Abstract
The processing and presentation of self-proteins is essential to develop an effective immune system, with almost all T cells only ever encountering self-peptide/MHC ligands. How positive selection in the thymus occurs with a weak interaction between the TCR and self-pMHC remains unresolved. The recent identification of a naturally occurring positive selecting self-peptide, gp250, for the MCC/I-Ek specific T cell, AND, has provided some key insights. Despite the weak 3D affinity of the positive selecting AND TCR: gp250/I-Ek interaction, it induces a sustained Ca2+ flux and Erk signaling. Transcriptional profiling revealed the unique expression of a voltage-gated sodium channel (VGSC) in DP thymocytes. Blocking of this channel with tetrodotoxin inhibited positive selection of AND and polyclonal CD4 T cells in vitro. VGSC sh-RNA knockdown inhibited the selection of CD4, but not CD8 T cells. Thus, the expression of a VGSC at the DP stage increases the sensitivity of signaling induced by positively selecting ligands, thereby, providing a mechanism by which a weak TCR: self-peptide interaction can result in a sustained developmental signal. One enigma regarding positive selection is that AND TCR recognizes gp250 self-peptide with a high degree of specificity, akin to what is seen with foreign antigen. The self-peptide repertoire is significantly smaller than the T cell repertoire, therefore, each self-peptide has to select many unrelated T cells. Other studies have shown that a single peptide/MHC can select a large number of T cells. To reconcile this dichotomy, we propose a model in which positive selection is not simply a live or die process, but that the strength of the interaction between a TCR and the positive selecting ligand is deterministic for the functional activity of the peripheral T cells.
Introduction
Initial studies on the processing and presentation of self-proteins
The essential balancing act of the immune system is developing a sufficiently broad T cell repertoire to recognize any potential pathogens, while avoiding autoimmunity. The seminal discovery of peptides binding to MHC molecules in 1985 provided the (intellectual) basis for the function of MHC molecules, the nature of the TCR ligand, self MHC restriction, and immune response genes, but let unresolved the question of self-tolerance (Babbitt et al., 1985). Thus, it was not known at this time if self-antigens were constitutively processed and presented in vivo, if all APCs process and present self-antigens, and if self-peptides were involved in T cell development. To address these questions, our laboratory developed the hemoglobin (Hb) self-antigen system (Lorenz and Allen, 1988). The basis of this system was the existence of allelic forms of murine Hb, and mouse strains with identical MHC (H-2k) expressed different alleles of Hb. We generated T cells specific for Hbd/I-Ek in Hbs mice to use as probes in ex vivo studies to detect self-Hb/I-Ek complexes. Importantly we demonstrated endogenous Hb/I-Ek complexes were presented constitutively by macrophages, dendritic cells, and B cells. These studies were also extended to non-professional APCs, where we also demonstrated that Hb was constitutively processed and presented by Kupffer cells and kidney proximal tubule cells. These studies were the first to directly demonstrate that the MHC molecules did not distinguish self from non-self at the APC level, and that this discrimination had to be occurring with the T cells.
Self-peptides and T cell selection in the thymus
Having established that MHC molecules presented peptides raised the issue of their role in selection of T cells. Self-peptide/MHC ligands are involved in both positive and negative selection of T cells. The Goldilocks model is well accepted, which posits that interactions with self-peptide/MHC too weak result in death by neglect. Interactions too strong result in death by activation induced cell death. It is only the “just right” interactions that lead to positive selection. In the 1980s one model was proposed to explain how a T cell repertoire could undergo both positive and negative selection by self-peptide if the TCR signaling thresholds were the same. In this model, the MHC molecules involved in positive selection on cortical thymic epithelial cells (cTEC) expressed a different set of self-peptides/MHC than medullary thymic epithelial cells (mTEC) and dendritic cells. This model would predict that Hb would not be presented by cTECs. Using our Hb reactive T cells, we directly demonstrated that cTECs, mTECs, and thymic DCs all constitutively presented Hb/I-Ek complexes. This finding disproved this cTEC lack of expression model, and necessitated differential signaling threshold models to explain how a cell could be positively selected and avoid negative selection by the same pMHC. The extremely low number of cTEC in an individual thymus makes it currently impossible to biochemically evaluate the self-peptides presented by cTECs, but for MHC class II molecules there is no evidence for any differential expression. For MHC class I and CD8 T cells the situation is less clear, in that the β5t proteasome is expressed specifically in cTECs as part of the thymoproteosome (Takahama et al., 2012), raising the possibility that class I could differ from class II in the peptides they display in cTECs.
Identification of a naturally occurring self-peptide for AND T cells
No previous studies had identified any bona fide naturally occurring positively selecting ligands for CD4 T cells, because the class II system has no equivalent tool such as organ culture of TAP-deficient or β2m-deficient mice as in class I system. For CD8 T cells, a few selecting ligands had been identified. To identify a naturally occurring self-peptide for one CD4 T cell, we developed a system in which we bred four I-Ek TCR transgenic mouse lines onto a Rag1-deficient and MHC non-selecting background, resulting in all of the thymocytes being DP (Lo et al., 2009). For the self-peptides we analyzed the peptides bound to I-Ek in the CH27 B cell line, and selected 95 peptides. Each of the peptides was selected to have canonical I-Ek binding residues, and only one member of each nested family was chosen. We then screened the 95 peptides for their abilities to positively select any of the 4 TCRtg mouse lines. We used CD69 upregulation using peptide loaded I-Ek Ig dimers and reaggregate cultures using the ANV41.2 cTEC line which had been transfected with I-Ek. This ANV41.2 cTEC line presents very few self peptides in the absence of IFNγ.
We identified one peptide, gp250, which was able to positively select AND T cells, which are specific for MCC/I-Ek. Somewhat surprisingly, we only had this single “hit” for a true positively selecting ligand, hinting at some degree of specificity in positive selection. The gp250 peptide (SAPGLIIATGSVGK) had no similarities at the TCR contact residues with MCC (RADLIAYLKQATK), showing that positive selection did not involve any type of molecular mimicry to the agonist peptide. The gp250 protein is from a sortilin-related endocytic receptor protein and the mRNA is expressed in all APCs including cTECs, mTECs, DCs, macrophages, and B cells. Being expressed in all APCs and being a component of the class II processing pathway makes gp250 highly likely to be expressed and presented in cTECs. The specificity of gp250-induced positive selection was explored by making single amino acid substitutions at each of the 4 TCR contact residues. We found a high degree of specificity, in that none of the singly substituted gp250 peptides could positively select AND T cells in the reaggregate cultures. The gp250 peptide did not stimulate peripheral AND T cells, but was able to act as a co-agonist. Several studies have shown that peripheral CD4 T cells require some type of self-peptide/MHC complexes to be maintained. To determine the role of the positively selecting ligand in peripheral T cell maintenance, we administered additional gp250 peptide to RAG-H-2k mice undergoing homeostatic proliferation of AND T cells. The presence of addition gp250 peptide enhanced the survival of the T cells. From these studies, we had identified a naturally occurring positive selecting ligand for the MCC specific CD4 T cell, AND, and showed a high degree of specificity in positive selection.
Biophysical nature of gp250/I-Ek: AND TCR interaction and Ca2+ signaling
The identification of gp250 as a positive selecting ligand for AND T cells permitted us to explore the nature of its recognition by the AND TCR, since the “strength” of this TCR: pMHC interaction, especially in relationship to that of agonist ligand, had not been ascertained (Lo et al., 2012). We generated soluble AND TCR and gp250/I-Ek quantified their interaction using surface plasmon resonance (SPR). We were able to easily detect the binding of AND to MCC/I-Ek to be ~13 μM. For gp250/I-Ek we could not detect any significant binding. Our lower limit of detect would put this binding at less than 500 μM. Another potentially more sensitive way to explore the nature of the AND: gp250/I-Ek interaction was through the use of MHC tetramers. We generated gp250/I-Ek and MCC/I-Ek tetramers and tested them for their ability to stain AND DP thymocytes. We could detect good binding with MCC/I-Ek tetramers, and obtain a half-life using tetramer decay (t1/2 = 43 minutes). For the gp250/I-Ek we did not detect any significant staining over a control tetramer. Thus, similar to our findings with SPR, the AND: gp250/I-Ek interaction was weak and undetectable even using MHC tetramers.
To determine if we could detect any biochemical signaling induced by gp250/I-Ek we examined Ca2+ influx, as a rapid and sensitive assay. Using gp250/I-Ek Ig dimers to stimulate preselection DP thymocytes, gp250 could stimulate a strong Ca2+ flux in a large number of preselection DP T cells. The pattern of the Ca2+ flux was very different from that induced by MCC/I-Ek. The gp250 pattern involved a rapid rise, and then a sustained Ca2+ influx. In contrast, MCC/I-Ek stimulation induced an initial high spike, but the level of was not sustained. We next tested for the ability of gp250 to induce downstream signaling, using phosphoERK staining. We observed that gp250 could induce a sustained Erk phosphorylation, whereas MCC induced a transient phosphorylation. Thus, gp250 indeed induces biochemical signaling, in a sustained manner, and the pattern is different from what was observed for agonist stimulation.
Current Status
Identification of a voltage-gated sodium channel expressed in DP thymocytes
Despite our progress with the gp250/AND positive selecting ligand system, the question still remained as to how a weak signal could be translated into the positive selection of T cells. To delve into this question, we performed transcriptional profiling of DP thymocytes stimulated with either gp250/I-Ek or MCC/I-Ek (Lo et al., 2012). We identified 28 genes that were upregulated by gp250/I-Ek and downregulated by MCC/I-Ek. Of these genes, SCN4b caught our attention. It is a β subunit of a voltage gated sodium channel (VGSC). VGSC have been well characterized in excitable cells such as neurons and muscle cells for their role in high frequency action potentials. Essentially nothing is known in the immune system. A VGSC is composed of one α chain pore and one or two regulatory β subunits. Na+ entry via the α chain pore is specifically inhibited by tetrodotoxin (TTX). There are 10 VGSCα chains and 4 VGSCβ chains in the mouse genome. We determined that DP thymocytes were expressing SCN5a and SCN4b as the only chains, and confirmed the expression by western blot analysis. The expression of these chains during T cell development was highly informative. They were uniquely expressed at the DN3 and DP stage, and not at any other T cell stage or peripheral T cell. These are two stages during T cell development involving weak self-ligand interactions, and we would contend that this expression pattern is too coincidental to be trivial.
Inhibition of the VGSC blocks positive selection
The functional role of the SCN5a/SCN4b VGSC in positive selection was assessed by blocking it with TTX. TTX inhibited a gp250-induced Ca2+ influx in DP thymocytes, and positive selection of AND cells in reaggregate cultures. If the cTEC cell line, ANV41.2, is treated with IFNγ, it can positively select polyclonal CD4 and CD8 T cells. When TTX was added to these cultures, CD4 T cell positive selection was inhibited, but not CD8, suggesting a differential requirement of the VGSC in CD4 versus CD8 T cells. The role of SCN4b was assessed using a SCN4b-human Ig fusion protein. When this fusion protein was added to the AND reaggregate cultures, we observed essentially a complete inhibition of positive selection. In the polyclonal reaggreate cultures, the SCN4b-human Ig fusion protein blocked CD4 development, but not CD8, consistent with the TTX effect.
To genetically test the role of SCN5a/SCN4b in vivo, we performed sh-RNA lentiviral knockdown. The SCN5a knockout mouse is embryonic lethal and there is no available SCN4b knockout mouse. In the SCN5a shRNA knockdown in B6 mice, we observed a significant decrease in the selection of CD4SP cells in the thymus and periphery, but did not see significant effect on CD8 development. Thus, SCN5a/SCN4b in multiple assays appears to be critically involved in the positive selection of CD4 T cells, but not CD8. How can we explain this? The kinetic signaling model of lineage commitment provides an explanation. In this model, the CD8 co-receptor is down regulated and the thymocyte determines if there is a persistent strong signaling. This would happen for class II restricted CD4 T cells. If the cell was class I restricted, it would require CD8, and in the absence of a strong persistent signal, the default pathway is to have co-receptor reversal. Because persistent signal is not required to positively select CD8SP T cells, the VGSC knockdown would not affect CD8SP selection. Thus, our finding the SCN5a/SCN4b is involved in strong sustained signaling and their mechanism of action differs between CD4 and CD8 is consistent with the kinetic signaling model.
Expression of SCN5a/SCN4b in peripheral T cells results in gain of function response to the positively selecting ligand
The tightly regulated expression of SCN5a/SCN4b empowers developing thymocytes to be highly sensitive to the relatively weak positive selecting ligand. The lack of expression of this VGSC in peripheral T cells would be predicted to prevent them from being autoreactive. To test this prediction, we transiently expressed SCN5a/SCN4b in peripheral AND T cells using GFP and DsRED to monitor expression. The SCN5a/SCN4b expressing cells now responded to gp250 as upregulating CD69. This gain of function response provides a clear explanation for the highly regulated expression of this VGSC.
Model for how a VGSC could induce a sustained Ca2+ flux
It is not known how Ca2+ enters thymocytes. The newly identified CRAC channels and STIM molecules do not appear to be involved since the knockouts of these channels/molecules have normal αβ T cell development. We propose a 3 stage model for how a VGSC could work. The TCR recognizes a positively selecting ligand, which results in the activation of the VGSC. This results in an influx of Na+ ion, depolarizing the membrane. This depolarization activates some voltage-gated Ca2+ channel, resulting in sustained Ca2+ entry. The identification of the voltage-gated Ca2+ channel involved in positive selection, and electrophysiology of the VGSC in thymocytes are the next frontiers in this field. Overall, our findings show that the expression of a VGSC in DP thymoyctes empowers them with greater sensitivity to recognize weak self-peptide/MHC ligands, resulting in sustained signaling and the maturation of the thymocytes into single positive T cells.
Future Perspectives
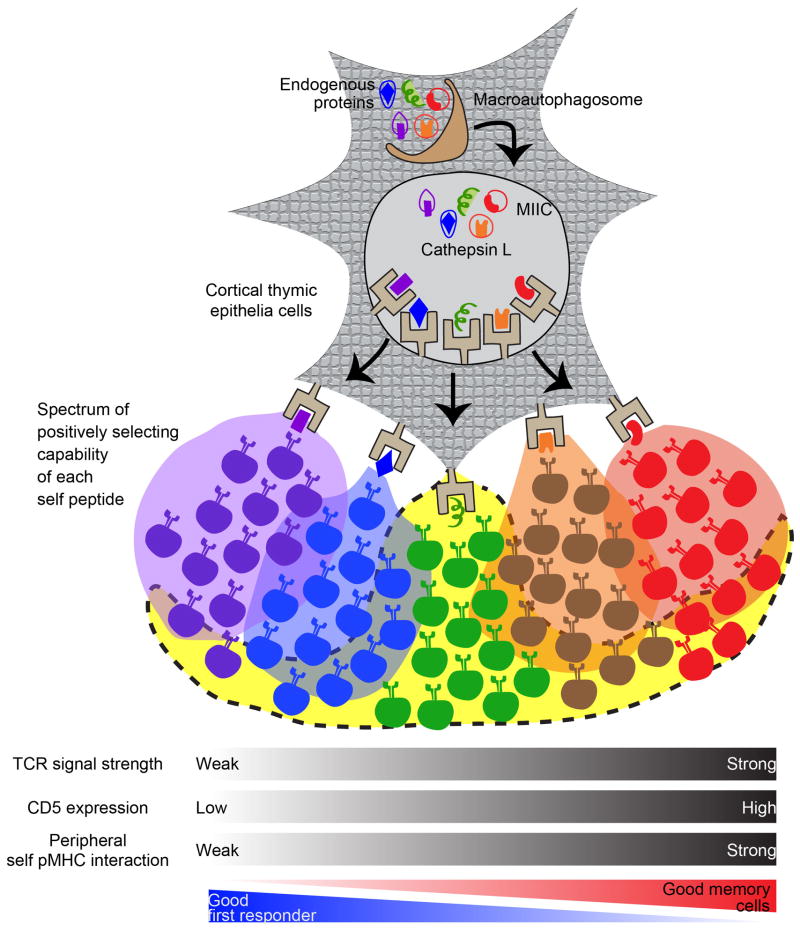
An open question is what is the relationship between the self-peptide pool and the T cell repertoire. Obviously there are many fewer self-peptide/MHC species displayed on a cTEC, than there are CD4 T cells. Therefore, each self-peptide must select hundreds or thousands of unrelated T cells. How then can we have specificity in positive selection as we observed for gp250 selection of AND T cells? An explanation of this is to propose that positive selection is not a simple binary “live or die” cell fate decision. It could be viewed as a “quality of life” decision, and that the strength of the TCR: self-peptide/MHC interaction is deterministic for the development and function of peripheral T cells (Figure 1). Thus, T cells on the high end of positive selection, as indicated by high CD5 levels, developing efficiently and make good memory T cells. T cells selected on the lower end of the spectrum do make it out into the periphery and participate in a primary response. We would propose that it is this type of T cell that are selected when there is only a single peptide/MHC molecule expressed in the thymus, thereby explaining why a single pMHC can select a large number of T cells. Also what needed to be incorporated in future studies would be where Tregs fits into this selection spectrum. The constitutively processing and presentation of self-proteins is critical for the development of an anticipatory immune system, and self-tolerance, and we are only becoming aware of how small nuanced changes in the handling of an self-protein by an APC can have profound effects on the development and function of an effective immune system.
Figure 1.
Proposed model for how the strength of the TCR: self-peptide interaction in the thymus dictates the function of peripheral T cells.
Highlights.
In this manuscript we briefly review the field of processing and presentation of self-proteins and then discuss the gp250/AND model of positive selection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Babbitt BP, Allen PM, Matsueda G, Haber E, Unanue ER. Binding of immunogenic peptides to Ia histocompatibility molecules. Nature. 1985;317:359–61. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009;10:1155–61. doi: 10.1038/ni.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo WL, Donermeyer DL, Allen PM. A voltage-gated sodium channel is essential for the positive selection of CD4(+) T cells. Nat Immunol. 2012;13:880–7. doi: 10.1038/ni.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz RG, Allen PM. Direct evidence for functional self-protein/Ia-molecule complexes in vivo. Proc Natl Acad Sci U S A. 1988;85:5220–3. doi: 10.1073/pnas.85.14.5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama Y, Takada K, Murata S, Tanaka K. beta5t-containing thymoproteasome: specific expression in thymic cortical epithelial cells and role in positive selection of CD8+ T cells. Curr Opin Immunol. 2012;24:92–8. doi: 10.1016/j.coi.2012.01.006. [DOI] [PubMed] [Google Scholar]