Abstract
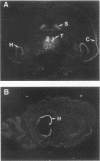
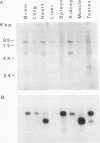
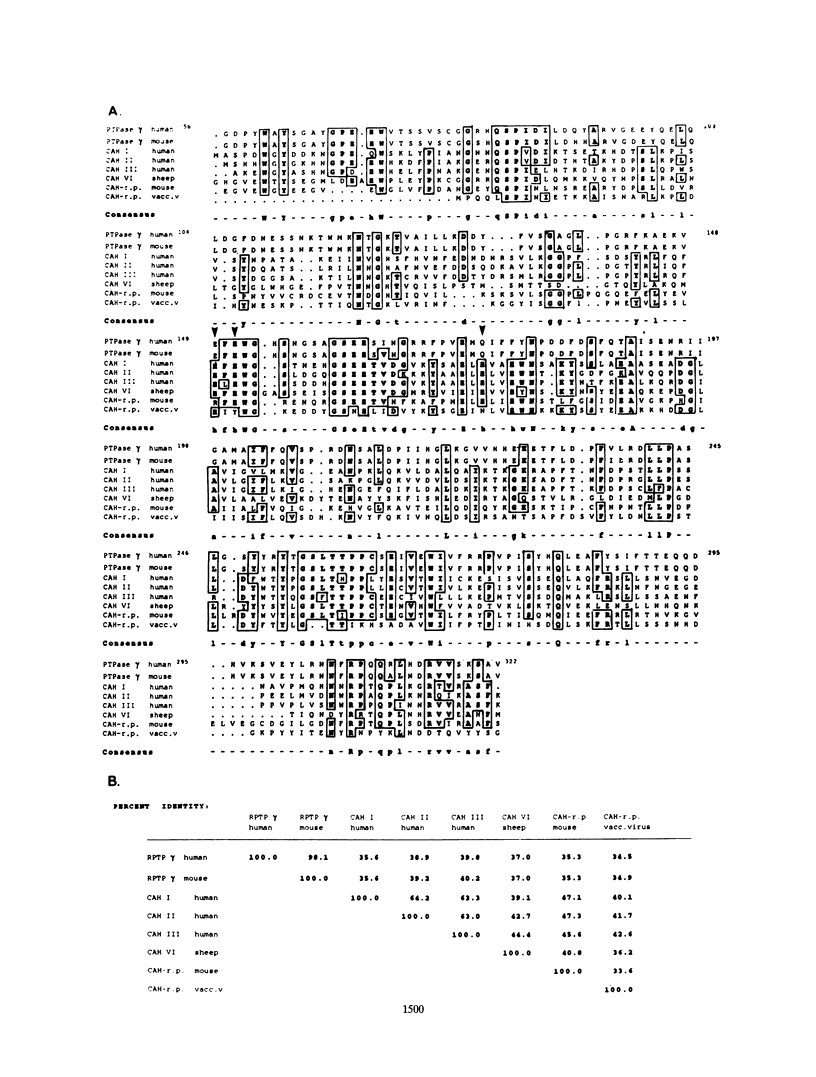
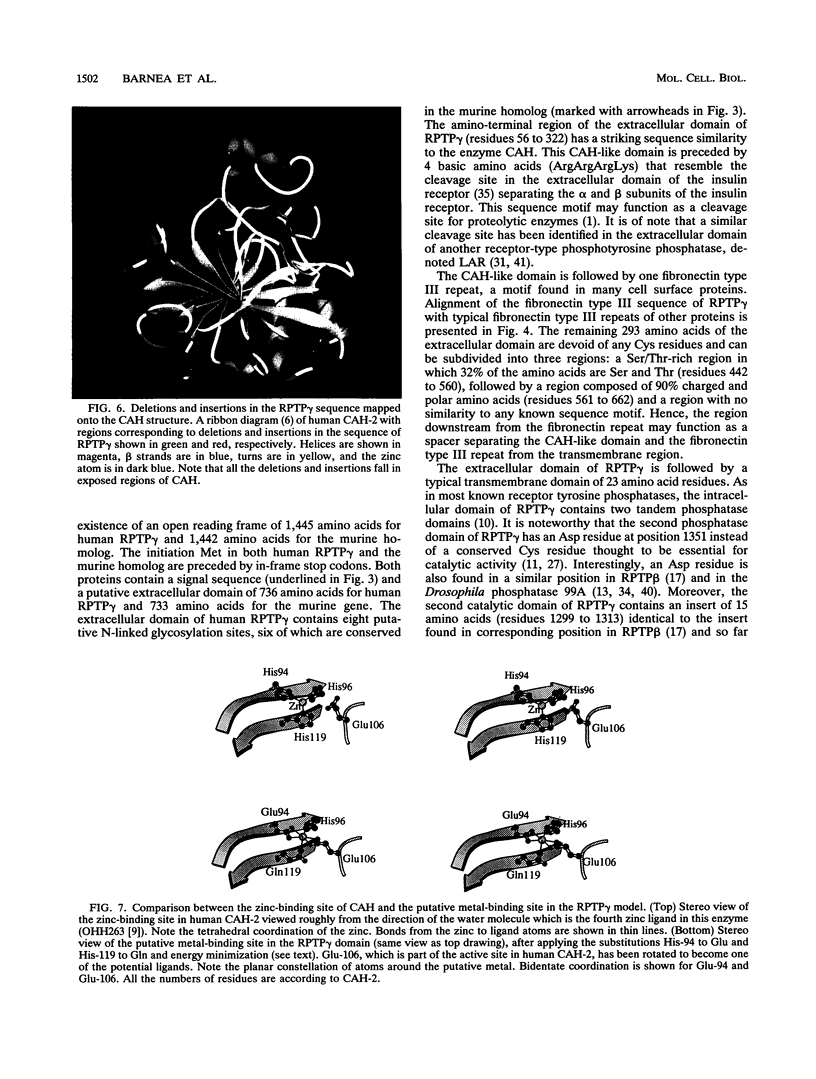
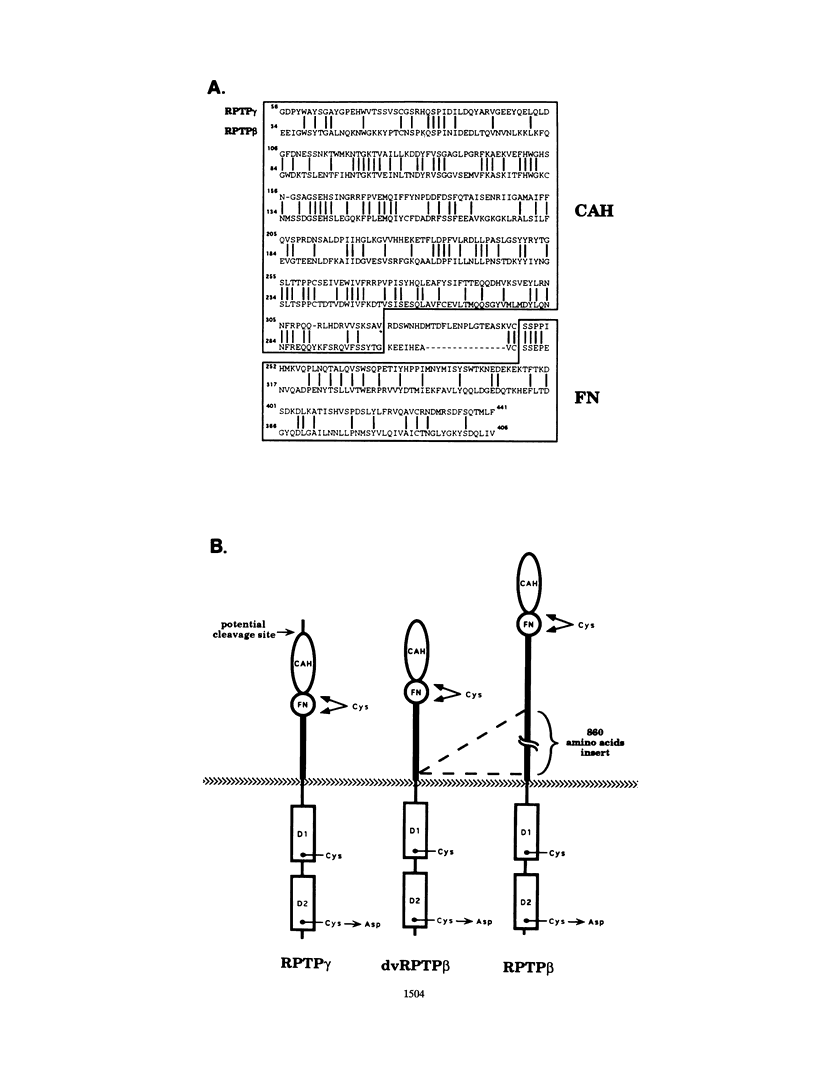
The tyrosine phosphatase RPTP gamma is a candidate tumor suppressor gene since it is located on human chromosome 3p14.2-p21 in a region frequently deleted in certain types of renal and lung carcinomas. In order to evaluate its oncogenic potential and to explore its normal in vivo functions, we have isolated cDNAs and deduced the complete sequences of both human and murine RPTP gamma. The murine RPTP gamma gene has been localized to chromosome 14 to a region syntenic to the location of the human gene. Northern (RNA) blot analysis reveals the presence of two major transcripts of 5.5 and 8.5 kb in a variety of murine tissues. In situ hybridization analysis reveals that RPTP gamma mRNA is expressed in specific regions of the brain and that the localization of RPTP gamma changes during brain development. RPTP gamma is composed of a putative extracellular domain, a single transmembrane domain, and a cytoplasmic portion with two tandem catalytic tyrosine phosphatase domains. The extracellular domain contains a stretch of 266 amino acids with striking homology to the zinc-containing enzyme carbonic anhydrase (CAH), indicating that RPTP gamma and RPTP beta (HPTP zeta) represent a subfamily of receptor tyrosine phosphatases. We have constructed a model for the CAH-like domain of RPTP gamma based upon the crystal structure of CAH. It appears that 11 of the 19 residues that form the active site of CAH are conserved in RPTP gamma. Yet only one of the three His residues that ligate the zinc atom and are required for catalytic activity is conserved. On the basis of this model we propose that the CAH-like domain of RPTP gamma may have a function other than catalysis of hydration of metabolic CO2.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Bordo D., Argos P. Evolution of protein cores. Constraints in point mutations as observed in globin tertiary structures. J Mol Biol. 1990 Feb 20;211(4):975–988. doi: 10.1016/0022-2836(90)90087-3. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Cunningham B. C., Bass S., Fuh G., Wells J. A. Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science. 1990 Dec 21;250(4988):1709–1712. doi: 10.1126/science.2270485. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson A. E., Jones T. A., Liljas A. Refined structure of human carbonic anhydrase II at 2.0 A resolution. Proteins. 1988;4(4):274–282. doi: 10.1002/prot.340040406. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Dixon J. E. Evidence for protein-tyrosine-phosphatase catalysis proceeding via a cysteine-phosphate intermediate. J Biol Chem. 1991 Sep 15;266(26):17026–17030. [PubMed] [Google Scholar]
- Hardman K. D., Agarwal R. C., Freiser M. J. Manganese and calcium binding sites of concanavalin A. J Mol Biol. 1982 May 5;157(1):69–86. doi: 10.1016/0022-2836(82)90513-7. [DOI] [PubMed] [Google Scholar]
- Hariharan I. K., Chuang P. T., Rubin G. M. Cloning and characterization of a receptor-class phosphotyrosine phosphatase gene expressed on central nervous system axons in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11266–11270. doi: 10.1073/pnas.88.24.11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Hunter T. Cooperation between oncogenes. Cell. 1991 Jan 25;64(2):249–270. doi: 10.1016/0092-8674(91)90637-e. [DOI] [PubMed] [Google Scholar]
- Jones T. A., Zou J. Y., Cowan S. W., Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991 Mar 1;47(Pt 2):110–119. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- Kaplan R., Morse B., Huebner K., Croce C., Howk R., Ravera M., Ricca G., Jaye M., Schlessinger J. Cloning of three human tyrosine phosphatases reveals a multigene family of receptor-linked protein-tyrosine-phosphatases expressed in brain. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7000–7004. doi: 10.1073/pnas.87.18.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornblihtt A. R., Umezawa K., Vibe-Pedersen K., Baralle F. E. Primary structure of human fibronectin: differential splicing may generate at least 10 polypeptides from a single gene. EMBO J. 1985 Jul;4(7):1755–1759. doi: 10.1002/j.1460-2075.1985.tb03847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Saito H. A human transmembrane protein-tyrosine-phosphatase, PTP zeta, is expressed in brain and has an N-terminal receptor domain homologous to carbonic anhydrases. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7417–7421. doi: 10.1073/pnas.89.16.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger N. X., Streuli M., Saito H. Structural diversity and evolution of human receptor-like protein tyrosine phosphatases. EMBO J. 1990 Oct;9(10):3241–3252. doi: 10.1002/j.1460-2075.1990.tb07523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForgia S., Morse B., Levy J., Barnea G., Cannizzaro L. A., Li F., Nowell P. C., Boghosian-Sell L., Glick J., Weston A. Receptor protein-tyrosine phosphatase gamma is a candidate tumor suppressor gene at human chromosome region 3p21. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):5036–5040. doi: 10.1073/pnas.88.11.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Tonks N. K., Fischer E. H., Clark E. A. CD45 regulates signal transduction and lymphocyte activation by specific association with receptor molecules on T or B cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8628–8632. doi: 10.1073/pnas.85.22.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews R. J., Cahir E. D., Thomas M. L. Identification of an additional member of the protein-tyrosine-phosphatase family: evidence for alternative splicing in the tyrosine phosphatase domain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4444–4448. doi: 10.1073/pnas.87.12.4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nada S., Okada M., MacAuley A., Cooper J. A., Nakagawa H. Cloning of a complementary DNA for a protein-tyrosine kinase that specifically phosphorylates a negative regulatory site of p60c-src. Nature. 1991 May 2;351(6321):69–72. doi: 10.1038/351069a0. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder J. W., Richards F. M. Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol. 1987 Feb 20;193(4):775–791. doi: 10.1016/0022-2836(87)90358-5. [DOI] [PubMed] [Google Scholar]
- Pot D. A., Dixon J. E. Active site labeling of a receptor-like protein tyrosine phosphatase. J Biol Chem. 1992 Jan 5;267(1):140–143. [PubMed] [Google Scholar]
- Ranscht B. Sequence of contactin, a 130-kD glycoprotein concentrated in areas of interneuronal contact, defines a new member of the immunoglobulin supergene family in the nervous system. J Cell Biol. 1988 Oct;107(4):1561–1573. doi: 10.1083/jcb.107.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Streuli M. Molecular characterization of protein tyrosine phosphatases. Cell Growth Differ. 1991 Jan;2(1):59–65. [PubMed] [Google Scholar]
- Sap J., D'Eustachio P., Givol D., Schlessinger J. Cloning and expression of a widely expressed receptor tyrosine phosphatase. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6112–6116. doi: 10.1073/pnas.87.16.6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Ariniello P. D., Tang M., Munro J. M., Blattler W. A., Adler D. A., Disteche C. M., Saito H. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992 Mar;11(3):897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashian R. E. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989 Jun;10(6):186–192. doi: 10.1002/bies.950100603. [DOI] [PubMed] [Google Scholar]
- Tian S. S., Tsoulfas P., Zinn K. Three receptor-linked protein-tyrosine phosphatases are selectively expressed on central nervous system axons in the Drosophila embryo. Cell. 1991 Nov 15;67(4):675–685. doi: 10.1016/0092-8674(91)90063-5. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vallee B. L., Auld D. S. Zinc coordination, function, and structure of zinc enzymes and other proteins. Biochemistry. 1990 Jun 19;29(24):5647–5659. doi: 10.1021/bi00476a001. [DOI] [PubMed] [Google Scholar]
- Vennström B., Bishop J. M. Isolation and characterization of chicken DNA homologous to the two putative oncogenes of avian erythroblastosis virus. Cell. 1982 Jan;28(1):135–143. doi: 10.1016/0092-8674(82)90383-x. [DOI] [PubMed] [Google Scholar]
- Yang X. H., Seow K. T., Bahri S. M., Oon S. H., Chia W. Two Drosophila receptor-like tyrosine phosphatase genes are expressed in a subset of developing axons and pioneer neurons in the embryonic CNS. Cell. 1991 Nov 15;67(4):661–673. doi: 10.1016/0092-8674(91)90062-4. [DOI] [PubMed] [Google Scholar]
- Yu Q., Lenardo T., Weinberg R. A. The N-terminal and C-terminal domains of a receptor tyrosine phosphatase are associated by non-covalent linkage. Oncogene. 1992 Jun;7(6):1051–1057. [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]