Abstract
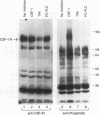
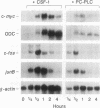
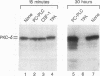
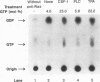
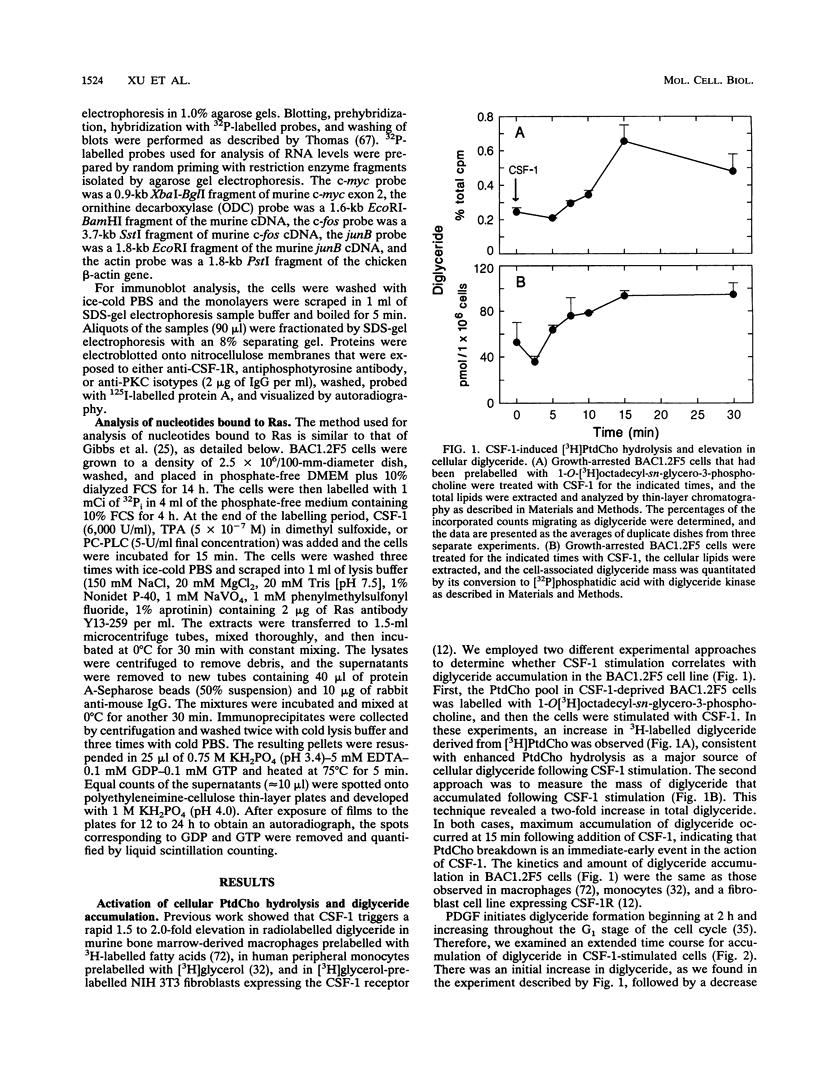
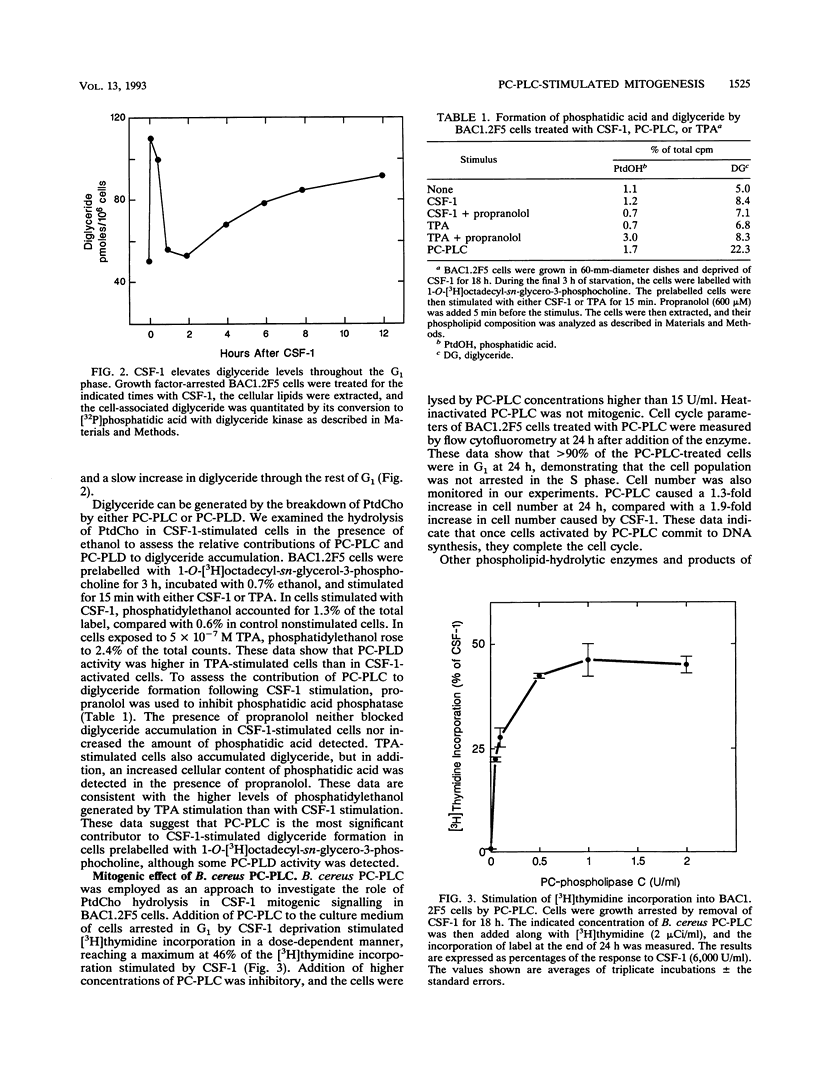
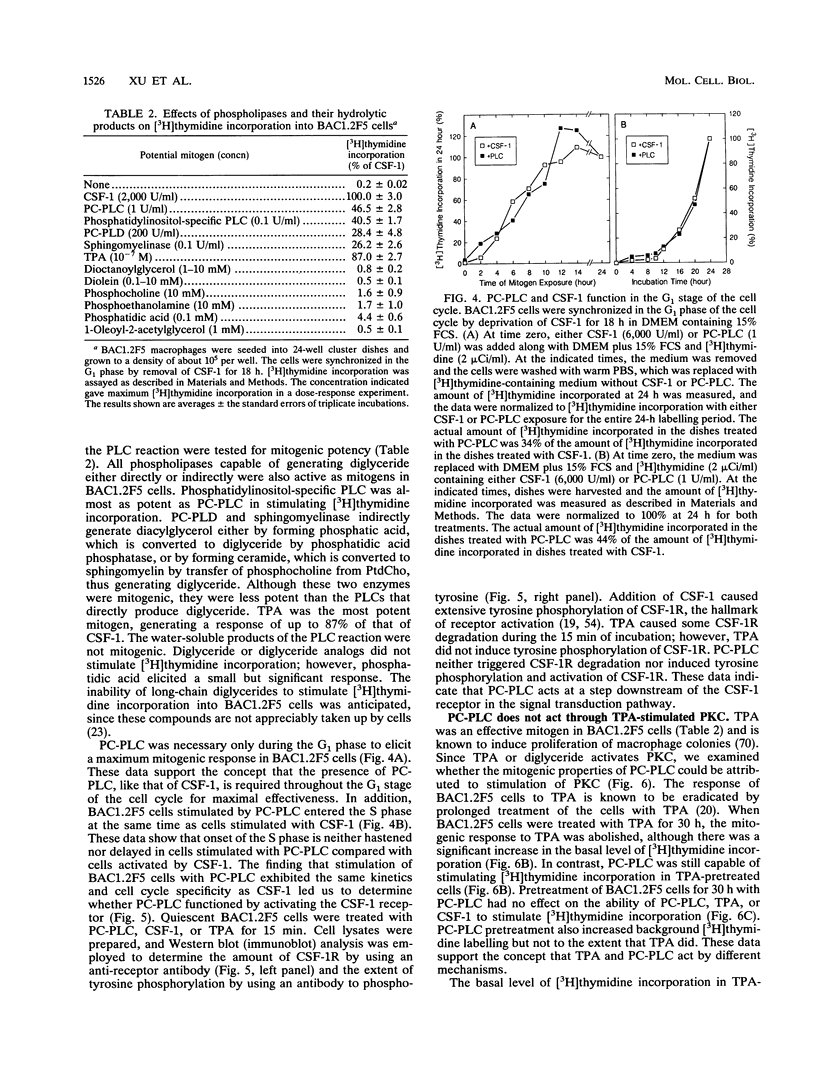
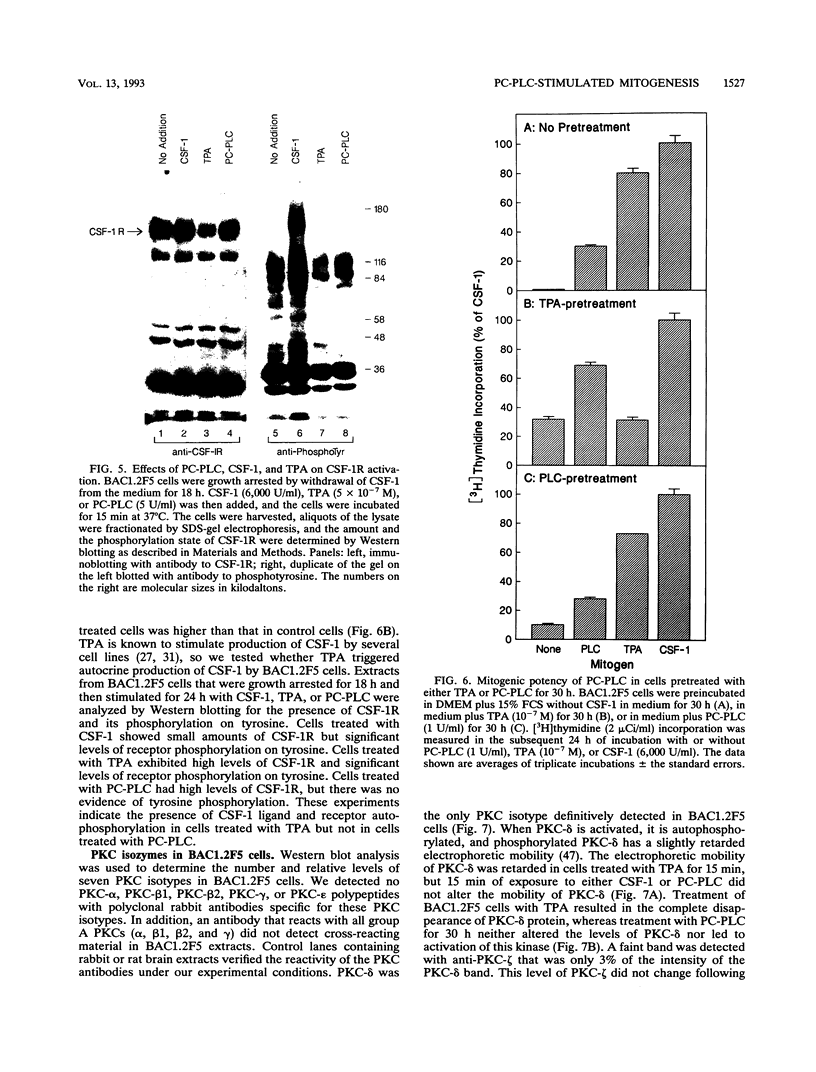
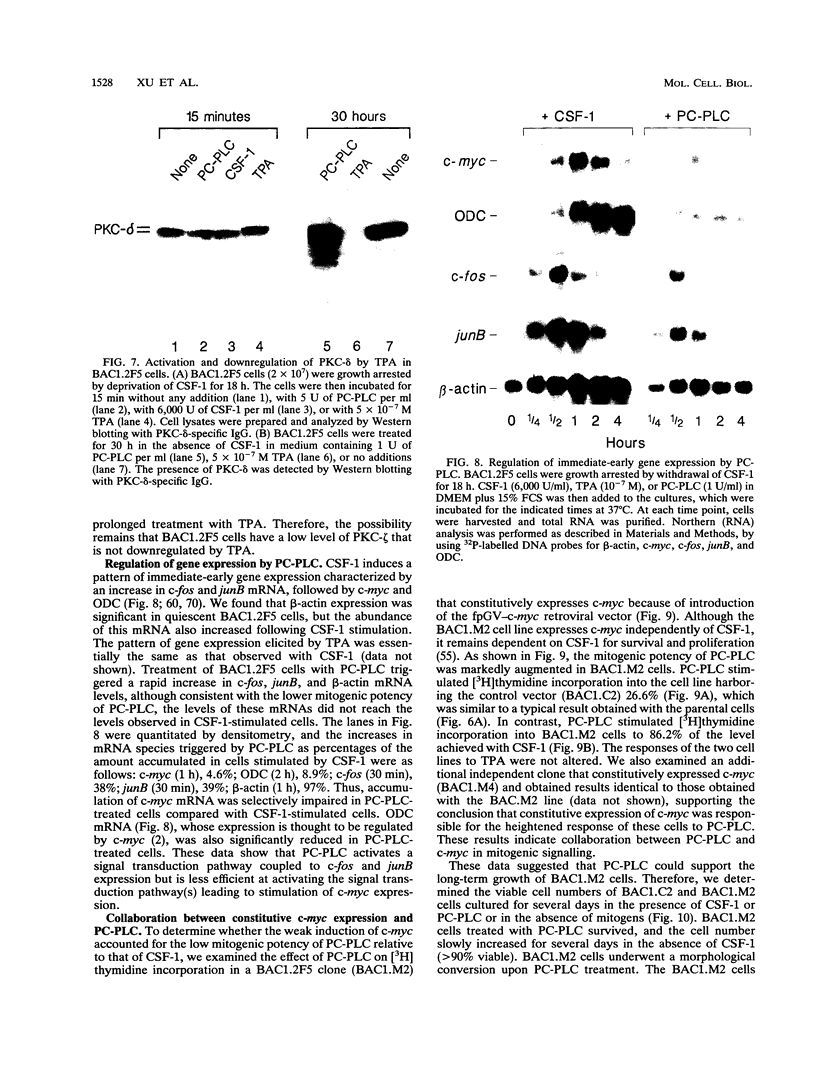
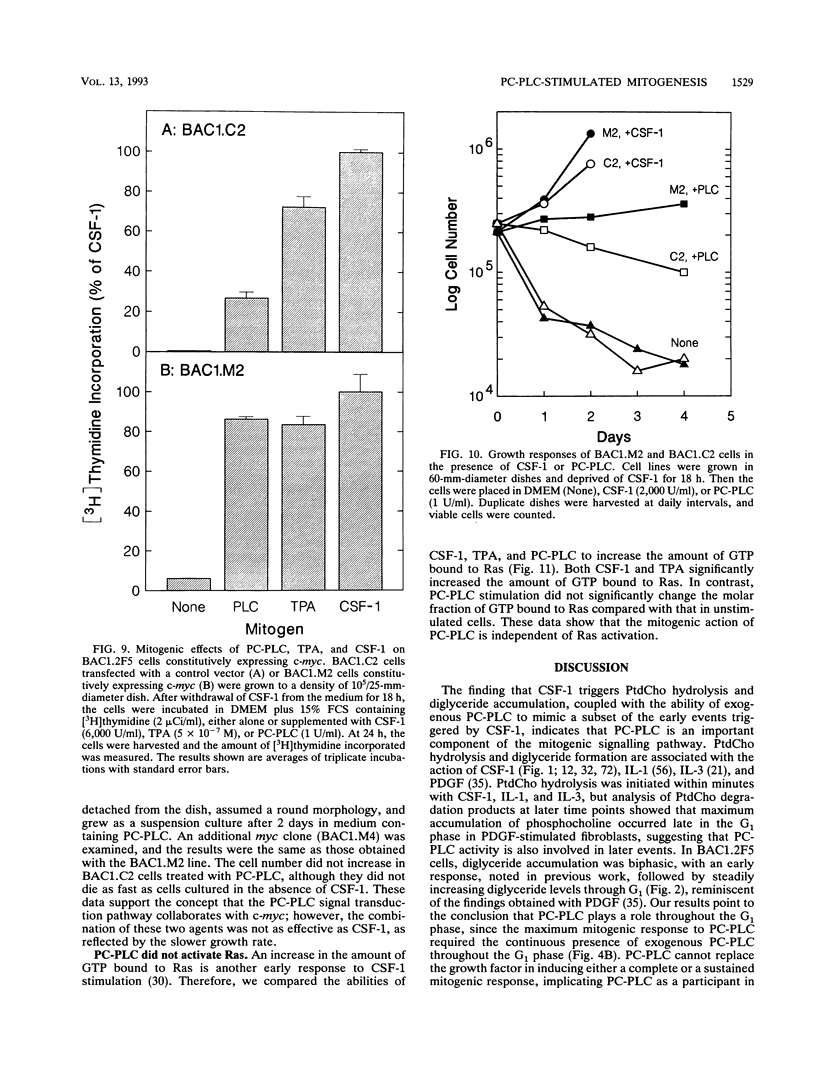
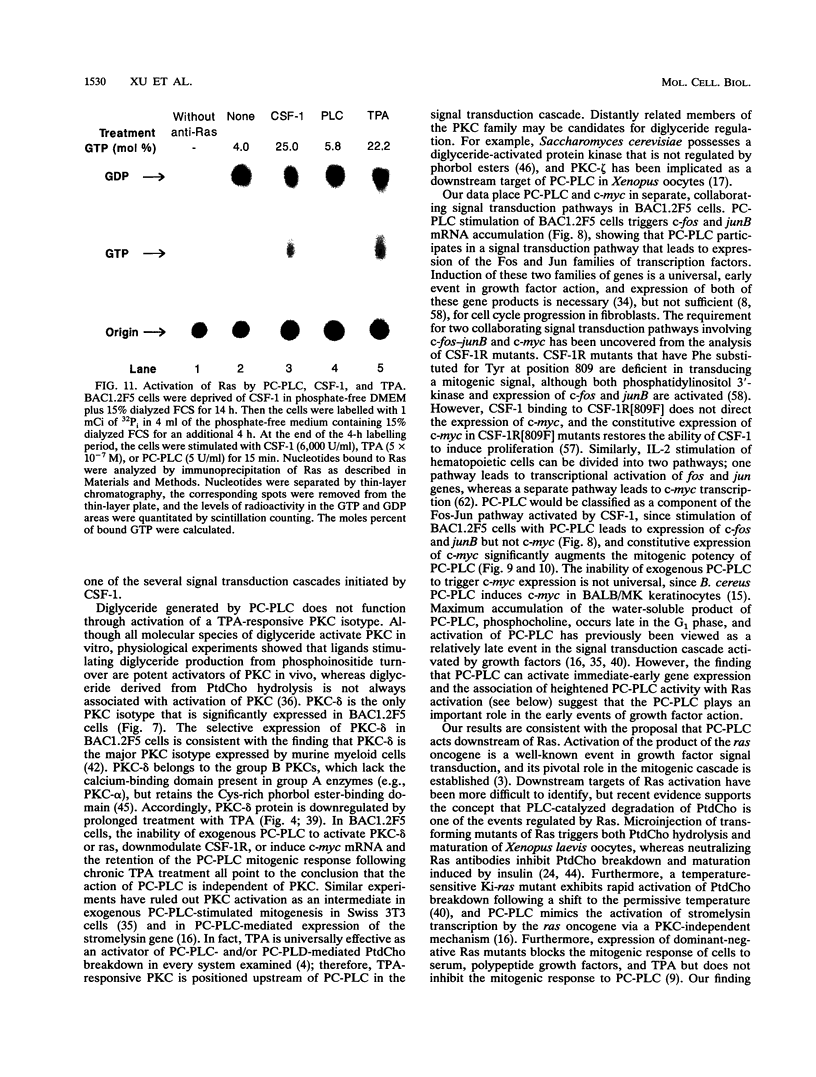
Stimulation of diglyceride production via phospholipase C (PLC) hydrolysis of phosphatidylcholine was an early event in the mitogenic action of colony-stimulating factor 1 (CSF-1) in the murine macrophage cell line BAC1.2F5 and was followed by a second phase of diglyceride production that persisted throughout the G1 phase of the cell cycle. Addition of phosphatidylcholine-specific PLC (PC-PLC) from Bacillus cereus to the medium of quiescent cells raised the intracellular diglyceride concentration and stimulated [3H]thymidine incorporation, although PC-PLC did not support continuous proliferation. PC-PLC treatment did not induce tyrosine phosphorylation or turnover of the CSF-1 receptor. The major protein kinase C (PKC) isotype in BAC1.2F5 cells was PKC-delta. Diglyceride production from PC-PLC did not target PKC-delta, since unlike phorbol esters, PC-PLC treatment neither decreased the electrophoretic mobility of PKC-delta nor increased the amount of GTP bound to Ras, and PC-PLC was mitogenically active in BAC1.2F5 cells in which PKC-delta was downregulated by prolonged treatment with phorbol ester. PC-PLC mimicked CSF-1 action by elevating c-fos and junB mRNAs to 40% of the level induced by CSF-1; however, PC-PLC induced c-myc mRNA to only 5% of the level in CSF-1-stimulated cells. PC-PLC addition to CSF-1-dependent BAC1.2F5 clones that constitutively express c-myc increased [3H]thymidine incorporation to 86% of the level evoked by CSF-1 and supported slow growth in the absence of CSF-1. Therefore, PC-PLC is a component of a signal transduction pathway leading to transcription of c-fos and junB that collaborates with c-myc and is independent of PKC-delta and Ras activation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Billah M. M., Anthes J. C. The regulation and cellular functions of phosphatidylcholine hydrolysis. Biochem J. 1990 Jul 15;269(2):281–291. doi: 10.1042/bj2690281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989 Oct 15;264(29):17069–17077. [PubMed] [Google Scholar]
- Bishop W. R., Bell R. M. Functions of diacylglycerol in glycerolipid metabolism, signal transduction and cellular transformation. Oncogene Res. 1988 Feb;2(3):205–218. [PubMed] [Google Scholar]
- Bravo R., Neuberg M., Burckhardt J., Almendral J., Wallich R., Müller R. Involvement of common and cell type-specific pathways in c-fos gene control: stable induction of cAMP in macrophages. Cell. 1987 Jan 30;48(2):251–260. doi: 10.1016/0092-8674(87)90428-4. [DOI] [PubMed] [Google Scholar]
- Cai H., Erhardt P., Szeberényi J., Diaz-Meco M. T., Johansen T., Moscat J., Cooper G. M. Hydrolysis of phosphatidylcholine is stimulated by Ras proteins during mitogenic signal transduction. Mol Cell Biol. 1992 Dec;12(12):5329–5335. doi: 10.1128/mcb.12.12.5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Choudhury G. G., Sylvia V. L., Sakaguchi A. Y. Activation of a phosphatidylcholine-specific phospholipase C by colony stimulating factor 1 receptor requires tyrosine phosphorylation and a guanine nucleotide-binding protein. J Biol Chem. 1991 Dec 5;266(34):23147–23151. [PubMed] [Google Scholar]
- Choudhury G. G., Sylvia V. L., Wang L. M., Pierce J., Sakaguchi A. Y. The kinase insert domain of colony stimulating factor-1 receptor is dispensable for CSF-1 induced phosphatidylcholine hydrolysis. FEBS Lett. 1991 May 6;282(2):351–354. doi: 10.1016/0014-5793(91)80511-z. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Dean P. N. A simplified method of DNA distribution analysis. Cell Tissue Kinet. 1980 May;13(3):299–308. doi: 10.1111/j.1365-2184.1980.tb00468.x. [DOI] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Dominguez I., Sanz L., Municio M. M., Berra E., Cornet M. E., Garcia de Herreros A., Johansen T., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is a target of transforming growth factor beta 1 inhibitory signals. Mol Cell Biol. 1992 Jan;12(1):302–308. doi: 10.1128/mcb.12.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Meco M. T., Quiñones S., Municio M. M., Sanz L., Bernal D., Cabrero E., Saus J., Moscat J. Protein kinase C-independent expression of stromelysin by platelet-derived growth factor, ras oncogene, and phosphatidylcholine-hydrolyzing phospholipase C. J Biol Chem. 1991 Nov 25;266(33):22597–22602. [PubMed] [Google Scholar]
- Dominguez I., Diaz-Meco M. T., Municio M. M., Berra E., García de Herreros A., Cornet M. E., Sanz L., Moscat J. Evidence for a role of protein kinase C zeta subspecies in maturation of Xenopus laevis oocytes. Mol Cell Biol. 1992 Sep;12(9):3776–3783. doi: 10.1128/mcb.12.9.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Margolis B. L., Zilberstein A., Ashmun R. A., Ullrich A., Sherr C. J., Schlessinger J. Phospholipase C-gamma, a substrate for PDGF receptor kinase, is not phosphorylated on tyrosine during the mitogenic response to CSF-1. EMBO J. 1989 Nov;8(11):3345–3350. doi: 10.1002/j.1460-2075.1989.tb08496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Roussel M. F., Sherr C. J. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol Cell Biol. 1989 Jul;9(7):2890–2896. doi: 10.1128/mcb.9.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio V., Nip L., Pelech S. L. Interleukin 3 stimulates phosphatidylcholine turnover in a mast/megakaryocyte cell line. Biochem Biophys Res Commun. 1989 Oct 31;164(2):804–808. doi: 10.1016/0006-291x(89)91530-1. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Florin-Christensen J., Florin-Christensen M., Delfino J. M., Stegmann T., Rasmussen H. Metabolic fate of plasma membrane diacylglycerols in NIH 3T3 fibroblasts. J Biol Chem. 1992 Jul 25;267(21):14783–14789. [PubMed] [Google Scholar]
- García de Herreros A., Dominguez I., Diaz-Meco M. T., Graziani G., Cornett M. E., Guddal P. H., Johansen T., Moscat J. Requirement of phospholipase C-catalyzed hydrolysis of phosphatidylcholine for maturation of Xenopus laevis oocytes in response to insulin and ras p21. J Biol Chem. 1991 Apr 15;266(11):6825–6829. [PubMed] [Google Scholar]
- Gibbs J. B., Schaber M. D., Marshall M. S., Scolnick E. M., Sigal I. S. Identification of guanine nucleotides bound to ras-encoded proteins in growing yeast cells. J Biol Chem. 1987 Aug 5;262(22):10426–10429. [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Hallet M. M., Praloran V., Vié H., Peyrat M. A., Wong G., Witek-Giannotti J., Soulillou J. P., Moreau J. F. Macrophage colony-stimulating factor (CSF-1) gene expression in human T-lymphocyte clones. Blood. 1991 Feb 15;77(4):780–786. [PubMed] [Google Scholar]
- Hamilton J. A., Veis N., Bordun A. M., Vairo G., Gonda T. J., Phillips W. A. Activation and proliferation signals in murine macrophages: relationships among c-fos and c-myc expression, phosphoinositide hydrolysis, superoxide formation, and DNA synthesis. J Cell Physiol. 1989 Dec;141(3):618–626. doi: 10.1002/jcp.1041410321. [DOI] [PubMed] [Google Scholar]
- Haynes J. R., Downing J. R. A recessive cellular mutation in v-fes-transformed mink cells restores contact inhibition and anchorage-dependent growth. Mol Cell Biol. 1988 Jun;8(6):2419–2427. doi: 10.1128/mcb.8.6.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi J., Warren M. K., Ralph P., Kufe D. Expression of the macrophage specific colony-stimulating factor (CSF-1) during human monocytic differentiation. Biochem Biophys Res Commun. 1986 Dec 30;141(3):924–930. doi: 10.1016/s0006-291x(86)80131-0. [DOI] [PubMed] [Google Scholar]
- Imamura K., Dianoux A., Nakamura T., Kufe D. Colony-stimulating factor 1 activates protein kinase C in human monocytes. EMBO J. 1990 Aug;9(8):2423-8, 2389. doi: 10.1002/j.1460-2075.1990.tb07418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner S. B., Reynolds A. B., Vines R. R., Parsons J. T. Monoclonal antibodies to individual tyrosine-phosphorylated protein substrates of oncogene-encoded tyrosine kinases. Proc Natl Acad Sci U S A. 1990 May;87(9):3328–3332. doi: 10.1073/pnas.87.9.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovary K., Bravo R. The jun and fos protein families are both required for cell cycle progression in fibroblasts. Mol Cell Biol. 1991 Sep;11(9):4466–4472. doi: 10.1128/mcb.11.9.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larrodera P., Cornet M. E., Diaz-Meco M. T., Lopez-Barahona M., Diaz-Laviada I., Guddal P. H., Johansen T., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is an important step in PDGF-stimulated DNA synthesis. Cell. 1990 Jun 15;61(6):1113–1120. doi: 10.1016/0092-8674(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Ruff V. A., Wright T. M., Pessin M. S., Raben D. M. Dissociation of protein kinase C activation and sn-1,2-diacylglycerol formation. Comparison of phosphatidylinositol- and phosphatidylcholine-derived diglycerides in alpha-thrombin-stimulated fibroblasts. J Biol Chem. 1991 Feb 15;266(5):3215–3221. [PubMed] [Google Scholar]
- Lee A. W., Nienhuis A. W. Mechanism of kinase activation in the receptor for colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7270–7274. doi: 10.1073/pnas.87.18.7270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Stanley E. R. Role of dimerization and modification of the CSF-1 receptor in its activation and internalization during the CSF-1 response. EMBO J. 1991 Feb;10(2):277–288. doi: 10.1002/j.1460-2075.1991.tb07948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liyanage M., Frith D., Livneh E., Stabel S. Protein kinase C group B members PKC-delta, -epsilon, -zeta and PKC-L(eta). Comparison of properties of recombinant proteins in vitro and in vivo. Biochem J. 1992 May 1;283(Pt 3):781–787. doi: 10.1042/bj2830781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barahona M., Kaplan P. L., Cornet M. E., Diaz-Meco M. T., Larrodera P., Diaz-Laviada I., Municio A. M., Moscat J. Kinetic evidence of a rapid activation of phosphatidylcholine hydrolysis by Ki-ras oncogene. Possible involvement in late steps of the mitogenic cascade. J Biol Chem. 1990 Jun 5;265(16):9022–9026. [PubMed] [Google Scholar]
- Majerus P. W., Ross T. S., Cunningham T. W., Caldwell K. K., Jefferson A. B., Bansal V. S. Recent insights in phosphatidylinositol signaling. Cell. 1990 Nov 2;63(3):459–465. doi: 10.1016/0092-8674(90)90442-h. [DOI] [PubMed] [Google Scholar]
- Mischak H., Bodenteich A., Kolch W., Goodnight J., Hofer F., Mushinski J. F. Mouse protein kinase C-delta, the major isoform expressed in mouse hemopoietic cells: sequence of the cDNA, expression patterns, and characterization of the protein. Biochemistry. 1991 Aug 13;30(32):7925–7931. doi: 10.1021/bi00246a008. [DOI] [PubMed] [Google Scholar]
- Morgan C., Pollard J. W., Stanley E. R. Isolation and characterization of a cloned growth factor dependent macrophage cell line, BAC1.2F5. J Cell Physiol. 1987 Mar;130(3):420–427. doi: 10.1002/jcp.1041300316. [DOI] [PubMed] [Google Scholar]
- Moscat J., Cornet M. E., Diaz-Meco M. T., Larrodera P., Lopez-Alanon D., Lopez-Barahona M. Activation of phosphatidylcholine-specific phospholipase C in cell growth and oncogene transformation. Biochem Soc Trans. 1989 Dec;17(6):988–991. doi: 10.1042/bst0170988. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Ogita K., Miyamoto S., Koide H., Iwai T., Oka M., Ando K., Kishimoto A., Ikeda K., Fukami Y., Nishizuka Y. Protein kinase C in Saccharomyces cerevisiae: comparison with the mammalian enzyme. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5011–5015. doi: 10.1073/pnas.87.13.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogita K., Miyamoto S., Yamaguchi K., Koide H., Fujisawa N., Kikkawa U., Sahara S., Fukami Y., Nishizuka Y. Isolation and characterization of delta-subspecies of protein kinase C from rat brain. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1592–1596. doi: 10.1073/pnas.89.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka M., Roussel M. F., Sherr C. J., Downing J. R. Ligand-induced phosphorylation of the colony-stimulating factor 1 receptor can occur through an intermolecular reaction that triggers receptor down modulation. Mol Cell Biol. 1990 Apr;10(4):1664–1671. doi: 10.1128/mcb.10.4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlofsky A., Stanley E. R. CSF-1-induced gene expression in macrophages: dissociation from the mitogenic response. EMBO J. 1987 Oct;6(10):2947–2952. doi: 10.1002/j.1460-2075.1987.tb02599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D. K., Hand W. L., Edmondson D. E., Lambeth J. D. Role of phospholipase D-derived diradylglycerol in the activation of the human neutrophil respiratory burst oxidase. Inhibition by phosphatidic acid phosphohydrolase inhibitors. J Immunol. 1992 Oct 15;149(8):2749–2758. [PubMed] [Google Scholar]
- Pollard J. W., Morgan C. J., Dello Sbarba P., Cheers C., Stanley E. R. Independently arising macrophage mutants dissociate growth factor-regulated survival and proliferation. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1474–1478. doi: 10.1073/pnas.88.4.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M., Liu X. Q., Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990 Nov;10(11):5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M., Liu X., van der Geer P., Letwin K., Waterfield M. D., Hunter T., Pawson T. Tyr721 regulates specific binding of the CSF-1 receptor kinase insert to PI 3'-kinase SH2 domains: a model for SH2-mediated receptor-target interactions. EMBO J. 1992 Apr;11(4):1365–1372. doi: 10.1002/j.1460-2075.1992.tb05181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Chen J. H., Roussel M. F., Sherr C. J. The product of the c-fms proto-oncogene: a glycoprotein with associated tyrosine kinase activity. Science. 1985 Apr 19;228(4697):320–322. doi: 10.1126/science.2580348. [DOI] [PubMed] [Google Scholar]
- Rock C. O., Cleveland J. L., Jackowski S. Macrophage growth arrest by cyclic AMP defines a distinct checkpoint in the mid-G1 stage of the cell cycle and overrides constitutive c-myc expression. Mol Cell Biol. 1992 May;12(5):2351–2358. doi: 10.1128/mcb.12.5.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosoff P. M., Savage N., Dinarello C. A. Interleukin-1 stimulates diacylglycerol production in T lymphocytes by a novel mechanism. Cell. 1988 Jul 1;54(1):73–81. doi: 10.1016/0092-8674(88)90181-x. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Cleveland J. L., Shurtleff S. A., Sherr C. J. Myc rescue of a mutant CSF-1 receptor impaired in mitogenic signalling. Nature. 1991 Sep 26;353(6342):361–363. doi: 10.1038/353361a0. [DOI] [PubMed] [Google Scholar]
- Roussel M. F., Shurtleff S. A., Downing J. R., Sherr C. J. A point mutation at tyrosine-809 in the human colony-stimulating factor 1 receptor impairs mitogenesis without abrogating tyrosine kinase activity, association with phosphatidylinositol 3-kinase, or induction of c-fos and junB genes. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6738–6742. doi: 10.1073/pnas.87.17.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbaum S., Halpern R., Diamond B. The generation of macrophage-like cell lines by transfection with SV40 origin defective DNA. J Immunol. 1984 Mar;132(3):1158–1162. [PubMed] [Google Scholar]
- Sherr C. J. Mitogenic response to colony-stimulating factor 1. Trends Genet. 1991 Nov-Dec;7(11-12):398–402. doi: 10.1016/0168-9525(91)90263-p. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shibuya H., Yoneyama M., Ninomiya-Tsuji J., Matsumoto K., Taniguchi T. IL-2 and EGF receptors stimulate the hematopoietic cell cycle via different signaling pathways: demonstration of a novel role for c-myc. Cell. 1992 Jul 10;70(1):57–67. doi: 10.1016/0092-8674(92)90533-i. [DOI] [PubMed] [Google Scholar]
- Shurtleff S. A., Downing J. R., Rock C. O., Hawkins S. A., Roussel M. F., Sherr C. J. Structural features of the colony-stimulating factor 1 receptor that affect its association with phosphatidylinositol 3-kinase. EMBO J. 1990 Aug;9(8):2415–2421. doi: 10.1002/j.1460-2075.1990.tb07417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Stanley E. R. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Taylor G. R., Reedijk M., Rothwell V., Rohrschneider L., Pawson T. The unique insert of cellular and viral fms protein tyrosine kinase domains is dispensable for enzymatic and transforming activities. EMBO J. 1989 Jul;8(7):2029–2037. doi: 10.1002/j.1460-2075.1989.tb03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tushinski R. J., Stanley E. R. The regulation of mononuclear phagocyte entry into S phase by the colony stimulating factor CSF-1. J Cell Physiol. 1985 Feb;122(2):221–228. doi: 10.1002/jcp.1041220210. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Vairo G., Hamilton J. A. Signalling through CSF receptors. Immunol Today. 1991 Oct;12(10):362–369. doi: 10.1016/0167-5699(91)90067-4. [DOI] [PubMed] [Google Scholar]
- Varticovski L., Druker B., Morrison D., Cantley L., Roberts T. The colony stimulating factor-1 receptor associates with and activates phosphatidylinositol-3 kinase. Nature. 1989 Dec 7;342(6250):699–702. doi: 10.1038/342699a0. [DOI] [PubMed] [Google Scholar]
- Veis N., Hamilton J. A. Colony stimulating factor-1 stimulates diacylglycerol generation in murine bone marrow-derived macrophages, but not in resident peritoneal macrophages. J Cell Physiol. 1991 May;147(2):298–305. doi: 10.1002/jcp.1041470215. [DOI] [PubMed] [Google Scholar]
- Veis N., Hamilton J. A. GM-CSF and IL-3 stimulate diacylglycerol generation in murine bone marrow-derived macrophages. Biochem Biophys Res Commun. 1991 Aug 30;179(1):586–591. doi: 10.1016/0006-291x(91)91411-5. [DOI] [PubMed] [Google Scholar]
- Whetton A. D., Monk P. N., Consalvey S. D., Downes C. P. The haemopoietic growth factors interleukin 3 and colony stimulating factor-1 stimulate proliferation but do not induce inositol lipid breakdown in murine bone-marrow-derived macrophages. EMBO J. 1986 Dec 1;5(12):3281–3286. doi: 10.1002/j.1460-2075.1986.tb04640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung Y. G., Jubinsky P. T., Sengupta A., Yeung D. C., Stanley E. R. Purification of the colony-stimulating factor 1 receptor and demonstration of its tyrosine kinase activity. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1268–1271. doi: 10.1073/pnas.84.5.1268. [DOI] [PMC free article] [PubMed] [Google Scholar]