Abstract
Maximum walking speed may offer an advantage over usual walking speed for clinical assessment of age-related declines in mobility function that are due to neuromuscular impairment. The objective of this study was to determine the extent to which maximum walking speed is affected by neuromuscular function of the lower extremities in older adults. We recruited two groups of healthy, well functioning older adults who differed primarily on maximum walking speed. We hypothesized that individuals with slower maximum walking speed would exhibit reduced lower extremity muscle size and impaired plantarflexion force production and neuromuscular activation during a rapid contraction of the triceps surae muscle group (soleus (SO) and gastrocnemius (MG)).
All participants were required to have usual 10-meter walking speed >1.0 m/s. If the difference between usual and maximum 10m walking speed was < 0.6 m/s, the individual was assigned to the “Slower” group (n=8). If the difference between usual and maximum 10-meter walking speed was > 0.6 m/s, the individual was assigned to the “Faster” group (n=12). Peak rate of force development (RFD) and rate of neuromuscular activation (rate of EMG rise) of the triceps surae muscle group were assessed during a rapid plantarflexion movement. Muscle cross sectional area of the right triceps surae, quadriceps and hamstrings muscle groups was determined by magnetic resonance imaging.
Across participants, the difference between usual and maximal walking speed was predominantly dictated by maximum walking speed (r=.85). We therefore report maximum walking speed (1.76 and 2.17 m/s in Slower and Faster, p<.001) rather than the difference between usual and maximal. Plantarflexion RFD was 38% lower (p=.002) in Slower compared to Faster. MG rate of EMG rise was 34% lower (p=.01) in Slower than Faster, but SO rate of EMG rise did not differ between groups (p=.73). Contrary to our hypothesis, muscle CSA was not lower in Slower than Faster for the muscle groups tested, which included triceps surae (p=.44), quadriceps (p=.76) and hamstrings (p=.98). MG rate of EMG rise was positively associated with RFD and maximum 10m walking speed, but not usual 10m walking speed.
These findings support the conclusion that maximum walking speed is limited by impaired neuromuscular force and activation of the triceps surae muscle group. Future research should further evaluate the utility of maximum walking speed for use in clinical assessment to detect and monitor age-related functional decline.
Keywords: aging, walking, mobility, muscle, electromyography
Introduction
Detection of functional decline using clinical assessments in older adults is important for facilitating the delivery of rehabilitation or other medical interventions in a timely manner. Earlier intervention is considered to be a crucial factor for optimizing long term functional outcomes (Manini and Pahor, 2009). There is growing interest in the use of walking speed as a clinic-based assessment of functional decline. Most discussion has focused on measuring an individual’s usual walking speed (Abellan van Kan et al., 2009; Cesari et al., 2005; Fielding et al., 2011; Morley et al., 2011; Studenski, 2009). However, maximum walking speed may also be a valuable assessment because it has been shown to decline more rapidly than usual walking speed with advancing age (Jahn et al., 2010; Ko et al., 2010; Tanaka et al., 1995). This finding is likely due in part to the presence of age-related changes in neuromuscular control (Kang and Dingwell, 2009; Schmitz et al., 2009), which limit the capability to produce the muscular force required for walking at fast speeds. Accordingly, assessing maximum walking speed may offer an advantage over usual walking speed for earlier detection of functional decline that is due to neuromuscular impairment.
Walking speed is highly dependent on neuromuscular function of the triceps surae muscle group (soleus and gastrocnemius muscles). Indeed, ankle plantarflexion power produced by the triceps surae is the largest contributor of energy into the gait cycle (Winter, 1987), and has been shown to be a crucial factor for modulating walking speed (Liu et al., 2008; Murray et al., 1978; Requiao et al., 2005). It is therefore important that a number of studies have demonstrated loss of triceps surae muscle size and/or neuromuscular activation in older adults (Morse et al., 2004; Morse et al., 2005; Runge et al., 2004; Simoneau et al., 2005; Thom et al., 2005). Furthermore, triceps surae weakness is directly associated with reduced usual and maximum walking speeds in older adults with functional limitations (Suzuki et al., 2001). The importance of assessing triceps surae neuromuscular function is further highlighted by evidence showing that age-related neurogenic adaptations (e.g., denervation/reinnervation) are more extensive in distal muscle groups, such as triceps surae (Jennekens et al., 1971; McComas et al., 1993; Stalberg and Fawcett, 1982). These adaptations have been linked to a decline in plantarflexion force production (Doherty et al., 1993b), which is consistent with reports that older adults use a walking control strategy in which force production is increased at proximal joints and reduced at distal joints (DeVita and Hortobagyi, 2000; Kerrigan et al., 1998; Monaco et al., 2009). Cumulatively, this evidence provides strong rationale for assessing triceps surae neuromuscular function as a determinant of mobility function in older adults.
The objective of this study was to determine the extent to which maximum walking speed is affected by neuromuscular function of the lower extremities in older adults. We examined two homogeneous groups of healthy, well-functioning older adults who were distinguished primarily by their maximum walking speed. We hypothesized that the group with slower maximum walking speed would exhibit impaired plantarflexion force production, reduced lower extremity muscle size and impaired neuromuscular activation of triceps surae muscles.
Methods
Experimental Design and Protocol
All study procedures were approved by the University of Florida Institutional Review Board and by the Malcom Randall VA Medical Center Human Research Protection Program. All participants provided their written informed consent. Healthy, well functioning older adults were recruited through local newspaper advertisements, with the objective of enrolling and evaluating twenty participants. Preliminary screening was conducted by telephone using the following exclusion criteria: age <65 years or >80 years; use of an assistive device for walking; affirmative response to the question “Do you find walking, climbing stairs or performing daily household chores to be physically challenging?”; experienced a fall within the previous year; pain, stiffness, numbness or range of motion limitations of the back or legs; involuntary weight gain or loss exceeding 10 pounds within the past six months; myocardial infarction or symptomatic cardiovascular disease in the past year; bone fracture in the past year; medical condition affecting movement; terminal illness; or contraindications to magnetic resonance imaging assessment. To limit variability of physical activity level, we did not enroll individuals who exercised more than one day per week (described as “physical activity that noticeably increases your breathing rate or makes your muscles feel tired”).
Individuals who met these initial criteria were invited to participate in a screening session at our research site. The same examiner conducted all assessments for all participants. The session lasted about 2.5 hours and included the following assessments: resting blood pressure (excluded if > 160/95); body mass index (excluded if <19 or >32); Berg Balance Test (BBT, excluded if <50 out of 56); Mini-Mental State Examination (MMSE, excluded if <25 out of 30), usual 10m walking speed (excluded if <1.0 m/s); maximum walking speed (no exclusion criterion); Short Physical Performance Battery (SPPB, no exclusion criterion); and usual 400m walking speed (no exclusion criterion). Walking tests were timed with a stopwatch. In order to ensure that we recorded steady state walking speed during the 10-meter tests, the beginning and end of the course included 5-meter acceleration/deceleration zones such that the total distance walked was 20 meters. The watch was started and stopped when the individual’s trunk passed over the lines marking the start and end, respectively, of the 10-meter course. Three trials were conducted for each 10-meter test, and the average was used for subsequent analysis. If the difference between usual and maximum 10m walking speed was < 0.6 m/s, the individual was assigned to the “Slower” group. If the difference between usual and maximum 10m walking speed was > 0.6 m/s, the individual was assigned to the “Faster” group. The 0.6 m/s criterion was defined prior to study initiation according to our best judgment, as there is no accepted criterion for which to differentiate individuals with better or worse maximum walking ability.
Participants who met all criteria were invited to return to our research site for a second visit, which involved assessment of plantarflexion dynamic strength as well as other biomechanical assessments not discussed in the present work. Plantarflexion force produced by the triceps surae muscle group was assessed with a rapid bilateral heel-rise task performed at maximal voluntary effort. Participants stood upright facing a wall 0.5 meters away, with feet shoulder width apart and with each foot resting on a separate force plate (Bertec Corporation, Columbus OH). Participants were permitted to touch the wall with their fingertips to assist with maintaining balance during the task. At the direction of study personnel, the participant performed the heel-rise movement (i.e., from flat-footed to standing on toes) by contracting the triceps surae muscle group as forcefully and rapidly as possible. The instruction to the participants was to “push up onto your toes as fast and as hard as possible”. After a brief rest, the test was repeated.
Plantarflexion force production was assessed by evaluating the vertical ground reaction force recorded by a pair of force plates (Bertec, Columbus OH). Neuromuscular activation was assessed by recording surface electromyography (EMG) signal from the right triceps surae muscles, which include soleus (SO) and medial gastrocnemius (MG). The electrode site for SO was distal to the belly of the gastrocnemius, medial and anterior to the Achilles tendon (Perroto, 1994). The site for MG was one hand breadth below the popliteal crease on the medial mass of the lower leg (Perroto, 1994). Each site was shaved and firmly rubbed with a sterile alcohol wipe prior to electrode placement. Disposable 1.5 inch surface gel electrodes (Versa-Trode, Vermed, Bellow Falls VT) with preamplifier (Motion Lab Systems MA-420, Baton Rouge LA) were placed on the skin with inter-electrode distance of approximately 2 cm. The EMG signals were amplified (Motion Lab Systems MA300-28, Baton Rouge LA) and, along with force, sampled at 1000 Hz and 200 Hz, respectively (VICON Nexus, Los Angeles CA). All data were saved to disk for offline analysis. Data from a representative heel-rise trial are shown in Figure 1. To mitigate the effects of inter-individual variability of electrode placement, subcutaneous adipose tissue thickness and other sources of variability, EMG magnitude during the maximal heel-risk task was normalized to a static submaximal reference contraction. For the reference contraction, participants stood on their toes with heels raised, exerting just enough force to support body weight and maintain a static posture.
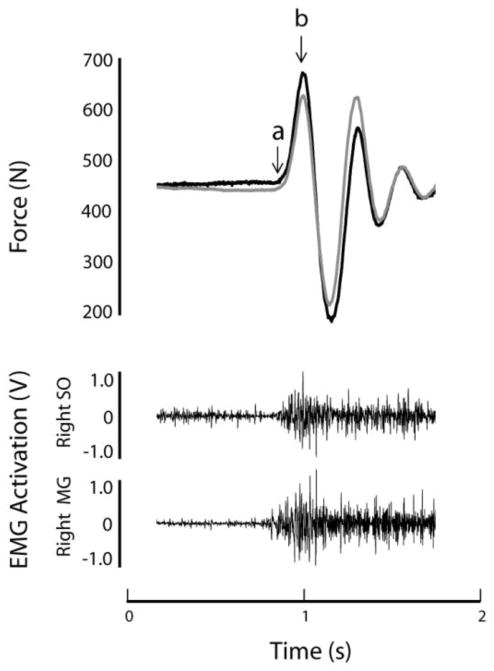
Figure 1. Representative force and EMG data.
Data from a representative heel-rise trial are shown for one participant. The top figure shows vertical force produced by the left (black) and right (gray) sides. Peak force occurs at point “b”. Rate of force production was calculated between points “a” and “b”. The lower set of figures shows unprocessed EMG data from the soleus (SO) and medial gastrocnemius (MG) muscles.
Muscle cross sectional area (CSA) of the right triceps surae, quadriceps and hamstrings muscle groups was determined by T1-weighted 3D-magnetic resonance imaging (MRI) using a Phillips 3.0 Tesla magnet (Philips Medical Systems, Bothell, WA). Data were collected using a fast gradient-echo sequence (TR=100 ms, TE=10 ms, flip angle of 30 ). Chemically-selective fat suppression was utilized, which enhanced muscle as high signal intensity (light gray) pixels and adipose tissue as low signal intensity (dark gray) pixels.
Data Analysis
Force and neuromuscular activation data were analyzed using custom software programs created in Matlab (The Mathworks, Natick MA). For each leg, the peak vertical force of the first positive force deflection during the heel-rise task was determined (force at point “b” in Figure 1). The values from each leg were then summed, and body weight was subtracted to provide bilateral peak force. The trial with the higher peak force was used for subsequent analysis. Time to peak force was calculated separately for each leg as the time between initial force onset and peak force production (i.e., duration of time between points “a” and “b” in Figure 1), then averaged for both legs. Plantarflexion rate of force development (RFD) was calculated as bilateral peak force divided by the time to peak force.
Neuromuscular activation was assessed by calculating the rate of EMG rise for the right triceps surae muscles, soleus (SO) and medial gastrocnemius (MG). Rate of EMG rise is an effective measure of neuromuscular activation capability, as demonstrated by us and others (Aagaard et al., 2002; Clark et al., 2011; Laroche et al., 2007). Figure 2 shows an example of the key steps of EMG data analysis. EMG signals were filtered with a 4th order, zero phase lag Butterworth band pass filter (10–500 Hz), de-biased (mean set to zero), rectified, smoothed with a 4th order Butterworth low pass filter (10 Hz), normalized to (i.e., expressed as a percent of) the EMG magnitude of the static reference trial, and the time derivative of the signal was calculated. For both the SO and MG muscles, the peak derivative was used to indicate the peak rate of EMG rise. The unit for rate of EMG rise is percent of the reference EMG magnitude per second.
Figure 2. EMG processing for rate of EMG rise.
The light gray line is EMG after rectification. The solid black line is the rectified signal after being smoothed with a low pass filter (10 Hz). The dashed black line is the derivative of the smoothed, rectified signal. The peak rate of rise is indicated by the arrow. These data are shown for illustrative purposes and are not drawn to scale.
Analysis of MRI images was conducted with MIPAV software (version 1.3; Medical Image Processing, Analysis and Visualization). Muscle cross sectional area was measured from a single axial slice at the point of greatest cross-sectional area of the mid-thigh and at the greatest cross-sectional area of the calf region as described previously (Manini et al., 2007). Tissue composition was quantified using automated pixel clustering by a fuzzy-c-means algorithm. This algorithm clusters pixels into different categories (e.g. muscle, adipose and background) based on differences in pixel intensity. If the pixel intensity is close to the centroid (median value) of the specified class then it receives a score of 1. If the pixel intensity is far from the centroid it receives a value close to zero. A membership function then determines which pixels belong to which category. Both the membership function and centroids are derived through an iterative process. Comparisons of results from hand-traced tissue segmentation to fuzzy-c-mean tissue segmentation show strong correlations for muscle (r = 0.99) and adipose tissue (r = 0.93). Isolation of individual muscle groups was accomplished by manual tracings. The technical error of drawing these regions is highly reliable among our technicians (CV=0.26%, n=10).
Statistics
Statistical analysis was conducted with JMP statistical software (v. 9.0.2, SAS Institute Inc, Cary, NC). Between-groups comparison of demographic and functional measures was conducted with two-tailed t-tests. Between-groups comparison of force, muscle size and rate of EMG rise was conducted with one-tailed t-tests based on the hypothesis that Slower < Faster. Associations between rate of EMG rise and mobility function were assessed with Pearson’s correlation (for walking speed) or Spearman’s correlation (for SPPB score). Statistical significance was set at α=.05. The strength of the differences observed between groups was further described by calculating effect sizes. Effect size larger than 0.5 is generally considered to be a large effect (Cohen, 1988).
Results
Comparison between groups
Twenty older adults participated in this study, with eight categorized into the Slower group and 12 categorized into the Faster group. Detailed group characteristics are presented in Table 1. Within each group there were an equal number of males and females. The groups did not differ for age, weight, height, BMI, functional balance (BBT) or cognitive function (MMSE). As discussed in the Methods, the groups were defined based on the difference between usual and maximum walking speed. However, we found this difference to be almost fully explained just by each individual’s maximum walking speed (r=.85, p<.0001). For simplicity, the study results and discussion therefore focus on maximum walking speed rather than the difference between usual and maximum.
Table 1.
Participant Characteristics and Functional
| Performance | |||
|---|---|---|---|
| Faster | Slower | p | |
| Age (years) | 70.8 ± 4.5 | 71.4 ± 5.0 | 0.77 |
| Sex (male/female) | 6/6 | 4/4 | 1.00 |
| Weight (kg) | 74.3 ± 13.1 | 78.3 ± 11.5 | 0.45 |
| Height (cm) | 167.4 ± 10.7 | 169.2 ± 10.4 | 0.81 |
| BMI(kg/m2) | 26.4 ± 2.4 | 27.1 ± 1.3 | 0.48 |
| 10m usual speed (m/s) | 1.37 ± 0.15 | 1.24 ± 0.15 | 0.08 |
| 10m maximum speed (m/s) | 2.17 ± 0.20 | 1.76 ± 0.16 | <0.001 |
| 400m usual speed (m/s) | 1.31 ± 0.15 | 1.10 ± 0.11 | 0.003 |
| SPPB (out of 12) | 11.8 ± 0.6 | 10.1 ± 1.2 | 0.001 |
| BBT (out of 56) | 54.7 ± 1.3 | 55.1 ± 1.1 | 0.44 |
| MMSE (out of 30) | 29.4 ± 1.5 | 29.1 ± 1.4 | 0.67 |
values are mean ± standard deviation
As expected due to the study design, both groups had usual 10m walking speeds that were well within the range of “normal” function (i.e., > 1.0 m/s) (Abellan van Kan et al., 2009; Cesari et al., 2005; Fielding et al., 2011; Morley et al., 2011; Studenski, 2009). Usual 10m walking speed was 1.37 and 1.24 m/s for Faster and Slower, respectively (p=.08). Also expected was a highly significant difference in maximum walking speed, with Faster walking at 2.17 m/s and Slower at 1.76 m/s (p<.001). Usual 400m walking speed was found to be within the “normal” range for both groups, but was significantly lower in Slower compared to Faster (1.10 m/s and 1.31 m/s, respectively, p=.003). SPPB score also differed between Slower and Faster (10.1 and 11.8 points, respectively, p=.001).
The results for force production, muscle size and rate of EMG rise are presented in Table 2. Contrary to our hypothesis, muscle CSA was not lower in Slower than Faster for any of the muscle groups tested, which included triceps surae (p=.44), quadriceps (p=.76) and hamstrings (p=.98). However, substantial differences were observed for rapid force and neuromuscular activation of the triceps surae. Consistent with our hypothesis, plantarflexion RFD was 38% lower (p=.002) in Slower compared to Faster. MG rate of EMG rise was also 34% lower (p=.01) in Slower than Faster, but SO rate of EMG rise did not differ between groups (p=.73). Across all participants, MG rate of EMG rise was significantly positively associated with plantarflexion RFD (p=.02, r=.62), maximum walking speed (p=.04, r=.54) and usual 400m walking speed (p=.01, r=.63), but not with usual 10m walking speed (p=.19) or SPPB score (p=.23).
Table 2.
Neuromuscular Measures
| Faster | Slower | p | Effect Size | |
|---|---|---|---|---|
| Rate of force production (N/sec) | 3218 ± 442 | 2010 ± 1112 | 0.002 | 1.26 |
| MG rate of EMG rise (% ref EMG/s) | 7400 ± 1960 | 4910 ± 1570 | 0.01 | 1.15 |
| SO rate of EMG rise (% ref EMG/s) | 5960 ± 1550 | 6750 ± 3280 | 0.73 | - |
| Plantarflexor CSA (cm2) | 347 ± 83 | 331 ± 65 | 0.44 | - |
| Quadriceps CSA (cm2) | 476 ± 130 | 496 ± 112 | 0.76 | - |
| Hamstrings CSA (cm2) | 275 ± 50 | 332 ± 53 | 0.98 | - |
values are mean ± standard deviation
Two minor adverse events occurred during this study. A participant who had arrived at our research site for the second visit (plantarflexor force assessment) began to feel light-headed prior to testing, but did not lose consciousness. It is not clear if this event was related to the study. Another participant who had already completed the study called us to describe soreness of the Achilles tendon that may have been associated with testing during the second visit. Both issues were resolved without medical intervention.
Discussion
The findings of this study support the conclusion that maximum walking speed in healthy, well-functioning older adults is associated with underlying impairments in neuromuscular activation and force production. We recruited two homogeneous groups of older adults who were primarily distinguished by a difference in maximum walking speed. This approach differs from most studies of age-related functional decline that compare groups with considerably different ages or functional abilities. Our approach is likely to provide a more direct indication of the neuromuscular factors that account for inter-individual differences in walking function. Plantarflexion RFD and was found to be considerably impaired in the Slower group relative to the Faster group. Importantly, these clear group differences were evident despite the fact that both the Slower and Faster groups had usual 10m walking speeds considered to be “normal” for healthy older adults (Abellan van Kan et al., 2009; Cesari et al., 2005; Fielding et al., 2011; Morley et al., 2011).
Few prior studies have investigated the link between impaired neuromuscular activation and decline of functional performance with aging. Brach and colleagues showed that neuromuscular activation of the quadriceps group during a chair stand task, particularly when quantified as the rate of EMG rise, was significantly predictive of better performance of usual walking speed and on a functional status questionnaire (Brach et al., 2001). Similarly, our own previous work has shown that rate of EMG rise in the quadriceps muscle group during a leg press movement is positively correlated with the SPPB score (Clark et al., 2011). Furthermore, we have shown considerable neuromuscular activation impairment in “mobility-limited” older adults (SPPB < 10) compared to healthy older adults (SPPB > 10) or middle aged adults (Clark et al., 2010; Clark et al., 2011). In the present study, we build upon these earlier findings by showing that MG rate of EMG rise is impaired in Slower versus Faster, and is positively associated with maximum walking speed and 400m walking speed. Impaired MG activation may affect walking speed due to its role in supporting body weight throughout the stance phase and accelerating the leg into swing during late stance (Neptune et al., 2001). A number of neural factors may be responsible for impaired rate of EMG rise with aging, such as reduced number of fast conducting motor neurons (Kawamura et al., 1977; Mittal and Logmani, 1987), reduced drive from the corticospinal pathway (Eisen et al., 1996; Oliviero et al., 2006; Smith et al., 2009), reduced motoneuronal conductivity (McNeil et al., 2005; Metter et al., 1998) and poorer transmission of activation signals at the neuromuscular junction (Deschenes et al., 2010). Unlike MG rate of EMG rise, SO rate of EMG rise did not differ between Slower and Faster. This finding is consistent with other studies that have shown preserved neuromuscular function in the SO of older adults (Dalton et al., 2009; Dalton et al., 2008; Fujiwara et al., 2010). One explanation for the differential findings between MG and SO is that innervation of MG is comprised of a higher proportion of large diameter motor neurons, as indicated by the higher proportion of fast twitch muscle fibers (Edgerton et al., 1975). Large diameter motor neurons are more susceptible than smaller diameter neurons to age-related cell death, which leads to a general slowing of neuromuscular activation (Doherty et al., 1993a). The differences might also be related to intensity of habitual muscle activity that helps to preserve SO activation capability to a greater extent than MG. For example, SO contributes more than MG to the demanding task of propelling the body forward during walking (McGowan et al., 2008; Neptune et al., 2001).
CSA of the triceps surae, quadriceps and hamstrings muscle groups did not differ between Slower and Faster, indicating that differences in muscle mass might not be a primary determinant of maximum walking ability in healthy older adults. While this finding is in agreement with a recent longitudinal study of healthy elderly women (Sergi et al., 2011), it does not rule out the possibility that some of our participants may have age-related loss of muscle mass that may eventually progress and contribute to impaired mobility function. Indeed, loss of muscle mass is known to be linked to the presence of overt mobility deficits and disability (Delmonico et al., 2007; Fielding et al., 2011; Janssen et al., 2002; Lauretani et al., 2003).
In addition to differences in maximum walking speed, Slower and Faster also exhibited significant differences in usual 400m walk speed and on the SPPB. The difference between groups to complete 400m was 59 seconds and the difference in SPPB score was 1.7 points. In both cases, this is considered a substantial, meaningful difference in functional performance (Kwon et al., 2009; Perera et al., 2006). Additional research would be necessary to determine the extent to which decrements in each test are caused by the same underlying impairments. For instance, aerobic fitness might be expected to be more important for the 400m walk than for the other tests, and the SPPB explicitly assesses strength (chair rise) and balance, which the walking tests do not. Importantly, maximum walking speed and usual 400m walking speed were found to be significantly correlated with MG rate of EMG rise, while usual 10m walking speed and SPPB score were not. This finding supports our main hypothesis that maximum walking speed is more affected by neuromuscular decline than is usual 10m walking speed. In the context of clinical assessment, it is also notable that maximum 10m walking speed offers the benefit of being less time consuming and simpler to administer than either the 400m walk test or SPPB.
There are a number of methodological issues that should be considered when interpreting the results of this study. Statistical power was limited by a small sample size. Nevertheless, statistical analysis revealed relatively large effect sizes and correlation coefficients, which suggest strong findings that can be further evaluated in future studies. The small sample size and restrictive inclusion/exclusion criteria (e.g., usual walking speed greater than 1.0 m/s) also limits the generalizability of the results, particularly with regard to neuromuscular determinants of maximum walking speed in lower functioning older adults. It should also be acknowledged that while EMG is a powerful tool for neuromuscular assessment, it can be affected by a variety of non-neural factors (De Luca, 1997). As described in the Methods section, we used the widely accepted approach of normalizing our EMG to a reference contraction in order to mitigate potential sources of variability (Burden, 2010). Despite the technical challenges of surface EMG, our data show a strong correlation between plantarflexor rate of EMG rise and RFD, which would be unlikely if the EMG recordings were of poor quality. Finally, it should be noted that we did not evaluate the intrinsic force generating characteristics of muscle fibers, which may have been another factor contributing to the differences in strength and function between our experimental groups.
Conclusions
The present findings support the conclusion that age-related neuromuscular activation impairment limits maximum 10-meter walking speed. Future research should further evaluate the utility of maximum walking speed for use in clinical assessment to detect and monitor age-related functional decline.
Figure 3.
Flow chart of participant screening and assessment.
Highlights.
Maximum walking speed may be valuable for clinical assessment of mobility function
Capability to rapidly activate muscle is correlated with maximum walking speed
Lower extremity muscle size does not explain maximum walking speed
Deficient maximum walking speed reveals impaired muscle activation in healthy elders
Acknowledgments
This work was supported by the University of Florida Claude D. Pepper Older Americans Independence Center (P30-AG028740-04) and by the U.S. Department of Veterans Affairs Rehabilitation Research and Development Service (B7176-W to DJC). This work was also supported by the U.S. Department of Agriculture, under agreement No. 58-1950-7-707, the Boston Claude D. Pepper Older Americans Independence Center and the Boston Rehabilitation Outcomes Center. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the U.S. Department of Veterans Affairs or U.S. Department of Agriculture.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aagaard P, Simonsen EB, Andersen JL, Magnusson P, Dyhre-Poulsen P. Increased rate of force development and neural drive of human skeletal muscle following resistance training. J Appl Physiol. 2002;93:1318–1326. doi: 10.1152/japplphysiol.00283.2002. [DOI] [PubMed] [Google Scholar]
- Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- Brach JS, Kriska AM, Newman AB, VanSwearingen JM. A new approach of measuring muscle impairment during a functional task: quadriceps muscle activity recorded during chair stand. J Gerontol A Biol Sci Med Sci. 2001;56:M767–770. doi: 10.1093/gerona/56.12.m767. [DOI] [PubMed] [Google Scholar]
- Burden A. How should we normalize electromyograms obtained from healthy participants? What we have learned from over 25 years of research. J Electromyogr Kinesiol. 2010;20:1023–1035. doi: 10.1016/j.jelekin.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Cesari M, Kritchevsky SB, Penninx BW, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky FA, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin SM, Harris TB, Pahor M. Prognostic value of usual gait speed in well-functioning older people--results from the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2005;53:1675–1680. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired Voluntary Neuromuscular Activation Limits Muscle Power in Mobility-Limited Older Adults. J Gerontol A Biol Sci Med Sci. 2010;65:495–502. doi: 10.1093/gerona/glq012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Muscle performance and physical function are associated with voluntary rate of neuromuscular activation in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:115–121. doi: 10.1093/gerona/glq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- Dalton BH, Harwood B, Davidson AW, Rice CL. Triceps surae contractile properties and firing rates in the soleus of young and old men. J Appl Physiol. 2009;107:1781–1788. doi: 10.1152/japplphysiol.00464.2009. [DOI] [PubMed] [Google Scholar]
- Dalton BH, McNeil CJ, Doherty TJ, Rice CL. Age-related reductions in the estimated numbers of motor units are minimal in the human soleus. Muscle Nerve. 2008;38:1108–1115. doi: 10.1002/mus.20984. [DOI] [PubMed] [Google Scholar]
- De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13:135–163. [Google Scholar]
- Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVita P, Hortobagyi T. Age causes a redistribution of joint torques and powers during gait. J Appl Physiol. 2000;88:1804–1811. doi: 10.1152/jappl.2000.88.5.1804. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Brown WF. Effects of ageing on the motor unit: a brief review. Can J Appl Physiol. 1993a;18:331–358. doi: 10.1139/h93-029. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993b;74:868–874. doi: 10.1152/jappl.1993.74.2.868. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Smith JL, Simpson DR. Muscle fibre type populations of human leg muscles. Histochem J. 1975;7:259–266. doi: 10.1007/BF01003594. [DOI] [PubMed] [Google Scholar]
- Eisen A, Entezari-Taher M, Stewart H. Cortical projections to spinal motoneurons: changes with aging and amyotrophic lateral sclerosis. Neurology. 1996;46:1396–1404. doi: 10.1212/wnl.46.5.1396. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan van Kan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara K, Asai H, Toyama H, Kunita K, Yaguchi C, Kiyota N, Tomita H, Jacobs JV. Changes in muscle thickness of gastrocnemius and soleus associated with age and sex. Aging Clin Exp Res. 2010;22:24–30. doi: 10.1007/BF03324811. [DOI] [PubMed] [Google Scholar]
- Jahn K, Zwergal A, Schniepp R. Gait disturbances in old age: classification, diagnosis, and treatment from a neurological perspective. Dtsch Arztebl Int. 2010;107:306–315. doi: 10.3238/arztebl.2010.0306. quiz 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Jennekens FG, Tomlinson BE, Walton JN. Histochemical aspects of five limb muscles in old age. An autopsy study. J Neurol Sci. 1971;14:259–276. doi: 10.1016/0022-510x(71)90216-4. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Dynamics and stability of muscle activations during walking in healthy young and older adults. J Biomech. 2009;42:2231–2237. doi: 10.1016/j.jbiomech.2009.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, O’Brien P, Okazaki H, Dyck PJ. Lumbar motoneurons of man II: the number and diameter distribution of large- and intermediate-diameter cytons in “motoneuron columns” of spinal cord of man. J Neuropathol Exp Neurol. 1977;36:861–870. doi: 10.1097/00005072-197709000-00010. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Todd MK, Della Croce U, Lipsitz LA, Collins JJ. Biomechanical gait alterations independent of speed in the healthy elderly: evidence for specific limiting impairments. Arch Phys Med Rehabil. 1998;79:317–322. doi: 10.1016/s0003-9993(98)90013-2. [DOI] [PubMed] [Google Scholar]
- Ko S-u, Hausdorff JM, Ferrucci L. Age-associated differences in the gait pattern changes of older adults during fast-speed and fatigue conditions: results from the Baltimore longitudinal study of ageing. Age Ageing. 2010;39:688–694. doi: 10.1093/ageing/afq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Perera S, Pahor M, Katula JA, King AC, Groessl EJ, Studenski SA. What is a meaningful change in physical performance? Findings from a clinical trial in older adults (the LIFE-P study) J Nutr Health Aging. 2009;13:538–544. doi: 10.1007/s12603-009-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroche DP, Knight CA, Dickie JL, Lussier M, Roy SJ. Explosive force and fractionated reaction time in elderly low- and high-active women. Med Sci Sports Exerc. 2007;39:1659–1665. doi: 10.1249/mss.0b013e318074ccd9. [DOI] [PubMed] [Google Scholar]
- Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Liu MQ, Anderson FC, Schwartz MH, Delp SL. Muscle contributions to support and progression over a range of walking speeds. J Biomech. 2008;41:3243–3252. doi: 10.1016/j.jbiomech.2008.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr. 2007;85:377–384. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- Manini TM, Pahor M. Physical activity and maintaining physical function in older adults. Br J Sports Med. 2009;43:28–31. doi: 10.1136/bjsm.2008.053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McComas AJ, Galea V, de Bruin H. Motor unit populations in healthy and diseased muscles. Phys Ther. 1993;73:868–877. doi: 10.1093/ptj/73.12.868. [DOI] [PubMed] [Google Scholar]
- McGowan CP, Neptune RR, Kram R. Independent effects of weight and mass on plantar flexor activity during walking: implications for their contributions to body support and forward propulsion. J Appl Physiol. 2008;105:486–494. doi: 10.1152/japplphysiol.90448.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Doherty TJ, Stashuk DW, Rice CL. Motor unit number estimates in the tibialis anterior muscle of young, old, and very old men. Muscle Nerve. 2005;31:461–467. doi: 10.1002/mus.20276. [DOI] [PubMed] [Google Scholar]
- Metter EJ, Conwit R, Metter B, Pacheco T, Tobin J. The relationship of peripheral motor nerve conduction velocity to age-associated loss of grip strength. Aging (Milano) 1998;10:471–478. doi: 10.1007/BF03340161. [DOI] [PubMed] [Google Scholar]
- Mittal KR, Logmani FH. Age-related reduction in 8th cervical ventral nerve root myelinated fiber diameters and numbers in man. J Gerontol. 1987;42:8–10. doi: 10.1093/geronj/42.1.8. [DOI] [PubMed] [Google Scholar]
- Monaco V, Rinaldi LA, Macri G, Micera S. During walking elders increase efforts at proximal joints and keep low kinetics at the ankle. Clin Biomech. 2009;24:493–498. doi: 10.1016/j.clinbiomech.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Morley JE, Abbatecola AM, Argiles JM, Baracos V, Bauer J, Bhasin S, Cederholm T, Coats AJ, Cummings SR, Evans WJ, Fearon K, Ferrucci L, Fielding RA, Guralnik JM, Harris TB, Inui A, Kalantar-Zadeh K, Kirwan BA, Mantovani G, Muscaritoli M, Newman AB, Rossi-Fanelli F, Rosano GM, Roubenoff R, Schambelan M, Sokol GH, Storer TW, Vellas B, von Haehling S, Yeh SS, Anker SD. Sarcopenia with limited mobility: an international consensus. J Am Med Dir Assoc. 2011;12:403–409. doi: 10.1016/j.jamda.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Davis MG, Fox KR, Birch KM, Narici MV. Reduced plantarflexor specific torque in the elderly is associated with a lower activation capacity. Eur J Appl Physiol. 2004;92:219–226. doi: 10.1007/s00421-004-1056-y. [DOI] [PubMed] [Google Scholar]
- Morse CI, Thom JM, Reeves ND, Birch KM, Narici MV. In vivo physiological cross-sectional area and specific force are reduced in the gastrocnemius of elderly men. J Appl Physiol. 2005;99:1050–1055. doi: 10.1152/japplphysiol.01186.2004. [DOI] [PubMed] [Google Scholar]
- Murray MP, Guten GN, Sepic SB, Gardner GM, Baldwin JM. Function of the triceps surae during gait. Compensatory mechanisms for unilateral loss. J Bone Joint Surg Am. 1978;60:473–476. [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V. Effects of aging on motor cortex excitability. Neurosci Res. 2006;55:74–77. doi: 10.1016/j.neures.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Perroto AO. Anatomical Guide for the Electromyographer. Springfield, IL: Charles C. Thomas; 1994. [Google Scholar]
- Requiao LF, Nadeau S, Milot MH, Gravel D, Bourbonnais D, Gagnon D. Quantification of level of effort at the plantarflexors and hip extensors and flexor muscles in healthy subjects walking at different cadences. J Electromyogr Kinesiol. 2005;15:393–405. doi: 10.1016/j.jelekin.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Runge M, Rittweger J, Russo CR, Schiessl H, Felsenberg D. Is muscle power output a key factor in the age-related decline in physical performance? A comparison of muscle cross section, chair-rising test and jumping power. Clin Physiol Funct Imaging. 2004;24:335–340. doi: 10.1111/j.1475-097X.2004.00567.x. [DOI] [PubMed] [Google Scholar]
- Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi G, Sarti S, Mosele M, Ruggiero E, Imoscopi A, Miotto F, Bolzetta F, Inelmen EM, Manzato E, Coin A. Changes in healthy elderly women’s physical performance: a 3-year follow-up. Exp Gerontol. 2011;46:929–933. doi: 10.1016/j.exger.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Simoneau E, Martin A, Van Hoecke J. Muscular performances at the ankle joint in young and elderly men. J Gerontol A Biol Sci Med Sci. 2005;60:439–447. doi: 10.1093/gerona/60.4.439. [DOI] [PubMed] [Google Scholar]
- Smith A, Ridding M, Higgins R, Wittert G, Pitcher J. Age-related changes in short-latency motor cortex inhibition. Exp Brain Res. 2009;198:489–500. doi: 10.1007/s00221-009-1945-8. [DOI] [PubMed] [Google Scholar]
- Stalberg E, Fawcett PR. Macro EMG in healthy subjects of different ages. J Neurol Neurosurg Psychiatry. 1982;45:870–878. doi: 10.1136/jnnp.45.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studenski S. Bradypedia: is gait speed ready for clinical use? J Nutr Health Aging. 2009;13:878–880. doi: 10.1007/s12603-009-0245-0. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Bean JF, Fielding RA. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- Tanaka A, Okuzumi H, Kobayashi I, Murai N, Nakamura T, Furuyama K, Shimizu Y. Age-related changes in natural and fast walking. Percept Mot Skills. 1995;80:217–218. doi: 10.2466/pms.1995.80.1.217. [DOI] [PubMed] [Google Scholar]
- Thom JM, Morse CI, Birch KM, Narici MV. Triceps surae muscle power, volume, and quality in older versus younger healthy men. J Gerontol A Biol Sci Med Sci. 2005;60:1111–1117. doi: 10.1093/gerona/60.9.1111. [DOI] [PubMed] [Google Scholar]
- Winter DA. The biomechanics and motor control of human gait. Waterloo, Ontario: University of Waterloo; 1987. [Google Scholar]