reference group (dotted, black line). LNR within the no RT groups (colored, solid lines) were compared to the 0% reference group (solid, black line).
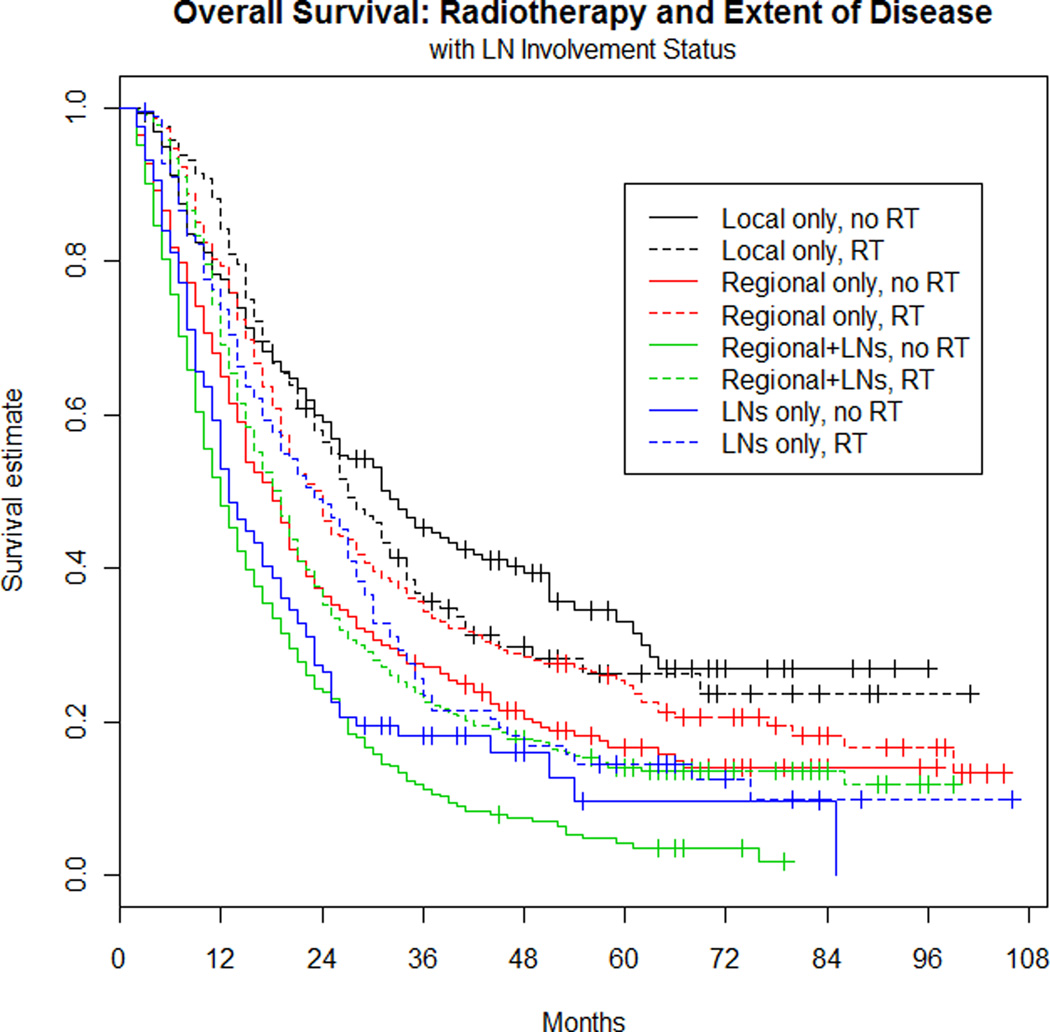
Extent of disease was originally determined to fall into one of four categories for all patients: (1) local disease and negative LN (14%), (2) regional extension with negative LN (25%), (3) local disease with involved LN (10%), and (4) regional extension and involved LN (51%). For estimating hazard ratios, the local disease and negative LN category was used as the reference group (see Figure 3). For patients receiving RT, extension of disease was significantly related to OS (p<0.001). Specifically, having LN involvement with either local disease or regional disease was significantly worse than local disease only. Patients with local disease and LN involvement had a 42% increased risk of death (HR=1.42; 95% CI: 1.09–1.86; p=0.01), and patients with regional disease and LN involvement had a 60% increased risk of death (HR=1.60; 95% CI: 1.29–1.98; p<0.001). Having regional disease only (no LN involvement) was not significantly different from having local disease only (HR=1.17; 95% CI: 0.93–1.49; p=0.20).
Figure 3. Overall survival: Radiotherapy and extent of disease with LN involvement status.
Local only subgroup was treated as the reference group (dotted, black line) within the RT patient group, and equally, the local only disease, no RT (solid, black line) as the reference group for the no RT group.
For patients that did not receive RT, any extension of disease (local with LN, regional only, regional with LN) was significantly worse than local only involvement. The risk of death in patients with local plus LN involvement was 2.27 times higher than the risk of death in patients with local extension and no LN involvement (HR=2.27; 95% CI: 1.77–2.90; p<0.001). Regional only extension had a 71% increased risk of death (HR=1.71; 95% CI: 1.40–2.09; p<0.001), and the risk of death in patients with regional plus LN involvement was 2.72 times higher than those with local extension only (HR=2.72; 95% CI: 2.26–3.28; p<0.001).
Multivariable Analysis
Table 2 reveals the results of the multivariable analyses. Among patients who did not receive RT, significant associations with OS were found for number of LN resected, LNR, year of diagnosis, regional extent of disease, and age at diagnosis. Number of positive LN was not significantly related to OS after adjusting for the other variables. Among the patients having received adjuvant RT, there were significant associations between OS and number of LN resected, number of positive resected LN, LNR, and age at diagnosis. Extent of disease and year of diagnosis were not found to be associated with OS in patients that received RT after adjusting for other factors.
Table 2.
Associations between clinical characteristics and overall survival based on Cox multiple regression models. Separate models were estimated in patients who did not (Model 1) and who did (Model 2) receive adjuvant radiotherapy. Hazard ratios represent associations with OS adjusting for other factors in the model.
| Model 1: PATIENTS NOT RECEIVING ADJUVANT RADIOTHERAPY | |||
| Variable | Hazard Ratio |
p-Value | 95% Confidence Interval |
| Number positive nodes | 1.0 | 0.053 | 1.00–1.07 |
| LNR*: >0–<20% | 1.65 | <0.001 | 1.40–1.93 |
| LNR*:20–40% | 1.53 | <0.001 | 1.24–1.89 |
| LNR*: >40% | 1.74 | <0.001 | 1.39–2.17 |
| Year of Diagnosis† | 0.93 | <0.001 | 0.90–0.96 |
| Extent of Disease‡ | 1.44 | <0.001 | 1.25–1.67 |
| ≥12 LN removed§ | 0.82 | 0.007 | 0.70–0.95 |
| Age at diagnosis | 1.01 | 0.017 | 1.00–1.01 |
| Model 2: PATIENTS RECEIVING ADJUVANT RADIOTHERAPY | |||
| Variable | Hazard Ratio |
p-Value | 95% Confidence Interval |
| Number positive nodes | 1.04 | 0.016 | 1.01–1.07 |
| LNR*: >0%–<20% | 1.20 | 0.032 | 1.02–1.41 |
| LNR*:20–40% | 1.44 | <0.001 | 1.18–1.74 |
| LNR*: >40% | 1.43 | 0.003 | 1.13–1.81 |
| Year of Diagnosis† | 0.98 | 0.11 | 0.95–1.01 |
| Extent of Disease‡ | 1.12 | 0.16 | 0.96–1.30 |
| ≥12 LN removed§ | 0.78 | 0.001 | 0.68–0.90 |
| Age at diagnosis | 1.01 | 0.043 | 1.00–1.01 |
Abbreviations: LNR, lymph node ratio (number of positive nodes/total number of nodes excised); LN, lymph node.
Reference category is LNR = 0%.
Continuous variable; hazard ratio <1 implies patients diagnosed in later years tended to have longer survival times.
Reference category is local disease.
Reference category is <12 LN removed.
Discussion
For pancreatic cancer, median OS rates with surgical resection alone have ranged from 11 months16 to 20.2 months17 and 5-year OS rates from 5% to 11.5%.16–18 Pancreatic cancer has a high propensity to fail locally. In a trial of pancreatic adenocarcinoma treated with resection, (85% R0 and 15% R1 margins), 41% had local recurrence.18 In a retrospective analysis of 78 patients having died after macroscopic curative resection for pancreatic cancer, receiving neither RT nor chemotherapy, 71.8% of patients had a local recurrence as a component of their pattern of failure.19
While the presence or absence of LN metastases has been known to be an important prognostic factor, few studies have addressed the LNR. The earliest and largest series reviewing this data originates from Johns Hopkins University School of Medicine where 905 patients having had pancreaticoduodenectomy were retrospectively reviewed to evaluate LNR as a prognostic factor11. The median number of nodes evaluated was 17. The majority of patients, 79.3%, had positive LN. Patients with LN metastases had a shorter median OS (16.5 months) compared with patients with negative LN (25.3 months). In reviewing LN data, LNR was the most compelling predictor of survival. As the LNR increased from 0%, >0% – <20%, 20% – 40% to > 40%, the median OS decreased. LNR remained an independent predictor of OS even after adjusting for other factors. This same study demonstrated that the number of lymph nodes resected (<12 versus ≥12) tended to be associated with a worse prognosis in N0 patients. This did not manifest as a prognostic factor in N1 patients (although virtually all patients with N1 disease received some form of adjuvant therapy.) This suggests that patients receiving dissection of fewer than 12 LN may be under staged. Adjuvant therapy, such as RT delivery and dose, was not analyzed as a potential prognostic factor. In a later analysis by researchers at Johns Hopkins, the pattern of recurrence was reviewed for 154 of the 905 patients previously assessed.2 A heterogeneous course of RT concurrent with 5-FU had been given based on institutional protocol from 1995 – 2005. The RT dose ranged from 34Gy – 57Gy delivered in fractionated doses of 1.8–2.4Gy. A portion of the patients had a 2-week break incorporated into the treatment. Among risk factors for local recurrence was the presence of metastatic lymph nodes. Among N1 patients, >5 metastatic LN and a LNR of >40% had the highest risk of local recurrence. Increasing LNR was associated with an incremental increased risk of local recurrence. An analysis of local failure and radiation dose found no association.
The SEER dataset was comprehensively evaluated for the impact of RT as adjuvant therapy in resectable pancreatic cancer by Hazard et al. However, this analysis only included patients from 1988 to 2002 and did not evaluate the impact of LNR. Forty-two percent of these patients received RT, and RT demonstrated a statistically significant improvement in OS as compared to no RT. In multivariable analysis, RT was associated with an improvement in OS in patients with either LN positive disease and/or direct extension but not in patients with T1–T2N0M0 disease.13
In a secondary analysis of RTOG 9704, the influence of LN factors (number of positive nodes, total nodes examined, and LNR) was assessed in regards to OS and disease-free survival (DFS). A higher number of positive LN was associated with worse OS and DFS. In multivariable analyses, both the number of positive LN and total nodes examined were associated with OS and DFS. Total nodes examined >12 and >15 were associated with increased OS for all patients. This, however, did not hold true for node-negative patients. Increased LNR was associated with worse OS and DFS.20
Our study of 3,314 patients from the 1998 – 2006 SEER database shows a significant benefit from adjuvant radiotherapy following resection for pancreatic cancer, despite the fact that patients who received radiotherapy tended to have more advanced disease. Among patients who did not receive RT, significant associations with OS were found for number of LN resected, LNR, year of diagnosis, regional extent of disease, and age at diagnosis. For patients that received adjuvant RT, significant associations were found between OS and number of LN resected, number of positive resected LN, LNR, and age at diagnosis; however, extent of disease and year of diagnosis were not found to be significant prognostic factors.
An important limitation of this study includes its observational design. There are likely other potential confounders not accounted for in this dataset. Margin status and use of chemotherapy may also influence outcomes but they are not tracked in the SEER database. Similarly, assignment of RT is not randomized. As a result of these design characteristics, the associations reported need to be interpreted with appropriate cautions.
Conclusions
This review of patients’ pancreatic cancer characteristics and treatment practices from the SEER database concludes LN status and extent of disease are strongly associated with survival. Age at diagnosis was significantly related with outcome, regardless of RT; thus, all models were adjusted for patient age at diagnosis. In patients not having received adjuvant RT, an earlier year of diagnosis and extension of disease outside of the pancreas were associated with worse OS in addition to fewer resected LN and LNR > 0. In patients that received adjuvant RT, only LN status (number resected, number of positive LNs, and LNR) remained statistically significantly related to OS. All patients tended to benefit from RT. A shortcoming of the SEER database, however, was that margin status and chemotherapy data were not recorded and thus, could not be evaluated in this analysis.
Acknowledgments
Supported in part by the Biostatistics Shared Resource, Hollings Cancer Center, Medical University of South Carolina (NIH P30 CA138313).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: American Society of Radiation Oncology (Annual Meeting) 2010
Conflicts of Interest: None
References
- 1.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363:1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 2.Asiyanbola B, Gleisner A, Herman JM, et al. Determining Pattern of Recurrence Following Pancreaticoduodenectomy and Adjuvant 5-Flurouracil-Based Chemoradiation Therapy: Effect of Number of Metastatic Lymph Nodes and Lymph Node Ratio. J Gastrointest Surg. 2008;13:752–759. doi: 10.1007/s11605-008-0762-x. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Stocken DD, Friess H, et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy after Resection of Pancreatic Cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 4.Garofalo MC, Regine WF, Tan MT. On Statistical Reanalysis, the EORTC Trial Is a Positive Trial for Adjuvant Chemoradiation in Pancreatic Cancer. Ann Surg. 2006;244(2):332–333. doi: 10.1097/01.sla.0000229980.81505.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolff RA, Varadhachary GR, Evans DB. Adjuvant Therapy for Adenocarcinoma of the Pancreas: Analysis of Reported Trials and Recommendations for Future Progress. Ann Surg Oncol. 2008;15:2773–2786. doi: 10.1245/s10434-008-0002-3. [DOI] [PubMed] [Google Scholar]
- 6.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant Radiotherapy and 5-Fluorouracil After Curative Resection of Cancer of the Pancreas and Periampullary Region. Ann Surg. 1999;230(6):776–776. doi: 10.1097/00000658-199912000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant Radiotherapy and Chemotherapy for Pancreatic Carcinoma: The Mayo Clinic Experience (1975–2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 8.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of Fluorouracil-Based Adjuvant Chemotherapy and Radiation After Pancreaticoduodenectomy for Ductal Adenocarcinoma of the Pancreas: Results of a Large, Prospectively Collected Database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farnell MB, Pearson RK, Sarr MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618–630. doi: 10.1016/j.surg.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 10.Michalski CW, Kleeff J, Wente MN, et al. Systematic review and meta-analysis of standard and extended lymphadenectomy in pancreaticoduodenectomy for pancreatic cancer. Br Surg. 2007;94:265–273. doi: 10.1002/bjs.5716. [DOI] [PubMed] [Google Scholar]
- 11.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Number of Persons by Race and Hispanic Ethnicity for SEER Participants - SEER Registries. [Accessed October 23, 2010]; Available at: http://seer.cancer.gov/registries/data.html.
- 13.Hazard L, Tward JD, Szabo A, Shrieve DC. Radiation therapy is associated with improved survival in patients with pancreatic adenocarcinoma: Results of a study from the surveillance, epidemiology, and end results (SEER) registry data. Cancer. 2007;110:2191–2201. doi: 10.1002/cncr.23047. [DOI] [PubMed] [Google Scholar]
- 14.American Joint Committee on Cancer: Publications & Electronic Products: Past Editions of the AJCC Cancer Staging Manual. [Accessed Oct 23, 2010]; Available at: http://www.cancerstaging.org/products/pasteditions.html. [Google Scholar]
- 15.SEER Coding and Staging Manuals. [Accessed Oct 23, 2010]; Available at: http://seer.cancer.gov/tools/codingmanuals/index.html.
- 16.Kaiser MH, Ellenberg SS. Pancreatic Cancer: Adjuvant Combined Radiation and Chemotherapy Following Curative Resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 17.Oettle H, Post S, Neuhaus P, et al. Adjuvant Chemotherapy With Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 18.Smeenk HG, van Eijck CHJ, Hop WC, et al. Long-term Survival and Metastatic Pattern of Pancreatic and Periampullary Cancer After Adjuvant Chemoradiation or Observation. Ann Surg. 2007;246:734–740. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 19.Sperti C, Pasquali C, Piccoli A, Pedrazzoli S. Recurrence after resection for ductal adenocarcinoma of the pancreas. World J Surg. 1997;21:195–200. doi: 10.1007/s002689900215. [DOI] [PubMed] [Google Scholar]
- 20.Showalter TN, Winter KA, Berger AC, et al. The Influence of Total Nodes Examined, Number of Positive Nodes, and Lymph Node Ratio on Survival after Surgical Resection and Adjuvant Chemoradiation for Pancreatic Cancer: A Secondary Analysis of RTOG 9704. Int J Radiat Oncol Biol Phys. 2010;81(5):1328–1335. doi: 10.1016/j.ijrobp.2010.07.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]