Abstract
A signal first arising in the dermis to initiate the development of hair follicles has been described for many decades. Wnt is the earliest signal known to be intimately involved in hair follicle induction. However, it is not clear whether the inductive signal of Wnt arises intradermally or intraepidermally. Whether Wnt acts as the first dermal signal to initiate hair follicle development also remains unclear. Here, we report that Wnt production mediated by Gpr177, the mouse Wls orthologue encoding a Wnt trafficking regulator, is essential for hair follicle induction. Cell-type specific abrogation of the signal reveals that only epidermal, but not dermal, production of Wnt is required. An intra-epidermal Wnt signal is necessary and sufficient for hair follicle initiation. But, the subsequent development depends on reciprocal signaling crosstalk of epidermal and dermal cells. Wnt signals within the epidermis and dermis, and crossing between the epidermis and dermis, have distinct roles and specific functions in skin development. This study not only defines the cell type responsible for Wnt production, but also reveals a highly dynamic regulation of Wnt signaling at different steps of hair follicle morphogenesis. Our findings uncover a mechanism underlying hair follicle development orchestrated by the Wnt pathway.
Keywords: Gpr177, Wntless, β-catenin, hair placode and skin
Introduction
The mammalian skin and its appendages are derived from ectoderm and mesoderm during embryogenesis (Fuchs, 2007; Hardy, 1992). The embryo surface emerges as a single layer of epithelial cells which give rise to the epidermis. The dermis is formed from the underlying mesoderm composed of mesenchymal cells. Subsequently, the epidermal-dermal interaction results in hair follicle formation (Fuchs, 2007; Hardy, 1992). Morphologically, the development of hair follicles begins with a local thickening of the epidermis, known as hair placodes. Upon successful initiation of the epithelial placode, a condensate of dermal cells is formed in the underlying mesenchyme (Driskell et al., 2011; Fuchs, 2007). The signals sent between this mesenchymal condensate and the overlying epithelial placode dictate the behavior of both cell populations, ultimately orchestrating formation of the hair follicle and dermal papilla (Fuchs, 2007; Hardy, 1992; Millar, 2002; Schmidt-Ullrich and Paus, 2005). Classical cross-species experiments indicate that mouse dermal tissue is capable of inducing the hair placode, feather bud or scale placode upon epithelial-mesenchymal recombination (Dhouailly, 1973; Hardy, 1992; Olivera-Martinez et al., 2004). The data thus suggest existence of an inducing factor arising initially in the dermis. However, the first dermal signal initiating the developmental programming of the epidermis remains elusive.
Several families of secreted signaling molecules have been implicated in the communication between epidermis and dermis during hair follicle development. Among them, the Wnt family appears to be the earliest and most critical regulator for early development of the epidermis (Fuchs, 2007; Millar, 2002; Noramly et al., 1999; Schmidt-Ullrich and Paus, 2005). Wnt blocks the ability of the ectoderm to respond to FGF. This in turn elevates BMP, leading to fate determination of the epidermis (Fuchs, 2007; Stern, 2005). During formation of the hair follicles, multiple Wnt proteins are expressed in the embryonic skin (Millar et al., 1999; Reddy et al., 2001). Activation of Wnt/β-catenin signaling is evident in both the epithelium and the underlying mesenchyme (Andl et al., 2002; DasGupta and Fuchs, 1999; Zhang et al., 2008). Deletion of β-catenin in the epidermis impairs the formation of hair placodes (Huelsken et al., 2001). β-catenin signaling is necessary and sufficient to initiate hair follicle development (Andl et al., 2002; Gat et al., 1998). Although the importance of Wnt signaling in hair follicle development is well established, it is not clear whether the Wnt signal arises intradermally or intraepidermally. Whether Wnt acts as the first dermal signal to initiate hair follicle development also remains to be determined.
Disruption of Wnt production in the signal-producing cells can yield important insights into the mechanism underlying hair follicle development. However, due to overlapping expression of Wnts in the developing skin, gene specific inactivation may not be practical, and is likely to encounter issues related to functional redundancy. We have recently identified Gpr177 as the mouse orthologue of Drosophila Wls/Evi/Srt essential for proper sorting and secretion of Wnt (Banziger et al., 2006; Bartscherer et al., 2006; Fu et al., 2011; Fu et al., 2009; Goodman et al., 2006). In flies, Wls/Evi/Srt regulates the secretion of all Wnts, except for WntD, due to its exclusion from lipid modifications (Ching et al., 2008; Herr and Basler, 2012). In mice, genetic studies have suggested that the Gpr177-mediated regulation of canonical and noncanonical Wnts is required for different cell types and tissues (Fu et al., 2011; Fu et al., 2009; Stefater et al., 2011). The abrogation of Wnt secretion caused by Gpr177 deficiency may provide an excellent strategy to determine the source of Wnt during organogenesis. To decipher Wnt signaling regulation in hair follicle development, we created mouse models with cell-type specific disruption of Gpr177. This study not only defines the cell type responsible for Wnt production, but also reveals a highly dynamic regulation of Wnt signaling, at various steps of hair follicle development. Our findings suggest a model for the role of Wnt signaling in hair follicle morphogenesis.
Results
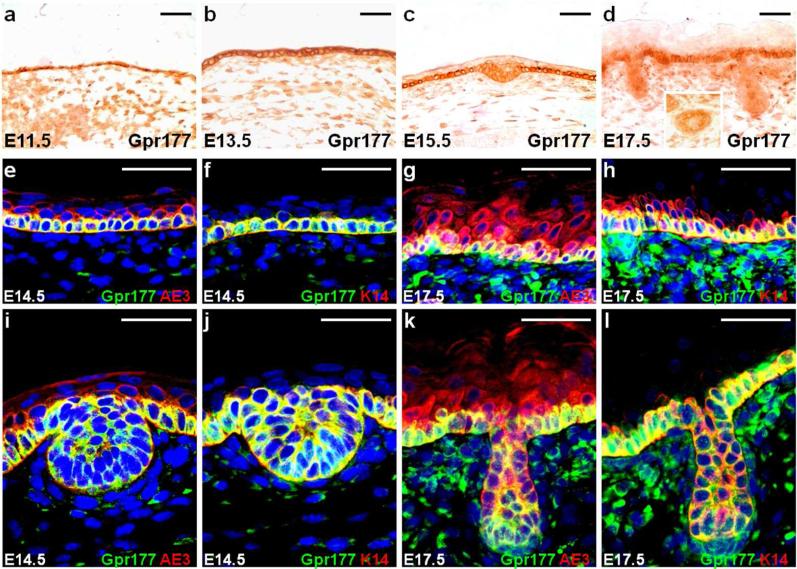
We examined the expression of Gpr177 to determine the cell type potentially responsible for Wnt production during hair follicle induction. Using a well-characterized antibody specifically recognizing Gpr177 (Fu et al., 2009), immunostaining analysis revealed that Gpr177 is expressed in the epithelium and the underlying mesenchyme at E11.5 and E13.5 (Fig. 1a, b). In the epithelium, Gpr177 expression was restricted to the developing hair follicles and the basal layer of epidermis at E15.5 and E17.5 (Fig. 1c, d). Co-labeling of Gpr177 with AE3, a marker for the entire epidermis, or K14, a marker for the epidermal basal layer, further indicated that the epidermal basal cells and the hair follicular cells express high levels of Gpr177 (Fig. 1e-l and Fig. S1).
Fig. 1.
Gpr177 is expressed in hair follicle development. Immunostaining of the E11.5 (a) and E13.5 (b) skins shows the expression of Gpr177 in the epithelium and underlying mesenchyme. A restricted elevation is found in the epidermal basal cells and hair follicular cells at E15.5 (c) and E17.5 (d), respectively. The inset shows non-uniform expression of Gpr177 in the hair follicle (d). Double labeling of Gpr177 (e-l) with AE3, a marker for the entire epidermis (e, g, i, k), or K14, a marker for the epidermal basal layer (f, h, j, l), identifies the Gpr177-expressing cells at E14.5 (e-f, i-j) and E17.5 (g-h, k-l). Scale bars, 50 μm (a-l).
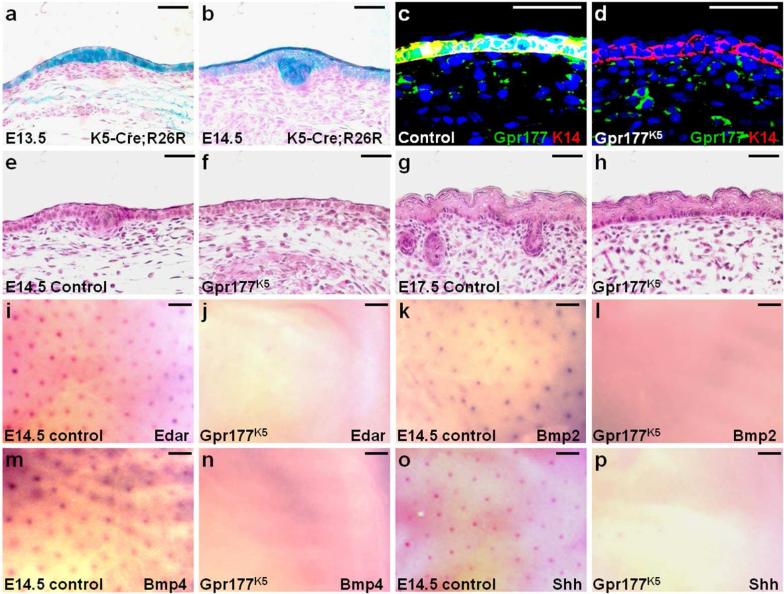
To determine the requirement of Gpr177 in the epidermis, we generated Gpr177K5 mutant mice in which Gpr177 was inactivated by K5-Cre (Ramirez et al., 2004). Using the R26R reporter allele, β-gal staining of the E13.5 and E14.5 skins showed the effectiveness of Cre recombination in the epidermis and developing hair follicles (Fig. 2a, b). Immunostaining for Gpr177 further indicated its successful ablation in the epidermis, but not dermis, of Gpr177K5 (Fig. 2c, d). Wnt secretion assays further examined epidermal Wnt production affected by the Gpr177 deletion. Primary epidermal cells of control and Gpr177K5 were used as the signal-producing cells, which were co-cultured with the signal-receiving cells harboring a TOPFLASH reporter for β-catenin and Lef/Tcf-dependent transcription. While the control epidermal cells were capable of activating the TOPFLASH reporter, this activation was significantly reduced and close to the background level in the Gpr177K5 dermal cells (Fig. S2a). Furthermore, activation of several Wnt signaling mediators was affected in the epidermis of Gpr177K5 (Fig. S2b). These results suggest that Gpr177 is essential for Wnt secretion in the epidermis. No hair placodes and follicles were detected in the Gpr177K5 mutants at E14.5 and E17.5, respectively, indicating an essential role of Gpr177 in the epidermis for hair follicle induction (Fig. 2e-h; 100%, n=15). Next, we examined the expression of placode/follicle-specific genes affected by the loss of Gpr177 (Botchkarev and Sharov, 2004; Laurikkala et al., 2002; Zhang et al., 2009). No expression of ectodysplasin receptor (Edar), BMP2, BMP4 and Shh was found, indicating no formation of the placodes in the Gpr177K5 mutants (Fig. 2i-p). The results imply that epidermal Gpr177 is involved in the regulation of Wnt, the earliest developmental signal known to turn on the programming for hair follicle induction (Fuchs, 2007; Millar, 2002; Noramly et al., 1999; Schmidt-Ullrich and Paus, 2005).
Fig. 2.
Epidermal deletion of Gpr177 abrogates the induction of hair follicles. β-gal staining of the E13.5 (a) and E14.5 (b) K5-Cre; R26R embryos analyzes the effectiveness of Cre recombination in the epidermis and hair placode. Sections of the control and Gpr177K5 embryos were analyzed by co-immunostaining of Gpr177 and K14 (c-d) and hematoxylin/eosin staining (e-h) at E14.5 (c-f) and E17.5 (g-h). Whole mount in situ hybridization of the control (i, k, m, o) and Gpr177K5 (j, l, n, p) embryos reveals the expression of Edar (i-j), Bmp2 (k-l), Bmp4 (m-n) and Shh (o-p) at E14.5. Control genotype: Gpr177Fx/Fx or K5-Cre; Gpr177Fx/+. Scale bars, 50 μm (a-h); 200 μm (i-p).
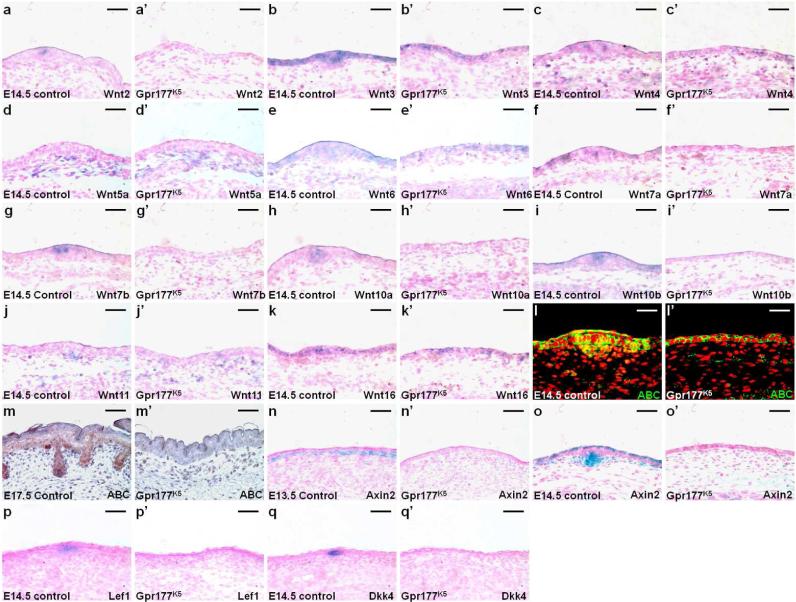
Multiple members of the Wnt family were expressed in distinct patterns in the developing skin with a few candidates, e.g. Wnt3, Wnt10a and Wnt10b, implicated in hair follicle morphogenesis (Andl et al., 2002; Millar et al., 1999; Reddy et al., 2001). We first examined the expression of all 19 mouse Wnt genes in the E14.5 skin by RT-PCR analysis, and detected transcripts of Wnts2, 3, 4, 5a, 6, 7a, 7b, 10a, 10b, 11 and 16, including those reported (Reddy et al., 2001), whose expression patterns were further characterized (Fig. S3). In situ hybridization revealed that Wnts2, 3, 7a, 7b, 10a and 16 are expressed in the epidermis (Fig. 3a, b, f, g, h); Wnts5a and 11 in the dermis (Fig. 3d, j); Wnts4, 6 and 10b in both layers (Fig. 3c-e, i, k). Furthermore, the Wnts3, 4 and 6 transcripts were evenly distributed throughout the epidermis, including the hair placode and interfollicular epithelium (Fig. 3b-c, e). While Wnts2, 7b, 10a and 10b showed elevated expression in the hair placode (Fig. 3a, g-i), Wnts7a and 16 were expressed mainly in the interfollicular epithelium (Fig. 3f, k). The inactivation of Gpr177 abolished the epidermal expression of Wnts2, 7a, 7b, 10a and 10b (Fig. 3a’, f’, g’-i’). In contrast, Wnts3, 4, 6 and 16 genes remained active in the Gpr177K5 mutants (Fig. 3b’-c’, e’, k’). Furthermore, the dermal expression of Wnt5a and Wnt11 genes was affected by the epidermal ablation of Gpr177 (Fig. 3d’ and j’). Their expression at low uniform levels was maintained in the dermis but absent in the dermal condensate due to the lack of hair placodes in the mutants. Based on these data, we could categorize the hair follicle-expressing Wnt genes into two groups: the first one, consisting of Wnts3, 4 and 6, was evenly expressed in the hair placode and interfollicular epithelium and the second one, consisting of Wnts2, 7b, 10a and 10b, exhibited elevated expression in the hair placode. The second, but not the first, group was affected by the epidermal deletion of Gpr177. The results imply a potential hierarchy of Wnt activation during hair follicle development.
Fig. 3.
Wnt expression and signaling are affected by the epidermal deletion of Gpr177. In situ hybridization in sections shows the expression of Wnt genes in the E14.5 control (a-k) and Gpr177K5 (a’-k’) skins. Sections of control (l-o) and Gpr177K5 (l’-o’) skins examine the signaling activity of Wnt by immunostaining of an activated form of β-catenin (ABC) and β-gal staining of the Axin2lacZ allele at E13.5 (n, n’), E14.5 (l, l’, o, o’) and E17.5 (m, m’). In situ hybridization analyzes the expression of Wnt downstream targets, Lef1 (p, p’) and Dkk4 (q, q’) in the E14.5 control (p-q) and Gpr177K5 (p’-q’) skins. Control genotype: Gpr177Fx/Fx or K5-Cre; Gpr177Fx/+. Scale bars, 50 μm (a-q, a’-q’).
Because β-catenin signaling is required for early onset of hair follicle morphogenesis (Huelsken et al., 2001; Zhang et al., 2009), we examined if the canonical Wnt pathway was affected by the Gpr177 deletion. Immunostaining of the control and Gpr177K5 skins revealed a drastic reduction of an activated form of β-catenin (ABC) in the hair placode and the underlying mesenchyme at E14.5 (Fig. 3l, l’), and in the hair follicular epithelial and dermal cells at E17.5 (Fig. 3m, m’). The reduction of active β-catenin was also accompanied by the loss of expression of its transcriptional targets, Axin2 (Fig. 3n-o, n’-o’), Lef1 (Fig. 3p, p’) and Dkk4 (Fig. 3q, q’), all of which are critically involved in hair follicle development (Bazzi et al., 2007; van Genderen et al., 1994; Yu et al., 2005a). An early activation of Wnt/β-catenin signaling in the upper dermis immediately adjacent to the epidermis occurs prior to the appearance of the placode at E13.5 (Fig. 3n). This early activation is totally disrupted by the epidermal deletion of Gpr177 (Fig. 3n’), suggesting that Wnt secretion from the epidermis drives the early response of β-catenin signaling in the dermis. Subsequently, the patterned signaling activity of Wnt/β-catenin necessary for hair follicle morphogenesis is impaired (Huelsken et al., 2001; Zhang et al., 2009). In addition to cell fate specification and differentiation, Wnt is also a key signal for cell proliferation and survival during skin organogenesis (Widelitz, 2008). BrdU incorporation analysis revealed that the number of proliferating cells was significantly reduced in the epidermis, but not dermis, of Gpr177K5, compared to the littermate control (Fig. S4a-c, mutant: 23.91±0.01% and control: 34.03±0.03%; p value <0.01, n=3). In contrast, no alteration in programmed cell death was detected (Fig. S4d-i). These results indicated a detrimental effect of the Gpr177 deletion on the canonical Wnt pathway, suggesting a mechanism underlying the induction of hair follicles mediated through Gpr177-dependent β-catenin signaling.
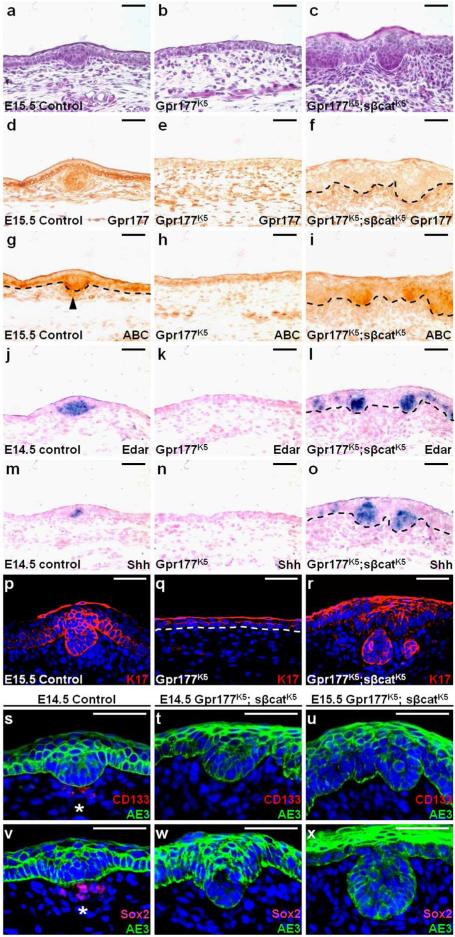
To definitively assess that the impairment of β-catenin signaling is responsible for the hair follicle defects, we introduced the β-catΔEx3Fx allele into the Gpr177K5 mice to generate the Gpr177K5; sβcatK5 model. In these mutants, a stabilized β-catenin mutant was expressed in the developing epidermis due to the deletion of exon 3 of β-catenin (Maruyama et al., 2010; Mirando et al., 2010). By restoring the signaling activity of β-catenin in the epidermal cells, we examined if the block in hair follicle induction caused by the Gpr177 deletion could be alleviated. Hair placode-like structures were apparent in the Gpr177K5; sβcatK5 skins, suggesting that induction has occurred at E15.5 (Fig. 4a-c; 100%, n=3). Immunostaining analysis further showed that epidermal activation of β-catenin takes place in the absence of Gpr177 (Fig. 4d-i). Nonetheless, dermal activation of β-catenin remained absent in the Gpr177K5; sβcatK5 skins (Fig. 4g-i). To demonstrate the proper induction of hair follicles, we first examined the expression of Edar and Shh, which are evident in the control but missing in the Gpr177K5 skins (Fig. 4j-k, m-n). However, expression of the β-catenin mutant was able to activate Edar and Shh in the placode-like structures of Gpr177K5; sβcatK5, suggesting a restoration of placode signals (Fig. 4l, o) and follicular epithelium positive for K17 (Fig. 4p-r). Therefore, β-catenin activation was able to overcome the block in hair follicle induction caused by the epidermal deletion of Gpr177. The formation of hair placodes then triggers a clustering of underlying mesenchymal cells to form dermal condensates, leading to the development of dermal papilla expressing CD133 (Hardy, 1992; Ito et al., 2007; Millar, 2002). The CD133 positive dermal papilla cells were present in the control, but absent in the Gpr177K5; sβcatK5 mutants (Fig. 4s-u). Analysis of another dermal papilla marker, Sox2, showed similar results (Fig. 4v-x). Although epidermal activation of β-catenin alleviated the defects associated with initial induction of hair follicles, it failed to induce the neighboring mesenchymal cells to form dermal condensates. These data indicate that Wnt/β-catenin signaling induced by the epidermal Wnt is essential for initial induction of hair follicles within the epidermis. They also imply that the subsequent development of dermal papilla requires Wnt secretion from the epithelial cells to induce Wnt signaling in the mesenchymal cells.
Fig. 4.
Epidermal stimulation of β-catenin alleviates the hair follicle defects of Gpr177K5. Sections of the control, Gpr177K5 and Gpr177K5; sβcatK5 were analyzed by H&E staining (a-c), immunostaining of Gpr177 (d-f), ABC (g-i) and K17 (p-r), in situ hybridization of Edar (j-l) and Shh (m-o), and double labeling of CD133 and AE3 (s-u) or Sox2 and AE3 (v-x) at E14.5 and E15.5. Arrowhead indicates the dermal activation of β-catenin in the control (g), but not in the Gpr177K5 and Gpr177K5; sβcatK5 mutants (h-i). Asterisks indicate the dermal papilla markers, CD133 and Sox2, are detected in the control (s, v), but absent in the Gpr177K5; sβcatK5 mutants (q-r, t-u). Control genotype: Gpr177Fx/Fx or K5-Cre. Scale bars, 50 μm (a-x).
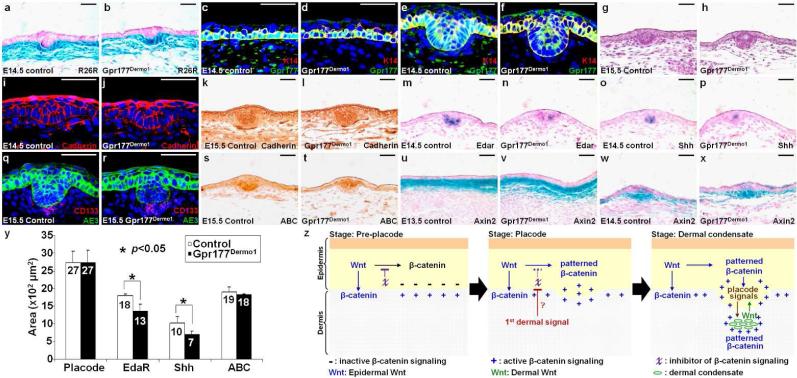
To investigate the requirement of Wnt production contributing to the first dermal signal essential for hair follicle induction, we generated the Gpr177Dermo1 model where Gpr177 was inactivated in the mesenchymal cells of the developing dermis by Dermo1-Cre (Tran et al., 2010). Using R26R, β-gal staining of the E14.5 skins showed the efficiency of Cre recombination in the mesenchymal cells of developing dermis (Fig. 5a-b). Immunostaining of Gpr177 further indicated its removal in the dermis, but not epidermis, of Gpr177Dermo1 (Fig. 5c-f). The dermal deletion of Gpr177 had no effect on the formation of hair placodes at E15.5, suggesting that Gpr177-mediated Wnt production may be dispensable in the dermis for hair follicle initiation (Fig. 5g-h; 100%, n=8). Immunostaining of Cadherin proteins showed that the morphology of follicular epithelial cells in the mutants is comparable to that of control (Fig. 5i-l). Although Edar and Shh were expressed in the hair placodes, their expression was affected by the mutation (Fig. 5m-p). Quantitative measurement confirmed their expression domains reduced in the mutants (Fig. 5y). Nonetheless, there were sufficient placode signals to promote the subsequent development in the underlying mesenchyme as the dermal condensate formation was not affected in the Gpr177Dermo1 mutants (Fig. 5q-r). Although the dermal deletion of Gpr177 had minimal effects on hair follicle induction, the dermal layer of Gpr177Dermo1 exhibited a loose structure with significant abnormalities (Fig. S5). The number of proliferating cells was significantly reduced in the dermis, but not the epidermis, of Gpr177Dermo1, compared to the littermate controls (Fig. S5a-c, mutant: 19.01±0.03% and control: 32.21±0.03%; p value <0.01, n=3). In contrast, no alteration in apoptosis was detected (Fig. S5d-i). The dermal deletion affected cell-type specification of mesenchymal cells into fibroblasts, smooth muscle cells and adipocytes modulated by Wnt (Wei et al., 2011). We detected an apparent shift of dermal cell identity to favor adipocytes in the Gpr177Dermo1 mutants (Fig. S5j-p). This is consistent with prior reports indicating the differential effects of Wnt on development of the mesenchymal derived cell types (Cristancho and Lazar, 2011; Wei et al., 2011).
Fig. 5.
The Gpr177-mediated regulation of Wnt in the dermis is dispensable for hair follicle initiation. β-gal staining of the E14.5 control and Gpr177Dermo1 skins carrying R26R shows Cre effectiveness (a-b). Sections are analyzed by co-labeling of Gpr177 and K14 (c-f) or CD133 and AE3 (q-r), H&E (g-h), immunostaining of Cadherin (i-l) and ABC (s-t) and in situ hybridization of Edar (m-n) and Shh (o-p), and β-gal staining of the Axin2lacZ allele (u-x) at E13.5, E14.5 and E15.5. (y) Graph indicates quantitative analysis for the placode region and the expression domain for ABC, Edar and Shh. Control genotype: Gpr177Fx/Fx or Dermo1-Cre; Gpr177Fx/+. Scale bars, 50 μm (a-x). (z) Model for epidermal Wnt in orchestrating signaling interaction between the epidermis and dermis during hair follicle development.
Analysis of ABC and Axin2 expression further indicated no significant difference in the pattern of Wnt/β-catenin signaling (Fig. 5s-y). In the upper dermis of Gpr177Dermo1, the early activation of Wnt/β-catenin signaling remained detectable prior to the appearance of hair placodes at E13.5 (Fig. 5u-v) and was unaffected in the placode and dermal condensate at E14.5 (Fig. 5w-x). To ensure that Gpr177 is required for Wnt production in the dermis, the Wnt secretion assay was performed. While the control dermal cells were capable of activating the TOPFLASH reporter, this activation was reduced by the Gpr177 deletion, suggesting an impairment of Wnt secretion (Fig. S6a). Furthermore, activation of several Wnt signaling effectors was not affected in the dermis and epidermis of Gpr177Dermo1 most likely due to the presence of epidermal Wnt (Fig. S6b). However, using the ex vivo culture of primary cells, we found that Wnt signaling activation is impaired in the Gpr177Dermo1 dermal cells (Fig. S6b). Although Gpr177 regulates Wnt secretion in the dermis, dermal Wnt is not required for hair follicle induction. Dermal Wnt seems to be dispensable for the early events of hair follicle formation. The findings thus do not support the idea that Wnt is secreted from the dermis to induce β-catenin signaling in the epidermis essential for hair follicle initiation.
Discussion
This study reveals that epidermal Gpr177 is essential for the Wnt-mediated induction of hair follicles. Epidermal, but not dermal, deletion of Gpr177 disrupts the early activation of Wnt/β-catenin signaling in the upper dermis prior to initiation of the hair follicle. Subsequently, the expression of Edar, Bmp2/4 and Shh, critical for hair follicle morphogenesis is disrupted (Botchkarev and Sharov, 2004; Laurikkala et al., 2002; St-Jacques et al., 1998), suggesting that Wnt signaling acts upstream of these pathways. These results are consistent with recent findings but provide additional insights into hair follicle induction mediated by Wnt signaling crosstalk between the epidermis and dermis. Our data also support the requirement of Gpr177 for secretion of all canonical Wnts, and imply a hierarchy of Wnt actions during hair follicle formation. Furthermore, the lack of hair placode formation upon deletion of Gpr177 in the epidermis can be alleviated by β-catenin stimulation, suggesting that epidermal autocrine signaling of Wnt promotes the induction process. However, epidermal restoration of β-catenin signaling fails to correct the subsequent formation of dermal condensates. Due to the lack of epidermal Wnt, the dermal papilla does not develop properly. Epidermal Wnt is therefore a part of the placode signals which induce the dermis through an extra-epidermal signaling mechanism. Our Dermo1-mediated deletion of Gpr177 causes defects in dermal cell proliferation and differentiation, as well as in full induction of the placode signal during hair follicle morphogenesis, not detected in the deletion mediated by En1-Cre (Chen et al., 2012). Although Wnt secretion is impaired, β-catenin signaling remains intact in both epidermis and dermis of the mutants. The induction of hair follicle and dermal papilla is mainly unaffected. These surprising results argue against Wnt being the initial dermal signal.
We propose a mechanism underlying hair follicle development mediated by the Wnt pathway based on previous evidence and current findings (Fig. 5z). β-catenin signaling is first uniformly activated in the upper dermis prior to its activation in the epithelium of hair placodes or feather buds (Noramly et al., 1999; Zhang et al., 2009). This is followed by activation in the placode epithelium, and then in the underlying mesenchyme and dermal condensate (DasGupta and Fuchs, 1999; Fuchs, 2007). Epidermal but not dermal production of Wnt is responsible for activating β-catenin signaling in the epithelium and mesenchyme during hair follicle and dermal papilla induction. Epidermal Wnt is required for activation of β-catenin signaling not only within the epidermis (epidermal autocrine) but also across the epidermis to the dermis (epidermal paracrine). Before the receipt of the initial dermal signal, β-catenin signaling is blocked by an inhibitor (χ) in the epidermis (Stage: Pre-placode). However, the epidermal Wnt signal permits a uniform activation of β-catenin signaling in the upper dermis via epidermal paracrine regulation. When the initial dermal signal arises, it prohibits the inhibitory effect on β-catenin to induce the placode-specific signaling in the epidermis, followed by induction of the hair placode via epidermal autocrine regulation (Stage: Placode). A reciprocal induction occurs subsequently between the placode and underlying mesenchyme (Stage: Dermal condensate). The placode signals including epidermal Wnt are sent back to the underlying mesenchyme to pattern the signaling activity of β-catenin for the formation of dermal condensate and papilla. Dermal Wnt as a feedback then promotes full induction of the placode signals.
The concept of the “first dermal message” as the initial signal for hair follicle induction was described decades ago (Hardy, 1992). Because of the essential role of β-catenin and its spatiotemporal expression pattern during hair follicle development, Wnt has been suggested to be the first signal in the dermis (Fuchs, 2007; Millar, 2002; Noramly et al., 1999; Schmidt-Ullrich and Paus, 2005). However, the identity of the initial dermal signal remains unknown. Our genetic studies imply that Wnt probably is not the “first dermal message”. Instead of activation, we hypothesize that the initial dermal signal is required for de-repression of β-catenin signaling in the epidermis. Smad7-mediated regulation of Smurf2 has been shown to modulate β-catenin in hair follicle formation (Han et al., 2006), suggesting a potential mechanism underlying the inhibition effect of Wnt. How the first signal arising in the dermis to regulate the Wnt pathway in the epidermis remains an important question to be addressed.
The dermal abnormalities detected in the Gpr177Dermo1 mutants suggest that the Gpr177-mediated secretion of Wnt play a critical role in the dermis. These abnormalities are most likely caused by alteration in noncanonical, but not canonical, Wnt because the β-catenin signaling activity is comparable in the dermis of control and Gpr177Dermo1. Furthermore, dermal Wnt is not required for hair follicle initiation, consistent with Wnt5a dispensable for this process (Hu et al., 2010).
Another question remaining to be addressed is which of the Wnt family members regulates the development of hair follicles. Our findings suggest that the epidermal deletion of Gpr177 interferes with the expression of Wnts2, 7b, 10a and 10b, which exhibit elevated expression in the placode. However, the expression of Wnts3, 4 and 6 uniformly in the interfollicular epithelium and placode is not affected in the mutants. As a trafficking regulator for Wnt proteins, the removal of Gpr177 does not usually affect their expression (Fu et al., 2011; Fu et al., 2009). Therefore, there is a hierarchy of Wnts controlling hair follicle development. The first group of Wnt genes, including Wnts3, 4 and 6, is unaffected by the Gpr177 deletion in the epidermis. Thus they represent candidates for the “primary Wnt” which mediates hair follicle initiation. The second group of Wnt genes, including Wnts2, 7b, 10a and 10b, whose expression is disrupted by the Gpr177 deletion, depends on epidermal activation of β-catenin signaling. This is supported by the observation that ectopic expression of the Wnt inhibitor Dkk1 and ectodermal deletion of β-catenin impair hair follicle development through disruption of patterning signaling mediated by Wnts 10a and 10b (Andl et al., 2002; Zhang et al., 2009). Therefore, Wnts 2, 7b, 10a and 10b likely act as the “secondary Wnt”, which is a part of the placode signal, essential for hair follicle development. Defining the specific role of these Wnts in hair follicle induction promises important insights into the mechanism underlying hair follicle morphogenesis.
Methods
Mouse strains and Cells
The Gpr177Fx, K5-Cre, Dermo1-Cre, R26R, β-catΔEx3Fx and Axin2lacZ mouse strains, and genotyping methods were reported previously (Fu et al., 2011; Harada et al., 1999; Soriano, 1999; Sosic et al., 2003; Tarutani et al., 1997; Yu et al., 2005a; Yu et al., 2003). Care and use of experimental animals described in this work comply with guidelines and policies of the University Committee on Animal Resources at the University of Rochester. Epidermal and dermal cells isolated from the specific skin layer were cultured in the CnT-PCT and DMEM media, respectively, with 10% FBS. For Wnt secretion assay, cells transfected with TOPFLASH were used as indicator for receiving of Wnt signals determined by relative luciferase activity.
Histology, β-gal staining, immunostaining, immunoblot and TUNEL analysis
For histology and immunostaining, samples were fixed and embedded for sections, stained with H&E and specific antibodies using the avidin:biotinlylated based method (Chiu et al., 2008; Fu et al., 2011; Fu et al., 2009; Maruyama et al., 2010; Yu et al., 2005a; Yu et al., 2010; Yu et al., 2005b). β-gal staining was performed with standard protocols described previously (Fu et al., 2009; Maruyama et al., 2010; Yu et al., 2005b). To detect apoptotic cells, TUNEL staining was performed as described (Maruyama et al., 2010; Yu et al., 2007).
RT-PCR and In situ hybridization
Total RNA isolated from E14.5 mouse skins was subject to RT-PCR analysis. In situ hybridization was performed to detect gene expression using the digoxygenin-labeled probes, followed by recognition with an alkaline phosphatase conjugated anti-digoxygenin antibody (Chiu et al., 2008; David and Wedlich, 2001; Fu et al., 2009; Yu et al., 2010).
Supplementary Material
Acknowledgements
We thank H-M Ivy Yu, Takamitsu Maruyama for assistance, and Alice Pentland, Dirk Bohmann, Catherine Ovitt, Anthony Mirando for comments. This work is supported by NIH grants CA106308 and DE15654 to W.H.
Footnotes
Experimental details are described in Supplemental Information
Conflict of Interest
The authors state no conflict of interest
References
- Andl T, Reddy ST, Gaddapara T, Millar SE. WNT signals are required for the initiation of hair follicle development. Developmental cell. 2002;2:643–653. doi: 10.1016/s1534-5807(02)00167-3. [DOI] [PubMed] [Google Scholar]
- Banziger C, Soldini D, Schutt C, Zipperlen P, Hausmann G, Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- Bartscherer K, Pelte N, Ingelfinger D, Boutros M. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Bazzi H, Fantauzzo KA, Richardson GD, Jahoda CA, Christiano AM. The Wnt inhibitor, Dickkopf 4, is induced by canonical Wnt signaling during ectodermal appendage morphogenesis. Developmental biology. 2007;305:498–507. doi: 10.1016/j.ydbio.2007.02.035. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Sharov AA. BMP signaling in the control of skin development and hair follicle growth. Differentiation; research in biological diversity. 2004;72:512–526. doi: 10.1111/j.1432-0436.2004.07209005.x. [DOI] [PubMed] [Google Scholar]
- Chen D, Jarrell A, Guo C, Lang R, Atit R. Dermal beta-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development (Cambridge, England) 2012;139:1522–1533. doi: 10.1242/dev.076463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching W, Hang HC, Nusse R. Lipid-independent secretion of a Drosophila Wnt protein. The Journal of biological chemistry. 2008;283:17092–17098. doi: 10.1074/jbc.M802059200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SY, Asai N, Costantini F, Hsu W. SUMO-Specific Protease 2 Is Essential for Modulating p53-Mdm2 in Development of Trophoblast Stem Cell Niches and Lineages. PLoS biology. 2008;6:e310. doi: 10.1371/journal.pbio.0060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nature reviews. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development (Cambridge, England) 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- David R, Wedlich D. PCR-based RNA probes: a quick and sensitive method to improve whole mount embryo in situ hybridizations. Biotechniques. 2001;30:769–772. 774. [PubMed] [Google Scholar]
- Dhouailly D. Dermo-epidermal interactions between birds and mammals: differentiation of cutaneous appendages. J Embryol Exp Morphol. 1973;30:587–603. [PubMed] [Google Scholar]
- Driskell RR, Clavel C, Rendl M, Watt FM. Hair follicle dermal papilla cells at a glance. Journal of cell science. 2011;124:1179–1182. doi: 10.1242/jcs.082446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Ivy Yu HM, Maruyama T, Mirando AJ, Hsu W. Gpr177/mouse Wntless is essential for Wnt-mediated craniofacial and brain development. Dev Dyn. 2011;240:365–371. doi: 10.1002/dvdy.22541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Jiang M, Mirando AJ, Yu HM, Hsu W. Reciprocal regulation of Wnt and Gpr177/mouse Wntless is required for embryonic axis formation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18598–18603. doi: 10.1073/pnas.0904894106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Scratching the surface of skin development. Nature. 2007;445:834–842. doi: 10.1038/nature05659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- Goodman RM, Thombre S, Firtina Z, Gray D, Betts D, Roebuck J, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development (Cambridge, England) 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- Han G, Li AG, Liang YY, Owens P, He W, Lu S, et al. Smad7-induced beta-catenin degradation alters epidermal appendage development. Developmental cell. 2006;11:301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. The EMBO journal. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy MH. The secret life of the hair follicle. Trends Genet. 1992;8:55–61. doi: 10.1016/0168-9525(92)90350-d. [DOI] [PubMed] [Google Scholar]
- Herr P, Basler K. Porcupine-mediated lipidation is required for Wnt recognition by Wls. Developmental biology. 2012;361:392–402. doi: 10.1016/j.ydbio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Hu B, Lefort K, Qiu W, Nguyen BC, Rajaram RD, Castillo E, et al. Control of hair follicle cell fate by underlying mesenchyme through a CSL-Wnt5a-FoxN1 regulatory axis. Genes & development. 2010;24:1519–1532. doi: 10.1101/gad.1886910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Erdmann B, Cotsarelis G, Birchmeier W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell. 2001;105:533–545. doi: 10.1016/s0092-8674(01)00336-1. [DOI] [PubMed] [Google Scholar]
- Ito Y, Hamazaki TS, Ohnuma K, Tamaki K, Asashima M, Okochi H. Isolation of murine hair-inducing cells using the cell surface marker prominin-1/CD133. J Invest Dermatol. 2007;127:1052–1060. doi: 10.1038/sj.jid.5700665. [DOI] [PubMed] [Google Scholar]
- Laurikkala J, Pispa J, Jung HS, Nieminen P, Mikkola M, Wang X, et al. Regulation of hair follicle development by the TNF signal ectodysplasin and its receptor Edar. Development (Cambridge, England) 2002;129:2541–2553. doi: 10.1242/dev.129.10.2541. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Mirando AJ, Deng CX, Hsu W. The balance of WNT and FGF signaling influences mesenchymal stem cell fate during skeletal development. Sci Signal. 2010;3:ra40. doi: 10.1126/scisignal.2000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar SE. Molecular mechanisms regulating hair follicle development. J Invest Dermatol. 2002;118:216–225. doi: 10.1046/j.0022-202x.2001.01670.x. [DOI] [PubMed] [Google Scholar]
- Millar SE, Willert K, Salinas PC, Roelink H, Nusse R, Sussman DJ, et al. WNT signaling in the control of hair growth and structure. Developmental biology. 1999;207:133–149. doi: 10.1006/dbio.1998.9140. [DOI] [PubMed] [Google Scholar]
- Mirando AJ, Maruyama T, Fu J, Yu HM, Hsu W. Beta-catenin/cyclin D1 mediated development of suture mesenchyme in calvarial morphogenesis. BMC Dev Biol. 2010;10:116. doi: 10.1186/1471-213X-10-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noramly S, Freeman A, Morgan BA. beta-catenin signaling can initiate feather bud development. Development (Cambridge, England) 1999;126:3509–3521. doi: 10.1242/dev.126.16.3509. [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I, Viallet JP, Michon F, Pearton DJ, Dhouailly D. The different steps of skin formation in vertebrates. Int J Dev Biol. 2004;48:107–115. doi: 10.1387/ijdb.15272376. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Page A, Gandarillas A, Zanet J, Pibre S, Vidal M, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- Reddy S, Andl T, Bagasra A, Lu MM, Epstein DJ, Morrisey EE, et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mechanisms of development. 2001;107:69–82. doi: 10.1016/s0925-4773(01)00452-x. [DOI] [PubMed] [Google Scholar]
- Schmidt-Ullrich R, Paus R. Molecular principles of hair follicle induction and morphogenesis. Bioessays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature genetics. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Sosic D, Richardson JA, Yu K, Ornitz DM, Olson EN. Twist regulates cytokine gene expression through a negative feedback loop that represses NF-kappaB activity. Cell. 2003;112:169–180. doi: 10.1016/s0092-8674(03)00002-3. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Dassule HR, Karavanova I, Botchkarev VA, Li J, Danielian PS, et al. Sonic hedgehog signaling is essential for hair development. Curr Biol. 1998;8:1058–1068. doi: 10.1016/s0960-9822(98)70443-9. [DOI] [PubMed] [Google Scholar]
- Stefater JA, 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD. Neural induction: old problem, new findings, yet more questions. Development (Cambridge, England) 2005;132:2007–2021. doi: 10.1242/dev.01794. [DOI] [PubMed] [Google Scholar]
- Tarutani M, Itami S, Okabe M, Ikawa M, Tezuka T, Yoshikawa K, et al. Tissue-specific knockout of the mouse Pig-a gene reveals important roles for GPI-anchored proteins in skin development. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7400–7405. doi: 10.1073/pnas.94.14.7400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran TH, Jarrell A, Zentner GE, Welsh A, Brownell I, Scacheri PC, et al. Role of canonical Wnt signaling/ss-catenin via Dermo1 in cranial dermal cell development. Development (Cambridge, England) 2010;137:3973–3984. doi: 10.1242/dev.056473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RG, Parslow TG, Bruhn L, et al. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes & development. 1994;8:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Wei J, Melichian D, Komura K, Hinchcliff M, Lam AP, Lafyatis R, et al. Canonical Wnt signaling induces skin fibrosis and subcutaneous lipoatrophy: a novel mouse model for scleroderma? Arthritis and rheumatism. 2011;63:1707–1717. doi: 10.1002/art.30312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widelitz RB. Wnt signaling in skin organogenesis. Organogenesis. 2008;4:123–133. doi: 10.4161/org.4.2.5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jerchow B, Sheu TJ, Liu B, Costantini F, Puzas JE, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development (Cambridge, England) 2005a;132:1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Jin Y, Fu J, Hsu W. Expression of Gpr177, a Wnt trafficking regulator, in mouse embryogenesis. Dev Dyn. 2010;239:2102–2109. doi: 10.1002/dvdy.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Liu B, Chiu SY, Costantini F, Hsu W. Development of a unique system for spatiotemporal and lineage-specific gene expression in mice. Proceedings of the National Academy of Sciences of the United States of America. 2005b;102:8615–8620. doi: 10.1073/pnas.0500124102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HM, Liu B, Costantini F, Hsu W. Impaired neural development caused by inducible expression of Axin in transgenic mice. Mechanisms of development. 2007;124:146–156. doi: 10.1016/j.mod.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Xu J, Liu Z, Sosic D, Shao J, Olson EN, et al. Conditional inactivation of FGF receptor 2 reveals an essential role for FGF signaling in the regulation of osteoblast function and bone growth. Development (Cambridge, England) 2003;130:3063–3074. doi: 10.1242/dev.00491. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Andl T, Yang SH, Teta M, Liu F, Seykora JT, et al. Activation of beta-catenin signaling programs embryonic epidermis to hair follicle fate. Development (Cambridge, England) 2008;135:2161–2172. doi: 10.1242/dev.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tomann P, Andl T, Gallant NM, Huelsken J, Jerchow B, et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Developmental cell. 2009;17:49–61. doi: 10.1016/j.devcel.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.