Abstract
Hypertension is a more serious disease in blacks. The determinants of the blood pressure (BP) may be uniquely different from those in whites. The characteristic low-renin, salt-sensitive hypertension of blacks is consistent with the kidney reabsorbing additional sodium (Na), which leads to an expanded plasma volume that drives the BP. Mechanisms considered are genetically based. These include: (1) the intra-renal renin-angiotensin system (RAS), one based on molecular variations in angiotensinogen; (2) the Na, K, 2Cl cotransporter (NKCC2) and its regulators in the thick ascending limb, which are associated with a variety of phenotypes consistent with a more active cotransporter in blacks; and (3) the genes for MYH9 and APOL 1, which have been associated with kidney disease in blacks. To achieve a state of hypertension, an increase in Na uptake in proximal nephron regions may require a distal nephron that does not fully adjust due to less than adequate suppression of aldosterone production.
Keywords: Race, Hypertension, Extracellular fluid volume, Angiotensinogen, Renin, Angiotensin II, Aldosterone, Proximal tubule, Thick ascending limb, Calcium-sensing receptor, NKCC2, MYH9, APOL 1, Epithelial sodium channel, Sodium, Potassium, Calcium
Introduction
Hypertension is the most commonly treated chronic disease. It is a major risk factor for heart disease, kidney failure and stroke. In blacks, the prevalence of hypertension is increased over that in whites [1] and the incident rates of complications are much higher in blacks than in whites [2, 3]. Blacks typically have hypertension that is low-renin [4] and salt-sensitive [5], consistent with an increase in sodium (Na) reabsorption by the kidney. This, in turn, leads to an expanded extracellular fluid volume that becomes the driving force behind development of what may be a race-specific form of hypertension. The expanded volume may in addition augment other pressor influences.
The pathophysiology of hypertension in blacks, what distinguishes it from that of other population groups, is grounded primarily in genetics. This template for proteins that regulate the kidney’s reabsorption of Na was selected for an adaption to an arid environment. That which was in low supply included not only Na, but also water. Environmental differences between race groups today would seem to play a more minor role. With respect to dietary differences, urinary excretion of Na has been shown repeatedly to be similar in blacks and whites, indicating similar dietary intakes of Na [6, 7]. Urinary excretion of potassium (K) is generally lower in blacks. Whether this is of dietary origin or is a manifestation of differences in the disposition of K is unclear [8]. Differences in handling of K by the kidney may be linked to a predisposition to hypertension [9]. Aviv et al. [10], in studies of the relative contributions of fecal versus urinary excretion of K, found evidence that relative rates of excretion could vary with differences in dietary K, as well as with individual differences in renal handling of K. The authors of this review subscribe to the notion that the race difference in K excretion represents more than an effect of diet; that racial difference in food preferences, if there are any, are insufficient to account for the consistently observed disparity in K excretion rates.
Whether the greater risk for hypertension in blacks resides with a unique blood pressure (BP) physiology or represents an amplification of the physiology that is characteristic of whites is not clear. In the following discussion of pathophysiology of hypertension in blacks, we consider an expansion of extracellular fluid volume as paramount, that much of the race difference can be explained by differences in volume. Over 30 years ago, volume was measured in blacks and whites and was indeed found to be greater in blacks [11]. Most evidence for an expanded plasma volume in blacks in comparison to other population groups rests on indirect evidence, most notably the level of plasma renin activity (PRA), which is lower with an expanded volume. Levels of aldosterone may also be lower [12]. In this review, we consider the intrarenal renin-angiotensin system and the medullary thick ascending limb (TAL) as potential sites for increases in Na reabsorption. The argument could easily be made that any transport system involved in reclaiming Na is likely more active in blacks. We further propose that there is an interaction of these sites with the epithelial Na channel (ENaC) in the distal nephron. Also, it is not our intention to suggest that a single transport system is tipping the scales in favor an elevated BP in blacks, but rather any one of them or all of them may be more operative in a given individual. This review is also not all-inclusive; we did not, for example, include discussion of the distal convoluted tubule, for the reason that its potential participation did not seem to us as well developed.
Angiotensinogen and the Renin-Angiotensin System in Kidney
Renin-angiotensin system (RAS) is involved at multiple levels in the regulation of BP. Race differences in volume and BP could stem from exposure to different intensities of RAS expressed in renal tissue. Extensive work by Navar and colleagues [13••, 14] demonstrated the presence of RAS operating within the kidney. Coffman’s laboratory showed in cross-transplantation experiments using mice deficient in the type 1 angiotensin (AT1) receptor (genetic knock-out models) and wild-type mice allowing for isolation of renal RAS from extra-renal RAS influences on BP [15••, 16]. About half the angiotensin II effect on BP was mediated by AT1 receptors expressed in kidney; the higher BP was a result of greater Na retention. A racial difference in the intra-renal RAS could contribute to differences in risk for hypertension. Genetic studies of angiotensinogen (AGT), the substrate from which angiotensin II is derived, suggest that such a mechanism could exist.
The concentration of AGT is at or near the Michaelis-Menten constant for its enzymatic reaction with renin, and therefore AGT is rate limiting for generation of angiotensin II. In a landmark paper published two decades ago, Lalouel and coworkers using a sib-pair analysis found a relationship of AGT to hypertension [17••]. In addition, an AGT molecular variant, M235T, was associated not only with the circulating level of AGT, but with hypertension in two separate populations, one from the US and the other from France. In blacks, the frequency of this allele is approximately 90 % (nearly 100 % in African blacks), raising the question of whether variations in AGT contribute to the race difference in susceptibility to hypertension. Subsequent studies using haplotypes that provided a more diverse range of genotypes, and therefore additional statistical power, showed that the level of AGT was related to the AGT genotype [18]. The original studies of AGT have now been replicated with several molecular variants, including M235T [19–21]. No other variation of a gene has so consistently shown significant associations with hypertension. The latest ‘twist’ to the relationship of AGT to hypertension is dietary intakes of Na and K (as determined from excretion rates) coupled to the plasma aldosterone in blacks residing in South Africa, as discussed in a subsequent section.
Regarding intra-renal RAS, which obviously is not easy to characterize or gauge clinically, its level of activity could play an important role, not just as a regulator of BP, but as a contributor to risk for chronic kidney disease in blacks. Indeed, a case can be made for treating the hypertension in blacks with RAS-lytic drugs apart from whether it improves BP. In keeping with this logic, blacks with renal insufficiency related to hypertension responded more favorably to use of ACE-inhibitors than beta-blockers or calcium channel blockers in the African American Study of Kidney Diseases [22].
Na, K, 2Cl Cotransporter in Thick Ascending Limb
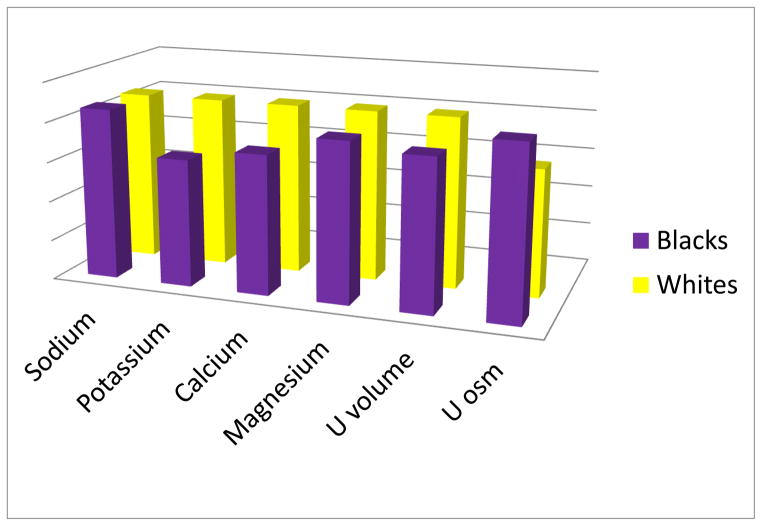
The best evidence for a specific site where Na reabsorption is increased in blacks comes from studies of Na, K, 2Cl cotransporter (NKCC2) in thick ascending limb (TAL). About a quarter of the Na filtered is reabsorbed in TAL, primarily by NKCC2 [23]. Regulation of NKCC2 is complex. For example, the severe salt-losing nephropathy known as Bartter syndrome [24] results from a loss of cotransporter function from mutations in NKCC2 itself (Type 1) [25] or mutations in four NKCC2 regulating genes (Types 2–5) [26–29]. Although components of ion transport systems in TAL are a rich source of candidate genes for hypertension, there has never been a monogenetic form of hypertension localized to TAL. Nonetheless, a large body of evidence favors the idea that NKCC2 is more active in blacks. This includes lower urinary excretion rates of Ca [30, 31], K and magnesium (Mg) [32] (with the exception of Mg, excretion rates of these ions have included identical intakes thus ruling out that differences are dietary in origin). Urinary volumes are lower and urinary osmolalities are higher in blacks in comparison to whites (Fig. 1). The direction of each of the race differences is in keeping with a more active NKCC2 in blacks.
Fig 1.
A comparative depiction of average urinary excretion rates of cations (Na, K, Ca and Mg), urine volumes and urine osmolalities in blacks and whites. Na excretion rates are the same in the two race groups, but K, Ca and Mg excretion rates are lower in blacks, consistent with a more active NKCC2 in TAL. Urine (U) volumes are smaller and urine osmolalities are higher in blacks, again consistent with NKCC2 being more active in blacks. Na excretion is the same in the two groups, pointing out similar dietary intakes of Na. Overall, however, blacks may retain more Na, but after coming into balance, excretion begins to equal intake
NKCC2–Dependent Reabsorption of Cations (Ca, Mg and K)
Paracellular uptake of Ca and Mg in TAL versus their downstream excretion is under the influence of the intra-luminal electrical charge [33], a by-product of the activity level of NKCC2. Cations, attracted to a negatively charged luminal milieu, remain in the lumen where they are more or less destined for excretion in urine. If the lumen holds a positive charge, the inclination of cations is to move out from the lumen by a paracellular route down an electrochemical gradient to the extracellular space. As NKCC2 becomes more active, the K that accumulates inside peritubular cells is returned to the lumen via the K channel ROMK. As more K recycles back to the lumen, the lumen takes on a positive charge, which then promotes Ca and Mg paracellular re-uptake. In blacks, Ca excretion, for example, is approximately 30 % less than it is in whites [31], in keeping with greater NKCC2 activity.
Ca-sensing receptor and NKCC2
Ca-sensing receptor (Ca-SR) expressed along the basolateral surface of cells lining the lumen of TAL may play a role in producing racial differences in BP regulation. When activated by an increase in extracellular Ca, the downstream effect to inhibit NKCC2. A loss of function variation in Ca-SR would lead to less inhibition of the cotransporter. Common genetic variations have now been shown to associate with a decrease in Ca excretion and an increase in BP in blacks, although not in whites [34], as if blacks were somehow (possibly through effects of an expanded volume) sensitized to the influences of Ca interacting with its receptor on NKCC2.
NKCC2 and Water Conservation
The Na reclaimed in TAL is used to build the osmotic gradient against which water is reabsorbed in collecting duct. Early on when living on the African continent, an exceptional ability to conserve water was important for survival, as was Na conservation (suggested first by L. Bankir; personal communication). This survival advantage could have been achieved by a NKCC2 functioning at a higher level. Bankir and coworkers have now shown that, indeed, in blacks, urine volumes are typically lower and urine concentrations higher in comparison to whites [35]. Interventional studies with furosemide [36] and with water loading [37] have provided additional evidence for a more active NKCC2 in blacks.
Interaction of Proximal Na Reabsorption and Aldosterone Production
If there is indeed increased Na reabsorption in blacks, by NKCC2 in TAL for example, for this to result in a higher BP, a built-in adjustment by the kidney would have to fail. The distal nephron is the target of aldosterone, lower levels of which accompany an expanded volume. Aldosterone activates mineralocorticoid receptors in principle cells in cortical collecting duct to produce the effect of prolonging the time ENaC resides at the apical surface. Although early genetic association studies suggested that common molecular variants of ENaC subunits that were more frequent in blacks might convey risk for hypertension [38, 39], replication of the findings has been mostly lacking. It would seem more likely that ‘failure to adjust’ on the part of the distal nephron results from aldosterone production that extends beyond what is needed to maintain Na and K homeostasis. Especially at the low end of aldosterone production, its regulation could depart from what is required—it could very easily be the case that too much is produced. Treatment with amiloride, an inhibitor of ENaC, was shown to reduce BP in whites more than in blacks. There appeared to be less ENaC activity that could be inhibited in blacks [40]. Greater Na reabsorption in a more proximal nephron region appeared to suppress aldosterone production, and in turn, level of ENaC activity in blacks. Inhibition of ENaC with either amiloride or spironolactone or both has been shown to benefit blacks with resistant hypertension [41•].
Primary aldosteronism, either from adrenal hyperplasia or adrenal adenoma, accounts for ~ 10 % of cases of essential hypertension [42], a formidable proportion. Normotensive individuals with a higher, but still within the normal range, plasma aldosterone concentration (PAC) are at significantly greater risk for future onset of hypertension [43]. In recent years, aldosterone has taken on new relevance as a contributor to the etiology of hypertension. More important is the PAC with respect to level of plasma renin activity (PRA). If individuals are already volume expanded, the case for blacks more than whites, then a non-elevated PAC may suffice to increase Na retention to where BP increases. PAC in relation to volume status can be expressed as the ‘aldosterone/renin ratio’ (ARR). Kotchen’s group showed that ARR was increased in blacks with hypertension and significantly higher in black hypertensives than white hypertensives [44•]. Most likely, the hypertension developed in response to a PAC that was too high for the extent of volume expansion.
There is considerable interest in finding variations in the regulation of aldosterone production that result in levels of aldosterone that exceed the restraints posed by a suppressed RAS. Recently, a study of blacks from South Africa showed that the level of AGT was related to the PAC when individuals consumed a diet high in Na and low in K [45•]. This may help to explain the relationship of AGT to BP. It was as if the high salt intake took away the contribution created by a need for restoring Na retention and volume normally conveyed by angiotensin II. The AGT concentration appeared to be driving the production of aldosterone to where it could seemingly raise BP.
K channels [46, 47] help to establish the transmembrane potential in adrenal glomerulosa cells that is critical for signaling secretion of aldosterone. Deficiencies in these channels have been associated with a primary aldosteronism-type of hypertension in genetic mouse models [48]. Single nucleotide polymorphism in one TASK channel gene, KCNK9, showed promise for an association with PAC and BP in blacks and whites [23]. It may just be that these kinds of modifiers of aldosterone synthesis provide the final piece to the puzzle for development of hypertension in many individuals. Blacks with what appears to be a pre-existing state of volume expansion may be particularly vulnerable.
Kidney Disease, the Genes MYH9 and APOL1, and Parasitic Protozoa (Trypanosoma brucei rhodesiense)
Genes for certain kidney diseases in blacks were found to reside in a region on chromosome 22 that included the MYH9 gene. Variations in MYH9 associated with focal-segmental-glomerulosclerosa (FSGS), hypertension-attributed end stage kidney disease (H-ESKD) and HIV-associated nephropathy [49, 50]. Causal mutations were, however, lacking. In the same region, the gene APOL1 encodes for apoprotein L-1. Two APOL1 variants, G1 and G2, had high odds ratios for associations with FSGS and H-ESKD. About 50 % of blacks are carriers of at least one or the other allele, 10–15 % are carriers of two alleles, whereas whites are carriers of neither [51 ••]. A multidiscipline team of researchers unfolded the evidence for positive selection of the risk allele, which today, with people living much longer, results in a negative selection for kidney disease. Specifically, ApoL1 circulates in blood where it lyses trypanosomes. Using an in vitro assay, the alleles that resulted in kidney disease were the same as those that lysed Trypanosoma brucei rhodesiense. The speculation is that the evolution of a critical factor for survival in Africa also contributes to high rates of renal disease in blacks residing in the US [52]. The role, if any, of these risk alleles to the development of hypertension is, to the best of our knowledge, unknown.
Epigenetics
In rat experiments, early in life stresses induced by maternal separation were accompanied by increased vascular sensitivity to vasopressors as adults [53]; there appeared to be evidence for epigenetic modifications affecting susceptibility to hypertension. The horrific injustices served on generations of blacks over many years: could they have contributed to the current predisposition to hypertension? Such is theoretically possible, through epigenetic mechanisms where DNA sequences are maintained, but phenotypes and gene expressions are altered by methylation of DNA as well as other reactions with DNA. This is an exciting area of investigation, but only recently has it been applied to an understanding of mechanisms for hypertension (see review by Cowley et al. [54]).
Conclusion
Blacks, in comparison to whites, appear to have a more expanded plasma volume due to an increase in Na reabsorption by the kidney. This may be what predisposes blacks to more severe hypertension. Potential sites where reuptake of Na may be excessive are discussed. What may be a critical component to the development of hypertension is a distal nephron that does not adjust because of a failure by aldosterone to sufficiently suppress its levels.
Footnotes
Disclosure W. Tu declares that he has no conflict of interest.
J.H. Pratt declares that he has no conflict of interest.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988–2008. JAMA. 2010;303:2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 2.Kissela B, Schneider A, Kleindorfer D, Khoury J, Miller R, Alwell K, Woo D, Szaflarski J, Gebel J, Moomaw C, Pancioli A, Jauch E, Shukla R, Broderick J. Stroke in a biracial population: The excess burden of stroke among blacks. Stroke. 2004;35:426–431. doi: 10.1161/01.STR.0000110982.74967.39. [DOI] [PubMed] [Google Scholar]
- 3.Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. New Engl J Med. 1982;306:1276–1279. doi: 10.1056/NEJM198205273062106. [DOI] [PubMed] [Google Scholar]
- 4.Helmer OM, Judson WE. Metabolic studies on hypertensive patients with suppressed plasma renin activity not due to hyperaldosternosm. Circulation. 1968;38:965–976. doi: 10.1161/01.cir.38.5.965. [DOI] [PubMed] [Google Scholar]
- 5.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8(Suppl II):II-127–II-134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 6.Pratt JH, Jones JJ, Miller JZ, Wagner MA, Fineberg NS. Racial differences in aldosterone excretion and plasma aldosterone concentrations in children. New Engl J Med. 1989;321:1152–1157. doi: 10.1056/NEJM198910263211703. [DOI] [PubMed] [Google Scholar]
- 7.Grim CE, Luft FC, Miller JZ, Meneely GR, Battarbee HD, Hames CG, Dahl LK. Racial differences in blood pressure in evans county, georgia: Relationship to sodium and potassium intake and plasma renin activity. J Chron Dis. 1979;33:87–94. doi: 10.1016/0021-9681(80)90032-6. [DOI] [PubMed] [Google Scholar]
- 8.Turban S, Miller ER, 3rd, Ange B, Appel LJ. Racial differences in urinary potassium excretion. J Am Soc Nephrol. 2008;19:1396–1402. doi: 10.1681/ASN.2007101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks. Hypertension. 2004;43:707–713. doi: 10.1161/01.HYP.0000120155.48024.6f. [DOI] [PubMed] [Google Scholar]
- 10.Klevay LM, Bogden JD, Aladjem M, Sandstead HH, Kemp FW, Li W, Skurnick J, Aviv A. Renal and gastrointestinal potassium excretion in humans: New insight based on new data and review and analysis of published studies. J Am Coll Nutr. 2007;26:103–110. doi: 10.1080/07315724.2007.10719591. [DOI] [PubMed] [Google Scholar]
- 11.Chrysant SG, Danisa K, Kem DC, Dillard BL, Smith WJ, Frohlich ED. Racial differences in pressure, volume and renin interrelationships in essential hypertension. Hypertension. 1979;1:136–141. doi: 10.1161/01.hyp.1.2.136. [DOI] [PubMed] [Google Scholar]
- 12.Pratt JH, Manatunga AK, Bloem LJ, Wei L. Racial differences in aldosterone excretion: A longitudinal study in children. J Clin Endocrinol Metabol. 1993;77:1512–1515. doi: 10.1210/jcem.77.6.8263135. [DOI] [PubMed] [Google Scholar]
- 13**.Zou LX, Imig JD, Von Thun AM, Hymel A, Ono H, Navar LG. Receptor-mediated intrarenal angiotensin ii augmentation in angiotensin ii-induced rats. Hypertension. 1996;28:669–677. doi: 10.1161/01.hyp.28.4.669. Work from this laboratory showed that there is an intra-renal RAS. [DOI] [PubMed] [Google Scholar]
- 14.Navar LG. The kidney in blood pressure regulation and development of hypertension. Med Clin. 1997;81:1165–1198. doi: 10.1016/s0025-7125(05)70573-3. [DOI] [PubMed] [Google Scholar]
- 15**.Ito M, Oliverio MI, Mannon PJ, Best CF, Maeda N, Smithies O, Coffman TM. Regulation of blood pressure by the type 1a angiotensin ii receptor gene. Proc Natl Acad Sci. 1995;92:3521–3525. doi: 10.1073/pnas.92.8.3521. Elegant mouse model studies that delineated a role for intra-renal RAS to regulate blood pressure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HS, Krege JH, Kluckman KD, Hagaman JR, Hodgin JB, Best CF, Jennette JC, Coffman TM, Maeda N, Smithies O. Genetic control of blood pressure and the angiotensinogen locus. Proc Natl Acad Sci. 1995;92:2735–2739. doi: 10.1073/pnas.92.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17**.Jeunemaitre X, Soubrier F, Kotelevtev YV, Lifton RP, Williams CS, Charru A, Hunt SC, Hopkins PN, Williams RR, Lalouel JM, Corvol P. Molecular basis of human hypertension: Role of angiotensinogen. Cell. 1992;71:169–180. doi: 10.1016/0092-8674(92)90275-h. A landmark paper demonstrating for the first time common variations in a gene associated with hypertension. [DOI] [PubMed] [Google Scholar]
- 18.Bloem LJ, Foroud TM, Ambrosius WT, Hanna MP, Tewksbury DA, Pratt JH. Association of the angiotensinogen gene to serum angiotensinogen in blacks and whites. Hypertension. 1997;29:1078–1082. doi: 10.1161/01.hyp.29.5.1078. [DOI] [PubMed] [Google Scholar]
- 19.Bloem LJ, Manatunga AK, Tewksbury DA, Pratt JH. The serum angiotensinogen concentration and variants of the angiotensinogen gene in white and black children. J Clin Investig. 1995;95:948–953. doi: 10.1172/JCI117803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caulfield M, Lavender P, Farrall M, Munroe P, Lawson M, Turner P, Clark AJ. Linkage of the angiotensinogen gene to essential hypertension. New Engl J Med. 1994;330:1629–1633. doi: 10.1056/NEJM199406093302301. [DOI] [PubMed] [Google Scholar]
- 21.Schorr U, Blaschke K, Beige J, Distler A, Sharma AM. Angiotensinogen m235t variant and salt sensitivity in young normotensive caucasians. J Hypertens. 1999;17:475–479. doi: 10.1097/00004872-199917040-00004. [DOI] [PubMed] [Google Scholar]
- 22.Wright JT, Jr, Bakris G, Aask, Greene T, Agodoa LY, Appel LJ, Charleston J, Cheek D, Douglas-Baltimore JG, Gassman J, Glassock R, Hebert L, Jamerson K, Lewis J, Phillips RA, Toto RD, Middleton JP, Rostand SG. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: Results from the aask trial. JAMA. 2002;288:2421–2431. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 23.Greger R. Ion transport mechanisms in thick ascending limb of henle’s loop of mammalian nephron. Physiol Rev. 1985;65:760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- 24.Bartter FC, Pronove P, Gill JR, MacCardle RC. Hyperplasia of the jutaglomerular complex wih hyperaldosteronism and hypokalemic alkalosis. Am J Med. 1962;33:811–828. doi: 10.1016/0002-9343(62)90214-0. [DOI] [PubMed] [Google Scholar]
- 25.Simon DB, Karet FE, Hamdan JM, DiPietro A, Sanjad SA, Lifton RP. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the na-k-2cl cotransporter nkcc2. Nat Genet. 1996;13:183–188. doi: 10.1038/ng0696-183. [DOI] [PubMed] [Google Scholar]
- 26.Simon DB, Karet FE, Rodriquez-Soriano J, Hamdan JH, DePietro A, Trachtman H, Sanjad SA, Lifton RP. Genetic heterogeneithy of bartter’s syndrome revealed by mutations in the k+ channel, romk. Nat Genet. 1996;14:152–156. doi: 10.1038/ng1096-152. [DOI] [PubMed] [Google Scholar]
- 27.Simon DB, Bindra RS, Mansfield TA, Nelson-Williams C, Mendonca E, Stone R, Schurman S, Nayir A, Alpay H, Bakkaloglu A, Rodriguez-Soriano J, Morales JM, Sanjad SA, Taylor CM, Pilz D, Brem A, Trachtman H, Griswold W, Richard GA, Jonh E, Lifton RP. Mutations in the chloride channel gene, clcnkb, cause bartter’s syndrome type iii. Nat Genet. 1997;17:171–178. doi: 10.1038/ng1097-171. [DOI] [PubMed] [Google Scholar]
- 28.Birkenhager R, Otto E, Schurmann MJ, Vollmer M, Ruf EM, Maier-Lutz I, Beekmann F, Fekete A, Omran H, Feldmann D, Milford DV, Jeck N, Konrad M, Landau D, Knoers NV, Antignac C, Sudbrak R, Kispert A, Hildebrandt F. Mutation of bsnd causes bartter syndrome with sensorineural deafness and kidney failure. Nat Genet. 2001;29:310–314. doi: 10.1038/ng752. [DOI] [PubMed] [Google Scholar]
- 29.Watanabe S, Fukumoto S, Chang H, Takeuchi Y, Hasegawa Y, Okazaki R, Chikatsu N, Fujita T. Association between activating mutations of calcium-sensing receptor and bartter’s syndrome. Lancet. 2002;360:692–694. doi: 10.1016/S0140-6736(02)09842-2. [DOI] [PubMed] [Google Scholar]
- 30.Wigertz K, Palacios C, Jackman LA, Martin BR, McCabe LD, McCabe GP, Peacock M, Pratt JH, Weaver CM. Racial differences in calcium retention in response to dietary salt in adolescent girls. Am J Clin Nutr. 2005;81:845–850. doi: 10.1093/ajcn/81.4.845. [DOI] [PubMed] [Google Scholar]
- 31.Pratt JH, Manatunga AK, Peacock M. A comparison of the urinary excretion of bone resorptive products in white and black children. J Lab Clin Med. 1996;127:67–70. doi: 10.1016/s0022-2143(96)90167-5. [DOI] [PubMed] [Google Scholar]
- 32.Chun TY, Bankir L, Eckert GJ, Bichet DG, Saha C, Zaidi SA, Wagner MA, Pratt JH. Ethnic differences in renal responses to furosemide. Hypertension. 2008;52:241–248. doi: 10.1161/HYPERTENSIONAHA.108.109801. [DOI] [PubMed] [Google Scholar]
- 33.Hebert SC, Andreoli TE. Control of nacl transport in the thick ascending limb. Am J Physiol. 1984;246:F745–F756. doi: 10.1152/ajprenal.1984.246.6.F745. [DOI] [PubMed] [Google Scholar]
- 34.Jung J, Foroud TM, Eckert GJ, Flury-Wetherill L, Edenberg HJ, Xuei X, Zaidi SA, Pratt JH. Association of the calcium-sensing receptor gene with blood pressure and urinary calcium in african-americans. J Clin Endocrinol Metab. 2009;94:1042–1048. doi: 10.1210/jc.2008-1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bankir L, Perucca J, Weinberger MH. Ethnic differences in urine concentration: Possible relationship to blood pressure. Clin J Am Soc Nephrol. 2007;2:304–312. doi: 10.2215/CJN.03401006. [DOI] [PubMed] [Google Scholar]
- 36.Hancock ML, 2nd, Bichet DG, Eckert GJ, Bankir L, Wagner MA, Pratt JH. Race, sex and the regulation of urine osmolality-observations made during water deprivation. Am J Physiol Regul Integr Comp Physiol. 2010 doi: 10.1152/ajpregu.00289.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weder AB, Gleiberman L, Sachdeva A. Whites excrete a water load more rapidly than blacks. Hypertension. 2009;53:715–718. doi: 10.1161/HYPERTENSIONAHA.108.121665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baker EH, Dong YB, Sagnella GA, Roghwell M, Onnipinla AK, Markandu ND, Cappuccio FP, Cook DG, Persu A, Corvol P, Jeunemaitre X, Carter ND, MacGregor GA. Association of hypertension with t594m mutation in á subunit of epithelial sodium channels in black people resident in london. Lancet. 1998;351:1388–1392. doi: 10.1016/s0140-6736(97)07306-6. [DOI] [PubMed] [Google Scholar]
- 39.Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, Guo C, Pratt JH. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension. 1999;34:631–637. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- 40.Pratt JH, Ambrosius WT, Agarwal R, Eckert GJ, Newman S. Racial difference in the activity of the amiloride-sensitive epithelial sodium channel. Hypertension. 2002;40:903–908. doi: 10.1161/01.hyp.0000039749.75068.f4. [DOI] [PubMed] [Google Scholar]
- 41*.Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q, Pratt JH. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–487. doi: 10.1161/01.HYP.0000179582.42830.1d. Demonstration of the effectiveness of ENaC inhibition to lower blood pressure in blacks. [DOI] [PubMed] [Google Scholar]
- 42.Funder JW, Carey RM, Fardella C, Gomez-Sanchez CE, Mantero F, Stowasser M, Young WF, Jr, Montori VM. Case detection, diagnosis, and treatment of patients with primary aldosteronism: An endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:3266–3281. doi: 10.1210/jc.2008-0104. [DOI] [PubMed] [Google Scholar]
- 43.Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. N Engl J Med. 2004;351:33–41. doi: 10.1056/NEJMoa033263. [DOI] [PubMed] [Google Scholar]
- 44*.El Gharbawy AH, Nadig VS, Kotchen JM, Grim CE, Sagar KB, Kaldunski M, Hamet P, Pausova Z, Gaudet D, Gossard F, Kotchen TA. Arterial pressure left ventricular mass and aldosterone in essential hypertension. Hypertension. 2001;37:845–850. doi: 10.1161/01.hyp.37.3.845. Important paper showing that development of hypertension in blacks, not whites, is coincident with excess aldosterone relative to volume, as expressed in the aldosterone/renin ratio. [DOI] [PubMed] [Google Scholar]
- 45*.Michel FS, Norton GR, Majane OH, Badenhorst M, Vengethasamy L, Paiker J, Maseko MJ, Sareli P, Woodiwiss AJ. Contribution of circulating angiotensinogen concentrations to variations in aldosterone and blood pressure in a group of african ancestry depends on salt intake. Hypertension. 2012;59:62–69. doi: 10.1161/HYPERTENSIONAHA.111.181230. New observation implicating a role for aldosterone to mediate the effects of angiotensinogen on blood pressure in blacks. [DOI] [PubMed] [Google Scholar]
- 46.Czirjak G, Fischer T, Spat A, Lesage F, Enyedi P. Task (twik-related acid-sensitive k+ channel) is expressed in glomerulosa cells of rat adrenal cortex and inhibited by angiotensin ii. Mol Endocrinol. 2000;14:863–874. doi: 10.1210/mend.14.6.0466. [DOI] [PubMed] [Google Scholar]
- 47.Czirjak G, Enyedi P. Task-3 dominates the background potassium conductance in rat adrenal glomerulosa cells. Mol Endocrinol. 2002;16:621–629. doi: 10.1210/mend.16.3.0788. [DOI] [PubMed] [Google Scholar]
- 48.Davies LA, Hu C, Guagliardo NA, Sen N, Chen X, Talley EM, Carey RM, Bayliss DA, Barrett PQ. Task channel deletion in mice causes primary hyperaldosteronism. Proc Natl Acad Sci. 2008;105:2203–2208. doi: 10.1073/pnas.0712000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, Coresh J, Patterson N, Tandon A, Powe NR, Fink NE, Sadler JH, Weir MR, Abboud HE, Adler SG, Divers J, Iyengar SK, Freedman BI, Kimmel PL, Knowler WC, Kohn OF, Kramp K, Leehey DJ, Nicholas SB, Pahl MV, Schelling JR, Sedor JR, Thornley-Brown D, Winkler CA, Smith MW, Parekh RS. Myh9 is associated with nondiabetic end-stage renal disease in african americans. Nat Genet. 2008;40:1185–1192. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, Oleksyk T, McKenzie LM, Kajiyama H, Ahuja TS, Berns JS, Briggs W, Cho ME, Dart RA, Kimmel PL, Korbet SM, Michel DM, Mokrzycki MH, Schelling JR, Simon E, Trachtman H, Vlahov D, Winkler CA. Myh9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–1184. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51**.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, Bowden DW, Langefeld CD, Oleksyk TK, Uscinski Knob AL, Bernhardy AJ, Hicks PJ, Nelson GW, Vanhollebeke B, Winkler CA, Kopp JB, Pays E, Pollak MR. Association of trypanolytic apol1 variants with kidney disease in african americans. Science. 2010;329:841–845. doi: 10.1126/science.1193032. Showed for the first time that a clear survival advantage led eventually to a high frequency kidney disease. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedman DJ, Pollak MR. Genetics of kidney failure and the evolving story of apol1. J Clin Invest. 2011;121:3367–3374. doi: 10.1172/JCI46263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loria AS, Pollock DM, Pollock JS. Early life stress sensitizes rats to angiotensin ii-induced hypertension and vascular inflammation in adult life. Hypertension. 2010;55:494–499. doi: 10.1161/HYPERTENSIONAHA.109.145391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cowley AW, Jr, Nadeau JH, Baccarelli A, Berecek K, Fornage M, Gibbons GH, Harrison DG, Liang M, Nathanielsz PW, O’Connor DT, Ordovas J, Peng W, Soares MB, Szyf M, Tolunay HE, Wood KC, Zhao K, Galis ZS. Report of the national heart, lung, and blood institute working group on epigenetics and hypertension. Hypertension. 2012;59:899–905. doi: 10.1161/HYPERTENSIONAHA.111.190116. [DOI] [PMC free article] [PubMed] [Google Scholar]