Abstract
Cell-surface CD25 expression is critical for maintaining immune function and homeostasis. As in few reported cases, CD25 deficiency manifests with severe autoimmune enteritis and viral infections. To dissect the underlying immunological mechanisms driving these symptoms, we analyzed the regulatory and effector T cell functions in a CD25 deficient patient harboring a novel IL2RA mutation. Pronounced lymphoproliferation, mainly of the CD8+ T cells, was detected together with an increase in T cell activation markers and elevated serum cytokines. However, Ag-specific responses were impaired in vivo and in vitro. Activated CD8+STAT5+ T cells with lytic potential infiltrated the skin, even though FOXP3+ Tregs were present and maintained a higher capacity to respond to IL-2 compared to other T-cell subsets. Thus, the complex pathogenesis of CD25 deficiency provides invaluable insight into the role of IL2/IL-2RA-dependent regulation in autoimmunity and inflammatory diseases.
Keywords: CD25;, IPEX-like;, Immunodeficiency;, Autoimmunity;, Tregs;, IL-2
Highlights
► CD25 deficiency leads to profound immune dysregulation. ► Preferential CD8+ T cell expansion and high cytokine serum levels were present. ► Proliferating CD8+ T cells infiltrated the skin but failed to respond to pathogens. ► CD4+FOXP3+CD127lowCD25null Tregs could be detected. ► Altered IL2 signaling events and failure of IL2 consumption contribute to autoimmunity.
1. Introduction
The interleukin-2 (IL-2) receptor is formed by the α (IL-2RA, CD25) [1], β (IL-2RB, CD122) [2] and γcommon (IL-2RG, CD132) [3] subunits, and plays a vital role in maintaining the immune system. Among the IL-2 receptors, CD25 is a unique subunit that exclusively binds IL-2, while CD132 binds the common γc family cytokines (IL-4, IL-7, IL-9, IL-15 and IL-21), and the CD122 subunit binds IL-15. CD25 is constitutively expressed at high levels by CD4+CD25+FOXP3+ regulatory T cells (Tregs), and enables them to be the first responders to IL-2 during an immune response [4] and promotes the transcription of FOXP3 by amplifying IL-2 signaling in a STAT5-dependent fashion [5]. Interestingly, single nucleotide polymorphism (SNP) studies of the IL2RA gene have been associated with several forms of autoimmunity [6–10] demonstrating that IL-2 signaling via CD25 is an important axis in regulating tolerance. CD25 is also critical for effector T cell expansion in response to IL-2 immediately after antigenic stimulation. Although both CD4+ and CD8+ T cells up regulate CD25 and IL-2RB upon activation, CD8+ T cells are more susceptible to IL-2 stimulation, probably due to their higher level of IL-2RB expression both in mice [11] and humans [12,13].
The immunological consequence resulting from the loss of CD25 has been ill-defined in man. Roifman's group was the first to describe a CD25 deficient patient who suffered from chronic infections and severe autoimmunity [14] resembling Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked (IPEX) syndrome, caused by mutations in FOXP3 gene [15]. This IPEX-like patient possessed a translation frameshift mutation in the IL2RA gene ablating its expression. Similarly, a second report described a patient with a different frameshift mutation in the IL2RA gene leading to a CD25 null phenotype with comparable clinical manifestations [16].
Here we describe the immunological findings of a patient carrying an IL2RA mutation not previously reported, selectively abrogating CD25 cell surface expression. Our results show, for the first time in human, the complex immunopathology associated with CD25 deficiency, and reveal a distinct pathogenetic mechanism of immune dysregulation.
2. Material and methods
2.1. IL2RA molecular analysis
Genomic DNA was extracted from peripheral blood mononuclear cells (PBMCs) using the QIAamp DNA Blood Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's recommendations. PCR for each of the 8 exons of the human IL2RA gene (including exon/intron boundaries) was performed using PCR techniques as previously reported [17] and sequence conservation analysis of mutations was performed using PolyPhen, SIFT and SNPs3D tools.
2.2. Flow cytometry
PBMCs were isolated using Lymphoprep (Axis-shield) density gradient centrifugation. Surface Ab staining was performed for 30 min on ice in the absence of light using a 2% bovine serum albumin PBS mixture. Cells were washed and fixed with either 2% paraformaldehyde (Pierce) for later acquisition or with FOXP3 perm/fix buffer (eBioscience) to be further stained for FOXP3 or Ki67 The following Abs (all antibodies purchased from BD Biosciences unless otherwise noted): CD4 (SK3), CD8 (SK1), CD25 (2A3; M-A251), CD45RA (HI100), CD49d (L25), CD62L (SK11), CD69 (FN50), CD122 (MIKB2), CD132 (TUGh4), Ki67 (B56), FOXP3 (eBioscience PCH101), HLA-DR (L243), FASL (NOK-1), and HELIOS (22F6) (Biolegend).
2.3. T cell line generation and stimulation
Healthy donor cell lines were generated by stimulating 1 × 106 PBMCs with PHA 1 μg/ml (Sigma) in X-Vivo media (Biowhitaker) containing 5% human serum (Biowhitaker), 1% penicillin and streptomycin (Lonza), IL-2 (40 U/ml, Proleukin (Novartis)). On days 9, 14 and 20 the cells were washed and plated in the presence of IL-2 (100 U/ml), IL-7 (10 ng/ml), and IL-15 (10 ng/ml). For the CD25 deficient patient, CD4+ T cells were enriched using CD4+ T cell negative selection beads (Miltenyi) and cultured with IL-2 (100 U/ml), IL-15 (10 ng/ml), IL-7 (10 ng/ml). Cells were washed and restimulated with the same conditions on days 7, 11, and 20. On day 24, cells were washed and stimulated in 24 well plates (Corning) containing plate bound anti-CD3 (10 μg/ml) (BD Pharmingen) and anti-CD28 (1 μg/ml) (BD Pharmingen) in the presence or absence of IL-2 (100 U/ml) and IL-15 (10 ng/ml) for 6 h.
2.4. Measurement of sCD25
Levels of sCD25 were evaluated using a commercially available ELISA kit (BD Pharmingen). To measure sCD25 from activated cells, PBMCs (1 × 105) were stimulated for 72 h in complete RPMI (Biowhitaker) with plate-bound anti-CD3 (OKT3) (10 μg/ml) and soluble anti-CD28 (2 μg/ml) in the presence or absence of IL-2 (1000U/ml), TPA (Sigma)/Ionomycin (Sigma), or left unstimulated.
2.5. Phospho flow cytometry
To determine the phosphorylation (p) status of STAT3 and STAT5 after cytokine stimulation, a barcode technique was employed as previously described [18]. Briefly, fresh PBMCs were rested overnight before stimulation with IL-2 (Low 10 U/ml, Med 100 U/ml, Hi 1000 U/ml), IL-15 (10 ng/ml), or IL-10 (10 ng/ml) for 0, 10, or 30 min. At the appropriate time point, the cells were fixed with 1.6% electron microscope grade paraformaldehyde (Pierce) for 10 min at 37° and then washed and permeabilized with 100% methanol (Sigma) for 10 min on ice. After washing, barcoding of the cells was performed using pacific blue succinimidyl ester (Invitrogen) suspended in PBS for 30 min. After washing, the individual wells were pulled into one tube and stained simultaneously with surface and intracellular-directed antibodies (CD4, CD8, pSTAT3 (pY705, 4/P-STAT3), pSTAT5 (Y694, clone 47) (BD Pharmingen)) for 30 min at room temperature. All samples were acquired on a FACScanto flow cytometer (BD Pharmingen).
2.6. Cytokine detection
To quantify cytokine levels in sera and plasma, the Bio-Plex protein array system was used according to the manufacturer's protocol. Levels of IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12(p70), IL-17, TNF-α, and IFN-γ cytokines (BioRad) were analyzed using the Luminex system (Luminex) and concentrations of each cytokine were obtained using Bio-Plex Manager version 3.0 software (Luminex).
2.7. Microscopy
Following informed consent, skin biopsies were taken under local anesthetic and divided for microscopic analysis. For light microscopy, samples were formalin-fixed, paraffin embedded and sectioned at 4 μM for staining. Skin biopsies for immunofluorescence were mounted in OCT compound (Tissue Tek) and immediately snap-frozen in liquid nitrogen. Cryostat sections (5 μm) were mounted on to microscopy slides (Fischer) and air-dried. Slides were stained with: CD8 (1:200), Ki-67 (1:100), and granzyme B (1:100) (BD Pharmingen). The tissues were blocked with 2% goat serum for 1 h and washed before incubating with the antibody for 1 h at 25 °C. The sections were then washed, mounted with vector shield (Sigma) and viewed under a Zeiss fluorescent microscope.
2.8. Proliferation assays
Polyclonal stimulation of PBMCs from healthy donors and the CD25 deficient patient was performed by culturing at 2 × 105 cells per well in duplicate in flat-bottom 96-well tissue culture plates (Corning) with plate bound anti-CD3 (10 μg/ml) and soluble anti-CD28 (1 μg/ml) in the presence of IL-2 low (10 U/ml), medium (100 U/ml), high (1000 U/ml), IL-2 (100 U/ml) + IL-15 (10 ng/ml), or with PHA alone (1 ug/ml). Ag-specific responses were measured using 1 × 105 PBMCs in 96-well round bottom tissue culture plates (Corning) containing the respective Ags. Each antigen (Candida albicans strain CA-2) (1.25 × 105 spores per well; kindly provided by Dr. L. Romani Perugia, Italy), Tetanus Toxoid (TT) (5 μg/ml (Enzo)), CMV (2.5 μg/ml Towne strain; kindly provided by Dr. Chiara Bonini, Milan, Italy), Varicella zoster (VZV) rod strain (2.5 μg/ml (Advanced Biotechnology)) and herpes simplex virus (HSV) MacIntyre strain (2.5 μg/ml (Advanced Biotechnology)) were cultured with the cells in the presence or absence of IL-2 (100 U/ml) and IL-15 (10 ng/ml). The cells were cultured for 3–4 days before pulsing with [3H]thymidine for 18 h. Plates were harvested and proliferation was recorded as counts per minute (cpm) using a gamma counter.
2.9. ELIspot
The frequency of interferon γ (IFNγ) producing cells was determined by ELIspot (BD Pharmingen) as previously described [19]. Briefly, 2 × 105 freshly isolated PBMCs were stimulated in the presence or absence of attenuated CMV (2.5 μg/ml Towne strain), IL-2 (100 U/ml), and or IL-15 (10 ng/ml). After 18–20 h of incubation, plates were washed and developed according to the established protocol. Spots were counted using an ELIspot reader adjusted to eliminate background spots (Aoelovis).
2.10. CMV proliferation
CMV-specific proliferative responses of T cells from the CD25 deficient patient and healthy controls were tested in vitro. PBMCs (4 × 106) were harvested from each donor and were labeled in 5 μM of carboxyfluorescein diacetate succinimidyl ester (CFSE) as previously described [20]. The cells were stimulated with live-attenuated CMV (2.5 μg/ml Towne strain), and/or IL-15 (10 ng/ml) for 5 days before harvesting. The resulting cells were stained with anti-CD4 and anti-CD8 antibodies and acquired by flow cytometry. The proliferation was determined by CFSE dilution for the respective populations indicated by decreasing MFI.
2.11. Suppression assay
The sensitivity to suppression of CD8+ effector T cells by Tregs was performed using allogeneic Treg cells as suppressors. CD8+ T cells were purified from the CD25 deficient patient, the patient's mother and father and a healthy donor using CD8+ T cell beads (Miltenyi) according to the manufacturer's protocol (CD8+ T cell purity was determined to be > 95% for all samples), and were labeled with 5 μM of CFSE. Tregs were isolated from the patient's mother and father and healthy donors using CD4+CD25+ Treg isolation kit (Miltenyi) to high purity and were seeded in a 96 well U bottom plate in triplicate at a 0.5:1 ratio (1.25 × 104 suppressor cells to 2.5 × 104 responder cells) in complete X-Vivo (Biowhitaker). The cells were then stimulated with inspector beads (Miltenyi) using 2 beads per cell in the presence or absence of IL-2 (100 U/ml) for 5 days. The cells were then harvested and stained for CD8 to determine the level of proliferation in each condition.
2.12. Statistics
Statistical significance of mean differences for each parameter was determined by 2-tailed Student's t test or by ANOVA followed by post-hoc Bonferroni test for multiple comparisons where appropriate. Data are expressed as mean ± SEM, and a p value less than 0.05 was considered significant. All calculations were done using GraphPad Prism software ver.5 (GraphPad Software).
3. Results
3.1. Case report
The patient is an 8 year-old female born to consanguineous parents (first cousins) of Italian descent, who developed symptoms resembling IPEX, such as diffuse eczema and severe diarrhea, during her first month of life. The enteropathy with severe villous atrophy was diagnosed as of autoimmune origin and complicated with CMV infection, which was treated by Gancyclovir, but became recurrent. The patient suffered from enteropathy until 5 years of age, often requiring parenteral nutrition and was treated with continuous combination therapy of steroids and Tacrolimus or Tacrolimus alone.
At 1 year of age, she developed a bullous pemphigoid as a reaction to hexavalent vaccination and was treated with plasmapheresis. She presented an autoimmune thyroiditis at 4 years of age, which was treated with hormone replacement therapy until the thyroiditis resolved at 7 years. She was constantly treated with immunosuppressive therapy over all 6 years, but only with partial and transient benefit. When she was 5 years old, she developed diffuse eczema and alopecia universalis despite the immunosuppressive therapies. The skin lesions became a severe form of psoriasiform dermatitis, which extended to the whole body and was characterized by erythroderma and scaling skin associated with multiple lymphadenopathies of inguinal, axillary, nucal and dorsal lymph nodes, diagnosed as hyper-reactive lymph nodes by ultrasonographic examination. During the last year of her follow-up, genital skin lesions were complicated by a severe cellulitis caused by Staphylococcus aureus and Pseudomonas aeruginosa, requiring multiple antibiotic therapy. At 8 years and 6 months of age, due to the evolving dermatitis, Methotrexate was also started with no benefit. Therefore, Mycophenolate Mophetil was given alone with limited improvement of skin lesions, but improvement of the lesions was only seen when combined with rapamycin. Additional therapies included prophylactic treatment, against Pneumocystis jiroveci and fungi, and Gancyclovir upon detection of CMV reactivation. Hematopoietic stem cell transplantation was indicated as the only curative treatment for the disease and since the patient did not have a matched family donor, a search for matched unrelated donors from the Registry was initiated.
While the patient was in our hospital between the ages of 8 years and 4 months and 8 years and 9 months, her WBC (8933/μL ± 3033; normal range: 5500–15,500/μL), and total lymphocyte counts (2821/μL ± 1194; normal range: 2000–8000/μL) were in the normal range. However, the patient showed an inverted CD4/CD8 ratio (0.8 ± 0.4; normal range: 0.9–2.6) with normal CD4 counts (1000/μL ± 341; normal range 300–1300/μL) and high CD8 counts (1574/μL ± 855; normal range 300–1300/μL). In addition, low B (77/μL ± 52; normal range 200–1600/μL) and NK (83/μL ± 72; normal range: 90–900/μL) cell counts persisted during her stay. C-reactive protein (CRP) was negative. Serum IgG and IgM were normal (1150 mg/dL — normal range: 633–1016 mg/dL — and 149 mg/dL — normal range: 56–261 mg/dL, respectively), while IgA and IgE were elevated (409 mg/dL — normal range: 41–315 mg/dL and > 5000 kUA/L — 1.6–60 kUA/L, respectively). Serum analysis showed the presence of protective titers of IgG anti-Tetanus, anti-Hepatitis B and anti-CMV, while she did not have anti-Herpes Simplex Virus-1 and anti-Herpes Simplex Virus-2 antibodies. The screening for celiac disease was negative. The serum screening for other auto-antibodies (anti-pancreatic insula (ICA), anti-glutamic acid decarboxylase (GAD), anti-insulin, anti-IA2, anti-ZNT8, anti-thyroglobulin, anti-thyroperoxidase, anti-liver kidney microsome (LKM), anti-adrenal glands, ANA, ENA) was negative. The NK activity measured as CD107a+ cells in the presence of IL-2 or IL-15 against K562, was preserved (data not shown).
3.2. Novel IL2RA mutation
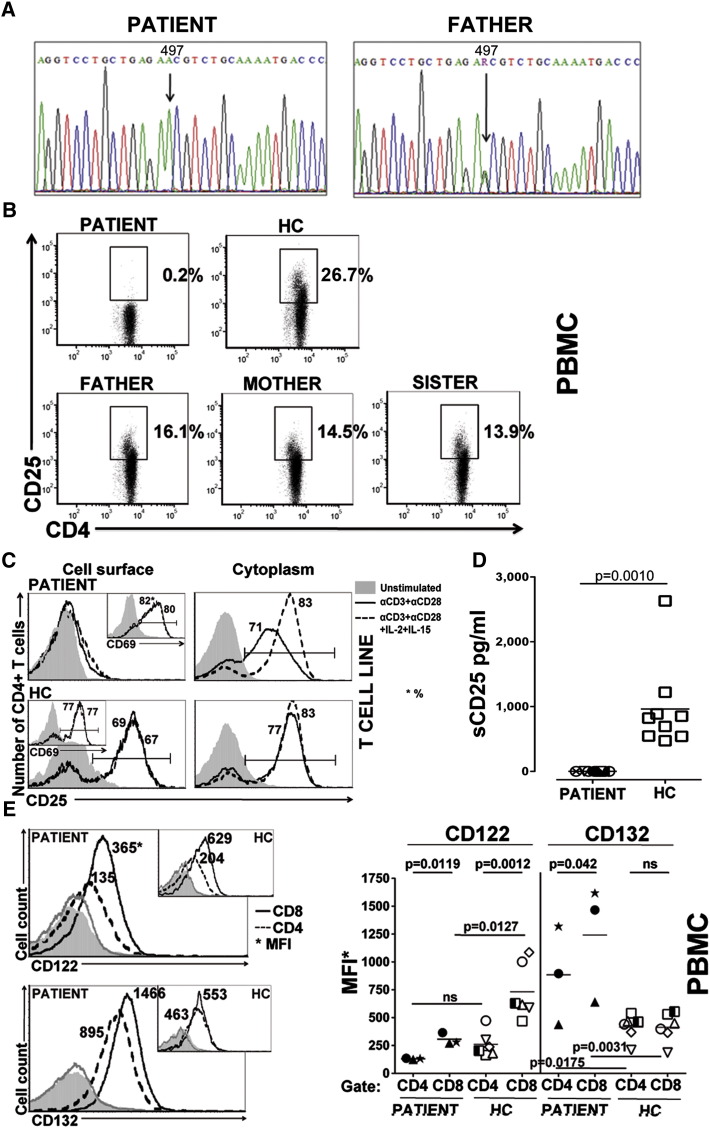
The molecular analysis of the IL2RA gene of the patient's DNA revealed the presence of a previously unreported mutation (Fig. 1A). The patient was homozygous for a c.497G>A transition in exon 4, leading to an amino acid substitution at codon 166 (S166N) of the protein. The patient's father (Fig. 1A), mother, and sister proved to be heterozygous for the same mutation (data not shown).
Figure. 1.
A c.497G>A mutation in the IL2RA gene inhibits surface and soluble CD25 expression. A, Sequence trace of genomic DNA of the patient (left panel) showing a G to A substitution at position 497 (down arrow) in exon 4 leading to the amino acid substitution S166N. Sequence trace of paternal genomic DNA shows a single copy of the G497A mutation (right panel). B, Representative dot plots from 3 independent experiments of CD25 expression on CD4+ T cells from PBMCs of the CD25 deficient patient, the patient's father, mother and sister and a healthy control (HC) (n = 6) as determined by flow cytometry. C, Representative histograms of surface (left column) and cytoplasmic (right column) expression of CD25 on T cell lines were determined for the patient (bottom histograms) and healthy controls (upper histograms) after stimulation with anti-CD3 and anti-CD28 alone (solid line) or in combination with IL-2 and IL-15 (dotted line) or left untreated (grayed area). Embedded histograms of CD69 expression for the patient and healthy controls are shown. D, Soluble CD25 (sCD25) was measured from the plasma at 8 different time points from the patient and from 9 healthy controls. E, Representative histograms showing the expression of CD122 (upper left) and CD132 (lower left) were determined on PBMCs of the patient (large histograms) and healthy controls (embedded histograms). The MFI of CD122 and CD132 on the CD4+ and CD8+ T cells of the patient (each symbol indicates a unique time point) and healthy controls (n = 6) was determined using FACS analysis on PBMCs.
3.3. CD25, CD122 and CD132 protein expression analysis
Immunophenotype analysis of freshly isolated PBMCs from the patient showed the absence of CD25 expression on the surface of CD4+ T cells (Fig. 1B). On the other hand, CD25 was detected on CD4+ T cells of the parents and the sister of the patient (16%, 14.5%, 13.9% for father, mother, and sister respectively), although at a lower frequency than the healthy controls (26.7 ± 6.5, n = 5), which might be explained by the patient's kin being heterozygous for the mutation (Fig. 1B).
CD25 surface expression on CD4+ T cell lines of the patient remained undetectable upon TCR mediated activation even in the presence of exogenous IL-2 and IL-15 (Fig. 1C). Control CD4+ T cell lines tested in parallel, showed increased surface CD25 expression. Importantly, TCR activated CD4+ T cells of the patient expressed CD25 in the cytoplasm, as well as at the mRNA level (data not shown), in a comparable amount to the healthy control (Fig. 1C). This raised the question whether CD25 was rapidly shed from patient's cell surface or remained intracellular. Soluble CD25 (sCD25) was undetectable in the sera (Fig. 1D) or in cell supernatants of activated PBMCs (Supplementary Fig. 1) from the patient compared to healthy control samples demonstrating that the IL2RA mutation of the patient inhibits plasma membrane expression and subsequent shedding of the CD25 protein.
To evaluate whether other IL-2R subunits were affected by the loss of CD25 surface expression, CD122 and CD132 expression was determined on CD4+ and CD8+ T cells. In healthy controls, CD8+ T cells expressed CD122 at higher intensity than CD4+ T cells as determined by Mean Florescence Intensity (MFI) (731.7 ± 249.5 and 261.1 ± 121.3, respectively, p = 0.0012) but there was no significant difference in CD132 expression between CD8+ and CD4+ T cells (497.9 ± 270.4 and 495.7 ± 308.9, respectively, n = 6) (Fig. 1E). Compared to healthy controls, CD8+ T cells of the patient expressed lower levels of CD122 (p = 0.0127), while, both CD8+ and CD4+ T cells of the patient expressed significantly higher levels of CD132 (p = 0.0031 and p = 0.0175, respectively). These data indicate that, despite the loss of CD25 cell surface expression, both CD122 and CD132 are still expressed on the patient's T cells and CD132 expression is elevated in the patient.
3.4. Lack of CD25 surface expression does not interfere with Treg development and is associated with predominant peripheral memory T cell phenotype
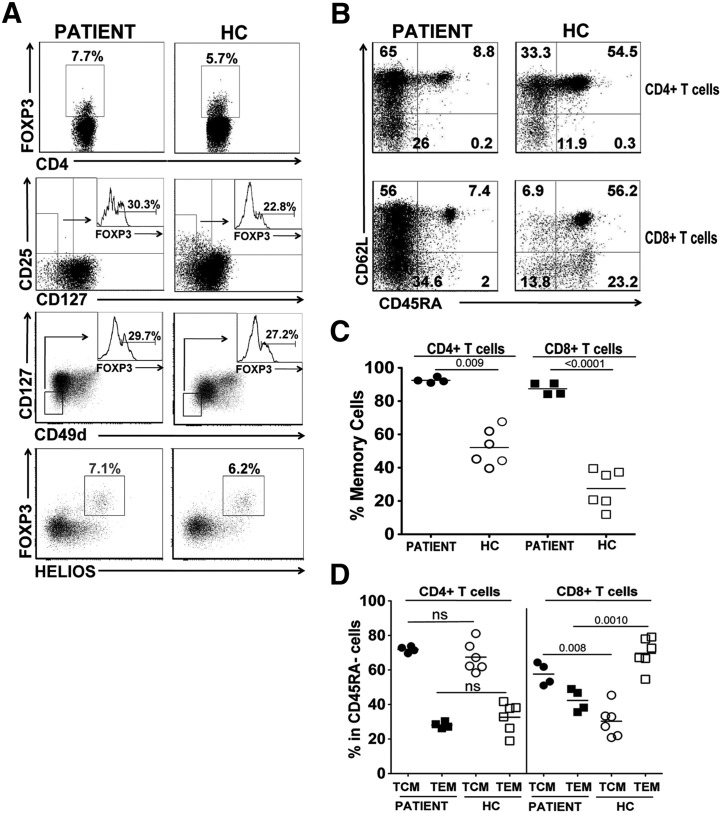
In the patient, the percentage of FOXP3-expressing CD4+ T cells was 7.7 ± 5% (n = 4 time points), which was comparable to those of healthy controls (5.7 ± 1.6%, n = 19, 7 months to 10 years) (Fig. 2A). This percentage did not seem to change with the stage of the disease. However, the highest percentage of CD4+FOXP3+ T cells (14.4%) was detected within three weeks of rapamycin treatment. We further characterized the Tregs using other Treg-specific markers (Fig. 2A). FOXP3 expressing cells were 30.3 ± 12.2 (n = 4 time points) in CD4+CD127low gate for the patient, which were comparable to healthy control values (22.8 ± 13.6%, n = 5). Additionally, FOXP3-expressing T cells of the patient in CD4+CD49dlowCD127low gate ranged between 12.5 and 62.1% (mean: 29.7%, n = 4 time points), which was similar to healthy control values with variability (22.2 ± 6.5%, n = 5). Similarly, the proportion of CD4+ T cells co-expressing HELIOShi and FOXP3+ showed no disparities with healthy controls (7.1 ± 4% (n = 2 time points) and 6.2 ± 4.5% n = 2 respectively), and the expression of CTLA4 and GITR on FOXP3+ T cells was found at comparable levels (data not shown). The presence of bona fide Tregs was confirmed at the molecular level by the analysis of the T-regulatory cell-specific demethylated region (TSDR) of FOXP3 [21]. To elucidate the relative proportion of Tregs in the CD3+ T cell population, we normalized for the percentage of total T-lymphocyte-specific demethylated region (TLSDR) of CD3 [22]. The patient displayed 3.80% of Tregs in CD3+ cell population, thus under the 25° percentile (< 4.21%) of the normal range (normal values 1.8–8.1%) [23], even if the percentage of Tregs determined in purified CD4+ T cells by TSDR demethylation analysis was 13.8%, a value slightly above those of healthy controls (6.5–12.2%, median: 7.5%, n = 7, 3 months to 13 years). Overall these results indicated that peripheral Tregs were present in the patient despite the absence of a functional CD25, albeit at an overall reduced level in total lymphocytes.
Figure. 2.
CD25 deficiency promotes a T memory cell phenotype. A, Representative FACS plots depicting the average percentage of FOXP3+ CD4+ T cells (upper panels), CD127low cells expressing FOXP3 (middle upper panels with embedded histograms), CD127lowCD49dlow cells expressing FOXP3 (middle bottom panels with embedded histograms), FOXP3+ and HELIOS+ staining were determined in the CD25 deficient patient (average of 2–4 different time points is shown) and healthy controls (HC; average percentage of 2–6 different donors is shown). B, The average percentage of naïve (CD45RA+CD62L+), central memory (TCM, CD45RA−CD62L+), effector memory (TEM; CD45RA−CD62L−) for CD4+ (upper row) and CD8+ (lower row) T cells for the patient (left column) and healthy controls (right columns). The percentages shown are the average of 4 different time points for the patient and the average of six healthy donors. C, The overall percentage of memory cells (CD45RA−) from the patient and healthy controls. D, The percentage of the effector populations within the memory pool (CD45A−) of the patient and healthy controls.
Further characterization of peripheral T cells showed increased CD45RA− memory CD4+ (92.5 ± 1.6%) and CD8+ (87.5 ± 3.6) T cells in the patient (n = 4 time points; Figs. 2B and C) compared to adult normal donors and to memory T cell values for age (CD4+ T cell memory: 24.0–43.4%; CD8+ T cell memory: 13.4–29.9%, [24]). Within the increased CD4+ memory T cell populations, CD45RA−CD62L+ central memory (TCM) cells were predominant over CD45RA−CD62L− effector memory (TEM) phenotype (Fig. 2D). CD4+ TCM and TEM cells of the patient showed a distribution pattern similar to those of healthy controls; however, the patient had a greater proportion of CD8+ TCM cells in CD8+ in the memory T cell population. These results indicated that IL2RA mutation in the patient led to predominant memory phenotype of both peripheral CD4+ and CD8+ T cells, with significant increase in CD8+TCM cells proportionally.
3.5. High doses of exogenous IL-2 are required to induce STAT5 signaling in CD25 null T cells while FOXP3+CD4+ Tregs remain the first responders
CD25 is a fundamental part of the high affinity IL-2R complex. To demonstrate that CD25 surface expression confers the ability of CD4+ T cells to respond rapidly to IL-2, phosphorylated (p) STAT5 was determined in CD4 T cells expressing different levels of CD25 in healthy donors: CD25 high (CD25hi), CD25+FOXP3+(which includes Tregs), CD25 medium (CD25med) and CD25 negative (CD25−) (Supplementary Fig. 2). The level of pSTAT5 was consistent with the intensity of CD25 expression. In the presence of IL-2 at low concentrations, CD4+CD25hi and CD4+CD25+FOXP3+ T cells had the highest percentage of pSTAT5+ cells while CD4+CD25med T cells were intermediate responders and CD4+CD25− responded poorly. Responses to increasing levels of IL-2 concentrations did not alter the sensitivity of each population to IL-2. Thus, this data demonstrates that high expression of CD25, including in FOXP3+ Treg, equips CD4+ T cells to respond preferentially to low levels of IL-2 and have a higher potential to signal through STAT5.
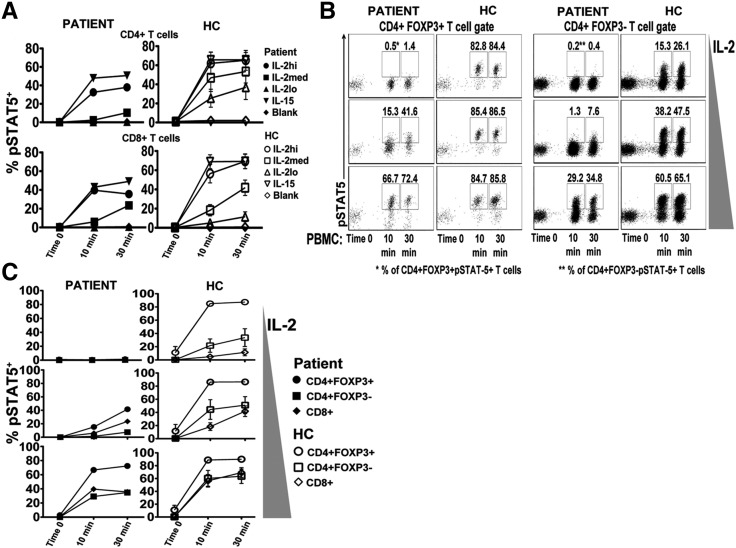
Since the patient's cells did not express CD25 on their surface, signaling through the intermediate affinity IL-2R (CD122 and CD132) was questioned. T cells from the CD25 null patient were tested for their ability to induce pSTAT5 in response to IL-15 and IL-2 (Fig. 3A). The patient's CD4+ and CD8+ T cells displayed a pSTAT5 pattern similar to healthy controls (n = 3) in the presence of IL-15, but with a slight decrease at all time points (10 and 30 min). These data demonstrate that in the absence of cell surface CD25, CD122 and CD132 are functional in the patient's T cells. To the contrary, CD4+ T cells of the patient had dramatically reduced pSTAT5 at all concentrations of IL-2 tested. CD4+ T cells of healthy controls (n = 3) induced pSTAT5 at all time points with IL-2 stimulation in a time and dose dependent manner, while the patient's CD4+ T cells responded only to the high concentration of IL-2. Overall, the percentages of pSTAT5+ CD4+ T cells from the patient, even stimulated at high concentrations of IL-2, were lower than those of healthy controls (n = 3), indicating an overall defect in IL-2 signaling in CD4+ T cells. CD8+ T cells of the patient were less affected by the loss of CD25 in response to IL-2 stimulation. The pSTAT5 levels in the patient's CD8+ T cells with high concentration of IL-2 were similar to the percentages of CD8+ T cells of healthy controls stimulated with high and medium concentrations. Together, these data show that CD4+ T cells from CD25 null patients are more affected than CD8+ T cells in response to IL-2 when CD25 surface expression is absent. The alteration in responsiveness results in the patient's CD8+ T cells becoming more reactive to IL-2 than CD4+ T cells at the medium doses tested.
Figure. 3.
Reduced IL-2 response of T cells from the CD25 deficient patient but FOXP3+ T cells remain first responders. A, The percentage of CD4+ (upper row) and CD8+ (lower row) T cells from PBMCs of the patient (left column) or healthy controls (right column; n = 5) responding to IL-2lo (10 U/ml), IL-2med (100 U/ml), IL-2hi (1000 U/ml), or IL-15 (10 ng/ml) at 0, 10 or 30 min. pSTAT5 was evaluated by flow cytometry from barcoded cells. B, Representative dot plots of the IL-2 responsiveness of CD4+FOXP3+ and CD4+FOXP3− T cells from the patient and healthy control were evaluated for using IL-2lo (10 U/ml), IL-2med (100 U/ml), IL-2hi (1000 U/ml) conditions at the indicated time points determined by pSTAT5. C, The hierarchy of IL-2 signaling was determined from PBMCs of the patient (left column; closed shapes) or healthy control (right column; open shapes (n = 3)) for CD4+FOXP3+, CD4+FOXP3− and CD8+ T cells in response to different concentrations of IL-2 as used in B.
The comparison of STAT5 signaling in CD4+FOXP3+ and FOXP3− T cells of the CD25 null patient versus normal donors shows that neither CD4+FOXP3+ nor CD4+FOXP3− T cells of patient responded to low concentration of IL-2 (Fig. 3B). Both CD4+FOXP3+ and CD4+FOXP3− T cells had pSTAT5 detection with medium and high concentration of IL-2 in a time and dose dependent manner (Fig. 3B). However, pSTAT5 levels in the patient's cells never reached those of healthy controls. Importantly, as seen in healthy controls, pSTAT5 response in CD4+FOXP3+ T cells was always higher than CD4+FOXP3− T cells. This indicated that even though CD4+FOXP3+ T cells lacked CD25 expression, they were able to transmit IL-2 signaling with medium and high IL-2 stimulation. Therefore, they were still the first CD4+ T cell subtype responding to IL-2, and their pSTAT5 status was always higher than CD4+FOXP3− T cells.
The IL-2 signaling hierarchy of CD4+FOXP3+, CD4+FOXP3− and CD8 T cells was also evaluated. In the CD25 null patient and in healthy donors, CD4+FOXP3+ T cells were always the highest responders to IL-2 in all conditions (Fig. 3C). The percentage of pSTAT5 in CD25 null CD8+ T cells was always higher than CD4+FOXP3− T cells in intermediate and high concentrations of IL-2, whereas the CD8+ T cells of healthy donors had significant levels of pSTAT5 only in the presence of high doses of IL-2 (Fig. 3C). This data indicates that in the absence of CD25 surface expression 1) the threshold required for IL-2 signaling in all T cell subsets is raised, 2) CD4+FOXP3+ are the first to respond to IL-2 despite the absence of CD25, and 3) unlike in healthy donors, CD8+ T cells from CD25 null patients preferentially respond to IL-2 compared to CD4+FOXP3−.
3.6. High serum cytokine levels associate with pSTAT5 and pSTAT3, hyperproliferation, and activation of CD25 null T lymphocytes in vivo
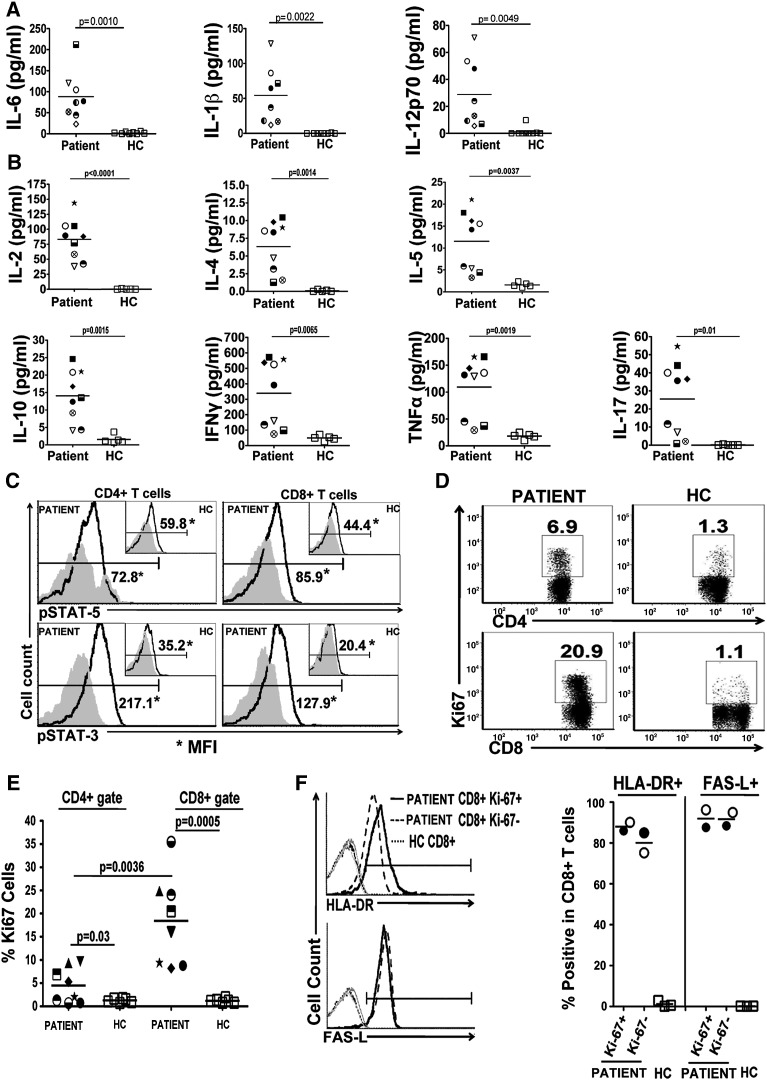
To determine why the CD25 null patient had high circulating T lymphocytes, cytokine levels were evaluated in serum. All cytokines tested, innate immune (IL-6, IL-1β, IL-12p70) (Fig. 4A) and Th1 (IL-2, IFNγ), Th2 (IL-4, IL-5, IL-10), Th17 (IL-17), and TNFα (Fig. 4B) were above that of age-matched control, tested over a period of nine months in the presence of varying levels of immunosuppressive drugs. This data demonstrates that the production of all cytokines tested was not impaired despite the absence of CD25 surface expression, suggesting that high cytokine levels found in circulation may sustain proliferation in vivo. To determine if CD25 null T cells are activated by the cytokines in vivo, freshly isolated peripheral CD4+ and CD8+ T cells of the patient were first evaluated for active STAT5 and STAT3 signaling (Fig. 4C). pSTAT5 levels in CD4+ T cells (72.8 MFI) and in CD8+ T cells (85.9 MFI) of CD25 null patient (59.8 MFI and 49.8 MFI, n = 2 time points) were higher than in healthy controls (44.4 MFI and 47.3 MFI, n = 2). Similarly, both CD4+ and CD8+ T cells possessed higher levels of pSTAT3 (217.1 MFI and 127.9 MFI respectively) than healthy controls (35.2 MFI and 77.4 MFI, n = 2; 20.4 MFI and 45.4 MFI, n = 2, respectively). This data indicates that cytokine signaling pathways in circulating T lymphocytes of the CD25 null patient had active in vivo signaling to both STAT3 and STAT5 pathways.
Figure. 4.
Abundant innate and adaptive cytokines in vivo correlate with predominate CD8+ activation and proliferation. Sera from the CD25 deficient patient (unique symbol for each time point) and healthy donors (each symbol represents a unique patient) were measured for innate (A) and adaptive (B) associated cytokines. C, Representative FACS plots of pSTAT5 (upper row; bold line) and pSTAT3 (lower row; bold line) in CD4+ and CD8+ T cells of the CD25 deficient patient (larger histogram) and healthy controls (embedded histogram) using isotype control antibody staining (grayed area) as a reference. D, Proliferating CD4+ and CD8+ T cells from fresh PBMCs were determined by staining for Ki-67+ (representative FACS plot shown). The average proliferative rate of CD4+ and CD8+ T cells from the CD25 deficient patient and healthy controls (E) was taken at different time points and averaged (p value; students t test). F, Representative histograms of HLA-DR and FAS-L expression on proliferating (Ki-67+; solid line) and non proliferating (Ki-67−; dashed line) CD8+ T cells from the CD25 deficient patient at two different time points or total CD8+ T cells from healthy controls (dotted lines). Gray line is the staining control on the CD8+ T cells.
Additionally, we found that peripheral CD8+ and CD4+ T cells of the patient were highly proliferative in vivo as evident by the expression of Ki-67 (Figs. 4D–E). Ki-67 expression ranged between 8.2 and 35.5% (n = 8) in CD8+ T cells which was significantly higher than healthy control levels (1.22 ± 0.45%, n = 6) at all different time points evaluated (p = 0.0005). CD4+ T cells did not always express Ki-67 at high levels (0.7–9.7%, n = 8), but overall Ki-67 expression was increased compared to healthy control values (1.28 ± 0.43%, n = 6), (p = 0.03). Importantly, an increase in Ki-67 expression was more prominent in CD8+ T cells than CD4+ T cells for all time points detected (p = 0.0036). Although Ki-67 expression remained high in CD8+ T cells the lowest point was during rapamycin treatment. Both proliferating and non-proliferating CD8+ T cells had high surface expression of HLA-DR (86% and 90.1%, n = 2; 85% and 75.2%, n = 2, respectively) and FAS-L on the cell surface consistent with the highly activated phenotype of CD8+ T cells (87.6% and 96.3%, n = 2; 88.4% and 95%, n = 2, respectively) (Fig. 4F). However, BCL-2 expression, an anti-apoptotic protein, was low in proliferating CD8+ T cells (40.7% and 52.1%, n = 2) but comparable to healthy donors in non-proliferating cells (data not shown). Irrespective of the BCL-2 levels, T cells from the CD25 deficient patient were not prone to apoptosis compared to healthy donors (data not shown). Decreased BCL-2 expression in proliferating peripheral CD8+ T cells and increased FAS-L expression in highly activated CD8+ T cells suggest that intrinsic and extrinsic apoptotic pathways in CD8+ T cells were not disrupted in the patient carrying CD25 mutation.
3.7. Proliferating CD8+ T cells mediate autoaggression in skin
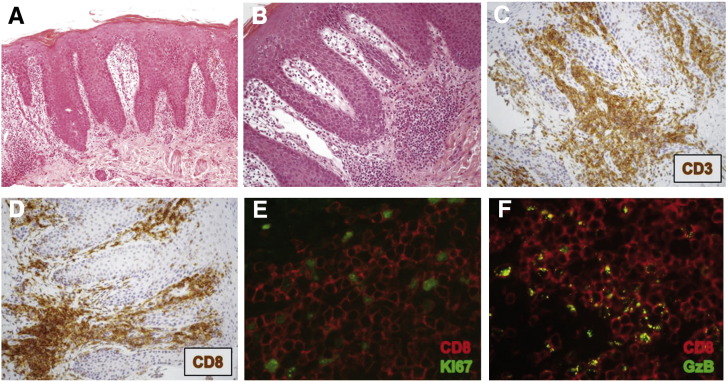
The morphological analysis of skin biopsy of the patient's, epidermis showed hyperplasia with hyper-orthokeratosis (Fig. 5A). A dense small to medium lymphocytic infiltrate was observed mainly in upper dermis (Fig. 5B). Infiltrating lymphocytes almost exclusively displayed a T cell phenotype (CD3+) (Fig. 5C), while B-cells (CD20+) were occasionally encountered, and staining for immunoglobulins was negative (data not shown). T cells were almost invariably CD8+ (Figs. 5D–F). Many of these skin infiltrating CD8+ T cells were proliferating as determined by double immunofluorescence staining with Ki-67 (Fig. 5E). Additionally, they were granzyme B+ (GrzB+) CD8+ T cells (Fig. 5F). TCR rearrangement assessed by PCR both on skin biopsy and peripheral blood revealed a polyclonal T-cell population (data not shown). These data show that CD8+ T cells highly infiltrate the skin, undergo proliferation, and present lytic capacity, suggesting that CD8+ T cells are mediating skin aggression manifesting as severe erythrodermia. However, the CD8+ T cells display normal sensitivity to suppression when co-cultured with Tregs from normal donors (Supplementary Fig. 3).
Figure. 5.
Epidermal hyperplasia due to CD8+ T cell infiltration, proliferation and granzyme B production. A, A skin biopsy of the patient showed hyperplasia with hyper-orthokeratosis. (B), Lymphocytic infiltrate was observed mainly in upper dermis, (C) displaying mostly a T cell phenotype (CD3+). Skin infiltrating cells were mostly CD8+ T cells (D–F) and many were proliferating as determined by double immunofluorescence staining with CD8 and Ki-67 (E). F, A high frequency of CD8+ T cells was also granzyme B+.
3.8. In vitro proliferation defect to polyclonal mitogens was rescued with exogenous IL-2, but viral-specific response was consistently absent
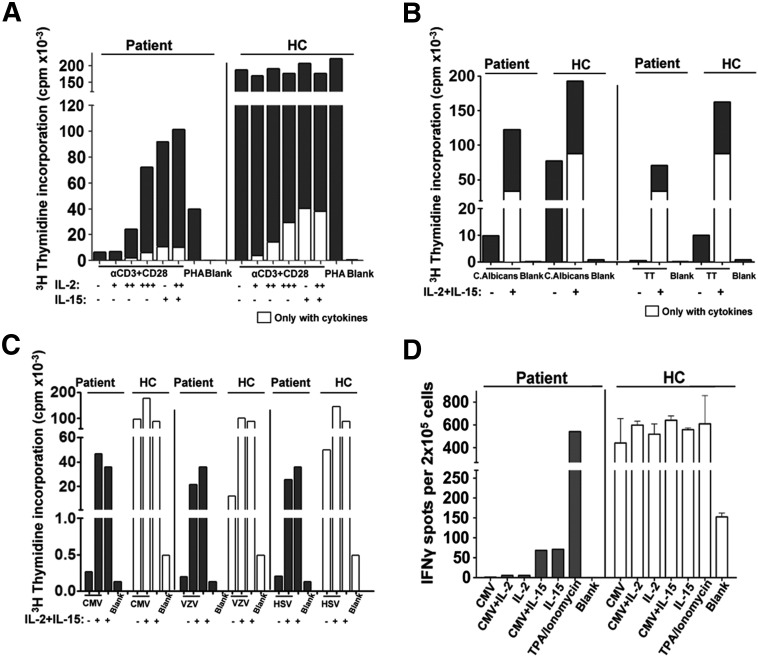
One of the main clinical manifestations of the patient, other than autoimmunity, was chronic viral infections especially CMV. We tested the ability of the patient's PBMCs to respond to Ag-specific and non-specific TCR activation. The patient's PBMCs had poor in vitro proliferation response to TCR-mediated activation compared to healthy controls (Fig. 6A), which was partially rescued by exogenous IL-2 and IL-15. T cell responses to C. albicans, Tetanus Toxoid (TT), and different viruses (CMV, VZV, and HSV) were also impaired (Figs. 6B–C). Similarly, the patient's PBMCs did not produce IFNγ upon CMV activation, as determined by ELISPOT assay. The addition of high concentrations of IL-2 or IL-15 did not rescue IFNγ production (Fig. 6D) or CD4 or CD8 CMV-specific proliferation (Supplementary Fig. 4). These results demonstrate that T cells from the CD25 null patient poorly respond to polyclonal mitogens and to microbial and viral Ags despite the persistent in vivo exposure to CMV.
Figure. 6.
Defective T cell responses to polyclonal mitogens and viral Ags. A, PBMC from the patient and healthy controls were stimulated with anti-CD3 and anti-CD28 in the presence or absence of IL-2 (+ 10 U/ml, ++ 100 U/ml, and +++ 1000 U/ml) and/or IL-15 (10 ng/ml) and/or IL-15 (10 ng/ml), or PHA and proliferation was determined by 3H-thymidine after 3 days. B, T cell responses to C. albicans, TT, were measured after 4 days and (C) different viruses (CMV, VZV, and HSV) were measured after 3 days of stimulation in the presence or absence of IL-2 and IL-15. D, PBMC ELIspot assays from the patient and healthy control were stimulated with CMV Ags alone or in the presence of IL-2 or IL-15, or with IL-2, IL-15, or TPA/Ionomyocin alone.
4. Discussion
Studies of primary immunodeficiencies have been fundamental in defining the function(s) of specific molecules in health and disease. The present work represents a detailed study on the immunological status of a patient lacking CD25 surface expression due to a novel point mutation. The chronic effect of this mutation led to the development of progressive manifestations of both autoimmunity, such as enteropathy, erythrodermia and severe alopecia, and immunodeficiency with chronic CMV infection. Profound alterations of the peripheral T cell subsets consisted of a complete skew towards increased CD8+ T cells over CD4+ T cells, expansion of the memory T cell compartments, with preservation of FOXP3+ T regulatory cells, whereas B cells and NK cells were persistently low. Increased expression of T cell activation markers and high serum levels of innate and adaptive cytokines were detected in parallel with high in vivo proliferation of T cells. Despite the lack of surface CD25, both Tregs and T effector cells remained able to respond to cytokines, although impaired in their sensitivity to IL-2, as demonstrated by pSTAT5. Among the peripheral T effector cells, CD8+ T cells of the CD25 deficient patient highly expressed CD132, and this increased expression made them more responsive to IL-2 than CD4+ T effector cells. Proliferating CD8+ GrzB+ T cells aggressively infiltrated the skin, suggesting that they were the main mediators of the tissue damage. However, Ag-specific T cell responses were deeply impaired in vitro and in vivo. Therefore, these data demonstrate that the persistent lack of CD25 surface expression hinders the development of effective Ag-specific T cell responses while cytokine driven polyclonal T cell proliferation and activation mediate tissue damage.
Similar to the reported CD25 deficient cases, CMV infection and severe early onset enteropathy were the first signs of the deficiency in the patient studied [14,16]. The failure to resolve CMV infection in CD25 deficient patients and other primary immunodeficiencies, could logically explain a skewed CD4/CD8 T cell ratio. Since CMV infection induces innate immune responses and leads to the expansion of CMV-specific effector T cells, one would predict that the expansion of the CD8+ T cell compartment in CD25 deficient patients is due to the response to CMV Ags [25]. Surprisingly, the patient's T cells responded poorly to viral, bacterial and fungal Ags. T cell exhaustion and increased sensitivity to apoptosis were ruled out as possible causes for decreased Ag-specific responsiveness (data not shown). The impairment of effector functions by the CD8+ T cells could be due to the lack of CD25 during priming. Bevan's group demonstrated in mouse that CD25 expression during a primary immune response is critical for programming protective memory cells to respond to Ag during re-exposure [26]. Thus, the absence of surface CD25 expression in the patient presumably impairs the induction of effective Ag-specific memory T cell responses thus explaining the patient's inability to clear infections. In addition, the reported dysfunction of CD25 deficient dendritic cells could contribute to ineffective Ag presentation and T cell activation [27]. It is tempting to speculate that the persistent infections (i.e. CMV) and the innate immune responses thereof, act in concert to promote non-specific CD8+ T cell proliferation probably as a result of the accumulation of the numerous cytokines like the ones found in the serum of the patient. Indeed, based upon our findings, CD25 deficiency altered the hierarchical signaling in response to IL-2 in favor of CD8+ T cells over the CD4+ T cells, contrary to what is observed in healthy subjects. This switch in IL-2 sensitivity together with increased IL-2 levels in serum could be the driving force in the expansion of CD8+ T cells in the absence of cell surface CD25 expression. Supporting this notion are studies performed in the CD25 KO mouse model that showed that the progressive expansion of memory CD8+ T cells is IL-2 driven [28,29].
Treg cells are clearly present in CD25 deficient patients. In line with the Verbsky study showing the presence of CD4+FOXP3+ cells [16], we detected a normal frequency of CD4+FOXP3+ Tregs that also had a similar phenotype to Tregs from normal donors (CD127LOCD49d−HELIOS+CTLA4+GITR+). However, we demonstrate that the proportion of bona fide Tregs with demethylated TSDR was low when normalized to the whole lymphocyte compartment. These findings are consistent with the possibility that CD25 is not necessary for FOXP3+ Treg development in humans, but suggest that in the absence of CD25, Tregs remain quantitatively insufficient for an appropriate regulation. Interestingly, CD25 null FOXP3+ Tregs from the patient were still the first to respond to IL-2 (in vitro) albeit at higher concentrations than required by healthy subject Tregs. Likely, the most affected function due to CD25 deficiency by FOXP3+ Tregs is the consumption of IL-2. Indeed, upon adoptive transfer of CD25+ FoxP3+ Tregs into CD25 knockout mice, IL-2 levels were dramatically reduced, normal CD4+ and CD8+ T cell ratios were restored and the memory phenotype of both CD4+ and CD8+ T cells returned to near normal levels [28]. Together this indicates that IL-2 consumption by FOXP3+ Tregs is a critical event to maintain the homeostasis of the immune system, and the loss of CD25 surface expression by FOXP3+ Tregs in CD25 deficient patients is likely a contributing factor in the preferential CD8+ T cell proliferation, which are the mediators of autoimmunity. Whether the tissue aggression by CD8+ T cells is auto-Ag driven remains to be clarified. However, the observation that the TCR repertoire of the proliferating T cells is polyclonal argues against it.
Even if CD25 deficiency is considered similar to IPEX syndrome, we clearly highlight major differences in the pathogenesis of the two diseases. Indeed, in IPEX lymphoproliferation involves mainly CD4+ T cells and it is mainly due to loss of regulation by intrinsically dysfunctional FOXP3mut Treg rather than being cytokine driven [30,31]. In the serum of IPEX patients, cytokines are not particularly elevated and Th2 rather than Th1 cytokines are detectable together with increased IL-17 and IL-22, directly related to autoimmunity [32]. In addition, effective immune response to pathogens is preserved in IPEX in which infections mostly occur as secondary to poor clinical conditions or drug dependent immunosuppression. Similarly, autoAb are not readily detectable in CD25 null patients. We therefore demonstrated that even though similarities exist, the pathophysiology of autoimmunity in CD25 deficiency is mechanistically distinct from that of IPEX syndrome, and also from that observed in other monogenic primary immunodeficiencies with autoimmunity, such as Omenn's syndrome, Wiskott–Aldrich or Autoimmune Lymphoproliferative syndrome (ALPS) due respectively to altered thymic deletion of autoreactive T cells, impaired cytoskeleton re-organization during activation of multiple cell type including Tregs [33,34] or defective apoptosis [35].
5. Conclusion
In conclusion, we show that CD25 expression is required to maintain immune homeostasis, and CD25 deficiency is a distinct immunological disease that leads to both an autoimmune and immunodeficiency syndrome that clinically resembles IPEX syndrome. Furthermore, CD25 expression is necessary in human T cells in order to establish a signaling paradigm where IL-2 is utilized in an ordered fashion, and alterations to the hierarchical signaling events can lead to the loss of immune homeostasis. The proposed concept that the failure to properly consume IL-2 and lack of optimal IL-2 signaling contributes to the development of autoimmunity in CD25 deficient patients, could be extended to different autoimmune pathologies in which a reduced CD25 expression occurs as the result of single nucleotide genetic variants of CD25 or of other molecules interfering with proper CD25 signaling. More in depth studies aimed at clarifying this possibility are desirable.
Conflict of interest statement
All authors listed have no conflict of interest regarding this manuscript.
Acknowledgments
We would like to thank the patient and the patient's family for their cooperation in this study, and the Pediatric Immunohematology team at San Raffaele Hospital for their clinical support. We are also grateful to Claudia Sartirana for her technical support and Dr. Laura Passerini for her helpful advice and discussion. This work was supported by the Telethon Foundation (Tele 10A4 to Rosa Bacchetta), the Italian Ministry of Health (Grant RF-2009-1485896 to Rosa Bacchetta), the Seventh Framework project (FP7) of the European Community (Cell-PID to Alessandro Aiuti and Rosa Bacchetta), and the Juvenile Diabetes Research Foundation (JDRF to Kevin Goudy). In addition, we give special thanks to Prof. Carlo Gelmetti and his team at Clinica Dermatologica at Ospedale Maggiore Policlinico, Milano for their help in caring for the patient and their expert advice in the treatment of the erythrodermia.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.clim.2013.01.004.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Leonard W.J., Depper J.M., Uchiyama T., Smith K.A., Waldmann T.A., Greene W.C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982;300:267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- 2.Sharon M., Klausner R.D., Cullen B.R., Chizzonite R., Leonard W.J. Novel interleukin-2 receptor subunit detected by cross-linking under high-affinity conditions. Science. 1986;234:859–863. doi: 10.1126/science.3095922. [DOI] [PubMed] [Google Scholar]
- 3.Teshigawara K., Wang H.M., Kato K., Smith K.A. Interleukin 2 high-affinity receptor expression requires two distinct binding proteins. J. Exp. Med. 1987;165:223–238. doi: 10.1084/jem.165.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Gorman W.E., Dooms H., Thorne S.H., Kuswanto W.F., Simonds E.F., Krutzik P.O., Nolan G.P., Abbas A.K. The initial phase of an immune response functions to activate regulatory T cells. J. Immunol. 2009;183:332–339. doi: 10.4049/jimmunol.0900691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zorn E., Nelson E.A., Mohseni M., Porcheray F., Kim H., Litsa D., Bellucci R., Raderschall E., Canning C., Soiffer R.J., Frank D.A., Ritz J. IL-2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108:1571–1579. doi: 10.1182/blood-2006-02-004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smyth D.J., Plagnol V., Walker N.M., Cooper J.D., Downes K., Yang J.H., Howson J.M., Stevens H., McManus R., Wijmenga C., Heap G.A., Dubois P.C., Clayton D.G., Hunt K.A., van Heel D.A., Todd J.A. Shared and distinct genetic variants in type 1 diabetes and celiac disease. N. Engl. J. Med. 2008;359:2767–2777. doi: 10.1056/NEJMoa0807917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alcina A., Fedetz M., Ndagire D., Fernandez O., Leyva L., Guerrero M., Abad-Grau M.M., Arnal C., Delgado C., Lucas M., Izquierdo G., Matesanz F. IL2RA/CD25 gene polymorphisms: uneven association with multiple sclerosis (MS) and type 1 diabetes (T1D) PLoS One. 2009;4:e4137. doi: 10.1371/journal.pone.0004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hinks A., Ke X., Barton A., Eyre S., Bowes J., Worthington J., Thompson S.D., Langefeld C.D., Glass D.N., Thomson W. Association of the IL2RA/CD25 gene with juvenile idiopathic arthritis. Arthritis Rheum. 2009;60:251–257. doi: 10.1002/art.24187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowe C.E., Cooper J.D., Brusko T., Walker N.M., Smyth D.J., Bailey R., Bourget K., Plagnol V., Field S., Atkinson M., Clayton D.G., Wicker L.S., Todd J.A. Large-scale genetic fine mapping and genotype–phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat. Genet. 2007;39:1074–1082. doi: 10.1038/ng2102. [DOI] [PubMed] [Google Scholar]
- 10.Dendrou C.A., Plagnol V., Fung E., Yang J.H., Downes K., Cooper J.D., Nutland S., Coleman G., Himsworth M., Hardy M., Burren O., Healy B., Walker N.M., Koch K., Ouwehand W.H., Bradley J.R., Wareham N.J., Todd J.A., Wicker L.S. Cell-specific protein phenotypes for the autoimmune locus IL2RA using a genotype-selectable human bioresource. Nat. Genet. 2009;41:1011–1015. doi: 10.1038/ng.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 12.Geginat J., Lanzavecchia A., Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 13.Geginat J., Sallusto F., Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J. Exp. Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharfe N., Dadi H.K., Shahar M., Roifman C.M. Human immune disorder arising from mutation of the alpha chain of the interleukin-2 receptor. Proc. Natl. Acad. Sci. U. S. A. 1997;94:3168–3171. doi: 10.1073/pnas.94.7.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barzaghi F., Passerini L., Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front. Immunol. 2012;3:211. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caudy A.A., Reddy S.T., Chatila T., Atkinson J.P., Verbsky J.W. CD25 deficiency causes an immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like syndrome, and defective IL-10 expression from CD4 lymphocytes. J. Allergy Clin. Immunol. 2007;119:482–487. doi: 10.1016/j.jaci.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Gambineri E., Perroni L., Passerini L., Bianchi L., Doglioni C., Meschi F., Bonfanti R., Sznajer Y., Tommasini A., Lawitschka A., Junker A., Dunstheimer D., Heidemann P.H., Cazzola G., Cipolli M., Friedrich W., Janic D., Azzi N., Richmond E., Vignola S., Barabino A., Chiumello G., Azzari C., Roncarolo M.G., Bacchetta R. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J. Allergy Clin. Immunol. 2008;122:1105–1112. doi: 10.1016/j.jaci.2008.09.027. (e1101) [DOI] [PubMed] [Google Scholar]
- 18.Krutzik P.O., Nolan G.P. Fluorescent cell barcoding in flow cytometry allows high-throughput drug screening and signaling profiling. Nat. Methods. 2006;3:361–368. doi: 10.1038/nmeth872. [DOI] [PubMed] [Google Scholar]
- 19.Vago L., Oliveira G., Bondanza A., Noviello M., Soldati C., Ghio D., Brigida I., Greco R., Lupo Stanghellini M.T., Peccatori J., Fracchia S., Del Fiacco M., Traversari C., Aiuti A., Del Maschio A., Bordignon C., Ciceri F., Bonini C. T-cell suicide gene therapy prompts thymic renewal in adults after hematopoietic stem cell transplantation. Blood. 2012;120:1820–1830. doi: 10.1182/blood-2012-01-405670. [DOI] [PubMed] [Google Scholar]
- 20.Lyons A.B., Parish C.R. Determination of lymphocyte division by flow cytometry. J. Immunol. Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 21.Polansky J.K., Kretschmer K., Freyer J., Floess S., Garbe A., Baron U., Olek S., Hamann A., von Boehmer H., Huehn J. DNA methylation controls Foxp3 gene expression. Eur. J. Immunol. 2008;38:1654–1663. doi: 10.1002/eji.200838105. [DOI] [PubMed] [Google Scholar]
- 22.Sehouli J., Loddenkemper C., Cornu T., Schwachula T., Hoffmuller U., Grutzkau A., Lohneis P., Dickhaus T., Grone J., Kruschewski M., Mustea A., Turbachova I., Baron U., Olek S. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics. 2011;6:236–246. doi: 10.4161/epi.6.2.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barzaghi F., Passerini L., Gambineri E., Ciullini Mannurita S., Cornu T., Kang E.S., Choe Y.H., Cancrini C., Corrente S., Ciccocioppo R., Cecconi M., Zuin G., Discepolo V., Sartirana C., Schmidtko J., Ikinciogullari A., Ambrosi A., Roncarolo M.G., Olek S., Bacchetta R. Demethylation analysis of the FOXP3 locus shows quantitative defects of regulatory T cells in IPEX-like syndrome. J. Autoimmun. 2012;38:49–58. doi: 10.1016/j.jaut.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Gent R., van Tilburg C.M., Nibbelke E.E., Otto S.A., Gaiser J.F., Janssens-Korpela P.L., Sanders E.A., Borghans J.A., Wulffraat N.M., Bierings M.B., Bloem A.C., Tesselaar K. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin. Immunol. 2009;133:95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie G.M., Wills M.R., Appay V., O'Callaghan C., Murphy M., Smith N., Sissons P., Rowland-Jones S., Bell J.I., Moss P.A. Functional heterogeneity and high frequencies of cytomegalovirus-specific CD8(+) T lymphocytes in healthy seropositive donors. J. Virol. 2000;74:8140–8150. doi: 10.1128/jvi.74.17.8140-8150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kamimura D., Bevan M.J. Naive CD8 + T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J. Exp. Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wuest S.C., Edwan J.H., Martin J.F., Han S., Perry J.S., Cartagena C.M., Matsuura E., Maric D., Waldmann T.A., Bielekova B. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 2011;17:604–609. doi: 10.1038/nm.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma R., Zheng L., Deshmukh U.S., Jarjour W.N., Sung S.S., Fu S.M., Ju S.T. A regulatory T cell-dependent novel function of CD25 (IL-2Ralpha) controlling memory CD8(+) T cell homeostasis. J. Immunol. 2007;178:1251–1255. doi: 10.4049/jimmunol.178.3.1251. [DOI] [PubMed] [Google Scholar]
- 29.Willerford D.M., Chen J., Ferry J.A., Davidson L., Ma A., Alt F.W. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 30.Bacchetta R., Passerini L., Gambineri E., Dai M., Allan S.E., Perroni L., Dagna-Bricarelli F., Sartirana C., Matthes-Martin S., Lawitschka A., Azzari C., Ziegler S.F., Levings M.K., Roncarolo M.G. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J. Clin. Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.d'Hennezel E., Ben-Shoshan M., Ochs H.D., Torgerson T.R., Russell L.J., Lejtenyi C., Noya F.J., Jabado N., Mazer B., Piccirillo C.A. FOXP3 forkhead domain mutation and regulatory T cells in the IPEX syndrome. N. Engl. J. Med. 2009;361:1710–1713. doi: 10.1056/NEJMc0907093. [DOI] [PubMed] [Google Scholar]
- 32.Passerini L., Olek S., Di Nunzio S., Barzaghi F., Hambleton S., Abinun M., Tommasini A., Vignola S., Cipolli M., Amendola M., Naldini L., Guidi L., Cecconi M., Roncarolo M.G., Bacchetta R. Forkhead box protein 3 (FOXP3) mutations lead to increased TH17 cell numbers and regulatory T-cell instability. J. Allergy Clin. Immunol. 2011;128:1376–1379. doi: 10.1016/j.jaci.2011.09.010. (e1371) [DOI] [PubMed] [Google Scholar]
- 33.Marangoni F., Trifari S., Scaramuzza S., Panaroni C., Martino S., Notarangelo L.D., Baz Z., Metin A., Cattaneo F., Villa A., Aiuti A., Battaglia M., Roncarolo M.G., Dupre L. WASP regulates suppressor activity of human and murine CD4(+)CD25(+)FOXP3(+) natural regulatory T cells. J. Exp. Med. 2007;204:369–380. doi: 10.1084/jem.20061334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park J.Y., Kob M., Prodeus A.P., Rosen F.S., Shcherbina A., Remold-O'Donnell E. Early deficit of lymphocytes in Wiskott–Aldrich syndrome: possible role of WASP in human lymphocyte maturation. Clin. Exp. Immunol. 2004;136:104–110. doi: 10.1111/j.1365-2249.2004.02409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puck J.M., Sneller M.C. ALPS: an autoimmune human lymphoproliferative syndrome associated with abnormal lymphocyte apoptosis. Semin. Immunol. 1997;9:77–84. doi: 10.1006/smim.1996.0056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.