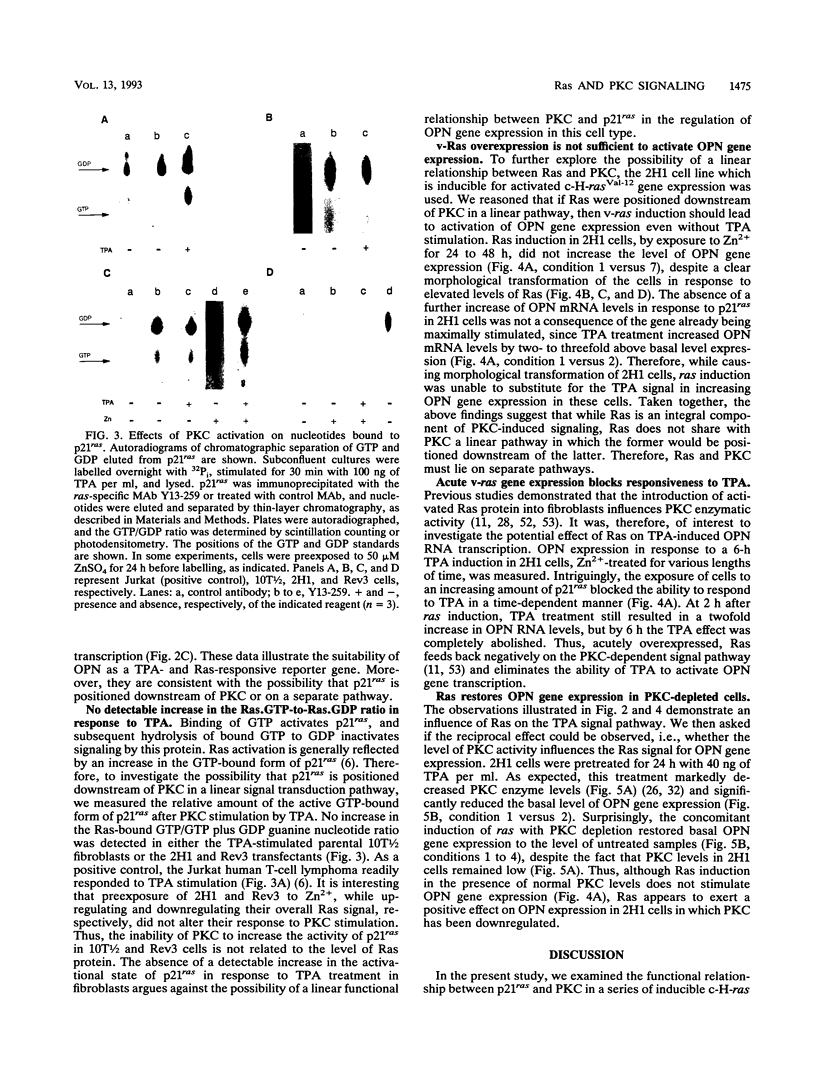
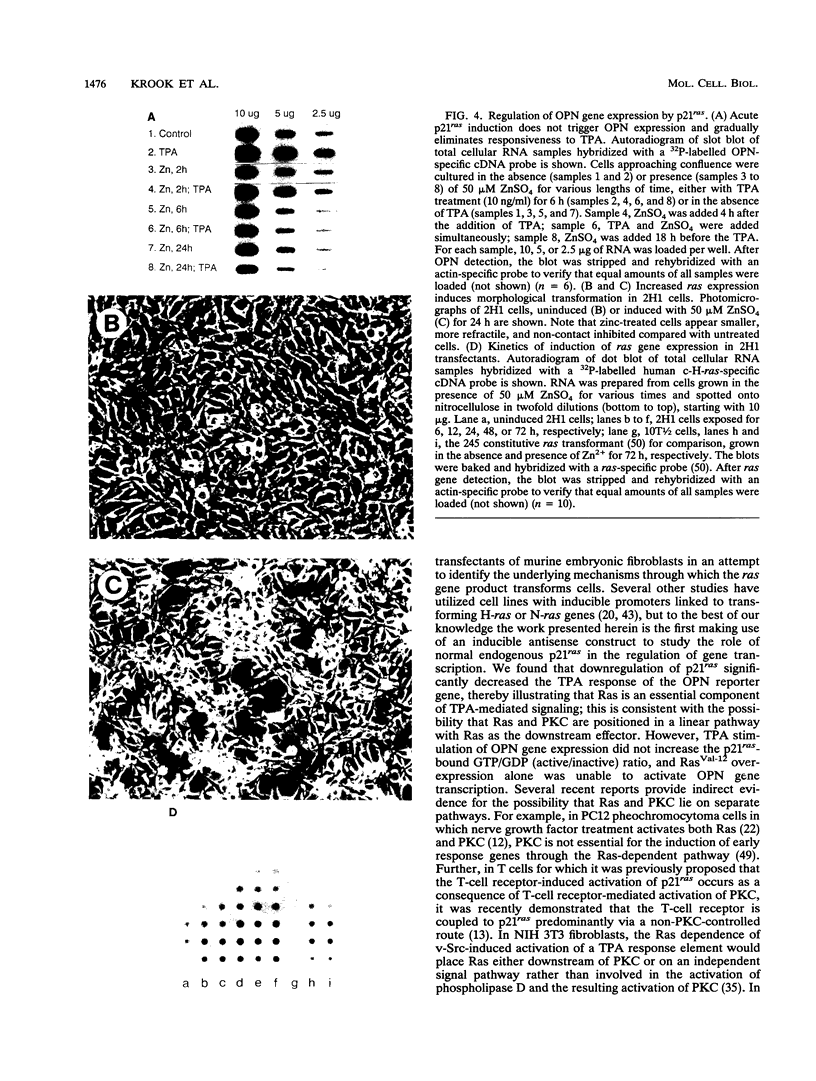
Abstract
Both p21ras and protein kinase C (PKC) are believed to function downstream of plasma membrane-associated tyrosine kinases in cellular signal transduction pathways. However, it has remained controversial whether they function in the same pathway and, if so, what their relative position and functional relationship in such a pathway are. We investigated the possibilities that p21ras and PKC function either upstream or downstream of each other in a common linear pathway or that they function independently in colinear signal pathways. Either decreased expression of endogenous normal ras in fibroblasts transfected with an inducible antisense ras construct or overexpression of a mutant ras gene reduced the capacity of the phorbol ester tetradecanoyl phorbol acetate to trigger expression of the tetradecanoyl phorbol acetate-responsive and ras-dependent reporter gene osteopontin (OPN). PKC depletion decreased basal OPN mRNA levels, and the overexpression of ras restored OPN expression to the level of non-PKC-depleted cells. We propose a model in which ras and PKC function in distinct and interdependent signaling pathways.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. K., Stankova J., Roder J. C. Decreased p21 levels in anti-sense ras transfectants augments NK sensitivity. Mol Immunol. 1989 Oct;26(10):985–991. doi: 10.1016/0161-5890(89)90117-x. [DOI] [PubMed] [Google Scholar]
- Burgering B. M., Medema R. H., Maassen J. A., van de Wetering M. L., van der Eb A. J., McCormick F., Bos J. L. Insulin stimulation of gene expression mediated by p21ras activation. EMBO J. 1991 May;10(5):1103–1109. doi: 10.1002/j.1460-2075.1991.tb08050.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig A. M., Denhardt D. T. The murine gene encoding secreted phosphoprotein 1 (osteopontin): promoter structure, activity, and induction in vivo by estrogen and progesterone. Gene. 1991 Apr;100:163–171. doi: 10.1016/0378-1119(91)90362-f. [DOI] [PubMed] [Google Scholar]
- Deeley R. G., Gordon J. I., Burns A. T., Mullinix K. P., Binastein M., Goldberg R. F. Primary activation of the vitellogenin gene in the rooster. J Biol Chem. 1977 Nov 25;252(22):8310–8319. [PubMed] [Google Scholar]
- Downward J., Graves J. D., Warne P. H., Rayter S., Cantrell D. A. Stimulation of p21ras upon T-cell activation. Nature. 1990 Aug 23;346(6286):719–723. doi: 10.1038/346719a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis C., Moran M., McCormick F., Pawson T. Phosphorylation of GAP and GAP-associated proteins by transforming and mitogenic tyrosine kinases. Nature. 1990 Jan 25;343(6256):377–381. doi: 10.1038/343377a0. [DOI] [PubMed] [Google Scholar]
- Frisch S. M., Clark E. J., Werb Z. Coordinate regulation of stromelysin and collagenase genes determined with cDNA probes. Proc Natl Acad Sci U S A. 1987 May;84(9):2600–2604. doi: 10.1073/pnas.84.9.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haliotis T., Trimble W., Chow S., Bull S., Mills G., Girard P., Kuo J. F., Hozumi N. Expression of ras oncogene leads to down-regulation of protein kinase C. Int J Cancer. 1990 Jun 15;45(6):1177–1183. doi: 10.1002/ijc.2910450631. [DOI] [PubMed] [Google Scholar]
- Heasley L. E., Johnson G. L. Regulation of protein kinase C by nerve growth factor, epidermal growth factor, and phorbol esters in PC12 pheochromocytoma cells. J Biol Chem. 1989 May 25;264(15):8646–8652. [PubMed] [Google Scholar]
- Izquierdo M., Downward J., Graves J. D., Cantrell D. A. Role of protein kinase C in T-cell antigen receptor regulation of p21ras: evidence that two p21ras regulatory pathways coexist in T cells. Mol Cell Biol. 1992 Jul;12(7):3305–3312. doi: 10.1128/mcb.12.7.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Samelson L. E. Increases in tyrosine phosphorylation are detectable before phospholipase C activation after T cell receptor stimulation. J Immunol. 1990 Mar 1;144(5):1591–1599. [PubMed] [Google Scholar]
- June C. H., Fletcher M. C., Ledbetter J. A., Schieven G. L., Siegel J. N., Phillips A. F., Samelson L. E. Inhibition of tyrosine phosphorylation prevents T-cell receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7722–7726. doi: 10.1073/pnas.87.19.7722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Samelson L. E. T cell antigen receptor activation pathways: the tyrosine kinase connection. Cell. 1991 Mar 8;64(5):875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Kolch W., Heidecker G., Lloyd P., Rapp U. R. Raf-1 protein kinase is required for growth of induced NIH/3T3 cells. Nature. 1991 Jan 31;349(6308):426–428. doi: 10.1038/349426a0. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Cuadrado A., Jones J. E., Trotta R., Burstein D. E., Thomson T., Pellicer A. Regulation of protein kinase C activity in neuronal differentiation induced by the N-ras oncogene in PC-12 cells. Mol Cell Biol. 1990 Jun;10(6):2983–2990. doi: 10.1128/mcb.10.6.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal J. C., Fleming T. P., Warren B. S., Blumberg P. M., Aaronson S. A. Involvement of functional protein kinase C in the mitogenic response to the H-ras oncogene product. Mol Cell Biol. 1987 Nov;7(11):4146–4149. doi: 10.1128/mcb.7.11.4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Thayer M. J., Overell R. W., Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell. 1989 Aug 25;58(4):659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Li B. Q., Kaplan D., Kung H. F., Kamata T. Nerve growth factor stimulation of the Ras-guanine nucleotide exchange factor and GAP activities. Science. 1992 Jun 5;256(5062):1456–1459. doi: 10.1126/science.1604323. [DOI] [PubMed] [Google Scholar]
- Lloyd A. C., Paterson H. F., Morris J. D., Hall A., Marshall C. J. p21H-ras-induced morphological transformation and increases in c-myc expression are independent of functional protein kinase C. EMBO J. 1989 Apr;8(4):1099–1104. doi: 10.1002/j.1460-2075.1989.tb03479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall C. J. How does p21ras transform cells? Trends Genet. 1991 Mar;7(3):91–95. doi: 10.1016/0168-9525(91)90278-X. [DOI] [PubMed] [Google Scholar]
- Marshall C. J., Lloyd A. C., Morris J. D., Paterson H., Price B., Hall A. Signal transduction by p21ras. Int J Cancer Suppl. 1989;4:29–31. doi: 10.1002/ijc.2910440708. [DOI] [PubMed] [Google Scholar]
- Miłoszewska J., Trawicki W., Janik P., Moraczewski J., Przybyszewska M., Szaniawska B. Protein kinase C translocation in relation to proliferative state of C3H 10T1/2 cells. FEBS Lett. 1986 Oct 6;206(2):283–286. doi: 10.1016/0014-5793(86)80997-8. [DOI] [PubMed] [Google Scholar]
- Molloy C. J., Bottaro D. P., Fleming T. P., Marshall M. S., Gibbs J. B., Aaronson S. A. PDGF induction of tyrosine phosphorylation of GTPase activating protein. Nature. 1989 Dec 7;342(6250):711–714. doi: 10.1038/342711a0. [DOI] [PubMed] [Google Scholar]
- Morris J. D., Price B., Lloyd A. C., Self A. J., Marshall C. J., Hall A. Scrape-loading of Swiss 3T3 cells with ras protein rapidly activates protein kinase C in the absence of phosphoinositide hydrolysis. Oncogene. 1989 Jan;4(1):27–31. [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Isakov N., Altman A. T cell antigen receptor-mediated activation of phospholipase C requires tyrosine phosphorylation. Science. 1990 Mar 30;247(4950):1584–1587. doi: 10.1126/science.2138816. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Ohno S., Akita Y., Hata A., Osada S., Kubo K., Konno Y., Akimoto K., Mizuno K., Saido T., Kuroki T. Structural and functional diversities of a family of signal transducing protein kinases, protein kinase C family; two distinct classes of PKC, conventional cPKC and novel nPKC. Adv Enzyme Regul. 1991;31:287–303. doi: 10.1016/0065-2571(91)90018-h. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Franzén A., Heinegård D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J., Loomis C. R., Bishop W. R., Stein R., Niedel J. E., Bell R. M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. J Biol Chem. 1986 Jul 5;261(19):8597–8600. [PubMed] [Google Scholar]
- Qureshi S. A., Alexandropoulos K., Rim M., Joseph C. K., Bruder J. T., Rapp U. R., Foster D. A. Evidence that Ha-Ras mediates two distinguishable intracellular signals activated by v-Src. J Biol Chem. 1992 Sep 5;267(25):17635–17639. [PubMed] [Google Scholar]
- Rapp U. R. Role of Raf-1 serine/threonine protein kinase in growth factor signal transduction. Oncogene. 1991 Apr;6(4):495–500. [PubMed] [Google Scholar]
- Reznikoff C. A., Brankow D. W., Heidelberger C. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to postconfluence inhibition of division. Cancer Res. 1973 Dec;33(12):3231–3238. [PubMed] [Google Scholar]
- Santos E., Nebreda A. R. Structural and functional properties of ras proteins. FASEB J. 1989 Aug;3(10):2151–2163. doi: 10.1096/fasebj.3.10.2666231. [DOI] [PubMed] [Google Scholar]
- Satake M., Ibaraki T., Yamaguchi Y., Ito Y. Loss of responsiveness of an AP1-related factor, PEBP1, to 12-O-tetradecanoylphorbol-13-acetate after transformation of NIH 3T3 cells by the Ha-ras oncogene. J Virol. 1989 Sep;63(9):3669–3677. doi: 10.1128/jvi.63.9.3669-3677.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Miyajima A., Kaziro Y. Involvement of ras p21 protein in signal-transduction pathways from interleukin 2, interleukin 3, and granulocyte/macrophage colony-stimulating factor, but not from interleukin 4. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3314–3318. doi: 10.1073/pnas.88.8.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Gracey C. F., Papadopoulos A., Tenen D. G. Secreted phosphoproteins associated with neoplastic transformation: close homology with plasma proteins cleaved during blood coagulation. Cancer Res. 1988 Oct 15;48(20):5770–5774. [PubMed] [Google Scholar]
- Senger D. R., Wirth D. F., Hynes R. O. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979 Apr;16(4):885–893. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- Sistonen L., Hölttä E., Mäkelä T. P., Keski-Oja J., Alitalo K. The cellular response to induction of the p21 c-Ha-ras oncoprotein includes stimulation of jun gene expression. EMBO J. 1989 Mar;8(3):815–822. doi: 10.1002/j.1460-2075.1989.tb03442.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. H., Denhardt D. T. Evidence for two pathways of protein kinase C induction of 2ar expression: correlation with mitogenesis. J Cell Physiol. 1989 Apr;139(1):189–195. doi: 10.1002/jcp.1041390126. [DOI] [PubMed] [Google Scholar]
- Smith J. H., Denhardt D. T. Molecular cloning of a tumor promoter-inducible mRNA found in JB6 mouse epidermal cells: induction is stable at high, but not at low, cell densities. J Cell Biochem. 1987 May;34(1):13–22. doi: 10.1002/jcb.240340103. [DOI] [PubMed] [Google Scholar]
- Smith M. R., DeGudicibus S. J., Stacey D. W. Requirement for c-ras proteins during viral oncogene transformation. Nature. 1986 Apr 10;320(6062):540–543. doi: 10.1038/320540a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Liu Y. L., Kim H., Rhee S. G., Kung H. F. Inhibition of serum- and ras-stimulated DNA synthesis by antibodies to phospholipase C. Science. 1990 Mar 2;247(4946):1074–1077. doi: 10.1126/science.2408147. [DOI] [PubMed] [Google Scholar]
- Somerman M. J., Prince C. W., Sauk J. J., Foster R. A., Butler W. T. Mechanism of fibroblast attachment to bone extracellular matrix: role of a 44 kilodalton bone phosphoprotein. J Bone Miner Res. 1987 Jun;2(3):259–265. doi: 10.1002/jbmr.5650020313. [DOI] [PubMed] [Google Scholar]
- Szeberényi J., Erhardt P., Cai H., Cooper G. M. Role of Ras in signal transduction from the nerve growth factor receptor: relationship to protein kinase C, calcium and cyclic AMP. Oncogene. 1992 Nov;7(11):2105–2113. [PubMed] [Google Scholar]
- Trimble W. S., Johnson P. W., Hozumi N., Roder J. C. Inducible cellular transformation by a metallothionein-ras hybrid oncogene leads to natural killer cell susceptibility. Nature. 1986 Jun 19;321(6072):782–784. doi: 10.1038/321782a0. [DOI] [PubMed] [Google Scholar]
- Tuck A. B., Wilson S. M., Khokha R., Chambers A. F. Different patterns of gene expression in ras-resistant and ras-sensitive cells. J Natl Cancer Inst. 1991 Apr 3;83(7):485–491. doi: 10.1093/jnci/83.7.485. [DOI] [PubMed] [Google Scholar]
- Weyman C. M., Taparowsky E. J., Wolfson M., Ashendel C. L. Partial down-regulation of protein kinase C in C3H 10T 1/2 mouse fibroblasts transfected with the human Ha-ras oncogene. Cancer Res. 1988 Nov 15;48(22):6535–6541. [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Wingrove T. G., Blackshear P. J., Macara I. G. Down-regulation of protein kinase C and of an endogenous 80-kDa substrate in transformed fibroblasts. J Biol Chem. 1987 Dec 5;262(34):16546–16552. [PubMed] [Google Scholar]
- Yu C. L., Tsai M. H., Stacey D. W. Cellular ras activity and phospholipid metabolism. Cell. 1988 Jan 15;52(1):63–71. doi: 10.1016/0092-8674(88)90531-4. [DOI] [PubMed] [Google Scholar]