Abstract
Objective
Musculoskeletal disorders are common and often lead to chronic pain in older adults. Since the efficacy of interventions varies with the duration of pain, the identification of early biomarkers for chronic pain would have important public health consequences. Imaging of functional connectivity differences between brain regions might identify some of the earliest functional consequences of a disease process. We tested the hypothesis that chronic musculoskeletal pain in older persons is associated with changes in functional brain connectivity.
Method
We used resting-state fMRI and a spherical seed-based region of interest approach to assess functional connectivity of brain regions on a sample of 128 (64 who reported chronic musculoskeletal pain and 64 demographically-matched, pain free) nondemented older adults from the Memory and Aging Project, a clinical-pathological cohort study of aging and dementia.
Results
Older adults with chronic pain showed greater functional connectivity between the posterior cingulate and left insula, left superior temporal gyrus, and left cerebellum.
Conclusion
Chronic musculoskeletal pain is associated with a specific pattern of functional connectivity between brain regions among older adults.
Keywords: chronic pain, functional connectivity, insula, posterior cingulate, resting-state fMRI
1. INTRODUCTION
Musculoskeletal pain is a common complaint among older adults (Mottram et al., 2008) and its prevalence has been found to increase with age (Wolfe et al., 1995; Andersson et al., 1993). Chronic musculoskeletal pain has been linked with mobility limitation and disability in old age (Shah et al., in 2011; Mailis-Gagnon et al., 2008) and its treatment costs billions of dollars (Bennett, 1999). The processing of acute musculoskeletal pain begins in the peripheral joints and impulses are conducted by the peripheral nerves to the central nervous system. Within the central nervous system, pain is processed in the dorsal horn of spinal cord prior to thalamic and cortical regions associated with the perception of pain. As the experience of pain becomes chronic, structural central nervous system changes occur, making treatment and intervention more difficult (May, 2008; Flor, 2003; Bennett, 1999). People age 65 years and older represent the fastest growing segment of the US population, underscoring the public health challenge of chronic pain. Identification of biomarkers to facilitate efficacious interventions for pain is critical to decreasing the development of disability and containing health costs in our rapidly aging population.
The ability to study network characteristics of broad brain systems may be one of the first and most sensitive neuroimaging biomarkers of a pathological state (Friston et al., 1994). Recent work has elucidated networks of functionally connected brain regions associated with the experience of chronic pain in younger and middle-age populations using resting-state fMRI functional connectivity approaches (Baliki et al., 2008; Tagliazucchi et al., 2010; Apkarian et al., 2004; Baliki et al., 2010). However, the functional connectivity of brain networks associated with chronic pain among older adults has not been examined.
Since structural changes in the brain have been reported in chronic pain (Farrell, 2012), the current study examined whether functional connectivity patterns differ between older adults with and without chronic pain. Utilizing a sample of 128 (64 who report chronic pain and 64 demographically-matched, pain free) nondemented older adults from a community-based epidemiologic study, we explored whether there were functional connectivity differences to the posterior cingulate cortex in older individuals with and without chronic musculoskeletal pain using resting-state fMRI. This region was chosen as a region of interest because of its role in networks sensitive to multiple pathological aspects of aging (Greicius et al, 2004; Wang et al., 2006; Buckner et al., 2008; Buckner et al., 2009; Sperling et al., 2009; Buckner et al., 2005; Hedden et al., 2009; Buckner and Vincent, 2007) and also pain (Brooks et al., 2005; Ostrowski et al., 2002). It was demonstrated that brain regions which commonly contribute to pain perception are more functionally connected in older adults with chronic pain. In addition, brain regions involved in affective and cognitive processing which may play an important role in chronic pain are also more functionally connected in older adults with chronic pain.
2. METHODS
2.1 Participants and Procedures
The current study included participants from the Rush Memory and Aging Project, a community-based clinical-pathologic cohort study of aging and dementia (Bennett et al., 2005). Participants are free of clinically-diagnosed dementia at baseline and agree to annual exam and autopsy at the time of death. They come from approximately 40 residential facilities across the greater Chicago metropolitan area, including subsidized senior housing facilities, retirement communities, retirement homes, local churches, and other community organizations. All participant procedures were approved by an Internal Review Board.
The Rush Memory and Aging Project has a rolling admission that started in 1997. Brain imaging was initiated in 2008. At the time of the current analyses, 1299 participants had enrolled and completed their baseline evaluation, 443 died, and 77 refused further participation before scan data collection began. Of the remaining 779, 260 had MRI contraindications or were unable to sign informed consent leaving 519 eligible for scanning. Of these, 155 (29.9%) refused, 214 were scanned, and the remaining 150 were still being scheduled for scanning. Of the 214 that were scanned, 133 completed our pain measure, and 64 were found to report chronic musculoskeletal pain. Of the remaining 69 that did not report pain, 64 individuals that were demographically-matched on age, education, and gender were randomly selected and used as a comparison group.
Musculoskeletal pain was assessed by asking the participant if he or she had experienced pain or aching in any joint (1 or more joints) on most days for at least one month during the year prior to brain imaging as previously described (Buchman et al., 2010). Positive responses to this question then prompted a clarification of pain being musculoskeletal and, for example, being in the back, neck, hands, hips, knees, or feet. Positive responses to this clarification assigned the participant to the “pain” group. Participants in the “pain” group reported pain in at least one region of the body, and some had multiple body regions with pain. These measures of pain were previously shown to be associated with incident disability in this and other cohorts supporting the clinical relevance of the scale (Buchman et al., 2010, Shah et al., 2011).
2.2 Image Acquisition and Processing
MRI scans were conducted on a 1.5 Tesla clinical scanner (General Electric, Waukesha, WI), equipped with a standard quadrature head coil, located within the community of the sample. Participants were instructed to keep their head still for the duration of imaging. High data quality was ensured through appropriate quality assurance tests. High-resolution T1-weighted anatomical images were collected with a 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence with the following parameters: TR = 6.3 ms; TE = 2.8 ms; preparation time = 1000 ms; flip angle = 8°; 160 sagittal slices; 1 mm slice thickness; field of view (FOV) = 24 cm × 24 cm; acquisition matrix 224 × 192, reconstructed to a 256 × 256 image matrix; scan time = 10 min and 56 secs. Two copies of the T1-weighted data were acquired on each subject and averaged. Resting state MRI data was acquired using a 2D spiral in/out echo-planar imaging (EPI) sequence with the following parameters: TR = 2000 ms; TE = 33 ms; flip angle = 85°; 26 oblique axial slices; 5 mm slice thickness; acquisition/reconstruction matrix 64 × 64; FOV = 24 cm × 24 cm; 240 time-points/volumes; scan time = 8 min. All of the participants included in the study had to satisfy exclusion criteria of head movement during fMRI scanning less than 2.5mm translation in any axis and less than 2.5° angular rotation in any axis.
The skull was removed from each averaged structural MRI dataset using FreeSurfer’s Hybrid Watershed Algorithm (Segonne et al., 2004). Structural scans were also manually edited when necessary to remove residual non-brain signals. Brain segmentation into gray matter, white matter and CSF was also performed using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/). Intracranial volume was also derived, and thus proportions of each compartment were calculated.
The first 5 image volumes of resting state data were discarded at the scanner to avoid using data collected before reaching signal equilibrium. Images were reconstructed on Linux machines from the acquired k-space data (Glover et al., 2004). Using the Statistical Parametric Mapping software (Friston et al., 1995; http://www.fil.ion.ucl.ac.uk/spm/) version 8 (SPM8), all resting-state volumes were corrected for motion, co-registered to the high-resolution T1-weighted data, and spatially normalized to the Montreal Neurological Institute (MNI) template. The normalized image volumes were spatially smoothed with a 4mm full-width half-maximum (FWHM) Gaussian kernel. Next, a band-pass filter of 0.01 to 0.08 Hz was applied to the data in temporal frequency space to minimize low-frequency signal drift and high frequency variations due to cardiac and respiratory effects.
A spherical seed ROI with a radius of 4mm was prescribed in the posterior cingulate cortex, with MNI coordinates of x=0, y=−53, z=26 in order to interrogate functional connectivity in a network of brain regions associated with the default mode network (Griecius et al., 2003). The size and location of the seed ROI was determined in consideration of previous work (Hedden et al., 2009). A mean signal time course for the seed was calculated and used as a reference. Correlation analysis was then conducted between the reference signal time course and the time series of each other voxel in the brain. The voxels showing significant functional connectivity to the posterior cingulate seed ROI were identified as those voxels whose cross-correlation differed significantly (alpha=0.005) from 0, based on one-sample t-tests applied to Fisher’s z-transformation of the correlation. In order to remove any residual effects of motion and other non-neuronal factors, 6 head motion parameters, as well as parameters for the white matter signal, global mean signal, and cerebrospinal fluid signal were used as nuisance variables (Buckner et al., 2009) in functional connectivity analysis using the Data Processing Assistant for Resting-State fMRI (DPARSF; http://restfmri.net/forum/DPARSF) and SPM8. Seed-based functional connectivity analysis was conducted with the Resting-State fMRI Data Analysis Toolkit (REST: http://restfmri.net/forum/REST).
2.3 Statistical Analyses
Statistical analyses proceeded in several steps. The 64 individuals who reported pain and the 64 demographically-matched individuals who did not report pain comprised two groups of contrast. We then examined between-group differences in demographic variables (age, education, sex, race, MMSE), cognitive performance data (global cognition), brain volumetry (total gray matter volume), and musculoskeletal pain (pain versus no pain) using two-tailed t-tests (age, education, MMSE, cognition, total gray matter volume) or Chi-square tests (sex, race). We then verified the functional connectivity of the posterior cingulate cortex to other regions by determining within-group whole brain Fisher z-transformed functional clusters of significance, for both pain categories (pain and no pain), after controlling for the effects of total gray matter volume. In order to control for multiple comparisons, within-group whole brain functional connectivity results were controlled by employing a false discovery rate (FDR) of 5%, cluster size > 5 voxels. Finally, we conducted voxel-wise, between-group comparisons of Fisher z-transformed functional connectivity values while adjusting for the effects of total gray matter volume. The chance of spurious findings was controlled by utilizing a conservative threshold of p <0.001 and a cluster-size > 5 voxels. This more conservative threshold was chosen based on previously published reports using similar resting-state fMRI approaches (e.g, Bluhm et al., 2007; Zhou et al., 2007; Bettus et al., 2009; Long et al., 2008).
3. RESULTS
3.1 Demographic, Cognitive, and Brain Volumetry Differences Between Those With Pain and Those Without Pain
Demographic, cognitive, and brain volumetry characteristics are shown in Table 1. Most participants were white. No other group differences were observed for age, education level, cognitive ability scores, or total brain gray matter volume. Of the 64 participants who reported joint pain, 21 reported 1 joint pain, 18 reported 2 joints pain, 18 reported 3 joints pain, and 7 reported 4 or more joints pain. In the same group, 23 reported 1 region of pain, 29 reported 2 regions of pain, and 12 reported 3 or more regions of pain. The pain group included 13 with lumbar radiculopathy, 51 with low back pain, 4 with cervical radiculopathy, and 14 with neck pain. There was no group difference between the use of any pain medication including anti-inflammatories, opioids, acetaminophen, and aspirin. Participants with chronic pain reported a higher number of pain medications (p<0.04). This latter difference did not affect neuroimaging differences observed between groups in subsequent analyses.
Table 1.
Statistics for demographic and brain volumetry variables. Data are summarized as Mean (Standard deviation=SD) or as number (%). Age and education are presented in years; MMSE is total score; global cognition is presented as z-scores; and total gray matter volume is presented as mm3.
| Pain | No Pain | t or X2 | p value | |
|---|---|---|---|---|
| N | 64 | 64 | ||
| Mean age (s.d.) | 81.3 (7.0) | 81.6 (3.5) | 0.28 | 0.78 |
| Mean education (s.d.) | 14.8 (3.3) | 14.7 (2.1) | −0.19 | 0.85 |
| Gender (F/M) | 47/17 | 53/11 | 1.14 | 0.29 |
| Mean MMSE score (s.d.) | 28.5 (1.5) | 28.3 (1.6) | −0.82 | 0.42 |
| Global cognition z-score (s.d.) | 0.36 (0.47) | 0.25 (0.56) | −1.25 | 0.22 |
| Mean total gray matter volume in mm3 (s.d.) | 285.19 (29.4) | 227.4 (26.6) | −1.57 | 0.12 |
| Depression symptoms (CES-D) | 1.06 (1.49) | 1.34 (1.71) | −0.96 | 0.33 |
| Use of pain medications | 53 | 49 | 0.77 | 0.38 |
| Number of pain medications | 1.39 (0.89) | 1.08 (0.76) | −2.063 | 0.04 |
3.2 Resting-State Functional Connectivity Differences Between Those With Pain and Those Without Pain
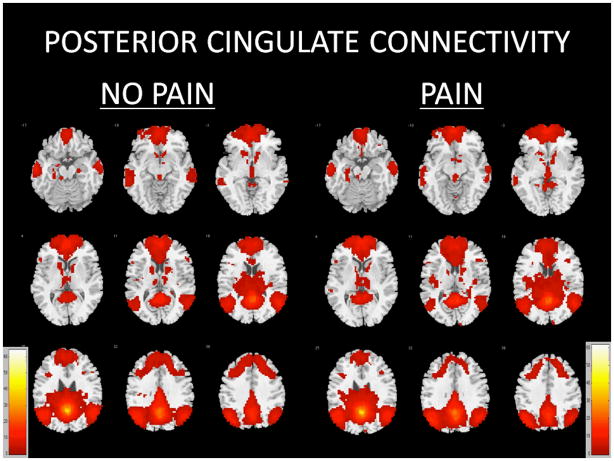
Seeding the posterior cingulate cortex yielded a network of functionally related regions after controlling for total gray matter volume among those with pain and those without pain (Figure 1, Table 2). Within-group analyses revealed two large clusters of significance incorporating bilateral frontal, temporal, parietal, and cerebellar regions (t=61.3778 and t=12.1484), as well as smaller clusters of significance within regions such as the left middle temporal gyrus (t=8.8546 and t=7.4509), right cerebellum (t=8.4453 and t=6.0997), left cerebellum (t=5.6949), left hippocampus (t=4.4624), right insula (t=4.1594), right middle temporal gyrus (t=4.1523), right middle frontal gyrus (t=4.1041), left fusiform (t=4.0631), left inferior frontal gyrus (t=4.0174), right superior temporal gyrus (t=3.8646), left superior frontal gyrus (t=3.7247), and left caudate (t=3.619) in older participants reporting pain. In those not reporting pain, two large clusters of significance incorporating bilateral frontal, temporal, parietal, and cerebellar regions (t=64.1825 and t=12.4576), as well as smaller cluster of significance within regions such as the left middle temporal gyrus (t=10.4737), right middle temporal gyrus (t=8.9566), right cerebellum (t=6.6093 and t=6.3507), left cerebellum (t=5.6873 and t=3.551), right caudate (t=4.8097 and t=4.2691), left middle frontal gyrus (t=4.0627), and left inferior frontal gyrus (t=3.6571).
Figure 1.
Functionally connected clusters indicated by a seed region of interest (ROI) prescribed in the posterior cingulate cortex for participants who experience no pain (N=64) and participants who report pain (N=64). Seed ROI MNI coordinates: x=0, y=53, z=−26; radius=4mm. Adjusted for total gray matter volume. False Discovery Rate (FDR) controlled at 5%. Cluster size > 5 voxels. p-value < 0.001.
Table 2.
Functionally connected clusters as indicated by a seed region of interest (ROI) prescribed in the posterior cingulate cortex and covarying for the effects of total gray matter volume. Seed ROI MNI coordinates: x=0, y=53, z=−26; radius= 4mm. Adjusted for total gray matter volume. False Discovery Rate (FDR) controlled at 5%. Cluster size > 5 voxels. p-value < 0.001.
| Pain | Region | Cluster Size (# voxels) | Maximum Intensity Voxel coordinates | t-value | ||
|---|---|---|---|---|---|---|
| Pain | ||||||
| Widespread Frontal, Temporal, Parietal, and Cerebellar | 5758 | 0 | −54 | 27 | 61.3778 | |
| Widespread Frontal, Temporal, Parietal, and Cerebellar | 3024 | 0 | 60 | 0 | 12.1484 | |
| L Middle Temporal Gyrus | 352 | −60 | −9 | −21 | 8.8546 | |
| R Cerebellum | 163 | 6 | −54 | −45 | 8.4453 | |
| L Middle Temporal Gyrus | 245 | 63 | −6 | −21 | 7.4509 | |
| R Cerebellum | 142 | 30 | −75 | −36 | 6.0997 | |
| L Cerebellum | 100 | −27 | −81 | −39 | 5.6949 | |
| L Hippocampus | 13 | −27 | −18 | −21 | 4.4624 | |
| R Insula | 8 | 39 | −18 | 15 | 4.1594 | |
| R Middle Temporal Gyrus | 13 | 60 | −36 | −9 | 4.1523 | |
| R Middle Frontal Gyrus | 11 | 39 | 24 | 21 | 4.1041 | |
| L Fusiform | 10 | −27 | −33 | −21 | 4.0631 | |
| L Inferior Frontal Gyrus | 6 | −48 | 21 | 6 | 4.0174 | |
| R Superior Temporal Gyrus | 11 | 45 | 15 | −36 | 3.8656 | |
| L Superior Frontal Gyrus | 5 | −9 | 33 | 54 | 3.7247 | |
| L Caudate | 5 | −18 | 18 | −3 | 3.619 | |
| No Pain | ||||||
| Widespread Frontal, Temporal, Parietal, and Cerebellar | 5510 | 0 | −54 | 27 | 64.1825 | |
| Widespread Frontal, Temporal, Parietal, and Cerebellar | 3693 | 3 | 51 | 6 | 12.4576 | |
| L Middle Temporal Gyrus | 398 | −60 | −12 | −18 | 10.4737 | |
| R Middle Temporal Gyrus | 313 | 63 | −6 | −21 | 8.9566 | |
| R Cerebellum | 115 | 6 | −54 | −42 | 6.6093 | |
| R Cerebellum | 201 | 27 | −81 | −33 | 6.3507 | |
| L Cerebellum | 75 | −27 | −84 | −42 | 5.6873 | |
| R Caudate | 29 | 15 | 0 | 15 | 4.8097 | |
| R Caudate | 42 | 9 | 6 | −3 | 4.2691 | |
| L Middle Frontal Gyrus | 12 | −39 | 57 | −12 | 4.0627 | |
| L Inferior Frontal Gyrus | 7 | −54 | 24 | 6 | 3.6571 | |
| L Cerebellum | 12 | −18 | −30 | −24 | 3.5511 | |
Regions of functional connectivity differences were observed between older participants with pain and without pain after between-group analyses (Figure 2, Table 3). Persons who reported pain showed greater connectivity between posterior cingulate and left insula (t=4.4295), left superior temporal gyrus (t=3.8694), and left cerebellum (t=3.7381). By contrast, persons who did not report pain showed greater connectivity between posterior cingulate and left middle frontal gyrus (t=3.9182).
Figure 2.
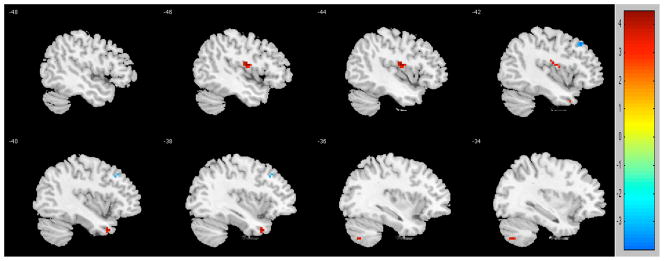
Regions of contrast between participants in pain (reds) and not in pain (blues). To control for multiple comparisons and spurious findings, we utilized the following: Cluster size > 5 voxels. p-value < 0.001.
Table 3.
Results of voxel-wise, between-group contrasts of z-transformed functional connectivity values (pain versus no-pain) while accounting for the effects of total gray matter volume. To control for multiple comparisons and spurious findings, we utilized the following: Cluster size > 5 voxels. p-value < 0.001.
| Pain | Region | Cluster size (# voxels) | Maximum Intensity Voxel coordinates | t-value | ||
|---|---|---|---|---|---|---|
| Pain> No Pain | ||||||
| L Insula | 13 | −45 | −9 | 15 | 4.4295 | |
| L Superior Temporal Gyrus | 5 | −39 | 12 | −36 | 3.8694 | |
| L Cerebellum | 5 | −33 | −69 | −48 | 3.7381 | |
| No Pain> Pain | ||||||
| L Middle Frontal Gyrus | 7 | −42 | 27 | 45 | 3.9182 | |
In subsequent post-hoc analyses, we investigated whether there were volumetric differences in the regions of functional connectivity difference that may be driving our findings. After correcting for intracranial volume in our regions of interest, we found no volumetric differences between groups in regions that showed functional connectivity differences (left insula, left middle frontal gyrus, left superior temporal gyrus, and left cerebellum). There were also no differences in posterior cingulate volume between groups.
An additional post-hoc analysis of the association between number of reported painful joints and functional connectivity data was conducted in the pain group. Controlling for age, education, gender, and total gray matter volume, results revealed regions that correlated directly and inversely with number of painful joints. Regions that correlated directly with number of reported painful joints included the left middle (t=4.7347) and superior (t=4.2537) temporal gyrus, right middle temporal gyrus (t=4.0782), and left middle occipital gyrus (t=4.0882). Regions that correlated inversely with number of reported painful joints include the bilateral middle cingulate (t=−3.9534), right precuneus (t=−4.4254), and the left inferior parietal gyrus (t=−3.8474).
4. DISCUSSION
We found that the pattern of functional brain connectivity is different in older adults with and without chronic musculoskeletal pain. Specifically, older adults with chronic musculoskeletal pain showed greater functional connectivity of the posterior cingulate to the left insula, left superior temporal gyrus, and the left cerebellum. These differences were observed after controlling for total gray matter volume. Furthermore, these functional connectivity differences were not driven by differences in region-specific volumetrics or the use of pain medication. Subsequent analyses with the pain group revealed a set of regions that showed functional connectivity associations with number of reported painful joints. Regions that showed a direct association included the left and right middle temporal gyrus, the middle occipital gyrus, and the left superior temporal gyrus. Regions that showed an inverse association included the bilateral middle cingulate, the right precuneus, and the left inferior parietal gyrus.
A model of chronic pain suggests greater sensitization of the central nervous system to both nociceptive and nonnociceptive input and the experience of nonnociceptive sensory input as nociceptive input via alterations in the dorsal horn of the spinal column affecting wide dynamic range neurons, which receive input from both nociceptive and nonnociceptive neurons (this general process termed “central sensitization”, see Bennett, 1999 for a review). Our findings of greater connectivity of the insula and temporal pole in older adults who report chronic musculoskeletal pain implicate a more significant role for these structures in the baseline state of older individuals with chronic pain. According to the notion of central sensitization, it is plausible that the experience of chronic pain affects the functional connectivity of these brain regions such that they become more active during the brain’s baseline state. The insula has been implicated as a region sensitive to the perception of negative emotional or physiological signals in event-related fMRI studies (e.g., Paulus et al., 2003), and most posterior regions of the insula have been described as “signaling the probability of aversive outcomes” (Clark et al., 2008). This understanding of insula function certainly supports its greater role in older persons who experience chronic musculoskeletal pain, perhaps as a result of emotional learning (Aprakrian, 2008). Activity in the temporal pole has been recently associated with neuroticism, or the tendency toward negative emotion (Jimura et al., 2010). It is possible that chronic pain may affect intrinsic connectivity networks in such a way as to predispose an older person to the greater probability of adverse pain outcomes and consequently a more neurotic or nociceptive-sensitive state even at rest. Since the insular cortex has been conceptualized as a “communication channel between the sensory-discriminative function of the somatosensory cortex and limbic cortical structures mediating the affective component of pain” (pg. 418, Matre and Tran, 2009), the prominence of the insula in our intrinsic baseline connectivity results generally suggests a greater network connectivity of pain-related brain structures in the context of chronic pain in old age.
Previous resting-state fMRI approaches have identified regions such as the insula, anterior cingulate, thalamus, basal ganglia, primary and secondary somatosensory, parietal, prefrontral cortex, and brainstem as involved in pain-associated network changes in young and middle-aged participants (Braz, et al., 2005; Apkarian et al., 2005; Baliki et al., 2008; Tagliazucchi et al., 2010; Buvanendran et al., 2007; Moulton et al., 2011; Rainville et al., 1997). In one of the first resting-state fMRI studies of pain (Baliki et al., 2008), evidence was presented for “disturbed network dynamics” in 15 pain participants (mean age=43.8, SD=4.11) and 15 non-pain participants (mean age=39.6, SD=3.43). In another study (Tagliazucchi et al., 2010) eight intrinsic connectivity networks were studied in 12 adults with chronic back pain (mean age=51.2) and 20 non-pain participants (mean age=38.4). An increase in the correlation between seed regions of interest and the insula and middle and superior temporal regions were found in this study among participants with chronic back pain. We also observed this increase in the insula and superior temporal pole in our between group analyses, and we observed direct associations between middle and superior temporal regions with reported number of painful joints in our post-hoc analyses. Our results may therefore be viewed as consistent with this earlier work and suggest that there may be a distinction between regions that may become more or less functionally connected based on whether (1) there is the experience of any pain, and (2) the magnitude of pain as assumed by number of painful joints. However, more prospective studies are needed to examine this.
Limitations of the present study include the use of a self-report measure of musculoskeletal pain. However, our results are consistent with previous neuroimaging results and self-reported measures commonly used in studies of pain. As there is evidence treatment of pain can affect brain function (Seifert & Maihöfner, 2011), we investigated whether use of pain medications differed between groups. The use of a pain medication (anti-inflammatory, opioid, acetaminophen, or aspirin) did not differ between the groups, but participants with pain were taking a higher number of pain medications, though this did not account for imaging differences between the groups. We did not have extensive information regarding pain medication dosage; therefore, further studies are needed to determine if there is a pain medication dose-response effect upon functional connectivity. We also did not have extensive information regarding the duration of pain in our participants, which may at least partially explain our observed differences. Certainly the use of other seed ROIs to interrogate other intrinsic connectivity networks would be of interest to explore in relation to pain in old age; however, we viewed the posterior cingulate and the network of regions associated with it as an essential network for aging-related conditions and pain perception based on previous literature. Therefore we chose to focus on this seed region of interest as our primary method of interrogating networks of interest. Another limitation was the restricted age range of our participants. Further studies which employ the same methodology are needed to determine whether there are subtle changes in functional brain connectivity across the lifespan. The consistency of our findings with previously published reports suggests that age may play less of a role in intrinsic network changes secondary to chronic pain; however, future studies should examine this issue more rigorously.
Strengths of the present study include the selection of participants from a community-based cohort of very old participants, and the size of the sample (64 in each group for a total sample size of 128). Our results support the notion of a network of regions continually ready to process the physiological and emotional modulations of pain, even at rest, among older adults who experience chronic musculoskeletal pain. These differences might serve as a potential biomarker for chronic pain in older adults and may contribute to the development of earlier and more effective treatments of pain in old age. To our knowledge, this is one of the first resting-state fMRI studies of musculoskeletal pain in old age.
Figure 3.
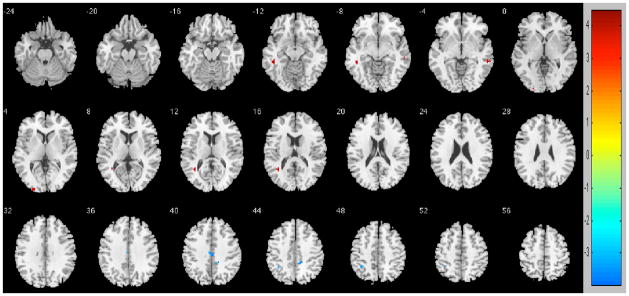
Regions that showed functional connectivity associations with number of reported painful joints. Direct associations are in reds and inverse associations are in blues. To control for multiple comparisons and spurious findings, we utilized the following: Cluster size > 5 voxels. p-value < 0.001.
Table 4.
Results of between-group analyses of cortical volume in regions of functional connectivity difference and the posterior cingulate cortex (seed region of interest). Results are corrected for intracranial volume (ICV) and presented as tenths of a percent (0.1% of ICV). There were no significance differences between groups.
| Volume | Pain | No Pain | t | p value |
|---|---|---|---|---|
| Total Posterior Cingulate | 3.40 (0.53) | 3.31 (0.48) | −0.92 | 0.35 |
| L insula | 3.89 (0.61) | 3.96 (0.51) | 0.28 | 0.78 |
| L middle frontal (rostral) | 7.72 (1.16) | 7.88 (0.98) | 0.76 | 0.44 |
| L middle frontal (caudal) | 3.13 (0.72) | 3.18 (0.61) | 0.39 | 0.69 |
| L superior temporal | 11.96 (1.79) | 11.86 (1.45) | 1.14 | 0.29 |
| L cerebellum | 58.62 (7.09) | 57.93 (4.93) | −0.60 | 0.54 |
Table 5.
Results of voxel-wise, within-group analysis of z-transformed functional connectivity values and number of reported painful joints while accounting for the effects of age, education, gender, and total gray matter volume. To control for multiple comparisons and spurious findings, we utilized the following: Cluster size > 5 voxels. p-value < 0.001.
| Association with Number of Joints | Region | Cluster size (#voxels) | Maximum Intensity Voxel coordinates | t-value | ||
|---|---|---|---|---|---|---|
| Direct | L middle temporal | 11 | −45 | −39 | −9 | 4.7347 |
| Direct | R middle temporal | 16 | 60 | −36 | −6 | 4.0782 |
| Direct | L middle occipital | 5 | −24 | −96 | 3 | 4.0882 |
| Direct | L superior temporal | 11 | −36 | −54 | 15 | 4.2537 |
| Inverse | Bilateral middle cingulate | 10 | 0 | −21 | 39 | −3.9534 |
| Inverse | R precuneus | 13 | 15 | −39 | 42 | −4.4254 |
| Inverse | L inferior parietal | 7 | −36 | −48 | 48 | −3.8474 |
Key points.
Chronic musculoskeletal pain is a significant problem for older adults.
Resting-state fMRI is a non-invasive method for neuroimaging of functional brain networks that may change in the context of chronic pain.
Older adults who endorse chronic pain have greater functional connectivity between the posterior cingulate and the left insula, left superior temporal gyrus, and left cerebellum.
Acknowledgments
Supported by National Institute on Aging Grant R01AG17917 and K23AG40625, the Illinois Department of Public Health, the American Federation for Aging Research, and The Marsha K. Dowd Philanthropic Fund. We gratefully acknowledge the assistance of Dr. Randy Buckner, Dr. Gary Glover, and Dr. Jeffrey Rosengarten with this project. We thank Niranjini Rajendran, M.S., and Woojeong Bang, M.S., for image post-processing and statistical analyses. We also thank the Rush Memory and Aging Project staff and participants.
Footnotes
Disclosures: There were no actual or potential conflicts of interest for any of the authors.
References
- 1.Alzheimer’s Association. 2009 Alzheimer’s Disease facts and figures. Alzheimer’s and Dementia. 2009;5(3):234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Andersson HI, Ejiertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9:174–182. doi: 10.1097/00002508-199309000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobio. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. Journal of Neuroscience. 2004;24:10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the Default-Mode Network dynamics. Journal of Neuroscience. 2008;28:1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: Nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD, Wilson RS. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- 9.Bennett DA, Schneider JA, Buchman AS, Mendes de Leon C, Bienias JL, Wilson RS. The Rush Memory and Aging Project: Study design and baseline characteristics of the study cohort. Neuroepidemiology. 2005;25(4):163–175. doi: 10.1159/000087446. [DOI] [PubMed] [Google Scholar]
- 10.Bennett DA, Wilson RS, Schneider JA, Evans DA, Beckett LA, Aggarwal NT, Barnes LL, Fox JH, Bach J. Natural history of mild cognitive impairment in older persons. Neurology. 2002;59:198–205. doi: 10.1212/wnl.59.2.198. [DOI] [PubMed] [Google Scholar]
- 11.Bennett RM. Emerging concepts in the neurobiology of chronic pain: Evidence of abnormal sensory processing in fibromyalgia. Mayo Clin Proc. 1999;74:385–398. doi: 10.4065/74.4.385. [DOI] [PubMed] [Google Scholar]
- 12.Bettus G, Guedj E, Joyeux F, Confort-Gouny S, Soulier E, Laguitton V, Cozzone PJ, Chauvel P, Ranjeva JP, Bartolomei F, Guye M. Decreased basal fMRI functional connectivity in epileptogenic networks and contralateral compensatory mechanisms. Human Brain Mapp. 2009;30:1580–1591. doi: 10.1002/hbm.20625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RW, Theberge J, Schaefer B, Williamson P. Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. doi: 10.1093/schbul/sbm052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology. 2005;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- 15.Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 16.Brooks JC, Zambreanu L, Godinez A, Craig AD, Tracey I. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201–209. doi: 10.1016/j.neuroimage.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 17.Buchman AS, Shah RC, Leurgans SE, Boyle PA, Wilson RS, Bennett DA. Musculoskeletal pain and incident disability in community-dwelling older adults. Arthritis Care & Research. 2010;62:1287–1293. doi: 10.1002/acr.20200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buckner RL, Andrews-Hanna JR, Schacter DL. The Brain’s Default Network: Anatomy, function, and relevance to disease. Ann NY Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 19.Buckner RL, Vincent JL. Unrest at rest: Default activity and spontaneous network correlations. NeuroImage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. The Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: Mapping, assessment of stability, and relation to Alzheimer’s disease. The Journal of Neuroscience. 2009;29(6):1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckner RL, Synder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: Evidence for a relationship between default activity, amyloid, and memory. The Journal of Neuroscience. 2005;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buvanendran A, Ali A, Stoub TR, Berger RA, Kroin JS. The use of brain positron emission tomography to identify sites of postoperative pain processing with and without epidural analgesia. Anesth Analg. 2007;105:1784–1786. doi: 10.1213/01.ane.0000270206.30333.cb. [DOI] [PubMed] [Google Scholar]
- 24.Clark L, Bechara A, Damasio H, Aitken MRF, Sahakian BJ, Robbins TW. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131:1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell MJ. Age-related changes in the structure and function of brain regions involved in pain processing. Pain Medicine. 2012;13:S37–S43. doi: 10.1111/j.1526-4637.2011.01287.x. [DOI] [PubMed] [Google Scholar]
- 26.Florr H. Cortical reorganization and chronic pain: Implications for rehabilitation. J Rehabil Med, Suppl. 2003;41:66–72. doi: 10.1080/16501960310010179. [DOI] [PubMed] [Google Scholar]
- 27.Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapping. 1994;2:56–78. [Google Scholar]
- 28.Friston KJ, Passingham RE, Hutt JG, Heather JD, Sawle GV, Frackowiak RSJ. Spatial registration and normalization of images. Human Brain Mapping. 1995;3(3):165–189. [Google Scholar]
- 29.Glover GH, Thomason ME. Improved combination of spiral-in/out images for BOLD fMRI. Magnetic Resonance Medicine. 2004;51:863–868. doi: 10.1002/mrm.20016. [DOI] [PubMed] [Google Scholar]
- 30.Greicius MD, Kiviniemi V, Tervonen O, Vainionpaa V, Alahuhta S, Reiss, Menon V. Pesistent default mode network connectivity during light sedation. Human Brain Mapping. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: Evidence from functional MRI. Proceedings of the National Academy of Sciences. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty. Arthritis & Rheumatism. 2010;62:2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 34.Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RA. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. The Journal of Neuroscience. 2009;29(40):12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodge CJ, Jr, Apkarian AV. The spinothalamic tract. Crit Rev Neurobiol. 1990;5:363–397. [PubMed] [Google Scholar]
- 36.Jimura K, Konishi S, Asari T, Miyashita Y. Temporal pole activity during understanding other persons’ mental states correlates with neuroticism trait. Brain Research. 2010;1328:104–112. doi: 10.1016/j.brainres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 37.Long XY, Zuo XN, Kiviniemi V, Yang Y, Zou QH, Zhu CZ, Jiang TZ, Yang H, Gong QY, Wang L, Li KC, Xie S, Zang YF. Default mode network as revealed with multiple methods for resting-state functional MRI analysis. J Neurosci Methods. 2008;30:349–355. doi: 10.1016/j.jneumeth.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 38.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease. Neurology. 1984;34:939. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 39.Mailis-Gagnon A, Nicholson K, Yegneswaran B, Zurowski M. Pain characteristics of adults 65 years of age and older referred to a tertiary care pain clinic. Pain Res Manag. 2008;13:389–394. doi: 10.1155/2008/541963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matre D, Tran TD. Imaging modalities for pain. In: Moore RJ, editor. Biobehavioral Approaches to Pain. New York: Springer Science+Business Media, LLC; 2009. pp. 409–446. [Google Scholar]
- 41.May A. Chronic pain may change the structure of the brain. Pain. 2008;137:7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 42.Melzack R, Casey KL. Placebo-induced changes in spinal cord pain processing. Journal of Neuroscience. 2006;26:559–563. doi: 10.1523/JNEUROSCI.4218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mottram S, Peat G, Thomas E, Wilkie R, Croft P. Patterns of pain and mbility limitation in older people: cross-sectional findings from a population survey of 18,497 adults aged 50 years and over. Qual Life Research. 2008;17:529–539. doi: 10.1007/s11136-008-9324-7. [DOI] [PubMed] [Google Scholar]
- 44.Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: Responses to noxious heat and unpleasant images. Journal of Neuroscience. 2011;31:3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ostrowski K, Magnin M, Ryvlin P, Isnard J, Guenot M, Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cerebral Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- 46.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 47.Raiche ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 49.Segonne F, et al. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22(3):1060–75. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 50.Shah RC, Buchman AS, Boyle PA, Leurgans SE, Wilson RS, Andersson GB, Bennett DA. Musculoskeletal pain is associated with incident mobility disability in community-dwelling elders. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences. 2011;66(1):82–88. doi: 10.1093/gerona/glq187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seifert F, Maihöfner C. Functional and structural imaging of pain-induced neuroplasticity. Current Opinion in Anesthesiology. 2011;24:515–523. doi: 10.1097/ACO.0b013e32834a1079. [DOI] [PubMed] [Google Scholar]
- 52.Tagliazucchi E, Balenzuela P, Fraiman D, Chialvo DR. Brain resting state is disrupted in chronic back pain patients. Neuroscience Letters. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson RS, Barnes LL, Bennett DA. Assessment of lifetime participation in cognitively stimulating activities. Journal of Clinical and Experimental Neuropsycholology. 2003;25:634–642. doi: 10.1076/jcen.25.5.634.14572. [DOI] [PubMed] [Google Scholar]
- 55.Wolfe F, Ross K, Anderson J, Russell IJ, Herbert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y, Liang M, Tian L, Wang K, Hao Y, Liu H, Liu Z, Jiang T. Functional disintegration in paranoid schizophrenia using resting-state fMRI. Schizophr Res. 2007;97:194–205. doi: 10.1016/j.schres.2007.05.029. [DOI] [PubMed] [Google Scholar]