Abstract
Background
Heart failure risk factors are diverse and likely to vary among world regions. Systematic review and pooled analysis were used describe contributions of major underlying risk factors for heart failure in six world regions.
Methods
Electronic databases were systematically searched, and 37 clinic-based studies representing 40 countries published 1980–2008 and reporting underlying risk factors for heart failure were included. Risk factors were classified as ischemic heart disease (IHD), hypertension, rheumatic/other valvular heart disease, cardiopulmonary disease, cardiomyopathy, and “other”. Crude and age- and sex-adjusted risk factor prevalence were estimated for each region using regression analysis, under specifications of overlapping as well as additive contributions.
Results
Many heart failure cases were assigned multiple underlying risk factors, leading to considerable overlap. Crude IHD prevalence among heart failure patients was >50% in Europe and North America, approximately 30–40% in East Asia and Latin America and the Caribbean, and <10% in sub-Saharan Africa. Age and sex adjustment attenuated regional differences in IHD-as-risk factor but IHD remained rare in sub-Saharan Africa. Hypertension prevalence was high in heart failure patients of all regions but highest in Eastern and Central Europe and sub-Saharan Africa (age- and sex-adjusted, 35.0% and 32.6%, respectively). Cardiomyopathy was most common in Latin American and the Caribbean and sub-Saharan Africa (age- and sex-adjusted, 19.8% and 25.7%).
Conclusions
Heart failure risk factors vary substantially among world regions. More detailed regional heart failure epidemiology studies are needed in order to quantify the global burden of heart failure and identify regional prevention and treatment strategies.
Keywords: heart failure, risk factors, epidemiology, global health
Introduction
Heart failure affects an estimated 23 million people worldwide,[1] and leads to substantial numbers of hospitalizations and health care costs.[2] Because heart failure is more common in older ages,[3] heart failure prevalence will continue to increase with aging of the world population.
Ischemic heart disease (IHD), hypertension, rheumatic fever and other valve disease, cardiomyopathy, cardiopulmonary disease, congenital heart disease, and other factors may all lead to heart failure, either alone or in concert with other risk factors. The pattern of heart failure risk factors are likely to vary across world regions based on risk factor prevalence and quality of health care. Past reviews found that IHD is the predominant cause of heart failure in Western high income nations, non-ischemic cardiomyopathies and rheumatic heart disease more common in developing regions, and IHD particularly rare in sub-Saharan Africa.[4, 5, 6] The standard of heart failure care has advanced dramatically in recent decades, but it is unclear if standard therapies, most of studied in patients with left ventricular systolic dysfunction, are equally effective in all types of heart failure patients and in all world regions. Data on regional prevalence of heart failure’s underlying risk factors are needed to develop region-specific priorities for heart failure research, prevention and early treatment.
Current International Classification of Disease (ICD) system classifies heart failure as an intermediate, not underlying cause of death, yet these rules are followed inconsistently around the world, even in high-income countries.[7] As a consequence, it is not possible to use routine vital statistics for valid or even comparable estimates of the upstream etiological causes of health failure worldwide. Past international literature reviews of heart failure causes were either limited in scope,[4, 5] or comprehensive but limited to one region,[6] and none used meta-analysis methods to pool studies or adjust for heart failure patient age or sex. To overcome these limitations, we conducted a systematic literature review and pooled analysis of studies with data on risk factors for heart failure in order to describe the worldwide prevalence of major underlying risk factors.
The author(s) of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology.[8]
Methods
Systematic literature review
Over the past three decades, most epidemiologic studies have based heart failure diagnosis on either the New York Heart Association (NYHA) functional classification[9] or Framingham Heart Study heart failure criteria (diagnosis based on symptoms and/or physical exam or radiologic signs of heart failure).[10] With the Framingham criteria as the standard, we included studies that described patients with a clinical diagnosis of heart failure based on typical symptoms (NYHA II-IV), physical examination findings, or cardiac imaging. Due to lack of a global sample of population-based studies of heart failure, clinic and hospital based studies were the main study type included. In order to avoid over-representing severe heart failure patients in some regions, all studies had to include participants with all of NYHA class II–IV functional status.
The major heart failure risk factors were IHD, hypertension, rheumatic and other valvular heart disease, cardiopulmonary disease, cardiomyopathy, and “other” (congenital heart disease and any other risk factors not included in the prior five categories). Cardiomyopathy was broadly defined according to the European Society of Cardiology 2007 definition as “a myocardial disorder in which the heart muscle is structurally and functionally abnormal, in the absence of coronary artery disease, hypertension, valvular disease and congenital heart disease sufficient to cause the observed myocardial abnormality.”[11] There are numerous causes of cardiomyopathy: familial, idiopathic, post-partum, auto-immune, infiltrative, infectious and other inflammatory, anemia and vitamin deficiency, heavy alcohol and other toxic exposures, and others. Because only one study used diagnostic testing in addition to self-report in order to identify hypertension as a unique cause (“hypertensive heart disease”, i.e. heart failure due to hypertension in the absence of IHD), [12] we treated all self-reported hypertension as a heart failure risk factor (with or without IHD). Alcohol was only recorded as a risk factor if it was reported as “heavy alcohol use” or “alcohol abuse”.
A research librarian (J.O.) and epidemiologist (A.M.) developed the search strategy. MEDLINE (via PubMed), EMBASE, and LILACS electronic literature databases were searched for heart failure papers, using controlled vocabulary (e.g., Medical Subject Heading, or MeSH) for heart failure plus key words related to heart failure, as well as either filters for clinical trials or the subheadings “epidemiology” and “mortality” (Appendix). A broad and inclusive search strategy was applied to low and middle income regions where heart failure data are scarce, and a restrictive strategy was used for high income regions where data are abundant. In the main searches, heart failure clinical trials were excluded because trial subjects are not likely representative of the general population, as subjects with certain etiological risk factors are commonly excluded.[3] In order to assess whether important heart failure papers were missed by focusing on epidemiologic studies and excluding clinical trials in the low and middle income region search, separate searches were also conducted omitting the “epidemiology” and “mortality” subheadings and filtering those results to low and middle income region and multinational clinical trial articles only.
The main search of “epidemiology” papers led to identification by one investigator (AM) of 87 papers for review. The “clinical trials” search led to identification of 30 clinical studies or trials from low and middle income regions. Because 87% of the trials and clinical studies had selection criteria that would bias assessment of risk factors among heart failure patients (37% selected participants with systolic dysfunction only, 23% selected only IHD patients, and 40% had other exclusions), clinical trials and clinical studies were not included in the review. However, the “clinical trials” search yielded 13 additional heart failure epidemiology papers which were reviewed (resulting total of 100). An additional 35 articles meeting inclusion criteria and additional studies identified in these papers’ bibliographies were considered for inclusion (Figure 1). Of 135 articles in the review, 53 cross-sectional studies with data on the etiology of heart failure were eligible for inclusion. Full text copies of eligible papers were obtained from the Columbia and Harvard University libraries and their affiliated libraries. Articles published in languages other than English or Spanish were translated. Two investigators (AM and SK) read all of the full text heart failure risk factor papers and reached consensus regarding inclusion of 38 papers (Figure 1). Included papers were original reports or reviews of clinic-based studies of unselected heart failure patients, inclusive of NYHA classes II–IV, that reported age and sex characteristics of the sample and heart failure risk factors (Table 1).
Figure 1.
Flow diagram describing the systematic review of heart failure risk factors among heart failure patients.
Table 1.
Prevalence of heart failure risk factors among heart failure patients in clinic or hospital-based studies, by world region. The seven main risk factor categories were ischemic heart disease (IHD), hypertension (HT), valvular disease (VHD), cardiopulmonary disease (CP), cardiomyopathy (CM), and other risk factors. NR = risk factor not reported in study.
| Region | Author(s) | Geographical region |
Data collection period |
Sample size |
Sex (%male) |
Age Mean (Range) |
%IHD | %HT | %VHD | %CP | %CM | Other | Type of CM |
Type of Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Western High Income | Baldasseroni et al.[26] | Italy | 1995–2000 | 5517 | 77% | 63.5 (14–96) | 46 | 13 | NR | NR | 36 | 5 | NR | Idiopathic/Other |
| Bourassa et al.[27] | Canada | 1988–1989 | 6273 | 74% | 62.2 (21<=) | 69 | 7 | NR | NR | NR | 24 | NR | Idiopathic/Other | |
| Cleland et al.[28] | Belgium | 2000–2001 | 41 | 63% | 70 | 76 | 59 | 51 | NR | 3 | 0 | NR | NR | |
| Denmark | 196 | 47% | 73 | 52 | 22 | 7 | NR | 1 | 0 | NR | NR | |||
| Finland | 569 | 43% | 75 | 66 | 47 | 24 | NR | 5 | 0 | NR | NR | |||
| France | 317 | 41% | 72 | 47 | 51 | 51 | NR | 22 | 0 | NR | NR | |||
| Germany | 702 | 65% | 67 | 82 | 65 | 35 | NR | 8 | 0 | NR | NR | |||
| Greece | 410 | 68% | 68 | 55 | 55 | 37 | NR | 12 | 0 | NR | NR | |||
| Ireland | 253 | 51% | 71 | 60 | 45 | 24 | NR | 10 | 0 | NR | NR | |||
| Israel | 584 | 57% | 75 | 76 | 58 | 18 | NR | 1 | 0 | NR | NR | |||
| Italy | 545 | 57% | 70 | 57 | 47 | 27 | NR | 11 | 0 | NR | NR | |||
| Netherlands | 101 | 57% | 68 | 72 | 35 | 48 | NR | 3 | 0 | NR | NR | |||
| Portugal | 347 | 48% | 71 | 39 | 47 | 12 | NR | NR | 0 | NR | NR | |||
| Spain | 633 | 53% | 73 | 37 | 56 | 28 | NR | 5 | 0 | NR | NR | |||
| Sweden | 553 | 47% | 77 | 59 | 39 | 30 | NR | 2 | 0 | NR | NR | |||
| Switzerland | 171 | 43% | 74 | 58 | 58 | 20 | NR | 4 | 0 | NR | NR | |||
| United Kingdom | 1700 | 48% | 75 | 66 | 47 | 17 | NR | 2 | 0 | NR | NR | |||
| Cowie et al.[29] | United Kingdom | 1995–1996 | 220 | 54% | 76 (29–95) | 36 | 14 | 7 | 2 | 3 | 34 | Alcohol, NR | Idiopathic, Other | |
| Davies et al.[30] | United Kingdom | 1995–1999 | 92 | 64% | 73(>=45) | 30 | 39 | 31.5 | NR | NR | 0 | NR | ||
| Fox et al.[12] | United Kingdom | 2001† | 332 | 54% | 76 (37–95) | 29 | 9 | 10 | NR | 2 | 46 | Alcohol | Idiopathic, Other | |
| González-Juanatey et al.[31] | Spain | 2005–2005 | 2249 | 55.492663 | 72(>=18) | 48 | 39 | 8 | 4 | 6 | 4 | NR | NR | |
| Hood et al.[32] | United Kingdom | 1997–1998 | 253 | 43.396226 | 74 (40–98) | 45 | 41 | 13 | 26 | NR | 0 | - | NR | |
| Adams et al.[33] | US | 2001–2004 | 105388 | 48 | 72.4 (>=18) | 57 | 73 | NR | NR | NR | 0 | - | NR | |
| Morgan et al.[34] | US | 2001–2002 | 522 | 77 | 61 (>=30) | 51 | 56 | NR | 19 | NR | 0 | - | NR | |
| Owan et al.[35] | US | 1987–2001 | 4596 | 55% | 73 | 59 | 55 | NR | NR | NR | 0 | - | NR | |
| Senni et al.[36] | US | 1991–1991 | 216 | 58% | 77.3 | 40 | 52 | NR | 23 | 1 | 0 | NR | NR | |
| 1981–1981 | 107 | 57% | 75 | 58 | 48 | 7 | NR | 4 | 0 | NR | NR | |||
| Siirilä-Waris et al.[33] | Finland | 2004–2004 | 620 | 50 | 75 | 55 | 55 | 13 | NR | 19 | 0 | NR | NR | |
| Teerlink et al.[37] | US | 1989–1990 | 1861 | 80% | 54.2 | 50 | 4 | 4 | NR | 3 | 39 | Alcohol, Viral, Postpartum Cardio-myopathy, Amyloidosis | Idiopathic/other | |
| van Veldhuisen et al.[38] | Belgium | 1992–1995 | 124 | 75% | 68 (18–80) | 65 | 5 | NR | NR | NR | 0 | - | NR | |
| Denmark | 81 | 89% | 65 (18–80) | 58 | 6 | NR | NR | NR | 0 | - | NR | |||
| France | 208 | 78% | 67 (18–80) | 40 | 4 | NR | NR | NR | 0 | - | NR | |||
| Germany | 223 | 82% | 61 (18–80) | 46 | 4 | NR | NR | NR | 0 | - | NR | |||
| Italy | 220 | 78% | 64 (18–80) | 39 | 13 | NR | NR | NR | 0 | - | NR | |||
| Netherlands | 427 | 77% | 67 (18–80) | 77 | 5 | NR | NR | NR | 0 | - | NR | |||
| Spain | 123 | 82% | 64 (18–80) | 54 | 6 | NR | NR | NR | 0 | - | NR | |||
| Switzerland | 82 | 87% | 61 (18–80) | 51 | 5 | NR | NR | NR | 0 | - | NR | |||
| United Kingdom | 337 | 82% | 65 (18–80) | 77 | 3 | NR | NR | NR | 0 | - | NR | |||
| Eastern and Central Europe | Bogdan et al.[39] | Poland | NR | 97 | 68% | 61(39–79) | 83 | NR | 12 | NR | 5 | 0 | NR | NR |
| Cleland et al.[28] | Austria | 2000–2001 | 335 | 50% | 73 | 53 | 54 | 41 | NR | 10 | 0 | NR | NR | |
| Czech Republic | 562 | 59% | 69 | 57 | 59 | 36 | NR | 10 | 0 | NR | NR | |||
| Georgia | 187 | 51% | 65 | 57 | 70 | 7 | NR | 3 | 0 | NR | NR | |||
| Hungary | 255 | 64% | 63 | 48 | 53 | 50 | NR | 17 | 0 | NR | NR | |||
| Lithuania | 226 | 46% | 67 | 84 | 69 | 41 | NR | 3 | 0 | NR | NR | |||
| Poland | 936 | 50% | 68 | 71 | 58 | 31 | NR | 3 | 0 | NR | NR | |||
| Russia | 370 | 56% | 65 | 77 | 67 | 29 | NR | 5 | 0 | NR | NR | |||
| Slovak Republic | 254 | 57% | 70 | 77 | 69 | 31 | NR | 2 | 0 | NR | NR | |||
| Slovenia | 454 | 51% | 71 | 51 | 50 | 45 | NR | 11 | 0 | NR | NR | |||
| Subsaharn Africa | Amoah et al.[40] | Ghana | 1992–1995 | 572 | 55% | 42 (0.04–95) | 10 | 23 | 20 | NR | 17 | 30 | NR | Congenital Heart Disease, Pericardial Disease, Idiopathic, Other |
| Antony[22] | Northern Nigeria | 1978–1978 | 315 | 45% | 36 (0–80) | NR | 12 | 14 | 6 | 60 | 9 | Anemia, Other | Idiopathic, Other | |
| Fofana et al.[41] | Guinea | 1981–1985 | 574 | 60% | 49 (15–80) | 1 | 37 | 14 | 7 | 19 | 21 | Syphlitic Heart Disease, NR | Congenital Heart Disease, Pericardial Disease, Senile Cardiomyopathy | |
| Karaye et al.[42] | Nigeria | 2007 | 79 | 56% | 46.9 | 8 | 57 | 13 | 3 | 24 | 13 | Postpartum Cardio-myopathy, NR | Pericardial Disease | |
| Kingue et al.[43] | Cameroon | 1998–2001 | 167 | 59% | 57 | 2 | 54 | 25 | 8 | 26 | 1 | NR | Congenital Heart Disease | |
| Ola et al.[44] | Nigeria | 2006† | 100 | 58% | 63 | NR | 58 | 10 | 15 | 11 | 8 | NR | Congenital Heart Disease | |
| Oyoo et al.[45] | Kenya | 1993–1993 | 91 | 48% | 38(13–90) | 2 | 18 | 32 | 8 | 25 | 15 | NR | Congenital Heart Disease, Pericardial Disease | |
| Stewart et al.[19] | South Africa | 2005–2006 | 844 | 43% | 55 | 9 | 33 | 8 | NR | 35 | 27 | NR | Right Heart Failure | |
| Middle East | Agarwal et al.[17] | Oman | 1992–1994 | 1164 | 61% | 58 (13–75) | 52 | 25 | 4 | 4 | 9 | 6 | Myocarditis, NR | Congenital Heart Disease, Pericardial Disease, Cardiac Arrhythmia, Pulmonary Embolism |
| East Asia | Cheng Kang'an, et al.[46] | China | 2000 | 6777 | 55% | 63.1 | 46 | 13 | 21 | NR | 8 | 10 | NR | Congenital Heart Disease, Idiopathic, Other |
| 1990 | 2181 | 60% | 63.8 | 34 | 10 | 37 | NR | 7 | 9 | NR | Congenital Heart Disease, Idiopathic, Other | |||
| 1980 | 1756 | 56% | 67.8 | 37 | 8 | 35 | NR | 6 | 11 | NR | Congenital Heart Disease, Idiopathic, Other | |||
| Chong et al.[47] | Malaysia | 2003† | 97 | 63% | 63.6 | 50 | 19 | 4 | 12 | 7 | 3 | Anemia, NR | Thyroid Disease | |
| Sanderson et al.[48] | Hong Kong | 1992–1992 | 409 | 0% | 75.6 (20–109) | 28 | 36 | 15 | 17 | NR | 4 | - | Idiopathic/Other | |
| 312 | 100% | 70.6 (20–109) | 27 | 29 | 11 | 30 | NR | 3 | - | Idiopathic/Other | ||||
| 730 | 44% | 73.5 (20–109) | 31 | 37 | 15 | 27 | NR | 14 | - | Idiopathic/Other | ||||
| Latin American & Caribbean | Barretto et al.[49] | Brazil | 1995–1995 | 903 | 60% | 52.6(0–98) | 33 | 7 | 22 | NR | 26 | 12 | NR | Congenital Heart Disease, Idiopathic, Other |
| Castro et al.[50] | Chile | 2002–2002 | 372 | 59% | 69 | 36 | 35 | 15 | NR | 9 | 5 | Alcohol, NR | LVH,‡ Idiopathic, Other | |
| de Campos Lopes et al.[51] | Brazil | 1998–2000 | 494 | 70% | 57.5 (15–90) | 40 | 23 | NR | NR | 25 | 12 | Chagas, Alcohol | Idiopathic, Other | |
| McSwain et al.[52] | Antigua and Barbuda | 1995–1996 | 138 | 37% | 69.2 (5 m-99) | 33 | 41 | 12 | NR | 2 | 12 | Alcohol | Mixed, Idiopathic, Other | |
| Oliveira et al.[53] | Brazil | 1993–1995 | 126 | 73% | 51.1 (18–82) | 17 | 10 | 6 | NR | 44 | 23 | Chagas | Idiopathic, Other | |
| Asia Pacific High Income | Itoh et al.[54] | Japan | 1978–1985 | 282 | 60% | (20–80) | 32 | 17 | 28 | NR | 16 | 7 | Other | Congenital Heart Disease, Idiopathic, Other |
| Seow et al.[18] | Singapore | 1998–1998 | 225 | 56% | 68.5 | 86 | 60 | 3 | NR | NR | 9 | NR | Idiopathic, Other | |
| Shiba et al.[55] | Japan | 2000–2005 | 1278 | 66% | 68.3 (>18) | 24 | NR | 25 | NR | 26 | 14 | Other | LVH | |
| Tsuchihashi et al.[56] | Japan | 1997–1997 | 230 | 60% | 69 (16–92) | 35 | 20 | 28 | NR | 19 | 17 | Other | Idiopathic/ Other | |
NR= not reported
observation years not reported, so publication year reported instead
LVH = left ventricular hypertrophy
Publication information and data on age, sex, heart failure risk factors, and country and region were extracted into a standard data extraction form. Risk factor prevalence was exclusively based on heart failure patients’ self-reported diagnosis history in almost all studies. All valvular heart disease was collapsed into a single category because the proportion of valvular disease due to rheumatic heart disease was not reported in all studies (including studies from some high rheumatic heart disease prevalence regions). Atrial fibrillation and diabetes mellitus were commonly reported “other” risk factors in high-income regions. Because evidence for an independent causal relationship between these risk factors and heart failure is relatively weak,[13, 14, 15] and it was impossible to determine whether atrial fibrillation or diabetes preceded or followed after heart failure diagnosis, we dropped these two risk factors from the main list of risk factors analyzed and analyzed the contributions of diabetes and atrial fibrillation separately (Appendix Table 2, Appendix Figures 1–3).
Study locations were collapsed into eight major world regions: 1) Western High Income (including North America and Western Europe), 2) Asia Pacific High Income (including Japan, South Korea and Singapore), 3) Eastern and Central Europe, 4) Sub-Saharan Africa, 5) Middle East, 6) East Asia, 7) South Asia and 8) Latin America and the Caribbean (Figure 2). South Asia was excluded from the analysis because the one paper screened from this region did not include data on participants’ age;[16] Middle East was excluded because only one study met inclusion criteria.[17] Therefore, results were estimated for six geographic regions. A paper from Singapore was excluded because 1) the relatively small size of the Singapore population compared with those of South Korea and Japan, 2) the participants’ ethnicities differed markedly from the other Asia Pacific High Income region studies (60% Chinese, 24% Malay, and 15% Indian ethnicities), and 3) a distinctly different heart failure risk factor pattern appeared (for example, 85% IHD).[18] Data for “non-black” South Africans from the Heart of Soweto Study[19] were excluded because the proportion of this group in the patient sample was higher than would be found in hospitals of the Sub-Saharan Africa region as a whole.
Figure 2.
Regions of the world with data published 1980–2008 and included in the review, according to the Global Burden of Disease 2005 classification (WHI = Western High Income, APHI = Asia Pacific High Income, C & E Europe = Central and Eastern Europe, East Asia, L America and Car = Latin America and Caribbean , and SS Africa = Sub-Saharan Africa).
Statistical Analysis
Analysis of crude and adjusted prevalence of underlying heart failure risk factors
For each region, distribution of reported prevalence was estimated for underlying risk factors as median and interquartile range. Mean age and percent of subjects who were male were compared across regions using one-way analysis-of-variance (ANOVA).
Multivariate analysis of underlying risk factors of heart failure
Underlying heart failure risk factors may vary by age and sex. Because mean participant age and proportion male varied across studies, relative to other studies and to the regional population, we sample-size weighted and adjusted the prevalence of different underlying risk factors for mean age and sex composition of the study populations within region. We conducted this analysis in two ways: first separately for each underlying risk factor and second for all underlying risk factors combined requiring their total contribution to become exactly 100%. The first analysis is relevant because it helps identify total contributions of each risk factor; the second is consistent with the ICD system which requires the identification of a single “underlying cause”.
We used logistic regression for the first analysis. In the second analysis, we used a multinomial logistic regression. Multinomial logistic regression estimates the probabilities of more than two outcome categories, relative to one another, as a function of independent covariates. Outcome variables in the model were probabilities of the six main heart failure risk factor categories. Independent variables were mean age of participants, percent of population who were male, and geographic region. A multinomial logistic model estimates the proportional contributions of each underlying risk factor–with contributions of different risk factors adding to 100%. Due to substantial overlap in heart failure risk factors in individual patients, multinomial logistic model estimates should not be interpreted as regional prevalence or exclusive clinical risk factor categories but rather as a pattern of adjusted relative probabilities of risk factors.
We estimated the uncertainty intervals of adjusted regional prevalence using Clarify software (http://gking.harvard.edu/clarify/docs/clarify.html) which uses repeated samples of the joint statistical distributions of regression model coefficients and performs a statistical simulation to estimate the uncertainty of the outcome. In this analysis 95% confidence intervals were based on 1000 simulations.
Results
The 38 studies initially included from the systematic review yield 74 data lines (risk factor sets) (Table 1, Appendix Table 2). Studies from Central and Eastern Europe region studies generally included older heart failure patients followed by those from Western High Income (mean ages 67.6 and 68.0 years, respectively), while mean age was lowest in the sub-Saharan Africa (48.5 years). Mean heart failure patient age was significantly different across regions (Appendix Table 3, one-way ANOVA p < 0.001). More male than female heart failure patients participated in almost all studies (overall percent male 52.5%, one-way ANOVA p < 0.001). All studies reported IHD and hypertension as distinct risk factors. Valvular heart disease, cardiopulmonary disease, and cardiomyopathy were reported as separate risk factors in some studies, but were included in “other risk factors” in others.
In crude analysis (Table 1, Figure 3), IHD was a risk factor for heart failure in >50% of patients in Western High Income and Eastern and Central Europe regions; 30–40% in East Asia, Asia Pacific High Income, and Latin American and Caribbean regions; and <10% in sub-Saharan Africa. Hypertension was a commonly reported risk factor in all regions, with an approximately 17% or more crude prevalence among heart failure cases. After adjustment for age and sex, variability in IHD-as-risk factor was attenuated, but IHD remained distinctly rare as a risk factor in sub-Saharan Africa [4.2% mean prevalence, 95% confidence interval (3.1%—5.5%), Figure 4]. After age and sex adjustment, hypertension was distinctly more prevalent among heart failure patients in Eastern and Central Europe [35.0% (32.7%—37.3%)], and in sub-Saharan Africa [32.6% (29.6%—35.7%)].
Figure 3.
Crude proportion of heart failure with underlying risk factors in six major world regions in included studies (median and interquartile range; WHI = Western High Income, APHI = Asia Pacific High Income, C & E Europe = Central and Eastern Europe, East Asia, L America and Car = Latin America and Caribbean , and SS Africa = Sub-Saharan Africa).
Figure 4.
Age and sex-adjusted absolute contributions of major underlying risk factors to heart failure in six world regions (vertical bars represent 95% confidence intervals; WHI = Western High Income, APHI = Asia Pacific High Income, C & E Europe = Central and Eastern Europe, East Asia, L America and Car = Latin America and Caribbean , and SS Africa = Sub-Saharan Africa).
Among the studies specifically reporting rheumatic heart disease prevalence, the highest median crude prevalences of rheumatic heart disease were 34% in East Asia 14% in sub-Saharan Africa (Table 1). Cardiopulmonary disease was a commonly reported risk factor for heart failure only in East Asia [Figures 3 and 4, age-, sex-adjusted prevalence 11.9% (9.5%—14.6%)]. Cardiomyopathy was a predominant risk factor of heart failure in Sub-Saharan Africa, Latin America and the Caribbean, and Asia Pacific High Income [age-, sex-adjusted prevalence 25.7% (22.8%—28.5%), [19.8% (16.5%—23.4%)], and 16.5% (12.8%—20.6%), respectively]. Cardiomyopathy was often attributed to Chagas’ disease in Latin American studies. The proportion of risk factors in the “other” category was largely driven by the presence of younger heart failure patients with congenital heart disease in the study sample.
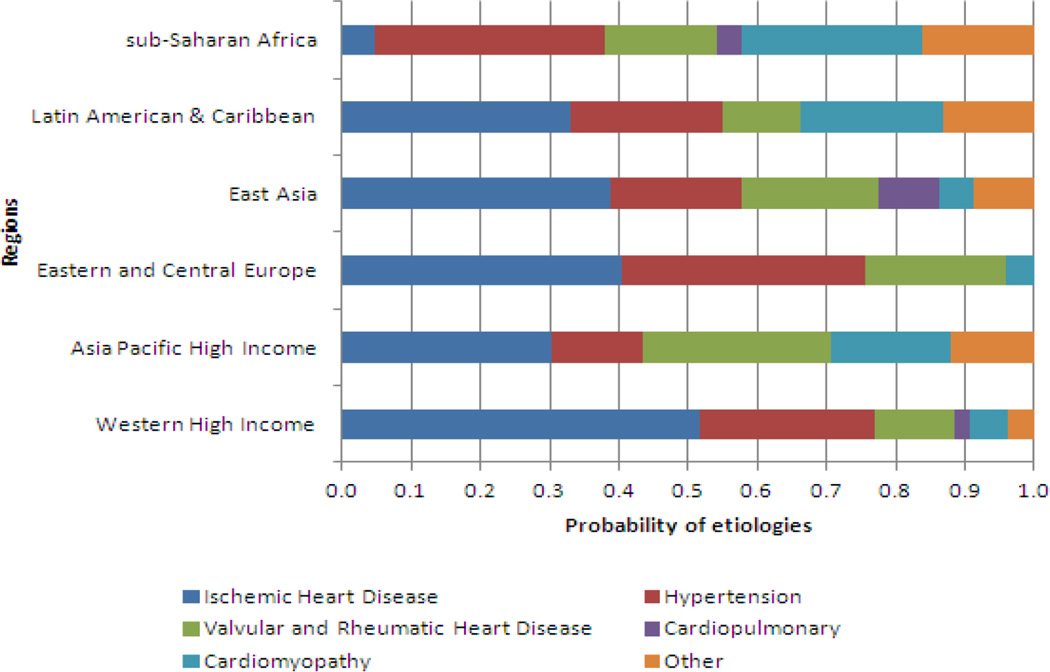
The adjusted multinomial analysis, which may be of interest to those who want to apportion the burden of heart failure among its underlying risk factors, showed risk factor probabilities that mapped roughly to the age- and sex-adjusted prevalence pattern of the risk factors in isolation (Figure 5). Proportional share of IHD as risk factor ranged from to 4.6% in sub-Saharan Africa to 51.6% in Western High Income. The lowest proportion of heart failure was apportioned to hypertension was in Asia Pacific High Income (13.2%) and the highest hypertension apportionments were in Eastern and Central Europe (35.0%) and in sub-Saharan Africa (33.5%).
Figure 5.
Age- and sex-adjusted relative contributions of different underlying risk factors of heart failure in six world regions.
Discussion
Our systematic review of the risk factors for heart failure demonstrated noticeably different risk factor patterns among regions, with patterns related to each region’s epidemiological characteristics in terms of risk factors, treatment access. Crude proportion of heart failure patients with an IHD history was highest in high income Western nations and Eastern and Central Europe, intermediate in East Asia and Latin America and the Caribbean, and very low in Sub-Saharan Africa. Adjustment for mean age of samples attenuated the IHD-as-risk factor differences among regions for all except sub-Saharan Africa, where IHD remained rare as a heart failure risk factor. Hypertension was a common risk factor for heart failure worldwide. Cardiopulmonary disease was found frequently only in heart failure patients in East Asia, where male smoking prevalence is approximately 60% and chronic obstructive pulmonary disease is common. Cardiomyopathy was most common in sub-Saharan Africa and Latin American and the Caribbean, where infectious cardiomyopathies are most common. Rheumatic heart disease, while not consistently reported as a risk factor of heart failure in the studies reviewed, appeared to be common in sub-Saharan Africa and East Asia, possibly due to limited treatment of endemic rheumatic fever.
The crude pattern of heart failure risk factors across regions was similar to that found in two past reviews of international differences in heart failure risk factors—a high proportion of heart failure attributed to IHD in the high income west, and a higher proportion of heart failure attributed to rheumatic heart disease and non-ischemic cardiomyopathies in developing regions.[4, 5] Both of the past studies were much smaller than ours and did not perform meta-analysis or adjust for age and sex variability within regions. For example, after adjustment for age and sex differences in our analysis, the contribution of IHD was lowered in Eastern and Central Europe and increased in East Asia. However, we did not age- or sex-standardize across regions, so regional variability in crude risk factor prevalence is partly due to the fact the heart failure patients tend to be younger or more likely to be male in some regions.
An important finding of our study was that many heart failure cases were associated with multiple risk factors. Under current ICD rules, each death related to heart failure must be assigned one underlying cause. This is analogous to the outcome of our multinomial logistic model, in which proportional contributions were estimated with the property that they added to 100% as they would in mortality statistics based on the ICD. Therefore, the results of this analysis may assist with allocating deaths recorded as heart failure deaths to the underlying causes most probable in specific regions. Despite the accounting and communication convenience of causes that add to 100%, in truth multiple overlapping risk factors contribute to heart failure. A complete epidemiologic model of heart failure risk requires accounting for the multiple underlying risk factors that may co-exist. Such an analysis would require access to individual record data, such as those from the Framingham Heart Study that have been used in multi-risk factor heart failure prediction tools.[20]
Our study is the largest systematic review of the global epidemiology of heart failure risk factors, and is the first to use multivariate adjustment to account for differences in age and sex of study samples. The review is limited because the available studies are clinic- and hospital based studies that may not represent the characteristics of heart failure patients in the community. For example, despite our requirement that studies include NYHA classes II–IV, mild and pre-clinical heart failure may not have been captured in these studies and heart failure duration was rarely described. In most of the studies included, younger patients were excluded. This explains why we found a crude prevalence of rheumatic heart disease among heart failure patients of only 14% in sub-Saharan Africa, a lower proportion than reported in most of the sub-Saharan African heart failure studies reviewed by Ntusi et al.[6] Sub-Saharan Africa studies published since 1980 that include the population <20 years old report a crude rheumatic heart disease prevalence ranging from 13.7—34.1%,[21] and one study reported a rheumatic heart disease prevalence of 44% in heart failure patients <20 years old.[22] Inclusion of heart failure clinical trials would have added many more high income region studies, and a few more low and middle income regions studies, but we found that at least in low and middle income regions, most trials used heart failure risk factors as selection criteria and would have biased the results. Studies included were heterogeneous in the list of risk factors measured and reported, case definitions, age, sex, and possibly in other unreported characteristics of study participants that may affect inter-region differences in risk factors for heart failure. It is likely that risk factors are screened for more often in some regions than in others, and that self report is biased by educational status and access to care. Imaging technology may assist with assigning specific risk factors to heart failure cases, but echocardiography, angiography and myocardial perfusion scanning were used in only one included study to distinguish between ischemic heart disease and hypertensive heart disease.[12] Last, because all of the studies reviewed were cross-sectional, there is no way to know if some of the risk factors preceded heart failure (i.e., were causal) or developed after heart failure (i.e,, were not causal but were co-morbid).
The epidemiology of heart failure is likely evolving rapidly across the globe. Ischemic heart disease may be increasing in places like China,[23] where it was relatively rare before, and hypertension has become the most prominent cause of heart failure in urban Africans.[24] Many populations are facing a “double burden” heart failure caused by communicable and noncommunicable diseases. Rheumatic heart disease prevalence may be higher than previously thought,[25] and HIV prevalence will remain high in many areas into the near future. Chaga’s disease, and endomyocardial fibrosis have not been eliminated as causes of cardiomyopathy in sub-Saharan Africa and South America, respectively. Unfortunately, we lacked sufficient data for estimating urban/rural differences or temporal changes in regional risk factor patterns.
Future research on the heart failure risk factors and progression should ideally be based on community-based longitudinal cohort studies in several world regions. Such studies are costly and would take years to complete. Multi-country case-control studies, would help provide information on the underlying risk factors for heart failure more immediately. Our study suggests that a case-control design incorporating physical exam, self-reported history, echocardiography, electrocardiogram, and pulmonary function tests in heart failure patients and controls might efficiently define the underlying risk factors for heart failure in different world regions. Future studies need to distinguish among causes of cardiopulmonary disease (for example, causes other than chronic obstructive lung disease), subtypes of valvular heart disease and cardiomyopathy, and discriminate between ischemic heart disease and hypertensive heart disease. The INTERCHF study will be a longitudinal case study of approximately 5,000 heart failure patients recruited from urban and rural areas of 13 low and middle income nations in Africa, Asia and South America. This study should contribute substantially to current knowledge about heart failure causes in those regions.
Heart failure is a large and global public health and health system problem that will become more important as the world population ages, and faces risk factors for cardiovascular diseases including heart failure. Using systematic review methods, we identified the regional patterns of heart failure risk factors, contributing to the development of region-specific heart failure prevention policies and estimation of heart failure mortality rates. Global heart failure prevention will require implementation of early prevention strategies: preventing heart failure’s communicable and emerging non-communicable causes, and identifying treatments targeting non-ischemic heart failure. However, more information is needed. The epidemiology of heart failure remains poorly described in most world regions, and geographically diverse population-based studies are needed.
Supplementary Material
Acknowledgments
None.
Acknowledgement of Grant Support: This study was funded by the Bill and Melinda Gates Foundation and Mentored Career Development Award number K08HL089675 from the United States National Heart, Lung, and Blood Institute of the United States National Institutes of Health to Dr. Moran.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.McMurray JJ, Petrie MC, Murdoch DR, et al. Clinical epidemiology of heart failure: public and private health burden. Eur Heart J. 1998;19(Suppl P):P9–P16. [PubMed] [Google Scholar]
- 2.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–289. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 3.Massie BM, Shah NB. Evolving trends in the epidemiologic factors of heart failure: rationale for preventive strategies and comprehensive disease management. Am Heart J. 1997;133:703–712. doi: 10.1016/s0002-8703(97)70173-x. [DOI] [PubMed] [Google Scholar]
- 4.Killip T. Epidemiology of congestive heart failure. The American journal of cardiology. 1985;56:2A–6A. doi: 10.1016/0002-9149(85)91198-1. [DOI] [PubMed] [Google Scholar]
- 5.Mendez GF, Cowie MR. The epidemiological features of heart failure in developing countries: a review of the literature. International journal of cardiology. 2001;80:213–219. doi: 10.1016/s0167-5273(01)00497-1. [DOI] [PubMed] [Google Scholar]
- 6.Ntusi NB, Mayosi BM. Epidemiology of heart failure in sub-Saharan Africa. Expert review of cardiovascular therapy. 2009;7:169–180. doi: 10.1586/14779072.7.2.169. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch DR, Love MP, Robb SD, et al. Importance of heart failure as a cause of death. Changing contribution to overall mortality and coronary heart disease mortality in Scotland 1979–1992. Eur Heart J. 1998;19:1829–1835. doi: 10.1053/euhj.1998.1269. [DOI] [PubMed] [Google Scholar]
- 8.Coats AJ, Shewan LG. Statement on authorship and publishing ethics in the international journal of cardiology. International journal of cardiology. 2011;153:239–240. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 9.The Criteria Committee of the New York Heart Association. Nomenclature and criteria for diagnosis of diseases of the heart and blood vessels. Boston: Little Brown; 1964. [Google Scholar]
- 10.McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 11.Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 12.Fox KF, Cowie MR, Wood DA, et al. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–236. doi: 10.1053/euhj.2000.2289. [DOI] [PubMed] [Google Scholar]
- 13.Cha YM, Redfield MM, Shen WK, et al. Atrial fibrillation and ventricular dysfunction: a vicious electromechanical cycle. Circulation. 2004;109:2839–2843. doi: 10.1161/01.CIR.0000132470.78896.A8. [DOI] [PubMed] [Google Scholar]
- 14.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. The American journal of cardiology. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 15.Nichols GA, Gullion CM, Koro CE, et al. The incidence of congestive heart failure in type-2 diabetes: an update. Diabetes care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 16.Joshi PP, Mohanan CJ, Sengupta SP, et al. Factors precipitating congestive heart failure-- role of patient non-compliance. J Assoc Physicians India. 1999;47:294–295. [PubMed] [Google Scholar]
- 17.Agarwal AK, Venugopalan P, de Bono D. Prevalence and aetiology of heart failure in an Arab population. Eur J Heart Fail. 2001;3:301–305. doi: 10.1016/s1388-9842(00)00149-5. [DOI] [PubMed] [Google Scholar]
- 18.Seow SC, Chai P, Lee YP, et al. Heart failure mortality in Southeast Asian patients with left ventricular systolic dysfunction. J Card Fail. 2007;13:476–481. doi: 10.1016/j.cardfail.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Stewart S, Wilkinson D, Hansen C, et al. Predominance of heart failure in the Heart of Soweto Study cohort: emerging challenges for urban African communities. Circulation. 2008;118:2360–2367. doi: 10.1161/CIRCULATIONAHA.108.786244. [DOI] [PubMed] [Google Scholar]
- 20.Kannel WB, D'Agostino RB, Silbershatz H, et al. Profile for estimating risk of heart failure. Arch Intern Med. 1999;159:1197–1204. doi: 10.1001/archinte.159.11.1197. [DOI] [PubMed] [Google Scholar]
- 21.Damasceno A, Cotter G, Dzudie A, et al. Heart failure in sub-saharan Africa: time for action. J Am Coll Cardiol. 2007;50:1688–1693. doi: 10.1016/j.jacc.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 22.Antony KK. Pattern of cardiac failure in Northern Savanna Nigeria. Trop Geogr Med. 1980;32:118–125. [PubMed] [Google Scholar]
- 23.Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China: twenty-oneyear observational study from the Sino-MONICA-Beijing Project. Stroke a journal of cerebral circulation. 2008;39:1668–1674. doi: 10.1161/STROKEAHA.107.502807. [DOI] [PubMed] [Google Scholar]
- 24.Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371:915–922. doi: 10.1016/S0140-6736(08)60417-1. [DOI] [PubMed] [Google Scholar]
- 25.Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. The New England journal of medicine. 2007;357:470–476. doi: 10.1056/NEJMoa065085. [DOI] [PubMed] [Google Scholar]
- 26.Baldasseroni S, Opasich C, Gorini M, et al. Left bundle-branch block is associated with increased 1-year sudden and total mortality rate in 5517 outpatients with congestive heart failure: a report from the Italian network on congestive heart failure. Am Heart J. 2002;143:398–405. doi: 10.1067/mhj.2002.121264. [DOI] [PubMed] [Google Scholar]
- 27.Bourassa MG, Gurne O, Bangdiwala SI, et al. Natural history and patterns of current practice in heart failure. The Studies of Left Ventricular Dysfunction (SOLVD) Investigators. J Am Coll Cardiol. 1993;22:14A–19A. doi: 10.1016/0735-1097(93)90456-b. [DOI] [PubMed] [Google Scholar]
- 28.Cleland JG, Swedberg K, Follath F, et al. The EuroHeart Failure survey programme--a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 29.Cowie MR, Wood DA, Coats AJ, et al. Incidence and aetiology of heart failure a population-based study. Eur Heart J. 1999;20:421–428. doi: 10.1053/euhj.1998.1280. [DOI] [PubMed] [Google Scholar]
- 30.Davies M, Hobbs F, Davis R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the Echocardiographic Heart of England Screening study: a population based study. Lancet. 2001;358:439–444. doi: 10.1016/s0140-6736(01)05620-3. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Juanatey JR, Alegria Ezquerra E, Bertomeu Martinez V, et al. [Heart failure in outpatients: comorbidities and management by different specialists. The EPISERVE Study] Rev Esp Cardiol. 2008;61:611–619. [PubMed] [Google Scholar]
- 32.Hood S, Taylor S, Roeves A, et al. Are there age and sex differences in the investigation and treatment of heart failure? A population-based study. Br J Gen Pract. 2000;50:559–563. [PMC free article] [PubMed] [Google Scholar]
- 33.Adams KF, Jr, Fonarow GC, Emerman CL, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Morgan AL, Masoudi FA, Havranek EP, et al. Difficulty taking medications, depression, and health status in heart failure patients. J Card Fail. 2006;12:54–60. doi: 10.1016/j.cardfail.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Owan TE, Hodge DO, Herges RM, et al. Trends in prevalence and outcome of heart failure with preserved ejection fraction. The New England journal of medicine. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 36.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29–34. doi: 10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 37.Teerlink JR, Goldhaber SZ, Pfeffer MA. An overview of contemporary etiologies of congestive heart failure. Am Heart J. 1991;121:1852–1853. doi: 10.1016/0002-8703(91)90072-p. [DOI] [PubMed] [Google Scholar]
- 38.van Veldhuisen DJ, Charlesworth A, Crijns HJ, et al. Differences in drug treatment of chronic heart failure between European countries. Eur Heart J. 1999;20:666–672. doi: 10.1053/euhj.1998.1343. [DOI] [PubMed] [Google Scholar]
- 39.Bogdan M, Nartowicz E, Grabczewska Z. [Retrospective analysis of prognostic factors for annual mortality in 97 patients with chronic congestive heart failure] Wiad Lek. 2000;53:372–380. [PubMed] [Google Scholar]
- 40.Amoah AG, Kallen C. Aetiology of heart failure as seen from a National Cardiac Referral Centre in Africa. Cardiology. 2000;93:11–18. doi: 10.1159/000006996. [DOI] [PubMed] [Google Scholar]
- 41.Fofana M, Toure S, Dadhi Balde M, et al. [Etiologic and nosologic considerations apropos of 574 cases of cardiac decompensation in Conakry] Ann Cardiol Angeiol (Paris) 1988;37:419–424. [PubMed] [Google Scholar]
- 42.Karaye KM, Sani MU. Factors associated with poor prognosis among patients admitted with heart failure in a Nigerian tertiary medical centre: a cross-sectional study. BMC Cardiovasc Disord. 2008;8:16. doi: 10.1186/1471-2261-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kingue S, Dzudie A, Menanga A, et al. [A new look at adult chronic heart failure in Africa in the age of the Doppler echocardiography: experience of the medicine department at Yaounde General Hospital] Ann Cardiol Angeiol (Paris) 2005;54:276–283. doi: 10.1016/j.ancard.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Ola BA, Adewuya AO, Ajayi OE, et al. Relationship between depression and quality of life in Nigerian outpatients with heart failure. J Psychosom Res. 2006;61:797–800. doi: 10.1016/j.jpsychores.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 45.Oyoo GO, Ogola EN. Clinical and socio demographic aspects of congestive heart failure patients at Kenyatta National Hospital, Nairobi. East Afr Med J. 1999;76:23–27. [PubMed] [Google Scholar]
- 46.Kang’an C. Retrospective investigation of hospitalized patients with heart failure in some parts of China in 1980, 1990 and 2000. Chin J Cardiol. 2002;30:450–454. [Google Scholar]
- 47.Chong AY, Rajaratnam R, Hussein NR, et al. Heart failure in a multiethnic population in Kuala Lumpur, Malaysia. Eur J Heart Fail. 2003;5:569–574. doi: 10.1016/s1388-9842(03)00013-8. [DOI] [PubMed] [Google Scholar]
- 48.Sanderson JE, Chan SK, Chan WW, et al. The aetiology of heart failure in the Chinese population of Hong Kong--a prospective study of 730 consecutive patients. International journal of cardiology. 1995;51:29–35. doi: 10.1016/0167-5273(95)02398-g. [DOI] [PubMed] [Google Scholar]
- 49.Barretto AC, Nobre MR, Wajngarten M, et al. [Heart failure at a large tertiary hospital of Sao Paulo] Arq Bras Cardiol. 1998;71:15–20. doi: 10.1590/s0066-782x1998000700004. [DOI] [PubMed] [Google Scholar]
- 50.Castro P, Vukasovic JL, Garces E, et al. [Cardiac failure in Chilean hospitals: results of the National Registry of Heart Failure, ICARO] Rev Med Chil. 2004;132:655–662. doi: 10.4067/s0034-98872004000600001. [DOI] [PubMed] [Google Scholar]
- 51.de Campos Lopes CB, Yamada AT, Araujo F, et al. Socioeconomic factors in the prognosis of heart failure in a Brazilian cohort. International journal of cardiology. 2006;113:181–187. doi: 10.1016/j.ijcard.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 52.McSwain M, Martin TC, Amaraswamy R. The prevalence, aetiology and treatment of congestive cardiac failure in Antigua and Barbuda. West Indian Med J. 1999;48:137–140. [PubMed] [Google Scholar]
- 53.Oliveira MT, Jr, Canesin MF, Munhoz RT, et al. [Major clinical characteristics of patients surviving 24 months or more after hospitalization due to decompensated heart failure] Arq Bras Cardiol. 2005;84:161–166. [PubMed] [Google Scholar]
- 54.Itoh A, Saito M, Haze K, et al. Prognosis of patients with congestive heart failure: its determinants in various heart diseases in Japan. Intern Med. 1992;31:304–309. doi: 10.2169/internalmedicine.31.304. [DOI] [PubMed] [Google Scholar]
- 55.Shiba N, Shimokawa H. Chronic heart failure in Japan: implications of the CHART studies. Vasc Health Risk Manag. 2008;4:103–113. doi: 10.2147/vhrm.2008.04.01.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuchihashi M, Tsutsui H, Kodama K, et al. Clinical characteristics and prognosis of hospitalized patients with congestive heart failure--a study in Fukuoka, Japan. Jpn Circ J. 2000;64:953–959. doi: 10.1253/jcj.64.953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.