Abstract
During histogenesis of the vertebrate central nervous system (CNS), neuronal progenitors must interact with germinal zone (GZ) niches, differentiate, and morphologically mature, and neurons must migrate to their final positions. The extrinsic cues that control neurogenesis, specify neurons, and guide their movement are relatively well understood. However, less is known about how neurons spatiotemporally modify cell-cell interactions and cell polarization to navigate through complex, distinct cellular environments during neuronal circuit formation. Here we examine the parallels between the mechanisms controlling epithelial morphogenesis and the cell adhesion events by which neural cells organize GZ niches and direct neuronal migration. We will focus on the emerging relationship between neuronal adhesive interactions and conserved cell polarity signaling cascades.
Keywords: cell adhesion, adherens junction, cell polarity, PAR complex
Introduction
Epithelial morphogenic events (ie. orientated cell division, directed cell migration, differentiation, and overall tissue structure) are regulated largely by the fine-tuning of specialized cell junctions by polarity signaling cascades[1-4]. As cells of the CNS arise from a neuroepithelium, and brain morphogenesis is linked to the spatiotemporal interaction of immature neurons with neighboring cells, similar events may shape CNS morphogenesis. We will first describe the architecture of polarized epithelia and the molecular and cellular players that establish adhesive events and apicobasal polarity, then review recent studies that demonstrate similar mechanisms in neural development. As adhesion is only one aspect of neural development controlled by cell polarity during vertebrate brain morphogenesis, we direct readers to recent reviews examining the role of the same cascades in other key events, such as asymmetric cell division[5-8], axon-dendrite specification[9-11], and polarized trafficking[12-14].
Signaling cascades that drive epithelial adhesion and polarization
Epithelial cells possesses apical-basal polarity and a series of specialized junctions (Figure 1A) that underlie 1) individual cells’ participation in epithelial morphogenesis and 2) the vectorial flow of substances across the epithelial surface[1, 3, 15]. Apical-basal polarity is established by recruitment of lipids and cell surface proteins (e.g., ion channels, transporters, pumps) to membrane domains essential for the epithelium’s functional polarity. Each membrane domain possesses distinct cellular contacts: the basal surface contacts basement membranes through integrin-dependent adhesion, while the basolateral and apical domains are separated by adherens junctions (AJs, containing cadherins and nectins) and tight junctions (TJs, containing occludins, claudins, or junctional adhesion molecules [JAMs]) that make cell-cell contacts[3, 16].
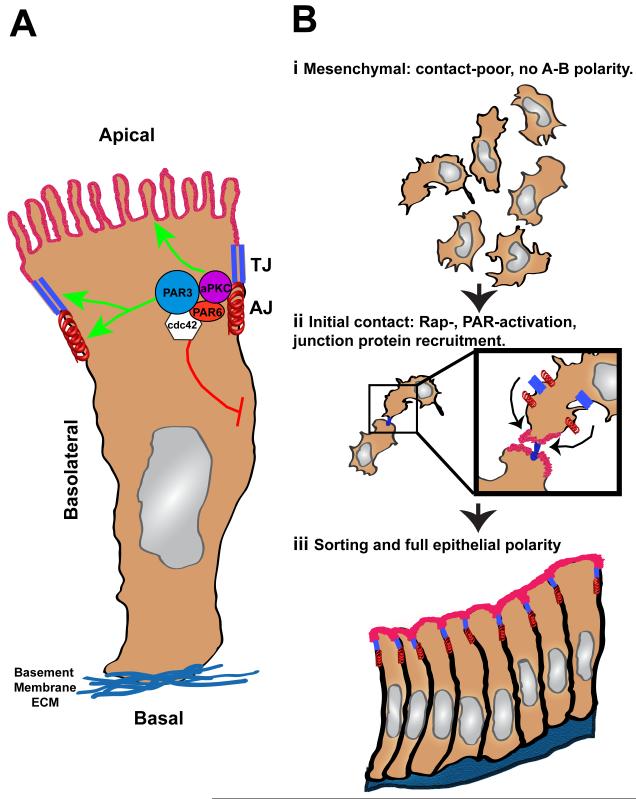
Figure 1.
Overview of epithelial polarity. (A) Polarized epithelial cells possess apical-basal polarity, in which an apical membrane (red) is separated from the basolateral and basal membranes (blue) by TJs (parallel blue rectangles) and AJs (red spring). While AJs and TJ represent cell-cell contact sites, the basal domain makes cell-matrix contacts through integrin receptor attachment to the basement membrane. The PAR complex (Pard3, Pard6, aPKC, cdc42) facilitates apical-basal polarity by promoting apical membrane specialization and TJ/AJ maturation (green arrows). The PAR complex also possesses inhibitory activities that limit the size of the basolateral membrane. (B) Primordial cell contacts promote MET. (i) Initially, epithelial cells in the mesenchymal state lack apical-basal (A-B) polarity and strong cell-cell contacts. (ii) Primordial contacts through adhesion receptors, like the nectins or JAMs, initiate a cascade of events, including Rap1 and PAR complex activation, that transform the primordial cell contacts into the nucleation site for TJ and AJ. (iii) Activation of the Rap1 and PAR complex signaling cascades, in cooperation with polarized trafficking events, promotes eventual full epithelial polarization.
Epithelial cells display remarkable plasticity of polarity during development[17-19]. They disconnect from neighboring cells, exit an epithelium via epithelial-mesenchymal transition (EMT), and reincorporate via mesenchymal-epithelial transition (MET). AJ formation during MET and its relation to key signal transduction cascades is a useful model for dissection of the mechanisms controlling polarity initiation (Figure 1B)[1]. Epithelial polarity in this context is initiated when nascent adhesions form, through molecules like nectins, linking cells that will incorporate into an epithelium and activating polarity-promoting signaling cascades. How do nascent cell contacts spur AJ formation and initiate epithelial polarity? The cytoplasmic domains of molecules at nascent adhesions act as scaffolds to recruit polarity-signaling molecules. For example, the nectin cytoplasmic domain provides a binding site for activators and effectors of the Ras-related protein 1 (Rap1) or Cell division control protein 42 (Cdc42) GTPases[20-24], which are essential for polarization and adhesion formation[25, 26].
Rap GTPases are key regulators of initial symmetry-breaking events[27]. The yeast Rap1 ortholog Ras-related 1 (Rsr1p) illustrates how Rap shapes the early phases of polarization[28, 29]. Rsr1p, which resides throughout the yeast plasma membrane, is locally activated by Bud site selection 5 (Bud5p), a Rsr1p GTP exchange factor (GEF) recruited by cell surface receptors near nascent bud sites. Activated Rsr1p then recruits the Cell division control protein 24 (cdc24) GEF to the bud site, leading to cdc42 GTPase activation and the site’s actin cytoskeleton–dependent maturation. In an analogous series of events, Rap1 is activated near nascent epithelial adhesions via recruitment of adaptor proteins such as afadin or postsynaptic density 95, PSD-85; discs large, Dlg; zonula occludens-1 (PDZ)-GEFs to the nectin or junctional adhesion molecules-A (JAM-A) cytoplasmic domains[30, 31] (Figure 2). While Rap1 activation promotes various pro-adhesive activities that recruit cadherins to AJs and JAMs or occludins to TJs [34, 35, 40-42], the available evidence suggests its function may not be strictly required for apical-basal polarization in metazoans. For example, Rap1-deficiency in Drosophila epithelial tissue severely impacts adhesion junction formation and structure without loss of apical or basal membrane specialization [32].
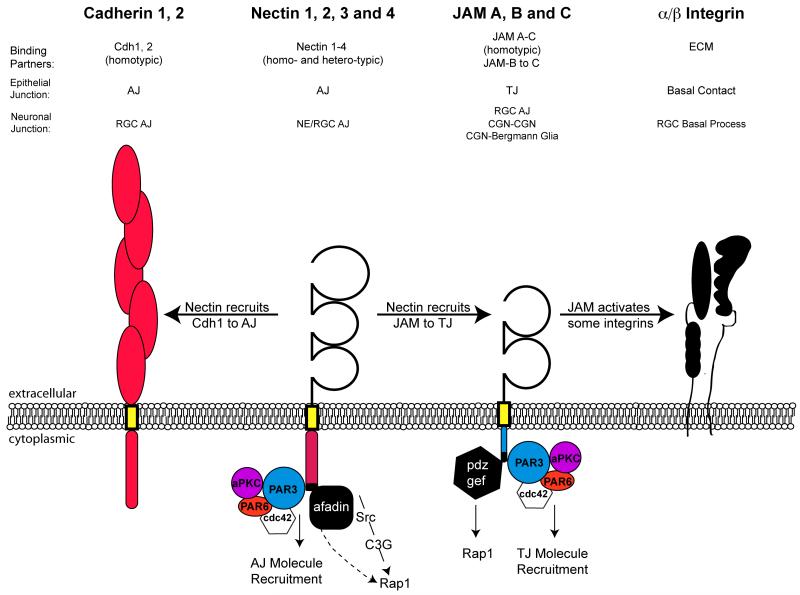
Figure 2.
Schematic illustrating common cell adhesion molecules involved in epithelial and neuronal cell adhesion. Four major adhesion molecules are recruited to both epithelial and neuronal cell contacts: Cadherins, Nectins, JAMs and Integrins. E- and N-cadherin are classic cadherin homotypic adhesion molecules possessing five extracellular cadherin domains (red ovals) that are localized to neuroepithelial and RGC AJs. Nectins possess three extracellular immunoglobulin domains (open loops) and a cytoplasmic domain that recruits Pard3, Afadin and serves as a scaffold for Rap1 activation. Cadherins are recruited to epithelial AJs by PAR complex recruitment and activated RAP1 signaling that are induced by primordial Nectin-based contacts. JAMs possess two extracellular immunoglobulin domains (open loops) and a cytoplasmic domain that recruits Pard3, PDZ-GEF and serves as a scaffold for Rap1 activation. JAMs are recruited to epithelial TJs by PAR complex recruitment and activated RAP1 signaling that are induced by primordial Nectin-based contacts. Integrins primarily bind extracellular matrix components (ECM) at the basal surface of epithelial cells and help anchor the basal process of RGC to the basement membrane produced by pial fibroblasts.
CDC42 and the evolutionarily conserved partitioning defective (PAR) proteins are perhaps the most crucial polarity signaling molecules recruited to nascent adhesions[16, 33-35]. The adaptor proteins Pard3 and Pard6 form a complex containing atypical protein kinase C (aPKC) and upstream activators, such as cdc42 and Rac1 Rho GTPases [35-40]. This multi-protein complex, first identified in C. elegans[41-44], is crucial for interpretation and execution of complex cell polarity programs, such as refinement of polarized actomyosin contractility, mitotic spindle orientation, front-to-rear polarization during directed cell migration, axon-dendrite specification, and epithelial polarization. Like Rap1, the PAR complex is recruited to nascent adhesions, perhaps through the interaction of Pard3 with nectin[22]. After activation of cdc42, the PAR complex near epithelial junctions supports diverse activities associated with apical-basal polarization and junctional maturation. First, it plays an integral role in apical-basal polarization, as aPKC defines and stabilizes the apical and basolateral membrane domains by phosphorylating and restricting the subcellular localization of members of the crumbs and scribble polarity complexes[45, 46]. Second, the PAR complex supports the maturation of AJs and TJs by promoting surface recruitment of the appropriate adhesion molecules to each junctional domain. Pard3 activity controls recruitment of E-cadherin to AJs[22, 47] and directly binds to the cytoplasmic domain of JAMs to regulate their recruitment to TJs[22, 48, 49].
Junctional transitions and brain morphogenesis
Parallels are increasingly found between junctional re-arrangements that shape developing epithelia and morphogenic events of nervous system development. This section will review some of the key transitions in junction formation and remodeling during brain morphogenesis; subsequent sections will describe how polarity-signaling cascades control junctions during neurogenesis and directed neuronal migration.
Early ultrastructural and more recent time-lapse imaging of the mouse neocortex revealed that an intricate balance of cell polarization and junction formation accompanies many key events during neural development (Figure 3). Radial glial cells (RGCs) in the ventricular zone (VZ) of the cortex, the progenitors of the neocortical output neurons, are morphologically polarized like epithelial cells. While they lack TJs[50], they possess apical cadherin-based AJs [51], strong apicobasal polarity, and a basal process that adheres through integrin-based junctions to the basal lamina produced by meningeal cells[52-54]. As RGCs divide asymmetrically to form transiently amplifying basal progenitors, signs of neuronal commitment include the loss of RGC apicobasal morphological characteristics, loss of AJ contacts with neighboring cells near the ventricular zone, and the appearance of a stellate multipolar morphology [55-57]. Polarized morphology is subsequently re-established, characterized by an organelle-enriched leading process containing the centrosome and Golgi complex, a thin axonal trailing process, and cell junctions (now neuron-glial), as the maturing neurons migrate radially to the upper neocortical layers [57-61]. It is worth noting that junctions ascribed to migrating neurons in early ultrastructural studies appeared as electron dense membrane specializations, later termed interstitial densities [61], that were not initially characterized at the molecular level and did not possess the same level of structural specialization of typical epithelial junctions but appeared more frequently at neuron-glial interfaces (as opposed to interfaces with neighboring axonal elements, see discussion in [59]). Finally, pyramidal neurons are sorted into their appropriate lamina, and their apical dendrites are polarized under the control of contacts between cortical plate (CP) neurons and the basal lamina extracellular matrix (i.e., laminin) or secreted signaling molecules (i.e., reelin) produced by pial cells and layer 1 neurons [62].
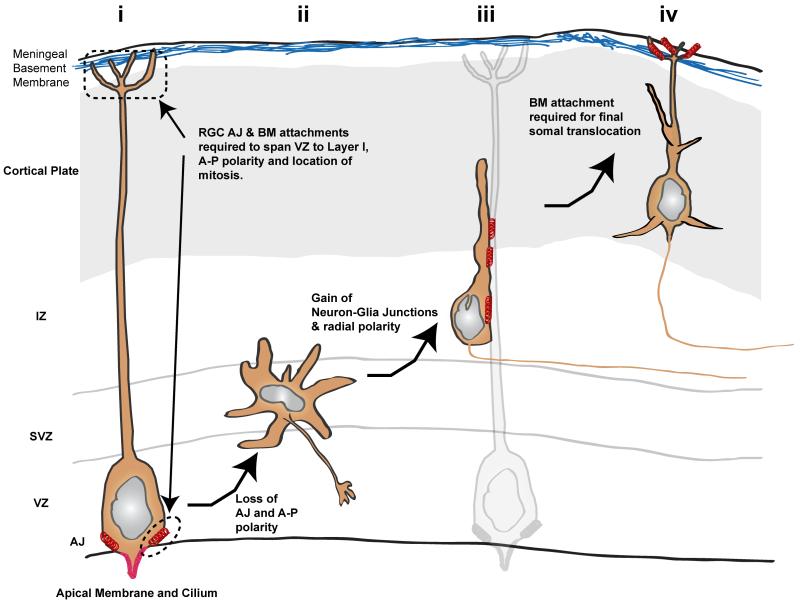
Figure 3.
Schematic illustrating junctional transition, using the developing mouse neocortex as a model. (i) Like epithelial cells, ventricular zone (VZ) RGCs possess strong apical-basal polarity. Their apical AJ contacts and adhesion to basement membrane produced by meningeal cells allow RGCs to span the width of the neocortex. (ii) Both AJs and apical-basal polarity are lost in multipolar neurons in the sub-ventricular zone (SVZ). (iii) Radial migration polarity and neuron-glial cell-cell junctions are re-established in neurons migrating along glial fibers. (iv) Finally, as maturing pyramidal neurons enter the cortical plate, they make Reelin- and N-cadherin–dependent contacts with the marginal zone, facilitating the somal translocation phase of migration. Red springs denote AJ, neuron-glial, or neuron-marginal zone contacts, most of which contain N-cadherin.
Aspects of this developmental sequence may be conserved in many regions of the vertebrate nervous system. Most RGCs display well defined epithelial-like polarity, and loss of junctional contact has been ultrastructurally observed in transiently amplifying progenitors in the retina [63] and spinal cord [64]. Cerebellar granule neuron precursors (GNPs) in the external granule layer (EGL) - another well studied model of neuronal differentiation and migration - also proceed through an unpolarized state. That is to say, they have limited contact with neighboring neurons or glia, before forming developmentally regulated neuron-glial junctions that facilitate their movement to the internal granule layer (IGL) and acquisition of the polarized, mature T-shape [65, 66].
Regulation of cell-cell junctions by polarity signaling shapes GZ structure and controls neurogenesis
Neurogenesis and subsequent stratification of the cortex are highly ordered events that require utmost precision in the timing and type of division of neuroepithelial cells and RGCs. Neuroepithelia initially divide symmetrically to generate a pool of neural stem cells. RGCs undergo asymmetric division to generate one daughter cell that remains a stem cell and another that differentiates. This strategy ensures that RGCs remain proliferative during later development and can still generate the latest-born neurons that occupy cortical laminae.
Neurogenesis of the vertebrate CNS depends on the formation of specialized junctions by neuroepithelial cells and RGCs to regulate the structure and lamination of GZs. A ring of AJs is formed at the apical membrane [50, 51], while the basal connections are required to ensure that RGC fibers span the entire cortical wall to serve as a template for subsequent neuronal migration [53]. The basal and apical attachments remain throughout the cell cycle, including mitosis [67, 68]. The site of mitosis is critical to maintain proper ratios of newborn neurons to proliferating progenitors. In the VZ, RGCs undergo mitosis exclusively at the apical membrane, ensuring that progenitor pools are maintained through asymmetric or symmetric progenitor division. At more basal locations, mitosis is more likely to be symmetric, thus depleting the progenitor pool. This spatial restriction is regulated by the process of interkinetic nuclear migration (IKNM) [69], in which the nucleus shuttles from the basal to the apical end of the cytoplasm during S/G2/M phase and returns to a more basal location upon re-entry into G1 [70]. IKNM results in neuroepithelial pseudostratification, in which nuclei reside in different layers but mitosis is restricted to the apical surface. IKNM requires establishment and maintenance of AJs and apicobasal polarity to ensure progenitor division at the apical surface [71-74].
Cdc42 and the PAR complex regulate RGC AJs during neocortex development
How are the AJs of neuroepithelial cells and RGCs controlled? As in epithelial cells, cdc42 occupies the apical surface in the neuroepithelium and RGCs. Conditional knockout of cdc42 results in major disruption of neuroepithelial architecture due to loss of PAR complex at AJs [71]. This disruption, the loss of AJs, improper IKNM, and increased early neurogenesis and basal mitosis phenotypically recapitulate the loss of PAR complex function [71, 75], underlining the indispensability of PAR-mediated establishment and maintenance of apicobasal polarity during neurogenesis. Finally, it was recently shown that RGCs are dynamic in vivo, interacting with each other via membrane protrusions and via growth cone–like end-feet at the apical and basal surfaces [76]. These interactions require cdc42 and GSK-3β, although the two appear to function independently. Polarity complexes may regulate not only polarity but also interactions between RGCs.
Like cdc42, the PAR complex is recruited to the apical membrane and AJs of RGCs [72, 77]. Down-regulation of Pard3 or Pard6 reduces proliferation and increases neuronal differentiation, while up-regulation has the opposite effect, causing mainly symmetrical division and increasing the progenitor pool [72, 77]. In vivo, Pard3 was asymmetrically inherited in ~ 50% of RGC divisions, consistent with the resulting ratio of neurons to progenitors [72]. Two studies have shed light on the apical recruitment of PAR complex in the developing neocortex. The first report demonstrated that ASPP2, a p53-interacting protein, interacts with Pard3 and functions in its recruitment to AJs [73]. Loss of ASPP2 results in loss of apical Pard3 and failure to form tight junctions. Loss of ASPP2 function led to gross disorganization of the neuroepithelium, loss of radial organization of RGCs, disruption of IKNM leading to more basal cell divisions, and ultimately a loss of later born neurons. The loss of ASPP2 also led to an increased progenitor pool due to a shortened cell cycle, which was offset by increased apoptosis [73]. The second report showed that myristolated alanine-rich C-kinase substrate protein (MARCKS) plays a role in recruitment of Pard3 to the apical domain of neuroepithelial cells. MARCKS−/− neuronal progenitors lack numerous apically-localized polarity proteins and no longer divide exclusively in the VZ, thus causing disorganization of the neuroepithelium and improper lamination [78]. Although MARCKS is a prominent substrate of PKC, myristoylation or membrane targeting of MARCKS is responsible for its function. Membrane targeting of MARCKS aids recruitment of Pard3 [78] and other cytoskeletal modulatory components [79], a combination that contributes toward AJ formation and subsequent apicobasal polarity.
In general, PAR complex–mediated apicobasal polarity is established and maintained through AJs at the apical surface. Although conditional knockout of the PAR component atypical PKC λ (aPKCλ) resulted in the loss of AJs at embryonic day 15.5 (E15.5), neurogenesis remained unaffected - despite a disorganized neuroepithelium and defects in IKNM [74]. This suggests that while early neurogenesis is dependent on apicobasal polarity, as outlined above, later developmental time points (E15.5 and onward) may be independent of AJ-mediated polarity.
While each of these studies defines roles for Cdc42 and the PAR complex in AJ regulation and GZ cell positioning through apical attachments and apicobasal polarity, it is unclear if AJ formation and the initiation of apical-basal polarity involves a similar set of primordial cell contacts demonstrated for epithelial cells. Although Nectins accumulate at neuroepithelial AJs in a complex with Pard3[80] and junctional adhesion molecule-C (JAM-C) is localized to RGC apical membrane and AJs [81], no studies have directly examined if these adhesion molecules support AJ formation that support apical-basal polarization. Intriguingly, Afadin deletion disrupts junctional organization and apical-basal polarization in neuroepithelial cells [82], suggesting deeper parallels to epithelial AJ formation may exist in the developing brain.
Notch Signaling is Downstream of the PAR complex in the developing neocortex
Until recently, the downstream molecular pathways downstream of AJ components that directly regulate progenitor fate have remained largely unknown. Interestingly, a connection between the PAR polarity complex and the cell fate determinant Notch may point to a downstream pathway for apicobasal polarity signaling in RGCs. Notch activation inhibits cell differentiation, and not surprisingly, Notch reporter constructs reveal that the VZ has the highest level of Notch activity in the developing neocortex. Notch activation is a prime candidate for the molecular mechanism driving asymmetric RGC division. Recent work in mice and zebrafish showed that Notch activity is directly regulated by Pard3 [72, 83]. Increased PAR complex activity increases Notch activation and a progenitor cell fate. Conversely, loss of Pard3 function decreases Notch activity and promotes neuronal differentiation. Mechanistically, the connection between Notch and the PAR complex involves the direct interaction of Pard3 and the Notch inhibitors Numb and Numbl. Regulation of Notch function via Pard3 levels is directly dependent on Numb and Numbl [72], which Pard3 likely sequesters from Notch. Numb and Numbl also directly influence the maintenance of cadherin-based AJs. Loss of Numb and Numbl results in neuroepithelial disorganization, like loss of PAR complex function [84]. Alternatively, Pard3-associated Numb and Numbl may contribute to AJ maintenance independently of their Notch regulatory activities [85, 86].
Adhesion control of substrate selectivity during GZ exit and migration
Regulated adhesion of neurons to substrates has been assumed to be important in guiding their migration since electron microscopy (EM) and Golgi staining studies first demonstrated the close association of neurons and glial fibers on this journey [58, 59, 87]. High-resolution time-lapse microscopy followed by EM examination of migrating cerebellar granule neurons (CGNs) demonstrated that neuron-glial adhesion junctions are dynamically remodeled as a neuron migrates along a glial fiber and that the junctions of neurons not actively migrating differ qualitatively from neuron-glial adhesion junctions [61]. The number of molecules that mediate this tightly regulated interaction has increased with the identification of astrotactin [88, 89], connexin 43 [90], neuregulin [91], and α3β1 or αV integrins [92]. However, it remains unknown how adhesion is rapidly and reversibly regulated, as the known mechanisms are too slow to account for dynamic adhesive events. Below we discuss recent developments showing dynamic regulation of cell-cell adhesion controls key transitions during GZ exit, pathway selection during neuronal migration and ultimately lamination. While neuron-neuron or neuron-glial junctions lack the classical ultra-structural organization of epithelial junctions [58, 59, 61], the paradigm emerging in the field is not only do these junctions contain molecules classically recruited to epithelial adhesions (e.g. cadherins and JAM) but also the cell-cell interactions containing these molecules are regulated by a set of conserved cell polarity signaling cascades, much as they are in epithelial cells.
Rap1 and N-cadherin regulate lamination of the developing neocortex
N-cadherin, a classical homotypic cadherin, is a major adhesion molecule in RGC apical junctions, developing excitatory synapses, neuronal growth cones, developing neuronal processes, and newly described junctions between pyramidal cell apical processes and the neocortical marginal zone (MZ)[51, 62, 84, 93, 94]. CNS deletion of N-cadherin causes disorganization of many brain regions, indicating that N-cadherin is essential for multiple adhesive events in CNS morphogenesis[95]. Given N-cadherin’s diverse adhesive activities, it has been challenging to dissect how its specificity is controlled for discrete morphogenic events. Two recent publications report that Rap1 GTPase activity regulates N-cadherin adhesive function during lamination of the mouse neocortex[62, 96].
During neocortical lamination, pyramidal neurons derived from RGCs undergo three discrete migration phases: multipolar migration in the SVZ, glial-guided locomotion through the intermediate zone, and a glial-independent terminal translocation that fine-tunes the layer-specific position in the CP[56, 57, 97]. Electroporation of Rap1 loss-of-function and Rap1 GAP gain-of-function constructs into developing mouse neocortex showed that Rap1 activity is required for appropriate CP positioning [62, 96]. Application of these tools at key neocortical development stages revealed stage-specific Rap1 function. Rap1 inhibition early in development showed that Rap1 is required for glial-independent terminal translocation, the predominant migration mode before neocortical expansion. At later stages, Rap1 activity is required for the transformation of multipolar neurons into radially oriented neurons that locomote along glial fibers, but not for locomotion itself. Rap1 inhibition at both stages eventually arrested neurons in a stellate morphology, with loss of normal apical process positioning and subcellular organelle orientation. Therefore, when Rap1 activity is lost, pyramidal neurons may be unable to undergo discrete polarity transitions at the multipolar and translocation phases.
How does Rap1 activity regulate the positioning of neocortical neurons? Both Rap1 and N-cadherin are required for terminal somal translocation in early neocortical development, multipolar-to-locomotion transformation, and apical process/organelle polarization, as over-expression of dominant-negative N-cadherin and acute N-cadherin silencing perturbs these events. The Rap1 loss-of-function phenotype at both the multipolar and translocation phases was rescued in vivo by over-expression of wild-type N-cadherin, suggesting that Rap1 is functionally linked to the specificity of N-cadherin adhesion. It has been proposed that Rap1 controls N-cadherin adhesive strength, as Rap1 loss of function 1) inhibits cortical neuron attachment to a pure N-cadherin substrate in a manner rescued by additional N-cadherin expression and 2) misdirects N-cadherin from the neuronal cell membrane[96]. While the cell biological mechanism downstream of Rap1 controlling N-cadherin recruitment remains unexplained, regulated N-cadherin endocytosis has been proposed to control neocortical interactions with RGCs [98]. Additional findings support the hypothesis that N-cadherin surface expression is required for initial polarization of CNS neurons[94]. Ectopic N-cadherin can induce generation of the first neurite, marking the transition from multipolar to bipolar migratory morphology. Thus, N-cadherin surface recruitment and adhesion, controlled by Rap1, may initiate a cascade of events that facilitates neuronal polarization during the multipolar and translocation phases, a process mechanistically similar to Rap1’s role of recruiting cadherins to AJs during epithelial polarization and MET.
The Reelin pathway has been implicated as a major cell-extrinsic factor controlling Rap1 activity. Reelin, which is produced by marginal zone cells and signals to migrating neurons though apoE receptor type 2 (Apoer2), very low density lipoprotein receptor (Vldlr), and Disabled-1 (Dab1), is a well-studied regulator of neuronal lamination[99, 100]. Various mechanisms have been proposed for Reelin’s action during lamination[101, 102]. Two recent studies used genetic, cell biology, and gene silencing tools to disrupt Apoer2, Vldlr, and Dab1 signaling; their findings indicate that Reelin regulates Rap1 activation and N-cadherin function [62, 96]. While the two groups differ on whether Reelin acts during the somal translocation or multipolar migration phase, they agree that Reelin activates the Rap1 pathway.
Control of JAM-C adhesion by PAR complex ubiquitination
Developing CGNs are an excellent model of the mechanisms regulating GZ exit and migration pathway selection, as they undergo two migration phases [103, 104]: (i) tangential migration near the cerebellar surface, followed by (ii) radial migration away from the EGL and then across the molecular layer (ML) to the IGL. Available evidence suggests that the differentiation and polarization status of CGNs is linked to their layer within the cerebellar cortex and their migration pathway selection. Unpolarized GNPs and nascent CGNs primarily migrate within the EGL but do not migrate radially. However, more mature CGNs migrate radially after terminal differentiation and this migration depends on PAR complex activity, as gain or loss of Pard6α function blocks radial migration in vitro and ex vivo by perturbing actomyosin organization and the two-stroke centrosome/nucleokinesis cycle [105, 106].
The emerging parallels between polarity-dependent adhesion control during epithelial polarization and brain morphogenesis are further supported by a recent study of the initiation of CGN radial migration. Specifically, an additional level of PAR complex involvement in CGN development has been reported [107]. Pard3A protein is expressed meagerly in immature CGNs in the EGL, where tangential migration predominates, but heavily in CGNs near the ML or migrating through it. Pard3A gain of function was shown to drive precocious radial migration, whereas loss of function arrested migration initiation [107]. Furthermore, Pard3A-deficient CGNs were motile but displayed random migration directionality and could not transition from tangential to radial migration in ex vivo cerebellar slices. What controls Pard3A expression during CGN differentiation? This same study reported that Pard3A is a target of the E3 ubiquitin ligases Siah (mouse seven in absentia homolog) 1 and 2[107], the vertebrate orthologs of the Drosophila sina gene[108]. Both Siah1 and 2 bind to Pard6 and Pard3A. Pard3A is degraded by Siah1/2 and is the only PAR complex member containing a Siah degron sequence. Siah2 expression and Siah activity are high in the GNPs of the outer EGL and diminish rapidly in newly differentiating CGNs. As a negative regulator of Pard3A, Siah gain of function inhibits GZ exit and radial migration initiation, which are rescued by Pard3A re-expression, while Siah2 loss of function promotes these activities.
Dynamic regulation of adhesion through JAM-C underlies Pard3A’s ability to control CGN migration. JAM-C, a member of the immunoglobulin superfamily, is an essential component of epithelial TJs. Earlier studies reported that Pard3A binds in a PDZ domain–dependent manner to the cytoplasmic domains of three members of the JAM family in epithelial cells [48, 109]. Subsequent studies showed that Pard3A and JAM are essential for TJ formation and that JAM recruitment requires Pard3A function[22]. Consistent with these earlier findings in epithelia, JAM-C and Pard3A are recruited to identical locations in the CGN leading process. Moreover, not only is the spatio-temporal expression of JAM-C and Pard3A identical in developing CGNs but also JAM-C adhesion is necessary and sufficient for CGN GZ exit and radial migration initiation. Interestingly, the regulatory interaction originally identified in epithelial cells appears to be conserved. In particular, Pard3A activity is essential to recruit JAM-C to neuron-neuron or neuron-glial cell contacts and disruption of Pard3A/JAM-C interaction or Siah gain of function blocks CGN movement to the IGL in a JAM-C dependent manner [107]. Thus, while neurons lack the classical TJs of an epithelial cell, Pard3A’s pro-adhesive function through JAM-C facilitates the PAR complex-dependent integration of new neurons into the developing cerebellar cortex.
Importantly, the Siah/Pard3A/JAM-C pathway is active not only in CGNs; Siah gain-of-function in MDCK polarized epithelial cells dissolved JAM-C–labeled tight junctions, and this effect was rescued by Pard3A re-expression [107]. Thus, the antagonistic interaction between Siah and Pard3A functions identically in neurons and epithelial cells as the competitive balance between Siah and Pard3A remodels cell-cell adhesions (Figure 4).
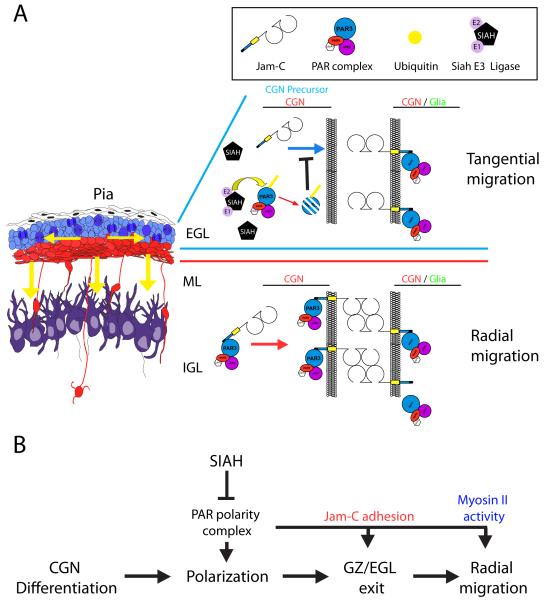
Figure 4.
Model of Siah E3 ligase regulation of germinal zone exit. (A) During cerebellar development, CGN precursors migrate tangentially within the EGL. Upon differentiation and polarization, CGNs exit the GZ/EGL and migrate radially to traverse the ML and assume their final position in the IGL. Within the developing postnatal cerebellum, Siah (E3 ubiquitin ligase) is highly expressed in the EGL, where it ubiquitinates Pard3A, targeting it for proteasome-mediated degradation, which inactivates the PAR polarity complex. This inactivation inhibits recruitment of the JAM-C adhesion molecule to glial cell contacts with CGNs and CGN precursors. The absence of JAM-C–mediated adhesion prevents GZ exit by restricting the radial migration of CGN precursors. (B) The PAR polarity complex is necessary to allow differentiated CGNs to polarize, exit the GZ via JAM-C–mediated adhesion, and migrate radially via activation of the myosin II motor. Siah negatively regulates CGN polarization, GZ exit, and radial migration by inactivating the PAR polarity complex.
Conclusions and future perspectives
As cells of the nervous system arise from a neuroepithelium, various epithelial metaphors have been proposed to explain aspects of neuronal polarization. While most of these models were limited, there is mounting evidence of parallels in the control of adhesive properties by conserved cell polarity signaling cascades in both neurons and epithelial cells. These parallels present a series of outstanding questions (see Box 1) and new lines for experimentation: What is the nascent adhesion/polarity–initiating equivalent of nectins in neurons? How are negative regulators of polarity (e.g. Siah activity) extrinsically controlled? How do polarity signaling cascades regulate adhesion receptor trafficking? Do adhesive events play a role in more classical aspects of neuronal polarization such as axon/dendrite specification? And finally, can firmer parallels be drawn between the polarity transitions experienced by neurons and the EMT and MET events that regulate plasticity in epithelial polarity (see Figure 5)? These are the remaining fundamental challenges in our understanding of the organization of GZs and the control of adhesive specificity by neurons migrating to their final positions.
Box 1. Outstanding questions.
How do we reconcile the functions of AJs during neurogenesis with those of mitotic spindle orientation and the primary cilia?
Besides the interface of Notch signaling with the PAR complex, there are two relatively unexplored mechanisms for the effect of junctional complexes on neurogenesis. First, junctions are known to play a critical role in orientating epithelial mitotic spindles during cell fate acquisition [110]. While there is extensive evidence that spindle orientation is related to the neurogenic output of RGC divisions [111] and that disruption of apical polarity complex components alters junctions or the orientation of RGC divisions, no direct link between junctions and RGC spindle orientation has been demonstrated. Second, the regulation of primary cilia location via junctions could influence neurogenesis. Primary cilia are usually anchored at the RGC apical membrane where they are exposed to cerebrospinal fluid[112], containing mitogenic stimuli [113]. Loss of AJs or polarity components frequently leads to apical process retraction; thus, perturbation of apical anchoring could impact neurogenesis by displacing the primary cilia from a mitogen-rich environment.
How are AJs disassembled during neuronal differentiation?
While it is clear that progeny of neuroepithelial cells and RGCs lose AJs and apical-basal polarity as they transition toward neuronal cell fates and delaminate from germinal zones, there is little insight into how AJ disassembly is controlled. A recent study showed that FoxP4 expression during this transition in spinal cord neuroepithelial progenitors inhibits N-cadherin adhesion, suggesting that AJ disassembly and delamination are active processes during neurogenesis [114]. In future studies, it will be important to determine whether similar mechanisms occur during neurogenesis in the brain. Moreover, given that perturbation of polarity signaling components frequently induces delamination phenotypes (i.e., AJ loss, apical process retraction, and cell displacement to superficial layers of the cerebral cortex), it will also be important to determine how signals that naturally induce AJ loss and delamination impinge on components like the PAR complex.
How do we reconcile the pro-adhesive function of PAR complex with its role in nucleokinesis?
During the two-stroke nucleokinesis cadence in migrating neurons - in which centrosomes enter the leading process before somal translocation - the PAR complex has been shown to contribute to centrosome positioning and subsequent somal translocation by controlling leading-process actomyosin [106]. An important question is how the newly discovered pro-adhesive functions of the PAR complex relate to previously established roles in nucleokinesis. At first glance, the functions of the PAR complex in actomyosin cytoskeletal dynamics, cell-cell adhesion, and centrosome positioning have little in common. Advances in general cell motility suggest that actomyosin acts in the leading portion of fibroblasts, called the lamellum, where contractile forces generated forward of the nucleus are required for adhesion maturation, the positioning of microtubule arrays, and cell body motility [115]. If the proximal leading process of migrating neurons (enriched for actomyosin, the PAR complex, and PAR- complex dependent adhesion molecules) is analogous to the lamellum, then the PAR complex may be well positioned to integrate the contractile and pro-adhesive functions of actomyosin.
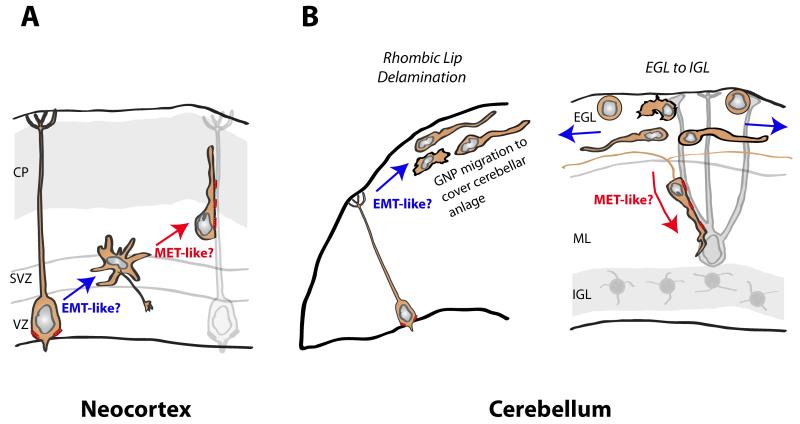
Figure 5.
Potential EMT and MET parallels in the developing neocortex and cerebellum. (A) In the neocortex, the transition between RGC polarity and the multipolar state may be EMT-like due to loss of junctions and apical-basal polarity, as proposed recently [116]. Transition between the multipolar state and radial migration may be MET-like as cell-cell junctions are re-established. Moreover, radial polarity may be associated with establishment of axonal and dendritic domains, a hallmark of classical neuronal polarity. (B) In the developing cerebellum, the delamination of GNPs from RGC progenitor cells in the rhombic lip may be EMT-like, as is the delamination of neural crest cells (another dorsal neural tube–derived cell type). Moreover, the transition from tangential migration within the EGL by GNPs and nascent CGNs (blue arrows depict migration paths) to radial migration by polarized T-shaped CGNs may be MET-like. As stated in the text, radial migration polarity, characterized by parallel fiber axons and prominent neuron-glial connections containing JAM-C, is stimulated by Pard3A activity. Red springs denote AJ and neuron glial junctions.
Acknowledgements
We thank Niraj Trivedi and Shalini Singh for critical reading of the manuscript. Sharon Naron provided expert editorial support. The Solecki Laboratory is funded by the American Lebanese Syrian Associated Charities (ALSAC), by grant #1-FY12-455 from the March of Dimes and by grant 1R01NS066936 from the National Institute of Neurological Disorders and Stroke (NINDS). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the National Institutes of Health. J.K.F. is the recipient of a Canadian Institutes of Health Research and Alberta Innovates Health Solutions postdoctoral fellowships.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nelson WJ. Remodeling epithelial cell organization: Transitions between Front-Rear and Apical-Basal polarity. Cold Spring Harbor Perspectives in Biology. 2009;1:a000513. doi: 10.1101/cshperspect.a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Etienne-Manneville S. Control of polarized cell morphology and motility by adherens junctions. Seminars in cell & developmental biology. 2011;22:850–857. doi: 10.1016/j.semcdb.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 3.Johnston DS, Sanson B. Epithelial polarity and morphogenesis. Current opinion in cell biology. 2011;23:540–546. doi: 10.1016/j.ceb.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Laprise P, Tepass U. Novel insights into epithelial polarity proteins in Drosophila. Trends in Cell Biology. 2011;21:401–408. doi: 10.1016/j.tcb.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Siller KH, Doe CQ. Spindle orientation during asymmetric cell division. Nature cell biology. 2009;11:365–374. doi: 10.1038/ncb0409-365. [DOI] [PubMed] [Google Scholar]
- 6.Knoblich JA. Asymmetric cell division: recent developments and their implications for tumour biology. Nature reviews. Molecular cell biology. 2010;11:849–860. doi: 10.1038/nrm3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fietz SA, Huttner WB. Cortical progenitor expansion, self-renewal and neurogenesis-a polarized perspective. Current opinion in neurobiology. 2011;21:23–35. doi: 10.1016/j.conb.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Lui JH, et al. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes AP, Polleux F. Establishment of axon-dendrite polarity in developing neurons. Annu Rev Neurosci. 2009;32:347–381. doi: 10.1146/annurev.neuro.31.060407.125536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Insolera R, et al. Par proteins and neuronal polarity. Developmental neurobiology. 2011;71:483–494. doi: 10.1002/dneu.20867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de la Torre-Ubieta L, Bonni A. Transcriptional regulation of neuronal polarity and morphogenesis in the mammalian brain. Neuron. 2011;72:22–40. doi: 10.1016/j.neuron.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasiecka ZM, Winckler B. Mechanisms of polarized membrane trafficking in neurons -- focusing in on endosomes. Molecular and cellular neurosciences. 2011;48:278–287. doi: 10.1016/j.mcn.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu C, Barry J. Function and mechanism of axonal targeting of voltage-sensitive potassium channels. Progress in neurobiology. 2011;94:115–132. doi: 10.1016/j.pneurobio.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kennedy MJ, Ehlers MD. Mechanisms and function of dendritic exocytosis. Neuron. 2011;69:856–875. doi: 10.1016/j.neuron.2011.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nature reviews. Molecular cell biology. 2008;9:29563–29571. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. The Journal of Cell Biology. 2011;192:907–917. doi: 10.1083/jcb.201009141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 18.Chaffer CL, et al. Mesenchymal to epithelial transition in development and disease. Cells Tissues Organs. 2007;185:7–19. doi: 10.1159/000101298. [DOI] [PubMed] [Google Scholar]
- 19.Baum B, et al. Transitions between epithelial and mesenchymal states in development and disease. Seminars in cell & developmental biology. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Takai Y, et al. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer science. 2003;94:655–667. doi: 10.1111/j.1349-7006.2003.tb01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuyama T, et al. Involvement of the c-Src-Crk-C3G-Rap1 signaling in the nectin-induced activation of Cdc42 and formation of adherens junctions. The Journal of biological chemistry. 2005;280:815–825. doi: 10.1074/jbc.M411099200. [DOI] [PubMed] [Google Scholar]
- 22.Ooshio T, et al. Cooperative roles of Par-3 and afadin in the formation of adherens and tight junctions. Journal of cell science. 2007;120:2352–2365. doi: 10.1242/jcs.03470. [DOI] [PubMed] [Google Scholar]
- 23.Kuramitsu K, et al. Novel role of nectin: implication in the co-localization of JAM-A and claudin-1 at the same cell-cell adhesion membrane domain. Genes to cells. 2008;13:797–805. doi: 10.1111/j.1365-2443.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- 24.Takai Y, et al. Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nature reviews. Molecular cell biology. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 25.Gerard A, et al. The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. The Journal of Cell Biology. 2007;176:863–875. doi: 10.1083/jcb.200608161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iden S, Collard JG. Crosstalk between small GTPases and polarity proteins in cell polarization. Nature reviews. Molecular cell biology. 2008;9:846–859. doi: 10.1038/nrm2521. [DOI] [PubMed] [Google Scholar]
- 27.Bos JL, et al. Rap1 signalling: adhering to new models. Nature reviews. Molecular cell biology. 2001;2:369–377. doi: 10.1038/35073073. [DOI] [PubMed] [Google Scholar]
- 28.Casamayor A, Snyder M. Bud-site selection and cell polarity in budding yeast. Current opinion in microbiology. 2002;5:179–186. doi: 10.1016/s1369-5274(02)00300-4. [DOI] [PubMed] [Google Scholar]
- 29.Gloerich M, Bos JL. Regulating Rap small G-proteins in time and space. Trends in Cell Biology. 2011;21:615–623. doi: 10.1016/j.tcb.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Boettner B, Van Aelst L. The Rap GTPase activator Drosophila PDZ-GEF regulates cell shape in epithelial migration and morphogenesis. Molecular and cellular biology. 2007;27:7966–7980. doi: 10.1128/MCB.01275-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severson EA, et al. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Molecular biology of the cell. 2009;20:1916–1925. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knox AL, Brown NH. Rap1 GTPase regulation of adherens junction positioning and cell adhesion. Science. 2002;295:1285–1288. doi: 10.1126/science.1067549. [DOI] [PubMed] [Google Scholar]
- 33.Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Current opinion in cell biology. 2008;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Nance J, Zallen JA. Elaborating polarity: PAR proteins and the cytoskeleton. Development. 2011;138:799–809. doi: 10.1242/dev.053538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohno S. Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Current opinion in cell biology. 2001;13:641–648. doi: 10.1016/s0955-0674(00)00264-7. [DOI] [PubMed] [Google Scholar]
- 36.Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol. 2003;15:67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 37.Munro EM. PAR proteins and the cytoskeleton: a marriage of equals. Curr Opin Cell Biol. 2006;18:86–94. doi: 10.1016/j.ceb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Joberty G, et al. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- 39.Hurd TW, et al. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- 40.Brazil DP, Hemmings BA. Cell polarity: Scaffold proteins par excellence. Current biology: CB. 2000;10:R592–594. doi: 10.1016/s0960-9822(00)00635-7. [DOI] [PubMed] [Google Scholar]
- 41.Hung TJ, Kemphues KJ. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development. 1999;126:127–135. doi: 10.1242/dev.126.1.127. [DOI] [PubMed] [Google Scholar]
- 42.Kemphues KJ, et al. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- 43.Tabuse Y, et al. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125:3607–3614. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- 44.Watts JL, et al. par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development. 1996;122:3133–3140. doi: 10.1242/dev.122.10.3133. [DOI] [PubMed] [Google Scholar]
- 45.Plant PJ, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- 46.Hutterer A, et al. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Developmental cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Simoes Sde M, et al. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Developmental cell. 2010;19:377–388. doi: 10.1016/j.devcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebnet K, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iden S, et al. aPKC phosphorylates JAM-A at Ser285 to promote cell contact maturation and tight junction formation. The Journal of cell biology. 2012;196:623–639. doi: 10.1083/jcb.201104143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aaku-Saraste E, et al. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure--remodeling of the neuroepithelium prior to neurogenesis. Developmental biology. 1996;180:664–679. doi: 10.1006/dbio.1996.0336. [DOI] [PubMed] [Google Scholar]
- 51.Chenn A, et al. Intrinsic polarity of mammalian neuroepithelial cells. Molecular and cellular neurosciences. 1998;11:183–193. doi: 10.1006/mcne.1998.0680. [DOI] [PubMed] [Google Scholar]
- 52.Graus-Porta D, et al. Beta1-class integrins regulate the development of laminae and folia in the cerebral and cerebellar cortex. Neuron. 2001;31:367–379. doi: 10.1016/s0896-6273(01)00374-9. [DOI] [PubMed] [Google Scholar]
- 53.Belvindrah R, et al. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27:13854–13865. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Radakovits R, et al. Regulation of radial glial survival by signals from the meninges. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:7694–7705. doi: 10.1523/JNEUROSCI.5537-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shoukimas GM, Hinds JW. The development of the cerebral cortex in the embryonic mouse: an electron microscopic serial section analysis. The Journal of comparative neurology. 1978;179:795–830. doi: 10.1002/cne.901790407. [DOI] [PubMed] [Google Scholar]
- 56.Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Noctor SC, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 58.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 59.Rakic P. Contact Regulation of Neuronal Migration. Wiley and Sons; 1985. [Google Scholar]
- 60.Rakic P, et al. Polarity of microtubule assemblies during neuronal cell migration. Proc Natl Acad Sci U S A. 1996;93:9218–9222. doi: 10.1073/pnas.93.17.9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gregory WA, et al. Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. J Neurosci. 1988;8:1728–1738. doi: 10.1523/JNEUROSCI.08-05-01728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Franco SJ, et al. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hinds JW, Hinds PL. Early ganglion cell differentiation in the mouse retina: an electron microscopic analysis utilizing serial sections. Developmental biology. 1974;37:381–416. doi: 10.1016/0012-1606(74)90156-0. [DOI] [PubMed] [Google Scholar]
- 64.Wentworth LE, Hinds JW. Early motoneuron formation in the cervical spinal cord of the mouse: an electron microscopic, serial section analysis. The Journal of comparative neurology. 1978;177:611–634. doi: 10.1002/cne.901770406. [DOI] [PubMed] [Google Scholar]
- 65.Rivas RJ, Hatten ME. Motility and cytoskeletal organization of migrating cerebellar granule neurons. J Neurosci. 1995;15:981–989. doi: 10.1523/JNEUROSCI.15-02-00981.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Powell SK, et al. Development of polarity in cerebellar granule neurons. J Neurobiol. 1997;32:223–236. doi: 10.1002/(sici)1097-4695(199702)32:2<223::aid-neu7>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 67.Miyata T, et al. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 68.Noctor SC, et al. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 69.Sauer ME, Walker BE. Radioautographic study of interkinetic nuclear migration in the neural tube. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine. 1959;101:557–560. doi: 10.3181/00379727-101-25014. [DOI] [PubMed] [Google Scholar]
- 70.Farkas LM, Huttner WB. The cell biology of neural stem and progenitor cells and its significance for their proliferation versus differentiation during mammalian brain development. Curr Opin Cell Biol. 2008;20:707–715. doi: 10.1016/j.ceb.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 71.Cappello S, et al. The Rho-GTPase cdc42 regulates neural progenitor fate at the apical surface. Nat Neurosci. 2006;9:1099–1107. doi: 10.1038/nn1744. [DOI] [PubMed] [Google Scholar]
- 72.Bultje RS, et al. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing neocortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sottocornola R, et al. ASPP2 binds Par-3 and controls the polarity and proliferation of neural progenitors during CNS development. Dev Cell. 2010;19:126–137. doi: 10.1016/j.devcel.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 74.Imai F, et al. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–1744. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, et al. Cdc42 deficiency causes Sonic hedgehog-independent holoprosencephaly. Proc Natl Acad Sci U S A. 2006;103:16520–16525. doi: 10.1073/pnas.0603533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yokota Y, et al. Cdc42 and Gsk3 modulate the dynamics of radial glial growth, inter-radial glial interactions and polarity in the developing cerebral cortex. Development. 2010;137:4101–4110. doi: 10.1242/dev.048637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Costa MR, et al. Par-complex proteins promote proliferative progenitor divisions in the developing mouse cerebral cortex. Development. 2008;135:11–22. doi: 10.1242/dev.009951. [DOI] [PubMed] [Google Scholar]
- 78.Weimer JM, et al. MARCKS modulates radial progenitor placement, proliferation and organization in the developing cerebral cortex. Development. 2009;136:2965–2975. doi: 10.1242/dev.036616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arbuzova A, et al. Cross-talk unfolded: MARCKS proteins. The Biochemical journal. 2002;362:1–12. doi: 10.1042/0264-6021:3620001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takekuni K, et al. Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. The Journal of biological chemistry. 2003;278:5497–5500. doi: 10.1074/jbc.C200707200. [DOI] [PubMed] [Google Scholar]
- 81.Stelzer S, et al. JAM-C is an Apical Surface Marker for Neural Stem Cells. Stem cells and development. 2012;2:757–766. doi: 10.1089/scd.2011.0274. [DOI] [PubMed] [Google Scholar]
- 82.Zhadanov AB, et al. Absence of the tight junctional protein AF-6 disrupts epithelial cell-cell junctions and cell polarity during mouse development. Current biology. 1999;9:880–888. doi: 10.1016/s0960-9822(99)80392-3. [DOI] [PubMed] [Google Scholar]
- 83.Ohata S, et al. Dual roles of Notch in regulation of apically restricted mitosis and apicobasal polarity of neuroepithelial cells. Neuron. 2011;69:215–230. doi: 10.1016/j.neuron.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 84.Rasin MR, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 85.Sestan N, et al. Contact-dependent inhibition of cortical neurite growth mediated by notch signaling. Science. 1999;286:741–746. doi: 10.1126/science.286.5440.741. [DOI] [PubMed] [Google Scholar]
- 86.Li HS, et al. Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron. 2003;40:1105–1118. doi: 10.1016/s0896-6273(03)00755-4. [DOI] [PubMed] [Google Scholar]
- 87.Rakic P, et al. Recognition, adhesion, transmembrane signaling and cell motility in guided neuronal migration. Curr Opin Neurobiol. 1994;4:63–69. doi: 10.1016/0959-4388(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 88.Fishell G, Hatten ME. Astrotactin provides a receptor system for CNS neuronal migration. Development. 1991;113:755–765. doi: 10.1242/dev.113.3.755. [DOI] [PubMed] [Google Scholar]
- 89.Wilson PM, et al. Astn2, a novel member of the astrotactin gene family, regulates the trafficking of ASTN1 during glial-guided neuronal migration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2010;30:8529–8540. doi: 10.1523/JNEUROSCI.0032-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Elias LA, et al. Gap junction adhesion is necessary for radial migration in the neocortex. Nature. 2007;448:901–907. doi: 10.1038/nature06063. [DOI] [PubMed] [Google Scholar]
- 91.Anton ES, et al. Role of GGF/neuregulin signaling in interactions between migrating neurons and radial glia in the developing cerebral cortex. Development. 1997;124:3501–3510. doi: 10.1242/dev.124.18.3501. [DOI] [PubMed] [Google Scholar]
- 92.Anton ES, et al. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. doi: 10.1016/s0896-6273(00)81089-2. [DOI] [PubMed] [Google Scholar]
- 93.Uchida N, et al. The catenin/cadherin adhesion system is localized in synaptic junctions bordering transmitter release zones. The Journal of cell biology. 1996;135:767–779. doi: 10.1083/jcb.135.3.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gartner A, et al. N-cadherin specifies first asymmetry in developing neurons. The EMBO journal. 2012;31:1893–1903. doi: 10.1038/emboj.2012.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kadowaki M, et al. N-cadherin mediates cortical organization in the mouse brain. Developmental biology. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 96.Jossin Y, Cooper JA. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nature neuroscience. 2011;14:697–703. doi: 10.1038/nn.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nadarajah B, et al. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- 98.Kawauchi T, et al. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 99.Rice DS, Curran T. Role of the reelin signaling pathway in central nervous system development. Annual review of neuroscience. 2001;24:1005–1039. doi: 10.1146/annurev.neuro.24.1.1005. [DOI] [PubMed] [Google Scholar]
- 100.Tissir F, Goffinet AM. Reelin and brain development. Nature reviews. Neuroscience. 2003;4:496–505. doi: 10.1038/nrn1113. [DOI] [PubMed] [Google Scholar]
- 101.Dulabon L, et al. Reelin binds alpha3beta1 integrin and inhibits neuronal migration. Neuron. 2000;27:33–44. doi: 10.1016/s0896-6273(00)00007-6. [DOI] [PubMed] [Google Scholar]
- 102.Tabata H, Nakajima K. Neurons tend to stop migration and differentiate along the cortical internal plexiform zones in the Reelin signal-deficient mice. Journal of neuroscience research. 2002;69:723–730. doi: 10.1002/jnr.10345. [DOI] [PubMed] [Google Scholar]
- 103.Ryder EF, Cepko CL. Migration patterns of clonally related granule cells and their progenitors in the developing chick cerebellum. Neuron. 1994;12:1011–1028. doi: 10.1016/0896-6273(94)90310-7. [DOI] [PubMed] [Google Scholar]
- 104.Komuro H, et al. Mode and tempo of tangential cell migration in the cerebellar external granular layer. J Neurosci. 2001;21:527–540. doi: 10.1523/JNEUROSCI.21-02-00527.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Solecki DJ, et al. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–1203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- 106.Solecki DJ, et al. Myosin II motors and F-actin dynamics drive the coordinated movement of the centrosome and soma during CNS glial-guided neuronal migration. Neuron. 2009;63:63–80. doi: 10.1016/j.neuron.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Famulski JK, et al. Siah Regulation of Pard3A Controls Neuronal Cell Adhesion During Germinal Zone Exit. Science. 2010;330:1834–1838. doi: 10.1126/science.1198480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carthew RW, Rubin GM. seven in absentia, a gene required for specification of R7 cell fate in the Drosophila eye. Cell. 1990;63:561–577. doi: 10.1016/0092-8674(90)90452-k. [DOI] [PubMed] [Google Scholar]
- 109.Ebnet K, et al. Junctional adhesion molecules (JAMs): more molecules with dual functions? J Cell Sci. 2004;117:19–29. doi: 10.1242/jcs.00930. [DOI] [PubMed] [Google Scholar]
- 110.Williams SE, et al. Asymmetric cell divisions promote Notch-dependent epidermal differentiation. Nature. 2011;470:353–358. doi: 10.1038/nature09793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lancaster MA, Knoblich JA. Spindle orientation in mammalian cerebral cortical development. Current opinion in neurobiology. 2012 doi: 10.1016/j.conb.2012.04.003. http://dx.doi.org/10.1016/j.conb.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hinds JW, Ruffett TL. Cell proliferation in the neural tube: an electron microscopic and golgi analysis in the mouse cerebral vesicle. Zeitschrift fur Zellforschung und mikroskopische Anatomie. 1971;115:226–264. doi: 10.1007/BF00391127. [DOI] [PubMed] [Google Scholar]
- 113.Lehtinen MK, et al. The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron. 2011;69:893–905. doi: 10.1016/j.neuron.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Rousso DL, et al. Foxp-mediated suppression of N-cadherin regulates neuroepithelial character and progenitor maintenance in the CNS. Neuron. 2012;74:314–330. doi: 10.1016/j.neuron.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gupton SL, Waterman-Storer CM. Spatiotemporal feedback between actomyosin and focal-adhesion systems optimizes rapid cell migration. Cell. 2006;125:1361–1374. doi: 10.1016/j.cell.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 116.Yi JJ, et al. TGF-beta signaling specifies axons during brain development. Cell. 2010;142:144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]