Abstract
Bioenergetic dysfunction is emerging as a cornerstone for establishing a framework for understanding the pathophysiology of cardiovascular disease, diabetes, cancer and neurodegeneration. Recent advances in cellular bioenergetics have shown that many cells maintain a substantial bioenergetic reserve capacity, which is a prospective index of “healthy” mitochondrial populations. The bioenergetics of the cell are likely regulated by energy requirements and substrate availability. Additionally, the overall quality of the mitochondrial population and the relative abundance of mitochondria in cells and tissues also impinge on overall bioenergetic capacity and resistance to stress. Because mitochondria are susceptible to damage mediated by reactive oxygen/nitrogen and lipid species, maintaining a “healthy” population of mitochondria through quality control mechanisms appears to be essential for cell survival under conditions of pathological stress. Accumulating evidence suggest that mitophagy is particularly important for preventing amplification of initial oxidative insults, which otherwise would further impair the respiratory chain or promote mutations in mitochondrial DNA (mtDNA). The processes underlying the regulation of mitophagy depend on several factors including the integrity of mtDNA, electron transport chain activity, and the interaction and regulation of the autophagic machinery. The integration and interpretation of cellular bioenergetics in the context of mitochondrial quality control and genetics is the theme of this review.
Keywords: spare respiratory capacity, xanthine oxidase, mitophagy, oxidative stress, haplotypes, aging, neurodegenerative disease, hepatotoxicity, cardiovascular disease
Introduction
It has long been known that mitochondria show diminished functional activity when isolated from a broad range of tissues subject to pathological stress. Well-established examples are alcohol-dependent hepatotoxicity, cardiac ischemia-reperfusion and neurodegenerative diseases (Bailey and Cunningham, 2002; Brookes et al., 2004; Carreira et al., 2011; Coskun et al., 2011; Jenner, 2003; Krzywanski et al., 2011; Lemasters et al., 2009; Pilsl and Winklhofer, 2012). In all of these cases a role for reactive oxygen or nitrogen species (ROS/RNS) has been proposed in causing mitochondrial dysfunction. Typically, mitochondrial damage is defined as a relative decrease in respiratory parameters such as a change in oxygen consumption in the presence of substrates and ADP (state 3 respiration), in the presence of substrates alone (state 4), or the ratio of these parameters (i.e., respiratory control ratio; RCR). Other parameters include decreased activity of specific enzymes, deletions in mtDNA and increases in oxidative markers such as protein oxidation. The challenge with these data is in integrating them into an overall model of cellular and tissue bioenergetic function. It is becoming apparent that mitochondria under pathological stress exhibit multiple defects which overall can be viewed as a decrease in mitochondrial quality (Karbowski and Neutzner, 2012; Piantadosi and Suliman, 2012; Pilsl and Winklhofer, 2012). Importantly, it has recently been shown that the cell possesses a complex machinery capable of surveying mitochondrial quality and scheduling sections of defective organelles for demolition through a specialized category of autophagy called mitophagy (Kim et al., 2007; Lee et al., 2012; Lemasters, 2005; Youle and Narendra, 2011). This method of mitochondrial surveillance is useful because it allows changes in mitochondrial parameters such as membrane potential, activity of oxidative phosphorylation, and mitochondrial damage to be placed in the dynamic context of cell function.
The controlled regulation of mitochondrial abundance through biogenesis or degradation is also critical for maintaining proper cell function. The formation of “new” mitochondria is a tightly controlled process involving several factors, including de novo synthesis of organelle components from cellular precursors, formation of mitochondrial membranes from pre-formed membranous structures, and division of pre-existing mitochondria (Michel et al., 2012). For maintenance and modulation of energy production, the cell then must coordinate the biogenic process: the transcription, import, and assembly of nuclear-encoded genes must match with the replication and transcription of the mitochondrial genome and with biogenesis of mitochondrial membranes. External stimuli have been shown to initiate this dynamic process. For example, caloric restriction, exercise, hypothermic conditions, and energy deprivation result in the activation of signaling pathways responsible for increasing mitochondrial abundance (Bordicchia et al., 2012; Cao et al., 2001; Nisoli et al., 2003; Wu et al., 2002). The PGC1 family, especially PGC1α, along with other multiple factors coordinate the biogenesis of mitochondria (Michel et al., 2012). Interesting examples of the pathological effects of a mismatch between energy demand and mitochondrial number was shown by studies where expression of PGC1α was manipulated in the heart. PGC-1α −/− mice develop signatures of heart failure, and constitutive, cardiac-specific overexpression of PGC-1α results in excessive mitochondrial biogenesis, leading ultimately to death from heart failure (Finck and Kelly, 2007). It appears then that mitochondrial abundance is finely tuned to the energetic needs of the cell—too few or too many mitochondria can result in similar pathological outcomes.
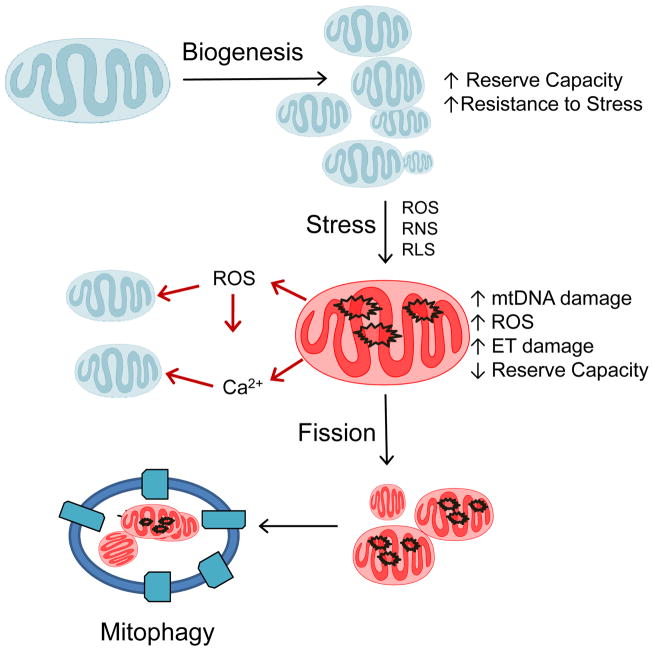
The obligation to match mitochondrial abundance and quality with energy demand is outlined in Figure 1. Once a stable population of mitochondria is established in a cell it must perform a series of integrated biological functions. These include the conversion of caloric energy into metabolic intermediates for cell differentiation, molecular energy (ATP), thermal energy (heat), and oxidants. The generation of ROS from mitochondria appears to serve a cell signaling function when controlled at low levels but can contribute to pathology if it leads to damage to electron transport proteins and “electron leak” to oxygen at a non-physiological locations (Gutierrez et al., 2006; Murphy, 2009). Under normal conditions mitochondrial populations in cells appear highly stable. For example, it has been shown that mitochondria have a half life of ~9 days in the liver, ~10 days in the kidney, ~16 days in the heart, and ~26 days in the brain (Menzies and Gold, 1971). Quality control of individual mitochondrial proteins, is mediated by intra-mitochondrial proteases (Bota and Davies, 2001; Ugarte et al., 2010). The ATP-stimulated mitochondrial Lon protease plays an important role in the degradation of oxidized proteins (Bayot et al., 2008). This process is important in the maintenance of proteins that are highly susceptible to damage and therefore need to be turned over more frequently to sustain normal mitochondrial function. For example, oxidatively damaged aconitase has been shown to be selectively degraded by Lon protease (Bota and Davies, 2002). In response to hypoxia, Lon protease degrades cytochrome c oxidase subunit IV isoform 1 to allow isoform 2 to replace its position (Fukuda et al., 2007). The mitochondrial AAA-proteases have been shown to be important for preventing mtDNA escape from mitochondria to the nucleus possibly through ensuring appropriate fission and fusion, as well as rapid proteolysis of non-assembled inner membrane proteins (Hori et al., 2002; Thorsness and Fox, 1990; Thorsness et al., 1993). In response to more extensive mitochondrial damage, increased mitochondrial fragmentation and decreased fusion can separate damaged mitochondrial proteins, lipids and DNA from functional components, and the entire damaged organelle is processed by mitophagy (Kim et al., 2007; Lee et al., 2012). It has recently been shown that the sub-populations of mitochondria destined for turnover are segregated through a dynamic process involving fission of areas of low functioning mitochondria and fusion of healthy populations (Kim and Lemasters, 2011; Twig et al., 2008b). This turnover of mitochondria by lysosomes is executed in a process now called autophagy of mitochondria, or mitophagy (Green et al., 2011; Kim et al., 2007; Lemasters, 2005; Youle and Narendra, 2011).
Figure 1. Regulation of mitochondrial quality control and the response to oxidative stress.
An existing mitochondrial population is shown subjected to a pathological stress with the formation of reactive oxygen, nitrogen and lipid species (ROS/RNS/RLS). This oxidative stress damages mtDNA impairing the ability of the organelle to replace damaged electron transport proteins and decreasing bioenergetic reserve capacity; the resulting increased mitochondrial ROS then oxidatively damages previously unmodified mitochondria. The damaged mitochondria are turned over by a mitophagic mechanism that then suppresses this vicious cycle. The mitochondrial population is now renewed through mitochondrial biogenesis. The bioenergetic reserve capacity is essential for resistance to oxidative stress and supplying ATP demand. Once the bioenergetic reserve is depleted, bioenergetic failure occurs and the cell is programmed for cell death.
Because of their importance in providing cellular energy, modulating ROS and controlling apoptosis, mitochondrial quality control is critical for normal cell function. Mitochondrial morphology and function are perturbed in genetically engineered autophagy-deficient animals, further supporting the hypothesis that autophagy of mitochondria (mitophagy) is essential for mitochondrial quality control (Kim et al., 2007). It therefore follows that damaged mitochondria arise during normal metabolism and are potentially hazardous because of their ability to amplify ROS-dependent damage (Figure 1). Under pathological stressors several interrelated mechanisms can contribute to decreased mitochondrial quality. These include mitochondrial calcium overload, increased levels of ROS/RNS, endoplasmic reticulum stress and accumulation of aggregated proteins. Where extra-mitochondrial sources of ROS/RNS are present, the quality of the mitochondrial population declines as evidenced by increased mtDNA damage, decreased membrane potential, and a diminished bioenergetic reserve capacity (Dranka et al., 2010; Higdon et al., 2012; Hill et al., 2009; Zelickson et al., 2011). Overwhelming the mitophagy pathway exacerbates the problem since the low quality mitochondria now impact on the remaining healthy mitochondrial population through release of ROS and calcium from the organelle. Thus, it is clear that the dynamics, turnover and biogenesis collectively maintain quality control. These processes are regulated by intracellular and extracellular signals that coordinate the biogenesis transcriptional program, cellular bioenergetics, redox signaling and mitophagy. In this review we will discuss emerging concepts in assessing cellular bioenergetic function, and how mitochondrial quality is maintained in a cellular setting. In particular, we address the following questions:
How can bioenergetics be measured and placed in context with disease?
What is the role of the mitochondrial genetic landscape in diseases based in bioenergetic dysfunction?; and
How does mitophagy contribute to mitochondrial quality control?
The cellular bioenergetic profile and its interpretation
Typically experiments with isolated mitochondria have been performed in the context of established protocols performed with oxygen electrodes. The outputs from such experiments are the rates of state 3 and 4 respiration and the ratio of these two rates—the RCR (Chance and Baltscheffsky, 1958a; Chance and Baltscheffsky, 1958b). Mitochondrial dysfunction has often been defined as a deviation of these parameters in a treatment or disease group relative to control. Classic experimentation with isolated mitochondria cannot effectively address this issue since cellular regulation of mitochondrial function is removed during isolation. To bridge this gap, methods have been developed to determine the behavior of mitochondria in a cellular setting using extracellular flux analysis (Brand and Nicholls, 2011; Dranka et al., 2010; Ferrick et al., 2008; Gerencser et al., 2009; Nicholls et al., 2010; Wu et al., 2007). Recent advances in this technology have allowed for real-time measurements of O2 consumption and acidification in cell culture with the capacity to inject respiratory inhibitors. This has quickly allowed for the establishment of methodical assays to interrogate bioenergetic function in cells under a broad range of conditions (Dranka et al., 2011; Ferrick et al.; Wu et al.). How the rates of O2 consumption (OCR) and extracellular acidification (ECAR), which are global properties of the cell preparations, can be ascribed to specific biological processes will be discussed next (Hill et al., 2009; Sridharan et al.).
Fundamentals of the bioenergetic profile assay
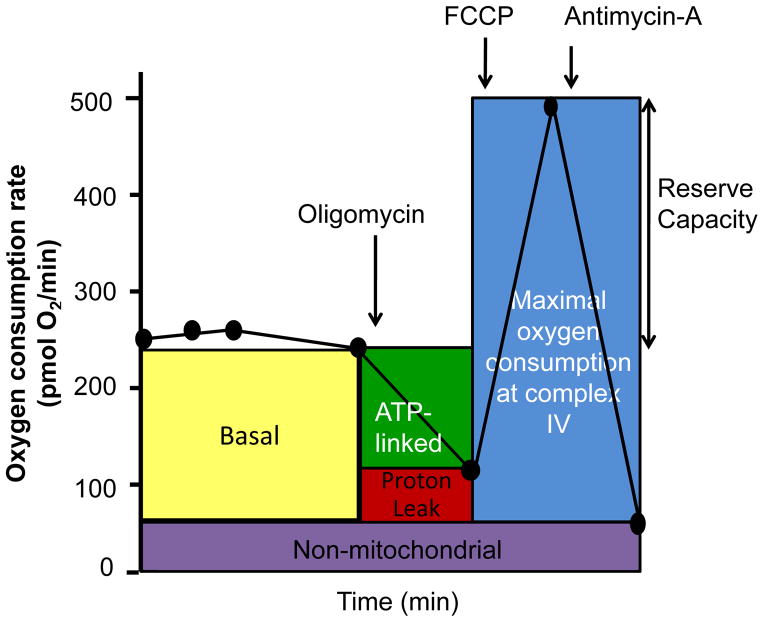
Information on how mitochondria function in intact cells has largely been derived from assays similar to that shown in Figure 2. Here, intracellular mitochondrial function can be determined by sequentially adding pharmacological inhibitors of oxidative phosphorylation (Brand and Nicholls, 2011; Dranka et al., 2011). In the first series of measurements, the basal oxygen consumption of the cells is established. The basal OCR represents the net sum of all processes in the cell capable of consuming O2, including mitochondria and other oxidases. Next, oligomycin, an inhibitor of mitochondrial ATP synthase, is injected. In support of the assumption that the basal respiration represents the demand on the proton motive force (for making either ATP or moving other ions across the inner membrane), the pharmacological inhibition of energy-requiring processes results in a decrease in basal respiration. The decrease in OCR in response to oligomycin is then related to the proportion of mitochondrial activity used to generate ATP.
Figure 2. The cellular bioenergetic profile.
This mitochondrial stress test can be used to measure several indices of mitochondrial function in intact cells in real time and allows for the identification of critical respiratory defects. A typical experiment is shown in which basal oxygen consumption rate (OCR) is allowed to stabilize before the sequential addition of oligomycin, FCCP, and antimycin A and/or rotenone with a measurement of changes in OCR as indicated. This time course is annotated to show the relative contribution of non-respiratory chain oxygen consumption, ATP-linked oxygen consumption, the maximal OCR after the addition of FCCP, and the reserve capacity of the cells.
In contrast to experiments with isolated mitochondria, respiration is sustained in these conditions by respiratory substrates provided by cellular metabolism. To estimate the maximal potential respiration sustainable by the cells, a proton ionophore (uncoupler) such as FCCP is used. Immediately upon exposure to the uncoupler, oxygen consumption increases as the mitochondrial inner membrane becomes permeable to protons, and electron transfer is no longer controlled by the proton gradient. An important feature emerging from such assays is the mitochondrial reserve capacity, which is calculated by subtracting the FCCP-stimulated rate from the basal OCR.
To determine the extent of non-mitochondrial oxygen-consuming processes, the respiratory chain is inhibited with antimycin A alone or in combination with rotenone. Such pharmacological inhibition of electron transport typically inhibits the majority of oxygen consumption in the cell, and we attribute the remaining O2 consumption to non-mitochondrial oxidases present in the cell. This value is subtracted from other OCR measurements to determine the mitochondrial-based parameters. It should be noted that for meaningful comparisons between different conditions or treatments, the basal OCR should be normalized to a factor to correct for different numbers of cells in the assay or changes in cell size, e.g., cell atrophy or hypertrophy. Insights into the specific activity of the mitochondria can be obtained by normalizing to a mitochondrial protein measured in the cell lysates after the assay. Taken together, these data have interesting implications for understanding mitochondrial function in a cellular context.
Interpretation of the bioenergetic profile
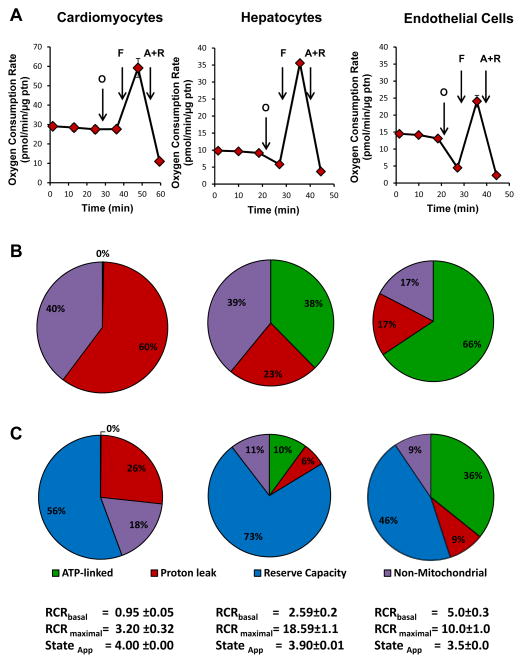
In Figure 3 we have selected examples with diverse energy requirements in vivo to illustrate how the bioenergetic profile differs between cell type. Aortic endothelial cells are typically regarded as having (low energetic demand), primary hepatocytes (moderately energetic), and adult cardiomyocytes (high energy demand). Differences in ATP-linked oxygen consumption and proton leak are clearly evident between the cell types, with endothelial cells having a much higher proportion of the basal OCR ascribed to ATP turnover. Both hepatocytes and cardiomyocytes show little change when treated with oligomycin. The FCCP-stimulated rate is highest in the more energetic hepatocytes and cardiomyocytes, which leads to a high reserve capacity in both cell. At first glance the profiles look remarkably similar but on closer examination differences are apparent. For example, the lack of sensitivity of cardiomyocytes to oligomycin is likely due to the fact that under these conditions the cells are not contracting. This means that the activity of ATP synthase required to supply the ATP demand is likely very low and therefore the effect of oligomycin is minimal. The pie chart in panel B represents Basal OCR distributed between ATP-linked OCR, proton leak and non-mitochondrial functions. It is interesting to note that proton leak (or ion movements requiring proton motive force) and non-mitochondrial consumption of oxygen is quite different between the different cell types. Data in Panel C are expressed as a % of the maximal respiration, and these data reinforce the conclusion that the contribution of mitochondria to oxygen consumption in cells is both diverse and distinct. This finding assumes an additional importance when we consider the potential role and extent of ROS generation in mitochondria both in relation to mitochondrial genetics and maintenance of organelle quality as will be discussed later.
Figure 3. Cellular bioenergetics in different cell types.
Extracellular flux analysis using the mitochondrial stress test assay: Panel A: Adult rat cardiomyocytes, primary hepatocytes or bovine aortic endothelial cells were subject to a metabolic stress test as shown, and the OCR rates were normalized to protein. Panel B: in this analysis basal OCR was established as 100% OCR and the proportion of ATP-linked OCR, proton leak and non-mitochondrial oxygen consumption is shown. Panel C: in this analysis maximal OCR is established as 100% and the oxygen consumption is apportioned between ATP-linked OCR, proton leak, reserve capacity and non-mitochondrial O2 consumption. The RCR for basal and maximal OCR is reported together with the stateapparent for each cell type.
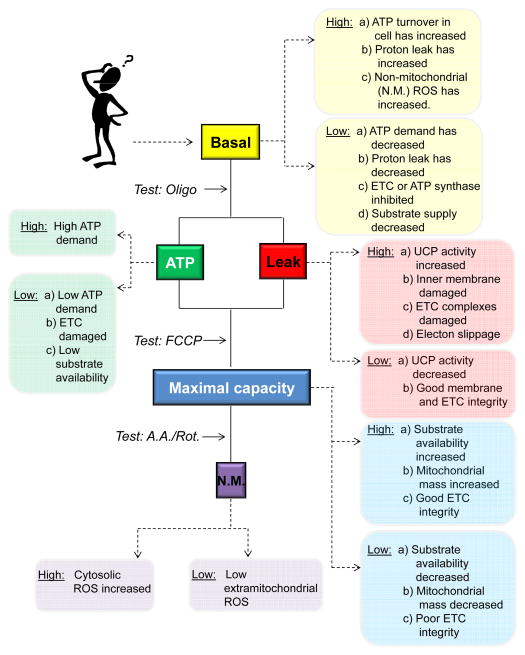
To assist the investigator Figure 4 shows a typical basis for interpretation of the most common changes in bioenergetic profile observed in routine experiments. Below we describe in detail these interpretations of cellular bioenergetic profiles and some of the principles that underlie changes in the key parameters of basal respiration, ATP-linked oxygen consumption, proton leak and reserve capacity (sometimes also called spare respiratory capacity).
Figure 4. Possible interpretation tree for the mitochondrial bioenergetic profile.
In cells, differences in basal oxygen consumption rate (OCR) could be due to several factors including ATP turnover reactions, proton leak, non-mitochondrial (N.M.) oxygen consumption, or damage to the electron transport chain. This can be tested by adding oligomycin, and some potential outcomes are shown. Next, the changes in the maximal capacity (and the reserve capacity) can be identified using the FCCP-stimulated rate. An increase or a decrease in this rate compared with controls could be due to changes in substrate availability, mitochondrial mass, or electron transport chain (ETC) integrity. Lastly, the effects of non-mitochondrial sources on oxygen consumption may be examined.
Basal oxygen consumption rate
Taken in isolation, a change in the value of basal OCR may be due to either a physiological adaptation or response to stress. An increase in basal OCR could be due to an increase in ATP turnover, an increase in proton leak, and/or an increase in non-mitochondrial oxygen consumption (e.g., ROS formation). Oligomycin treatment gives insight into the former two possibilities (Figure 4).
ATP-linked OCR and proton leak
The addition of oligomycin is assumed to eliminate completely control of respiration by oxidative phosphorylation, and the remaining rate of mitochondrial respiration is then taken to represent the cumulative proton leak that was present prior to addition of oligomycin. An increase in the ATP-linked rate of O2 consumption would indicate an increase in ATP demand, and a decrease would indicate either low ATP demand, a lack of substrate availability, or severe damage to the ETC, which would impede the flow of electrons and result in a lower OCR (Figure 4); of note, this would also decrease the maximal capacity as well (see below).
An increase in apparent proton leak could be due to a number of factors: uncoupling protein (UCP) activity could be increased (Divakaruni and Brand; Echtay et al.); the inner mitochondrial membrane and/or ETC complexes could be damaged, allowing H+ to leak into the matrix; or there could be electron slippage, which would result in oxygen consumption in the absence of proton translocation (Brown, 1992). Treatment of cardiomyocytes with 4-hydroxynonenal (HNE), for example, results in an increase in both ATP-linked oxygen consumption and proton leak (Hill et al.; Sansbury et al., 2011a; Sansbury et al., 2011b).
In such a result, it is important to consider that the pharmacological interrogation of the metabolic profile can bias data output and interpretation. For example, oligomycin increases the mitochondrial membrane potential (Δψm) and induces a State 4-like respiratory condition (Brown et al.), which will also increase proton leak through the mitochondrial membrane (Brand; Brown). The value for ATP-linked respiration is then modestly under-estimated and proton leak over-estimated. It is important to consider also that the rate of apparent proton leak could represent the passage of any ion, e.g., calcium, across the inner membrane which can dissipate the proton gradient. Technologies that allow the simultaneous measurement of mitochondrial membrane potential in conjunction with cellular respiration would need to be developed to test this experimentally.
Maximal OCR
Experimentally, the use of FCCP to estimate the maximal respiratory rate has several limitations. In each cell type the FCCP concentration has to be titrated since at high concentrations it can be inhibitory (Dranka et al., 2011). A lower maximal capacity could indicate decreased substrate availability or that mitochondrial mass or integrity is compromised. A high FCCP-stimulated rate compared with the basal rate suggests that the cell has a substantial mitochondrial reserve capacity, which could be important for responding to acute insults.
Non-mitochondrial OCR
Very few measurements have been performed in cells to determine the contribution of non-mitochondrial sources of oxygen consumption. Using isolated mitochondria and respiratory chain inhibitors it was calculated in the early 1970’s that mitochondrial superoxide production was 1–2% of oxygen consumption (Boveris and Chance, 1973; Turrens, 2003). Using cell fractionation and the measurement of hydrogen peroxide generation in hepatocytes, it was calculated that hydrogen peroxide production was 10% of the total oxygen consumption by the cell (Boveris et al., 1972). In Figure 3B we show the distribution of oxygen consumption in the cells calculated assuming basal respiration is 100%; in Figure 3C this is calculated assuming maximal respiration is 100%. In the hepatocytes and cardiomyocytes, non-mitochondrial oxygen consumption constitutes approximately 40% of basal OCR, and in endothelial cells approximately 15%. A more detailed investigation of the potential sources of these oxygen consuming pathways needs to be performed since not all of these pathways will generate hydrogen peroxide. However, it is intriguing that this value is different between cell types. The widely held view that mitochondria consume over 95% of the oxygen in the body should be reevaluated.
Cellular respiratory stateapparent
It is becoming clear that in most cells mitochondria function at an intermediate respiratory state between State 3 and State 4 and have RCR values far in excess of those found in isolated mitochondria (Brand and Nicholls, 2011; Dranka et al., 2011; Dranka et al., 2010; Sansbury et al., 2011a). The fact that cells have measurable respiratory state values that vary with insult or injury suggests that the redox status of individual components of the respiratory chain populate a dynamic range of intermediate metabolic conformations. When comparing the state 4 condition (oligomycin) to the state 3/uncoupled condition (FCCP-stimulated), cytochrome c oxidase appears to exhibit a potential range of activity of approximately 5–10 fold. This is interesting since experiments with the isolated enzyme have shown the existence of intermediate active forms of cytochrome c oxidase that depend on electron flux, and these forms of the enzyme are differentially reactive with respiratory modulators such as CO (carbon monoxide), NO (nitric oxide) and NO2− (nitrite) (Cooper and Giulivi, 2007; Sarti et al., 2003).
We have proposed that the cellular Stateapparent can be calculated as follows:
The Stateapparent therefore can be modulated by changes in the basal OCR, the coupling status, and/or the maximal respiratory rate.
It is important to note that the calculation of Stateapparent simplifies the underlying and highly interactive phenomena that act together to determine overall flux through the respiratory chain. Since the factors that determine respiration are multiparametric then it is clear that several different conditions could lead to similar values for these parameters. The underlying molecular mechanisms for changes in Stateapparent could involve changes in activation or levels of expression of the metabolic proteins controlling these processes which then must then be determined in independent experiments.
Cellular RCR
The respiratory control ratio was originally defined for isolated rat heart muscle sarcosomes and referred to the extent to which respiration is slowed after added ADP is phosphorylated completely (State 3 to State 4 transition) (Chance and Baltscheffsky, 1958b). This ratio, in its various forms, is generally regarded as the “gold standard” for measuring mitochondrial coupling, and the concept has been extended to cells. In the original paradigm, respiratory control can be viewed as a result of the buildup of a high-energy intermediate that links respiration to ATP synthesis, which occurs because all ADP is phosphorylated.
In reality, the phenomena that are responsible for respiratory control are much more complex. Metabolic control analysis (MCA) has undoubtedly been the most useful and quantitative framework for examining mitochondrial respiratory control in cells. The overall approach involves determining the relative extent to which one individual step in a pathway, such as oxidative phosphorylation, exerts control of overall flux through the entire pathway. The parameter that quantitates this control is the control coefficient and can assume a value between 0 and 1. Importantly, if the overall flux is at steady-state the sum of control coefficients for all steps of a pathway is equal to 1. Functionally, the closer the value of a control coefficient for an individual step is to 1 the more control this single step has over the flux through the entire pathway. Metabolic control analysis has been applied to both isolated mitochondria and also to mitochondrial respiration in intact cells and has revealed information which can be used for the quantitative and mechanistic interpretation of the reserve (Pan-Zhou et al., 2000) or spare respiratory (Choi et al., 2009) capacity in cells under various conditions (Brand and Nicholls, 2011).
In terms of interpreting reserve capacity, the assignment of each component of OCR to the biological activities depicted in Figure 2 makes specific assumptions regarding the control coefficients for the various components under the different experimental conditions. Specifically, the control coefficient for the proton leak component must dominate respiration after addition of oligomycin and the coefficient for electron transfer (implicating the integrity of respiratory complexes and/or substrate conditions) must dominate after addition of uncoupler. Previous application of MCA to studies with hepatocytes has demonstrated that under baseline conditions (similar to the basal OCR) control of oxidation is distributed among all three components (ΔψM-generating processes, ATP production, and proton leak) (Brown et al., 1990). Comparison with Figure 3 indeed supports the concept that under basal conditions control of respiration is distributed among these components but this differs between different cell types.
Biological regulation of the RCR is also complex, and it is now known that the RCR varies with both the respiratory substrate used and the activation of uncoupling processes in the inner mitochondrial membrane. These can be specifically regulated by channels known as uncoupling proteins, proton slip in respiratory chain complexes, or non-specific damage to the mitochondrial membrane such as would occur with lipid peroxidation.
The bioenergetic profiles shown in Figures 2 and 3 can be used to calculate a respiratory control ratio (RCR) for oxidative phosphorylation in a cellular setting. To do this, we assume that the OCR after the addition of oligomycin represents State 4 respiration and that after FCCP is equivalent to State 3. The cellular RCR without (Basal RCR) and with (maximal RCR) the addition of FCCP is then calculated as the State 3 rate divided by the State 4 rate. The non-cytochrome c oxidase OCR (i.e., in the presence of antimycin A; Anti A) is subtracted from all rates.
The RCR calculations for the three cell types are shown in Figure 3. The high RCR values calculated using these data are clearly much above that reported for isolated mitochondria. There could be several reasons for this difference.
The isolation process damages mitochondria and detaches them from the cellular matrix, which results in higher proton leak.
The mitochondria respond dynamically to substrate demand in a cellular setting through metabolic regulation which is lost in the isolated mitochondria.
Both factors likely contribute. In any event, it is clear that mitochondria in many cells are normally functioning at a sub-maximal capacity under basal conditions.
Bioenergetic reserve capacity
In addition to the RCR, calculation of the mitochondrial reserve capacity (FCCPrate – Basalrate) is a useful qualitative indicator of mitochondrial energetic status in cells. The factors controlling the mitochondrial reserve capacity are complex. Substrate supply, coupling, ATP demand and allosteric regulation of key metabolic enzymes, and the integrity or level of expression of respiratory chain components are critical features that regulate oxygen consumption.
Substrate supply appears to be extremely important in regulating the bioenergetic reserve in the cellular context. In contrast to experiments using isolated mitochondria, substrate delivery in intact cells is subject to more complex regulation. Allosterism of key metabolic enzymes and the cadence of metabolic cell signaling are two fundamental ways in which respiration is controlled. For example, pyruvate dehydrogenase (PDH), which is the rate limiting step in glucose oxidation, is regulated allosterically by NAD+/NADH, ATP and AMP, CoA/Acetyl CoA, and calcium as well as by both PDH phosphatase and kinase (Patel and Korotchkina). Furthermore, the TCA cycle is regulated allosterically at several points including isocitrate dehydrogenase, α-ketoglutarate dehydrogenase, and malate dehydrogenase (Williamson and Cooper). This is even more important since anaplerotic metabolism, for instance through metabolism of the amino acids glutamine and aspartate entering at the levels of α-ketoglutarate and oxaloacetate, respectively, would influence basal metabolism. Fatty acids can also enter the TCA cycle through anaplerotic metabolism to succinyl CoA (Brunengraber and Roe). These selected examples illustrate how basal metabolism could be altered by different substrates. The use of alternative pathways may also differentially modulate the control coefficients for mitochondrial respiration.
Substrate utilization is cell- and context-specific. Experimentally, this has been validated by simply providing different concentrations, types, or combinations of substrate(s) in the extracellular medium and examining their effects on respiration. For example, hepatocyte respiration is increased by fatty acids, pyruvate and lactate (Korzeniewski et al.; Nobes et al.; Nobes et al.), while insulinoma basal respiration depends primarily on glucose concentration (Affourtit and Brand). In synaptosomes, inhibition of pyruvate uptake leads to a decrease in the reserve capacity by inhibiting the maximal respiratory rate (Brand and Nicholls, 2011; Kauppinen and Nicholls, 1986). Such a result would indicate that glycolysis, in some cells, can exert considerable control over respiration rate. In cardiomyocytes, it appears that glycolysis can negatively regulate mitochondrial respiration and that pyruvate is the substrate of choice (Sansbury et al., 2011a). Interestingly, neonatal myocytes in the presence of pyruvate alone have a 20% higher reserve capacity compared with cells having both pyruvate and glucose as substrate, and this difference is due to a suppression of the maximal respiratory rate when glucose is present. The reasons for such inhibition are unclear but may be due to allosteric regulation of the electron transport chain by glycolytic intermediates (Diaz-Ruiz et al.). Recent data suggests that bioenergetic reserve capacity may be particularly important in the cell cycle and related to mitochondrial fission/fusion (Mitra and Lippincott-Schwartz, 2010; Mitra et al., 2009). In addition, it has recently been shown that the regulation of glycolysis is central to controlling the cell cycle suggesting a complex interaction between mitochondrial dynamics and metabolism (Garedew et al., 2012). Collectively, these results exemplify the importance of substrate availability in regulating the reserve capacity, which, as discussed below, translate to the cellular response to differentiation, division and stress.
The bioenergetic reserve capacity is also influenced by overall mitochondrial mass, the activity of individual mitochondrial complexes and the mitochondrial network. It is important to note that an underlying bioenergetic dysfunction can counter-intuitively increase the apparent reserve capacity. For example, conditions which act to inhibit the ATP synthase (or any of its associated functions) would decrease the basal OCR, and, assuming no change in the maximally uncoupled rate, this would produce a “false-positive” increase in the reserve capacity. However, this effect would in fact compromise the production of ATP and actually result in a decreased bioenergetic capacity. This can be identified and tested for as described in Figure 4. The ability of a cell to meet a sustained but high ATP demand may be a less vulnerable metabolic state than a transient but severe ATP demand in which reserve capacity is exceeded (Brand and Nicholls, 2011). It is then important to consider not just the magnitude of the reserve capacity but the physiological context in which the mitochondria are functioning.
The challenge as we move forward is to develop methods to compare different conditions quantitatively and draw mechanistic conclusions. A limitation in the analysis is the assumption that addition of inhibitors/uncouplers can be used to selectively eliminate certain “blocks or metabolic units,” leaving intact and unchanged the functions of the other “components.” This leads to some error in assignment of metabolic functions such as ATP-linked respiration and proton leak as discussed above. Similarly, the presence of respiratory substrates capable of increasing NADH may increase the control coefficient for chain control of respiration, perhaps increasing the respiration rate. This effect could decrease the apparent reserve capacity when actually proton flux (and thus ATP synthesis) increases, resulting in a true increase in bioenergetic capacity. Lastly, it has been shown that under conditions of increased bioenergetic stress (ATP consumption) a substantial amount of ATP production is via glycolysis in addition to mitochondrial phosphorylation, and this can be estimated by measurement of ECAR under basal or stressed conditions (Dranka et al., 2011).
Role for a glycolytic reserve in the response to stress
It is well known that glucose metabolism plays a fundamental role in maintaining metabolic homeostasis. Branching off of the primary glycolysis conduit are pathways regulating multiple cellular processes and functions, including: (1) the biosynthesis of nucleotides and NADPH (pentose phosphate pathway); (2) cellular osmolarity (polyol pathway); (3) the synthesis of amino sugars (hexosamine biosynthetic pathway); and (4) the synthesis of glycerophospholipids (dihydroxyacetone phosphate pathway). Metabolism of glucose to pyruvate results in two primary possibilities: the metabolism of pyruvate to lactate or the oxidative decarboxylation of pyruvate to acetyl CoA for entry into the TCA cycle. It is the former of these two possibilities that can be measured as an index of glycolytic flux. While lactate assays can give a snapshot view of whether glycolytic flux is increased or decreased over a period of time, XF ECAR analysis gives a time-resolved view of changes in glycolysis (Ferrick et al.). This has allowed for new concepts to develop concerning how glycolysis could regulate cell function.
Interestingly, in hypertrophied neonatal myocytes, it appears that the glycolytic reserve rather than the mitochondrial reserve capacity helps the cell survive HNE-induced injury and cell death. Cellular hypertrophy driven by adrenergic stimuli resulted in a decrease in the mitochondrial reserve capacity concomitant with an increase in the glycolytic reserve, and this reserve appeared to help the cell through the oxidative insult. Extant evidence suggests that the hypertrophy of the heart in vivo also leads to an increase in glycolytic capacity. For example, in a rodent model of pressure-overload left ventricular hypertrophy (LVH), insulin-independent glucose uptake was increased 3-fold and activators of phosphofructokinase were increased up to 10-fold, leading to a 2-fold increase in glycolysis (Nascimben et al.).
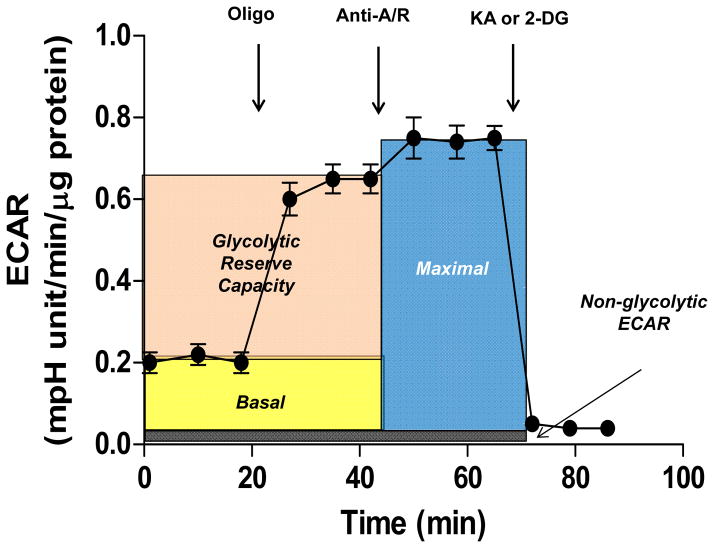
In extracellular flux assays, the glycolytic reserve capacity can be examined through an assay analogous to the mitochondrial function assay where oligomycin and the GAPDH inhibitor koningic acid (KA) are used to examine glycolysis and non-glycolytic ECAR. An example of how this assay could be used is shown in Figure 5. Oligomycin is used in this assay to examine how the glycolytic pathway responds to loss of mitochondrial ATP production. To further inhibit mitochondrial electron flux and remove proton leak, which could act a small sink for reducing equivalents derived from glycolysis (e.g., pyruvate), antimycin-A and rotenone can be added. This can give a maximal rate of glycolysis that could possibly occur if all mitochondrial electron flux is inhibited. Inhibitors of glycolysis such as KA or 2-deoxyglucose (2-DG) can then be used to measure non-glycolytic ECAR, which is comparable to the use of antimycin-A and rotenone in the mitochondrial function assay.
Figure 5. Glycolysis stress test.
This extracellular flux assay demonstrates how basal glycolytic rate, maximal glycolytic rate, and the glycolytic reserve capacity can be determined. After measurement of the basal extracellular acidification rate (ECAR), oligomycin can be added, which generally increases glycolytic rate in response to loss of mitochondrial ATP production. The addition antimycin A/rotenone, depending on experimental conditions and cell type, may further increase the ECAR, giving an index of lactate production occurring when all mitochondrial electron transport is inhibited. Non-glycolytic extracellular acidification (i.e., acidification not due to lactate production) can then be measured by introducing koningic acid (KA; an inhibitor of glyceraldehyde-3-dehydrogenase) to inhibit glycolysis; alternatively, 2-deoxyglucose (2-DG) may be used. This assay may be prefaced by addition of oxidants or other stressors.
The impact of mitochondrial DNA haplotype and damage on mitochondrial quality
A unique feature of the molecular machinery controlling cellular bioenergetics is that the proteins necessary for electron transport and oxidative phosphorylation are encoded by both nuclear and mitochondrial genomes. Each cell contains hundreds to thousands of mitochondria and each mitochondrion contains 5 – 10 copies of maternally inherited mitochondrial DNA (mtDNA). The mammalian mtDNA encodes for the 13 key structural subunits required for the catalytic activity of four of the five OXPHOS enzyme complexes (I, III, IV and V). Because of the pivotal role of these proteins in bioenergetics it has been proposed that mutations in the mtDNA-encoded subunits modulate the economics of oxidative phosphorylation in controlling the relative distribution of electrons to ATP generation, heat and the formation of ROS. We and others are now exploring the hypothesis that these features of mitochondrial economy, which have evolved over thousands of years, can ultimately affect mitochondrial quality and the susceptibility to diseases in which the interface between bioenergetics and inflammation are playing a key role (Figure 6)(Krzywanski et al., 2011; Wallace, 2010, 2011).
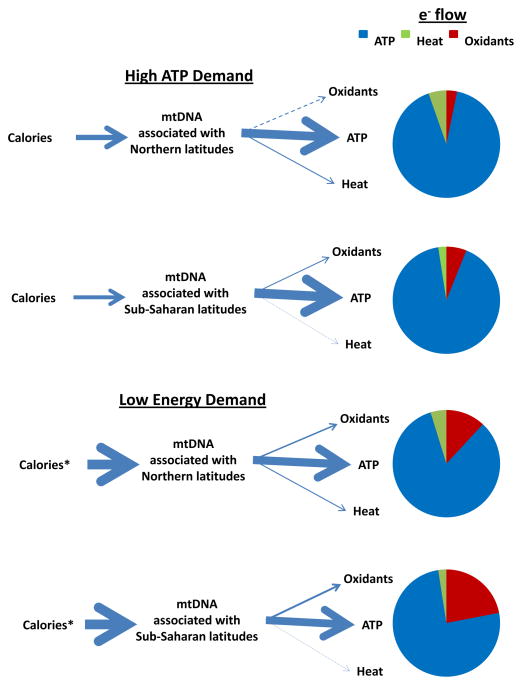
Figure 6. Hypothetical presentation of electronic energy utilization between mitochondria having mtDNA haplotypes representing Northern or Sub-Saharan origins in terms of latitude.
Under conditions of high energy (ATP) demand, differences in energy utilization exist between mitochondria having different mtDNAs that manifest in differences in electrons used to generate ATP, oxidants and heat as illustrated by the arrows and pie charts. Under conditions of low energy (ATP) demand and excessive caloric intake (*), these profiles shift to decreased use of electronic energy for ATP generation and greater production of oxidants, with those mitochondria having sub-Saharan latitude mtDNAs generating more oxidants compared with those with Northern latitude mtDNAs as indicated by the arrows and pie charts.
As the ancestors for contemporary humans migrated out of sub-Saharan Africa, they accumulated missense mutations in their mtDNAs. It is now becoming clear that some of these mutations may have had a selective advantage through their impact on mitochondrial economy. For example, if these mutations altered mitochondrial economy such that caloric utilization for ATP generation was decreased while that used for generating heat increased then this would have been a metabolic advantage for our ancestors as they migrated into northern climates (Ruiz-Pesini et al., 2004; Wallace, 2005). Although these mutations redistributed the economics of electron transfer with a decrease in efficiency in ATP generation, these changes could be accommodated in our migratory ancestors due to changes in their diet (increased caloric intake associated with animal fats) (Cordain et al., 2000). In contrast, the mtDNA haplotypes associated with an economic distribution of electrons to ATP synthesis (e.g., those having sub-Saharan origins) are better adapted to low calorie warmer climates. In the modern era with massive and rapid migration of populations over decades, the mitochondrial haplotype can frequently be maladapted an excessive caloric availability and low energetic demand lifestyle. Figure 6 illustrates this concept by showing that under conditions of high ATP demands (e.g., adequate diet and exercise), electron flow energetics predominately manifests in ATP generation with subtle differences existing in mitochondrial oxidant and heat generation between individuals harboring mtDNA haplotypes originally associated with northern or sub-Saharan latitudes. In contrast, under conditions of low ATP demand (e.g., excess calorie intake and physical inactivity) mitochondrial oxidant production will be differentially increased between individuals with distinct patterns of electron distribution between ATP/ROS and heat (associated with mtDNA haplotype). Specifically, under conditions of excess caloric intake and sedentary lifestyle individuals with sub-Saharan maternal origins will have greater mitochondrial oxidant production relative to those having origins associated with more northern latitudes since excess electron flow in those individuals (sub-Saharan origins) are less likely to be utilized for heat generation and will therefore be diverted to ROS formation.
While cybrid cell culture studies have provided conflicting data regarding the role of mtDNA haplotype on in vitro bioenergetics, other studies have shown that mtDNA haplotype can influence tumor growth, age-related deafness, cognition, behavior, reproductive behavior, and susceptibility to autoimmune disease in mice (Amo and Brand, 2007; Amo et al., 2008; Gomez-Duran et al., 2010; Moreno-Loshuertos et al., 2006). Similarly, molecular epidemiologic studies have shown that human longevity correlates with mtDNA haplotypes having temperate and arctic origins (yet have increased risk for illnesses related with energetic insufficiency) whereas those of sub-Saharan origins appear to be more frequently associated with certain types of cancer and diseases thought to be due to increased oxidative stress and/or mutagenesis (Bhopal and Rafnsson, 2009; De Benedictis et al., 1999; Petros et al., 2005; Rose et al., 2001; Wallace, 2005).
Mitochondrial quality, mtDNA damage and disease
From the previous discussion it is clear that since mitochondrial function declines with the accumulation of mitochondrial damage, then assessment of overall mitochondrial quality can serve as a prognostic indicator for disease risk. For example, studies on primary vascular endothelial and smooth muscle cells have shown preferential and sustained mtDNA damage, altered mitochondrial transcript levels, and decreased mitochondrial protein synthesis in response to oxidant treatments (Ballinger et al., 2000). High levels of nitric oxide have also been shown to decrease the levels of mitochondrial respiratory proteins in endothelial cells and increase the susceptibility to cell death (Ramachandran et al., 2004). In vivo studies have shown increased mtDNA damage in atherosclerotic and aged tissues from both humans and animals, and moreover, that vascular mtDNA damage occurs prior to or coincidental to pathologic changes (atherogenesis) in mice and non-human primates (Ballinger et al., 2002; Westbrook et al., 2010). It is also well established that numerous environmental oxidants, cardiotoxicants and disease risk factors (e.g. hypercholesterolemia, hyperglycemia) cause significant mtDNA damage, and it has been suggested that developmental exposure to these factors can significantly increase the risk for adult disease development by causing early damage to the mtDNA (Cakir et al., 2007; Chuang et al., 2009; Gutierrez et al., 2006; Yang et al., 2007). In addition to the obvious impact of damaged mtDNA on organelle function, oxidant production, and energy generation, studies are now suggesting that the damaged mtDNA can serve as a Damage Associated Molecular Pattern (DAMP) which contributes to a pro-inflammatory cellular environment (Krysko et al., 2011). Finally, certain mtDNA haplotypes may be more prone to mtDNA damage by virtue of mitochondrial oxidant generation associated with conditions of chronic excess energy balance (e.g., mtDNA haplotypes associated with greater oxidant production will sustain more mtDNA damage compared with those that generate less oxidants). Consequently, the mitochondrial bioenergetics and organelle quality are likely dependent upon organelle economy that may be influenced by mtDNA haplotype which in turn may influence the accumulation of mtDNA damage.
Regulation of mitochondrial quality control and the role of mitophagy
Mitochondrial turnover is stimulated by a broad range of conditions including nutrient deprivation. Electron microscopy studies in the early 1960s showed that lysosomes are increased in number in glucagon-treated rat liver and many of the lysosomal structures contained mitochondria or remnants which appeared to be in various stages of breakdown or hydrolysis (Ashford and Porter, 1962). During development, mitophagy plays a key role in eliminating the paternal mitochondria after fertilization in C. elegans (Sato and Sato, 2011). As maternal inheritance of mtDNA is shared by many organisms, including humans, mitophagy may play a critical role in the genetics and diversity of mtDNA haplotype and, as discussed previously, disease susceptibility.
Mitophagy and mitochondrial quality
In addition to the developmental process, tissues that are highly dependent on mitochondrial quality, including liver, heart and brain, maintain active mitophagy that appears to be protective against toxins, hypoxia, and age-dependent diseases (Karbowski and Neutzner, 2012; Lee et al., 2012; Okamoto and Kondo-Okamoto, 2012; Osiewacz, 2011). Oxidative stress may be a key mechanism through which mitochondria are damaged and targeted for mitophagy (Apostolova et al., 2011). We have recently shown in heme-dependent toxicity in endothelial cells that one of the earliest events is bioenergetic dysfunction and the engagement of mitophagy (Higdon et al., 2012). In a number of human diseases with mitochondrial deficits, mitochondrial respiratory chain activities and CoQ10 levels are decreased, and oxidative stress and mitochondrial membrane permeabilization are increased (Cotan et al., 2011). Mitophagic turnover in these cells is important for preventing accumulation of additional damage. For example, in MELAS fibroblasts prevention of autophagy resulted in apoptotic cell death, supporting the idea that mitophagy plays a protective role in cell survival (James et al., 1996).
Mitophagy is important for control of mitochondrial quality and ROS. Key factors in the mitophagy pathway include Atg1/Atg13/FIP200 activation, an ubiquitin-like conjugation pathway involving Atg 3, 5, 7 and 22 leading to lipidation of LC3I and its conversion to LC3II, and LC3II insertion into autophagosomal membranes (Figure 7). Autophagosomal expansion is also stimulated by Beclin/VPS34 complex activities. Some of the key molecular mediators are shown in Figure 7 and are important for mitochondrial quality control. Deformed mitochondria have been observed in Atg3−/− (Jia and He, 2011), Atg5−/−, Atg7−/− (Pua et al., 2009), and Beclin (Atg6)+/− cells. These effects also impact on pathology since livers of VPS34 knockout mice exhibit significant increases in mitochondrial mass and steatosis (Jaber et al., 2012). Significantly increased levels of ROS have been found in various cells in FIP200, Atg3, Atg5, and Atg7 knockout mice (Jia and He, 2011). LC3B knockout macrophages generate more superoxide and in response to LPS, these macrophages displayed more swollen mitochondria (Xu et al., 2011). It is important to note that inhibiting mitophagy has not always been shown to be detrimental. For instance, a mouse model with a targeted deletion of Atg7 in adipose tissue had increased amounts of brown-like adipose tissue containing higher levels of mitochondria and increased fatty acid oxidation. The mice were resistant to diet-induced obesity and insulin resistance (Singh et al.; Zhang et al.). These results illustrate that, in some organs and under certain pathological conditions, a dysfunctional mitochondrial quality control system may have unexpected advantages.
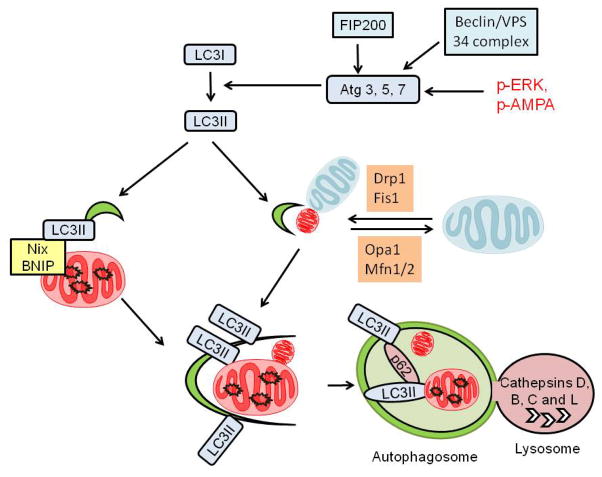
Figure 7. General pathway for activation of autophagy.
Autophagy is mediated by the interaction or activation of several components including Atg1/Atg13/FIP200 and a ubiquitin-like conjugation pathway involving Atg 3, 5, 7 and 22. This leads to the lipidation of LC3I, converting it to LC3II. LC3II is inserted into autophagosomal membranes and is essential for autophagosomal formation. Autophagosomal expansion is also stimulated by Beclin/VPS34 complex activities. Autophagosomes then fuse with lysosomes and the content of autophagosomes are degraded in the acidic environment of the lysosomes. Nix and BNIP can target to mitochondria and bind to LC3II via a LIR consensus sequence and are thus important for mitophagy. ERK and AMPA activities have been shown to stimulate mitophagy. Mitochondrial fission and fusion proteins such as Drp1, Fis1, Opa1, and Mfn1/2 are also critical in regulating mitophagy.
Molecular control of mitophagy
Because mitophagy impacts mitochondrial homeostasis, it is not surprising that it is a tightly regulated process. Depending on the cell type, genetic makeup and environmental stimuli, mitophagic regulatory mechanisms include: mitochondrial membrane depolarization followed by recruitment of Parkin (Elmore et al., 2001; Jin and Youle, 2012), fission and fusion activities, phosphorylated ERK targeting to the mitochondria, and cell signaling processes that detect nutrient conditions and energy status (Figure 7). Importantly, there exists a module of key proteins that regulate the interaction of mitochondria with autophagosomes, and considerable progress has been made in identifying these proteins and their mechanisms of mitochondrial recognition. BNIP3 and Nix (BNIP3L), both BH3-domain containing Bcl-2 family of proteins, have been shown to play critical roles in mitophagy (Band et al.; Ding et al., 2010; Zhang and Ney). These proteins localize to mitochondria and are important in targeting mitochondria for degradation. The significance of the involvement of Nix in mitochondrial clearance is supported by deficient reticulocyte maturation in Nix knockout mice (Zhang and Ney, 2009a). Another important regulator of mitochondria-autophagosome targeting is FUNDC1, which is an outer mitochondrial membrane protein that binds to LC3, and may be important in mitochondrial quality control in response to hypoxia (Liu et al.).
The rapid pace of investigations concerning mitophagy has led to a detailed understanding of two particularly important components of the mitophagic machinery—PTEN-induced putative kinase 1 (PINK1) and Parkin. It is clear that PINK1 is critical in identifying mitochondria destined for autophagy. In “healthy” mitochondria, PINK1 is rapidly turned over by proteolysis; however, mitochondria that become damaged appear to allow mitochondrial accumulation of PINK, which leads to the recruitment of Parkin (Matsuda et al.; Narendra et al., 2010b). How membrane potential and bioenergetic function regulate this PINK-Parkin mechanism is unknown; nevertheless, it is well-established that the machinery is acutely sensitive to changes in mitochondrial function. Within one hour after administration of uncoupling agents, Parkin translocates to mitochondria; this translocation can also be induced by mitochondrial poisons such as azide (Narendra et al., 2008; Suen et al.). The induction of mitophagy following this recruitment is thought to involve the ubiquitin ligase activity of Parkin, which ubiquitinates substrates such as mitofusin 1 (Mfn1), mitofusin 2 (Mfn 2) (Gegg et al.; Tanaka et al.), and voltage-dependent anion channel (VDAC) (Geisler et al.). The ubiquitin-binding adaptor p62 may then be involved in recruiting the mitochondrial cargo into autophagosomes (Ding et al., 2010; Geisler et al.; Pankiv et al.), although contradictory reports exist concerning whether p62 is indeed essential for mitophagy (Narendra et al.; Okatsu et al.). Parkin also interacts with Ambra1; this is important for Ambra1 to activate class III PI3K which is needed for subsequent mitochondrial clearance (Van Humbeeck et al., 2011).
Role of fission and fusion in mitophagy
The shape and functional attributes of mitochondria appear to be important for determining whether they are targeted for mitophagy (Gomes et al., 2011). In hepatocytes, fission is associated with mitophagy (Kim and Lemasters, 2011). The blocking of mitochondrial fusion results in loss of membrane potential in distinct mitochondrial subpopulations and recruitment of parkin (Youle and Narendra, 2011). Parkin, once localized to mitochondria, develops an increase in its E3 ubiquitin ligase activity (Matsuda et al.), and ubiquitinylates mitofusins (Gegg et al.; Tanaka et al.). In turn, this appears to mediate the proteolytic degradation of mitofusin and to promote mitochondrial fragmentation. Hence, parkin may interact dynamically with the fission/fusion machinery and facilitate the removal of only damaged mitochondria by autophagy.
Other proteins affecting fission and fusion, including Fis1, Drp1, and Opa1, have been shown to be important as well (Chan, 2006). For example, in INS1 β-cells, Fis1 knockdown, overexpression of Opa1 or dominant negative Drp1 resulted in decreased mitophagy as assayed by colocalization of mitochondria with autophagosomes (Twig et al., 2008a). It is important to draw attention to the fact that all mitophagic events appear to be exquisitely sensitive to changes in mitochondrial function. In most cases, mitochondrial membrane depolarization appears to serve as a mechanism to recruit signaling molecules that target damaged mitochondria to be degraded. This may then help prevent injured mitochondria from releasing pro-apoptotic agents and producing undesirable ROS (Figure 8). Additional evidence suggests that the bioenergetic reserve capacity may be sensed by the mitophagic machinery (Higdon et al., 2012). An attractive hypothesis that integrates all of these findings is that loss of the ability to proteolyze initial recruitment proteins such as PINK occurs once the bioenergetic reserve is breached. Such a hypothesis integrates the bioenergetic status of each mitochondrion tightly with targeted mitophagic processes.
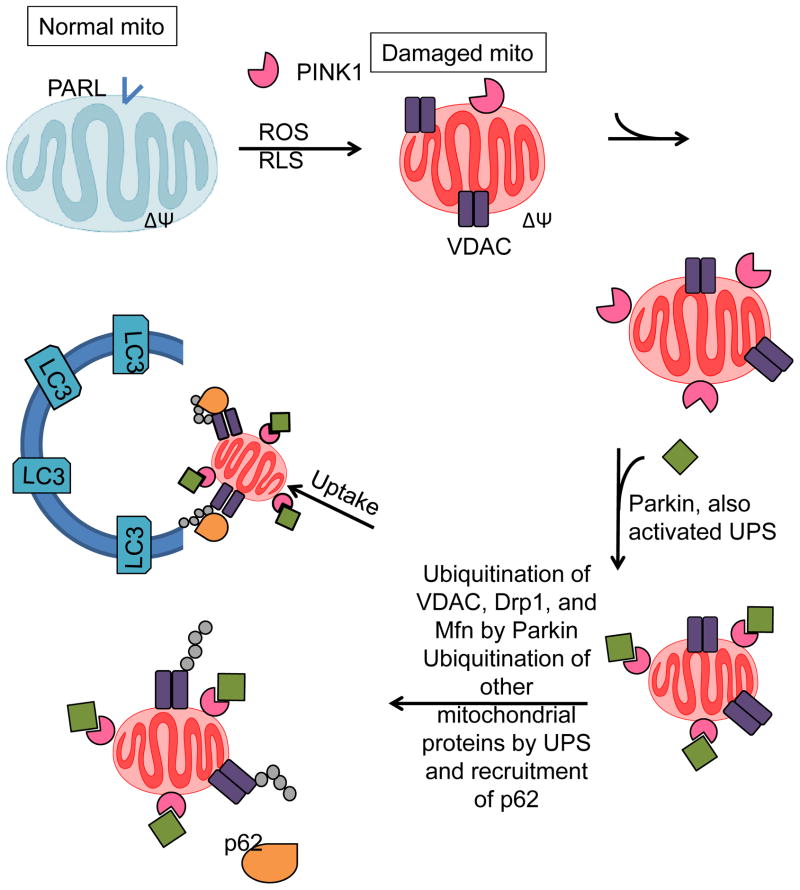
Figure 8. Regulation of mitophagy in response to mitochondrial membrane depolarization.
Mitochondria with normal membrane potential and active presenilin-associated rhomboid-like protein (PARL) prevent mitochondrial PINK1 accumulation. When mitochondria become damaged, mitochondrial membrane potential decreases, PARL is inactivated, and mitochondrial PINK1 is stabilized and accumulates in the affected mitochondria. This recruits Parkin, which promotes the ubiquitination of Drp1 and Mfn, as well as a large number of other mitochondrial proteins. Ubiquitinated proteins can be recognized by p62 which binds LC3 and may help traffic damaged mitochondria to autophagosomes for degradation.
Regulation of Mitochondrial quality in disease
The concept that mitochondrial quality control is a critical element in the role the organelle plays in the pathogenesis of disease is gaining support. Some of selected examples are discussed below.
The reserve capacity and its regulation of cell (patho)physiology
An emerging concept in the energetics field is that the reserve capacity can be used as an index of mitochondrial health (Brand and Nicholls, 2011; Dranka et al., 2010; Higdon et al., 2012; Hill et al., 2009; Zelickson et al., 2011). By indicating how close a cell is to operating at its bioenergetic limit, predictions can be made regarding the cellular response to stress or increased energy demand. This has been demonstrated in the response of cells to oxidative stress. In intact myocytes, stressors that are both causes and consequences of oxidative stress can impact the reserve capacity. For example, the lipid peroxidation product, HNE, increases ATP demand and proton leak to a point where the reserve capacity is depleted (Hill et al.). It appears that a higher bioenergetic reserve capacity results in a greater ability to withstand oxidative stress. In cells given only glucose to respire on, the reserve capacity was only 30% of that of cells provided with both glucose and pyruvate, and the cells given only glucose showed a much earlier HNE-induced bioenergetic collapse compared with cells having extracellular pyruvate available (Sansbury et al., 2011a). Another example was shown in neurons, where glutamate excitotoxicity similarly depleted the reserve respiratory capacity, resulting in respiratory failure (Johnson-Cadwell et al.; Yadava and Nicholls). In the development of resistance to chemotherapy, changes to the mitochondrial respiratory chain (predominantly at cytochrome c oxidase) were associated with an increase in reserve capacity (Oliva et al., 2010). In endothelial cells, exposure to nontoxic concentrations of NO or low levels of hydrogen peroxide reversibly decreased mitochondrial reserve capacity. However, combined NO and superoxide and hydrogen peroxide treatment resulted in an irreversible loss of reserve capacity and was associated with cell death (Dranka et al.). These findings suggest that the reversibility of deleterious changes in the reserve capacity may also be critical; the mechanisms controlling the recoverability of the reserve capacity are unclear, but likely relate with irreversible oxidative protein modification to metabolic and respiratory chain components or non-repairable damage to proteins or organelles that regulate ion fluxes.
The role of the bioenergetic reserve has been extended beyond its relationship simply with cell death. In particular, emerging evidence implicates the mitochondrial respiratory capacity in immunity. For example, it was shown that CD8+ memory T-cells possessed a substantial reserve capacity that was responsive to interleukin-15 (IL-15), which regulated the stability of memory T-cells after infection (van der Windt et al.). A high reserve capacity may also be linked to proliferation of certain cell types, which could result in pathology. For example, it was shown that cancer cells proliferate more rapidly in the presence of exogenous pyruvate when compared with lactate and that pyruvate supplementation increased the reserve respiratory capacity. Inhibition of cellular uptake of pyruvate led to decreased mitochondrial respiration and cell growth (Diers et al.). Hence, cells that transform to a phenotype with high proliferative potential could use the mitochondrial reserve capacity to increase energy for biosynthesis reactions that drive cell division.
Neurodegenerative Diseases
Some of the most interesting mechanisms implicating mitochondrial quality control in pathologies are associated with bioenergetic dysfunction characteristic of many neurodegenerative diseases.
Mutations in Parkin, Pink1 and DJ-1 have been shown to be responsible for a small subset of autosomal-recessive Parkinson’s disease cases. The recent finding that these proteins also play an important role for mitochondrial quality control and mitophagy in a variety of cell types have stimulated new research regarding neurodegeneration pathogenesis in Parkinson’s disease (Jin and Youle, 2012). As discussed above, studies in HEK294, HeLa cells, cardiomyocytes and primary cortical neurons have shown that Parkin accumulates in depolarized mitochondria (Narendra et al., 2008). PINK1-Parkin interactions lead to ubiquitination of VDAC and involve changes in the activity of fission and fusion proteins such as Drp1 and Mfn (Chu, 2010; Geisler et al., 2010b; Rakovic et al., 2011). In addition, Parkin activates the ubiquitin-proteasome system (UPS) which in turn ubiquitinates a large number of mitochondrial proteins (Chan et al., 2011), which may serve as signals for mitophagy. DJ-1, has been shown to act in a parallel pathway to Parkin and PINK1 to regulate mitochondrial fragmentation and is critical in Parkinson’s disease (Schapira and Gegg, 2011). Knockout of DJ-1 in flies decreased the respiratory control ratio (RCR) and mitochondrial DNA/nuclear DNA ratio without changing membrane potential or complex I subunit NDUFS3 level (Hao et al., 2010). DJ-1−/− mice exhibit decreased skeletal muscle ATP (Hao et al., 2010), associated with down regulation of uncoupling protein 4 and 5 (and decreased mitochondrial length and fusion rate. DJ-1−/− MEFs exhibit a profound decrease in mitochondrial quality. This is evident from impaired mitochondrial respiration, increased mitochondrial ROS, decreased mitochondrial membrane potential and characteristic alterations of mitochondrial shape, and depression of autophagy (Krebiehl et al., 2010).
The concepts of reserve capacity and cellular RCR can be applied to mitochondrial quality control via fusion/fission in the context of these Parkinson’s disease associated proteins. The sensing of mitochondrial depolarization during mitophagy occurs via PINK1 whereby depolarization prevents the engagement of the N-terminus mitochondrial targeting sequence (MTS) with the Tim23 pathway and prevents localization to the mitochondrial matrix side of the inner mitochondrial membrane (Narendra and Youle, 2011). This interruption in localization inhibits the normally active proteolytic cleavage of PINK1 and it accumulates at the outer mitochondrial membrane where it recruits and activates Parkin resulting in polyubiquitination of multiple mitochondrial protein targets and subsequent targeting for mitophagy. It is noteworthy that either uncoupler (which will collapse both the pH and ΔψM component of the proton motive force) or valinomycin plus potassium (which collapses only the ΔψM component) (Narendra et al., 2010b; Youle and Narendra, 2011) will prevent processing of PINK1, suggesting that it is the transmembrane electrical field component of the proton motive force that is responsible. Studies with isolated rat hepatocytes (Hafner et al., 1990) indicate that under normal conditions there is only modest control of the proton motive force exerted by the respiratory chain, phosphorylating system, or proton leaks, illustrating the high degree of homeostasis of this component of mitochondrial quality. This result supports the idea that under these normal conditions mitochondrial ΔψM homeostasis is maintained and mitophagy is avoided, even under changing metabolic conditions (e.g., “State3–4”-like transitions).
Alcohol-dependent hepatotoxicity
In the liver, an early event in response to ethanol, endotoxin and cytokines are released, this depresses mitochondrial function, leading to hepatotoxicity (Arteel et al., 2003; Bailey, 2003; Scaglioni et al., 2011). The reserve capacity of the mitochondria after chronic alcohol exposure is decreased and this is exacerbated by the combined exposure of the cells to hypoxia and nitric oxide. Nitric oxide (NO) in combination with reactive oxygen species (ROS) and the associated formation of potent prooxidants such as peroxynitrite contributes to steatosis and decreased mitochondrial quality (Jaeschke et al., 2002; Pacher et al., 2007; Shiva et al., 2005). For example, alcohol consumption is associated with increased mtDNA damage, enhanced sensitivity to the mitochondrial permeability transition, increased sensitivity to the NO-dependent control of respiration, extensive modification of the mitochondrial proteome, protein nitration and lipid peroxidation (Venkatraman et al., 2004a; Venkatraman et al., 2004b; Venkatraman et al., 2004c; Zelickson et al., 2011). Consistent with these findings, a mitochondrially targeted antioxidant decreases oxidative stress and steatosis (Chacko et al., 2011). It is now becoming clear that repair of damaged mitochondria is a complex process, mediated by the autophagy-lysosomal system, which is defective in EtOH toxicity (Ding et al., 2011; Donohue, 2009; Lemasters, 2007). Interestingly, enhancing autophagy with the therapeutic agent rapamycin decreased acute EtOH dependent hepatotoxicity (Ding et al., 2011). In this context, autophagy is protective since inhibition of the autophagic process resulted in increased mitochondrial-dependent apoptosis. These data also suggest that inhibition of the autophagic process occurs and is associated with increased steatosis and marked deterioration of mitochondrial quality probably through a Cyp2E1 mediated mechanism(Wu et al., 2010). This is consistent with the observation that damaged mitochondria which are not removed by mitophagy are susceptible to uncoupling and may promote cell death. The formation of Mallory-Denk bodies is characteristic of alcoholic steatohepatitis in human patients and are comprised of protein aggregates containing cytokeratins, ubiquitin and p62 (Rautou et al., 2010; Zatloukal et al., 2007). These findings suggest that therapeutic strategies that link mitochondrial oxidative damage and autophagy may be beneficial in alcoholic liver disease.
Cardiovascular disease
Given the high energy demands of the cardiovascular system it is not surprising that defects in bioenergetics are associated with the pathology of ischemia-reperfusion and heart failure. An example of how the Stateapparent changes based on cell phenotype can be drawn from our previous work, where cardiomyocytes were exposed to phenylephrine (Sansbury et al., 2011b). This resulted in hypertrophy of the cells and changes in cellular bioenergetics. Notably, the hypertrophied cardiomyocytes demonstrated a small movement toward state 3 compared with non-hypertrophied cells. In this instance, the difference in respiratory state was driven by a single factor, i.e., a higher basal OCR due to increased ATP demand. A similar response was shown in vivo in hypertrophied and failing hearts, where failure was shown to deplete bioenergetic capacity almost completely (Gong et al.), which would be thought to push the Stateapparent to near 3.0. These are several examples illustrating that an intermediate respiratory state exists under physiological conditions and that intracellular mitochondria possess the ability to move toward a more energetic condition using the available reserve cellular bioenergetic capacity. Evidence for a reserve capacity in functioning organs and tissues has also been presented using 31P-NMR techniques in tissues under metabolic stress (Sako et al.).
During heart failure, the heart decompensates when there is an imbalance between energy production and use, leading to a decreased energy reserve in the heart (Ingwall, 2009; Liao et al., 1996). For example, with volume overload (VO), there is a twofold increase in myocardial oxygen consumption and increased ATP consumption (Strauer, 1987; Yun et al., 1992). As shown in Figure 9, the increased ATP demand in VO is also associated with the activation of the ROS-generating enzyme xanthine oxidase (XO) (Gladden et al., 2011a; Gladden et al., 2011b). This finding was translated to the clinic in a recent study which showed that left ventricular biopsies taken from patients with isolated mitral regurgitation had significant cardiomyocyte myofibrillar degeneration along with increased protein nitration and lipofuscin accumulation, consistent with increased formation of ROS/RNS (Ahmed et al., 2010). These oxidants are generated from a number of sources including XO. In addition to being a major source of reactive oxygen species, XO is linked to bioenergetic dysfunction since its substrates derive from ATP catabolism (Figure 9). Correspondingly, there was also evidence of aggregates of small mitochondria in cardiomyocytes, which is generally considered a response to bioenergetic deficit in cells. This increase pro-oxidant environment could decrease mitochondrial quality and underlie the finding that mitochondria isolated from rats with chronic VO have a greater sensitivity to Ca2+-induced PTP opening, and these volume-overloaded hearts have increased ischemia-reperfusion injury (Marcil et al., 2006).
Figure 9. Mitochondrial quality control and xanthine oxidase in the heart.
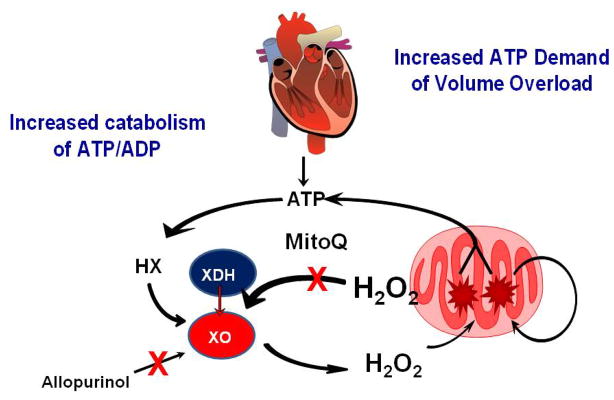
The increase in VO leads to increased usage of ATP, resulting in increased levels of ADP and AMP. ADP and AMP are then degraded to hypoxanthine (HX) via purine catabolism. XO reacts with HX forming superoxide and hydrogen peroxide (H2O2) which damage mitochondria, leading to bioenergetic dysfunction and increased ATP catabolism. Collectively, this causes increased electron leak to form more ROS and decreased ATP production. The increase in mitochondrial ROS leads to increased conversion of xanthine dehydrogenase (XDH) to xanthine oxidase. The enhanced ROS generation from mitochondria and XO cause further damage to the mitochondria and left ventricular dysfunction. Mitochondrially targeted ubiquinone (Mito Q) inhibits the conversion of XDH to XO which may depend on mitochondrial H2O2.
To gain further insight into changes from oxidative stress and bioenergetic dysfunction in VO, we have shown that cyclical stretch of adult rat cardiomyocytes causes increased oxidative stress, mitochondrial swelling, and cytoskeletal disruption—all of which are prevented by an XO inhibitor or the mitochondrial targeted drug MitoQ (Gladden et al., 2011b). More importantly, MitoQ prevented XO activation, suggesting that the mitochondria themselves represent a more direct target in preventing oxidative stress, XO activation, and cellular remodeling in cardiomyocyte stretch. In a similar fashion, 24 hours of VO in vivo also results in increased cardiomyocyte XO activity with decreased state 3 mitochondrial respiration and decreased LV contractility, which are significantly improved with allopurinol (Gladden et al., 2011b; Ulasova et al., 2011). Most recently, ROS production from XO has been linked to myocardial high energy phosphates and ATP flux through creatine kinase in the failing human heart (Hirsch et al., 2012). Treatment of heart failure patients with an XO inhibitor increased phosphocreatine to ATP ratio and creatine kinase flux (measured using in vivo 31P magnetic resonance spectroscopy), which is consistent with the high susceptibility of creatine kinase to oxidative modifications resulting in decreased activity and efficiency of high energy phosphate transfer from mitochondria to the contractile apparatus.
Obesity and Diabetes
An excess consumption of high calorie foods appears to be one of the key factors in the epidemic of obesity (Briefel and Johnson; Duffey and Popkin; Kant and Graubard; Nielsen and Popkin; Popkin et al.; Wang et al.), which increases the risk for developing diseases with a bioenergetic component (Haslam and James). As described above, these include cardiovascular disease (Roger et al.), hepatotoxicity (Zakhari and Li), and neurodegenerative diseases (Bruce-Keller et al.). While a high caloric intake can be offset by exercise (for example, the gold medalist Michael Phelps remains lean despite consuming ~12,000 calories per day!), increasing evidence suggests that physical activity, on average, is declining (Hu et al.; Robinson; Roger et al.; Smith et al.; Williamson et al.). This has led to heightened efforts to promote increased energy utilization and to better understand how altered intermediary metabolism, perturbations in cellular bioenergetics, and genetic differences contribute to the risk for becoming obese and insulin resistant.
Mitochondria lie at the heart of systemic metabolic regulation. They act as endpoint regulators of metabolic rate and affect thermogenesis primarily by increasing proton leak. In brown fat, the relatively high expression of uncoupling protein 1 (UCP1) allows re-entry of protons into the mitochondrial matrix without generating ATP. This uncoupling therefore generates an increase in substrate utilization and electron transport chain turnover as well as energy in the form of heat (Tseng et al.). Despite the low amounts of brown fat in humans, as little as 50 g of brown fat has been estimated to be capable of utilizing up to 20% of basal caloric needs (Rothwell and Stock). This suggests that decreasing mitochondrial efficiency is an attractive therapeutic option for obesity. Indeed, synthetic uncouplers such as 2,4-dinitrophenol were used in the 1930s as a weight loss drug, and it was capable of increasing metabolic rate by up to 40% (Cutting; Harper et al.). However, DNP has a narrow therapeutic window (similar to the narrow dose-response curves observed in XF experiments), and side effects led to removal from the market (Harper et al.). Nevertheless, it is clear that decreasing mitochondrial efficiency may be a robust treatment option for obesity.
The obvious drawback for such an approach is its systemic effects. Mitochondria in organs with high energetic needs (e.g., the heart) should likely maintain a high level of mitochondrial coupling, while other organs such as adipose tissue could afford to be less economical. Indeed, overexpression of UCP1 in adipose tissue (Kopecky et al.) and skeletal muscle (Li et al.) prevented diet-induced obesity in mice, suggesting that uncoupling of oxidative phosphorylation in these two organs is beneficial. Interestingly, oxidative phosphorylation is more uncoupled in endurance athletes compared with sedentary subjects (Befroy et al.), and this appears to enhance fatty acid oxidation and diminish oxidative stress. Moreover, genes encoding fatty acid oxidation are increased in athletes compared with sedentary subjects (Schrauwen-Hinderling et al.), which display an increased gene profile favoring fat storage (Schrauwen-Hinderling et al.). However, it is important to note that a higher metabolic capacity does not always equate with a decreased risk for metabolic disease. For example, American subjects of Asian Indian ancestry are predisposed to obesity and type 2 diabetes and have higher mitochondrial genomic content and oxidative phosphorylation capacity compared with Americans of European descent (Nair et al.). The South Asian population does appear to have more coupled mitochondria, which has led to a “mitochondrial efficiency hypothesis” that could explain the propensity for obesity in some populations (Bhopal et al, 2009). Other hypotheses relating to genetic predispositions such as the “thrifty gene” hypothesis and the “predation release” hypothesis have also been postulated (Speakman, 2008). The fact that mtDNA variants relate with the risk of type 2 diabetes does support a role for genetic factors relating to energetics and metabolism (Irwin et al.; Sun et al.; Wallace, 2011). However, the sudden increase in obesity in areas that have genetically dissimilar populations (such as the Southeastern United States) does not appear to support any of these hypotheses exclusively. It appears that a stronger hypothesis integrating physiological, genetic, and environmental frameworks is required to explain the relatively abrupt increase in the prevalence of obesity and diabetes.
The regulation of autophagy also appears to be important in the context of metabolic diseases such as obesity. Interestingly, autophagy appears to be upregulated in adipose tissues of obese humans (Kovsan et al.), suggesting that it may be involved in adipocyte hypertrophy, and inhibiting autophagy appears to have beneficial effects in the context of obesity. Conditional knock-out of atg7 in adipocytes was shown to prevent diet-induced obesity and to increase insulin sensitivity in mice (Singh et al.; Zhang et al.). Interestingly, deletion of atg7 was associated with significantly increased levels of mitochondria in white adipocytes, supporting a critical role of autophagy in regulating mitochondrial number in adipose tissue. These changes were also associated with an increase in adipocyte fatty acid oxidation as well as changes in whole body metabolism (Singh et al.; Zhang et al.). Inactivation of other autophagy genes has resulted in similar results in vitro. For example, mouse embryonic fibroblasts (MEFs) isolated from atg5−/− mice accumulate less lipid when stimulated to develop into adipocytes, and atg7−/− MEFs similarly cannot undergo adipogenesis to the same extent as their wild-type counterpart cells. This suggests that inhibiting autophagy may be a therapeutic option for treating or preventing obesity or type 2 diabetes although the impact on mitochondrial quality is unclear. In line with this view, a prospective, observational multicenter clinical study showed that hydroxychloroquine, an inhibitor of autophagy, is associated with a reduced risk of diabetes (Wasko et al.).
While the above-mentioned studies suggest that inhibiting autophagy is beneficial in the context of obesity, the role of autophagy and components critical to the autophagic machinery remain unclear in the context of insulin resistance. For example, an interesting recent study demonstrated that autophagy deficits undermine the benefit of exercise in protection against high-fat-diet-induced glucose intolerance (He et al., 2012). Moreover, systemic knock-out of the parkin gene, which as described in the previous sections is involved in mitophagy, has also been shown to prevent diet-induced obesity, steatohepatitis, and insulin resistance. However, the effect of parkin deletion did not appear to be due to regulation of mitochondria or intermediary metabolism. A lack of parkin expression prevented high fat diet-induced upregulation of lipid transport proteins such as CD36, Sr-B1, and FABP and the uptake of fat from the diet (Kim et al.). Hence, it appears that the metabolic effects of parkin are complex and regulate much more than autophagy and mitophagy.
Summary
Collectively, these studies illustrate how differences in mitochondrial economy, bioenergetic reserve capacity, and autophagy regulate health and disease. The control of mitochondrial quality in which biogenesis is balanced with mitophagy is likely to serve as a productive framework for the design of mitochondrial therapeutics. As expected, many of the factors controlling metabolism and susceptibility to disease appear to be driven by genetics and the environment. It is now also becoming clear that physiological processes such as cell differentiation, are associated with profound changes in cellular bioenergetics including reserve capacity (Schneider et al., 2011). The challenge for us is to continue to strive to more fully understand how bioenergetics regulates cell and tissue function. Certainly, this would best position us to develop better and more targeted therapies.
Acknowledgments
The authors acknowledge funding from the following sources: BGH was supported by a grant from the NIH-NCRR (P20 RR024489). JL is supported by NIH CA131653; SB is supported by NIH HL94518, HL103859. LD-I was supported by NIH HL097176. JZ was supported by NIHR01-NS064090 and a VA merit award. VDU was supported by NIH Grants (ES10167, AA 13395 and DK 75865).
Abbreviations
- Ambra1
activating molecule in Beclin 1-regulated autophagy
- AMPA
AMP-activated protein kinase
- Anti A
antimycin A
- Atg
autophagy related
- BNIP
Bcl-2/adenovirus E1B 19-kDa interacting protein 3; DJ-1/PARK7 (mutated in Parkinson’s disease)
- DNP
Dinitrophenol
- Drp1
dynamin-related protein 1
- FIP200
focal adhesion kinase (FAK) family interacting protein of 200 kDa
- Fis1
fission 1
- FCCP
carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- LC3
microtubule-associated protein 1 light chain 3
- MEF
mouse embryonic fibrobalsts
- Mfn
mitofusin
- LIR
LC3-interacting region
- Nix
NIP3-like X, BNIP3L
- NO
nitric oxide
- Opa1
optic atrophy 1
- Parkin
PARK2 (mutated in Parkinson’s disease)
- PARL
presenilin-associated rhomboid-like
- PINK1
PTEN-induced kinase 1/PARK6 (mutated in Parkinson’s disease)
- PTP
permeability transition pore
- RCR
Respiratory Control Ratio
- ROS/RNS
Reactive Oxygen Species/Reactive Nitrogen Species
- UCP
uncoupling proteins
- UPS
ubiquitin-proteasome system
- VPS
vacuolar protein sorting
- XO
xanthine oxidase
- PVA
pressure volume area
- HX
hypoxanthine
- LV
left ventricle
Literature Cited
- Affourtit C, Brand MD. Uncoupling protein-2 contributes significantly to high mitochondrial proton leak in INS-1E insulinoma cells and attenuates glucose-stimulated insulin secretion. Biochem J. 2008;409:199–204. doi: 10.1042/BJ20070954. [DOI] [PubMed] [Google Scholar]
- Ahmed MI, Gladden JD, Litovsky SH, Lloyd SG, Gupta H, Inusah S, Denney T, Jr, Powell P, McGiffin DC, Dell’Italia LJ. Increased oxidative stress and cardiomyocyte myofibrillar degeneration in patients with chronic isolated mitral regurgitation and ejection fraction >60% Journal of the American College of Cardiology. 2010;55:671–679. doi: 10.1016/j.jacc.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo T, Brand MD. Were inefficient mitochondrial haplogroups selected during migrations of modern humans? A test using modular kinetic analysis of coupling in mitochondria from cybrid cell lines. The Biochemical journal. 2007;404:345–351. doi: 10.1042/BJ20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amo T, Yadava N, Oh R, Nicholls DG, Brand MD. Experimental assessment of bioenergetic differences caused by the common European mitochondrial DNA haplogroups H and T. Gene. 2008;411:69–76. doi: 10.1016/j.gene.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolova N, Blas-Garcia A, Esplugues JV. Mitochondria sentencing about cellular life and death: a matter of oxidative stress. Current pharmaceutical design. 2011;17:4047–4060. doi: 10.2174/138161211798764924. [DOI] [PubMed] [Google Scholar]
- Arteel G, Marsano L, Mendez C, Bentley F, McClain CJ. Advances in alcoholic liver disease. Best Pract Res Clin Gastroenterol. 2003;17:625–647. doi: 10.1016/s1521-6918(03)00053-2. [DOI] [PubMed] [Google Scholar]
- Ashford TP, Porter KR. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey SM. A review of the role of reactive oxygen and nitrogen species in alcohol-induced mitochondrial dysfunction. Free Radic Res. 2003;37:585–596. doi: 10.1080/1071576031000091711. [DOI] [PubMed] [Google Scholar]
- Bailey SM, Cunningham CC. Contribution of mitochondria to oxidative stress associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:11–16. doi: 10.1016/s0891-5849(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, et al. Mitochondrial integrity and function in atherogenesis. Circulation. 2002;106:544–549. doi: 10.1161/01.cir.0000023921.93743.89. [DOI] [PubMed] [Google Scholar]
- Ballinger SW, Patterson C, Yan CN, Doan R, Burow DL, Young CG, Yakes FM, Van Houten B, Ballinger CA, Freeman BA, et al. Hydrogen peroxide-and peroxynitrite-induced mitochondrial DNA damage and dysfunction in vascular endothelial and smooth muscle cells. Circ Res. 2000;86:960–966. doi: 10.1161/01.res.86.9.960. [DOI] [PubMed] [Google Scholar]
- Band M, Joel A, Hernandez A, Avivi A. Hypoxia-induced BNIP3 expression and mitophagy: in vivo comparison of the rat and the hypoxia-tolerant mole rat, Spalax ehrenbergi. FASEB J. 2009;23:2327–2335. doi: 10.1096/fj.08-122978. [DOI] [PubMed] [Google Scholar]
- Bayot A, Basse N, Lee I, Gareil M, Pirotte B, Bulteau AL, Friguet B, Reboud-Ravaux M. Towards the control of intracellular protein turnover: mitochondrial Lon protease inhibitors versus proteasome inhibitors. Biochimie. 2008;90:260–269. doi: 10.1016/j.biochi.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Befroy DE, Petersen KF, Dufour S, Mason GF, Rothman DL, Shulman GI. Increased substrate oxidation and mitochondrial uncoupling in skeletal muscle of endurance-trained individuals. Proc Natl Acad Sci U S A. 2008;105:16701–16706. doi: 10.1073/pnas.0808889105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhopal RS, Rafnsson SB. Could mitochondrial efficiency explain the susceptibility to adiposity, metabolic syndrome, diabetes and cardiovascular diseases in South Asian populations? Int J Epidemiol. 2009;38:1072–1081. doi: 10.1093/ije/dyp202. [DOI] [PubMed] [Google Scholar]
- Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. The Journal of clinical investigation. 2012;122:1022–1036. doi: 10.1172/JCI59701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bota DA, Davies KJ. Protein degradation in mitochondria: implications for oxidative stress, aging and disease: a novel etiological classification of mitochondrial proteolytic disorders. Mitochondrion. 2001;1:33–49. doi: 10.1016/s1567-7249(01)00005-8. [DOI] [PubMed] [Google Scholar]
- Bota DA, Davies KJ. Lon protease preferentially degrades oxidized mitochondrial aconitase by an ATP-stimulated mechanism. Nature cell biology. 2002;4:674–680. doi: 10.1038/ncb836. [DOI] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Oshino N, Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972;128:617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand MD. The proton leak across the mitochondrial inner membrane. Biochim Biophys Acta. 1990;1018:128–133. doi: 10.1016/0005-2728(90)90232-s. [DOI] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. The Biochemical journal. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briefel RR, Johnson CL. Secular trends in dietary intake in the United States. Annu Rev Nutr. 2004;24:401–431. doi: 10.1146/annurev.nutr.23.011702.073349. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Brown GC. The leaks and slips of bioenergetic membranes. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1992;6:2961–2965. doi: 10.1096/fasebj.6.11.1644259. [DOI] [PubMed] [Google Scholar]
- Brown GC, Lakin-Thomas PL, Brand MD. Control of respiration and oxidative phosphorylation in isolated rat liver cells. European journal of biochemistry / FEBS. 1990;192:355–362. doi: 10.1111/j.1432-1033.1990.tb19234.x. [DOI] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keller JN, Morrison CD. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 2009;1792:395–400. doi: 10.1016/j.bbadis.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunengraber H, Roe CR. Anaplerotic molecules: current and future. J Inherit Metab Dis. 2006;29:327–331. doi: 10.1007/s10545-006-0320-1. [DOI] [PubMed] [Google Scholar]
- Cakir Y, Yang Z, Knight CA, Pompilius M, Westbrook D, Bailey SM, Pinkerton KE, Ballinger SW. Effect of alcohol and tobacco smoke on mtDNA damage and atherogenesis. Free radical biology & medicine. 2007;43:1279–1288. doi: 10.1016/j.freeradbiomed.2007.07.015. [DOI] [PubMed] [Google Scholar]
- Cao W, Medvedev AV, Daniel KW, Collins S. beta-Adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. The Journal of biological chemistry. 2001;276:27077–27082. doi: 10.1074/jbc.M101049200. [DOI] [PubMed] [Google Scholar]
- Carreira RS, Lee P, Gottlieb RA. Mitochondrial therapeutics for cardioprotection. Current pharmaceutical design. 2011;17:2017–2035. doi: 10.2174/138161211796904777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DC. Mitochondrial fusion and fission in mammals. Annu Rev Cell Dev Biol. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Human molecular genetics. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Baltscheffsky H. Respiratory enzymes in oxidative phosphorylation. VII. Binding of intramitochondrial reduced pyridine nucleotide. The Journal of biological chemistry. 1958a;233:736–739. [PubMed] [Google Scholar]
- Chance B, Baltscheffsky M. Spectroscopic effects of adenosine diphosphate upon the respiratory pigments of rat-heart-muscle sarcosomes. The Biochemical journal. 1958b;68:283–295. doi: 10.1042/bj0680283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls DG. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. Journal of neurochemistry. 2009;109:1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT. A pivotal role for PINK1 and autophagy in mitochondrial quality control: implications for Parkinson disease. Human molecular genetics. 2010;19:R28–37. doi: 10.1093/hmg/ddq143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang GC, Yang Z, Westbrook DG, Pompilius M, Ballinger CA, White CR, Krzywanski DM, Postlethwait EM, Ballinger SW. Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. American journal of physiology Lung cellular and molecular physiology. 2009;297:L209–216. doi: 10.1152/ajplung.00102.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CE, Giulivi C. Nitric oxide regulation of mitochondrial oxygen consumption II: Molecular mechanism and tissue physiology. Am J Physiol Cell Physiol. 2007;292:C1993–2003. doi: 10.1152/ajpcell.00310.2006. [DOI] [PubMed] [Google Scholar]
- Cordain L, Miller JB, Eaton SB, Mann N, Holt SH, Speth JD. Plant-animal subsistence ratios and macronutrient energy estimations in worldwide hunter-gatherer diets. The American journal of clinical nutrition. 2000;71:682–692. doi: 10.1093/ajcn/71.3.682. [DOI] [PubMed] [Google Scholar]
- Coskun P, Wyrembak J, Schriner S, Chen HW, Marciniack C, Laferla F, Wallace DC. A mitochondrial etiology of Alzheimer and Parkinson disease. Biochimica et biophysica acta 2011 [Google Scholar]
- Cotan D, Cordero MD, Garrido-Maraver J, Oropesa-Avila M, Rodriguez-Hernandez A, Gomez Izquierdo L, De la Mata M, De Miguel M, Lorite JB, Infante ER, et al. Secondary coenzyme Q10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:2669–2687. doi: 10.1096/fj.10-165340. [DOI] [PubMed] [Google Scholar]
- Cutting WLT, ML Actions and uses of dinitrophenol: promising metabolic applications. JAMA. 1933;101:1472–1475. [Google Scholar]
- De Benedictis G, Rose G, Carrieri G, De Luca M, Falcone E, Passarino G, Bonafe M, Monti D, Baggio G, Bertolini S, et al. Mitochondrial DNA inherited variants are associated with successful aging and longevity in humans. The FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 1999;13:1532–1536. doi: 10.1096/fasebj.13.12.1532. [DOI] [PubMed] [Google Scholar]
- Diaz-Ruiz R, Averet N, Araiza D, Pinson B, Uribe-Carvajal S, Devin A, Rigoulet M. Mitochondrial oxidative phosphorylation is regulated by fructose 1,6-bisphosphate. A possible role in Crabtree effect induction? J Biol Chem. 2008;283:26948–26955. doi: 10.1074/jbc.M800408200. [DOI] [PubMed] [Google Scholar]
- Diers AR, Broniowska KA, Chang CF, Hogg N. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition. Biochem J. 2012 doi: 10.1042/BJ20120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Li M, Yin XM. Selective taste of ethanol-induced autophagy for mitochondria and lipid droplets. Autophagy. 2011;7:248–249. doi: 10.4161/auto.7.2.14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Ni HM, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, 2nd, Yin XM. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. The Journal of biological chemistry. 2010;285:27879–27890. doi: 10.1074/jbc.M110.119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaruni AS, Brand MD. The regulation and physiology of mitochondrial proton leak. Physiology (Bethesda) 2011;26:192–205. doi: 10.1152/physiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- Donohue TM., Jr Autophagy and ethanol-induced liver injury. World journal of gastroenterology : WJG. 2009;15:1178–1185. doi: 10.3748/wjg.15.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranka BP, Benavides GA, Diers AR, Giordano S, Zelickson BR, Reily C, Zou L, Chatham JC, Hill BG, Zhang J, et al. Assessing bioenergetic function in response to oxidative stress by metabolic profiling. Free radical biology & medicine. 2011;51:1621–1635. doi: 10.1016/j.freeradbiomed.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranka BP, Hill BG, Darley-Usmar VM. Mitochondrial reserve capacity in endothelial cells: The impact of nitric oxide and reactive oxygen species. Free radical biology & medicine. 2010;48:905–914. doi: 10.1016/j.freeradbiomed.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffey KJ, Popkin BM. Shifts in patterns and consumption of beverages between 1965 and 2002. Obesity (Silver Spring) 2007;15:2739–2747. doi: 10.1038/oby.2007.326. [DOI] [PubMed] [Google Scholar]
- Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, Pamplona R, Vidal-Puig AJ, Wang S, Roebuck SJ, et al. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. Embo J. 2003;22:4103–4110. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore SP, Qian T, Grissom SF, Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. Faseb J. 2001;15:2286–2287. doi: 10.1096/fj.01-0206fje. [DOI] [PubMed] [Google Scholar]
- Ferrick DA, Neilson A, Beeson C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discov Today. 2008;13:268–274. doi: 10.1016/j.drudis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–122. doi: 10.1016/j.cell.2007.01.047. [DOI] [PubMed] [Google Scholar]
- Garedew A, Andreassi C, Moncada S. Mitochondrial dynamics, biogenesis, and function are coordinated with the cell cycle by APC/C CDH1. Cell metabolism. 2012;15:466–479. doi: 10.1016/j.cmet.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol. 2010a;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature cell biology. 2010b;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- Gerencser AA, Neilson A, Choi SW, Edman U, Yadava N, Oh RJ, Ferrick DA, Nicholls DG, Brand MD. Quantitative microplate-based respirometry with correction for oxygen diffusion. Analytical chemistry. 2009;81:6868–6878. doi: 10.1021/ac900881z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladden JD, Ahmed MI, Litovsky SH, Schiros CG, Lloyd SG, Gupta H, Denney TS, Jr, Darley-Usmar V, McGiffin DC, Dell’Italia LJ. Oxidative stress and myocardial remodeling in chronic mitral regurgitation. The American journal of the medical sciences. 2011a;342:114–119. doi: 10.1097/MAJ.0b013e318224ab93. [DOI] [PubMed] [Google Scholar]
- Gladden JD, Zelickson BR, Wei CC, Ulasova E, Zheng J, Ahmed MI, Chen Y, Bamman M, Ballinger S, Darley-Usmar V, et al. Novel insights into interactions between mitochondria and xanthine oxidase in acute cardiac volume overload. Free radical biology & medicine. 2011b;51:1975–1984. doi: 10.1016/j.freeradbiomed.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Duran A, Pacheu-Grau D, Lopez-Gallardo E, Diez-Sanchez C, Montoya J, Lopez-Perez MJ, Ruiz-Pesini E. Unmasking the causes of multifactorial disorders: OXPHOS differences between mitochondrial haplogroups. Human molecular genetics. 2010;19:3343–3353. doi: 10.1093/hmg/ddq246. [DOI] [PubMed] [Google Scholar]
- Gong G, Liu J, Liang P, Guo T, Hu Q, Ochiai K, Hou M, Ye Y, Wu X, Mansoor A, et al. Oxidative capacity in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H541–548. doi: 10.1152/ajpheart.01142.2002. [DOI] [PubMed] [Google Scholar]
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112. doi: 10.1126/science.1201940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Ballinger SW, Darley-Usmar VM, Landar A. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circulation research. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- Hafner RP, Brown GC, Brand MD. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the ‘top-down’ approach of metabolic control theory. European journal of biochemistry / FEBS. 1990;188:313–319. doi: 10.1111/j.1432-1033.1990.tb15405.x. [DOI] [PubMed] [Google Scholar]
- Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci U S A. 2010;107:9747–9752. doi: 10.1073/pnas.0911175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper ME, Green K, Brand MD. The efficiency of cellular energy transduction and its implications for obesity. Annu Rev Nutr. 2008;28:13–33. doi: 10.1146/annurev.nutr.28.061807.155357. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP. Obesity. Lancet. 2005;366:1197–1209. doi: 10.1016/S0140-6736(05)67483-1. [DOI] [PubMed] [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, et al. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature. 2012;481:511–515. doi: 10.1038/nature10758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higdon AN, Benavides GA, Chacko BK, Ouyang X, Johnson MS, Landar A, Zhang J, Darley-Usmar VM. Hemin causes mitochondrial dysfunction in endothelial cells through promoting lipid peroxidation: the protective role of autophagy. American journal of physiology Heart and circulatory physiology. 2012;302:H1394–1409. doi: 10.1152/ajpheart.00584.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol acutely increases adenosine triphospate energy delivery in failing human hearts. Journal of the American College of Cardiology. 2012;59:802–808. doi: 10.1016/j.jacc.2011.10.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori O, Ichinoda F, Tamatani T, Yamaguchi A, Sato N, Ozawa K, Kitao Y, Miyazaki M, Harding HP, Ron D, et al. Transmission of cell stress from endoplasmic reticulum to mitochondria: enhanced expression of Lon protease. The Journal of cell biology. 2002;157:1151–1160. doi: 10.1083/jcb.200108103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin JA, Saunier JL, Niederstatter H, Strouss KM, Sturk KA, Diegoli TM, Brandstatter A, Parson W, Parsons TJ. Investigation of heteroplasmy in the human mitochondrial DNA control region: a synthesis of observations from more than 5000 global population samples. J Mol Evol. 2009;68:516–527. doi: 10.1007/s00239-009-9227-4. [DOI] [PubMed] [Google Scholar]
- Jaber N, Dou Z, Chen J-S, Catanzaro J, Jiang Y-P, Ballou LM, Selinger E, Ouyang X, Lin RZ, Zhang J, et al. Class III PI3K Vps34 plays an essential role in autophagy and in heart and liver function. Proceedings of the National Academy of Sciences; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicological sciences : an official journal of the Society of Toxicology. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- James AM, Wei YH, Pang CY, Murphy MP. Altered mitochondrial function in fibroblasts containing MELAS or MERRF mitochondrial DNA mutations. Biochem J. 1996;318(Pt 2):401–407. doi: 10.1042/bj3180401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–36. doi: 10.1002/ana.10483. discussion S36–28. [DOI] [PubMed] [Google Scholar]
- Jia W, He YW. Temporal regulation of intracellular organelle homeostasis in T lymphocytes by autophagy. J Immunol. 2011;186:5313–5322. doi: 10.4049/jimmunol.1002404. [DOI] [PubMed] [Google Scholar]
- Jin SM, Youle RJ. PINK1- and Parkin-mediated mitophagy at a glance. Journal of cell science. 2012;125:795–799. doi: 10.1242/jcs.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Cadwell LI, Jekabsons MB, Wang A, Polster BM, Nicholls DG. ‘Mild Uncoupling’ does not decrease mitochondrial superoxide levels in cultured cerebellar granule neurons but decreases spare respiratory capacity and increases toxicity to glutamate and oxidative stress. J Neurochem. 2007;101:1619–1631. doi: 10.1111/j.1471-4159.2007.04516.x. [DOI] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Eating out in America, 1987–2000: trends and nutritional correlates. Prev Med. 2004;38:243–249. doi: 10.1016/j.ypmed.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Kant AK, Graubard BI. Secular trends in patterns of self-reported food consumption of adult Americans: NHANES 1971–1975 to NHANES 1999–2002. Am J Clin Nutr. 2006;84:1215–1223. doi: 10.1093/ajcn/84.5.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Neutzner A. Neurodegeneration as a consequence of failed mitochondrial maintenance. Acta Neuropathol. 2012;123:157–171. doi: 10.1007/s00401-011-0921-0. [DOI] [PubMed] [Google Scholar]
- Kauppinen RA, Nicholls DG. Synaptosomal bioenergetics. The role of glycolysis, pyruvate oxidation and responses to hypoglycaemia. Eur J Biochem. 1986;158:159–165. doi: 10.1111/j.1432-1033.1986.tb09733.x. [DOI] [PubMed] [Google Scholar]
- Kim I, Lemasters JJ. Mitochondrial degradation by autophagy (mitophagy) in GFP-LC3 transgenic hepatocytes during nutrient deprivation. American journal of physiology Cell physiology. 2011;300:C308–317. doi: 10.1152/ajpcell.00056.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Rodriguez-Enriquez S, Lemasters JJ. Selective degradation of mitochondria by mitophagy. ArchBiochemBiophys. 2007;462:245–253. doi: 10.1016/j.abb.2007.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Stevens MV, Akter MH, Rusk SE, Huang RJ, Cohen A, Noguchi A, Springer D, Bocharov AV, Eggerman TL, et al. Parkin is a lipid-responsive regulator of fat uptake in mice and mutant human cells. J Clin Invest. 2011;121:3701–3712. doi: 10.1172/JCI44736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopecky J, Rossmeisl M, Hodny Z, Syrovy I, Horakova M, Kolarova P. Reduction of dietary obesity in aP2-Ucp transgenic mice: mechanism and adipose tissue morphology. Am J Physiol. 1996;270:E776–786. doi: 10.1152/ajpendo.1996.270.5.E776. [DOI] [PubMed] [Google Scholar]
- Korzeniewski B, Harper ME, Brand MD. Proportional activation coefficients during stimulation of oxidative phosphorylation by lactate and pyruvate or by vasopressin. Biochim Biophys Acta. 1995;1229:315–322. doi: 10.1016/0005-2728(95)00008-7. [DOI] [PubMed] [Google Scholar]
- Kovsan J, Bluher M, Tarnovscki T, Kloting N, Kirshtein B, Madar L, Shai I, Golan R, Harman-Boehm I, Schon MR, et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96:E268–277. doi: 10.1210/jc.2010-1681. [DOI] [PubMed] [Google Scholar]
- Krebiehl G, Ruckerbauer S, Burbulla LF, Kieper N, Maurer B, Waak J, Wolburg H, Gizatullina Z, Gellerich FN, Woitalla D, et al. Reduced basal autophagy and impaired mitochondrial dynamics due to loss of Parkinson’s disease-associated protein DJ-1. PLoS One. 2010;5:e9367. doi: 10.1371/journal.pone.0009367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, Vandenabeele P. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends in immunology. 2011;32:157–164. doi: 10.1016/j.it.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Krzywanski DM, Moellering DR, Fetterman JL, Dunham-Snary KJ, Sammy MJ, Ballinger SW. The mitochondrial paradigm for cardiovascular disease susceptibility and cellular function: a complementary concept to Mendelian genetics. Laboratory investigation; a journal of technical methods and pathology. 2011;91:1122–1135. doi: 10.1038/labinvest.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. The Biochemical journal. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation research. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ. Modulation of mitochondrial membrane permeability in pathogenesis, autophagy and control of metabolism. Journal of gastroenterology and hepatology. 2007;22(Suppl 1):S31–37. doi: 10.1111/j.1440-1746.2006.04643.x. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Theruvath TP, Zhong Z, Nieminen AL. Mitochondrial calcium and the permeability transition in cell death. Biochimica et biophysica acta. 2009;1787:1395–1401. doi: 10.1016/j.bbabio.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Nolte LA, Ju JS, Han DH, Coleman T, Holloszy JO, Semenkovich CF. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000;6:1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- Liao R, Nascimben L, Friedrich J, Gwathmey JK, Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ Res. 1996;78:893–902. doi: 10.1161/01.res.78.5.893. [DOI] [PubMed] [Google Scholar]
- Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, et al. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- Marcil M, Ascah A, Matas J, Belanger S, Deschepper CF, Burelle Y. Compensated volume overload increases the vulnerability of heart mitochondria without affecting their functions in the absence of stress. Journal of molecular and cellular cardiology. 2006;41:998–1009. doi: 10.1016/j.yjmcc.2006.08.117. [DOI] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, et al. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies RA, Gold PH. The turnover of mitochondria in a variety of tissues of young adult and aged rats. The Journal of biological chemistry. 1971;246:2425–2429. [PubMed] [Google Scholar]
- Michel S, Wanet A, De Pauw A, Rommelaere G, Arnould T, Renard P. Crosstalk between mitochondrial (dys)function and mitochondrial abundance. Journal of cellular physiology. 2012;227:2297–2310. doi: 10.1002/jcp.23021. [DOI] [PubMed] [Google Scholar]
- Mitra K, Lippincott-Schwartz J. Analysis of mitochondrial dynamics and functions using imaging approaches. Curr Protoc Cell Biol. 2010;Chapter 4(Unit 4 25):21–21. doi: 10.1002/0471143030.cb0425s46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra K, Wunder C, Roysam B, Lin G, Lippincott-Schwartz J. A hyperfused mitochondrial state achieved at G1-S regulates cyclin E buildup and entry into S phase. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11960–11965. doi: 10.1073/pnas.0904875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Loshuertos R, Acin-Perez R, Fernandez-Silva P, Movilla N, Perez-Martos A, Rodriguez de Cordoba S, Gallardo ME, Enriquez JA. Differences in reactive oxygen species production explain the phenotypes associated with common mouse mitochondrial DNA variants. Nature genetics. 2006;38:1261–1268. doi: 10.1038/ng1897. [DOI] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair KS, Bigelow ML, Asmann YW, Chow LS, Coenen-Schimke JM, Klaus KA, Guo ZK, Sreekumar R, Irving BA. Asian Indians have enhanced skeletal muscle mitochondrial capacity to produce ATP in association with severe insulin resistance. Diabetes. 2008;57:1166–1175. doi: 10.2337/db07-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Kane LA, Hauser DN, Fearnley IM, Youle RJ. p62/SQSTM1 is required for Parkin-induced mitochondrial clustering but not mitophagy; VDAC1 is dispensable for both. Autophagy. 2010a;6:1090–1106. doi: 10.4161/auto.6.8.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra D, Tanaka A, Suen DF, Youle RJ. Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. The Journal of cell biology. 2008;183:795–803. doi: 10.1083/jcb.200809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010b;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendra DP, Youle RJ. Targeting mitochondrial dysfunction: role for PINK1 and Parkin in mitochondrial quality control. Antioxidants & redox signaling. 2011;14:1929–1938. doi: 10.1089/ars.2010.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- Nicholls DG, Darley-Usmar VM, Wu M, Jensen PB, Rogers GW, Ferrick DA. Bioenergetic profile experiment using C2C12 myoblast cells. J Vis Exp. Nielsen, S.J., and Popkin, B.M. (2003). Patterns and trends in food portion sizes, 1977–1998. JAMA. 2010;289:450–453. [Google Scholar]
- Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hay WW, Jr, Brand MD. The mechanism of stimulation of respiration by fatty acids in isolated hepatocytes. J Biol Chem. 1990;265:12910–12915. [PubMed] [Google Scholar]
- Nobes CD, Lakin-Thomas PL, Brand MD. The contribution of ATP turnover by the Na+/K+-ATPase to the rate of respiration of hepatocytes. Effects of thyroid status and fatty acids. Biochim Biophys Acta. 1989;976:241–245. doi: 10.1016/s0005-2728(89)80236-1. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Kondo-Okamoto N. Mitochondria and autophagy: Critical interplay between the two homeostats. Biochimica et biophysica acta. 2012;1820:595–600. doi: 10.1016/j.bbagen.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Okatsu K, Saisho K, Shimanuki M, Nakada K, Shitara H, Sou YS, Kimura M, Sato S, Hattori N, Komatsu M, et al. p62/SQSTM1 cooperates with Parkin for perinuclear clustering of depolarized mitochondria. Genes Cells. 2010;15:887–900. doi: 10.1111/j.1365-2443.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva CR, Nozell SE, Diers A, McClugage SG, Sarkaria JN, 3rd, Markert JM, Darley-Usmar VM, Bailey SM, Gillespie GY, Landar A, et al. Acquisition of temozolomide chemoresistance in gliomas leads to remodeling of mitochondrial electron transport chain. The Journal of biological chemistry. 2010;285:39759–39767. doi: 10.1074/jbc.M110.147504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiewacz HD. Mitochondrial quality control in aging and lifespan control of the fungal aging model Podospora anserina. Biochemical Society transactions. 2011;39:1488–1492. doi: 10.1042/BST0391488. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan-Zhou XR, Cui L, Zhou XJ, Sommadossi JP, Darley-Usmar VM. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrob Agents Chemother. 2000;44:496–503. doi: 10.1128/aac.44.3.496-503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Patel MS, Korotchkina LG. Regulation of the pyruvate dehydrogenase complex. Biochem Soc Trans. 2006;34:217–222. doi: 10.1042/BST20060217. [DOI] [PubMed] [Google Scholar]
- Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, Lim S, Issa MM, Flanders WD, Hosseini SH, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi CA, Suliman HB. Transcriptional control of mitochondrial biogenesis and its interface with inflammatory processes. Biochimica et biophysica acta. 2012;1820:532–541. doi: 10.1016/j.bbagen.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilsl A, Winklhofer KF. Parkin, PINK1 and mitochondrial integrity: emerging concepts of mitochondrial dysfunction in Parkinson’s disease. Acta Neuropathol. 2012;123:173–188. doi: 10.1007/s00401-011-0902-3. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Armstrong LE, Bray GM, Caballero B, Frei B, Willett WC. A new proposed guidance system for beverage consumption in the United States. Am J Clin Nutr. 2006;83:529–542. doi: 10.1093/ajcn.83.3.529. [DOI] [PubMed] [Google Scholar]
- Pua HH, Guo J, Komatsu M, He YW. Autophagy is essential for mitochondrial clearance in mature T lymphocytes. J Immunol. 2009;182:4046–4055. doi: 10.4049/jimmunol.0801143. [DOI] [PubMed] [Google Scholar]
- Rakovic A, Grunewald A, Kottwitz J, Bruggemann N, Pramstaller PP, Lohmann K, Klein C. Mutations in PINK1 and Parkin impair ubiquitination of Mitofusins in human fibroblasts. PLoS One. 2011;6:e16746. doi: 10.1371/journal.pone.0016746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Ceaser E, Darley-Usmar VM. Chronic exposure to nitric oxide alters the free iron pool in endothelial cells: role of mitochondrial respiratory complexes and heat shock proteins. Proc Natl Acad Sci U S A. 2004;101:384–389. doi: 10.1073/pnas.0304653101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautou PE, Mansouri A, Lebrec D, Durand F, Valla D, Moreau R. Autophagy in liver diseases. Journal of hepatology. 2010;53:1123–1134. doi: 10.1016/j.jhep.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Robinson TN. Reducing children’s television viewing to prevent obesity: a randomized controlled trial. JAMA. 1999;282:1561–1567. doi: 10.1001/jama.282.16.1561. [DOI] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart Disease and Stroke Statistics--2012 Update: A Report From the American Heart Association. Circulation. 2011 doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose G, Passarino G, Carrieri G, Altomare K, Greco V, Bertolini S, Bonafe M, Franceschi C, De Benedictis G. Paradoxes in longevity: sequence analysis of mtDNA haplogroup J in centenarians. Eur J Hum Genet. 2001;9:701–707. doi: 10.1038/sj.ejhg.5200703. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. Luxuskonsumption, diet-induced thermogenesis and brown fat: the case in favour. Clin Sci (Lond) 1983;64:19–23. doi: 10.1042/cs0640019. [DOI] [PubMed] [Google Scholar]
- Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004;303:223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- Sako EY, Kingsley-Hickman PB, From AH, Foker JE, Ugurbil K. ATP synthesis kinetics and mitochondrial function in the postischemic myocardium as studied by 31P NMR. J Biol Chem. 1988;263:10600–10607. [PubMed] [Google Scholar]
- Sansbury BE, Jones SP, Riggs DW, Darley-Usmar VM, Hill BG. Bioenergetic function in cardiovascular cells: the importance of the reserve capacity and its biological regulation. Chemico-biological interactions. 2011a;191:288–295. doi: 10.1016/j.cbi.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansbury BE, Riggs DW, Brainard RE, Salabei JK, Jones SP, Hill BG. Responses of hypertrophied myocytes to reactive species: implications for glycolysis and electrophile metabolism. The Biochemical journal. 2011b;435:519–528. doi: 10.1042/BJ20101390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarti P, Giuffre A, Barone MC, Forte E, Mastronicola D, Brunori M. Nitric oxide and cytochrome oxidase: reaction mechanisms from the enzyme to the cell. Free Radic Biol Med. 2003;34:509–520. doi: 10.1016/s0891-5849(02)01326-6. [DOI] [PubMed] [Google Scholar]
- Sato M, Sato K. Degradation of paternal mitochondria by fertilization-triggered autophagy in C. elegans embryos. Science. 2011;334:1141–1144. doi: 10.1126/science.1210333. [DOI] [PubMed] [Google Scholar]
- Scaglioni F, Ciccia S, Marino M, Bedogni G, Bellentani S. ASH and NASH. Dig Dis. 2011;29:202–210. doi: 10.1159/000323886. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Gegg M. Mitochondrial contribution to Parkinson’s disease pathogenesis. Parkinsons Dis. 2011;2011:159160. doi: 10.4061/2011/159160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, Giordano S, Zelickson BR, MSJ, GAB, Ouyang X, Fineberg N, Darley-Usmar VM, Zhang J. Differentiation of SH-SY5Y cells to a neuronal phenotype changes cellular bioenergetics and the response to oxidative stress. Free radical biology & medicine. 2011;51:2007–2017. doi: 10.1016/j.freeradbiomed.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Hesselink MK, Moonen-Kornips E, Schaart G, Kooi ME, Saris WH, Schrauwen P. Short-term training is accompanied by a down regulation of ACC2 mRNA in skeletal muscle. Int J Sports Med. 2006;27:786–791. doi: 10.1055/s-2005-873020. [DOI] [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Kooi ME, Hesselink MK, Moonen-Kornips E, Schaart G, Mustard KJ, Hardie DG, Saris WH, Nicolay K, Schrauwen P. Intramyocellular lipid content and molecular adaptations in response to a 1-week high-fat diet. Obes Res. 2005;13:2088–2094. doi: 10.1038/oby.2005.259. [DOI] [PubMed] [Google Scholar]
- Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic Biol Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DE, Lewis CE, Caveny JL, Perkins LL, Burke GL, Bild DE. Longitudinal changes in adiposity associated with pregnancy. The CARDIA Study. Coronary Artery Risk Development in Young Adults Study. JAMA. 1994;271:1747–1751. [PubMed] [Google Scholar]
- Speakman JR. Thrifty genes for obesity, an attractive but flawed idea, and an alternative perspective: the ‘drifty gene’ hypothesis. International journal of obesity. 2008;32:1611–1617. doi: 10.1038/ijo.2008.161. [DOI] [PubMed] [Google Scholar]
- Sridharan V, Guichard J, Li CY, Muise-Helmericks R, Beeson CC, Wright GL. O(2)-sensing signal cascade: clamping of O(2) respiration, reduced ATP utilization, and inducible fumarate respiration. Am J Physiol Cell Physiol. 2008;295:C29–37. doi: 10.1152/ajpcell.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauer BE. Cardiac energetics in clinical heart disease. Basic Res Cardiol. 1987;82(Suppl 2):389–402. doi: 10.1007/978-3-662-11289-2_38. [DOI] [PubMed] [Google Scholar]
- Suen DF, Narendra DP, Tanaka A, Manfredi G, Youle RJ. Parkin overexpression selects against a deleterious mtDNA mutation in heteroplasmic cybrid cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:11835–11840. doi: 10.1073/pnas.0914569107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, Cui J, Gavras H, Schwartz F. A novel class of tests for the detection of mitochondrial DNA-mutation involvement in diseases. Am J Hum Genet. 2003;72:1515–1526. doi: 10.1086/375656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsness PE, Fox TD. Escape of DNA from mitochondria to the nucleus in Saccharomyces cerevisiae. Nature. 1990;346:376–379. doi: 10.1038/346376a0. [DOI] [PubMed] [Google Scholar]
- Thorsness PE, White KH, Fox TD. Inactivation of YME1, a member of the ftsH-SEC18-PAS1-CDC48 family of putative ATPase-encoding genes, causes increased escape of DNA from mitochondria in Saccharomyces cerevisiae. Molecular and cellular biology. 1993;13:5418–5426. doi: 10.1128/mcb.13.9.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. The EMBO journal. 2008a;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochimica et biophysica acta. 2008b;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugarte N, Petropoulos I, Friguet B. Oxidized mitochondrial protein degradation and repair in aging and oxidative stress. Antioxidants & redox signaling. 2010;13:539–549. doi: 10.1089/ars.2009.2998. [DOI] [PubMed] [Google Scholar]
- Ulasova E, Gladden JD, Chen Y, Zheng J, Pat B, Bradley W, Powell P, Zmijewski JW, Zelickson BR, Ballinger SW, et al. Loss of interstitial collagen causes structural and functional alterations of cardiomyocyte subsarcolemmal mitochondria in acute volume overload. Journal of molecular and cellular cardiology. 2011;50:147–156. doi: 10.1016/j.yjmcc.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Humbeeck C, Cornelissen T, Hofkens H, Mandemakers W, Gevaert K, De Strooper B, Vandenberghe W. Parkin interacts with Ambra1 to induce mitophagy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10249–10261. doi: 10.1523/JNEUROSCI.1917-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, et al. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. The Journal of biological chemistry. 2004a;279:22092–22101. doi: 10.1074/jbc.M402245200. [DOI] [PubMed] [Google Scholar]
- Venkatraman A, Landar A, Davis AJ, Ulasova E, Page G, Murphy MP, Darley-Usmar V, Bailey SM. Oxidative modification of hepatic mitochondria protein thiols: effect of chronic alcohol consumption. American journal of physiology Gastrointestinal and liver physiology. 2004b;286:G521–527. doi: 10.1152/ajpgi.00399.2003. [DOI] [PubMed] [Google Scholar]
- Venkatraman A, Shiva S, Wigley A, Ulasova E, Chhieng D, Bailey SM, Darley-Usmar VM. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004c;40:565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DC. Mitochondrial DNA mutations in disease and aging. Environmental and molecular mutagenesis. 2010;51:440–450. doi: 10.1002/em.20586. [DOI] [PubMed] [Google Scholar]
- Wallace, D.C. Bioenergetic Origins of Complexity and Disease. Cold Spring Harb Symp Quant Biol. 2011 doi: 10.1101/sqb.2011.76.010462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Bleich SN, Gortmaker SL. Increasing caloric contribution from sugar-sweetened beverages and 100% fruit juices among US children and adolescents, 1988–2004. Pediatrics. 2008;121:e1604–1614. doi: 10.1542/peds.2007-2834. [DOI] [PubMed] [Google Scholar]
- Wasko MC, Hubert HB, Lingala VB, Elliott JR, Luggen ME, Fries JF, Ward MM. Hydroxychloroquine and risk of diabetes in patients with rheumatoid arthritis. JAMA. 2007;298:187–193. doi: 10.1001/jama.298.2.187. [DOI] [PubMed] [Google Scholar]
- Westbrook DG, Anderson PG, Pinkerton KE, Ballinger SW. Perinatal tobacco smoke exposure increases vascular oxidative stress and mitochondrial damage in non-human primates. Cardiovascular toxicology. 2010;10:216–226. doi: 10.1007/s12012-010-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson DF, Madans J, Anda RF, Kleinman JC, Kahn HS, Byers T. Recreational physical activity and ten-year weight change in a US national cohort. Int J Obes Relat Metab Disord. 1993;17:279–286. [PubMed] [Google Scholar]
- Williamson JR, Cooper RH. Regulation of the citric acid cycle in mammalian systems. FEBS Lett. 1980;117(Suppl):K73–85. doi: 10.1016/0014-5793(80)80572-2. [DOI] [PubMed] [Google Scholar]
- Wu D, Wang X, Zhou R, Cederbaum A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochemical and biophysical research communications. 2010;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Kanatous SB, Thurmond FA, Gallardo T, Isotani E, Bassel-Duby R, Williams RS. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science. 2002;296:349–352. doi: 10.1126/science.1071163. [DOI] [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- Xu YN, Cui XS, Sun SC, Lee SE, Li YH, Kwon JS, Lee SH, Hwang KC, Kim NH. Mitochondrial dysfunction influences apoptosis and autophagy in porcine parthenotes developing in vitro. J Reprod Dev. 2011;57:143–150. doi: 10.1262/jrd.10-110h. [DOI] [PubMed] [Google Scholar]
- Yadava N, Nicholls DG. Spare respiratory capacity rather than oxidative stress regulates glutamate excitotoxicity after partial respiratory inhibition of mitochondrial complex I with rotenone. J Neurosci. 2007;27:7310–7317. doi: 10.1523/JNEUROSCI.0212-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Harrison CM, Chuang GC, Ballinger SW. The role of tobacco smoke induced mitochondrial damage in vascular dysfunction and atherosclerosis. Mutation research. 2007;621:61–74. doi: 10.1016/j.mrfmmm.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nature reviews Molecular cell biology. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun KL, Rayhill SC, Niczporuk MA, Fann JI, Derby GC, Daughters GT, Ingels NB, Jr, Miller DC. Left ventricular mechanics and energetics in the dilated canine heart: acute versus chronic mitral regurgitation. The Journal of thoracic and cardiovascular surgery. 1992;104:26–39. [PubMed] [Google Scholar]
- Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]
- Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. From Mallory to Mallory-Denk bodies: what, how and why? Exp Cell Res. 2007;313:2033–2049. doi: 10.1016/j.yexcr.2007.04.024. [DOI] [PubMed] [Google Scholar]
- Zelickson BR, Benavides GA, Johnson MS, Chacko BK, Venkatraman A, Landar A, Betancourt AM, Bailey SM, Darley-Usmar VM. Nitric oxide and hypoxia exacerbate alcohol-induced mitochondrial dysfunction in hepatocytes. Biochimica et biophysica acta. 2011;1807:1573–1582. doi: 10.1016/j.bbabio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell death and differentiation. 2009a;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Ney PA. Role of BNIP3 and NIX in cell death, autophagy, and mitophagy. Cell Death Differ. 2009b;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]