Abstract
Autologous stem cell transplantation (ASCT) is widely used in first-line treatment of multiple myeloma (MM). However, most patients eventually have relapse or progression of disease (R/POD). While precise knowledge of R/POD patterns would be important to generate evidence-based surveillance recommendations after ASCT, such data is limited in the literature, especially after introduction of the free light chain assay (FLCA). This retrospective study examined the patterns of R/POD after first-line ASCT in 273 patients, using established criteria. At time of R/POD, only 2% of patients had no associated serologic evidence of R/POD. Eighty five percent had asymptomatic R/POD first detected by serologic testing, while 15% had symptomatic R/POD with aggressive disease, early R/POD, and short survival, with poor cytogenetics and younger age identified as risk factors. While occult skeletal lesions were found in 40% of asymptomatic patients tested following serologic R/POD, yearly skeletal surveys and urine testing were poor at heralding R/POD. We found a consistent association between paraprotein types at diagnosis and R/POD allowing informed recommendations for appropriate serologic monitoring and propose a new needed criterion using FLCA for patients relapsing by FLC only. Our findings provide important evidence-based recommendations that strengthen current monitoring guidelines after first-line ASCT in MM.
Keywords: Multiple Myeloma, Autologous Transplantation, Relapse, Free Light Chain Assay
INTRODUCTION
Treatment guidelines endorsed by the International Myeloma Working Group (IMWG) and the National Comprehensive Cancer Network (NCCN) for patients with multiple myeloma (MM) under the age of 65 include an induction treatment followed by autologous stem cell transplantation (ASCT)1-4. Over the past decade, the incorporation of novel therapeutic agents such as bortezomib, thalidomide, and lenalidomide in this treatment schema has significantly improved response rate and progression-free survival (PFS)5,6. However, despite these encouraging achievements, most, if not all, patients eventually develop relapse or progression of disease (R/POD)4,7.
While precise knowledge of R/POD patterns would be important to generate evidence-based surveillance recommendations after ASCT, such data is limited in the literature, especially in the era of novel therapies and following the introduction of the free light chain (FLC) assay 8-10. In a retrospective analysis of 280 patients, Alegre et al. recognized four distinct R/POD patterns after ASCT: extramedullary disease (14%); plasma cell leukemia (2%); clinically symptomatic disease (66%); and asymptomatic disease with insidious increase in serum monoclonal protein (18%)11. Another study by Lenhoff et al. prospectively followed 313 patients who underwent ASCT for MM between 1994 and 1997 12. The authors identified four similar patterns of relapse: Insidious or asymptomatic disease (31%); classical or symptomatic disease along with increase in serum and/or urine monoclonal component (51%); extramedullary disease (14%); and transformed disease such as plasmacytic leukemia and immunoblastic lymphoma (4%) 11, 12. Both studies present drawbacks that may limit their relevance to currently treated patients with MM. While both studies identified distinct patterns of relapse, neither study provided a clear definition of R/POD, the modality of identification, and frequency of patients’ monitoring. Furthermore, the development of novel treatment regimens and the introduction of the serum FLC assay may limit the applicability of these studies to MM patients treated in the current era. This retrospective study examines the patterns of R/POD after first-line ASCT in the current era, incorporating novel treatment regimens, current consensus R/POD definitions, and the availability of FLC monitoring. We evaluate the utility of different diagnostic monitoring modalities with the ultimate goal of deriving evidence-based recommendations for optimal surveillance of patients post ASCT.
METHODS
Patient Population
The study population consisted of all patients with MM who underwent ASCT at Memorial Sloan Kettering Cancer Center (MSKCC) between 2001 and 2009, as part of first line therapy. We excluded from the analysis: patients transplanted more than one year after initiation of induction treatment, which we considered inconsistent with current standard of care; patients whose response to transplant could not be assessed at 2-3 months post transplantation due to transfer of care to other institutions, lack of a baseline evaluation, or death during the first three months post-transplantation; patients with associated malignancies; and patients who received tandem autologous-allogeneic transplantation. Patients who transferred care after a limited length of follow up at MSKCC were retained for analysis, but their follow up was limited to the period of monitoring at MSKCC.
Retrospective Data Collection
This study was approved by the Institutional Review Board. Data on baseline and treatment characteristics, response status, and monitoring data prior to R/POD were collected from a database of all MM patients who underwent ASCT. Baseline and treatment characteristics analyzed are detailed in Table 1. Risk assessment by cytogenetics used karyotyping and fluorescent in situ hybridization (FISH) analyses. High risk patients included those with deletion 13, deletion p53, translocations t(4:14) and t(14:16), amplification 1q23, and complex cytogenetics 13. Patients received various induction regimen and some received maintenance therapy post-ASCT. Best response to ASCT was assessed 2-3 months after ASCT. Time of R/POD was the time of serologic or clinical R/POD.
Table 1.
Baseline and treatment characteristics for all patients included in the analysis.
| Mean age at diagnosis (SD) | 57 (9) | |
| Male Sex, N (%) | 163 (60) | |
| Cytogenetic risk, N (%) | Standard High N/A |
146 (53) 39 (14) 88 (32) |
| ISS stage, N (%) | Stage I Stage II Stage III N/A |
93 (34) 72 (26) 46 (17) 57 (21) |
| LDH, Mean (SD) | 175 (70) | |
| Monoclonal component, N (%) |
IgG IgA IgM FLC only |
154 (56) 51 (19) 2 (1) 66 (24) |
| Kappa/Lambda ratio, N (%) | Normal Abnormal N/A |
17(6) 199 (73) 57 (21) |
| Mean time from diagnosis to transplantation, months (SD) |
11 (10) | |
| Mean time from initial treatment to transplantation, months (SD) |
8 (4) | |
| *Induction agents | Thalidomide Lenalidomide Bortezomib Other |
128 58 74 70 |
| Maintenance agents | Thalidomide Lenalidomide Interferon Cyclophosphamide None |
22 33 2 1 215 |
| Type of transplant, N (%) | Single auto Tandem auto-auto |
235 (86) 38 (14) |
| Best response after transplantation (IMWG), N (%) |
CR nCR VGPR PR SD POD |
146 (53) 39 (14) 41 (15) 33 (12) 1 (0.3) 3 (1) |
Agents overlapped in some regimens
Criteria used for determination of R/POD
Standard IMWG criteria for disease response, relapse and progression were used for determination of serologic, urinary, and clinical R/POD 9, 14-16. Since immunohistochemical or flow cytometric analyses were not available on all patients who achieved a CR, patients with confirmed sCR and CR were grouped together into the CR group. Furthermore, flow cytometry and molecular studies were not used for detection of minimal residual disease or R/POD. Since the IMWG criteria do not have provisions pertaining to patients relapsing from CR whose relapse is detected solely by the FLC assay, we adopted a new relapse criterion found to be common to all such patients: to be considered relapsing from CR, a patient had to have an abnormal FLC ratio and an elevated level of involved FLC (iFLC) preceded by a normal FLC ratio on at least 2 prior measurements. The abnormal findings had to be confirmed on a subsequent measurement that additionally showed a rise in the abnormal iFLC level by at least 50%.
Strategy used for determination of R/POD
The strategy adopted in this study consisted of several steps (Figure 1): First, we reviewed electronic medical records to identify patients who had serologic R/POD and determine the date of serologic R/POD. Second, all patients, those with and those without serologic R/POD, were examined to determine whether clinical R/POD (anemia, renal failure, hypercalcemia, soft tissue or symptomatic bone lesions) had coincided with or anteceded serologic R/POD, in which case the patients were labeled symptomatic R/POD. All other patients with serologic R/POD only were labeled asymptomatic R/POD. Further analysis was done on these subgroups of patients.
Figure 1.
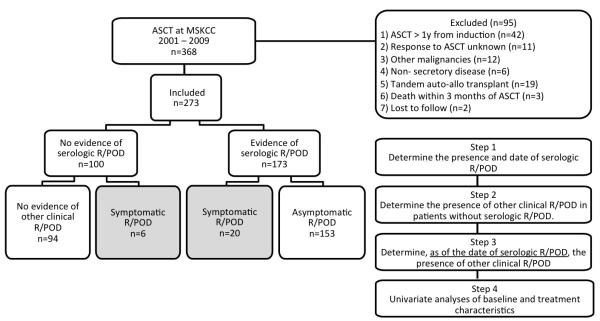
Overall study scheme.
Statistical Analysis
Among patients with R/POD, the univariate association between the type of R/POD and key patient and clinical characteristics were assessed using t-tests or Fisher’s exact tests as appropriate. Kaplan-Meier survival curves and the logrank test were used to compare the time from R/POD to death for the asymptomatic and symptomatic groups. Cox proportional hazards regression was implemented to compare the risk of death adjusted for the potential difference in lag time between ASCT and R/POD in these two groups. Significance was defined as P < 0.05 based on 2-sided tests. Statistical analyses were conducted using R statistical software17.
RESULTS AND DISCUSSION
Patient baseline and treatment characteristics
Among 368 patients transplanted between 2001 and 2009, 95 were excluded from analysis for reasons detailed in Figure 1. The clinical characteristics of the remaining 273 patients are summarized in Table 1. Cytogenetic studies for del17p and t(4;14) were only available after 2005. Therefore complete cytogenetic testing was only available in 185 patients. The majority of patients received induction treatments based on thalidomide, lenalidomide, bortezomib, or combination of these drugs (Table 1).
The majority of R/POD is associated with serologic evidence of R/POD
Among 273 eligible patients, 173 had serologic R/POD post-transplant. Analysis of 100 remaining patients revealed 6 additional patients who met IMWG clinical criteria for R/POD without evidence of serologic R/POD, though 2 among them had no serologic data available on the date of R/POD and therefore had inadequate serologic follow up. In addition, one patient relapsed with leptomeningeal disease, one with plasma cell leukemia, and 2 had oligo-secretory disease. Interestingly, one of the latter 2 patients had IgA gammopathy and exhibited steadily rising IgA levels preceding symptomatic relapse without meeting IMWG serologic criteria for R/POD. Excluding patients with no serologic follow-up, only 4 patients (2 %) had symptomatic R/POD without evidence of serologic R/POD.
The majority of patients with R/POD have asymptomatic R/POD
In addition to the 6 patients who had symptomatic R/POD in the absence of serologic findings, 20 patients with serologic R/POD had concurrent evidence of clinical/radiologic R/POD (symptomatic group). These 26 patients exhibited often coinciding clinical findings consistent with aggressive disease including acute severe anemia (n=5), leptomeningeal relapse (n=2), soft tissue involvement (n=14), plasma cell leukemia (n=1), symptomatic skeletal lesions (n=21), renal failure (n=1), and hypercalcemia (n=1). In this group, a total of 4 out of 5 patients with IgA gammopathy had steady rise in their IgA level from baseline without meeting IMWG criteria for serologic R/POD prior to symptomatic R/POD. Although this study is not designed to determine the optimal serologic follow-up interval post-transplantation, 5 of the 26 symptomatic patients had intervals ranging from 143 to 469 days, probably contributing to their symptomatic presentation. The remaining 153 out of 179 patients with R/POD (85%) had isolated serologic R/POD without conspicuous evidence of concomitant clinical R/POD (asymptomatic group). There was evidence by univariate association that symptomatic patients tend to have younger age, high-risk cytogenetics, and shorter time from ASCT to R/POD (Table 2). Further attesting to the aggressive nature of the disease in symptomatic patients, the survival curves in Figure 2 show considerable separation in the time from relapse to death. Adjusting for the lag time between ASCT and relapse, the risk of death was significantly higher for the symptomatic group (HR: 3.43 (2.09, 5.63), p< 0.001).
Table 2.
Univariate associations between the type of R/POD and key baseline and treatment characteristics.
| Baseline and Treatment Characteristics | Asymptomatic N = 153 |
Symptomatic N = 26 |
P-value* |
|---|---|---|---|
| Age at Diagnosis, Mean (SD) | 58 (9) | 53 (7) | 0.01 |
| Male Sex, N (%) | 90 (59) | 17 (65) | 0.53 |
| High Risk Cytogenetics**, N (%) | 24 (24) | 8 (53) | 0.03 |
| M-Protein, N (%) | |||
| FLC-only | 35 (23) | 3 (12) | 0.48 |
| IgA | 30 (20) | 5 (19) | |
| IgG | 87 (57) | 18 (69) | |
| IgM | 1 (1) | 0 (0) | |
| Abnormal Kappa/Lambda**, N (%) | 112 (90) | 16 (89) | 0.69 |
| Advanced ISS Stage (II+III)**, N (%) | 72 (56) | 10 (50) | 0.64 |
| LDH, Mean (SD) | 168 (61) | 199 (106) | 0.21 |
| Single Auto Infusion, N (%) | 129 (84) | 22 (85) | 0.99 |
| Months from Diagnosis to Transplant, Mean (SD) | 10.5 (7.9) | 11.9 (13.8) | 0.60 |
| Months from Treatment to Transplant, Mean (SD) | 7.5 (3.4) | 7.7 (3.4) | 0.74 |
| CR Best Response, N (%) | 77 (50) | 11 (42) | 0.45 |
| Any Maintenance Therapy N (%) | 27 (18) | 5 (19) | 0.79 |
| Time from Infusion to Serologic R/POD, Mean (SD) | 25.6 (18.2) | 14.2 (11.1) | |
| Median (IQR) | 20.9 (13.3-33.4) | 10.4 (4.8-21.2) | < 0.001 |
| Months from R/POD to prior Serologic Evaluation, Mean (SD) |
3.7 (4.6) | 3.6 (3.7) | 0.95 |
for differences in means/proportions across the two R/POD groups
denominator includes only those with an observed value
Figure 2. Kaplan-Meier estimates of overall survival from the time of R/POD for the symptomatic and asymptomatic groups.
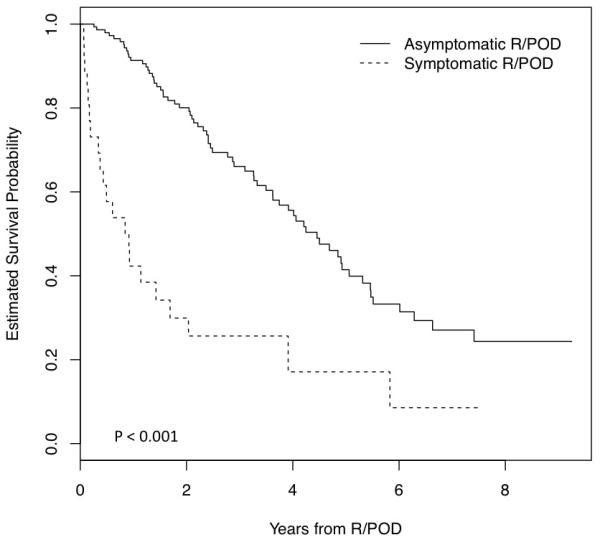
The survival distributions for the two groups were compared using the logrank test.
Role of imaging at the time of serologic R/POD in patients with asymptomatic R/POD
To determine whether patients in the asymptomatic group exhibited occult radiographic R/POD despite lack of suspicion of clinical R/POD, we examined all patients who had radiologic imaging within 4 weeks following the date of serologic R/POD. Sixty-four out of 153 asymptomatic patients had routine imaging available. Twenty-five (40%) among them had new bone lesions by SS (n = 12), PET/CT (n = 11), or MRI (n = 2) despite absence of any prior suspicion.
Role of yearly routine skeletal survey in the monitoring of patient with MM after ASCT
To determine whether yearly routine skeletal survey (SS) is useful to monitor patients after ASCT, we reviewed SS within one year prior to the date of serologic R/POD, available in 95 of the 179 patients with R/POD. Only 3 patients (3%) had evidence of radiologic R/POD anteceding serologic R/POD. All 3 patients were in the symptomatic group with no serologic follow-up on the day of radiologic R/POD and had poor prior serologic follow up, ranging from 208 to 252 days.
Association between paraprotein types at times of diagnosis and R/POD
To determine whether one could predict the pattern of serologic R/POD in individual patients, and thereby identify the most appropriate serologic testing for monitoring, we examined the association between the type of paraprotein at times of R/POD and diagnosis in patients who had complete serologic data available. Serologic paraprotein types singularized included intact immunoglobulin only (Ig-only) detected by serum immunofixation (IF) and/or protein electrophoresis (PEP), FLC only (FLC-only) detected by FLC assay, and both (Ig/FLC). Among 66 evaluable patients with CR, 13 had FLC-only at diagnosis, 7 Ig-only, and 47 Ig/FLC. All patients with FLC-only relapsed with FLC-only; 6 of 7 with Ig-only relapsed with Ig-only and 1 with Ig/FLC; 5 of 47 with Ig/FLC relapsed with FLC-only, 23 with Ig-only, and 19 with Ig/FLC. Among 54 evaluable patients achieving <CR, 13 had FLC-only at diagnosis, 6 Ig-only, and 35 Ig/FLC. All patients with FLC-only progressed with FLC-only; 5 of 6 with Ig-only progressed with Ig-only and 1 with Ig/FLC; 13 of 38 with Ig/FLC progressed with FLC-only, 9 with Ig-only, and 13 with Ig/FLC (Figure 3).
Figure 3. Association between the types of monoclonal protein present at diagnosis and R/POD.
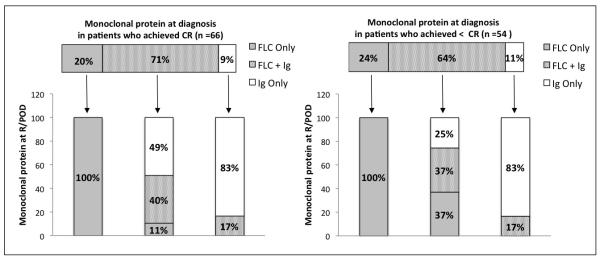
Only the patients who had complete set of serologic testing available at diagnosis and R/POD were included for the analysis. Patients were analyzed on the basis of the best response achieved after ASCT (CR or less than CR) and the type of paraprotein present at diagnosis and R/POD: FLC only, intact immunoglobulin only (Ig), and FLC with intact immunoglobulin (FLC+Ig).
Clinical significance of R/POD by FLC-only disease
To determine whether detection of R/POD by FLC testing is clinically relevant, we examined the records of the 51 patients who met R/POD criteria by FLC-only. Four had symptomatic R/POD and 6 additional patients among 22 with imaging available within 4 weeks following serologic R/POD, had new radiographic findings. Importantly, when focusing on the 21 patients who relapsed with FLC-only according to the proposed criterion in methods, 1 was symptomatic and 4 additional patients of 12 with available imaging within 4 weeks following serologic R/POD had new radiographic findings. Thus, at least 23% of patients meeting the proposed FLC criterion had radiographic/clinical relapse.
Role of urine studies in the monitoring of patients with MM after ASCT
Among patients with urine studies available at the time of serologic R/POD, 12 of 59 (20%) with CR and 4 of 47 (8%) with <CR had evidence of R/POD by Urine IF and/or PEP. Among patients who had urine studies available within 3 months prior to serologic R/POD, 5 of 52 (9%) with CR and none of 43 with <CR demonstrated evidence of R/POD anteceding serologic R/POD by UIF. Overall, only 1 of the 95 patients had symptomatic relapse raising uncertainty regarding the clinical relevance of urine testing. Interestingly, this patient also had an IgA gammopathy.
Discussion
In this retrospective study, we examined the patterns of R/POD in patients treated with induction therapy followed by ASCT. We have made several observations that could be relevant to current monitoring guidelines of patients after ASCT.
Based on this analysis, the overwhelming majority of R/POD is associated with concurrent serologic R/POD, with only a small percentage of patients (2%) presenting with symptomatic clinical disease in the absence of serologic R/POD. Furthermore, we find that the vast majority of patients with R/POD present with serologic abnormalities as the initial manifestation of R/POD. Only 15 % present with symptomatic overt clinical disease. Although the small number of patients in the latter group precludes a multivariate analysis, the univariate analysis indicates that poor cytogenetics, aggressive disease with early R/POD, and younger age are associated with symptomatic R/POD. Further examination of this group also shows leukemic transformation, soft tissue involvement, and leptomeningeal disease prominently featured at the time of R/POD. An important corollary of these observations would be that monitoring patients after ASCT with serologic testing appears to be adequate in heralding the occurrence of R/POD, except for a minority of patients who have biologically aggressive disease, predicted by poor cytogenetics and manifested by aggressive patterns of transformed R/POD, early R/POD, and poor survival as similarly described by others18.
Interestingly, we observed retrospectively that patients with IgA gammopathy often tend to have progressive elevation in IgA levels, without meeting IMWG criteria for R/POD by SPEP prior to symptomatic R/POD 15. We therefore caution that serologic monitoring of these patients using IMWG criteria alone may not be sufficient. IgA level measurements in these patients may be more revealing. The development and incorporation of the new Hevylite assay may well remedy this inadequacy of the currently available tests, as suggested by others.19, 20
Although it appears that serologic testing alone may well be adequate for monitoring patients after ASCT, it is also interesting to highlight that our observations may not fully support the IMWG recommendations regarding the specific type of laboratory testing needed during monitoring. Indeed, the IMWG consensus recommends the use of serum FLC assay for serial monitoring and assessment of response in patients with oligo-secretory disease only and does not advocate its use for serial follow-up or relapse assessment in patients with measurable M spike “due to lack of sufficient data to suggest that addition of FLC assay to serum IFE and SPEP may improve patient outcomes”9. Furthermore, the IMWG consensus also states that “it is important for a particular patient to use the same method for follow-up of disease”14. In contrast, our observations indicate that all patients should be followed using all available serologic testing including SPEP, IF, and FLC assay, except for patients with FLC-only disease who always have serologic R/POD detected by FLC assay only. Indeed, among patients who had Ig-only or Ig/FLC disease at diagnosis, a substantial percentage present with abnormal FLC as first serologic indicator of R/POD, which we show to be clinically relevant. This testing would particularly be useful for patients who lose expression of immunoglobulin heavy chain, a phenomenon coined by others “FLC-escape”21, 22.
In this study, we further propose a new FLC criterion for patients who relapse from CR since the IMWG criteria have no provision pertaining to such patients 9, 14. It consists of an abnormal FLC ratio and involved FLC level after at least 2 prior normal measurements, with subsequent confirmation of the abnormal measurements and demonstration of >50% further increase in the involved FLC. Supporting the clinical relevance of such a criterion, we find that a least 23% of the patients meeting this criterion have concurrent evidence of radiographic R/POD.
In this study, yearly imaging with SS was not useful for earlier detection of R/POD in any patients who had a SS within a year prior to serologic R/POD. Thus, yearly SS cannot be recommended for routine follow-up in the absence of other evidence for R/POD. This observation supports the IMWG recommendation that was not previously clearly substantiated 14. This statement however, may not apply to MRI, which was found useful by investigators who showed a poor correlation between clinical CR and MRI CR in patients with more than 7 focal lesions and superior survival with resolution of MRI focal lesions 23. Based on these findings, the authors recommended routine use of MRI every 6 months until resolution of focal lesions and annually thereafter, a recommendation awaiting validation 23.
On the other hand, among patients with asymptomatic R/POD, a significant percentage had evidence of occult new skeletal findings by MRI, PET or SS obtained within 4 weeks after serologic R/POD, despite the complete lack of suspicion of progressive clinical disease. These findings suggest that imaging should be recommended in patients with serologic R/POD to uncover potential occult lesions. This recommendation is in contrast with that of the IMWG consensus stating that in patients with relapsed disease, “a SS may be indicated to detect possible lesions at risk for fracture. Other imaging studies (CT, MRI, and PET-CT) may be indicated according to clinical circumstances” 14. Uncovering these occult lesions is important since it would signify clinical relapse/progression and would compel physicians, in agreement with the IMWG consensus, to initiate salvage therapy15
The role of urine testing has been controversial in the literature, with some arguing that it may be obsolete in the era of FLC assay 24-27. In this study, urine testing was far less sensitive to detect R/POD since only a small percentage of patients with serologic R/POD had evidence of concomitant UIF/UPEP abnormalities. However, UIF did herald serologic R/POD in a small percentage of patients with CR, an observation of uncertain clinical relevance. We would therefore conclude that UIF/UPEP could be helpful in the subset of patients achieving CR post-ASCT, although clinical relevance needs to be further examined.
In summary, we have described the patterns of R/POD in patients with MM after ASCT and propose evidence based recommendations for monitoring and management (Table 3). Our findings differ from previous reports in the literature. This discrepancy may reflect the use of the FLC assay and better-defined criteria for R/POD established by the IMWG. Although we acknowledge the limitations of the study due to the retrospective nature of the analysis and the heterogeneity of the patient population, we believe that our findings may strengthen the current guidelines for monitoring patients with MM after ASCT and provide a blueprint for further validation studies addressing our findings in large prospective populations.
Table 3.
Summary of observations and recommendations
| Pertinent Observations in Current Study | Potential Impact of Observation on Current Practice and Guidelines |
|---|---|
| The majority (98%) of R/POD is associated with serologic evidence of R/POD |
Serologic follow up may be sufficient to monitor patients |
| The majority (85%) of patients with R/POD have asymptomatic R/POD. Symptomatic disease is associated with younger age, poor cytogenetics and shorter PFS and post-R/POD survival |
Younger patients with poor cytogenetics may need closer monitoring |
| New proposed criteria for relapse in patients with FLC only disease (Currently there are no IMWG criteria available) |
New criteria using FLC assay could be used to detect relapse even in patients with measurable M spike |
| Annual skeletal survey was not useful in any patients to predict R/POD |
Annual skeletal survey is not recommended for routine monitoring |
| Urine testing was not useful to predict R/POD except in a few patients in CR |
Routine urine testing is possibly not recommended for routine monitoring |
| The association between patterns of paraprotein at diagnosis and relapse is predictable and versatile |
Allows to predict patterns of paraprotein at relapse and mitigates the current IMWG recommendation to “follow patients using the same method” as at diagnosis |
| A significant percentage of patients with asymptomatic serologic R/POD actually have occult bone lesions |
Imaging at serologic R/POD is recommended in asymptomatic patients, recommendation that departs from the current IMWG recommendation that “CT, MRI, and PET may be indicated according to clinical circumstances” at R/POD |
Acknowledgements and authorship contributions
D.Z. has been supported by the NIH T32 training grant (CA009207).
Footnotes
D.Z. and H.H. designed the study, collected and analyzed the data, and wrote the manuscript. D.C., D.B., M.B. collected the data. S.D., E.R., S.G., H.L., N.L., A.L., D.C., and G.C. analyzed the data and assisted in manuscript preparation. The authors have no relevant disclosures.
Conflict of Interest The authors have no relevant conflicts of interest to declare.
Disclosures: None
REFERENCES
- 1.Cavo M, Rajkumar SV, Palumbo A, Moreau P, Orlowski R, Blade J, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117(23):6063–73. doi: 10.1182/blood-2011-02-297325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelhardt M, Udi J, Kleber M, Spencer A, Rocci A, Knop S, et al. European Myeloma Network: the 3rd Trialist Forum Consensus Statement from the European experts meeting on multiple myeloma. Leuk Lymphoma. 2010;51(11):2006–11. doi: 10.3109/10428194.2010.516378. [DOI] [PubMed] [Google Scholar]
- 3.Anderson KC, Alsina M, Bensinger W, Biermann JS, Chanan-Khan A, Cohen AD, et al. NCCN clinical practice guidelines in oncology: multiple myeloma. Journal of the National Comprehensive Cancer Network : JNCCN. 2009;7(9):908–42. doi: 10.6004/jnccn.2009.0061. [DOI] [PubMed] [Google Scholar]
- 4.Harousseau JL, Moreau P. Autologous hematopoietic stem-cell transplantation for multiple myeloma. The New England journal of medicine. 2009;360(25):2645–54. doi: 10.1056/NEJMct0805626. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Anderson K. Multiple myeloma. The New England journal of medicine. 2011;364(11):1046–60. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 6.Laubach JP, Schlossman RL, Mitsiades CS, Anderson KC, Richardson PG. Thalidomide, lenalidomide and bortezomib in the management of newly diagnosed multiple myeloma. Expert Rev Hematol. 2011;4(1):51–60. doi: 10.1586/ehm.10.83. [DOI] [PubMed] [Google Scholar]
- 7.Laubach J, Richardson P, Anderson K. Multiple myeloma. Annu Rev Med. 2011;62:249–64. doi: 10.1146/annurev-med-070209-175325. [DOI] [PubMed] [Google Scholar]
- 8.Bradwell AR, Carr-Smith HD, Mead GP, Tang LX, Showell PJ, Drayson MT, et al. Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clinical chemistry. 2001;47(4):673–80. [PubMed] [Google Scholar]
- 9.Dispenzieri A, Kyle R, Merlini G, Miguel JS, Ludwig H, Hajek R, et al. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215–24. doi: 10.1038/leu.2008.307. [DOI] [PubMed] [Google Scholar]
- 10.Katzmann JA, Clark RJ, Abraham RS, Bryant S, Lymp JF, Bradwell AR, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clinical chemistry. 2002;48(9):1437–44. [PubMed] [Google Scholar]
- 11.Alegre A, Granda A, Martinez-Chamorro C, Diaz-Mediavilla J, Martinez R, Garcia-Larana J, et al. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002;87(6):609–14. [PubMed] [Google Scholar]
- 12.Lenhoff S, Hjorth M, Turesson I, Westin J, Gimsing P, Wisloff F, et al. Intensive therapy for multiple myeloma in patients younger than 60 years. Long-term results focusing on the effect of the degree of response on survival and relapse pattern after transplantation. Haematologica. 2006;91(9):1228–33. [PubMed] [Google Scholar]
- 13.Avet-Loiseau H. Role of genetics in prognostication in myeloma. Best Pract Res Clin Haematol. 2007;20(4):625–35. doi: 10.1016/j.beha.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 14.Dimopoulos M, Kyle R, Fermand JP, Rajkumar SV, San Miguel J, Chanan-Khan A, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701–5. doi: 10.1182/blood-2010-10-299529. [DOI] [PubMed] [Google Scholar]
- 15.Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, et al. Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood. 2011;117(18):4691–5. doi: 10.1182/blood-2010-10-299487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, et al. International uniform response criteria for multiple myeloma. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, U.K. 2006;20(9):1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 17.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2011. [Google Scholar]
- 18.Kumar S, Mahmood ST, Lacy MQ, Dispenzieri A, Hayman SR, Buadi FK, et al. Impact of early relapse after auto-SCT for multiple myeloma. Bone marrow transplantation. 2008;42(6):413–20. doi: 10.1038/bmt.2008.180. [DOI] [PubMed] [Google Scholar]
- 19.Bradwell AR, Harding SJ, Fourrier NJ, Wallis GL, Drayson MT, Carr-Smith HD, et al. Assessment of monoclonal gammopathies by nephelometric measurement of individual immunoglobulin kappa/lambda ratios. Clinical chemistry. 2009;55(9):1646–55. doi: 10.1373/clinchem.2009.123828. [DOI] [PubMed] [Google Scholar]
- 20.Donato LJ, Zeldenrust SR, Murray DL, Katzmann JA. A 71-year-old woman with multiple myeloma status after stem cell transplantation. Clinical chemistry. 2011;57(12):1645–8. doi: 10.1373/clinchem.2011.163766. [DOI] [PubMed] [Google Scholar]
- 21.Dawson MA, Patil S, Spencer A. Extramedullary relapse of multiple myeloma associated with a shift in secretion from intact immunoglobulin to light chains. Haematologica. 2007;92(1):143–4. doi: 10.3324/haematol.10297. [DOI] [PubMed] [Google Scholar]
- 22.Kuhnemund A, Liebisch P, Bauchmuller K, zur Hausen A, Veelken H, Wasch R, et al. ’Light-chain escape-multiple myeloma’-an escape phenomenon from plateau phase: report of the largest patient series using LC-monitoring. J Cancer Res Clin Oncol. 2009;135(3):477–84. doi: 10.1007/s00432-008-0470-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walker R, Barlogie B, Haessler J, Tricot G, Anaissie E, Shaughnessy JD, Jr., et al. Magnetic resonance imaging in multiple myeloma: diagnostic and clinical implications. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25(9):1121–8. doi: 10.1200/JCO.2006.08.5803. [DOI] [PubMed] [Google Scholar]
- 24.Nowrousian MR, Brandhorst D, Sammet C, Kellert M, Daniels R, Schuett P, et al. Serum free light chain analysis and urine immunofixation electrophoresis in patients with multiple myeloma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2005;11(24 Pt 1):8706–14. doi: 10.1158/1078-0432.CCR-05-0486. [DOI] [PubMed] [Google Scholar]
- 25.Singhal S, Stein R, Vickrey E, Mehta J. The serum-free light chain assay cannot replace 24-hour urine protein estimation in patients with plasma cell dyscrasias. Blood. 2007;109(8):3611–2. doi: 10.1182/blood-2006-11-060368. [DOI] [PubMed] [Google Scholar]
- 26.Dispenzieri A, Zhang L, Katzmann JA, Snyder M, Blood E, Degoey R, et al. Appraisal of immunoglobulin free light chain as a marker of response. Blood. 2008;111(10):4908–15. doi: 10.1182/blood-2008-02-138602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katzmann JA, Dispenzieri A, Kyle RA, Snyder MR, Plevak MF, Larson DR, et al. Elimination of the need for urine studies in the screening algorithm for monoclonal gammopathies by using serum immunofixation and free light chain assays. Mayo Clinic proceedings. Mayo Clinic. 2006;81(12):1575–8. doi: 10.4065/81.12.1575. [DOI] [PubMed] [Google Scholar]


