Abstract
Endothelium-derived hyperpolarizing factors (EDHFs) regulate vascular tone by contributing to the vasorelaxations to shear stress and endothelial agonists such as bradykinin and acetylcholine. 15(S)-Hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA) and 11(R),12(S),15(S)-trihydroxyeicosatrienoic acid (11,12,15-THETA) are endothelial metabolites of the 15-lipoxygenase (15-LO) pathway of arachidonic acid metabolism and are EDHFs. 11,12,15-THETA activates small conductance, calcium-activated potassium channels on smooth muscle cells causing membrane hyperpolarization and relaxation. Expression levels of 15-LO in the endothelium regulate the activity of the 15-LO/15-H-11,12-EETA/11,12,15-THETA pathway and its contribution to vascular tone. Regulation of its expression is by transcriptional, translational and epigenetic mechanisms. Hypoxia, hypercholesterolemia, atherosclerosis, anemia, estrogen, interleukins and possibly other hormones increase 15-LO expression. An increase in 15-LO results in increased synthesis of 15-H-11,12-EETA and 11,12,15-THETA, increased membrane hyperpolarization and enhanced contribution to relaxation by endothelial agonists. Thus, the 15-LO pathway represents the first example of an inducible EDHF. In addition to 15-LO metabolites, a number of chemicals have been identified as EDHFs and their contributions to vascular tone vary with species and vascular bed. The reason for multiple EDHFs has evaded explanation. However, EDHFs functioning as constitutive EDHFs or inducible EDHFs may explain the need for chemically and biochemically distinct pathways for EDHF activity and the variation in EDHFs between species and vascular beds. This new EDHF classification provides a framework for understanding EDHF activity in physiological and pathological conditions.
Keywords: Endothelium-derived hyperpolarizing factor, 15-Lipoxygenase, vascular relaxation, potassium channels, THETA, HEETA
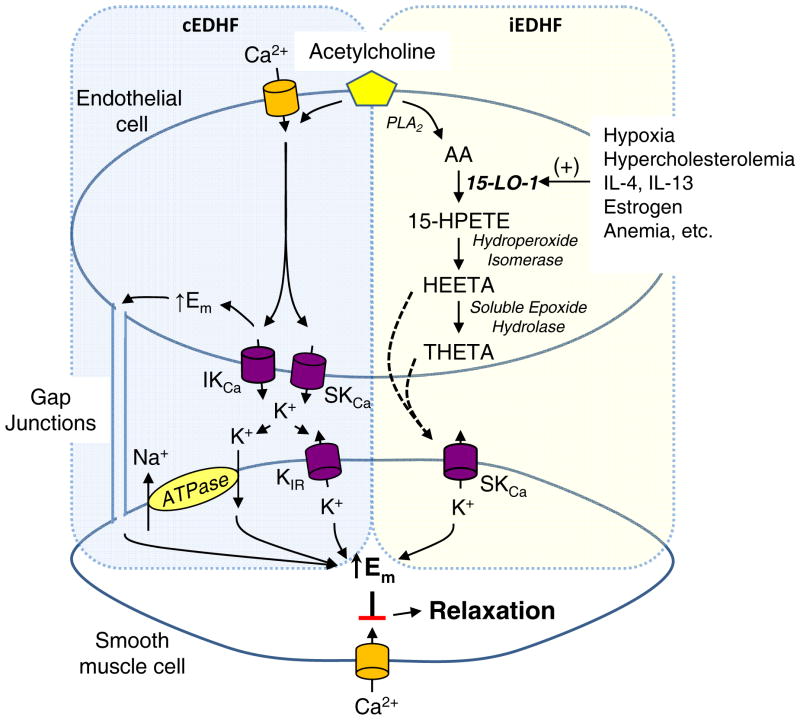
Endothelial cells regulate vascular tone through the release of several soluble mediators 1–4. These mediators include nitric oxide (NO), prostaglandin I2 (PGI2) and endothelium-derived hyperpolarizing factors (EDHFs). They are released by a number of stimuli including endothelial agonists such as acetylcholine and bradykinin and shear stress and mediate vasorelaxation. When the synthesis of NO and PGI2 are inhibited by NO synthase (NOS) and cyclooxygenase (COX) blockers, a component of the endothelium-dependent relaxations to acetylcholine or bradykinin persists and is associated with hyperpolarization of the smooth muscle cell membrane 2–4. These activities are attributed to EDHF. Interestingly, the contribution of EDHF to vascular tone is more prominent in resistance arteries than conduit arteries 5. Unlike NO and PGI2, EDHF is not a single chemical compound but a family of endothelial factors that hyperpolarize and relax smooth muscle. Several endogenous compounds mediate endothelium-dependent hyperpolarization in various arteries and species (Figure 1). These include metabolites of arachidonic acid (AA) such as epoxyeicosatrienoic acids (EETs), 15-hydroxy-11,12-epoxyeicosatrienoic acids (15-H-11,12-EETA) and 11,12,15-trihdyroxyeicosatrienoic acid (THETA), potassium (K) ion, hydrogen peroxide and C-type natriuretic peptide (CNP)(Figure 1)3, 4, 6–11. Despite differences in their chemical structure, these EDHFs act on vascular smooth muscle cells to activate calcium-activated K (KCa) channels or inward rectifying K (Kir) channels to cause membrane hyperpolarization and inhibition of calcium influx through voltage-activated calcium channels resulting in vasorelaxation. Gap junctions between endothelial cells, smooth muscle cells and the two cell types transmit the hyperpolarization along the vascular wall. Alternatively, endothelium-dependent hyperpolarization may not require a soluble mediator 4, 8, 12, 13. Acetylcholine stimulates calcium influx into endothelial cells activating KCa channels and causing the membrane of endothelial cells to hyperpolarize. Myo-endothelial gap junctions transfer the endothelial hyperpolarization to the smooth muscle cells resulting in relaxation. Since a factor is not involved, this mechanism has been called endothelium-dependent hyperpolarizations (EDH). Thus, endothelium-dependent hyperpolarization may occur by the transfer of a soluble factor (EDHF) or the transfer of hyperpolarization between endothelial cells and smooth muscle cells (EDH).
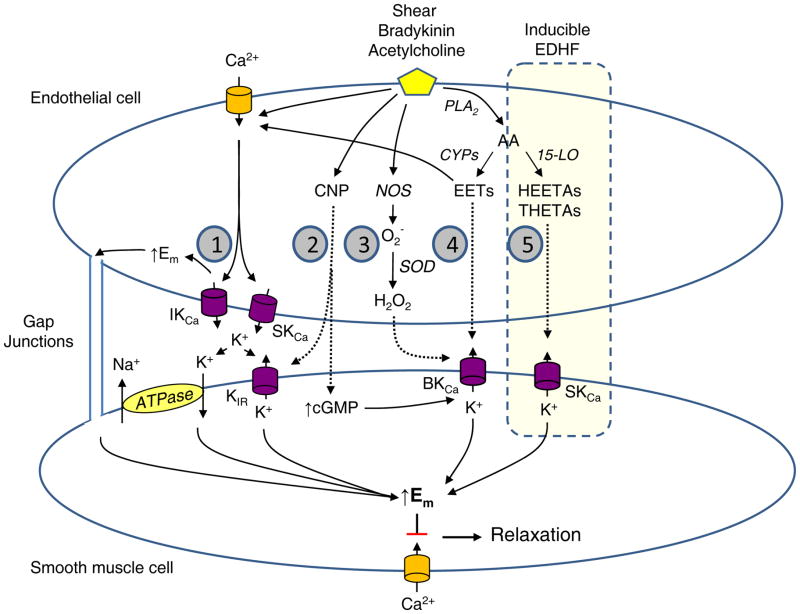
Figure 1.
Signaling mechanisms of the five major endothelium-derived hyperpolarizing factors (EDHFs). Shear stress or endothelium-dependent agonists including acetylcholine and bradykinin stimulate EDHF-dependent vascular relaxation. EDHF mediators include: (1) electrical transmission of endothelial hyperpolarization through myoendothelial gap junctions, (1) K ion, (2) C-type natriuretic peptide (CNP), (3) hydrogen peroxide (H2O2), (4) epoxyeicosatrienoic acids (EETs) and (5) 15-lipoxygenase-1 (15-LO-1) metabolites, 15-H-11,12-EETA and 11,12,15-THETA. The 15-LO-1 inducible EDHF pathway is highlighted. The shaded numbers in the figure refer to the Classification of EDHF section of the text and correspond to numbered descriptions of 5 major EDHFs.
Regulation of the synthesis and release of endothelial factors
In addition to acetylcholine, bradykinin and shear stress, endothelial mediators of vasorelaxation are regulated in other ways. NO is synthesized from L-arginine by NOS. There are three NOS isozymes: endothelial NOS (eNOS), inducible NOS (iNOS) and neuronal NOS (nNOS)14. Of these enzymes, eNOS and nNOS are constitutively expressed in various cells and tissues while the expression of iNOS is regulated. Similarly, there are two isoforms of COX involved in the synthesis of PGs including PGI2 15. COX-1 is constitutively expressed in most cells whereas COX-2 is an inducible form of the enzyme. Cytokines and growth factors induce the expression of both COX-2 and iNOS. It would seem logical that analogous dual regulatory pathways exist for EDHF. We present evidence in this review that constitutive EDHF (cEDHF) or cEDH and inducible EDHF (iEDHF) exist in arteries. A cEDHF is expressed in the endothelium of most blood vessels under basal conditions, is released by endothelial agonists and mediates a portion of endothelium-dependent relaxation; however, the chemical or electrical nature may vary with vascular beds and with species. iEDHF is dormant under normal circumstances so does not contribute to endothelial agonist-stimulated relaxations. However, iEDHF is induced under various physiological and pathological conditions to participate in endothelium-dependent relaxation by endothelial agonists. Thus, the contribution to iEDHF to endothelium-dependent relaxation may be absent under basal conditions but its contribution is enhanced when induced. While both COX-1 and COX-2 produce PGI2 and eNOS, nNOS and iNOS produce NO, cEDHF and iEDHF are different chemical entities or chemical and electrical entities. Since cEDHF and iEDHF may activate different K channels, a synergistic or additive interaction is possible and may result in greater vasodilation with their combination.
Classification of EDHFs
The EDHFs can be divided into cEDHFs and iEDHFs based on our current knowledge (Figure 1)(The shaded numbers in Figure 1 refer to the corresponding numbered descriptions of major EDHFs below.):
Activation of endothelial intermediate conductance KCa (IKCa) and small conductance KCa (SKCa) channels hyperpolarizes endothelial cells and releases K ion into the sub-endothelial space 8, 10, 12, 13. This results in hyperpolarization of smooth muscle cells through gap junctional transfer of the hyperpolarization (EDH) and/or K ion activating Kir channels or the sodium-potassium ATPase on smooth muscle cells (EDHF). This EDH or EDHF is constitutive in several vascular beds. There is no current evidence of enhanced expression of its components.
Several EDHFs are constitutively expressed; however, their synthesis or expression may also increase. For example, CNP is constitutively expressed in endothelial cells, is released by endothelial agonists and relaxes smooth muscle by increasing cyclic GMP and membrane hyperpolarization 9. The endothelial expression and secretion of CNP is increased by transforming growth factor-β (TGF-β), tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1)16–18. In some, but not all, reports, platelet-derived growth factor (PDGF) and basic fibroblast growth factor (bFGF) increase CNP expression in smooth muscle cells. The enhanced secretion of CNP with these cytokines and growth factors is regulated by its synthesis and not endothelial agonists.
Hydrogen peroxide is formed by hydration of superoxide by superoxide dismutase 19, 20. It is constitutively present in some arteries and mediates a portion of the relaxation to endothelial agonists. The enzymatic source of superoxide varies with vascular bed. For example, superoxide may arise from NADPH oxidase, mitochondria or NOS 20–22. Although not studied in detail, the superoxide generating enzyme(s) rather than superoxide dismutase appears to be rate limiting in vascular hydrogen peroxide formation. The three NOS isozymes are sources of vascular superoxide and hydrogen peroxide in the mouse mesenteric artery 20. Thus, eNOS and nNOS represent constitutive sources of hydrogen peroxide and iNOS as an inducible source. However, iNOS is not regulated by endothelial agonists.
EETs are cytochrome P450 (CYP) metabolites of AA 23, 24. CYPs are a large family of enzymes with subsets that metabolize xenobiotics and drugs, synthesize steroid hormones and metabolize fatty acids. Several CYPs are constitutively expressed in endothelial cells including CYP2C and CYP2J. The EETs are synthesized by the endothelium and released by acetylcholine, bradykinin and shear stress 4, 6, 25. They hyperpolarize and relax vascular smooth muscle by activating large conductance KCa (BKCa) channels. Drugs, xenobiotics and hypoxia increase the expression of certain CYP epoxygenases and increase EET-mediated hyperpolarization and relaxation 26–29. Inflammatory cytokines reduce the expression and activity of CYP epoxygenases by increasing iNOS and NO synthesis 30. Thus, EETs function as cEDHFs; however, the synthesis of endothelial EETs may be increased by conditions or treatments that increase the expression of CYP epoxygenases.
15-H-11,12-EETA and 11,12,15-THETA are endothelial 15-lipoxygenase (15-LO) metabolites of AA 11, 31. They mediate a portion of the relaxations to acetylcholine in several arteries by activating smooth muscle cell SKCa-like channels and causing hyperpolarization. They may also act through other mechanisms. Basal expression of 15-LO is elevated in arteries of neonatal and young animals but is absent in adolescent and adult animals 32, 33. However, the expression of 15-LO is increased by cytokines, estrogen, hypoxia and hypercholesterolemia resulting in enhanced production of HEETA/THETAs and enhanced relaxations to acetylcholine 34–37. The 15-LO/HEETA/THETA pathway is an iEDHF and will be examined in more detail.
Role of LO metabolites of AA as EDHFs
Furchgott and Zawadzki first showed that endothelium-dependent relaxations to acetylcholine were mediated by a LO metabolite of AA 38. In the rabbit aorta, LO inhibitors and inhibitors of AA release by phospholipase A2 attenuated the relaxations to acetylcholine. COX inhibitors were without effect. Using the same pharmacological approach, a role for LO metabolites in the endothelium-dependent relaxations to acetylcholine, bradykinin, histamine, thrombin, substance P and other agonists was demonstrated in arteries from a variety of species and vascular beds 39–47. When the synthesis of NO and PGI2 are inhibited, the relaxations to acetylcholine are inhibited, but not blocked, indicating a component of the relaxations is mediated by EDHF 11, 31, 47. A role for EDHF was confirmed by showing that the residual relaxations to acetylcholine were inhibited by high extracellular K that blocks K channels and by the SKCa channel inhibitor, apamin. These relaxations are also inhibited by LO inhibitors. When antisense oligonucleotides were used to suppress the expression of 15-LO in rabbit aorta, the non- NO, non-PGI2-mediated relaxations to acetylcholine were inhibited 48. Scrambled oligonucleotides that did not affect 15-LO expression did not alter the relaxations to acetylcholine. These studies support a role for LO metabolites as EDHFs causing endothelium-dependent relaxations through activation of apamin-sensitive K channels. These K channels have properties of SKCa channels 49; however, SKCa channels are localized to the endothelium rather than smooth muscle so the term SKCa-like channels will be used 13.
Additionally, AA caused endothelium-dependent relaxations in a number of species and arteries 34, 39, 50–55. These relaxations were inhibited by LO inhibitors and enhanced by COX inhibitors. Inhibition of COX provided more AA for metabolism to vasoactive metabolites by LO 34. As with acetylcholine, the relaxations to AA were inhibited by high extracellular K and by apamin indicating a role for SKCa-like channels in the relaxations 49. In rabbit aorta, AA also hyperpolarized smooth muscle cells if the endothelium was intact but not when the endothelium was removed 31, 49, 56. Endothelium-dependent hyperpolarizations to AA were inhibited with the SKCa channel inhibitor, apamin. Thus, like acetylcholine, endothelium-dependent relaxations to AA are mediated by LO metabolites that activate apamin-sensitive SKCa-like channels, to cause smooth muscle membrane hyperpolarization and relaxation. These studies with acetylcholine and AA support a role for LO metabolites as EDHFs.
Endothelial metabolism of AA by 15-LO
LOs are a family of non-heme containing oxygenases that add molecular oxygen at double bonds of polyunsaturated fatty acids such as AA 57–59. In the case of AA, the LO product is a hydroperoxyeicosatetraenoic acid (HPETE) that is reduced by glutathione peroxidase to a hydroxyeicosatetraenoic acid (HETE). The LOs are named for the position of the hydroperoxy or hydroxy group on AA. In most cases, the vascular LOs were identified by immunoblotting or PCR. However, the rabbit aortic LO was cloned, sequenced and identified as 15-LO-1 48. It is localized to the endothelium. The two main LOs in arteries and endothelial cells of humans, dogs, pigs, cows and rabbits are 12-LO and 15-LO 11. 12-LO synthesizes predominately 12-HPETE, and 15-LO synthesizes predominately 15-HPETE 58, 59. The major vasoactive LO metabolites arise from 15-LO 11. Rats and mice have a single LO that synthesizes both 12- and 15-HPETE 60, 61. The rodent 12/15-LOs synthesize 12-HPETE in a three-fold greater amount than 15-HPETE. Thus, there are clear species differences in the LOs and AA products from vascular LOs. The vascular AA metabolites from rodent 12/15-LO differ sufficiently from human 15-LO that they may not be comparable.
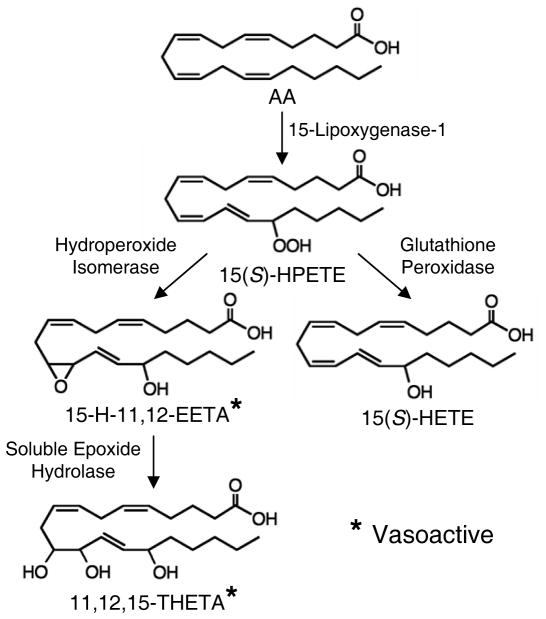
The vascular metabolism of AA by 15-LO is outlined in Figure 2. The endothelium is the major source of AA metabolites. Agonists such as acetylcholine or bradykinin regulate AA release from membrane phospholipids by activating either phospholipase A2 or the phospholipase C pathway 62, 63. Free AA is metabolized by 15-LO to 15(S)-HPETE 64. Two competing pathways metabolize 15(S)-HPETE. Reduction of 15(S)-HPETE by glutathione peroxidase produces 15(S)-HETE that has little or no vasoactivity. Alternatively, 15(S)-HPETE is rearranged by a hydroperoxide isomerase to 15-H-11,12-EETA 56, 64. The epoxy group is unstable in acid with a half-life of less than 10 s at pH 3 but 33 h at pH 7.4. Hydration of the epoxide also occurs enzymatically in arteries. 15-H-11,12-EETA is metabolized by soluble epoxide hydrolase (sEH) to 11,12,15-THETA 56. With three hydroxyl groups, many stereoisomers of 11,12,15-THETA are possible. However, of the possible isomers, only 11(R),12(S),15(S)-THETA comigrated with the 11,12,15-THETA produced by the aorta 65. The stereochemical configuration of endogenous 15-H-11,12-EETA is not known. Both 15-H-11,12-EETA and 11(R),12(S),15(S)-THETA cause relaxation 56, 65. In contrast, AA is metabolized by 12-LO to 12-HPETE and 12-HETE 57. 12-HETE is also the major metabolite of rodent 12/15-LO 60. 12-HETE relaxes some arteries but not others 66, 67. There is currently no evidence for the synthesis of other vasoactive metabolites from 12-HPETE by arteries.
Figure 2.
15-Lipoxygenase-1 pathway of endothelial cell arachidonic acid (AA) metabolism. 15(S)-hydroperoxyeicosatetraenoic acid (15(S)-HPETE), 15-hydroxy-11,12-epoxyeicosatrienoic acid (15-H-11,12-EETA), 15(S)-hydroxyeicosatetraenoic acid (15(S)-HETE), 11,12,15-trihydroxyeicosatrienoic acid (11,12,15-THETA). * 15-H-11,12-EETA and 11,12,15-THETA cause vascular relaxation and function as EDHFs.
Mechanism of action of endothelial 15-LO metabolites
As indicated above, AA causes endothelium-dependent hyperpolarizations and relaxations that are inhibited by blocking K channels with high extracellular K and by the SKCa channel inhibitor apamin but not by the IKCa/BKCa channel inhibitor charybdotoxin 31, 49. Aortic smooth muscle cells have a 24 pS K channel that is activated by calcium and inhibited by apamin. This is consistent with the presence of a SKCa-like channel. These findings suggest that AA is metabolized to a LO metabolite(s) that activate smooth muscle SKCa channels causing membrane hyperpolarization and relaxation. 15-H-11,12-EETA and 11,12,15-THETA as the major endothelial 15-LO metabolites would be expected to act by this same mechanism. Due to its instability, 15-H-11,12-EETA has not been tested directly on arteries. However, inhibition of the metabolism of 15-H-11,12-EETA with a sEH inhibitor enhances the relaxations to acetylcholine 56. Inhibition of sEH also enhances the endothelium-dependent hyperpolarization and relaxation to AA. These enhanced hyperpolarizations and relaxations to AA are blocked by LO inhibition. These indirect studies indicate that 15-H-11,12-EETA causes smooth muscle hyperpolarization and vasorelaxation.
11,12,15-THETA also causes relaxation of rabbit and mouse arteries 31, 65. This vasoactivity is limited to the specific 11,12,15-THETA stereoisomer that is produced by the vascular endothelium. 11(R),12(S),15(S)-THETA relaxes rabbit arteries while seven other stereoisomers are inactive 65. 11(R),12(S),15(S)-THETA also increases the activity of an apamin-sensitive K channel in aortic smooth muscle cells. The finding that a specific stereoisomer of 11,12,15-THETA activates smooth muscle SKCa-like channels and causes relaxation indicates that a specific binding site or receptor must mediate these effects. This binding site/receptor has not been characterized further.
Regulation of Vascular 15-LO
While the release of AA from membrane lipids by agonists is essential for 15-LO synthesis of eicosanoids, 15-LO is the key enzyme in regulating the activity of this pathway and regulating its contribution to vascular tone. Thus, variation in the expression of 15-LO is an important determinant of the pathway’s role in physiological and pathological conditions. Increasing the expression of 15-LO enhances endothelium-dependent relaxations whereas inhibition of 15-LO has the opposite effect. For example, rabbit arteries were transduced with a 15-LO-containing adenovirus to increase the endothelial expression of 15-LO 68, 69. In transduced arteries, the increased expression of endothelial 15-LO increased the synthesis of the 15-LO metabolites, 15-H-11,12-EETA and 11,12,15-THETA and the relaxations to acetylcholine and AA when compared to non-transduced arteries. Pharmacological inhibition of 15-LO activity or decreasing endothelial 15-LO expression with 15-LO antisense oligonucleotides reduced the synthesis of the vasoactive AA metabolites and inhibited relaxations to AA and acetylcholine 31, 48, 50, 65. Thus, variations in the expression of 15-LO are important to the endothelium-dependent regulation of vascular tone and vascular EDHF activity.
The expression of 15-LO is regulated at several levels: epigenetic modifications, transcription and translation 70, 71. Transcription is stimulated by cytokines such as interleukin (IL)-4 and IL-13 through activation of the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) transcription factor pathway or the p38 mitogen-activated protein kinase (MAPK)-STAT or protein kinase C (PKC)-STAT pathways 72, 73. Steroid hormone receptors and hormone response elements, Ku70/80 and GATA transcription factors and methylation and acetylation of the 15-LO promoter region also regulate 15-LO transcription 71, 74, 75. The 3′-untranslated region of the 15-LO mRNA contains a differentiation control element 76. When the translational repressor proteins heterogeneous nuclear ribonucleoproteins (hnRNP)-E1 or -K are bound to this element, translation of the 15-LO mRNA is prevented. Activities of hnRNP proteins are regulated by phosphorylation 77. When phosphorylated by MAP kinase or src kinase, hnRNP localizes to the cytosol, rather than the nucleus, so translation is inhibited 78. Growth factors and agonist acting on G-protein coupled receptors may regulate 15-LO mRNA transcription through src kinase- or MAP kinase-hnRNP pathway. Thus, there are multiple regulators of 15-LO expression and many mechanisms of regulation.
Conditions and endogenous chemicals regulating 15-LO and iEDHF activity
A number of pathological conditions and endogenous compounds induce the expression of 15-LO. While there are many studies on the regulation of 15-LO in cultured cell lines or inflammatory cells, investigations in endothelial cells, smooth muscle cells and arteries are more limited. In arteries, the increase in endothelial 15-LO is accompanied by an increase in the synthesis of vasodilatory eicosanoids, smooth muscle membrane hyperpolarization and enhanced endothelium-dependent relaxations 68, 69.
1. Changes in 15-LO with age
EDHF activity decreases with age in rodents and rabbits 32, 33, 79. 15-LO expression was very high in aortas from 1 week old rabbits and declined by 10% at 4 weeks and 40% by 8 weeks of age 32, 33. This was paralleled by a decline in the synthesis of AA metabolites of 15-LO by aortic and mesenteric rings over the same time period. In the presence of a COX inhibitor, the major AA metabolites were THETAs, HEETAs and HETEs and the synthesis of these metabolites were blocked by the LO inhibitor BW755C. At 8 weeks of age, the synthesis of HEETA, THETA and HETEs were not detectable. The EDHF-mediated relaxations to acetylcholine were reduced in aortas and mesenteric arteries of 4, 8 and 16 week old rabbits compared to 1 week old rabbits 32. Maximal relaxations to acetylcholine were reduced by approximately 50% in arteries from 16 week old rabbits compared to 1 week old rabbits. Responses to AA were similarly reduced with age whereas endothelium-independent relaxations to sodium nitroprusside were not changed. In arteries from 1 week old rabbits, EDHF-mediated relaxations to acetylcholine were inhibited by 40–50% by either LO inhibition by BW755C or IKCa/BKCa channel inhibition by charybdotoxin 33. The combination of BW755C and charybdotoxin blocked the relaxations. Thus, a component is mediated by 15-LO and HEETA and THETA and a component by IKCa channels and K ion. In contrast, LO inhibition did not alter the relaxations to acetylcholine in aortas from 16 week old rabbits whereas IKCa/BKCa channel inhibition blocked the relaxations. Similar results were obtained in vivo when blood pressure decreases to acetylcholine were measured 33. Thus, at birth, vascular 15-LO expression is high and 15-LO metabolites contribute to endothelium-dependent dilation. The mechanism responsible for the increased expression is not known. The 15-LO-mediated component of EDHF is lost in the months following birth while the IKCa component is unchanged and becomes the sole mediator. These findings are consistent with the 15-LO pathway functioning as an iEDHF and the IKCa-K ion pathway functioning as cEDHF. iEDHF and cEDHF function together in early life to maintain dilation and organ perfusion. Over the next months, the stimulus inducing 15-LO expression is reduced so the pathway becomes dormant and cEDHF mediates endothelium-dependent relaxations.
2. Regulation of 15-LO by hypoxia
Hypoxia induced the expression of 15-LO in rabbit lung microsomes when compared to normoxia 36. In contrast, 12-LO was unchanged. 15-LO was localized in the lung to pulmonary arteries. Lung microsomes synthesized THETAs, HEETAs and 15-HETE, and the synthesis of these AA metabolites was 3-fold greater with hypoxia than normoxia. 15-HETE synthesis was blocked by LO inhibition but not by COX or CYP inhibitors. Hypoxia also increased the expression of 15-LO-1 and the synthesis of 15-HETE compared to normoxia in human microvascular endothelial cells 80. Thus, both in vivo and in vitro hypoxic conditions increase vascular 15-LO. The mechanism for hypoxia inducing 15-LO expression is not known. The promoter region of 15-LO does not contain the hypoxia response element suggesting that hypoxia acts indirectly to induce the enzyme, possibly through platelet-derived growth factor (PDGF)81.
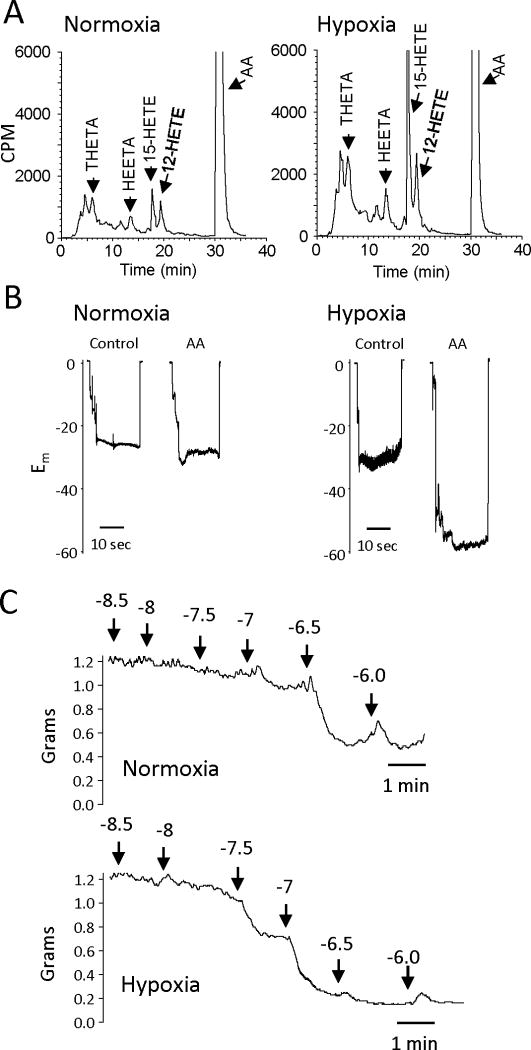
In rabbit aortic endothelial cells, hypoxia increased the metabolism of AA by 15-LO. The synthesis of THETAs, HEETAs and HETEs was greater in cells exposed to hypoxic compared to normoxic conditions (Figure 3). The membrane potential did not differ in aortic smooth muscle cells of normoxic or hypoxic rabbits. However, hyperpolarization to AA was enhanced in smooth muscle cells of hypoxic rabbits. The acetylcholine-induced relaxations mediated by EDHF were also greater in arterial rings from hypoxic compared to normoxic rabbits. Thus, hypoxia induces the expression of 15-LO resulting in the increased synthesis of THETA and HEETA and increased hyperpolarization and relaxation. Hypoxia activates this iEDHF.
Figure 3.
Effect of hypoxia on 15-LO activity in the rabbit vasculature.
(A) AA metabolism of rabbit aortic endothelial cells exposed to normal oxygen (21% O2, normoxia) or reduced oxygen (0.7% O2, hypoxia). Endothelial cells were incubated with 14C-AA in the presence of indomethacin (10 μM) for 8 h under normoxic or hypoxic conditions. The media was removed, extracted and metabolites resolved by reverse-phase HPLC. Radioactivity of column fractions was measured by scintillation counting. Migration times of known standards are noted on each chromatogram.
(B) AA-induced hyperpolarization responses in mesenteric arteries from normoxic or hypoxic rabbits. Male 8 week old rabbits were exposed to either normoxic conditions (21% O2) or hypoxic conditions (12% O2) for 5 days. Membrane potential cell impalement recordings were made in the freshly dissected arterial segments incubated with indomethacin (10 μM) and phenylephrine (100 nM) with or without AA (10 μM).
(C) Acetylcholine relaxations of mesenteric arteries from normoxic (top trace) or hypoxic (bottom trace) rabbits. Arterial rings were mounted in a myograph, stretched to a basal tension of 1 gram, treated with indomethacin (10 μM) and N-nitro-L-arginine (30 μM) and constricted with phenylephrine (0.1 – 1.0 μM). Increasing concentrations of acetylcholine were added and relaxation responses recorded.
3. Regulation of 15-LO by cholesterol, anemia and atherosclerosis
The expression of 15-LO is increased in atherosclerotic lesions in arteries of rabbits and human 82, 83. The synthesis of THETAs, HEETAs and HETEs was increased in aortas of rabbits fed a high cholesterol diet for 2 weeks compared to a normal diet 34. The synthesis of the THETAs was inhibited by LO inhibitors but enhanced by COX inhibition. Hypercholesterolemia and elevated 15-LO were associated with a 40% lowering of the blood hematocrit 84. Lowering the hematocrit by other means and experimental anemia increase 15-LO expression 70, 84.
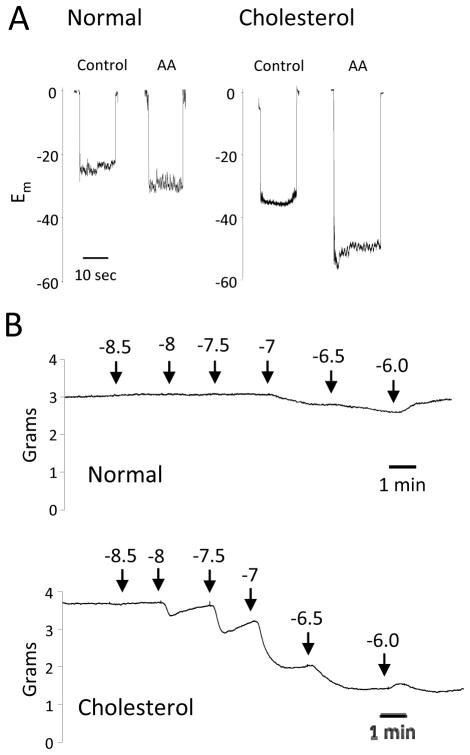
In addition to the increase in 15-LO expression, relaxations to AA were enhanced in aortas of cholesterol-fed rabbits compared to controls 34. Similarly, the relaxations were enhanced by COX inhibition and reduced by LO inhibition or removal of the endothelium. While the membrane potential was unchanged in smooth muscle cells of aortas from normal and cholesterol-fed rabbits, hyperpolarization to AA was greater in smooth muscle of cholesterol-fed rabbits (Figure 4). The EDHF component of relaxation is also greater with cholesterol feeding. In NOS- and COX-inhibited aortic rings, acetylcholine relaxations were enhanced in rings from cholesterol-fed rabbits compared to rabbits on a normal diet. Thus, the 15-LO component of EDHF is increased with hypercholesterolemia.
Figure 4.
Effect of a high cholesterol diet on aortic vascular activity. Male 8 week old rabbits were fed either normal chow (normal) or cholesterol enriched (2% cholesterol) chow (cholesterol) for 2 weeks.
(A) AA-induced hyperpolarization responses. Membrane potential cell impalement recordings were made in the freshly dissected aortic segments incubated with indomethacin (10 μM) and phenylephrine (100 nM) with or without AA (10 μM).
(B) Acetylcholine relaxations. Arterial rings from rabbits fed normal chow (top trace) or rabbits fed cholesterol-enriched chow (bottom trace) were mounted in a myograph, stretched to a basal tension of 2 grams, treated with indomethacin (10 μM) and N-nitro-L-arginine (30 μM) and constricted with phenylephrine (0.1 – 1.0 μM). Increasing concentrations of acetylcholine were added and relaxation responses recorded.
4. Regulation of 15-LO by interleukins
Both IL-4 and IL-13 stimulate 15-LO expression in a variety of cell types 35, 73, 85. As indicated above, IL-4 and IL-13 activate transcription of the 15-LO gene through the JAK-STAT, src-STAT or PKC-STAT pathways 73. Transgenic mice overexpressing IL-4 show increased 15-LO activity in the lung, spleen, kidney and heart 72. IL-4 induced the transcription of the 15-LO-1 gene in human endothelial cells; however, an increase in the expression of the 15-LO-1 enzyme was not detected 85. The reason for this dissociation between gene activation and protein expression is not known. In monocytes and other cell types, IL-4 increases 15-LO-1 mRNA, protein and AA metabolites 72. IL-13 increased the transcription of the 15-LO-1 gene and expression of the 15-LO-1 protein in rabbit aortic endothelial cells and aortic rings 35. In aortic rings, 15-LO was localized predominately to the endothelium. Following IL-13 treatment, immunohistochemical staining for 15-LO markedly increased in the endothelium and in smooth muscle cell layers next to the endothelium. Metabolism of AA to THETAs, HEETAs and HETEs by aortic rings was greater in IL-13-treated rings compared to control rings. Similarly, relaxations to AA were enhanced in rings treated with IL-13 compared to untreated rings. These studies indicate that the 15-LO-EDHF pathway is induced by Th2 cytokines, IL-4 and IL-13.
5. Regulation of 15-LO by estrogen
Gender and estrogen clearly influence EDHF activity. The EDHF-mediated relaxations to acetylcholine in femoral arteries were greater in female than male mice 86. This difference was absent in female mice lacking the estrogen receptor. Acetylcholine-induced relaxations that are mediated by EDHF were greater in estrogen-treated male rats than control rats 87. When estrogen deficiency is produced by ovariectomy of female rats, the opposite effect is observed 88. Ovariectomy reduced EDHF-mediated relaxations in mesenteric arteries. Estrogen treatment of the ovariectomized rats restored the EDHF component to relaxation.
The expression of 15-LO and 5-LO, but not 12-LO, increased in pulmonary arteries of female rabbits compared to male rabbits 37. This was associated with an increased metabolism of AA to 15-HETE and 5-HETE by arteries from females compared to males. When male pulmonary arteries were treated with estrogen, the expression of 15-LO was increased in estrogen-treated arteries compared with control arteries. Additionally, the metabolism of AA to THETAs, HEETAs and HETEs was enhanced in estrogen-treated pulmonary arteries. EDHF activity was not evaluated in these arteries. Estrogen treatment of human endothelial cells also increased the expression of 15-LO by three-fold 89. These studies indicate that estrogen increases the expression of the 15-LO pathway in arteries and endothelial cells.
6. Other regulators of 15-LO
A number of other endogenous mediators increase the cellular expression of 15-LO; however, these mediators were tested in cultured cells and not in endothelial cells or arteries. Thus, their ability to induce the endothelial 15-LO pathway of iEDHF is in need of investigation. For example, angiotensin II, aldosterone, PDGF, TGF-β, IL-8, growth hormone-releasing peptide-2, peroxisome proliferator-activated receptor-γ agonist and high glucose increase 15-LO expression or rodent 12/15-LO expression in cultured smooth muscle cells or mesangial cells when compared with untreated cells 90–96. 15-LO expression is also increased in human umbilical arteries from patients with pre-eclampsia compared to normal patients 97.
In summary, many conditions, cytokines, growth factors and hormones regulate 15-LO and/or 12/15-LO expression in vascular and non-vascular cells; however, their role in regulating iEDHF activity has not been investigated. Clearly, age, hypoxia, hypercholesterolemia and atherosclerosis, Th2 cytokines and estrogen increase the expression of 15-LO and enhance the synthesis of 15-LO-derived vasodilatory eicosanoids, smooth muscle cell hyperpolarization and EDHF-mediated relaxation. Thus, these conditions and mediators induce 15-LO-mediated iEDHF activity.
Interactions between iEDHF and other endothelial mediators
PGI2, NO, cEDHF and iEDHF may be co-release from the endothelium by endothelial agonists. Thus, interactions between these endothelial mediators in regulating vascular tone are important. COX and 15-LO complete for the same substrate, AA. As a result, when COX was inhibited, more AA was available for metabolism by 15-LO and the synthesis of 11,12,15-THETA and 15-H-11,12-EETA increased 34. In rabbit mesenteric arteries, relaxations to AA were partially inhibited by COX inhibition and blocked by the combination of COX and LO inhibition suggesting near equal contributions to relaxation by COX and 15-LO metabolites 54. The effect of PGI2 on relaxations to 11,12,15-THETA or 15-H-11,12-EETA and visa versa has not been determined. The relaxations to acetylcholine were significantly inhibited by LO and SKCa-like channel inhibition but not by COX inhibition. Thus, the 15-LO pathway of AA metabolism contributes more to acetylcholine-induced relaxations than the COX pathway. The reason for the difference in the contributions of these pathways to the relaxations to AA and acetylcholine is not known.
The interaction between the 15-LO and NOS pathways has been investigated 98. The NO donor DPTA-NONOate relaxed the rabbit aorta, and the relaxations were blocked by the soluble guanylyl cyclase inhibitor ODQ but not by LO inhibition. The relaxations to acetylcholine were inhibited by ODQ, NOS inhibition or LO inhibition and blocked by the combination of ODQ and LO inhibition. Thus, the NOS and LO pathways mediate the relaxations to acetylcholine. In contrast, ODQ did not alter relaxations to AA whereas the combination of COX and LO inhibition blocked the relaxations. Consistent with this finding, the metabolism of AA to THETA, HEETA and 15-HETE by rabbit aorta was not altered by NOS inhibition or by physiological concentrations of DPTA-NONOate. When endogenous NO synthesis was inhibited, DPTA-NONOate was added to restore the NO contribution. The amount of relaxation to acetylcholine was the same in the presence or absence of DPTA-NONOate. Thus, the NOS and 15-LO pathways act in parallel to mediate the relaxations to acetylcholine. Also, NO does not alter the activity of the 15-LO pathway.
iEDHF enhanced relaxations mediated by cEDHF (Figure 5)55. In rabbit mesenteric arteries, acetylcholine stimulated the endothelial release of THETAs and voltage-dependent outward K currents from endothelial cells. Thus, THETAs and K ion are co-released from the endothelium by acetylcholine. In the presence of COX and NOS blockade, inhibition of the synthesis or action of the THETA with a LO inhibitor or SKCa channel inhibitor partially inhibited the relaxations to acetylcholine as did inhibition of K release or K action with an IKCa/BKCa channel inhibitor or Kir channel inhibitor. Combining inhibitors of both pathways blocked the relaxations of acetylcholine indicating their combined participation in the relaxations. Addition of 10.9 mM K ion potentiated the relaxations to both AA and 11,12,15-THETA. Thus, acetylcholine-induced, EDHF-dependent relaxation of mesenteric arteries involves two separate and parallel mechanisms: K ion, a cEDHF and 11,12,15-THETA, an iEDHF.
Figure 5.
Co-release of constitutive (c) and inducible (i) EDHFs mediate synergistic vasorelaxation. In vascular endothelial cells, acetylcholine activates; 1) calcium influx which stimulates IKCa and SKCa channels resulting in K ion efflux (the cEDHF pathway) and 2) PLA2 release of AA from membrane phospholipids. AA is metabolized by 15-LO-1 to HEETA and THETA (the iEDHF pathway). 15-LO-1 expression is increased by hypoxia, hypercholesterolemia, interleukin-4 (IL-4), interleukin-13 (IL-13), estrogen and anemia. The cEDHF and iEDHF pathways cause smooth muscle hyperpolarization via distinct synergistic mechanisms. For the cEDHF pathway, endothelial cell hyperpolarization from K ion efflux is transmitted to the smooth muscle through myoendothelial gap junctions or K ions activate smooth muscle KIR channels and the Na/K ATPase. HEETAs and THEETAs from the iEDHF pathway activate smooth muscle SKCa channels.
Role of EDHF/EDH in the endothelial dysfunction of pathological conditions
Many cardiovascular diseases such as hypertension, diabetes, ischemic heart disease, renal failure and congestive heart failure are associated with reductions in endothelium-dependent relaxations allowing endothelium-dependent constrictors or circulating constrictors to act unopposed 99. This has been termed endothelial dysfunction and is thought to contribute to the pathology of these diseases. Correction of endothelial dysfunction may represent a new therapeutic approach. The diminished endothelium-dependent relaxations may be due to a reduced contribution of NO and/or EDHF. The role(s) of specific EDHFs or EDH in endothelial dysfunction may vary in specific diseases but comprehensive investigations are lacking.
1. Hypertension
In isolated arteries from renal or spontaneously hypertensive rats, endothelium-dependent relaxations and hyperpolarizations to acetylcholine were reduced 100–103. The contributions of both NO and EDHF/EDH were diminished. In spontaneously hypertensive rats, the cEDHF/EDH pathway mediated by potassium ion was reduced 103. While the contribution by endothelial IKCa channels was unaltered, the contributions of both SKCa and Kir channels were reduced. The iEDHF pathway was not investigated; however, iEDHF did not compensate for the reduction of cEDHF to maintain endothelial function. Endothelium-dependent dilation was also decreased in patients with essential hypertension 104–106. Measuring endothelium-dependent dilation by forearm blood flow, the NO contribution to dilation was lost in hypertensive patients; however, the EDHF component mediated by EETs was retained and partially compensated for the loss of NO 104. In contrast, both the NO and EET components were reduced in hypertensive patients when endothelium-dependent dilation was measured by changes in radial artery diameter 105, 106. The 15-LO iEDHF pathway has not been assessed in essential hypertension. Clearly, in hypertension, cEDHF and iEDHF do not compensate for the loss of NO so endothelial dysfunction ensues.
2. Diabetes mellitus and insulin resistance
Endothelium-dependent dilations and hyperpolarizations were reduced in arteries from rats with streptozotocin-induced diabetes and in Zucker diabetic fatty rats 107–110. Experimental studies differ regarding whether reductions in NO and EDHF or only EDHF are responsible for the endothelial dysfunction. The relaxations and hyperpolarizations to IKCa channel activation with EBIO or IKCa and SKCa channel activation with NS309 were reduced in diabetic rats 108, 109. Thus, the reduction in EDHF/EDH in diabetes may be attributed to attenuation of K channels of the cEDHF pathway. The iEDHF pathway has not been investigated. However, as with hypertension, iEDHF does not prevent the endothelial dysfunction of diabetes.
Endothelium-dependent relaxations were also reduced in insulin-resistant rats 111. The EDHF-mediated relaxations were inhibited by the cytochrome P450 inhibitor miconazole in normal rats, but miconazole was without effect in insulin-resistant rats. Induction of cytochrome P450 with 14-days of pentobarbital treatment increased the EDHF-mediated relaxations in insulin-resistant rats and improved endothelial dysfunction. Phenobarbital treatment also reduced blood pressure in insulin-resistant rats. The 15-LO iEDHF pathway was not studied, and the effect of phenobarbital on 15-LO is not known. Under these experimental conditions, induction of cytochrome P450-derived EETs restores endothelial function in insulin-resistant rats.
3. Congestive heart failure and ischemic preconditioning
Congestive heart failure (CHF) was induced in rats by left coronary artery ligation 112, 113. Endothelium-dependent relaxations did not differ in arteries of control and CHF rats. However, the EDHF-mediated contribution was increased, and the NO-mediated contribution was reduced. Upregulation of EDHF compensated for the loss of NO. The EDHF pathway, cEDHF or iEDHF, that increased in CHF was not determined. Similar results were obtained in patients with CHF 114. Increases in forearm blood flow with acetylcholine were the same in normal subjects and CHF patients. Inhibition of NO synthesis reduced the dilation to acetylcholine in normal subjects but had no effect in CHF patients. Thus, EDHF is the major mediator of endothelium-dependent dilation in CHF patients. The EDHF mediating dilation in CHF is not known.
Similarly, in mice, ischemia-reperfusion injury reduced the endothelium-dependent relaxations to acetylcholine 115. A reduction in the NO-mediated component was responsible. The EDHF component was increased. Hypoxic pre-conditioning enhanced both the NO- and EDHF-mediated relaxations and eliminated the endothelial dysfunction. The expression of endothelial TRPV4 and connexins and phosphorylation of eNOS increased with preconditioning. The increase in TRPV4 resulted in increased intracellular calcium in endothelial cells that activates eNOS and KCa channels. Increases in connexins enhance gap junctions to increase EDH and the spread of the EDHF response along the artery. Hypoxia increases the expression of 15-LO; however, the role of 15-LO iEDHF was not determined.
In summary, endothelial dysfunction is observed in many cardiovascular diseases. In some cases such as hypertension and diabetes, EDHF is impaired and unable to compensate for a loss of NO so endothelium-dependent dilation is reduced. However, in other conditions such as CHF, EDHF compensates for the loss of NO so endothelial function is normal. The role of cEDHF and iEDHF in these diseases merits further study.
Conclusion
The endothelium releases a number of chemicals that mediate endothelium-dependent hyperpolarization. The reasons for redundancy are not apparent. Likely, the EDHFs serve different physiological and/or pathological functions. We suggest that some EDHFs such as the 15-LO pathway function as an iEDHF while others are cEDHFs. The 15-LO pathway has many characteristics of an inducible pathway. The principle enzyme regulating the activity of the pathway is 15-LO. It is regulated in multiple ways including transcription, translation and epigenetics, and by many conditions and hormones such as hypoxia, anemia, hypercholesterolemia, atherosclerosis, estrogen, ILs and likely others (angiotensin, aldosterone, cytokines and growth factors). While elevated in newborn and young animals, the 15-LO pathway is suppressed and dormant in older animals but can be reactivated. Hypoxia, hypercholesterolemia, estrogen and IL-13 increase the expression of 15-LO, the synthesis of vasoactive 15-H-11,12-EETA and 11,12,15-THETA, increases membrane hyperpolarization to AA and increases relaxations to AA and acetylcholine. When activated, the 15-LO pathway is additive with the COX and NOS vasodilatory pathways and synergistic with a cEDHF. Additional studies are needed to fully define the regulation of 15-LO-iEDHF and its role in pathological and physiological settings. It is possible that EDHFs do not vary with the vascular bed and species but are suppressed in some vascular beds and species and induced in others. Understanding the mechanisms regulating the expression of various EDHFs may define which are cEDHFs and iEDHFs and clarify the vascular and species distribution of EDHFs and their relative activities.
Acknowledgments
The authors thank Dr. Sandra Pfister for her insightful discussions and Ms. Gretchen Barg for her secretarial assistance. These studies were supported by grants from the National Heart, Lung and Blood Institute (HL-103673 and HL-37981).
References
- 1.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- 2.Cohen RA, Vanhoutte PM. Endothelium-dependent hyperpolarization: Beyond nitric oxide and cyclic gmp. Circulation. 1995;92:3337–3349. doi: 10.1161/01.cir.92.11.3337. [DOI] [PubMed] [Google Scholar]
- 3.Feletou M, Vanhoutte PM. Endothelium-derived hyperpolaizing factor. Where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- 4.Campbell WB, Fleming I. Epoxyeicosatrienoic acids and endothelium-dependent responses. Pflugers Arch. 2010;459:881–895. doi: 10.1007/s00424-010-0804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagao T, Illiano S, Vanhoutte PM. Heterogenous distribution of endothelium-dependent relaxations resistant to n-nitro-l-arginine in rats. Am J Physiol. 1992;263:H1090–H1094. doi: 10.1152/ajpheart.1992.263.4.H1090. [DOI] [PubMed] [Google Scholar]
- 6.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 7.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kandaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: Do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan SD, Nilsson H, Ahluwalia A, Hobbs AJ. Release of c-type natriuretic peptide accounts for the biological activity of endothelium-derived hyperpolarizing factor. Proc Natl Acad Sci USA. 2003;100:1426–1431. doi: 10.1073/pnas.0336365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- 11.Chawengsub Y, Gauthier KM, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in the regulation of vascular tone. Am J Physiol. 2009;297:H495–H507. doi: 10.1152/ajpheart.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edwards G, Feletou M, Gardener MJ, Thollon C, Vanhoutte PM, Weston AH. Role of gap junctions in the responses to edhf in rat and guinea-pig small arteries. Brit J Pharmacol. 1999;128:1788–1794. doi: 10.1038/sj.bjp.0703009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarizing factors and associated pathways: A synopsis. Pfluegers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- 14.Griffith OW, Stuehr DJ. Nitric oxide synthases: Properties and catalytic mechanism. Ann Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 15.Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 16.Suga S, Itoh H, Komatsu H, Ogawa Y, Hama N, Yoshimasa T, Nakao K. Cytokine-induced c-type natriuretic peptide (cnp) secretion from vascular endothelial cells. Endocrinology. 1993;133:3038–3041. doi: 10.1210/endo.133.6.8243333. [DOI] [PubMed] [Google Scholar]
- 17.Suga S, Nakao K, Itoh H, Komatsu Y, Ogawa Y, Hama N, Imura H. Endothelial production of c-type natriuretic peptide and its marked augmentation by transforming growth factor-beta. J Clin, Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellitti DF, Koles N, Mendonca MC. Regulation of c-type natriuretic peptide expression. Peptides. 2011;32:1964–1971. doi: 10.1016/j.peptides.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Faraci FM, Didion SP. Vascular protection: Superoxide dismutase isoforms in the vessel wall. Arterioscler Thromb Vasc Biol. 2004;24:1367–1273. doi: 10.1161/01.ATV.0000133604.20182.cf. [DOI] [PubMed] [Google Scholar]
- 20.Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch. 2010;459:915–922. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- 21.Gorlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing nadph oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res. 2000;87:26–32. doi: 10.1161/01.res.87.1.26. [DOI] [PubMed] [Google Scholar]
- 22.Xi Q, Cheranov SY, Jaggar JH. Mitochrondria-derived reactive oxygen species dilate cerebral arteries by activating ca sparks. Circ Res. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Capdevila J, Chacos N, Werringloer J, Prough RA, Estabrook RW. Liver microsomal cytochrome p450 and the oxidative metabolism of archidonic acid. Proc Natl Acad Sci USA. 1981;78:5362–5366. doi: 10.1073/pnas.78.9.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeldin DC. Epoxygenase pathways of arachidonic acid metabolism. J Biol Chem. 2001;276:36059–36062. doi: 10.1074/jbc.R100030200. [DOI] [PubMed] [Google Scholar]
- 25.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 26.Fissllthaler B, Hinsch N, Chataigneau T, Popp R, Kiss L, Busse R, Fleming I. Nifedipine increases cytochrome p4502c expression and endothelium-derived hyperpolarizing factor-mediated responses in coronary arteries. Hypertension. 2000;36:270–275. doi: 10.1161/01.hyp.36.2.270. [DOI] [PubMed] [Google Scholar]
- 27.Bauersachs J, Christ M, Ertl G, Michaelis UR, Fissllthaler B, Busse R, Fleming I. Cytochrome p450 2c expression and edhf-mediated relaxation in porcine coronary arteries is increased by cortisol. Cardiovas Res. 2002;54:669–675. doi: 10.1016/s0008-6363(02)00257-2. [DOI] [PubMed] [Google Scholar]
- 28.Earley S, Pastuszyn A, Walker BR. Cytochrome p-450 epoxygenase products contribute to attenuated vasoconstriction after chronic hypoxia. Am J Physiol Heart Circ Physiol. 2003;285:H127–136. doi: 10.1152/ajpheart.01052.2002. [DOI] [PubMed] [Google Scholar]
- 29.Fissllthaler B, Michaelis UR, Randriamboavonjy V, Busse R, Fleming I. Cytochrome p450 epoxygenases and vascular tone: Novel role for hmg-coa reductase inhibitors in the regulation of cyp 2c expression. Biochim Biophy Acta. 2003;1619:332–339. doi: 10.1016/s0304-4165(02)00492-0. [DOI] [PubMed] [Google Scholar]
- 30.Kessler P, Popp R, Busse R, Schini-Kerth VB. Proinflammatory mediators chronically downregulate the formation of the endothelium-derived hyperpolarizing factor in arteries via a nitric oxide/cyclic gmp-dependent mechanism. Circulation. 1999;99:1878–1884. doi: 10.1161/01.cir.99.14.1878. [DOI] [PubMed] [Google Scholar]
- 31.Campbell WB, Spitzbarth N, Gauthier KM, Pfister SL. 11,12,15-trihydroxyeicosatrienoic acid mediates acetylcholine-induced relaxations in the rabbit aorta. Am J Physiol. 2003;285:H2648–H2656. doi: 10.1152/ajpheart.00412.2003. [DOI] [PubMed] [Google Scholar]
- 32.Tang X, Aggarwal N, Holmes BB, Kuhn H, Campbell WB. Age-related decrease in 15-lipoxygenase contributes to reduced vasorelaxation in rabbit aorta. Am J Physiol. 2008;294:H679–H687. doi: 10.1152/ajpheart.01053.2007. [DOI] [PubMed] [Google Scholar]
- 33.Aggarwal NT, Gauthier KM, Campbell WB. 15-lipoxygenase metabolites contribute to age-related reduction in acetylcholine-induced hypotension in rabbits. Am J Physiol. 2008;295:H89–H96. doi: 10.1152/ajpheart.00054.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pfister SL, Spitzbarth N, Edgemond W, Campbell WB. Vasorelaxation by an endothelium-derived metabolite of arachidonic acid. Am J Physiol. 1996;270:H1021–H1030. doi: 10.1152/ajpheart.1996.270.3.H1021. [DOI] [PubMed] [Google Scholar]
- 35.Tang X, Spitzbarth N, Kuhn H, Chaitidis P, Campbell WB. Interleukin-13 upregulates vasodilatory 15-lipoxygenase eicosanoids in rabbit aorta. Arterioscler Thromb Vasc Biol. 2003;23:1768–1774. doi: 10.1161/01.ATV.0000092915.03128.73. [DOI] [PubMed] [Google Scholar]
- 36.Zhu D, Medhora M, Campbell WB, Spitzbarth N, Baker JE, Jacobs ER. Chronic hypoxia activates lung 15-lipoxygenase, which catalyzes production of 15-hete and enhances constriction in neonatal rabbit pulmonary arteries. Circ Res. 2003;92:992–1000. doi: 10.1161/01.RES.0000070881.65194.8F. [DOI] [PubMed] [Google Scholar]
- 37.Pfister SL. Role of lipoxygenase metabolites of arachidonic acid in enhanced pulmonary artery contractions of female rabbits. Hypertension. 2011;57:825–832. doi: 10.1161/HYPERTENSIONAHA.110.168716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 39.DeMey JG, Claeys M, Vanhoutte PM. Endothelium-dependent inhibitory effects of acetylcholine, adenosine triphosphate, thrombin and arachidonic acid in the canine femoral artery. J Pharmacol Exp Ther. 1982;222:166–173. [PubMed] [Google Scholar]
- 40.Van de Voorde J, Leusen I. Role fo the endothelium in the vasodilator response of rat thoracic aorta to histamine. Europ J Pharmacol. 1983;28:113–120. doi: 10.1016/0014-2999(83)90056-0. [DOI] [PubMed] [Google Scholar]
- 41.Forstermann U, Mugge A, Frolich JC. Endothelium-dependent relaxations of human epicardial coronary arteries: Frequent lack of effect of acetylcholine. Europ J Pharmacol. 1986;128:277–281. doi: 10.1016/0014-2999(86)90778-8. [DOI] [PubMed] [Google Scholar]
- 42.Förstermann U, Alheid U, Frolich JG, Mulsch A. Mechanisms of action of lipoxygenase and cytochrome p-450-mono-oxygenase inhibitors in blocking endothelium-dependent vasodilation. Brit J Pharmacol. 1988;93:569–578. doi: 10.1111/j.1476-5381.1988.tb10312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Förstermann U, Hertting G, Neufang B. The role of endothelial and nonendothelial prostaglandins in the relaxation of isolated blood vessels of the rabbit induced by bradykinin and acetylcholine. Brit J Pharmacol. 1986;87:521–532. doi: 10.1111/j.1476-5381.1986.tb10194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minami Y, Toda N. Possible involvement of 5-lipoxygenase products in the generation of endothelium derived relaxing factor. J Pharmacol Exp Ther. 1989;250:1055–1060. [PubMed] [Google Scholar]
- 45.Miller AW, Katakam PVG, Lee H-C, Tulbert CD, Busija DW, Weintraub NL. Arachidonic acid-induced vasodilation of rat small mesenteric arteries is lipoxygenase-dependent. J Pharmacol Exp Ther. 2003;304:139–144. doi: 10.1124/jpet.102.041780. [DOI] [PubMed] [Google Scholar]
- 46.Stapleton PA, Goodwill AG, James ME, Frisbee JC. Altered mechanisms of endothelium-dependent dilation in skeletal muscle arterioles with genetic hypercholesterolemia. Am J Physiol. 2007;293:R1110–R1119. doi: 10.1152/ajpregu.00410.2007. [DOI] [PubMed] [Google Scholar]
- 47.Gauthier KM, Goldman DH, Aggarwal NT, Chawengsub Y, Falck JR, Campbell WB. Role of arachidonic acid lipoxygenase metabolites in acetylcholine-induced relaxations in mouse arteries. Am J Physiol. 2010;300:H725–H735. doi: 10.1152/ajpheart.00696.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tang X, Holmes BB, Nithipatikom K, Hillard CJ, Kuhn H, Campbell WB. Reticulocyte 15-lipoxygenase-1 is important in acetylcholine-induced endothelium-dependent vasorelaxation in rabbit aorta. Arterioscler Thromb Vasc Biol. 2006;26:78–84. doi: 10.1161/01.ATV.0000191640.73313.ad. [DOI] [PubMed] [Google Scholar]
- 49.Gauthier KM, Spitzbarth N, Edwards EM, Campbell WB. Apamin-sensitive k+ currents mediate arachidonic acid-induced relaxations of rabbit aorta. Hypertension. 2004;43:413–419. doi: 10.1161/01.HYP.0000110945.84443.d2. [DOI] [PubMed] [Google Scholar]
- 50.Pfister SL, Campbell WB. Arachidonic acid- and acetylcholine-induced relaxations of rabbit aorta. Hypertension. 1992;20:682–689. doi: 10.1161/01.hyp.20.5.682. [DOI] [PubMed] [Google Scholar]
- 51.Singer HA, Peach MJ. Endothelium-dependent relaxation of rabbit aorta. I. Relaxation stimulated by arachidonic acid. J Pharmacol Exp Ther. 1983;226:790–795. [PubMed] [Google Scholar]
- 52.Förstermann U, Neufang B. The endothelium-dependent vasodilator effect of acetylcholine: Characterization of the endothelial relaxing factor with inhibitors of arachidonic acid metabolism. Europ J Pharmacol. 1984;103:65–70. doi: 10.1016/0014-2999(84)90190-0. [DOI] [PubMed] [Google Scholar]
- 53.Rosolowsky M, Campbell WB. Role of pgi2 and eets in the relaxation of bovine coronary arteries to arachidonic acid. Am J Physiol. 1993;264:H327–H335. doi: 10.1152/ajpheart.1993.264.2.H327. [DOI] [PubMed] [Google Scholar]
- 54.Zhang DX, Gauthier KM, Chawengsub Y, Holmes BB, Campbell WB. Cyclooxygenase- and lipoxygenase-dependent relaxations to arachidonic acid in rabbit small mesenteric arteries. Am J Physiol. 2005;288:H302–H309. doi: 10.1152/ajpheart.00661.2004. [DOI] [PubMed] [Google Scholar]
- 55.Zhang DX, Gauthier KM, Chawengsub Y, Campbell WB. Ach-induced relaxations of rabbit small mesenteric arteries: Role of arachidonic acid metabolites and k. Am J Physiol. 2007;293:H152–H159. doi: 10.1152/ajpheart.00268.2006. [DOI] [PubMed] [Google Scholar]
- 56.Chawengsub Y, Aggarwal NT, Nithipatikom K, Gauthier KM, Anjaiah S, Hammock BD, Falck JR, Campbell WB. Identification of 15-hydroxy-11,12-epoxyeicosatrienoic acid as a vasoactive 15-lipoxygenase metabolite in rabbit aorta. Am J Physiol. 2008;294:H1348–H1356. doi: 10.1152/ajpheart.01326.2007. [DOI] [PubMed] [Google Scholar]
- 57.Yamamoto S. Mammalian lipoxygenases: Molecular structures and functions. Biochimica et Biophysica Acta. 1992;1128:117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- 58.Kuhn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 59.Yoshimoto T, Takahashi Y. Arachidonate 12-lipoxygenases. Prostaglandins Other Lipid Mediat. 2002;68–69:245–262. doi: 10.1016/s0090-6980(02)00034-5. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe T, Medina JF, Haeggstrom JZ, Radmark O, Samuelsson B. Molecular cloning of a 12-lipoxygenase cdna from rat brain. Europ J Biochem. 1993;212:605–612. doi: 10.1111/j.1432-1033.1993.tb17699.x. [DOI] [PubMed] [Google Scholar]
- 61.Freire-Moar J, Alavi-Nassab A, Ng M, Mulkins M, Sigal E. Cloning and characterization of a murine macrophage lipoxygenase. Biochim Biophys Acta. 1995;1254:112–116. doi: 10.1016/0005-2760(94)00199-9. [DOI] [PubMed] [Google Scholar]
- 62.Fulton D, McGiff JC, Quilley J. Role of phospholipase c and phospholipase a2 in the nitric oxide-independent vasodilator effect of bradykinin in the rat perfused heart. J Pharmacol Exp Ther. 1996;278:518–526. [PubMed] [Google Scholar]
- 63.Tang X, Edwards EM, Holmes BB, Falck JR, Campbell WB. Role of phospholipase c and diacylglyceride lipase pathway in arachidonic acid release and acetylcholine-induced vascular relaxation in rabbit aorta. Am J Physiol. 2006;290:H37–H45. doi: 10.1152/ajpheart.00491.2005. [DOI] [PubMed] [Google Scholar]
- 64.Pfister SL, Spitzbarth N, Nithipatikom K, Edgemond WS, Falck JR, Campbell WB. Identification of 11,14,15- and 11,12,15-trihydroxyeicosatrienoic acids as endothelium-derived relaxing factors of rabbit aorta. J Biol Chem. 1998;273:30879–30887. doi: 10.1074/jbc.273.47.30879. [DOI] [PubMed] [Google Scholar]
- 65.Gauthier KM, Chawengsub Y, Goldman DM, Conrow RE, Anjaiah S, Falck JR, Campbell WB. 11(r),12(s),15(s)-trihydroxyeicosa-5(z),8(z),13(e)-trienoic acid: An endothelium-derived 15-lipoxygenase metabolite that relaxes rabbit aorta. Am J Physiol. 2008;294:H1467–H1472. doi: 10.1152/ajpheart.01052.2007. [DOI] [PubMed] [Google Scholar]
- 66.Zink MH, Oltman CL, Lu T, Katakam PVG, Kaduce TL, Lee H-C, Dellsperger KC, Spector AA, Myers PR, Weintraub NL. 12-lipoxygenase in porcine coronary microcirculation: Implications for coronary vasoregulation. Am J Physiol. 2001;280:H693–H704. doi: 10.1152/ajpheart.2001.280.2.H693. [DOI] [PubMed] [Google Scholar]
- 67.Ma YH, Harder DR, Clark JE, Roman RJ. Effects of 12-hete on isolated dog renal arcuate arteries. Am J Physiol. 1991;261:H451–456. doi: 10.1152/ajpheart.1991.261.2.H451. [DOI] [PubMed] [Google Scholar]
- 68.Aggarwal N, Chawengsub Y, Gauthier KM, Viita H, Yla-Herttuala S, Campbell WB. Endothelial 15-lipoxygenase-1 overexpression increases acetylcholine-induced hypotension and vasorelaxation in rabbits. Hypertension. 2008;51:246–251. doi: 10.1161/HYPERTENSIONAHA.107.104125. [DOI] [PubMed] [Google Scholar]
- 69.Aggarwal N, Holmes BB, Cui L, Viita H, Yla-Herttuala S, Campbell WB. Adenoviral expression of 15-lipoxygenase-1 in rabbit aortic endothelium: Role in arachidonic acid-induced relaxation. Am J Physiol. 2007;292:H1033–H1041. doi: 10.1152/ajpheart.00624.2006. [DOI] [PubMed] [Google Scholar]
- 70.Kuhn H, Heydeck D, Brinckman R, Trebus F. Regulation of cellular 15-lipoxygenase activity on pretranslational, translational and posttranslational levels. Lipids. 1999;34:s2273–s2279. doi: 10.1007/BF02562317. [DOI] [PubMed] [Google Scholar]
- 71.Kelavkar UP, Harya NS, Hutzley J, Bacich DJ, Monzon FA, Chandran U, Dhir R, O’Keefe DS. DNA methylation paradigm shift: 15-lipoxygenase-1 upregulation in prostatic intraepithelial neoplasia and prostate cancer by atypical promoter hypermethylation. Prostaglandins Other Lipid Mediat. 2007;82:185–197. doi: 10.1016/j.prostaglandins.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 72.Heydeck D, Thomas L, Schnurr K, Trebus F, Thierfelder WE, Ihle JN, Kuhn H. Interleukin-4 and -13 induce upregulation of the murine macrophage 12/15-lipoxygenase activity: Evidence for the involvement of transcription factor stat6. Blood. 1998;92:2503–2510. [PubMed] [Google Scholar]
- 73.Xu B, Bhattacharjee A, Roy B, Feldman GM, Cathcart MK. Role of protein kinase c isoforms in the regulation of interleukin-13-induced 15-lipoxygenase gene expression in human monocytes. J Biol Chem. 2004;279:15954–15960. doi: 10.1074/jbc.M400413200. [DOI] [PubMed] [Google Scholar]
- 74.Kelavkar UP, Wang S, Badr KF. Ku autoantigen (DNA helicase) is required for interleukins-13/-4-induction of 15-lipoxygenase-1 gene expression in human epithelial cells. Genes Immun. 2000;1:237–250. doi: 10.1038/sj.gene.6363665. [DOI] [PubMed] [Google Scholar]
- 75.Shureiqi I, Zuo X, Broaddus R, Wu Y, Guan B, Morris JS, Lippman SM. The transcription factor gata-6 is overexpressed in vivo and contributes to silencing 15-lox-1 in vitro in human colon cancer. FASEB J. 2007;21:743–753. doi: 10.1096/fj.06-6830com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ostareck DH, Ostareck-Lederer A, Wilm M, Thiele BJ, Mann M, Hentze MW. Mrna silencing in erythroid differentiation: Hnrnp k and hnrnp e1 regulate 15-lipoxygenase translation from the 3′ end. Cell. 1997;89:597–606. doi: 10.1016/s0092-8674(00)80241-x. [DOI] [PubMed] [Google Scholar]
- 77.Bomsztyk K, Denisenko O, Ostrowski J. Hnrnpk: One protein multiple processes. BioEssays. 2004;26:629–638. doi: 10.1002/bies.20048. [DOI] [PubMed] [Google Scholar]
- 78.Habelhah H, Shah K, Huang L, Ostareck-Lederer A, Burlingame AL, Shokat KM, Hentze MW, Ronai Z. Erk phosphorylation drives cytoplasmic accumulation of hnrnp-k and inhibition of mrna translation. Nat Cell Biol. 2001;3:325–330. doi: 10.1038/35060131. [DOI] [PubMed] [Google Scholar]
- 79.Goto K, Fujii K, Onaka U, Abe I, Fujishima M. Angiotensin-converting enzyme inhibitor prevents age-related endothelial dysfunction. Hypertension. 2000;36:581–587. doi: 10.1161/01.hyp.36.4.581. [DOI] [PubMed] [Google Scholar]
- 80.Bajpai AK, Blaskova E, Pakala SB, Zhao T, Glasgow WC, Penn JS, Johnson DA, Rao GN. 15(s)-hete production in human retinal microvascular endothelial cells by hypoxia: Novel role for mek1 in 15(s)-hete induced angiogenesis. Invest Ophthalmol Vis Sci. 2007;48:4930–4938. doi: 10.1167/iovs.07-0617. [DOI] [PubMed] [Google Scholar]
- 81.Zhang L, Ma J, Shen T, Wang S, Ma C, Liu Y, Ran Y, Wang L, Liu L, Zhu D. Platelet-derived growth factor (pdgf) induces pulmonary vascular remodeling through 15-lo/15-hete pathway under hypoxic conditions. Cell Signal. 2012;24:1931–1939. doi: 10.1016/j.cellsig.2012.06.007. [DOI] [PubMed] [Google Scholar]
- 82.Yla-Herttuala S, Rosenfeld ME, Parthasarathy S, Sigal E, Sarkioja T, Witztum JL, Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger rna colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991;87:1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hiltunen T, Luoma J, Nikkari T, Yla-Herttuala S. Induction of 15-lipoxygenase mrna and protein in early atherosclerotic lesions. Circulation. 1995;92:3297–3303. doi: 10.1161/01.cir.92.11.3297. [DOI] [PubMed] [Google Scholar]
- 84.Trebus F, Heydeck D, Schimke I, Gerth C, Kuhn H. Transient experimental anemia in cholesterol-fed rabbits induces systemic overexpression of the reticulocyte-type 15-lipoxygenase and protects from aortic lipid deposition. Prostaglandins Leukot Essent Fatty Acids. 2002;67:419–428. doi: 10.1054/plef.2002.0452. [DOI] [PubMed] [Google Scholar]
- 85.Lee YW, Kuhn H, Kaiser S, Hennig B, Daugherty A, Toborek M. Interleukin-4 induces transcription of the 15-lipoxygenase-i gene in human endothelial cells. J Lipid Res. 2001;42:783–791. [PubMed] [Google Scholar]
- 86.Luksha L, Poston L, Gustafsson JA, Hultenby K, Kublickiene K. The oestrogen receptor beta contributes to sex related differences in endothelial function of murine small arteries via edhf. J Physiol. 2006;577:945–955. doi: 10.1113/jphysiol.2006.121939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Woodman OL, Boujaoude M. Chronic treatment of male rats with daidzein or 17 beta-oestradiol induces the contribution of edhf to endothelium-dependent relaxation. Brit J Pharmacol. 2004;141:322–328. doi: 10.1038/sj.bjp.0705603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nawate S, Fukao M, Sakuma I, Soma T, Nagai K, Takikawa O, Miwa S, Kitabatake A. Reciprocal changes in endothelium-derived hyperpolarizing factor- and nitric oxide-system in the mesenteric artery of adult female rats following ovariectomy. Brit J Pharmacol. 2005;144:178–189. doi: 10.1038/sj.bjp.0706091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Maccarrone M, Bari M, Battista N, Finazzi-Agro A. Estrogen stimulates arachidonylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- 90.Natarajan R, Gu J-L, Rossi J, Gonzales N, Lanting L, Xu L, Nadler J. Elevated glucose and angiotensin ii increase 12-lipoxygenase activity and expression in porcine aortic smooth muscle cells. Proc Natl Acad Sci USA. 1993;90:4947–4951. doi: 10.1073/pnas.90.11.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Natarajan R, Bai W, Rangarajan V, Gonzales N, Gu J-L, Lanting L, Nadler J. Platelet-derived growth factor bb mediated regulation of 12-lipoxygenase in porcine aortic smooth muscle cells. J Cell Physiol. 1996;169:391–400. doi: 10.1002/(SICI)1097-4652(199611)169:2<391::AID-JCP19>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 92.Limor R, Kaplan M, Sharon O, Knoll E, Naidich M, Weisinger G, Keidar S, Stern N. Aldosterone up-regulates 12- and 15-lipoxygenase expression and ldl oxidation in human vascular smooth muscle cells. J Cell Biochem. 2009;108:1203–1210. doi: 10.1002/jcb.22352. [DOI] [PubMed] [Google Scholar]
- 93.Kim Y-S, Xu Z-G, Reddy MA, Li S-L, Lanting L, Sharma K, Adler SG, Natarajan R. Novel interactions between tgf-beta1 actions and the 12/15-lipoxygenase pathway in mesangial cells. J Am Soc Nephrol. 2005;16:352–362. doi: 10.1681/ASN.2004070568. [DOI] [PubMed] [Google Scholar]
- 94.Kim JH, Kang YJ, Kim HS. Il-8/cxcl8 upregulates 12-lipoxygenase expression in vascular smooth muscle cells from spontaneously hypertensive rats. Immune Network. 2009;9:106–113. doi: 10.4110/in.2009.9.3.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Titterington JS, Sukhanov S, Higashi Y, Vaughan C, Bowers C, Delafontaine P. Growth hormone-releasing peptide-2 suppresses vascular oxidative stress in apoe−/− mice but does not reduce atherosclerosis. Endocrinology. 2009;150:5478–5487. doi: 10.1210/en.2009-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Limor R, Sharon O, Knoll E, Many A, Weisinger G, Stern N. Lipoxygenase-derived metabolites are regulators of peroxisome proliferator-activated receptor gamma-2 expression in human vascular smooth muscle cells. Am J Hyperten. 2008;21:219–223. doi: 10.1038/ajh.2007.39. [DOI] [PubMed] [Google Scholar]
- 97.Wang Y, Zhu D, An Y, Sun J, Cai L, Zheng J. Preeclampsia activates 15-lipoxygenase and its metabolite 15-hydroxyeicosatetraenoic acid enhances constriction in umbilical arteries. Prost Leuk and Ess Fatty Acids. 2012;86:79–84. doi: 10.1016/j.plefa.2011.10.006. [DOI] [PubMed] [Google Scholar]
- 98.Aggarwal NT, Gauthier KM, Campbell WB. Endothelial nitric oxide and 15-lipoxygenase metabolites independently mediate relaxations of the rabbit aorta. Vascul Pharmacol. 2012;56:106–112. doi: 10.1016/j.vph.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feletou M, Vanhoutte PM. Endothelial dysfunction: A multifaceted disorder. Am J Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 100.Van de Voorde J, Vanheel B, Leusen I. Endothelium-dependent relaxation and hyperpolarization in aorta from control and renal hypertensive rats. Circ Res. 1992;70:1–8. doi: 10.1161/01.res.70.1.1. [DOI] [PubMed] [Google Scholar]
- 101.Fujii K, Tominaga M, Ohmori S, Kobayashi K, Koga T, Takata Y, Fujishima M. Decreased endothelium-dependent hyperpolarizations to acetylcholine in smooth muscle of the mesenteric artery of spontaneously hypertensive rats. Circ Res. 1992;70:660–669. doi: 10.1161/01.res.70.4.660. [DOI] [PubMed] [Google Scholar]
- 102.Michel FS, Mann GS, Man RYK, Vanhoutte PM. Hypertension and the absence of edhf-mediated respones favour endothelium-dependent contractions in renal arteries fo rats. Brit J Pharmacol. 2008;155:217–226. doi: 10.1038/bjp.2008.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weston AH, Porter EL, Harno E, Edwards G. Impairment of endothelial skca channels and of downstream hyperpolarizing pathways in mesenteric arteries from spontaneously hypertensive rats. Brit J Pharmacol. 2010;160:836–843. doi: 10.1111/j.1476-5381.2010.00657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Taddei S, Varsari D, Cipriano A, Ghiadoni L, Glaetta F, Franzoni F, Magagna A, Virdis A, Salvetti A. Identification of a cytochrome p450 2c9-derived endothelium-derived hyperpolarizing factor in human essential hypertensive patients. J Am Coll Cardiol. 2006;48:508–515. doi: 10.1016/j.jacc.2006.04.074. [DOI] [PubMed] [Google Scholar]
- 105.Bellien J, Joannides R. Epoxyeicosatrienoic acid pathway in human health and disease. J Cardiovas Pharmacol. 2012 doi: 10.1097/FJC.0b013e318273b007. [DOI] [PubMed] [Google Scholar]
- 106.Bellien J, Iacob M, Remy-Jouet I, Lucas D, Montell C, Gutierrez L, Vendeville C, Dreano Y, Mercier A, Thuillez C, Joannides R. Epoxyeicosatrienoic acids contribute with altered nitric oxide and endothelin-1 pathways to conduit artery endothelial dysfunction in essential hypertension. Circulation. 2012;125:1266–1275. doi: 10.1161/CIRCULATIONAHA.111.070680. [DOI] [PubMed] [Google Scholar]
- 107.Fukao M, Hattori Y, Kanno M, Sakuma I, Kitabatake A. Alterations in endothelium-dependent hyperpolaization and relaxation in mesenteric arteries from streptozotocin-induced diabetic rats. Brit J Pharmacol. 1997;121:1383–1391. doi: 10.1038/sj.bjp.0701258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wigg SJ, Tare M, Tonta MA, O’Brien RC, Meredith IT, Parkington HC. Comparison of effects of diabetes mellitus on an edhf-dependent and an edhf-independent artery. Am J Physiol. 2001;281:H232–H240. doi: 10.1152/ajpheart.2001.281.1.H232. [DOI] [PubMed] [Google Scholar]
- 109.Brondum E, Kold-Petersen H, Simonsen U, Aalkjaer C. Ns309 restores edhf-type relaxation in mesenteric small arteries from type 2 diabetic zdf rats. Brit J Pharmacol. 2010;159:154–165. doi: 10.1111/j.1476-5381.2009.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leo CH, Hart JL, Woodman OL. Impairment of both nitric oxide-mediated and edhf-type relaxation in small mesenteric arteries from rats with streptozotocin-induced diabetes. Brit J Pharmacol. 2011;162:365–377. doi: 10.1111/j.1476-5381.2010.01023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katakam PVG, Hoenig M, Ujhelyi MR, Miller AW. Cytochrome p450 activity and endothelial dysfunction in insulin resistance. J Vasc Res. 2000;37:426–434. doi: 10.1159/000025759. [DOI] [PubMed] [Google Scholar]
- 112.Malmsjo M, Bergdahl A, Zhao XH, Sun XY, Hedner T, Edvinsson L, Erlinge D. Enhanced acetylcholine and p2y-receptor stimulated vascular edhf-dilatation in congestive heart failure. Cardiovasc Res. 1999;43:200–209. doi: 10.1016/s0008-6363(99)00062-0. [DOI] [PubMed] [Google Scholar]
- 113.Ueda A, Ohyanagi M, Koida S, Iwasaki T. Enhanced release of endothelium-derived hyperpolarizing factor in small coronary arteries from rats with congestive heart failure. Clin Exp Pharmacol Physiol. 2005;32:615–621. doi: 10.1111/j.0305-1870.2005.04240.x. [DOI] [PubMed] [Google Scholar]
- 114.Katz SD, Krum H. Acetylcholine-mediated vasodilation in the forearm circulation of patients with heart failure: Indirect evidence for the role of endothelium-derived hyperpolarizing factor. Am J Cardiol. 2001;87:1089–1092. doi: 10.1016/s0002-9149(01)01466-7. [DOI] [PubMed] [Google Scholar]
- 115.Rath G, Saliez J, Behets G, Romero-Perez M, Leon-Gomez E, Bouzin C, Vriens J, Nilius B, Feron O, Dessy C. Vacular hypoxic preconditioning relies on trpv4-dependent calcium influx and proper intercellular gap junctions communication. Arterio Thromb Vasc Biol. 2012;32:2241–2249. doi: 10.1161/ATVBAHA.112.252783. [DOI] [PubMed] [Google Scholar]