Abstract
We aimed to investigate the significance of microalbuminuria and its relationship with subclinical atherosclerosis in nonhypertensive and nondiabetic patients, by using coronary artery computed tomography (CT). A total of 1,318 nonhypertensive and nondiabetic subjects who had taken coronary artery CT and measured spot urine albumin to creatinine ratio (UACR) were evaluated. The atherosclerotic changes of coronary arteries were greater in subjects with microalbuminuria, reflected by coronary artery calcium score (CACS) and significant coronary artery stenosis (CACS ≥ 100 in 15.3% vs 7.6% and stenosis ≥ 50% in 11.5% vs 4.9% of patients with vs without microalbuminuria, P = 0.008 and P = 0.011, respectively). Among various parameters that are known as a risk factor or possible biomarkers of coronary artery disease, presence of microalbuminuria, age and Framingham risk score were significantly related to coronary artery stenosis. Among them the presence of microalbuminuria showed stronger correlation than others to the coronary artery stenosis detected by CT, even after adjusting confounding factors (OR 3.397, 95% confidence interval 1.138 to 10.140, P = 0.028). The presence of microalbuminuria by UACR was significantly associated with presence of coronary artery stenosis ≥ 50% in asymptomatic, nonhypertensive and nondiabetic general population. Our study suggests that the presence of microalbuminuria may imply subclinical coronary artery disease, even in asymptomatic population.
Keywords: Microalbuminuria; Coronary Artery; Tomography, X-Ray Computed
INTRODUCTION
Microalbuminuria is a well-established risk factor for cardiovascular morbidity and mortality in individuals with cardiovascular risk factors such as diabetes mellitus or hypertension (1-4). It indicates underlying generalized vascular dysfunction (5, 6), and screening for microalbuminuria is recommended in patients with cardiovascular risk factors (7, 8). Furthermore, the results from Heart Outcomes Prevention Evaluation (HOPE) study have postulated that any degree of albuminuria is a risk factor for cardiovascular events, in both diabetic and non-diabetic patients, starting well below the known microalbuminuria cutoff, defined as urine albumin to creatinine ratio of 2 mg/mM or more (9). The significance of microalbuminuria in cardiovascular outcome has also been studied in apparently healthy population who are normotensive and without diabetes mellitus (10). Even in healthy patients, microalbuminuria has been suggested as a marker of cardiovascular risk, as it reflects earlier vascular damage in the kidneys and systemic endothelial dysfunction (6, 11). Although the pathophysiological basis of the association between microalbuminuria and cardiovascular disease still remains to be elucidated, microalbuminuria and atherosclerosis appear to have certain pathogenetic mechanisms in common.
Although much has been studied about clinical significance of microalbuminuria in predicting cardiovascular outcome, most of the studies targeted population with risk factors such as hypertension or diabetes mellitus, and less is known about the significance of microalbuminuria in relation to subclinical atherosclerosis, especially in asymptomatic patients without known cardiovascular risk factors.
In this study, we aimed to investigate the significance of microalbuminuria and its relationship with subclinical atherosclerosis in nonhypertensive and nondiabetic patients, by using coronary artery computed tomography (CT).
MATERIALS AND METHODS
Study population
The current study was performed retrospectively. A total of 2,456 asymptomatic patients who visited Seoul National University Hospital Healthcare System Gangnam Center between January 2006 and December 2010 for comprehensive medical examinations and had coronary artery CT and ratio of spot urine albumin to creatinine (UACR) were evaluated. The coronary CT was performed for screening purpose on patients' demand. Past medical histories and current medications were derived from medical questionnaires, at the time of the medical examinations. The patients with known hypertension, diabetes mellitus or coronary artery disease were excluded. To exclude patients with decreased renal function, those with serum creatinine level of greater than 1.5 mg/dL or those with previously known chronic kidney disease were also excluded in the analysis. The patients with macroalbuminuria, defined by UACR of 200 mg/g or greater were excluded. In the final analysis, 1,318 patients were included.
Laboratory evaluation
Spot determination of UACR was used for screening. Based on the previous results showing high diagnostic accuracy of spot UACR compared to 24-hr urine collection, spot UACR was used in the data analysis (5, 12-14). The presence of microalbuminuria was defined as UACR of greater than 20 and less than 200 mg/g (9, 15). Other biomarkers such as high sensitive C-reactive protein (hsCRP), homocysteine, insulin and fibrinogen were also evaluated. Insulin resistance was calculated using Homeostasis model assessment of insulin resistance (HOMA-IR) formula. Glomerular filtration rate was estimated using Modification of Diet in Renal Disease (MDRD) formula;
| MDRD GFR (mL/min/1.73m2) = 186 × SCr-1.154 × age-0.203 × 0.742 (in females), |
where SCr is serum creatinine.
Framingham risk score was derived from on-line National Cholesterol Education Program Adult Treatment Panel III (NCEP/ATP-III) guideline (16).
Computed tomography
Coronary artery was scanned using a 16-row multi-slice CT scanner (Sensation 16, Siemens Medical Systems, Erlangen, Germany). After obtaining a topogram of the chest, a calcium score scan and angiography were performed using retrospective method with tube voltage of 120 kV, and 110 effective mAs with a 200 mm field of view. Data was reconstructed to 3-mm slice thickness at -400 ms acquisition window. Calcium score analysis was performed onsite using a dedicated workstation and analysis soft-ware using Wizard VB10B (Somaris/5 VB10B-W, SynGo, Siemens, Germany), and quantitative CACS were calculated according to the method described by Agatston et al.(17) Coronary artery stenosis of 50% or more by CT was considered significant.
Among the study population, 748 patients who had abdominal CT were also evaluated for amount of intraperitoneal fat. The cross-sectional surface areas of abdominal fat compartments were measured by a software program (Rapidia 2.8; INFINITT, Seoul, Korea) with attenuation ranging from -250 to -50 Hounsfield units. The intraperitoneal fat area was defined as intraperitoneal part fat bound by the parietal peritoneum or transversalis fascia, excluding the vertebral column and paraspinal muscles.
Statistical analysis
Data are expressed as mean ± standard deviation. For comparing continuous parameters in patients with microalbuminuria and without microalbuminuria, t-test was performed. Bivariate analyses were used for correlation analysis, and to determine the factors that predict significant coronary artery stenosis of 50% or more, multivariate analysis was performed using binary logistic regression analysis. For all statistical analyses, statistical software package (SPSS 17.0, SPSS Inc, Chicago, IL, USA) was used and a P value of less than 0.05 was considered statistically significant.
Ethics statement
The study protocol was reviewed and approved by the institutional review board of Seoul National University Hospital (IRB No.H-1104-087-359). Since the current study was performed as a retrospective study using the database and medical records, informed consent was waived by the board.
RESULTS
The baseline characteristics of the study population are shown in Table 1. The mean age of the study population was 54 yr and 72.6% were males. The average systolic and diastolic blood pressures were 117 and 78 mmHg and mean low-density lipoprotein (LDL) cholesterol was 124 mg/dL. The prevalence of microalbuminuria was 7.9% in asymptomatic nonhypertensive and nondiabetic Korean patients. As shown in Table 1, 8.1% of the general Korean population had coronary artery calcification of greater than 100, and 10.6% of the apparently healthy patients had any coronary artery stenosis, defined as stenosis > 0%.
Table 1.
Baseline characteristics of the study population
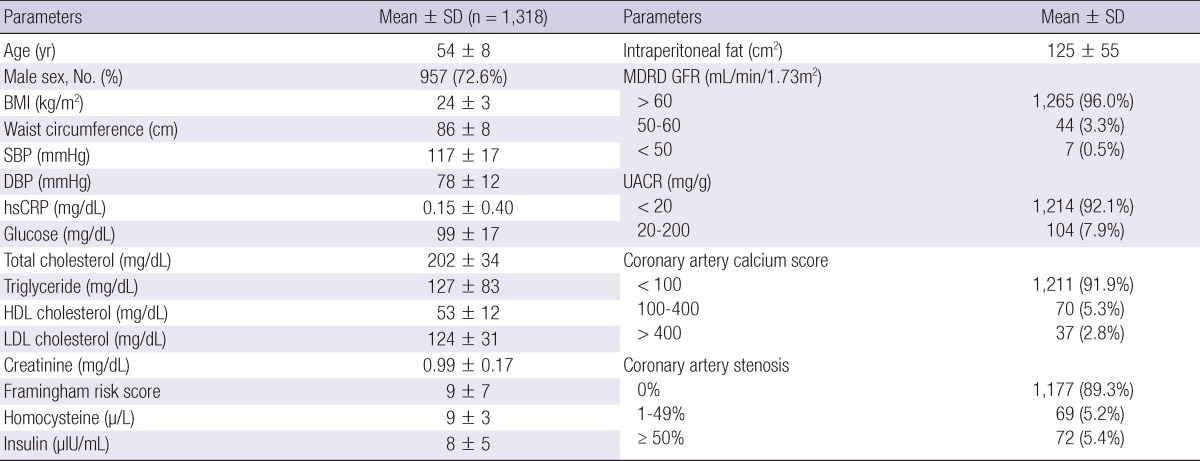
BMI, body mass index; DBP, diastolic blood pressure; GFR, glomerular fitration rate; HDL cholesterol, high-density lipoprotein cholesterol; hsCRP, high sensitivity C-reactive protein; LDL cholesterol, low-density lipoprotein cholesterol; MDRD, Modification of Diet in Renal Disease; SBP, systolic blood pressure; SD, standard deviation; UACR, urine albumin to creatinine ratio.
Differences in patients with versus without microalbuminuria
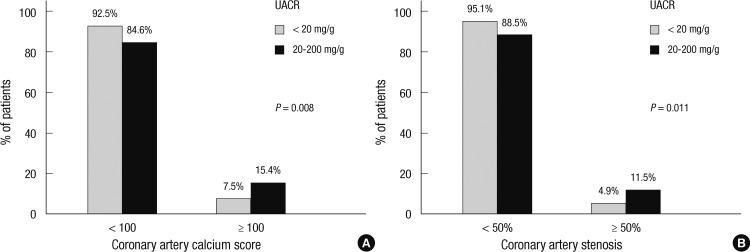
The characteristics of patients with and without microalbuminuria are shown in Table 2. Cardiovascular risk, assessed by Framingham risk score was not different in patients with and without microalbuminuria. The patients with microalbuminuria were older, and had higher fasting serum glucose. Total cholesterol and LDL-cholesterol levels were not significantly different in the two groups. Serum level of homocysteine was not significantly different. The renal function evaluated by MDRD GFR showed no difference according to the presence of microalbuminuria. The systolic and diastolic blood pressures were also higher in patients with microalbuminuria, but pulse pressure did not show significant difference. The atherosclerotic changes of coronary arteries were greater in patients with microalbuminuria, reflected by coronary artery calcium score and significant coronary artery stenosis (CACS ≥ 100 in 15.3% vs 7.6% and stenosis ≥ 50% in 11.5% vs 4.9% of patients with vs without microalbuminuria, P = 0.008 and P = 0.011, respectively, Fig. 1).
Table 2.
Comparisons of clinical parameters in patients with and without microalbuminuria
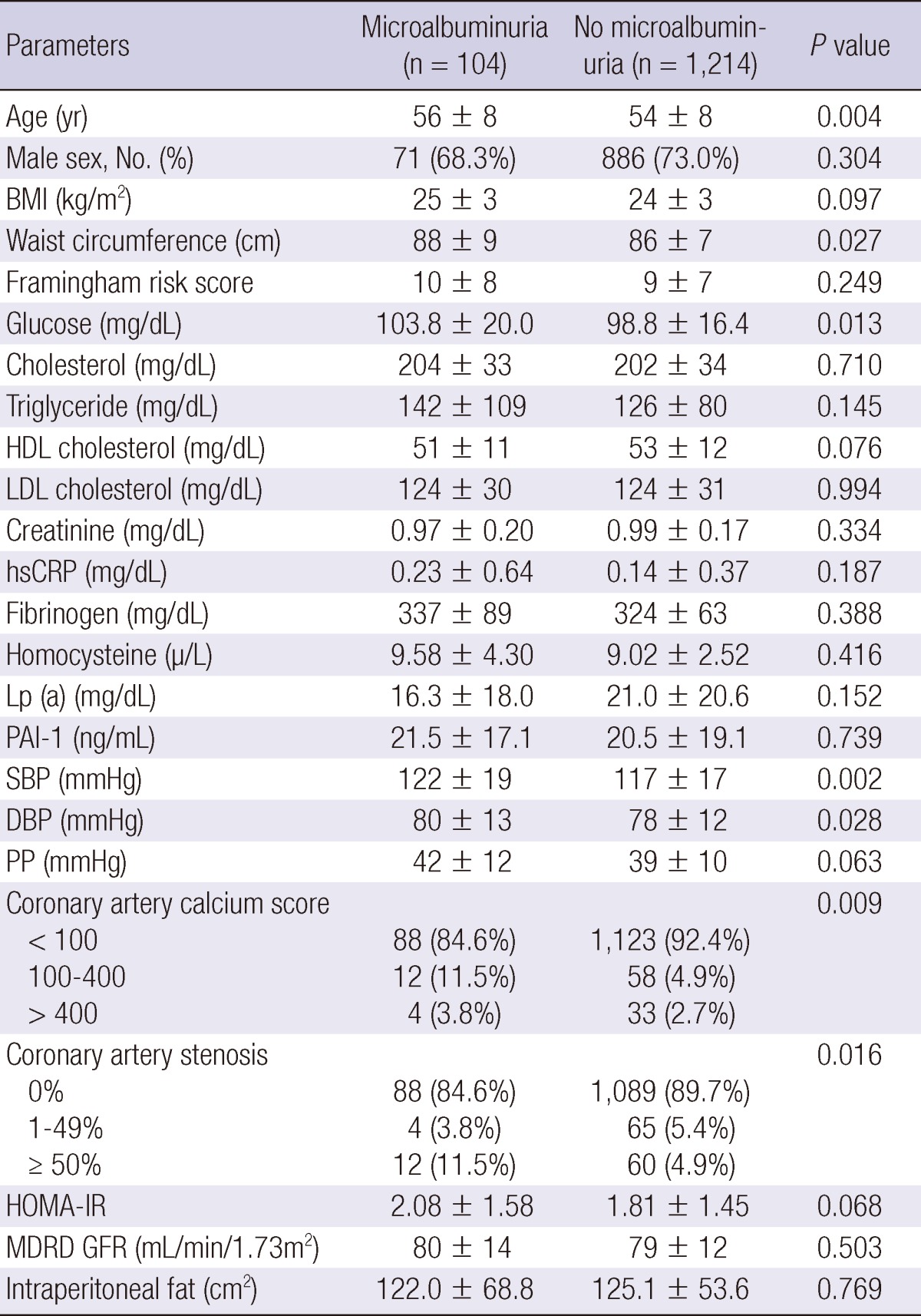
BMI, body mass index; DBP, diastolic blood pressure; GFR, glomerular fitration rate; HDL cholesterol, high-density lipoprotein cholesterol; HOMA-IR, Homeostasis model assessment of insulin resistance; hsCRP, high sensitivity C-reactive protein; IDMS, Isotope Dilution Mass Spectrometry; LDL cholesterol, low-density lipoprotein cholesterol; MDRD, Modification of Diet in Renal Disease; PAI-1, plasminogen activator inhibitor-1; PP, pulse pressure; SBP, systolic blood pressure.
Fig. 1.
Proportion of subjects with coronary atherosclerosis in patients with versus without microalbuminuria. Reflected by (A) coronary artery calcium score and (B) significant coronary artery stenosis (≥ 50%) (UACR,urine albumin to creatinine ratio; ▒, % of patients with UACR < 20 mg/g; █, % of patients with UACR 20-200 mg/g).
Correlation of urine albumin to creatinine ratio with other parameters
Using bivariate correlation analysis, correlation of UACR and other parameters were assessed. UACR showed significant correlation with age (r = 0.075, P = 0.006), BMI (r = 0.068, P = 0.015), waist circumference (r = 0.070, P = 0.011), serum fasting glucose (r = 0.087, P = 0.002), triglyceride (r = 0.066, P = 0.016), hsCRP (r = 0.120, P < 0.001), fibrinogen (r = 0.095, P = 0.024), homocysteine (r = 0.095, P = 0.026), and insulin resistance (r = 0.072, P = 0.009). Total cholesterol, HDL-cholesterol and LDL-cholesterol levels did not show significant correlation with UACR (P = 0.276, 0.133 and 0.631 for total cholesterol, HDL-cholesterol and LDL-cholesterol). The parameters of renal function were not associated with degree of microalbuminuria (P = 0.081 for serum creatinine; P = 0.201 for MDRD GFR).
The systolic and diastolic blood pressures were both significantly correlated with increasing level of UACR (r = 0.087, P = 0.002 for systolic blood pressure, r = 0.091, P = 0.001 for diastolic blood pressure). When assessed as continuous variables, coronary artery calcium score did not show correlation with UACR (P = 0.130), but the degree of coronary artery stenosis correlated significantly with increasing UACR (r = 0.076, P = 0.006).
Factors determining significant coronary artery stenosis
Multivariate analysis was performed to find the factors that determine coronary artery stenosis of 50% or more. Age, sex, serum fasting glucose, total cholesterol, LDL-cholesterol, systolic and diastolic blood pressure, pulse pressure, presence of microalbuminuria, Framingham risk score, amount of intraperitoneal fat, MDRD GFR and HOMA-IR were included in the multivariate analysis (Table 3). Among these parameters, age (OR, 1.088; 95% CI, 1.032-1.146, P=0.002), Framingham risk score (OR, 1.090; 95% CI, 1.028-1.155, P=0.004) and presence of microalbuminuria (OR, 3.397; 95% CI, 1.138-10.140, P=0.028) showed significant relationship with coronary artery stenosis ≥50%. The presence of microalbuminuria showed strongest relationship with coronary artery stenosis ≥50% from CT.
Table 3.
Multiple regression analysis showing correlation with coronary artery stenosis of 50% or more*
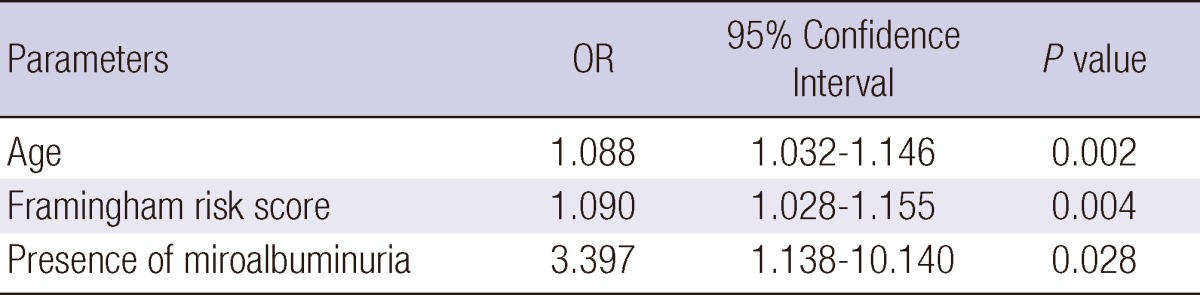
*adjusted variables: sex, fasting blood sugar level, total cholesterol, LDL cholesterol, systolic and diastolic blood pressure, pulse pressure, insulin resistance by Homeostasis model assessment of insulin resistance (HOMA-IR), intraperitoneal fat, glomerular filtration rate by Modification of Diet in Renal Disease (MDRD) formula.
DISCUSSION
The key finding of our study is that microalbuminuria detected by UACR is associated with coronary atherosclerosis, detected by coronary artery CT, in nonhypertensive, nondiabetic subjects. Not only greater coronary artery calcification, but more patients with significant coronary artery stenosis were found in asymptomatic patients with microalbuminuria.
Although the significance of microalbuminuria had been studied in depth in various populations, most of the studies focused on its value as a prognostic marker of cardiovascular events (2, 9, 10). Multi-ethnic study of atherosclerosis (MESA) study has shown coronary atherosclerosis in relation with various renal functions and biomarkers associated with chronic kidney disease, and has reported that patients with microalbuminuria are at increased risk of greater coronary artery calcification progression at follow up (18). Another substudy from MESA study also showed that microalbuminuria is an independent risk factor for incident chronic kidney disease and thus could be useful as a screening tool to identify patients at risk (19). However, the diagnostic value of microalbuminuria in relation to coronary atherosclerosis in asymptomatic, apparently healthy individuals without hypertension or diabetes mellitus had not been studied, in comparison with degree of coronary artery stenosis detected by CT. From our results, we provide a considerable insight into the relationship of microalbuminuria and coronary artery disease, especially degree of stenosis detected by CT, in asymptomatic nonhypertensive and nondiabetic patients. Among various parameters that are known as a risk factor or possible biomarkers of coronary artery disease, age, Framingham risk score and presence of microalbuminuria were significantly related to significant coronary artery stenosis, among which the microalbuminuria showed strongest correlation, even after adjusting confounding factors.
Other known cardiovascular risk factors, such as degree of hyperglycemia (1, 20-22), blood pressure (23), hypercholesterolemia (20) and insulin resistance (22) are known to be associated with increasing level of UACR, which were also shown in our results. Although this study was a cross-sectional study, the strength of our study is that we provide degree of coronary artery stenosis and coronary calcium score measured using coronary artery CT, in association with UACR and other known cardiovascular risk factors and biomarker, in a large number of apparently healthy patients.
We have found significant correlations among hyperhomocysteinemia, insulin resistance, and microalbuminuria, which may be related to changes in glomerular albumin filtration and renal endothealial and mesangial cell functions (24, 25). Insulin resistance and accompanied hyperinsulinemia in patients without impaired insulin secretion capacity may also cause renal vasodilatation, increase plasma flow and glomerular hydrostatic pressure gradient (26, 27), which may be suggested as a mechanism of microalbuminuria in relation to insulin resistance (22).
This study, to our knowledge, is the first large-numbered study to evaluate atherosclerotic changes of coronary artery in asymptomatic patients, in association with microalbuminuria. It is easy and noninvasive to detect microalbuminuria, and may be used as one of the tools to evaluate risk of subclinical coronary atherosclerosis.
In conclusion, the present study demonstrates diagnostic significance of microalbuminuria in asymptomatic population with low cardiovascular risk. In apparently healthy subjects without hypertension or diabetes mellitus, the presence of low grade urinary albumin excretion is significantly associated with subclinical coronary artery disease. Thus, the clinical significance of microalbuminuria should not be neglected or under-estimated, even in low risk asymptomatic subjects.
ACKNOWLEDGMENT
Nothing is declared for the conflict of interest.
Footnotes
The Current study was supported by Seoul National University Hospital (0420100700, 2010-1096).
References
- 1.Van der Tol A, Van Biesen W, De Groote G, Verbeke P, Vermeiren F, Eeckhaut K, Vanholder R. Microalbuminuria is more consistent in the presence of cardiovascular risk factors. J Nephrol. 2012 doi: 10.5301/jn.5000194. doi: 10.5301/jn.5000194. [DOI] [PubMed] [Google Scholar]
- 2.Dinneen SF, Gerstein HC. The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus: a systematic overview of the literature. Arch Intern Med. 1997;157:1413–1418. [PubMed] [Google Scholar]
- 3.Woo J, Cockram CS, Swaminathan R, Lau E, Chan A, Cheung R. Microalbuminuria and other cardiovascular risk factors in nondiabetic subjects. Int J Cardiol. 1992;37:345–350. doi: 10.1016/0167-5273(92)90265-5. [DOI] [PubMed] [Google Scholar]
- 4.Kang DG, Jeong MH, Lim SY, Yun KH, Kim KH, Lee SH, Lee YS, Hong YJ, Park HW, Kim JH, et al. The relationship between microalbuminuria and coronary artery stenosis or inflammatory markers in patients with angina pectoris. Korean Circ J. 2005;35:49–54. [Google Scholar]
- 5.Stehouwer CD, Nauta JJ, Zeldenrust GC, Hackeng WH, Donker AJ, den Ottolander GJ. Urinary albumin excretion, cardiovascular disease, and endothelial dysfunction in non-insulin-dependent diabetes mellitus. Lancet. 1992;340:319–323. doi: 10.1016/0140-6736(92)91401-s. [DOI] [PubMed] [Google Scholar]
- 6.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 7.Bennett PH, Haffner S, Kasiske BL, Keane WF, Mogensen CE, Parving HH, Steffes MW, Striker GE. Screening and management of microalbuminuria in patients with diabetes mellitus: recommendations to the Scientific Advisory Board of the National Kidney Foundation from an ad hoc committee of the Council on Diabetes Mellitus of the National Kidney Foundation. Am J Kidney Dis. 1995;25:107–112. doi: 10.1016/0272-6386(95)90636-3. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 9.Gerstein HC, Mann JF, Yi Q, Zinman B, Dinneen SF, Hoogwerf B, Hallé JP, Young J, Rashkow A, Joyce C, et al. Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals. JAMA. 2001;286:421–426. doi: 10.1001/jama.286.4.421. [DOI] [PubMed] [Google Scholar]
- 10.Arnlöv J, Evans JC, Meigs JB, Wang TJ, Fox CS, Levy D, Benjamin EJ, D'Agostino RB, Vasan RS. Low-grade albuminuria and incidence of cardiovascular disease events in nonhypertensive and nondiabetic individuals: the Framingham Heart Study. Circulation. 2005;112:969–975. doi: 10.1161/CIRCULATIONAHA.105.538132. [DOI] [PubMed] [Google Scholar]
- 11.Jensen JS, Borch-Johnsen K, Jensen G, Feldt-Rasmussen B. Microalbuminuria reflects a generalized transvascular albumin leakiness in clinically healthy subjects. Clin Sci (Lond) 1995;88:629–633. doi: 10.1042/cs0880629. [DOI] [PubMed] [Google Scholar]
- 12.Zelmanovitz T, Gross JL, Oliveira JR, Paggi A, Tatsch M, Azevedo MJ. The receiver operating characteristics curve in the evaluation of a random urine specimen as a screening test for diabetic nephropathy. Diabetes Care. 1997;20:516–519. doi: 10.2337/diacare.20.4.516. [DOI] [PubMed] [Google Scholar]
- 13.Chaiken RL, Khawaja R, Bard M, Eckert-Norton M, Banerji MA, Lebovitz HE. Utility of untimed urinary albumin measurements in assessing albuminuria in black NIDDM subjects. Diabetes Care. 1997;20:709–713. doi: 10.2337/diacare.20.5.709. [DOI] [PubMed] [Google Scholar]
- 14.Vestbo E, Damsgaard EM, Frøland A, Mogensen CE. Urinary albumin excretion in a population based cohort. Diabet Med. 1995;12:488–493. doi: 10.1111/j.1464-5491.1995.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 15.Tichet J, Vol S, Hallab M, Caces E, Marre M. Epidemiology of microalbuminuria in a French population. J Diabetes Complications. 1994;8:174–175. doi: 10.1016/1056-8727(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 16.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 17.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 18.DeFilippis AP, Kramer HJ, Katz R, Wong ND, Bertoni AG, Carr J, Budoff MJ, Blumenthal RS, Nasir K. Association between coronary artery calcification progression and microalbuminuria: the MESA Study. JACC Cardiovasc Imaging. 2010;3:595–604. doi: 10.1016/j.jcmg.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shastri S, Katz R, Shlipak MG, Kestenbaum B, Peralta CA, Kramer H, Jacobs DR, Jr, de Boer IH, Cushman M, Siscovick D, et al. Cystatin C and albuminuria as risk factors for development of CKD stage 3: the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Kidney Dis. 2011;57:832–840. doi: 10.1053/j.ajkd.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried LF, Orchard TJ, Kasiske BL. Effect of lipid reduction on the progression of renal disease: a meta-analysis. Kidney Int. 2001;59:260–269. doi: 10.1046/j.1523-1755.2001.00487.x. [DOI] [PubMed] [Google Scholar]
- 21.Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, Parikh CR. Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med. 2012;172:761–769. doi: 10.1001/archinternmed.2011.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mykkänen L, Zaccaro DJ, Wagenknecht LE, Robbins DC, Gabriel M, Haffner SM. Microalbuminuria is associated with insulin resistance in nondiabetic subjects: the insulin resistance atherosclerosis study. Diabetes. 1998;47:793–800. doi: 10.2337/diabetes.47.5.793. [DOI] [PubMed] [Google Scholar]
- 23.Park IS, Rho TH, Park JW, Jeon DS, Yoon HJ, Choi EJ, Bang BK, Hong SJ. Prevalence of urinary microalbuminuria in normal and hypertensive Koreans and its correlation with blood pressure measured by 24 hours ambulatory blood pressure monitoring. Korean Circ J. 1994;24:834–840. [Google Scholar]
- 24.Hoogeveen EK, Kostense PJ, Jager A, Heine RJ, Jakobs C, Bouter LM, Donker AJ, Stehouwer CD. Serum homocysteine level and protein intake are related to risk of microalbuminuria: the Hoorn Study. Kidney Int. 1998;54:203–209. doi: 10.1038/sj.ki.4495353. [DOI] [PubMed] [Google Scholar]
- 25.Deckert T, Kofoed-Enevoldsen A, Nørgaard K, Borch-Johnsen K, Feldt-Rasmussen B, Jensen T. Microalbuminuria. Implications for micro- and macrovascular disease. Diabetes Care. 1992;15:1181–1191. doi: 10.2337/diacare.15.9.1181. [DOI] [PubMed] [Google Scholar]
- 26.Tucker BJ, Anderson CM, Thies RS, Collins RC, Blantz RC. Glomerular hemodynamic alterations during acute hyperinsulinemia in normal and diabetic rats. Kidney Int. 1992;42:1160–1168. doi: 10.1038/ki.1992.400. [DOI] [PubMed] [Google Scholar]
- 27.Chagnac A, Weinstein T, Korzets A, Ramadan E, Hirsch J, Gafter U. Glomerular hemodynamics in severe obesity. Am J Physiol Renal Physiol. 2000;278:F817–F822. doi: 10.1152/ajprenal.2000.278.5.F817. [DOI] [PubMed] [Google Scholar]