Abstract
Crry and DAF are two murine membrane C3 complement regulators with overlapping functions. Crry deletion is embryonically lethal while DAF-deficient mice are generally healthy. Crry−/−DAF−/− mice were viable on a C3−/− background, but platelets from such mice were rapidly destroyed when transfused into C3-sufficient mice. Here we used the cre-lox system to delete platelet Crry in DAF−/− mice and studied Crry/DAF-deficient platelet development in vivo. Rather than displaying thrombocytopenia, Pf4-Cre+-Crryflox/flox mice had normal platelet counts and their peripheral platelets were resistant to complement attack. However, chimera mice generated with Pf4-Cre+-Crryflox/flox bone marrows showed platelets from C3−/− but not C3+/+ recipients to be sensitive to complement activation, suggesting that circulating platelets in Pf4-Cre+-Crryflox/flox mice were naturally selected in a complement-sufficient environment. Notably, Pf4-Cre+-Crryflox/flox mouse platelets became complement susceptible when fH function was blocked. Examination of Pf4-Cre+-Crryflox/flox mouse bone marrows revealed exceedingly active thrombopoiesis. Thus, under in vivo conditions, Crry/DAF deficiency on platelets led to abnormal platelet turnover but peripheral platelet count was compensated for by increased thrombopoiesis. Selective survival of Crry/DAF-deficient platelets aided by fH protection and compensatory thrombopoiesis demonstrate the cooperation between membrane and fluid phase complement inhibitors and the body’s ability to adaptively respond to complement regulator deficiencies.
Introduction
Complement plays an important role in host defense as part of the innate immune system (1, 2). However, spontaneous complement activation via the alternative pathway (AP3) can lead to inflammatory tissue injury (1, 3). Avoidance of complement-mediated autologous injury is achieved by host cell-specific complement regulatory proteins on the cell membrane and in the plasma (4, 5). Two of the most critical membrane complement regulators in humans are decay-accelerating factor (DAF) and membrane cofactor protein (MCP), and a key plasma complement regulator is factor H (fH) (4–6). DAF and fH accelerate the decay of C3 convertases while MCP and fH serve as cofactors for factor I-mediated cleavage and inactivation of C3b, a central component of the AP C3 convertase. Expression of MCP in rodents is restricted to the testis (7, 8) and a rodent-specific membrane protein, complement receptor 1-related gene/protein y (Crry), is considered a functional homolog of human MCP in the rat and mouse (9, 10). Unlike human MCP, but similar to fH, Crry has both cofactor and decay-accelerating activities (9, 10).
Dysregulation of complement in humans is associated with a number of complement-dependent diseases including paroxysmal nocturnal hemoglobinuria, atypical hemolytic uremic syndrome and C3 glomerulopathy (4, 6, 11, 12). In these conditions, cells with close contact with the plasma complement system, e.g. red blood cells (RBCs), platelets and kidney glomerular endothelial cells, are the primary targets of complement injury. Gene mutations leading to reduced or absent expression or function of regulators, and gain of function mutations in C3 and factor B that enhance the C3 convertase activity have been found in PNH, aHUS or C3 glomerulopathy (6, 11–14). Substantial heterogeneity has been noted in the underlying defects of these diseases and mutations in the relevant complement genes are often not fully penetrant (6, 11–14). Health-related risk factors such as infection, pregnancy and organ transplantation are thought to be important modifiers in determining disease susceptibility (15). Additionally, the expression level and overlapping function of various complement regulators in different tissues and individuals may be relevant as well, but current knowledge on this aspect of human complement physiology is still limited.
Studies in mice have revealed considerable insight into this issue. Crry gene deletion in mice is embryonically lethal while DAF-deficient mice are generally healthy (16, 17). Crry knockout mice are viable on a C3−/− background and this has allowed us to study the relevance of Crry on blood cells in transfusion experiments (18, 19). Using these mutant mice, we have demonstrated that DAF and Crry are functionally interchangeable, and tissue sensitivity to complement attack is a function of relative DAF and Crry expression levels (20, 21). Thus, Crry is the predominant membrane C3 regulator on mouse embryos and RBCs, accounting for the embryonic lethality phenotype of Crry knockout and the fact that Crry-deficient, but not DAF-deficient, RBCs were susceptible to complement-mediated elimination (18, 19). On the other hand, we found that only Crry/DAF-deficient, but not Crry- or DAF-deficient, mouse platelets were susceptible to complement attack (21), suggesting that both regulators are sufficiently expressed on platelets and there is redundancy between the two. In the present study, we used the cre-lox system to circumvent the lethal phenotype of global Crry deficiency and to selectively delete Crry from endothelial cells or platelets in DAF−/− mice. While the use of Tie-2-Cre transgenic mouse resulted in Crry deletion from endothelial as well as hematopoietic cells, leading to secondary complement insufficiency and preventing us from studying an endothelial injury phenotype, the use of Pf4-Cre transgenic mouse allowed us to study Crry/DAF-deficient platelet development under in vivo conditions. Here we show that Crry/DAF deficiency on platelets led to abnormal platelet turnover which was compensated for by increased thrombopoiesis. It also resulted in selective survival of Crry/DAF-deficient platelets that were resistant to complement attack under the protection of fH. Our data demonstrate the cooperation between membrane and fluid phase inhibitors and the body’s ability to respond adaptively to complement regulator deficiency.
Methods
Mice
The sources of C3−/−, fB−/−, DAF−/−, Crry−/−C3−/−, DAF−/−/Crry−/−/C3−/−, and Crryflox/flox mice were described previously (19, 22–24). Apart from Crryflox/flox mice which were on a mixed 129/C57BL6 background, all other mutant mice were of C57BL6. Tie2-Cre transgenic breeder mice (25) were kindly provided by the laboratory of Dr Garret FitzGerald (University of Pennsylvania, USA). Pf4-Cre transgenic mice were from the Jackson Laboratory (Bar Harbor, ME). DAF−/− mice were crossed with Crryflox/flox mice to generate Crryflox/flox/DAF−/− mice and the latter were then crossed with Tie-2-Cre transgenic mice. For crossing with Pf4-Cre transgenic mice, a line of Crryflox/flox mice with the Neo gene excised by FLPe recombinase (22) were first crossed with DAF−/− mice and then with Pf4-Cre transgenic mice. Thus, unless otherwise specified, all Crryflox/flox mice were on a DAF−/− background. A summary of all Cre transgenic Crryflox/flox mice used in this study is given in Table 1. Wild-type (WT) and floxed Crry gene alleles (with or without Neo) and the Cre transgene were screened by PCR using tail DNA as described (22). Both male and female mice aged between 6–12 weeks were used. Mice were maintained under specific pathogen-free conditions and all animal experiments were approved by the Institutional Animal Care and Use Committees of the University of Pennsylvania.
Table 1.
Summary of Cre transgenic Crryflox/flox mouse strains described in the text
| Strain Name | Crry deletion | DAF deletion | Plasma AP complement | Phenotype |
|---|---|---|---|---|
| Tie-2-Cre+ -Crryflox/flox | endothelial cells, blood cells | global | low | not apparent |
| Tie-2-Cre+ -Crryflox/Δ | endothelial cells, blood cells | global | low | not apparent |
| Tie-2-Cre− -CrryΔ/Δ | global | global | low | not apparent |
| Tie-2-Cre− -Crryflox/Δ | - | global | slightly lower | normal |
| Pf4-Cre+ -Crryflox/flox | Platelets | global | normal | increased thrombopoiesis |
| Pf4-Cre− -Crryflox/flox | - | global | normal | normal |
Antibodies and flow cytometry reagents
Polyclonal rabbit anti-mouse C3a and Crry antibodies were generated at Cocalical Biologicals, Inc (Reamstown, PA) by immunizing rabbits with recombinant mouse C3a or a Crry fragment corresponding to short-consensus repeat (SCR) 3 and 4, respectively. The following reagents were from BD PharMingen (San Diengo, CA): Phycoerythrin (PE)-conjugated hamster anti-mouse DAF (CD55) mAb, allophycocyanin (APC)-conjugated streptavidin, rat anti-mouse C3a mAb (clone I87-1162), biotinylated rat anti-mouse C3a mAb (clone I87-419), horseradish peroxidase (HRP)-conjugated polyclonal rat anti-mouse C3a, HRP-conjugated avidin. Fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse C3 was from Cappel, ICN Pharmaceuticals (Aurora, OH). PE-conjugated goat anti-rabbit IgG was from Sigma (St. Louis, MO). Rat anti-mouse C3 mAb (clone 11H9) was from Hycult biothech (Uden, The Netherlands). HRP-conjugated polyclonal goat anti-rabbit IgG was from BioRad (Hercules, CA). HRP-conjugated goat anti-mouse C3 was from MP Biomedicals (Solon, OH). Polyclonal rabbit anti-human Von Willebrand factor (vWF) was from Dako (Glostrup, Denmark). Alexa fluor 488-conjugated goat anti-rabbit IgG was from Invitrogen (Eugene, OR).
Expression of recombinant mouse fH SCR19/20, C3a and Crry SCR3/4
A cDNA (mFHSP-mFH19/20-8×His) encoding mouse fH signal peptide, SCR19/20 and an eight-histidine (8×His) tag at its carboxyl terminus was amplified by nested PCR using as template a full-length mouse fH cDNA (kindly provided by Dr M. Nonaka, University of Tokyo, Japan). First, SCR19/20 was amplified using the following two primers: M6 (upstream), 5′-agaattatttggcttatattatggactgtttgtgcagcaGCGGCCGCTGGGAAATGTGGGCCTCCTCCACCT-3′ (lower case: partial sequence of fH signal peptide; underlined: NotI sequence; italicized: SCR19 sequence); M3 (downstream), 5′-atgatgatgatgCTTATCGTCATCGTCCCCGGGTACACAAGTGGGATAATTGATGGTGCC-3′ (lower case: 4×His tag sequence; double underlined: enterokinase cleavage site sequence (26); underlined: SmaI sequence, italicized: SCR20 sequence). The PCR product from the above was used as a template in a second round PCR using the following two primers: M5 (upstream), 5′-CGCGGATCCGAATTCGATATCATCCAAATTatgagactgtcagcaagaattatttggcttatattatggact-3′ (underlined: EcoRI sequence; lower case: partial fH signal peptide sequence); M4 (downstream), 5′-CCCAAGCTTGAATTCGATATCCGATCTTTATTAAGATCCACTatgatgatgatgatgatgatgatgCTTATCGTCATCGTC-3′ (underlined: EcoRI sequence; lower case: 8×His tag sequence; double underlined: enterokinase cleavage site sequence). The final PCR product was cloned into the pCR2.1 vector and then sub-cloned at EcoRI site into the pCAGGS expression vector (kindly provided by Dr. J. Miyazaki, Osaka University, Japan). HEK cells (ATCC, Rockville, MD) were cultured in DMEM medium containing 10% fetal bovine serum. They were seeded at 95% confluence in 100 mm dishes and transfected with 24 µg of pCAGGS-mFHSP-mFH19/20-8×His using linear polyethylenimines (Polysciences Inc, Warrington, PA). At 48 hrs after transfection, cells were switched to serum-free medium and cultured for two more days. The cell culture medium was then collected and recombinant fH SCR19/20 protein was purified by Ni2+-chelate chromatography.
Mouse C3a and Crry SCR3/4 cDNAs were amplified from liver RNA by RT-PCR, cloned into the pCR2.1 vector (Invitrogen) and then sub-cloned into the pCAGGS-mFHSP-mFH19/20-8×His plasmid at NotI and SmaI sites (i.e. replacing fH19/20 with C3a or Crry SCR3/4, respectively). The following primers were used in RT-PCR: for mouse C3a cDNA, C3a-NotI (upstream), 5′- GCAGCGGCCGCTTC-AGTACAGTTGATGGAAAGAAGG-3′ (underlined: NotI sequence, italicized: 5’ sequence of C3a); C3a-SmaI (downstream), 5′-TATCCCGGGCCTGGCCAGGCCC-AGCACGTG-GTCGC-3′ (underlined: SmaI sequence, italicized: 3’ sequence of C3a); for Crry SCR3/4, Crry-NotI (upstream), 5′-GCAGCGGCCGCTATTCCTTGTGAGATACCCCCAGGCA-3′ (underlined: NotI sequence, italicized: SCR3 sequence of Crry); Crry-SmaI (downstream), 5′-TATCCCGGGCTTGAAGCAGCTTGGTAACTCTGGC-3′ (underlined: SmaI sequence, italicized: SCR4 sequence of Crry). C3a and Crry SCR3/4 expression in HEK cells and purification by Ni2+ column were the same as described above for fH SCR19/20.
Immunohistochemistry staining of Crry on arterial endothelial cells
The Vectastin Elite ABC Kit (Burlingame, CA) was used for immunohistochemistry. Mouse kidneys were fixed in 10% formalin solution overnight and then embedded in paraffin. Tissue sections of 4 µm thickness were prepared. The slides were treated sequentially in 100% xylene, 100%, 95%, 70% ethanol and distilled water twice for 5 minutes. After treatment with Target Retrieval Solution as described by manufacturer (Dako, Glostrup, Denmark), the sections were serum-blocked and stained for 1h with a polyclonal rabbit anti-mouse Crry absorbed to retain specificity to SCR3/4 only (22). Samples were developed using the DAB substrate.
Platelets isolation
Mouse blood, collected from the vena cava using an heparinized syringe, was diluted (1:1.5) in HEN buffer (150 mM NaCl, 1 mM Na2EDTA, 10 mM HEPES, pH 6.5) and centrifuged at 200g for 6 minutes to obtain platelet-rich plasma (PRP). Platelets were collected from PRP by centrifugation at 1200g for 10 minutes at room temperature (RT) and re-suspended in modified Tyrode buffer (20mM HEPES, 137 mM NaCl, 2.7 mM KCl, 1mM MgCl2, 12 mM NaHCO3, 3.3 mM Na2PO4, 0.1% BSA, 5.6 mM glucose, pH 7.4). Platelets numbers were determined using an automated cell counter (Beckman Coulter), set to count cells between 2 and 5µm, after appropriately diluting the isolated platelets.
FACS analysis
For Crry expression, platelets and erythrocytes were stained with rabbit anti-mouse Crry SCR3/4 polyclonal antibody followed by PE-conjugated goat anti-rabbit IgG. For DAF expression, cells were stained with PE-conjugated hamster anti-mouse DAF (CD55) mAb. C3 deposition on platelets was assessed by staining with FITC-conjugated goat anti-mouse C3. Cell staining was performed in FACS buffer (0.1% albumin and sodium azide in phosphate-buffered saline) for 30 minutes at RT. Samples were analyzed on a FACS Calibur flow cytometer (Bedon Dickson Franklin Lakes, NJ) connected to CellQuest (Becton Dickson) and data were processed with FlowJo (TullStar, Ashland, OR).
Enzyme-linked immunoasorbent assays (ELISA)
For plasma intact C3 levels, ELISA plates were coated with rat anti-mouse C3 mAb (2.5 µg/ml, clone 11H9). Serially diluted (1:50 to 1:25600) mouse plasma was added to the plate (50 µl per well) and incubated at RT for 1 hour. Plate-captured intact C3 was detected using a polyclonal rabbit anti-mouse C3a antibody (1:4000) and HRP-conjugated goat anti-rabbit IgG (1:4000). To measure plasma total C3 and fB levels, serially diluted mouse plasma (1:400 to 1:102400) was added to ELISA plates directly (50 µl per well). After overnight incubation at 4°C, plates were blocked with 1% BSA (Sigma) at RT for 1 hour. After washing, C3 ELISA plates were incubated with HRP-conjugated goat anti-mouse C3 (3.2 µg/ml) for 1 hour at RT. For fB ELISA, goat anti-human factor B (18µg/ml; CompTech, Tyler, Tx), which has been shown to cross-react with mouse factor B in plasma(27), was added and the plates were incubated at RT for 1 hour. After washing, fB plates were incubated with HRP-conjugated rabbit anti-goat IgG (1:2000; Bio-Rad Laboratories, Hercules, CA). C3- or fB-bound plates were developed by adding the HRP substrate (0.05 % 2,2'-azino-di-(3 ethylbenzthiazoline sulfonic acid) (ABTS; Roche, Indianapolis, IN). In the above ELISA assays, C3−/− or fB−/− mouse plasma was used as a negative control. C3 and fB levels were normalized to a WT mouse plasma sample based on OD values and using the Non-Linear Curve Fit Function from the Origin® 7 SR2 v7.0383 (B383) curve fitting program (OriginLab® Corporation Northampton, MA). For plasma C3a/C3a-desArg levels, ELISA plates were coated with a rat anti-mouse C3a mAb (clone I87-1162), blocked with assay diluents (BD PharMingen) at RT for 1h, and incubated with plasma samples or C3a standard (150 pg-300 ng/mL in assay diluents) at RT for 1h. Following incubation with biotinylated rat anti-mouse C3a (clone I87-419) for 1h, plates were developed with avidin-conjugated HRP.
Assessment of in vivo alternative pathway complement activity
To assess the functional activity of AP complement in mice, we used an extravascular hemolysis test as described in previous publications (18, 21). RBCs (equivalent to 100 µl of blood, 5–10 × 108 cells) from Crry−/−/C3−/− mice were collected, labeled with FITC and transfused through the tail vein into Tie2-Cre+-Crryflox/Δ (or Tie2-Cre+-Crryflox/flox), Tie2-Cre−-Crryflox/Δ and WT mice. Blood samples were collected at 0, 1 and 20 hrs after transfusion, and the percentages of FITC-labeled RBCs were determined by FACS. The percentage of transfused cells remaining at 1 and 20 hrs was normalized to that of 0 hr (immediately after transfusion) (18, 21).
Measurement of serum alternative pathway complement activity in vitro
Serum AP complement activity was measured using an LPS-based ELISA assay (27). Briefly, LPS was coated onto ELISA plates (2 µg/well in 50 µl) at 4 C overnight. After washing, mouse serum (50 µL per well), diluted in gelatin veronal buffered saline containing Mg2+/EGTA (Sigma), was added and incubated at 37°C for 1 hour. After washing, plate-bound activated C3 was detected using HRP-conjugated goat anti-mouse C3 antibody (1:4000).
In vitro assay of platelet sensitivity to complement activation
Isolated mouse platelets (final concentration 5 × 108 platelets/ ml) were incubated with 30% wild-type male mouse serum diluted in modified Tyrode buffer, to a total reaction volume of 100 µl, at 37 °C for 20 minutes. In some experiments, fH function was blocked by including 5µM fH SCR19/20 in the incubation reactions. After washing in FACS buffer, C3 deposition on the platelets was analyzed by FACS.
Generation of bone marrow chimera mice
Bone marrow (BM) chimera mice were generated as previously described (20, 22) using Pf4-Cre+-Crryflox/flox mice as donors and WT (C3+/+) or C3−/− mice as recipients. Briefly, BM cells were flushed out from tibia and femur bones in PBS, passed through a nylon mesh and treated with ACK buffer to clear erythrocytes. Before receiving donor BM cells, recipient mice were lethally irradiated (2 doses of 525 Rads, separated by 3 hrs). Each mouse received 5 ×107 total BM cells through the tail vein. Chimera mice were used after 2 months of BM cell transfer. Success of BM engraftment was confirmed by FACS analysis of DAF expression on peripheral RBCs.
Evaluation of megakaryocytopoiesis
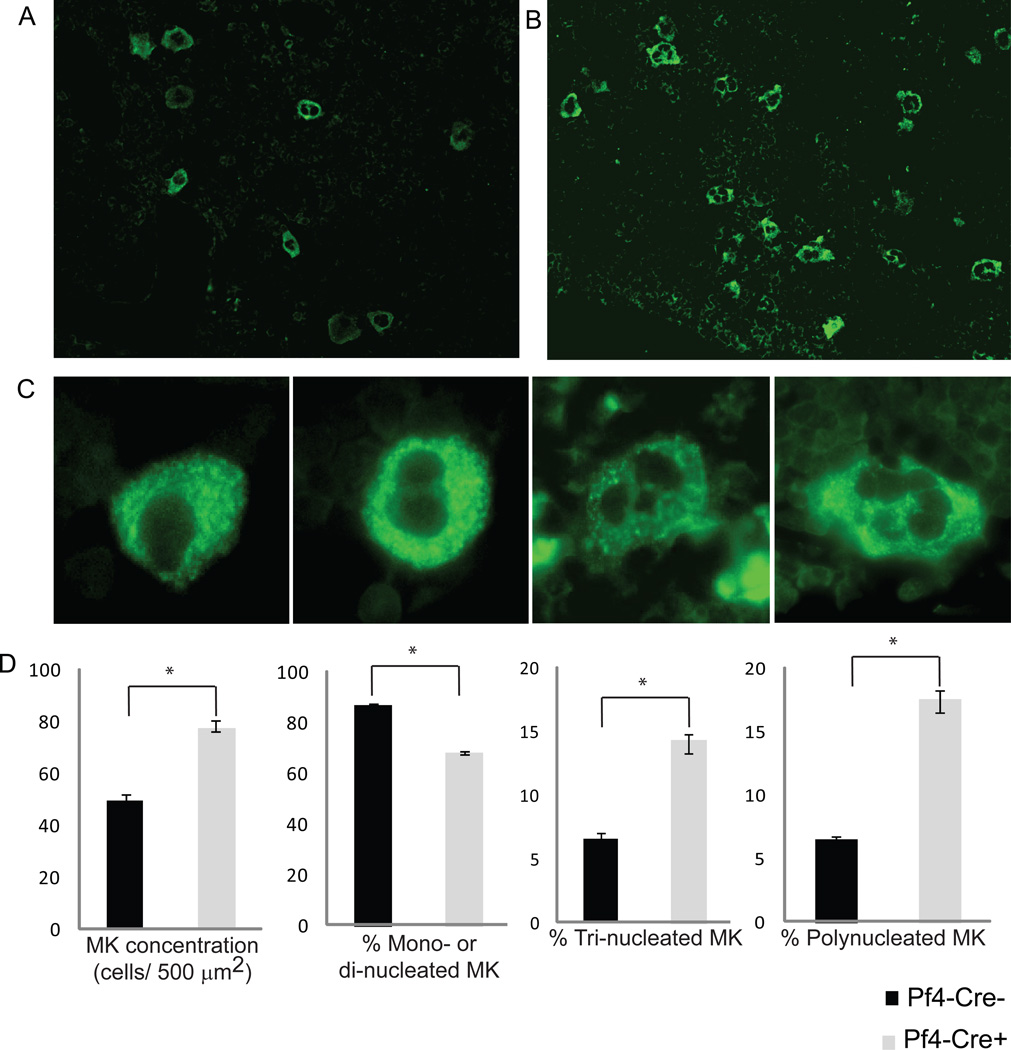
Tibia and femur bones were fixed for 1h in 4% paraformaldehyde. After decalcification in 20 ml of 390 mM EDTA for 7 days, the bones were embedded in paraffin and cut into 5µm sections. The bone sections were deparaffinized, rehydrated (using xilol for 15min, ethanol 100% twice for 10 min, ethanol 70% for 10min) and treated with 6% hydrogen peroxide for 10 min to quench endogenous peroxide activity. Antigen retrieval was performed in 10 mM citrate buffer pH 6, at 80°C for 2h, for efficient restoration of the antigen immunoreactivity and conservation of the fragile BM tissue. After blocking with 10% BSA, rabbit anti-human vWF was used as a primary antibody to stain for megakaryocytes (28). Alexa 488-conjugated goat anti-rabbit IgG was used as a secondary antibody. The number of megakaryocytes was counted in a blind fashion. Megakaryocytes were counted from two different microscopic viewing fields (10×) of each slide section and 4 different sections from each bone were examined. The area of the microscopic field under 10× amplification was calculated to be 500 µm2 using a ruler scale. Megakaryocyte abundance, calculated as the average number of eight independent counts, was then expressed as the number of vWF+ cells per 500µm2 of BM section. Megakaryocytes were divided into three groups according to the number of nucleus observed: mono/di-nucleated, tri-nucleated and poly-nucleated. The total number of each subtype of megakaryocytes was counted for each of the eight BM sections per mouse. Relative abundance of each sub-group of megakaryocytes was expressed as a percentage of the total number of megakaryocytes present in the BM section.
Results
Tie-2-Cre-mediated Crry deletion from endothelial and blood cells in DAF−/− mice caused systemic complement consumption
In previous blood cell transfusion experiments, we showed that endogenous or over-expressed DAF and Crry can compensate for each other on mouse RBCs and platelets (18, 20, 21). To determine the role of these two complement regulators on the vascular wall, we used the cre-lox system to delete endothelial Crry in DAF−/− mice. We first crossed DAF−/− (17) and Crryflox/flox (22) mice and generated DAF−/−Crryflox/flox mice and then crossed the latter with the Tie-2-Cre transgenic mouse (26). As reported by others (29, 30), we found that Cre recombinase expression driven by the Tie-2 gene promoter was leaky, being active in endothelial cells and in hematopoietic cells, as well as in some germ cells. Cre activity in germ cells was indicated by evidence of Crry deletion (delta allele, Δ) in some Tie-2-Cre− offspring. Tie-2-Cre+-Crryflox/flox and Tie-2-Cre+-Crryflox/Δ animals could not be easily distinguished as a mutant Crry allele could indicate germline deletion (Δ) or Cre-mediated deletion of floxed Crry alleles, therefore these mice were grouped together for study. As shown in Fig 1A, endothelial Crry staining was reduced in Tie-2-Cre−-Crryflox/Δ mice and absent in Tie-2-Cre+Crryflox/Δ (or Crryflox/flox) mice. FACS analysis of Crry expression on RBCs (Fig 1B) and platelets (Fig 1C) showed similar patterns, i.e. it was reduced on Tie-2-Cre−-Crryflox/Δ mouse cells and absent on Tie-2-Cre+-Crryflox/Δ (or Crryflox/flox) cells. These results indicated that Tie-2-Cre-mediated Crry deletion occurred both on endothelial and hematopoietic cells.
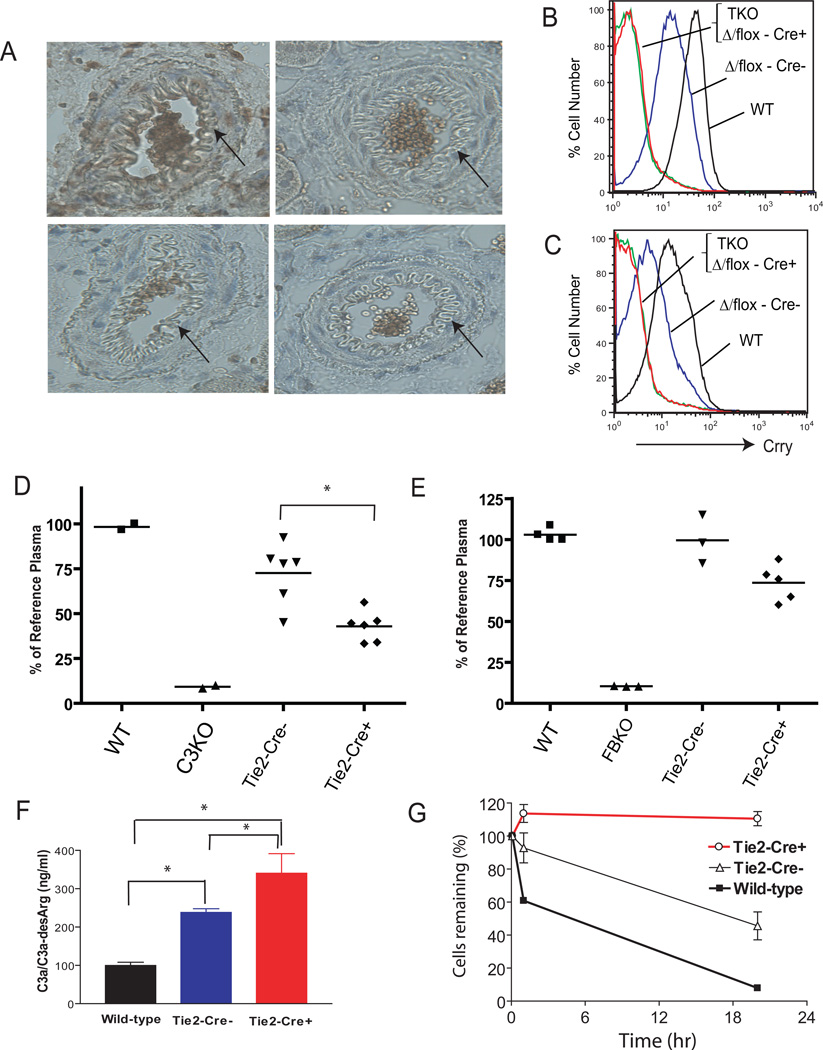
Figure 1. Tie-2-Cre-mediated Crry deletion from endothelial and hematopoietic cells of Crryflox/flox mice led to secondary complement insufficiency.
(A) Immunohistochemical staining shows that Crry is present on WT mouse kidney arterial endothelial cells (a) but is absent from that of DAF−/−/Crry−/−/C3−/− (b) and Tie2-Cre+-Crryflox/Δ (or Tie2-Cre+-Crryflox/flox) mice (c). Arrows indicate endothelial cells. (B, C) FACS analysis of Crry expression on erythrocytes (B) and platelets (C). TKO: DAF−/−/Crry−/−/C3−/−, Δ/flox-Cre+: Tie2-Cre+-Crryflox/Δ (or Tie2-Cre+-Crryflox/flox), Δ/flox-Cre−: Tie2-Cre−-Crryflox/Δ, WT: wild-type. (D, E) ELISA assay of total plasma C3 (D) and factor B (E) levels in mice (n= 3–6 mice per group). C3KO: C3−/−, FBKO: fB−/−, Tie-2-Cre−: Tie2-Cre−-Crryflox/Δ, Tie-2-Cre+: Tie2-Cre+-Crryflox/Δ (or Tie2-Cre+-Crryflox/flox). Levels are normalized to a reference plasma sample from a randomly chosen WT mouse. (F) ELISA assay of plasma C3a/C3a-desArg levels (n= 3 mice per group). (G) In vivo AP complement activity assay. Crry−/−C3−/− mouse erythrocytes were rapidly eliminated in a wild-type mouse as we demonstrated before (21) or in Tie2-Cre−-Crryflox/Δ mice (n=4), but such cells persisted when transfused into Tie2-Cre+-Crryflox/Δ (or Tie2-Cre+-Crryflox/flox) mice (n= 3 mice). All error bars represent SEM. *P < 0.05, Student t test.
Tie-2-Cre+-Crryflox/Δ (or Crryflox/flox) mice appeared healthy and histological examination of their kidneys showed no glomerular and vascular C3 deposition or injury (data not shown). ELISA assays revealed significantly reduced plasma C3 and factor B (fB) (Fig 1D, E), and elevated C3a/C3a-desArg levels (Fig 1F) in these mice, suggesting that they had secondary complement insufficiency, most likely as a result of excessive AP complement activation and consumption. Because the ELISA assays used in Fig 1D and 1E did not differentiate between intact and activated C3 or fB, the intact plasma C3 and fB levels in Tie-2-Cre+-Crryflox/Δ (or Crryflox/flox) mice were likely to be much lower. This was confirmed by a functional assay in an RBC transfusion experiment. RBCs from Crry−/−/C3−/− mice were rapidly eliminated by AP complement when transfused into wild-type (WT) or Tie-2-Cre−-Crryflox/Δ recipients but they survived normally when transfused into Tie-2-Cre+-Crryflox/Δ (or Crryflox/flox) mice (Fig 1G). AP complement depletion was also indicated by the fact that CrryΔ/Δ mice were produced from Tie-2-Cre+-Crryflox/Δ (or Crryflox/flox) mouse intercrosses, which was in clear contrast to the observation that no live Crry−/− mice could be obtained from Crry+/− mating on a complement-sufficient background (16). These results suggested that Crry deletion on endothelial and hematopoitic cells in DAF−/− mice caused systemic complement consumption.
Platelet-specific deletion of Crry in DAF−/− mice using Pf4-Cre transgenic mice
We next studied the physiological role of DAF and Crry on platelets. We crossed Pf4-Cre transgenic mice, which expressed the Cre recombinase under the Platelet Factor 4 gene promoter (31), with Crryflox/flox DAF−/− mice to specifically delete Crry from platelets. The Cre transgene and WT, floxed or mutant Crry gene alleles were identified by PCR using tail DNA (Fig 2A). As expected, the mutant Crry gene allele was detected only in Pf4-Cre+-Crryflox/flox mice (Fig 2A). FACS analysis confirmed that Crry expression was detected on WT (positive control) and Pf4-Cre−- Crryflox/flox mouse platelets but not on Pf4-Cre+-Crryflox/flox or Crry−/−/ DAF−/−/C3−/− (negative control) mouse platelets (Fig 2B). Pf4-Cre-mediated Crry deletion was specific to platelets as there was no difference in Crry staining on RBCs of WT, Pf4-Cre− and Pf4-Cre+ mice (Fig 2C). Lack of platelet DAF expression in Pf4-Cre−-Crryflox/flox and Pf4-Cre+-Crryflox/flox mice was also confirmed by FACS (Fig 2D).
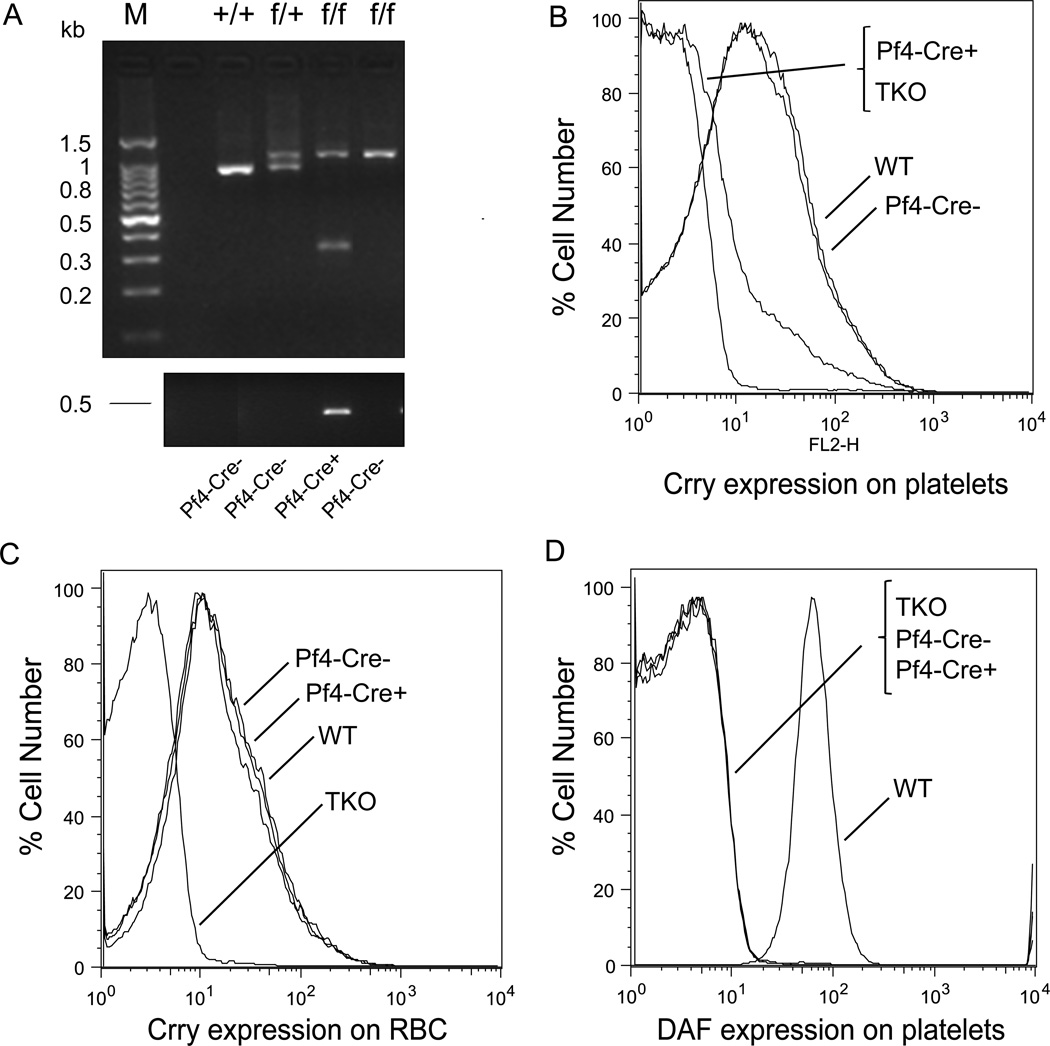
Figure 2. Platelet-specific deletion of Crry by the Pf4-Cre transgene.
(A) PCR genotyping of WT (+/+), heterozygous (f/+) and homozygous (f/f) floxed Crry gene (upper panel), and of the Pf4-Cre transgene (lower panel). Tail DNA was used as a template. WT Crry allele corresponded to a 970 bp band, whereas the floxed allele corresponded to a 1100 bp band. A 350 bp fragment, corresponding to a mutated Crry allele, was present in floxed and Pf4-Cre+ mice. (B) FACS analysis showing that Crry was expressed on the platelets of WT and Pf4-Cre−-Crryflox/flox mice but not of Pf4-Cre+-Crryflox/flox or DAF−/−/Crry−/−/C3−/− (TKO) mice. (C) FACS analysis demonstrating that Crry deletion was specific to the platelets of Pf4-Cre+-Crryflox/flox mice as erythrocyte expression of Crry on these mice was not affected. (D) FACS analysis confirming that DAF was absent on the platelets of TKO, Pf4-Cre−-Crryflox/flox and Pf4-Cre+-Crryflox/flox mice.
Pf4-Cre+-Crryflox/flox mice had normal platelet counts and their platelets were resistant to complement attack
Our previous work has demonstrated that both DAF and Crry are highly expressed on mouse platelets, and while deficiency of either regulator was inconsequential, Crry/DAF double deficiency rendered platelets susceptible to complement-mediated elimination when transfused to C3-sufficient recipients (21). Thus, we speculated that Pf4-Cre+-Crryflox/flox mice on a DAF−/− background might suffer from complement-mediated thrombocytopenia. However, we found peripheral platelet counts in Pf4-Cre+-Crryflox/flox mice to be completely normal (Fig 3A). FACS analysis also detected no C3 fragment deposition on their platelets (Fig 3B), suggesting that circulating platelets were not attacked by complement in vivo. These observations led us to wonder if Pf4-Cre+-Crryflox/flox mice, like Tie-2-Cre+-Crryflox/Δ (or Crryflox/flox) mice, might have developed secondary complement insufficiency. However, ELISA measurement of plasma intact C3 levels (Fig 3C) and functional AP complement activity assay (Fig 3D) showed this not to be the case. We next compared platelets from Pf4-Cre+-Crryflox/flox and Crry−/−/ DAF−/−/C3−/− mice for their sensitivity to AP complement attack in vitro. Fig 3E shows that, as we demonstrated before (21), platelets from Crry−/− DAF−/−/C3−/− mice were heavily opsonized with C3 fragments after in vitro incubation with WT mouse serum, but there was little C3 deposition on similarly treated Pf4-Cre+-Crryflox/flox mouse platelets. Because a truncated Crry protein was still produced from the cre/lox-mediated mutant Crry gene (22), to exclude the possibility that the truncated Crry protein still possessed some activity, we also tested platelets from CrryΔ/Δ DAF−/− mice generated from Tie-2-Cre+-Crryflox/flox/DAF−/− intercrosses that were viable but had secondary complement insufficiency (data not shown). Fig 3F shows that platelets from CrryΔ/Δ DAF−/− mice were also susceptible to C3 opsonization in vitro. Collectively, these results suggested that Crry/DAF-deficient platelets developed in a C3-sufficient environment were resistant to AP complement attack, whereas those produced in mice with no or compromised AP complement were not.
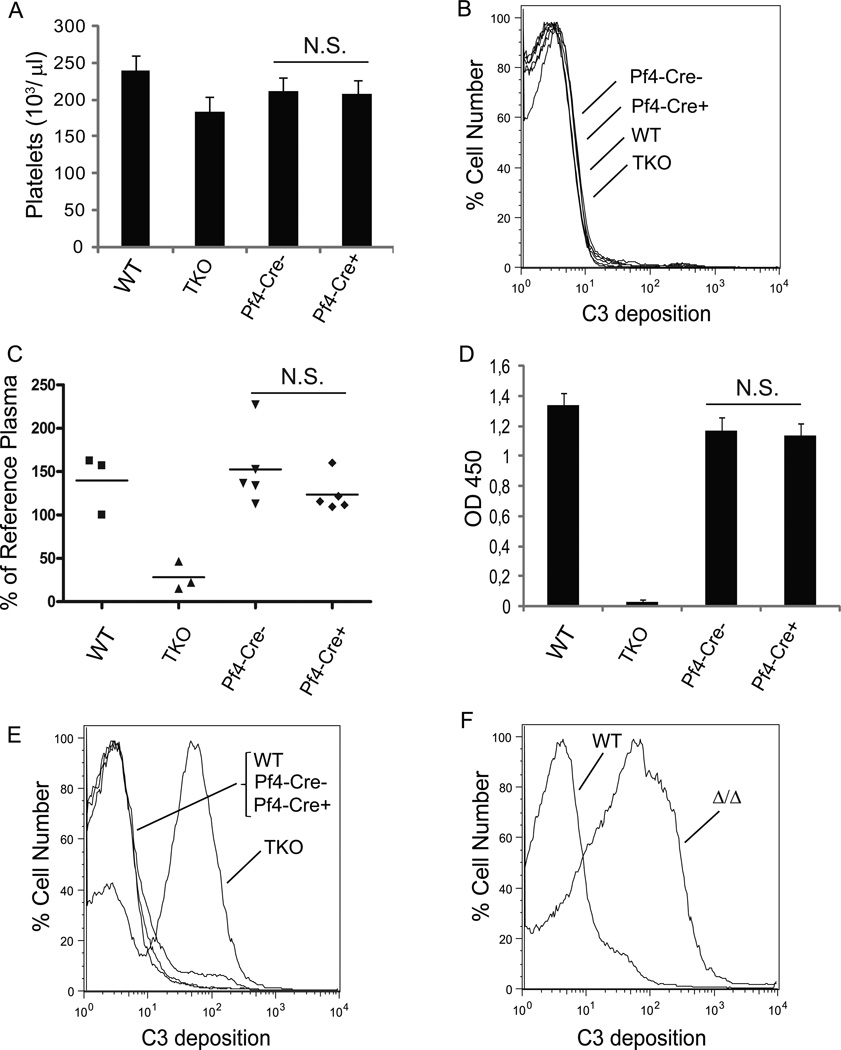
Figure 3. Platelets from Pf4-Cre+-Crryflox/flox mice were resistant to complement attack.
(A) Peripheral platelet count in Pf4-Cre+-Crryflox/flox mice was normal (n= 17–20 mice per group). Error bars represent SEM. (B) FACS analysis showing no C3 deposition on Pf4-Cre+-Crryflox/flox mouse platelets in vivo. (C) ELISA assay showing normal plasma intact C3 levels in Pf4-Cre+-Crryflox/flox mice. Each data point represents a single mouse. Levels are normalized to a reference plasma sample from a randomly chosen WT mouse. (D) ELISA assay of LPS-induced AP complement activation showing normal complement activity in the sera (1: 10 dilution) of Pf4-Cre+-Crryflox/flox mice (n= 6 mice per group). Error bars represent SEM. (E) FACS analysis showing that DAF−/−/Crry−/−/C3−/− (TKO) mouse platelets were sensitive to AP complementmediated C3 deposition, while Pf4-Cre+-Crryflox/flox platelets were resistant. (F) FACS analysis showing that platelets from Pf4-Cre+-CrryΔ/Δ mice (on DAF−/− background), which had secondary complement insufficiency, were also sensitive to AP complement-mediated C3 deposition. Results in E and F were representative of >3 independent experiments. TKO: DAF−/−/Crry−/−C3−/−, Pf4-Cre−: Pf4-Cre−-Crryflox/flox, Pf4-Cre+: Pf4-Cre+-Crryflox/flox mice. N.S. p > 0.05, Student t test.
Platelets from bone marrow (BM) chimera mice generated with C3+/+ but not C3−/− mice as graft recipients were resistant to complement attack
To verify this conclusion, we produced BM chimera mice using Pf4-Cre+-Crryflox/flox mice as BM donors and WT (C3+/+) or C3−/− mice as recipients. We used DAF expression on RBCs to track BM graft success. As shown in Fig 4A and C, DAF was detected on RBCs of both naïve WT and C3−/− mice but not on RBCs of WT or C3−/− chimera mice 2 months after they received Pf4-Cre+-Crryflox/flox mouse BM cells, confirming that the donor BM had successfully engrafted. When platelets from the chimera mice were treated with WT mouse serum in vitro, platelets from WT recipients but not C3−/− recipients were found to be resistant to AP complement attack (Fig 4B, D). This experiment thus established that either complement-resistant or -susceptible DAF/Crry-deficient platelets were produced from the same engrafted Pf4-Cre+-Crryflox/flox mouse BM, depending on the presence or absence of a functional AP complement system in the recipient animals.
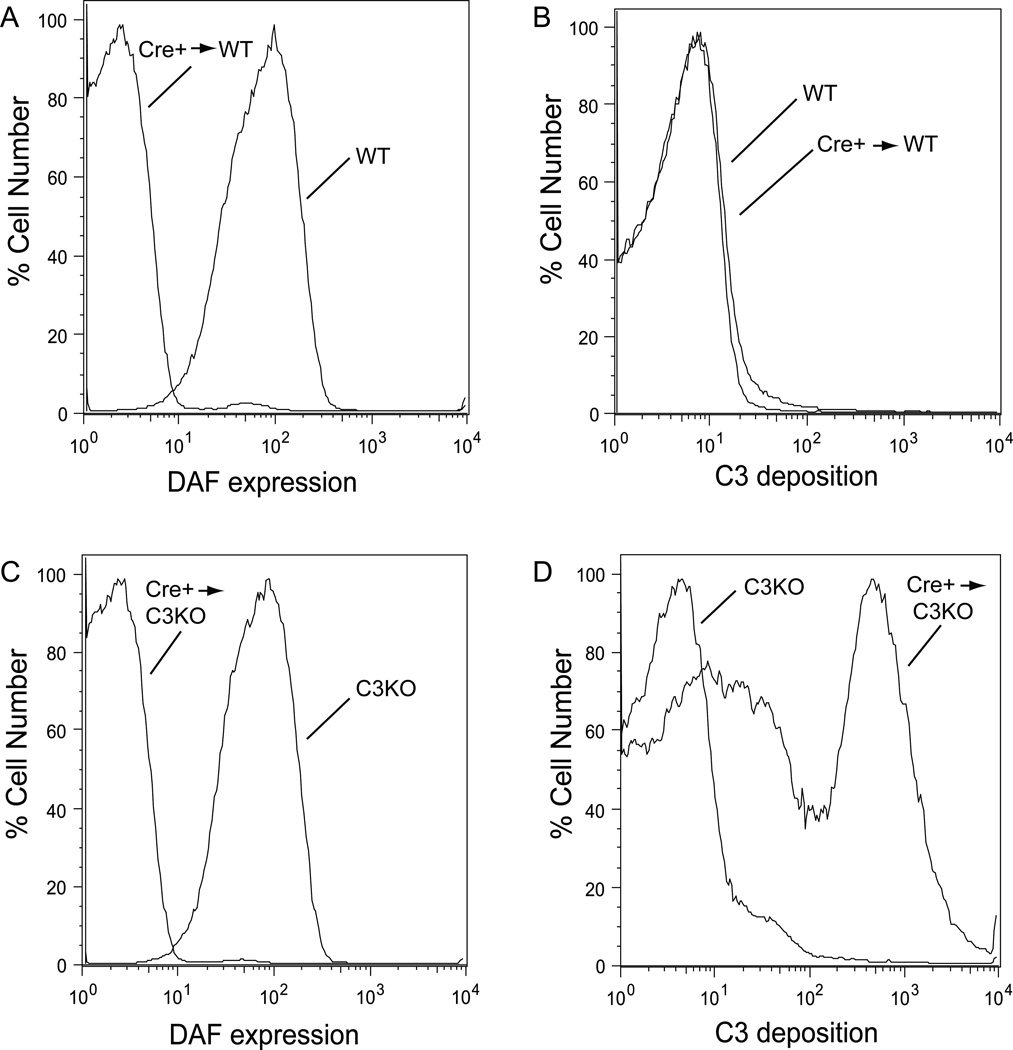
Figure 4. Pf4-Cre+-Crryflox/flox mouse platelets were naturally selected to resist complement attack in a complement-sufficient environment.
(A, C) FACS analysis of DAF on erythrocytes to confirm successful engraftment of Pf4-Cre+-Crryflox/flox BM in lethally irradiated C3+/+ (WT, A) and C3−/− (C3KO, C) recipients. (B, D) Platelets from chimera mice generated with WT mice as recipients were resistant to AP complement attack (B), whereas those with C3−/− mice as recipients were susceptible to AP complement attack (D). Results were representative of 3–4 independent experiments using chimera mice.
Pf4-Cre+-Crryflox/flox mice had increased thrombopoiesis
To explain this interesting phenomenon, we hypothesized that there is heterogeneity in complement sensitivity among Crry/DAF-deficient platelets when they first reach circulation. In the presence of a functional AP complement system, those platelets that are complement susceptible are eliminated while those that are resistant survive. Such a natural selection process would not have occurred in mice with a compromised or deficient complement system. Since peripheral platelet count in Pf4-Cre+-Crryflox/flox mice was normal, we reasoned that loss of complement-sensitive Crry/DAF-deficient platelets may have been compensated for by increased thrombopoiesis. Indeed, by staining vWF as a marker for megakaryocytes, the precursors of platelets, we found evidence of increased thrombopoiesis in Pf4-Cre+-Crryflox/flox mice compared with Pf4-Cre−-Crryflox/flox mice (Fig 5). On BM tissue sections, there was an increased number and size of megakaryocytes in Pf4-Cre+-Crryflox/flox mice (Fig 5A, B, D). Furthermore, there were fewer mono- or di-nucleated, but dramatically more numbers of tri- or poly-nucleated megakaryocytes in the BM of Pf4-Cre+-Crryflox/flox mice (Fig 5C,D).
Figure 5. Increased bone marrow thrombopoiesis in Pf4-Cre+-Crryflox/flox mice.
(A, B) Representative photomicrograph showing more megakaryocytes (positive for vWF, original magnification 200×) in the BMs of Pf4-Cre+-Crryflox/flox mice (B) than in Pf4-Cre−-Crryflox/flox mice (A). (C) Photomicrograph showing mono-, di-, tri- and poly-nucleated megakaryocytes expressing vWF (original magnification 600×) in the BM of a Pf4-Cre+-Crryflox/flox mouse. (D) Enumeration of different types of megakaryocytes confirmed that there were more megakaryocytes in the BMs of Pf4-Cre+-Crryflox/flox mice. The percentage of mono/di-nucleated megakaryocytes was significantly lower in Pf4-Cre+-Crryflox/flox mice but the percentage of tri- or poly-nucleated megakaryocytes was significantly higher in Pf4-Cre+-Crryflox/flox mice (n= 4 mice per group). Error bars represent ±SEM. *P < 0.05, Student t test.
Peripheral platelets in Pf4-Cre+-Crryflox/flox mice were protected by factor H
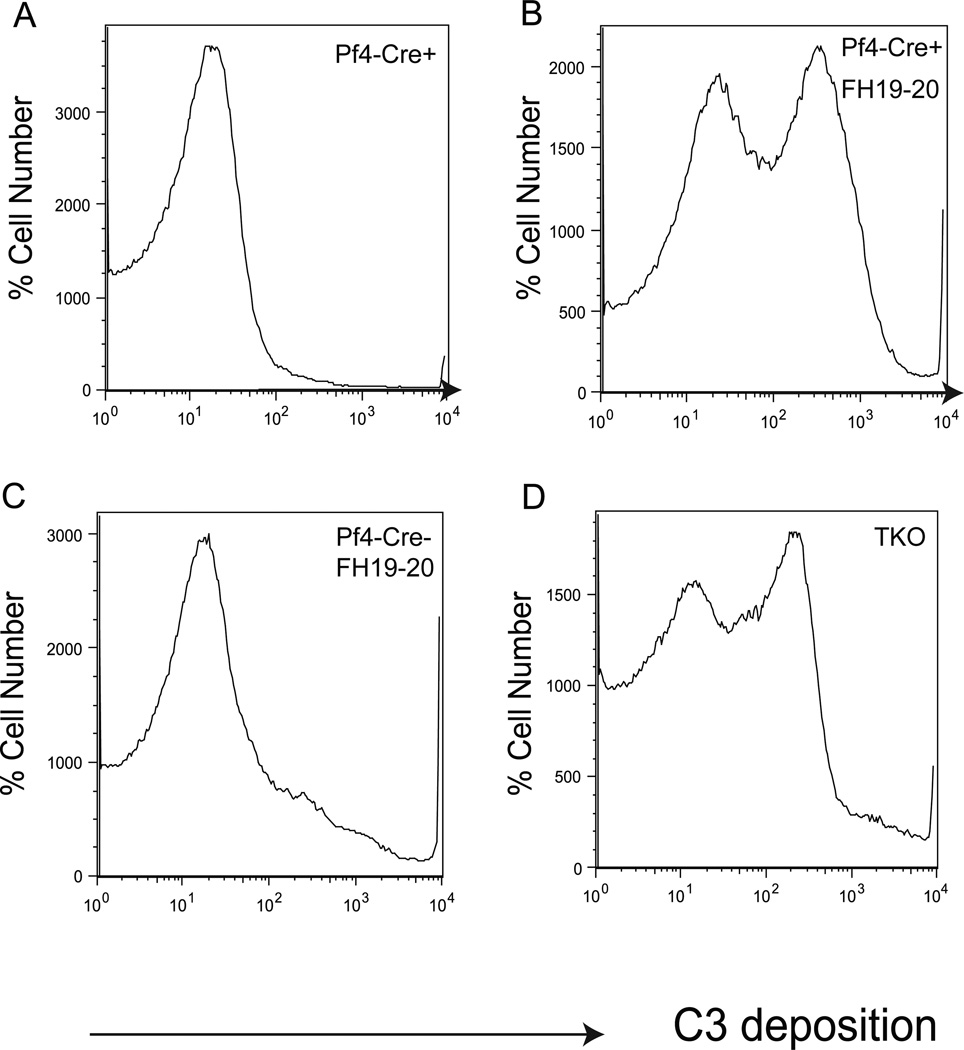
In addition to membrane C3 regulators such as Crry and DAF, host tissues are also protected by the fluid phase complement regulator fH (5, 6). Additionally, although murine MCP is constitutively expressed only in the testis (7), it may be abnormally expressed on Pf4-Cre+-Crryflox/flox mouse platelets to compensate for the lack of Crry and DAF. However, Western blot analysis using a polyclonal or monoclonal anti-mouse MCP antibody (kindly provided by Dr John Atkinson and Dr Paul Morgan, respectively) failed to detect MCP up-regulation (data not shown). By ELISA assays, we also found no increase in plasma fH levels in Pf4-Cre+-Crryflox/flox mice (data not shown). To determine if fH was nevertheless critical in preventing Crry/DAF-deficient platelets in Pf4-Cre+-Crryflox/flox mice from complement attack, we blocked fH function by using a recombinant protein, fH19/20, which corresponds to the C-terminal domain of mouse fH. fH19/20 is composed of short consensus repeat 19 and 20 of fH and lacks complement regulating function. However, it competes with fH for cell surface binding and thus can work as an inhibitor of fH activity on the cell surface (32). Fig 6A and B show that although Pf4-Cre+-Crryflox/flox platelets were resistant to complement attack when incubated with WT mouse serum in vitro, they became susceptible if fH19/20 was added to the serum to block fH. In contrast, fH19/20 treatment did not significantly increase complement sensitivity of Pf4-Cre−-Crryflox/flox platelets (Fig 6C), suggesting that Crry alone was still sufficient to protect the platelets. Notably, blocking fH function with fH19/20 rendered Pf4-Cre+-Crryflox/flox platelets as sensitive to complement attack as Crry−/−/ DAF−/−/C3−/− platelets (Fig 6B,D), implying that protection by normal plasma levels of fH was the main driving force behind the ‘survival of the fittest’ selection process for Pf4-Cre+-Crryflox/flox platelets.
Figure 6. Factor H protected Pf4-Cre+-Crryflox/flox mouse platelets from alternative pathway complement attack.
(A, B) FACS analysis of C3 deposition demonstrating that Pf4-Cre+-Crryflox/flox mouse platelets were resistant to AP complement attack in vitro (A) but they became complement sensitive if fH19/20 was added WT mouse serum to block fH function on the cell surface (B). The degree of complement sensitivity of Pf4-Cre+-Crryflox/flox platelets in the presence of fH19/20 was similar to that of DAF−/−/Crry−/−/C3−/− (TKO) mouse platelets (D). Addition of fH19/20 to the incubation of Pf4-Cre−-Crryflox/flox mouse platelets with WT serum did not make them hyper-sensitive to AP complement attack (C), suggesting that Crry alone was sufficient to protect the platelets from AP complement. Results were representative of at least 3 independent experiments.
Discussion
In this study, we used the cre-lox system to study the physiological function of Crry and DAF on cells of the vasculature. Crry is a rodent-specific complement regulator but is regarded as a functional homolog of human MCP (10). Thus, its study in murine models sheds light on the biology of human MCP in health and disease. One of the difficulties in the study of Crry in vivo is that global Crry gene knockout is embryonically lethal (16). Although Crry−/−C3−/− mice are viable, and studies of blood cell transfusion (RBCs, platelets) and solid organ transplantation (kidney) between them and WT mice have been carried out and proven informative (18–21, 33), it has not been possible to directly assess the in vivo biology of Crry in the vasculature in mice. Through breeding using female mice with compromised AP complement or the use of an anti-C5 mAb during pregnancy, Crry−/− or Crry−/−DAF−/− mice with one or both copies of the C3 gene have been obtained (34–36). However, these mice had secondary complement insufficiency, making them also less suitable for direct in vivo studies. We have previously generated a Crry gene floxed mouse (22) which, when used in combination with tissue-specific Cre transgenic mice, circumvents the embryonic lethality problem and allows for the study of Crry function in specific mouse tissues in vivo.
We used the Tie-2-Cre transgenic mouse in the current study with an intention to delete endothelial Crry in DAF−/− mice. However, we found that Tie-2-Cre caused Crry deletion from endothelial as well as hematopoietic cells of Crryflox/flox mice. Like Crry−/− mice (34, 35), Tie-2-Cre+-Crryflox/flox mice, and a line of CrryΔ/Δ mice derived from them, had secondary AP complement insufficiency. Thus, deficiency of Crry from endothelial and hematopoietic cells on the DAF−/− background was sufficient to cause systemic complement consumption. That Tie-2-Cre+-Crryflox/flox and CrryΔ/Δ mice were grossly normal and lacked apparent vascular injury, despite evidence of continuous AP complement activation, was remarkable and contrasted with the phenotype of fH-deficient mice that had similar systemic AP complement activation but developed C3 glomerulonephritis (37). Lack of renal injury and C3 deposition was also noted previously in Crry−/− mice (34, 35). It is possible that Crry deficiency-related AP complement activation occurred on cells throughout the body and this might have helped to dissipate its injurious impact. In fH deficiency, the kidney bore the brunt of injury because of trapping of fluid-phase activated C3 fragments and/or the possibility that fH is the only AP complement regulator working on the non-cellular and heparan sulfate-rich glomerular basement membrane where membrane inhibitors are not expressed.
Use of the more specific Pf4-Cre transgenic mouse has allowed us to study Crry/DAF-deficiency on platelet development in vivo. Our data suggested that DAF and Crry normally are the main regulators protecting platelets from complement attack, as Crry deletion from DAF−/− mouse platelets caused abnormal platelet turnover, leading to compensatory increase in thrombopoiesis. That fH usually played an insignificant role on murine platelets was supported by evidence from two separate experiments. First, platelets from Crry−/−DAF−/−C3−/− mice were highly susceptible to C3 deposition when incubated in vitro with WT mouse serum even though fH was present in the serum (Fig 3E). Second, blocking fH function with fH19/20 did not significantly change complement sensitivity of Pf4-Cre−-Crryflox/flox platelets (Fig 6C), suggesting that Crry alone was sufficient to protect the platelets and both DAF and fH were normally dispensable.
The finding that platelets from C3++ but not C3−/− chimera mice resisted AP complement attack suggested that Pf4-Cre+-Crryflox/flox platelets underwent a natural selection process in complement-sufficient animals and only those that had high affinity for fH survived. The site of this selection process is unknown. We assume that the selection process occurred in the periphery where AP complement was most active but cannot rule out the possibility that selection may have occurred at the megakaryocyte stage. Regardless, it is clear that there was heterogeneity in newly formed platelets or megakaryocytes in their ability to interact with fH. The nature of this heterogeneity remains to be established, but is likely to be related to the level or composition of polyanionic constituents such as sialic acid, heparin sulfate or other glycosaminoglycans (GAGs) on the platelet surface that are known to be important for fH interaction with other types of host cells (38, 39).
Our results shed new light on the relative roles of membrane and fluid phase C3 complement regulators in vivo. It has been a unresolved question why dysfunction of a given complement regulator often causes injury in one tissue or organ but not others. As alluded to above, fH deficiency caused C3 glomerulopathy but spared other tissues (37), and Crry deficiency resulted in fetal death but once passing through the gestation stage, Crry−/− mice displayed no apparent tissue injury even through AP complement was continuously activated and consumed (34, 35). We previously have demonstrated that Crry and DAF are functionally interchangeable, and their relative importance on a give cell type depended on their expression levels (20, 21). Thus, Crry level was nearly 4 times as high as DAF on murine RBCs but they were present at similar levels on murine platelets (20, 21). This explained why Crry-deficient but not DAF-deficient RBCs, and Crry/DAF-deficient platelets but not Crry-deficient or DAF-deficient platelets, were susceptible to AP complement-mediated elimination (21). We propose here that a combination of membrane regulator expression level and affinity for fH interaction determines host cell sensitivity to AP complement attack. The fact that Crry/DAF-deficient platelets in Pf4-Cre+-Crryflox/flox mice were able to resist AP complement attack in a fH-dependent manner demonstrated that, at least for the surviving and selected platelets, fH alone was sufficient in the absence of membrane regulators. Together with our previous findings (20, 21), this observation suggested that quantitative rather than qualitative characteristics of Crry (MCP in humans), DAF and fH determine host cell sensitivity to AP complement attack. Thus, variations in membrane C3 complement regulator expression may contribute to the partial penetrance of fH-related mutations and vice versa. The finding of a natural selection process leading to ‘survival of the fittest’ platelets and a compensatory increase in thrombopoiesis has also highlighted the body’s ability to respond adaptively to complement regulator deficiencies, which may also contribute to the partial penetrance of many complement gene mutations.
Acknowledgement
We thank Dr John Atkinson of Washington University at St Louis and Drs Timothy Hughes and Paul Morgan of Cardiff University for providing anti-mouse MCP antibodies.
Footnotes
Supported by National Institutes of Health grants AI44970, GM92108 and AI49344 (to W.-C.S.).
Abbreviations used:
AP, alternative pathway; DAF, decay-accelerating factor; Crry, complement receptor 1-related gene/protein y; BM, bone marrow; DC, dendritic cells; NEO, neomycin; WT, wild-type; RBC, red blood cell; MCP, membrane cofactor protein; fH, factor H; aHUS, atypical hemolytic uremic syndrome; PNH, paroxysmal nocturnal hemoglobinuria; GAG, glycosylaminoglycan
References
- 1.Dunkelberger JR, Song W-C. Complement and its role in innate and adaptive immune responses. Cell research. 20:34–50. doi: 10.1038/cr.2009.139. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 3.Thurman JM, Holers VM. The central role of the alternative complement pathway in human disease. J Immunol. 2006;176:1305–1310. doi: 10.4049/jimmunol.176.3.1305. [DOI] [PubMed] [Google Scholar]
- 4.Miwa T, Song WC. Membrane complement regulatory proteins: insight from animal studies and relevance to human diseases. Int Immunopharmacol. 2001;1:445–459. doi: 10.1016/s1567-5769(00)00043-6. [DOI] [PubMed] [Google Scholar]
- 5.Kim DD, Song W-C. Membrane complement regulatory proteins. Clinical immunology (Orlando, Fla) 2006;118:127–136. doi: 10.1016/j.clim.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Jozsi M, Zipfel PF. Factor H family proteins and human diseases. Trends in immunology. 2008;29:380–387. doi: 10.1016/j.it.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Tsujimura A, Shida K, Kitamura M, Nomura M, Takeda J, Tanaka H, Matsumoto M, Matsumiya K, Okuyama A, Nishimune Y, Okabe M, Seya T. Molecular cloning of a murine homologue of membrane cofactor protein (CD46): preferential expression in testicular germ cells. The Biochemical journal. 1998;330(Pt 1):163–168. doi: 10.1042/bj3300163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miwa T, Nonaka M, Okada N, Wakana S, Shiroishi T, Okada H. Molecular cloning of rat and mouse membrane cofactor protein (MCP, CD46): preferential expression in testis and close linkage between the mouse Mcp and Cr2 genes on distal chromosome 1. Immunogenetics. 1998;48:363–371. doi: 10.1007/s002510050447. [DOI] [PubMed] [Google Scholar]
- 9.Molina H. The murine complement regulator Crry: new insights into the immunobiology of complement regulation. Cellular and molecular life sciences : CMLS. 2002;59:220–229. doi: 10.1007/s00018-002-8418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holers VM, Kinoshita T, Molina H. The evolution of mouse and human complement C3-binding proteins: divergence of form but conservation of function. Immunology today. 1992;13:231–236. doi: 10.1016/0167-5699(92)90160-9. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez de Cordoba S, Esparza-Gordillo J, Goicoechea de Jorge E, Lopez-Trascasa M, Sanchez-Corral P. The human complement factor H: functional roles, genetic variations and disease associations. Molecular immunology. 2004;41:355–367. doi: 10.1016/j.molimm.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Rosse WF, Parker CJ. Paroxysmal nocturnal haemoglobinuria. Clinics in haematology. 1985;14:105–125. [PubMed] [Google Scholar]
- 13.Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, Hurault de Ligny B, Fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJC, Goodship THJ, Atkinson JP. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goicoechea de Jorge E, Harris CL, Esparza-Gordillo J, Carreras L, Arranz EA, Garrido CA, Lopez-Trascasa M, Sanchez-Corral P, Morgan BP, Rodriguez de Cordoba S. Gain-of-function mutations in complement factor B are associated with atypical hemolytic uremic syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:240–245. doi: 10.1073/pnas.0603420103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown JHTJ, Wilson V, Mackie IJ, Scully M, Tredger MM, Moore I, McDougall NI, Strain L, Marchbank KJ, Sheerin NS, O'Grady J, Harris CL, Goodship TH. Postpartum aHUS secondary to a genetic abnormality in factor H acquired through liver transplantation. Proc Natl Acad Sci U S A. 2012;13:4187–4190. doi: 10.1111/j.1600-6143.2012.03991.x. [DOI] [PubMed] [Google Scholar]
- 16.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Funk CD, Deng C, Sahu A, Lambris JD, Song WC. Role of decay-accelerating factor in regulating complement activation on the erythrocyte surface as revealed by gene targeting. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:628–633. doi: 10.1073/pnas.96.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miwa T, Zhou L, Hilliard B, Molina H, Song WC. Crry, but not CD59 and DAF, is indispensable for murine erythrocyte protection in vivo from spontaneous complement attack. Blood. 2002;99:3707–3716. doi: 10.1182/blood.v99.10.3707. [DOI] [PubMed] [Google Scholar]
- 19.Molina H, Miwa T, Zhou L, Hilliard B, Mastellos D, Maldonado MA, Lambris JD, Song WC. Complement-mediated clearance of erythrocytes: mechanism and delineation of the regulatory roles of Crry and DAF. Decay-accelerating factor. Blood. 2002;100:4544–4549. doi: 10.1182/blood-2002-06-1875. Epub 2002 Aug 4541. [DOI] [PubMed] [Google Scholar]
- 20.Kim DD, Miwa T, Song W-C. Retrovirus-mediated over-expression of decay-accelerating factor rescues Crry-deficient erythrocytes from acute alternative pathway complement attack. Journal of immunology (Baltimore, Md : 1950) 2006;177:5558–5566. doi: 10.4049/jimmunol.177.8.5558. [DOI] [PubMed] [Google Scholar]
- 21.Kim DD, Miwa T, Kimura Y, Schwendener RA, van Lookeren Campagne M, Song WC. Deficiency of decay-accelerating factor and complement receptor 1-related gene/protein y on murine platelets leads to complement-dependent clearance by the macrophage phagocytic receptor CRIg. Blood. 2008;112:1109–1119. doi: 10.1182/blood-2008-01-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miwa T, Zhou L, Kimura Y, Kim D, Bhandoola A, Song WC. Complement-dependent T-cell lymphopenia caused by thymocyte deletion of the membrane complement regulator Crry. Blood. 2009;113:2684–2694. doi: 10.1182/blood-2008-05-157966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X, Kimura Y, Fang C, Zhou L, Sfyroera G, Lambris JD, Wetsel RA, Miwa T, Song WC. Regulation of Toll-like receptor-mediated inflammatory response by complement in vivo. Blood. 2007;110:228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lesher AM, Zhou L, Kimura Y, Sato S, Gullipalli D, Herbert AP, Barlow PN, Eberhardt HU, Skerka C, Zipfel PF, Hamano T, Miwa T, Tung KS, Song WC. Combination of Factor H Mutation and Properdin Deficiency Causes Severe C3 Glomerulonephritis. J Am Soc Nephrol. 2012 Nov 30; doi: 10.1681/ASN.2012060570. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Developmental biology. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 26.Kuhn S, Zipfel PF. The baculovirus expression vector pBSV-8His directs secretion of histidine-tagged proteins. Gene. 1995;162:225–229. doi: 10.1016/0378-1119(95)00360-i. [DOI] [PubMed] [Google Scholar]
- 27.Kimura Y, Miwa T, Zhou L, Song W-C. Activator-specific requirement of properdin in the initiation and amplification of the alternative pathway complement. Blood. 2008;111:732–740. doi: 10.1182/blood-2007-05-089821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu Z, Slayton WB, Rimsza LM, Bailey M, Sallmon H, Sola-Visner MC. Differences between newborn and adult mice in their response to immune thrombocytopenia. Neonatology. 98:100–108. doi: 10.1159/000280413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gitler AD, Kong Y, Choi JK, Zhu Y, Pear WS, Epstein JA. Tie2-Cre-induced inactivation of a conditional mutant Nf1 allele in mouse results in a myeloproliferative disorder that models juvenile myelomonocytic leukemia. Pediatric research. 2004;55:581–584. doi: 10.1203/01.PDR.0000113462.98851.2E. [DOI] [PubMed] [Google Scholar]
- 30.de Lange WJ, Halabi CM, Beyer AM, Sigmund CD. Germ line activation of the Tie2 and SMMHC promoters causes noncell-specific deletion of floxed alleles. Physiological genomics. 2008;35:1–4. doi: 10.1152/physiolgenomics.90284.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. Journal of immunology (Baltimore, Md : 1950) 2006;177:6308–6316. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- 33.Bao L, Wang Y, Chang A, Minto AW, Zhou J, Kang H, Haas M, Quigg RJ. Unrestricted C3 activation occurs in Crry-deficient kidneys and rapidly leads to chronic renal failure. Journal of the American Society of Nephrology : JASN. 2007;18:811–822. doi: 10.1681/ASN.2006101176. [DOI] [PubMed] [Google Scholar]
- 34.Wu X, Spitzer D, Mao D, Peng SL, Molina H, Atkinson JP. Membrane protein Crry maintains homeostasis of the complement system. Journal of immunology (Baltimore, Md : 1950) 2008;181:2732–2740. doi: 10.4049/jimmunol.181.4.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruseva MM, Hughes TR, Donev RM, Sivasankar B, Pickering MC, Wu X, Harris CL, Morgan BP. Crry deficiency in complement sufficient mice: C3 consumption occurs without associated renal injury. Molecular immunology. 2009;46:803–811. doi: 10.1016/j.molimm.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Miwa T, Zhou L, Tudoran R, Lambris JD, Madaio MP, Nangaku M, Molina H, Song WC. DAF/Crry double deficiency in mice exacerbates nephrotoxic serum-induced proteinuria despite markedly reduced systemic complement activity. Mol Immunol. 2007;44:139–146. doi: 10.1016/j.molimm.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 37.Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nature genetics. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- 38.Kajander T, Lehtinen MJ, Hyvarinen S, Bhattacharjee A, Leung E, Isenman DE, Meri S, Goldman A, Jokiranta TS. Dual interaction of factor H with C3d and glycosaminoglycans in host-nonhost discrimination by complement. Proceedings of the National Academy of Sciences of the United States of America. 108:2897–2902. doi: 10.1073/pnas.1017087108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Z-Z, Hellwage J, Seeberger H, Zipfel PF, Meri S, Jokiranta TS. Comparison of surface recognition and C3b binding properties of mouse and human complement factor H. Molecular immunology. 2006;43:972–979. doi: 10.1016/j.molimm.2005.05.011. [DOI] [PubMed] [Google Scholar]