Abstract
The importance of the medial temporal lobe to episodic memory has been recognized for decades. Recent human fMRI findings have begun to delineate the functional roles of different MTL regions, most notably the hippocampus, in the retrieval of episodic memories. Importantly, these studies have also identified a network of cortical regions – each interconnected with the MTL – that are also consistently engaged during successful episodic retrieval. Along with the MTL these regions appear to constitute a content-independent network that acts in concert with cortical regions representing the contents of retrieval to support consciously accessible representations of prior experiences.
Introduction
Episodic memory – consciously accessible memory for unique events – allows us to represent past experiences and to flexibly employ these representations in service of current and future goals [1]. The present review focuses on recent human fMRI findings relevant to the functional neuroanatomy of successful episodic memory retrieval. The majority of the reviewed studies took as their starting point a ‘dual-process’ model of memory [2, 3]. These models posit that a retrieval cue (such as a recognition memory test item) can elicit two qualitatively distinct kinds of mnemonic information: a multi-dimensional recollection signal that provides information about qualitative aspects of a prior event, including its context, and a scalar familiarity signal that can support simple judgments of prior occurrence. From this perspective, identifying the neural bases of episodic retrieval requires experimental designs that permit recollection- and familiarity-driven memory to be dissociated (Box 1). Current evidence suggests that the distinction between recollection and familiarity holds both within the MTL and at the level of the cerebral cortex, where a network of regions that appears to be preferentially engaged during successful recollection can be identified.
Box 1. Dissociating the Neural Correlates of Recollection and Familiarity.
To identify neural activity selectively associated with successful recollection it is necessary to employ memory tests that allow the activity to be distinguished from the neural correlates of other forms of memory, most notably, familiarity (see text). Two variants of recognition memory tests have frequently been employed in efforts to dissociate recollection and familiarity. In the ‘Remember/Know’ procedure subjects report whether recognition of a test item is accompanied (Remember) or unaccompanied (Know) by retrieval of one or more contextual details about the study presentation. It is assumed that items endorsed as Remembered were both recollected and familiar, whereas items endorsed Know were recognized on the basis of familiarity alone. Thus, by contrasting the fMRI activity elicited by these two classes of item the neural correlates of recollection can be identified. More complex versions of the procedure have required subjects to respond differentially depending on the number of details recollected [e.g., 57], or to rate unrecollected items on a confidence (definitely old’ to ‘definitely new) or familiarity scale (highly familiar to highly unfamiliar), allowing items to be segregated by the strength of the underlying familiarity signal [5, 55]. A second popular procedure for identifying recollected items requires an explicit judgment to be made about a specific contextual feature of the study episode (a ‘source memory’ judgment), for example, whether a test word was studied in a red or a green font. It is assumed that retrieval of source information signifies successful recollection. Failure to retrieve a source feature does not, however, necessarily mean that recollection failed, as it is difficult to discount the possibility that recollection occurred but did not include information diagnostic of the source judgment (‘non-criterial recollection’).
Memory signals within the MTL
The MTL - the hippocampus and surrounding perirhinal, entorhinal and parahippocampal cortices - has long been recognized as a key brain area supporting episodic memory. Reminiscent of electrophysiological findings in primates [4], fMRI studies have reported that perirhinal activity covaries inversely with the familiarity of recognition memory test items [e.g., 5]. These fMRI results are consistent with evidence from animal lesion studies [6] and a human single-case study [7] that suggest a pre-eminent role for perirhinal cortex in familiarity-based recognition.
Perirhinal cortex is not, however, the only MTL region to demonstrate activity reductions for familiar recognition memory items, with several studies reporting similar findings for the hippocampus, in some cases seemingly in the same hippocampal regions that also manifested recollection-related enhancement (see below) [8]. Hippocampal ‘novelty effects’ have usually been interpreted as reflecting a bias toward the encoding of novel information [9] rather than as a familiarity signal. In keeping with the idea that perirhinal cortex plays the more important role in familiarity-driven recognition, a recent study [10••] reported that, as indexed by both fMRI and local field potentials, perirhinal activity differentiated familiar and novel test items at an earlier latency than did hippocampal activity.
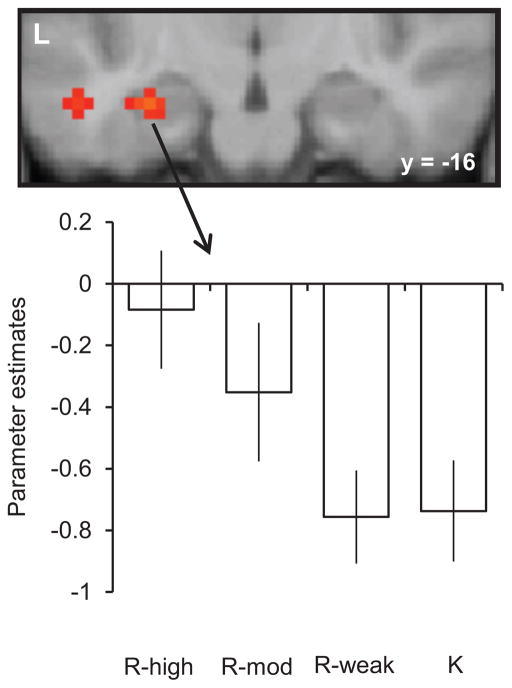
Relative to test items judged to be familiar, but for which recollection seemingly failed, successful recollection is associated with enhancement of fMRI activity in the hippocampus and parahippocampal cortex [11]. These findings converge with some [e.g., 12], but by no means all [e.g., 13], human lesion studies to suggest a selective role for the hippocampus in memory for qualitative information. It has been reported that fMRI hippocampal recollection effects are sensitive not to whether a test item elicits a subjective sense of recollection, but to the amount of contextual information retrieved about the study episode [14•; see Figure 1].
Figure 1.
Retrieval-related hippocampal activity co-varies with amount of retrieved contextual information [56]. The data are shown for test items endorsed as familiar (K) or recollected (R), further segregated by the confidence and accuracy of a subsequent source memory judgment made on recollected items. R-high and R-mod refer to accurate source judgments made with high and moderate levels of confidence respectively. R-weak refers to source judgments made with low confidence or that were inaccurate.
Recollection-related activity in parahippocampal cortex has been interpreted in light of proposals that it has a central role in the representation of contextual information [15, see below], retrieval of which is a defining feature of successful recollection. Whereas the information represented in parahippocampal cortex was initially conceived of as predominantly spatial, it has been argued that the region may also represent non-spatial contextual information [16]. It has been proposed that the hippocampus acts in concert with the parahippocampal and perirhinal cortices to support recollection, the hippocampus ‘binding’ contextual information from the parahippocampal cortex with object information from the perirhinal cortex to form an integrated episodic representation [15, 17]. Consistent with this proposal, it was recently reported that hippocampal-perirhinal connectivity is greater during successful than unsuccessful source memory judgments [10••]. In another study, successful recall and recognition were accompanied by enhanced connectivity between the hippocampus and both perirhinal and parahippocampal cortices [18]. Interestingly, the connectivity analyses suggested that the directions of inter-regional influence differed between the two types of test, perirhinal cortex modulating the hippocampus during recognition, but being modulated by the hippocampus during recall.
The view that fMRI findings indicate a selective role for the hippocampus in recollection has been challenged [19]. According to this alternative proposal retrieval-related hippocampal activity covaries with memory ‘strength’ – indexed by the accuracy and confidence of simple recognition judgments – regardless of whether memory is based on recollection, familiarity, or a mixture of the two signals. Findings consistent with this proposal were reported in two recent studies [20, 21•]. The strategy in each case was to contrast the hippocampal activity elicited by recollected test items with activity elicited by items for which recollection failed but which were equated for memory strength. In both studies hippocampal activity elicited by the two classes of item was of comparable magnitude, and exceeded the activity elicited by studied items misidentified as new (misses). In one of these studies [20] recollection was operationalized by accurate source memory judgments, leaving open the possibility that items designated as unrecollected were associated with recollection of ‘non-criterial’ details of the study episode (Box 1). This criticism does not apply to the second study [21•], in which items matched for strength were contrasted according to whether they were given a ‘Remember’ or a ‘Know’ judgment (Box 1). Two other studies conducted along similar lines reported different findings, however, in that items endorsed as recollected elicited greater hippocampal (and parahippocampal) activity than items matched for memory strength but endorsed as ‘strongly familiar’ [17, 22]. In another study [23], recollection was indexed by the accurate discrimination of word pairs according to whether the constituent words had been studied on the same or on different study trials (an associative recognition test). Hippocampal activity was greater when elicited by recollected than by unrecollected pairs, even when the two classes of pairs were equated for memory strength. Together with other evidence [e.g., 14•], these findings [17, 22, 23] suggest that the construct of memory strength does not provide a full account of retrieval-related hippocampal activity.
Cortical recollection effects
In addition to enhancement of hippocampal and parahippocampal activity, successful recollection is characteristically associated with engagement of several cortical regions, including retrosplenial/posterior cingulate cortex (BA 23/29/30/31), ventral posterior parietal cortex centered on the angular gyrus (BA 39) and mPFC (BA 10/32) [11]. Because of the density of its connections with the hippocampus and the memory impairments that accompany lesions to the region, retrosplenial cortex has been proposed to be a component of an ‘extended hippocampal system’ [24]. Recent evidence suggests that, like parahippocampal cortex, both this region and the mPFC (which shares connections with the hippocampus and parahippocampal and retrosplenial cortices [24]) may play a role in the processing of contextual information [25].
Unlike retrosplenial cortex and mPFC, there is little evidence from animal studies to implicate the angular gyrus in episodic memory (although a putative homologous region in the macaque demonstrates a pattern of resting state connectivity similar to that shown in humans [26]). Evidence from human resting state connectivity and DTI tractography indicates that the region is interconnected with the hippocampus, parahippocampal cortex and retrosplenial/posterior cingulate cortices [27, 28•], suggesting that it might form part of a common functional network. This possibility is buttressed by the finding that recollection-based recognition is associated with enhanced connectivity between the angular gyrus and hippocampus [29].
Based largely on fMRI evidence, a number of proposals have been advanced regarding the functional significance of recollection-related activity in the angular gyrus. According to one idea [30], the sensitivity of the region to recollection reflects its role in ‘bottom-up’ attentional re-orienting. By this account, recollection is a salient internal event that triggers the re-direction of attention from a retrieval cue toward the contents of retrieval. A somewhat related account [31] - based on the finding that angular gyrus activity is sensitive to whether a recognition test item is congruent or incongruent with a cue that predicts its study status - proposes that recollection-related activity in this region is a consequence of ‘expectancy violation’. A third proposal [32] is that the angular gyrus contributes to the representation of recollected information, perhaps acting as a component of the ‘episodic buffer’ posited to act as an interface between episodic memory and executive processes [33]. Another proposal [34] is that the region acts as a convergence zone, binding the different features of an episode into an integrated representation. The dual findings that recollection-related activity in this region scales with the amount of information recollected [e.g., 35], and tracks the time over which recollected information is maintained [36•], arguably favor some form of representational account. Neither these nor the re-orienting or expectancy violation accounts are easily reconcilable with the results of lesion studies, however, which indicate that lateral parietal damage has little or no impact on the accuracy of either recognition or source memory [37, 38] judgments. Rather, deficits are observed with respect to self-initiated retrieval of autobiographical memory [39], and in the proportions of test items accorded Remember [40, 41] or highly confident source judgments [42]. Thus, parietal lesions seem to have more of an impact on subjective or metacognitive aspects of recollection than on objective indices of accuracy.
A general recollection network?
As was just reviewed, recollection-sensitive fMRI effects have consistently been identified in the hippocampus, parahippocampal, retrosplenial/posterior cingulate and lateral parietal cortices, and mPFC (Figure 2). The robustness of these effects in the face of wide variation in test materials and procedures for operationalizing recollection have led to the proposal that the regions constitute a content-independent network engaged whenever a retrieval cue elicits recollection [43, 44]. In keeping with this proposal, successful cued recall – held to depend on the same processes that support recollection-based recognition – is associated with enhanced activity in the same regions [e.g., 45]. In one recent study [43] the recall effects in parahippocampal cortex, retrosplenial/posterior cingulate cortices and the left angular gyrus were potentiated when recall was accompanied by an accurate rather than an inaccurate source memory judgment on the recalled item, suggesting that these regions were responding to the amount of information recollected rather than to its content.
Figure 2.
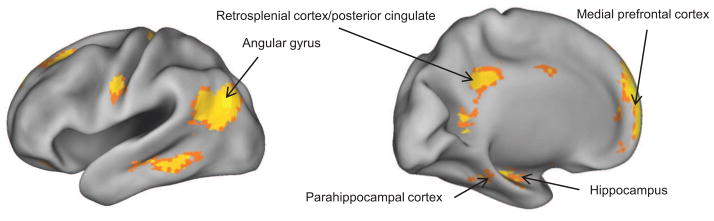
The general recollection network. The figure illustrates the outcome of the contrast between accurately recognized test words endorsed as ‘Remember’ or ‘Know’ in an unpublished study (n=19) of Wang and Rugg. The words had been studied either as pictures or as words in the context of two different encoding tasks. Shown are regions where recollection was associated with enhanced activity at test regardless of the encoding condition.
The putative general recollection network partially overlaps with the heavily-investigated ‘default mode network’, originally identified by its tendency to demonstrate relatively greater activity during periods of ‘rest’ than during stimulus-directed tasks [46]. The recollection network also overlaps with regions that are characteristically active when individuals mentally construct future-oriented, self-relevant scenarios (‘episodic future thought’ [e.g., 47]). While much remains to be understood about the reasons for these overlaps, they likely reflect the common engagement of processes that support the retrieval and representation of episodic information and its employment in self-directed cognition [48•].
Content-selective recollection effects
According to an influential class of models [e.g., 49, 50], a key role of the hippocampus is to store non-overlapping representations of the distributed patterns of cortical activity elicited when different events are encoded. When an effective retrieval cue is present, the appropriate hippocampal representation is reactivated, causing the reinstatement of the original pattern of activity in the cortex and the event to be ‘re-experienced’. Thus, successful recollection should be associated not only with the engagement of the content-independent general network discussed above, but also with patterns of activity that vary in their location according to the content of what is recollected. Furthermore, this content-dependent activity should overlap with the activity elicited when the recollected event was initially experienced. These predictions have been the subject of numerous studies [reviewed in 51], with findings that are largely supportive of what is often referred to as the ‘cortical reinstatement hypothesis.’ In recent studies [52, 53•] these predictions have been addressed using MVPA. This method allows measurement of the similarity between patterns of fMRI activity distributed across a population of voxels, even when effects at the single voxel level are not statistically significant or spatially contiguous. In one such study [52], subjects undertook one of three encoding tasks on a series of words. Consistent with the cortical reinstatement hypothesis, a multivariate classifier trained to distinguish the fMRI activity elicited by the three different classes of study word was able to reliably classify recollected test words according to the task in which they were encoded. The classifier was also able to discriminate highly familiar but unrecollected words, albeit less accurately and in fewer regions than was the case for recollected items. This finding might indicate that, like familiarity, recollection varies continuously, and can be weakly present even when a test item fails to elicit a ‘Remember’ judgment [2]. A second MVPA study [53•] also identified cortical reinstatement effects (differentiating cued recall of images of faces, scenes and objects). Interestingly, the strength with which a retrieval cue elicited reinstatement of the image it had originally been paired with was inversely related to the accuracy with which the cue elicited retrieval of a subsequently learned image.
In the studies reviewed above, reinstatement effects were investigated by contrasting activity common to a set of test items. A more recent experiment [54•] addressed the question of whether reinstatement effects could be detected at the single item level. Subjects studied trial-unique scenes, and then discriminated between studied and new scenes using a modified Remember/Know procedure [see 55]. The across-voxel similarity in the patterns of activity elicited by each scene during the study and test phases was computed. In several cortical regions of interest the similarity index was greater for scenes that were recollected or confidently endorsed old than it was for scenes misclassified as new. Furthermore, degree of study-test similarity correlated positively with hippocampal activity. These findings are consistent with the proposal that episodic retrieval involves the hippocampally-mediated reinstatement of encoding-related activity.
Summary and open questions
Recollection of a prior experience is associated with engagement of a general network, centered on the hippocampus, in concert with cortical regions that, collectively, represent the contents of recollection. Among the many questions raised by this framework, three stand out. First, what are the specific functional roles of the different regions comprising this network? Second, how does the network interact with content-sensitive regions thought to represent the contents of recollection? Third, and relatedly, if recollected content is represented by distributed patterns of cortical activity, how does the information represented in these patterns become integrated or ‘bound’ into a coherent, consciously accessible representation of a prior experience?
Highlights.
Recollection depends on a content-insensitive network centered on the hippocampus.
The recollection network comprises both medial temporal and neocortical regions.
The network interacts with cortical regions that represent retrieved content.
Acknowledgments
Preparation of this article and some of the research described in it was supported by NIMH grant 5R01MH072966.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tulving E. Elements of episodic memory. Oxford University Press; 1983. [Google Scholar]
- 2.Wixted JT, Mickes L. A continuous dual-process model of remember/know judgments. Psychol Rev. 2010;117:1025–54. doi: 10.1037/a0020874. [DOI] [PubMed] [Google Scholar]
- 3.Yonelinas AP. The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language. 2002;46:441–517. [Google Scholar]
- 4.Brown MW, Xiang JZ. Recognition memory: neuronal substrates of the judgement of prior occurrence. Prog Neurobiol. 1998;55:149–89. doi: 10.1016/s0301-0082(98)00002-1. [DOI] [PubMed] [Google Scholar]
- 5.Montaldi D, Spencer TJ, Roberts N, Mayes AR. The neural system that mediates familiarity memory. Hippocampus. 2006;16:504–20. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- 6.Brown MW, Aggleton JP. Recognition memory: what are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2:51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 7.Bowles B, Crupi C, Mirsattari SM, Pigott SE, Parrent AG, Pruessner JC, Yonelinas AP, K(o)hler S. Impaired familiarity with preserved recollection after anterior temporal-lobe resection that spares the hippocampus. Proc Natl Acad Sci U S A. 2007;104:16382–7. doi: 10.1073/pnas.0705273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suzuki M, Johnson JD, Rugg MD. Recollection-related hippocampal activity during continuous recognition: a high-resolution fMRI study. Hippocampus. 2011;21:575–83. doi: 10.1002/hipo.20781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark CE, Okado Y. Making memories without trying: medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–53. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10••.Staresina BP, Fell J, Do Lam AT, Axmacher N, Henson RN. Memory signals are temporally dissociated in and across human hippocampus and perirhinal cortex. Nat Neurosci. 2012;15:1167–73. doi: 10.1038/nn.3154. Using time-resolved fMRI in healthy subjects, and local field recordings from depth electrodes in epilepsy patients, the authors report convergent evidence that perirhinal cortex discriminates between familiar and novel items more rapidly than the hippocampus, but that this temporal ordering is reversed for discrimination between items attracting accurate versus inaccurate source judgments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H. Dissociating the roles of the default-mode, dorsal, and ventral networks in episodic memory retrieval. Neuroimage. 2010;50:1648–57. doi: 10.1016/j.neuroimage.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 12.Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Sparing of the familiarity component of recognition memory in a patient with hippocampal pathology. Neuropsychologia. 2005;43:1810–23. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Song Z, Wixted JT, Hopkins RO, Squire LR. Impaired capacity for familiarity after hippocampal damage. Proc Natl Acad Sci U S A. 2011;108:9655–60. doi: 10.1073/pnas.1107247108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14•.Rugg MD, Vilberg KL, Mattson JT, Yu SS, Johnson JD, Suzuki M. Item memory, context memory and the hippocampus: fMRI evidence. Neuropsychologia. in press. This paper summarizes findings from a series of studies suggesting that retrieval-related activity in the hippocampus co-varies with the amount of information retrieved about a studied episode and not merely whether a test item elicits a subjective sense of recollection rather than familiarity. [Google Scholar]
- 15.Diana RA, Yonelinas AP, Ranganath C. Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn Sci. 2007;11:379–86. doi: 10.1016/j.tics.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–58. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- 17.Montaldi D, Mayes AR. The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus. 2010;20:1291–314. doi: 10.1002/hipo.20853. [DOI] [PubMed] [Google Scholar]
- 18.Sadeh T, Maril A, Bitan T, Goshen-Gottstein Y. Putting Humpty together and pulling him apart: accessing and unbinding the hippocampal item-context engram. Neuroimage. doi: 10.1016/j.neuroimage.2011.12.004. in press. [DOI] [PubMed] [Google Scholar]
- 19.Squire LR, Wixted JT, Clark RE. Recognition memory and the medial temporal lobe: a new perspective. Nat Rev Neurosci. 2007;8:872–83. doi: 10.1038/nrn2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wais PE, Squire LR, Wixted JT. In search of recollection and familiarity signals in the hippocampus. Journal of Cognitive Neuroscience. 2010;22:109–23. doi: 10.1162/jocn.2009.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21•.Smith CN, Wixted JT, Squire LR. The hippocampus supports both recollection and familiarity when memories are strong. J Neurosci. 2011;44:15693–702. doi: 10.1523/JNEUROSCI.3438-11.2011. Retrieval-related hippocampal activity was contrasted between test items endorsed as Remember or Know that had been recognized with equivalent levels of accuracy and confidence. Relative to unrecognized items, the two classes of recognized item elicited comparably enhanced hippocampal activity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kafkas A, Montaldi D. Familiarity and recollection produce distinct eye movement, pupil and medial temporal lobe responses when memory strength is matched. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2012.08.001. in press. [DOI] [PubMed] [Google Scholar]
- 23.Wais PE. Hippocampal signals for strong memory when associative memory is available and when it is not. Hippocampus. 2011;21:9–21. doi: 10.1002/hipo.20716. [DOI] [PubMed] [Google Scholar]
- 24.Aggleton JP. Multiple anatomical systems embedded within the primate medial temporal lobe: Implications for hippocampal function. Neurosci Biobehav Rev. 2012;36:1579–96. doi: 10.1016/j.neubiorev.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Kveraga K, Ghuman AS, Kassam KS, Aminoff EA, Hamalainen MS, Chaumon M, Bar M. Early onset of neural synchronization in the contextual associations network. Proc Natl Acad Sci U S A. 2011;108:3389–94. doi: 10.1073/pnas.1013760108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mars RB, Jbabdi S, Sallet J, O’Reilly JX, Croxson PL, Olivier E, Noonan MP, Bergmann C, Mitchell AS, Baxter MG, Behrens TE, Johansen-Berg H, Tomassini V, Miller KL, Rushworth MF. Diffusion-weighted imaging tractography-based parcellation of the human parietal cortex and comparison with human and macaque resting-state functional connectivity. J Neurosci. 2011;31:4087–100. doi: 10.1523/JNEUROSCI.5102-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable connectivity within human angular gyrus and intraparietal sulcus: evidence from functional and structural connectivity. Cereb Cortex. 2010;20:2636–46. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.Sestieri C, Corbetta M, Romani GL, Shulman GL. Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J Neurosci. 2011;31:4407–20. doi: 10.1523/JNEUROSCI.3335-10.2011. Using time-resolved fMRI to temporally dissociate the activity elicited during the search, retrieval, and post-retrieval phases of a cued recall task, it was reported that, alone among lateral parietal regions, the angular gyrus was active at the time of retrieval, as were two regions with which it showed significant resting state connectivity, the posterior cingulate and parahippocampal cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick C, Moscovitch M, Protzner AB, Huber CG, McAndrews MP. Hippocampal-neocortical networks differ during encoding and retrieval of relational memory: functional and effective connectivity analyses. Neuropsychologia. 2010;48:3272–81. doi: 10.1016/j.neuropsychologia.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: an integrative theoretical account. Trends Cogn Sci. 2012;16:338–52. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Connor AR, Han S, Dobbins IG. The inferior parietal lobule and recognition memory: expectancy violation or successful retrieval? J Neurosci. 2010;30:2924–34. doi: 10.1523/JNEUROSCI.4225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilberg KL, Rugg MD. Memory retrieval and the parietal cortex: a review of evidence from a dual-process perspective. Neuropsychologia. 2008;46:1787–99. doi: 10.1016/j.neuropsychologia.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baddeley A. The episodic buffer: a new component of working memory? Trends Cogn Sci. 2000;4:417–23. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- 34.Shimamura AP. Episodic retrieval and the cortical binding of relational activity. Cogn Affect Behav Neurosci. 2011;11:277–91. doi: 10.3758/s13415-011-0031-4. [DOI] [PubMed] [Google Scholar]
- 35.Vilberg KL, Rugg MD. Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia. 2007;45:2216–25. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36•.Vilberg KL, Rugg MD. The neural correlates of recollection: transient versus sustained fMRI effects. J Neurosci. in press. Subjects were required to recollect studied associates and maintain them in mind across a variable delay period. Recollection-related activity was segregated according to whether it was transient (unaffected by the delay duration) or sustained (tracked the delay period). Whereas some regions of the general recollection network, including the hippocampus, demonstrated transient recollection effects, the effects in the left angular gyrus were sustained. [Google Scholar]
- 37.Haramati S, Soroker N, Dudai Y, Levy DA. The posterior parietal cortex in recognition memory: a neuropsychological study. Neuropsychologia. 2008;46:1756–66. doi: 10.1016/j.neuropsychologia.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Simons JS, Peers PV, Hwang DW, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. 2008;46:1185–91. doi: 10.1016/j.neuropsychologia.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. J Neurosci. 2007;27:14415–23. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drowos DB, Berryhill M, Andre JM, Olson IR. True memory, false memory, and subjective recollection deficits after focal parietal lobe lesions. Neuropsychology. 2010;24:465–75. doi: 10.1037/a0018902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davidson PS, Anaki D, Ciaramelli E, Cohn M, Kim AS, Murphy KJ, Troyer AK, Moscovitch M, Levine B. Does lateral parietal cortex support episodic memory? Evidence from focal lesion patients. Neuropsychologia. 2008;46:1743–55. doi: 10.1016/j.neuropsychologia.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simons JS, Peers PV, Mazuz YS, Berryhill ME, Olson IR. Dissociation between memory accuracy and memory confidence following bilateral parietal lesions. Cereb Cortex. 2010;20:479–85. doi: 10.1093/cercor/bhp116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hayama HR, Vilberg KL, Rugg MD. Overlap between the neural correlates of cued recall and source memory: evidence for a generic recollection network? J Cogn Neurosci. 2012;24:1127–37. doi: 10.1162/jocn_a_00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duarte A, Henson RN, Graham KS. Stimulus content and the neural correlates of source memory. Brain Res. 2011;1373:110–23. doi: 10.1016/j.brainres.2010.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schott BH, Henson RN, Richardson-Klavehn A, Becker C, Thoma V, Heinze HJ, D(u)zel E. Redefining implicit and explicit memory: the functional neuroanatomy of priming, remembering, and control of retrieval. Proc Natl Acad Sci U S A. 2005;102:1257–62. doi: 10.1073/pnas.0409070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schacter DL, Addis DR, Buckner RL. Episodic simulation of future events: concepts, data, and applications. Ann N Y Acad Sci. 2008;1124:39–60. doi: 10.1196/annals.1440.001. [DOI] [PubMed] [Google Scholar]
- 47.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45:1363–77. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48•.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–62. doi: 10.1016/j.neuron.2010.02.005. Using hierarchical clustering methods to decompose patterns of inter-regional functional connectivity, it is reported that the default mode network can be dissociated into at least two major sub-systems, associated with episodic memory and self-referential cognition respectively. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norman KA, O’Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–46. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- 50.Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- 51.Danker JF, Anderson JR. The ghosts of brain states past: Remembering reactivates the brain regions engaged during encoding. Psychological Bulletin. 2010;136:87–102. doi: 10.1037/a0017937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson JD, McDuff SG, Rugg MD, Norman KA. Recollection, familiarity, and cortical reinstatement: a multivoxel pattern analysis. Neuron. 2009;63:697–708. doi: 10.1016/j.neuron.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53•.Kuhl BA, Bainbridge WA, Chun MM. Neural reactivation reveals mechanisms for updating memory. J Neurosci. 2012;32:3453–61. doi: 10.1523/JNEUROSCI.5846-11.2012. MVPA is employed to demonstrate that a single cue can elicit reactivation of multiple memories, which compete with one another. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54•.Ritchey M, Wing EA, Labar KS, Cabeza R. Neural similarity between encoding and retrieval is related to memory via hippocampal interactions. Cereb Cortex. in press. Representation similarity analysis (RSA) was employed to estimate the similarity between patterns of activity elicited by scenes during an encoding and a subsequent retrieval task in each of a large set of cortical regions. Study-test similarity at the single-item level was greater for scenes endorsed as recollected or confidently judged old than it was for scenes that were recognized with low confidence or that were forgotten. Retrieval-related activity in the hippocampus covaried with degree of similarity. [Google Scholar]
- 55.Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–8. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu SS, Johnson JD, Rugg MD. Hippocampal activity during recognition memory covaries with the accuracy and confidence of source memory judgments. Hippocampus. 2012;22:1429–37. doi: 10.1002/hipo.20982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Res. 2006;1122:161–70. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]