Abstract
Depression involves plasticity of brain reward neurons, although the mechanisms and behavioral relevance are unknown. Transcriptional profiling of nucleus accumbens (NAc) for RhoGTPase related genes, known regulators of synaptic structure, following chronic social defeat stress, revealed a long-term reduction in Rac1 transcription. This was marked by a repressive chromatin state surrounding its proximal promoter. Inhibition of class 1 HDACs with MS-275 rescued both decreased Rac1 transcription and social avoidance behavior. A similar repressive chromatin state was found surrounding the Rac1 promoter in human postmortem NAc from depressed subjects, which corresponded with reduced Rac1 transcription. We show Rac1 is necessary and sufficient for social avoidance and anhedonia, and the formation of stubby excitatory spines by redistributing synaptic cofilin, an actin severing protein downstream of Rac1. Our data identifies epigenetic regulation of Rac1 in NAc as a bona fide disease mechanism in depression and reveals a functional role in regulating stress-related behaviors.
Keywords: synaptic plasticity, nucleus accumbens, actin, RhoGTPases, cytoskeleton, stress disorders, mood disorders, social defeat, dendritic spines, structural plasticity, medium spiny neuron, chromatin, methylation, acetylation, histone, Rac1, transcription
The treatment of major depressive disorder (MDD) is confounded by high rates of treatment resistance, coupled with a low probability of achieving lasting remission1. Classically prescribed monoaminergic modulators regularly lead to measurable improvement in only half of the depressed clinical population, and remission in less than 30–40%2. Such clinical realities, paired with an estimated depression-related economic burden in excess of US$80 billion dollars annually3,4, has led to a concerted effort to identify and develop novel alternative therapeutic approaches based on validated disease mechanisms to treat depression and related mood disorders.
Although the monoamine hypothesis of depression5 has dominated depression treatment strategies, recent work suggests that the neuropathology of depression is stratified across a number of biological domains such as inflammatory cytokines6, neurotrophic factors7, and glutamate8. One commonality observed between these divergent systems is that they all robustly regulate excitatory synaptic structure9–11 and have been shown functionally to play an important role in the etiology of depression and anxiety-like behavior7,12. It has been suggested that classical antidepressant drugs may produce their effects through rewiring of excitatory synapses in mood circuitry13. Interestingly, the NMDA antagonist ketamine rapidly and transiently produces antidepressant effects in concert with alterations in synaptogenesis14,15.
While it is clear that synaptic loss occurs in cortical regions in depression16, a major question has been whether depression or anxiety is caused by an underlying synaptic pathology in reward circuits. Evidence in rodent models show that chronic stress regulates synaptic and structural plasticity most profoundly in reward-related limbic regions17. Recent work from our laboratory showed that chronic social defeat stress induces structural and functional synaptic plasticity on medium spiny neurons (MSNs) in the nucleus accumbens (NAc)17, a brain region well known for controlling reward dysfunction in depression18. Although the phenomena of stress-induced structural plasticity have been observed across multiple species and stress models, the intracellular mechanisms underlying these neuronal alterations and their relevance to human depression are poorly understood.
Under non-pathological conditions, small RhoGTPases, such as RAS-related C3 botulinum substrate 1 (Rac1), act as critical modulators of synaptic structure. Here we report that Rac1 is transcriptionally downregulated in NAc through an epigenetic mechanism in major depressive disorder subjects, an effect that is recapitulated in the chronic social defeat stress model of depression-like behaviors. Functionally, Rac1 modulates depression-related behaviors such as social avoidance and anhedonia, as well as MSN structural plasticity. Either a viral gene therapy based, or epigenetic rescue of, Rac1 downregulation has strong anti-stress efficacy.
RESULTS
Stress-induced transcriptional regulation of Rac1 in pre-clinical models of depression
Pathway analysis of gene ontology groups from micro-array data commonly identify cytoskeleton-related and small RhoGTPase signaling pathways as highly modulated by stress and depression-like behavioral paradigms, both in the NAc19,20 and other limbic regions21,22. This is not unexpected due to the well understood phenomenological role of structural plasticity in stress and depression. Small GTPases are responsible, to a large extent, for regulation and reorganization of the actin cytoskeleton in a number of biological systems, including neuronal populations during synaptogenesis and dendritic spine maintenance23. They are modulated by upstream factors such as guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs), leading ultimately to the severing of the underlying actin cytoskeleton via cofilin23,24.
Although a number of pre-clinical animal models of depression have been developed, prolonged social stress has proven especially useful in revealing the molecular and cellular mechanisms underlying mood- or anxiety-related disorders25. In this model, male C57BL/6J mice are repeatedly subjected to bouts of social defeat by a larger and more aggressive CD-1 mouse, resulting in a majority of animals (termed susceptible) developing a syndrome highlighted by enduring social avoidance and anhedonia to previously pleasurable activities. Non-susceptible animals (termed resilient), which fail to show social avoidance and anhedonic response, generally constitute one-third of mice subjected to social defeat26. Therefore, the behavioral syndrome induced by social defeat allows for detailed dissection of individual variations in the molecular mechanisms that underlie stress-related behavioral phenotypes. It is important to note that social defeat stress results in the development of anxiety-like behaviors in both the susceptible and resilient populations, reinforcing the likelihood that this model captures components of depression, post-traumatic stress disorder or even social anxiety19.
Based on the lines of evidence outlined above, we utilized a transcriptional profiling procedure to identify transcriptional regulation of RhoGTPase-related genes in the NAc of susceptible and resilient mice following social defeat. Although we paneled 13 RhoGTPase-related genes, only Rac1 showed selective stress-induced transcriptional regulation in susceptible, but not resilient, mice (Fig. 1a; Supplementary Table 1). Forty-eight hr following the final defeat episode, Rac1 expression is strongly downregulated (Fig. 1b) and correlated with social avoidance behavior (Fig. 1c) (see Supplemental Fig. 1a–p for detailed behavioral data). The selective downregulation of Rac1 in the NAc of susceptible mice is not generally observed throughout other limbic structures. For example, in the prefrontal cortex (PFC) Rac1 is unaffected by social defeat stress, and in the dorsal striatum Rac1 mRNA is decreased in both susceptible and resilient mice (Supplemental Table 2). Importantly, Rac1 deletion in PFC or dorsal striatum has no effect on social avoidance behavior (Supplemental Table 2).
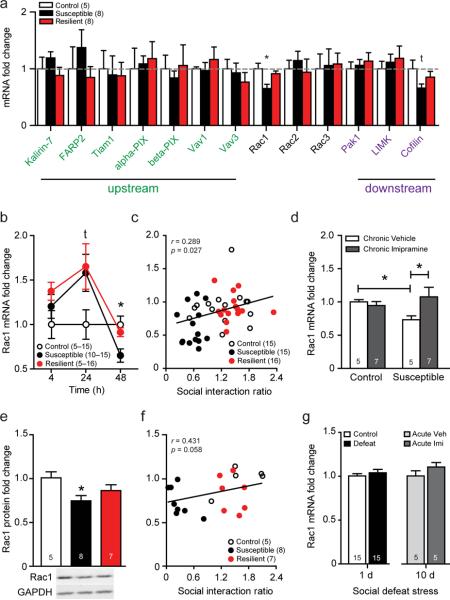
Figure 1.
Transcriptional profiling reveals that chronic social defeat stress produces a long-lasting and selective decrease in Rac1 mRNA expression in the NAc of susceptible, but not resilient, mice. (a) mRNA expression of small RhoGTPase-related genes in the NAc of C57BL/6J mice 48 hrs following their last social defeat exposure. (b) Temporal profile of Rac1 mRNA expression in the NAc 4, 24 and 48 hrs following chronic social defeat stress. (c) Correlation of 48 hr Rac1 mRNA expression with social avoidance behavior. (d) Rac1 mRNA expression following a 35 d schedule of once daily antidepressant administration (20 mg kg−1 imipramine or vehicle, i.p). (e) Total Rac1 protein levels 48 hr after chronic social defeat stress in the NAc. (f) Correlation of total Rac1 protein with social avoidance behavior. (g) Left bars represent NAc Rac1 mRNA expression 48 hr following a single acute social defeat stress. Right bars indicate NAc Rac1 mRNA expression 48 hr following an acute injection of imipramine (20 mg kg−1, i.p.) or vehicle in previously susceptible mice. All data presented as mean ± SEM, group size is indicated in either figure legends or within graph bars; (g) *P <0.05 compared by students t-test, (a, b, e) *P <0.05 by one-way ANOVA, (d) *P <0.05 by two-way ANOVA, (c,f) P <0.05 compared by Pearson's r.
Within the NAc, Rac1 transcriptional downregulation and social avoidance behavior are both long-lasting, for at least 35 days, and partially reversible by chronic anti-depressant treatment (20 mg kg−1 imipramine i.p., once-daily for 35 d) (Fig. 1d; Supplemental Fig. 2a–b). A single acute imipramine treatment following chronic social defeat stress neither regulated Rac1 mRNA expression in the NAc (Fig. 1g), nor reversed social avoidance in susceptible mice (Supplemental Fig. 2c–d). The robust transcriptional downregulation of Rac1 by chronic social defeat stress is translated into decreased total Rac1 protein levels in susceptible, but not resilient, mice 48 hr following the final defeat session (Fig. 1e) and there is a trend for this measure to correlate with social avoidance behavior (Fig. 1f). Neither mRNA or protein levels of downstream effectors of the Rac1 signaling cascade, such as p21-activated kinase 1 (Pak1) and LIM-domain containing protein kinase (LIMK), were affected by social defeat at similar time points (Fig. 1a, Supplemental Fig. 3a–c). Notably, a single social defeat episode does not regulate Rac1 transcription (Fig. 1g), indicating that only prolonged or severe stressors that induce significant depression-like behaviors are capable of inducing downregulation of Rac1.
Stress-induced epigenetic regulation of Rac1 in a pre-clinical depression model
Genome-wide promoter analysis using chromatin immunoprecipitation paired to microarray analysis (ChIP-Chip) has recently identified increased repressive histone H3 lysine 9 (H3K9me3) and 27 (H3K27me3) methylation in the NAc of mice along the promoter region of Rac1 following social isolation stress27. Based on our finding that Rac1 transcription is downregulated for extended periods following chronic social defeat stress, we next explored the possibility that this transcriptional decrease is controlled through an epigenetic mechanism that selectively modifies the chromatin architecture surrounding the Rac1 promoter region. Our mouse brain-tissue ChIP assay utilizes sonication for genomic DNA fragmentation resulting in 350–750bp fragments28. We then designed primer pairs (Supplemental Table 3) sequentially aligned from the promoter region and extending ~2000bp upstream of the transcription start site (TSS) on Rac1 (Fig. 2a). Using site-directed quantitative chromatin immunoprecitation (qChIP) we examined permissive histone 3 acetylation (acH3) and repressive H3K27me3. It is important to note that, unlike acH3, methylation at H3K27 has been shown to be either repressive or permissive under varying biological conditions. However, recent evidence suggests that in adult mouse NAc, levels of H3K27me3 within the gene promoter region correlates with repressed gene expression (J.F., M.W., X.L., D.F., V.V, P.K, J.K, C.D, N.S., I.M., B.L., Q.L., M.C., L.S, E.J.N, SfN 2012 Abstract 458.12/T7). Across both the promoter and 2000bp upstream region, permissive acetylation is significantly reduced in the susceptible, but not resilient, populations (Fig. 2b). Conversely, methylation is differentially modulated at the proximal promoter and immediate 1000bp upstream region; within the promoter region resilient mice show reduced methylation, and directly upstream of the promoter, susceptible mice have enhanced methylation (Fig. 2c). These patterns of histone architecture surrounding the Rac1 promoter and upstream regulatory regions are strongly correlated with social avoidance behavior (Supplemental Fig. 3a–d) for both acetylation and methylation.
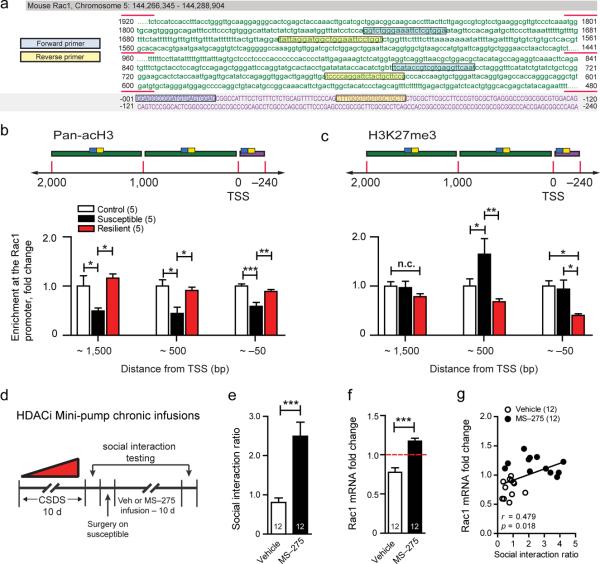
Figure 2.
Epigenetic regulation of Rac1 following chronic social defeat stress. (a) A schematic of the mouse Rac1 gene promoter and upstream regulatory region. Purple upper-case text represents the promoter region and green lower-case text represents the untranslated region upstream of the TSS. Blue and yellow boxes represent the promoter primer pair locations sequentially aligned throughout the sequence. Profile of (b) permissive H3 acetylation and (c) repressive tri-methylation on H3K27 along the mouse Rac1 promoter and upstream gene sequence in the NAc 48 hrs following chronic social defeat stress. (d) Schematic detailing experimental protocol for intra-NAc minipump infusion of the class 1 HDAC inhibitor, MS-275. Infusion of MS-275 (100 μm) in the NAc of previously susceptible mice reverses (e) social avoidance and (f) increases Rac1 mRNA expression. (g) Correlation of social avoidance behavior and NAc Rac1 mRNA expression. All data presented as mean ± SEM, group size is indicated in either figure legend or within graph bars; (b,c) *P < 0.05, **P < 0.01, ***P < 0.001 by one-way ANOVA, (e,f) ***P < 0.001 by students t-test, (g) P <0.05 compared by Pearson's r.
Given that we found the largest and widest-spanning decreases in H3 acetylation in susceptible mice, we administered the class 1 HDAC inhibitor, MS-275, locally into the NAc for 10 days via osmotic minipump (Fig. 2d). This results in a robust reversal of social avoidance in previously susceptible mice (Fig. 2e). Importantly, Rac1 mRNA was normalized and levels were positively correlated with social interaction, suggesting that there is indeed a functional link between epigenetic regulation of Rac1 and social defeat behavior (Fig. 2e,g).
Transcriptional and epigenetic regulation of Rac1 in MDD subjects
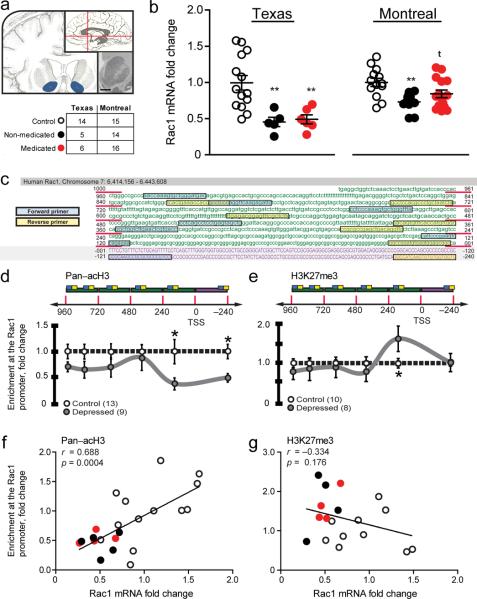
Having identified a robust epigenetic mechanism in our chronic social defeat model, we next extended these findings to post-mortem NAc tissue derived from MDD subjects (Fig. 3a). We utilized samples from two independently collected MDD cohorts, one from UT Southwestern in Texas and the other from McGill University in Montreal. Although all subjects in both cohorts had been on antidepressant medication at some point throughout their life, roughly half in each cohort were on medication at their time of death as determined by toxicology reports (complete demographics in Supplemental Table 4 and 5). As observed in rodent models, Rac1 expression is strongly down regulated in non-medicated depressed subjects from both cohorts (Fig. 3b). Results for the depressed medicated subjects were somewhat mixed. In the Texas cohort there was no significant effects of medication status on Rac1 mRNA, however, in the larger Montreal cohort, though the trend for reduced Rac1 mRNA was still evident, there was a bimodal distribution with some subjects exhibiting Rac1 levels similar to controls. These findings are consistent with our mouse model, which shows that Rac1 decreases in susceptible mice are only normalized by chronic antidepressant treatment in about 50% (4 of 7 exhibit social interactions ratios above 1.0; see Supplemental Fig. 2a). In addition, Rac1 mRNA was not reduced in a separate human cohort of cocaine addiction cases, suggesting that this is at least partly specific to MDD (Supplementary Fig. 5). Interestingly, these data are consistent with previous work29, showing that chronic cocaine in mice transiently decreases NAc Rac1 protein activity, with no effect on Rac1 gene transcription. Thus, we feel that this is compelling evidence to suggest that Rac1 downregulation is not an artifact of addictive drugs or antidepressant medication, nor is it a general marker of all psychiatric illnesses.
Figure 3.
Epigenetic regulation of Rac1 mRNA expression in subjects with MDD. (a) Coronal and sagittal schematic of the human brain highlighting regions of NAc dissection in MDD subjects. Scale bars represent 10 mm. (b) Rac1 mRNA levels from the NAc of depressed subjects in two separate cohorts. Number of samples per cohort is shown in the table (complete demographics available in Supplemental Tables 4 and 5). (c) Schematic of the human Rac1 gene promoter region and its ~1000bp upstream sequence. Purple upper-case text represents the promoter region and green lower-case text represents the untranslated region upstream of the TSS. Blue and yellow boxes represent the promoter primer pair locations sequentially aligned throughout the sequence. (d) Profile of permissive H3 acetylation along the human Rac1 promoter and upstream sequence in the NAc. (e) Profile of repressive tri-methylation on H3K27 along the human Rac1 promoter and upstream sequence in the NAc. (f) Correlation of human NAc H3 acetylation levels with Rac1 mRNA expression. (g) Correlation of human NAc H3K27 trimethylation levels with Rac1 mRNA expression. *P < 0.05, **P < 0.01 by one-way ANOVA (b) and students t-test (d,e), P <0.05 compared by Pearson's r (f,g).
To examine chromatin structure around the Rac1 gene, we adapted our qChIP protocol for use in fresh frozen human post-mortem NAc tissue, through the use of a micrococcal nuclease (MNase) based assay30, which reliably results in 146 to 165bp fragments (Supplemental Fig. 6a–b). We used this to create a high-resolution map of the human Rac1 promoter and 1000bp upstream regulatory region, by designing closely grouped sequentially aligned primer pairs (Supplemental Table 6) and site-directed qChIP (Fig. 3c). This sequence length was selected due to our observation that Rac1 chromatin landscape modification was most strongly regulated in mouse NAc tissue within the first 1000bp. We found decreased acetylation of Rac1 promoter sequence ~200bp up- and downstream of the TSS (Fig. 3d), while increased methylation is observed only ~200bp upstream of the transcription start site (Fig. 3e). Since qChIP was performed from the same human tissue samples as the Texas cohort cDNA library, we next directly correlated Rac1 mRNA expression with promoter acetylation (Fig. 3f) and methylation (Fig. 3g) values. As shown, acH3 enrichment ~200bp upstream of the TSS significantly correlated with Rac1 mRNA values. Importantly, re-analysis of qChIP data using medication status at time of death as a co-factor revealed no effect of medication (Supplemental Fig. 7a–b), similar to Rac1 transcription.
Rac1 modulates depression-related behavior
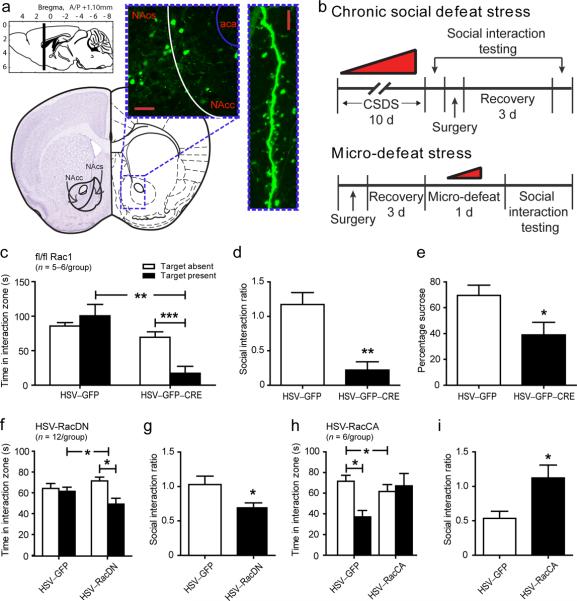
Based on our initial findings that Rac1 is selectively transcriptionally regulated in NAc following stress in an animal model, and in MDD subjects, we next directly examined its role in regulating depression-like behavior following social defeat stress. Using a combination of viral gene transfer and targeted genetic Rac1 knockout mice, we tested whether Rac1 in NAc is both necessary and sufficient for the expression of depression-like behaviors following either chronic social defeat or a subthreshold microdefeat (Fig. 4). Unlike chronic social defeat, microdefeats do not result in the development of any social avoidance and anhedonia in control mice, which can help to reveal pro-susceptibility factors.
Figure 4.
Regulation of Rac1 by viral-mediated gene transfer and gene knockout modulates stress-related behavior in mice. (a) Representative schematic of the mouse NAc region and HSV expression. GFP-labeled neurons in green show the spread of viral infection, which is limited to the NAc region. Scale bar represents 50 μm. GFP-infected neurons strongly express GFP in the distal dendrites of MSNs. Scale bar represents 5 μm (b) Experimental schematic of chronic social defeat stress and microdefeat protocols, detailing timing for viral infection and behavioral testing. (c,d,e) Removal of Rac1 via infection of a CRE expressing HSV in the NAc of fl/fl Rac1 mice promotes susceptibility to depression-like behavior as measured by increased social avoidance and decreased sucrose preference following microdefeat. (f,g) Viral-mediated gene transfer of RacDN similarly induces social avoidance behavior following microdefeat. (h,i) Viral-mediated gene transfer of RacCA into the NAc of susceptible mice is sufficient to reverse social avoidance behavior after chronic social defeat. **P < 0.05, **P < 0.01, ***P < 0.001 by two-way ANOVA (c,f,h) and students t-test (d,e,g,i).
To manipulate Rac1, we utilized herpes-simplex virus (HSV)-mediated gene transfer of bicistronic constructs containing green fluorescent protein (GFP) and either a dominant negative Rac1 (RacDN), a constitutively active Rac1 (RacCA), or Cre recombinase infused directly into the NAc (Fig. 4a,b). Viral over-expression of HSV-CRE in the NAc of floxed Rac1 (fl/fl Rac1) mice to locally knockout the Rac1 gene (Supplemental Fig. 8a) strongly induces susceptibility to social avoidance (Fig. 4c–d; Supplemental Fig. 9a–b) and anhedonia, measured by reduced sucrose preference (Fig. 4e), following a microdefeat. Similarly, HSV-mediated overexpression of RacDN recapitulates this pro-susceptibility phenotype (Fig. 4f–g; Supplemental Fig. 9c–d). Conversely, HSV-mediated overexpression of RacCA to restore Rac1 levels following chronic social defeat, rescues the susceptible phenotype, reverting previously susceptible mice to control and resilient levels of social avoidance behavior (Fig. 4h–I; Supplemental Fig. 9e–f). These data indicated that direct reversal of stress-induced downregulation of Rac1 has strong anti-stress efficacy.
Rac1 controls stress-induced formation of immature spine structures
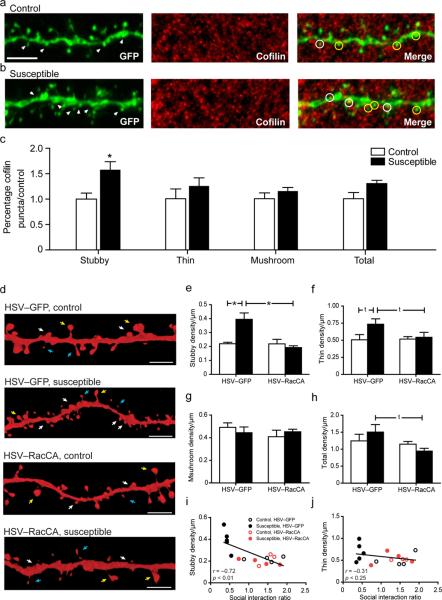
Chronic social defeat stress induces the formation of highly plastic and immature stubby dendritic spines on MSNs in the NAc. These dendritic alterations are associated with a smaller post synaptic density and a greatly increased frequency of excitatory drive that seems to be necessary and sufficient for the expression of depression-related phenotypes in mice17. In order to understand the mechanism leading to the induction of immature stubby dendritic spines on NAc MSNs, we first examined cofilin, a critical downstream Rac1 target important for actin severing and shown previously to regulate immature spine structure31. NAc MSN dendritic segments were examined 48 hrs following chronic social defeat stress through a combinatorial approach utilizing automated 3D dendritic spine reconstruction from HSV-GFP infected neurons overlaid with cofilin immunohistochemistry (Fig. 5a–b; Supplemental Fig. 10a). Chronic social defeat stress resulted in a selective increase in total cofilin puncta colocalization with immature stubby spines, but not thin, mushroom, or total spine density (Fig. 5c). Within the tissue set used for immunohistochemical analysis, we also observed a replication of previous data where chronic social defeat stress resulted in an induction in total stubby spine density on MSN dendrites (Supplemental Fig. 9b).
Figure 5.
Chronic social defeat regulates immature stubby spine formation through a Rac1 dependent mechanism. (a–b) Representative 63x confocal z-stack images of MSN dendritic segments from control and susceptible mice following chronic social defeat. White arrows in the GFP panel indicate dendritic stubby spines. White circles in merge panel highlight colocalization of GFP stubby spines and cofilin puncta, while yellow circles indicate non-co-localized stubby spines. Measurement bar is 10 μm. (c) Chronic social defeat stress results in increased cofilin puncta in stubby dendritic spines, but not in thin, mushroom, or by total spine density. *P < 0.05 by students t-test, 48 neurons, 4–5 mice per group. (d) Representative 3D reconstructions of HSV-infected MSN dendritic segments. White arrows delineate stubby spines, yellow arrows delineate mushroom spines, and blue arrows delineate thins spines. (e,i) Over expression of RacCA is sufficient to reverse chronic social defeat stress induction of stubby spines, and is significantly correlated with social avoidance behavior. (f–h,j) Neither chronic social defeat nor RacCA modulates thin, mushroom or total dendritic spine density. *P < 0.05 by two-way ANOVA, 61 neurons, 3–5 mice per group (e–h), P <0.05 compared by Pearson's r (i,j); measurement bar is 5 μm.
Having observed Rac1 dependent control over depression-associated behavior, we next wanted to determine if a rescue of the downregulated Rac1 with HSV-RacCA following chronic stress is sufficient to prune away these stubby spines (Fig. 5d). We injected HSV vectors following 10 days of chronic social defeat stress and found that overexpression of RacCA reversed the induction of stubby spines previously seen in susceptible mice (Fig. 5e), and there was a strong trend for reduced thin spines (Fig. 5f), suggesting that RacCA may selectively target immature spine structures. Importantly, stubby spine number correlated with social avoidance behavior (Fig. 5i–j). We observed no effect of social defeat or Rac1 overexpression on mushroom spines (Fig. 5g) or total dendritic spine (Fig. 5h) density. To determine whether Rac1 is sufficient for immature dendritic spine formation on medium spiny neurons in the NAc, we performed an identical dendritic spine morphology analysis in conditional fl/fl Rac1 mice following a subthreshold microdefeat stress (Fig. 6a). Removal of Rac1 causes a large induction of both thin and stubby spine density in control and microdefeated mice (Fig. 6b–e), while having no effect on mushroom spine density (Fig. 6d). Interestingly, an additive effect is observed on stubby spines where acute social defeat almost doubles the spine density of fl/fl Rac1 mice (Fig. 6b), and this induction in both stubby and thin spines is correlated with social interaction ratio (Fig. 6f–g). While future studies will need to examine these morphological changes in human post mortem tissue from depressed subjects, new methodologies and techniques will need to be developed for this purpose.
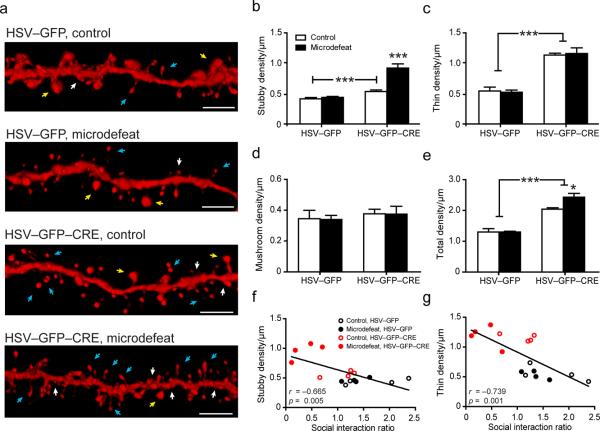
Figure 6.
Conditional deletion of Rac1 gene in NAc medium spiny neurons in fl/fl Rac1 mice leads to the induction of immature synaptic structures. (a) Representative 3D reconstructions of HSV-infected MSN dendritic segments. White arrows delineate stubby spines, yellow arrows delineate mushroom spines, and blue arrows delineate thins spines. (b) Stubby spine density is increased in mice after Rac1 gene deletion and further increased by microdefeat stress. (c) Rac1 deletion similarly increases thin dendritic spine density in control and microdefeated mice. (d) Neither microdefeat stress nor Rac1 deletion regulates mushroom spine density. (e) Rac1 deletion also increases total spine density. (f,g) Both stubby and thin spine density are strongly correlated with social avoidance behavior. *P < 0.05, ***P < 0.001 by two-way ANOVA, 60 neurons, 4 mice per group (b–e), P <0.05 compared by Pearson's r (f,g); measurement bar is 5 μm.
DISCUSSION
Our data shows a novel mechanism whereby chronic stress or depression regulates the transcriptional profile of Rac1 in the NAc of rodents and humans through an epigenetic mechanism. Intriguingly, Rac1 transcriptional tone is normalized by chronic imipramine treatment in mice following chronic social defeat and is positively correlated with treatment response, suggesting that this adaptation may be necessary for effective antidepressant treatment. However, in the majority of MDD subjects found to be on antidepressant at the time of suicide, normal Rac1 expression and altered chromatin modifications along the Rac1 promoter and upstream regions were not restored, suggesting a need for more direct Rac1 targeting strategies to achieve therapeutic effects in this patient population. Functionally, in mice, selective genetic deletion of Rac1 and viral mediated gene-transfer of Rac1 mutants, or epigenetic modulation through class 1 HDAC inhibition, reveals that accumbal Rac1 signaling is both sufficient and necessary for social avoidance and anhedonia behavioral responses and synaptic structural plasticity. Together, these data suggest that small RhoGTPase signaling pathways, and Rac1 specifically, are viable candidates for future development as antidepressant therapeutic agents when targeted to the nucleus accumbens.
Indeed, small RhoGTPases have been heavily implicated in the neuropathogenesis of several psychiatric disorders including fragile X mental retardation32, Rett syndrome33 and schizophrenia34. Under non-pathological conditions, Rac1 plays an important role in neuronal development35, axon guidance36 and learning and memory processes37. In regard to dendritic spines, as a general rule, increased synaptogenesis and functional synaptic plasticity is tightly correlated with the size and shape of a dendritic spine; as spines shift along a continuum from immature (stubby and thin) to mature (mushroom) they also shift towards greater synaptic strength and stability38,39. Within neuronal populations, there is wealth of evidence supporting a role for Rac1 in dendritic spine morphogenesis and maintenance34,40–42 via modulation of cofilin spatiotemporal dynamics43,44.
This signaling cascade is therefore exceptionally positioned to regulate the delicate interface between extra-cellular stimuli and dynamic reorganization of the actin cytoskeleton to accommodate neuronal transduction. The transcriptional downregulation of this system consequently plays an important role in setting the threshold for subsequent experience-dependant plasticity within NAc. Our data suggest that stress-induced decreases in Rac1 expression result in concurrent increases in immature stubby spine formation and cofilin localization within these spines. Recent findings have confirmed that decreased p21-activated kinase 3 (PAK3) activity, an immediate downstream effecter of Rac1, leads to both an increase in the growth of new, immature spines and an impairment of plasticity-mediated spine stabilization that interferes with the formation of persistent stable spines45. A feature of chronic stress exposure is a shift towards synaptic instability17,46. Such synaptic instability is relieved through the application of low-doses of ketamine14,46, which has rapid antidepressant efficacy. Ketamine treatments normalize stress-induced immature spine formation resulting in a greater proportion of mature dendritic spines in pyramidal neurons of the prefrontal cortex46. This “plasticity consolidation” treatment strategy11 may be occurring through a RhoGTPase-dependent mechanism, however, the specifics of this need to be teased apart in future studies.
It is noteworthy that within the NAc both cold water forced swim47 and chronic social defeat stress17,48 result in enhanced glutamatergic synaptic plasticity. In the case of social defeat, this coincides with the formation of immature stubby spines with smaller post-synaptic densities (PSDs) and a higher frequency of mini excitory postsynaptic currents (mEPSCs). The finding of increased excitatory drive in NAc is somewhat surprising since previous work shows that deep brain stimulation (DBS) in the NAc of treatment-resistant depression subjects produces a profound antidepressant response49,50. However, DBS stimulates both excitatory and inhibitory neuronal populations, as well as fibers of passage that transit through the generalized region of stimulation. Further, the stimulation parameters used in such treatments are extremely super-physiological in frequency and amplitude that, based on Channel Rhodopsin studies, may result in de-sensitization of excitatory responses51. How this ultimately regulates the diverse cell populations within the NAc is still unknown.
While there are clearly many acute biochemical events acting directly in the synapse that may mediate NAc plasticity, data here points to a more sustained long-lasting mechanism through downregulation of Rac1, as a result of stressful experience, to cause social avoidance and anhedonia. Thus, epigenetic mechanisms of sustained transcriptional disruptions provide a logical basis for such long-lasting synaptic restructuring events. While more general strategies of epigenetic regulation, through administration of histone deactelyase inhibitors20 or histone demethylases52, may show significant antidepressant efficacy by enhancing Rac1 expression, still further refinement is necessary. A recent advance in targeting gene specific chromatin states using zinc finger artificial transcription factors that bind specific consensus sequences53 allow for targeted epigenetic therapeutics to act on specific genes to reverse long-term chromatin disruptions, for example, on Rac1. However, until these tools are more widely available for testing in humans, a more feasible therapeutic strategy may be to screen compounds that target either Rac1 activity, or more generally, excitatory synapse consolidation.
In summary, we provide strong evidence that chronic stress induces long-term transcriptional downregulation of Rac1 through an epigenetic mechanism, robustly regulating NAc MSN synaptic structural and behavioral plasticity. Reversal of this endophenotype may present a novel therapeutic venue for exploration, and guide future therapeutics to target adaptive intracellular plasticity mechanisms.
Supplementary Material
Acknowledgements
This research was supported by US National Institute of Mental Health grant R01 MH090264 and Janssen IMHRO Rising Star Award (SJR) and P50 MH66172 and P50MH096890 (CAT).
Footnotes
Author Constributions S.A.G., and S.J.R. contributed to study design, data collection, analysis and writing. D.J.C., M.H., G.E.H., J.M., K.D., M.E.C., C.D., E.R., A.J.R, P.J.K and J.L.A. contributed to data collection. J.M-G. contributed to data analysis. R.L.N. contributed to viral design and packaging. G.T., S.G. and C.A.T. collected human brain samples and contributed to data analysis.
Competing Financial Interests The authors declare no competing financial interests.
References
- 1.Krishnan V, Nestler EJ. Linking molecules to mood: new insight into the biology of depression. Am J Psychiatry. 2010;167:1305–1320. doi: 10.1176/appi.ajp.2009.10030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trivedi MH, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg PE, et al. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 5.Tissot R. The common pathophysiology of monaminergic psychoses: a new hypothesis. Neuropsychobiology. 1975;1:243–260. doi: 10.1159/000117498. [DOI] [PubMed] [Google Scholar]
- 6.Smith RS. The macrophage theory of depression. Med Hypotheses. 1991;35:298–306. doi: 10.1016/0306-9877(91)90272-z. [DOI] [PubMed] [Google Scholar]
- 7.Duman RS, Heninger GR, Nestler EJ. A molecular and cellular theory of depression. Arch Gen Psychiatry. 1997;54:597–606. doi: 10.1001/archpsyc.1997.01830190015002. [DOI] [PubMed] [Google Scholar]
- 8.Palucha A, Pilc A. The involvement of glutamate in the pathophysiology of depression. Drug News Perspect. 2005;18:262–268. doi: 10.1358/dnp.2005.18.4.908661. [DOI] [PubMed] [Google Scholar]
- 9.Christoffel DJ, Golden SA, Russo SJ. Structural and synaptic plasticity in stress-related disorders. Rev Neurosci. 2011;22:535–549. doi: 10.1515/RNS.2011.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacol Ther. 2012;130:226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Manji HK, et al. Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression. Biol Psychiatry. 2003;53:707–742. doi: 10.1016/s0006-3223(03)00117-3. [DOI] [PubMed] [Google Scholar]
- 12.Vidal R, et al. New strategies in the development of antidepressants: towards the modulation of neuroplasticity pathways. Curr Pharm Des. 2011;17:521–533. doi: 10.2174/138161211795164086. [DOI] [PubMed] [Google Scholar]
- 13.Racagni G, Popoli M. Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci. 2008;10:385–400. doi: 10.31887/DCNS.2008.10.4/gracagni. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li N, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Autry AE, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang HJ, et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med. 2012;18:1413–1417. doi: 10.1038/nm.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christoffel DJ, et al. IkappaB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nestler EJ, Carlezon WA., Jr. The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Krishnan V, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 20.Covington HE, 3rd, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei Q, et al. Early-life forebrain glucocorticoid receptor overexpression increases anxiety behavior and cocaine sensitization. Biol Psychiatry. 2012;71:224–231. doi: 10.1016/j.biopsych.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andrus BM, et al. Gene expression patterns in the hippocampus and amygdala of endogenous depression and chronic stress models. Mol Psychiatry. 2012;17:49–61. doi: 10.1038/mp.2010.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tolias KF, Duman JG, Um K. Control of synapse development and plasticity by Rho GTPase regulatory proteins. Prog Neurobiol. 2011;94:133–148. doi: 10.1016/j.pneurobio.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiraly DD, Eipper-Mains JE, Mains RE, Eipper BA. Synaptic plasticity, a symphony in GEF. ACS Chem Neurosci. 2010;1:348–365. doi: 10.1021/cn100012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13:1161–1169. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilkinson MB, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar A, et al. Chromatin remodeling is a key mechanism underlying cocaine-induced plasticity in striatum. Neuron. 2005;48:303–314. doi: 10.1016/j.neuron.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Dietz DM, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012;15:891–896. doi: 10.1038/nn.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiang Y, Matevossian A, Huang H-S, Straubhaar J, Akbarian S. Isolation of neuronal chromatin from brain tissue. BMC neuroscience. 2008;9:42. doi: 10.1186/1471-2202-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pontrello CG, et al. Cofilin under control of beta-arrestin-2 in NMDA-dependent dendritic spine plasticity, long-term depression (LTD), and learning. Proc Natl Acad Sci USA. 2012;109:E442–451. doi: 10.1073/pnas.1118803109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bongmba O, Martinez L, Elhardt M, Butler K, Tejada-Simon M. Modulation of dendritic spines and synaptic function by Rac1: a possible link to Fragile X syndrome pathology. Brain research. 2011;1399:79–174. doi: 10.1016/j.brainres.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Q, et al. CDKL5, a protein associated with rett syndrome, regulates neuronal morphogenesis via Rac1 signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;30:12777–12863. doi: 10.1523/JNEUROSCI.1102-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi-Takagi A, et al. Disrupted-in-Schizophrenia 1 (DISC1) regulates spines of the glutamate synapse via Rac1. Nat Neurosci. 2010;13:327–332. doi: 10.1038/nn.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Curtis I. Functions of Rac GTPases during neuronal development. Developmental neuroscience. 2008;30:47–105. doi: 10.1159/000109851. [DOI] [PubMed] [Google Scholar]
- 36.Koh C-G. Rho GTPases and their regulators in neuronal functions and development. Neuro-Signals. 2006;15:228–265. doi: 10.1159/000101527. [DOI] [PubMed] [Google Scholar]
- 37.Martinez L, Tejada-Simon M. Pharmacological inactivation of the small GTPase Rac1 impairs long-term plasticity in the mouse hippocampus. Neuropharmacology. 2011;61:305–317. doi: 10.1016/j.neuropharm.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 39.Yoshihara Y, De Roo M, Muller D. Dendritic spine formation and stabilization. Curr Opin Neurobiol. 2009;19:146–153. doi: 10.1016/j.conb.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 40.Luo L, et al. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–840. doi: 10.1038/379837a0. [DOI] [PubMed] [Google Scholar]
- 41.Nakayama AY, Harms MB, Luo L. Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–5338. doi: 10.1523/JNEUROSCI.20-14-05329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tashiro A, Yuste R. Regulation of dendritic spine motility and stability by Rac1 and Rho kinase: evidence for two forms of spine motility. Mol Cell Neurosci. 2004;26:429–440. doi: 10.1016/j.mcn.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 43.Kuhn TB, et al. Regulating actin dynamics in neuronal growth cones by ADF/cofilin and rho family GTPases. J Neurobiol. 2000;44:126–144. [PubMed] [Google Scholar]
- 44.Rex CS, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubos A, et al. Alteration of synaptic network dynamics by the intellectual disability protein PAK3. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:519–546. doi: 10.1523/JNEUROSCI.3252-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li N, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campioni MR, Xu M, McGehee DS. Stress-induced changes in nucleus accumbens glutamate synaptic plasticity. J Neurophysiol. 2009;101:3192–3198. doi: 10.1152/jn.91111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vialou V, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schlaepfer TE, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 50.Grubert C, et al. Neuropsychological safety of nucleus accumbens deep brain stimulation for major depression: effects of 12-month stimulation. World J Biol Psychiatry. 2011;12:516–527. doi: 10.3109/15622975.2011.583940. [DOI] [PubMed] [Google Scholar]
- 51.Gunaydin LA, et al. Ultrafast optogenetic control. Nat Neurosci. 2010;13:387–392. doi: 10.1038/nn.2495. [DOI] [PubMed] [Google Scholar]
- 52.LaPlant Q, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stolzenburg S, Bilsland A, Keith WN, Rots MG. Modulation of gene expression using zinc finger-based artificial transcription factors. Methods Mol Biol. 2010;649:117–132. doi: 10.1007/978-1-60761-753-2_7. [DOI] [PubMed] [Google Scholar]
- 54.Tsankova N, et al. Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nature neuroscience. 2006;9:519–544. doi: 10.1038/nn1659. [DOI] [PubMed] [Google Scholar]
- 55.Berton O, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 56.Stan AD, et al. Human postmortem tissue: what quality markers matter? Brain Res. 2006;1123:1–11. doi: 10.1016/j.brainres.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Radley JJ, et al. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez A, Ehlenberger DB, Dickstein DL, Hof PR, Wearne SL. Automated three-dimensional detection and shape classification of dendritic spines from fluorescence microscopy images. PLoS One. 2008;3:e1997. doi: 10.1371/journal.pone.0001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russo SJ, et al. Nuclear factor kappa B signaling regulates neuronal morphology and cocaine reward. J Neurosci. 2009;29:3529–3537. doi: 10.1523/JNEUROSCI.6173-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.