Abstract
Purpose
The aim of this study was to investigate the current status of the use of antiadhesive agents (AAdAs) via a questionnaire and to discuss the availability of AAdAs.
Methods
The survey was sent to a list of members that was approved by the Korean Gastric Association. The survey included questions on AAdA use by surgeons, the type of AAdAs used, and the reasons for not using AAdAs. Surgeons were also asked to describe complications related to AAdAs, and the reliability of its use.
Results
The response rate was 21%. The rates of frequent use stratified by procedure were 26.9% (14/52) for open gastrectomy, 5.9% (3/51) for laparoscopic gastrectomy, and 31.5% (17/54) for surgery for postoperative bowel obstruction (P < 0.01). After including data from the occasional use group, the corresponding values were 51.9% (27/52), 19.6% (10/51), and 70.4% (38/54), respectively (P < 0.01). Sefrafilm and Guardix were most commonly used for open procedures. Guardix and Interceed were most commonly used for laparoscopic surgery. The primary reasons for nonuse of AAdAs were ineffectiveness and high cost. Ten percent (4/40) of surgeons observed complications associated with AAdAs. A minority (17.3%, 9/52) had positive attitudes toward AAdAs. The majority of respondents expressed neutral (73.1%, 38/52) or negative (9.6%, 5/52) attitudes toward AAdAs.
Conclusion
The low use rates of AAdAs in gastric cancer surgery may be attributable to perceptions that AAdAs are ineffective, unreliable, and costly. We anticipate the emergence of promising antiadhesive strategies that reach far beyond the limitations of current products.
Keywords: Peritoneal adhesions, Questionnaires, Adhesion barriers, Postoperative complication, Stomach neoplasms
INTRODUCTION
Postoperative intraabdominal adhesions are pathological bonds, usually formed between the omentum, loops of the bowel, and the abdominal wall. These bonds may be formed by a thin film of connective tissue, a thick fibrous bridge containing blood vessels and nerve tissue, or direct contact between 2 organ surfaces [1]. Adhesions occur in more than 90% of patients following abdomino-pelvic surgery [2,3]. In the United States (US), an epidemiological study estimated adhesion-related health care costs at 1.3 billion US Dollars in 1994 [4]. One study reported that a 130-Euro antiadhesion product with an efficacy rate of 25% could save over 40 million Euro over a 10-year period in the United Kingdom [5]. Peritoneal adhesions are common and costly sources of postoperative morbidity.
There are several classical strategies for adhesion prevention or reduction. These include avoiding the use of powdered gloves, minimizing tissue handling and trauma, using sufficient irrigation, minimizing use of electrocoagulation, performing meticulous hemostasis, using small, biocompatible suture materials, and avoiding desiccation of the tissue [2,6,7]. Most surgeons endeavor to follow these strategies, but clearly, adhesions still occur. Myriad abdominal adhesion prevention strategies (e.g., solid barriers, fluid and gel barriers, cellular strategies, pharmaceuticals) have been developed over the past decades. However, selection of the most appropriate preventive modality remains controversial.
In gastric surgeries, the incidence of bowel obstruction ranges from 11.7% to 38.5% [8,9]. Despite the high risk associated with these obstructions, there have been very few reports of preventive strategies for this complication, especially in the English literature [10,11]. Several preclinical studies of the use of antiadhesive agents (AAdAs) in abdominal surgery have been reported in South Korea [12,13]. However, there is little consensus regarding the use of AAdAs.
The aim of this study was to investigate the current status and controversies associated with the use AAdAs via a questionnaire survey conducted among Korean gastric cancer surgeons.
METHODS
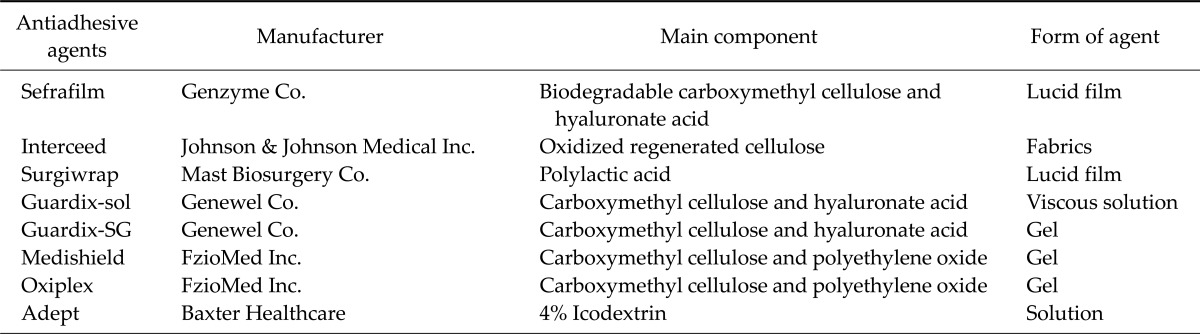
The survey was sent to a list of members of the Korean Gastric Association that was approved by the Korean Gastric Association throughout Korea on November 11, 2011. Responses were received over a period of 2 weeks. The questionnaire as translated into English is shown as Table 1. We obtained the following data using the questionnaire: 1) age of the respondent; 2) annual number of patients who underwent gastrectomy at respondent's hospital; 3) AAdAs use by the respondent; 4) the type of AAdAs used (Sefrafilm, Genzyme Co., Cambridge, MA, USA; Interceed, Johnson & Johnson Medical Inc., Arlington, TX, USA; Surgiwrap, Mast Biosurgery Co., San Diego, CA, USA; Guardix, Genewel Co., Seoul, Korea; Medishield, FzioMed Inc., San Luis Obispo, CA, USA; Oxiplex, FzioMed Inc., San Luis Obispo, CA, USA; Adept, Baxter Healthcare, Utrecht, The Netherlands; others); 5) reasons for not using AAdAs (high cost, ineffectiveness, worries about anastomotic failure, aggravated adhesion, and others); 6) the incidence of complications after application of AAdAs (wound dehiscence, anastomotic failure, aggravation of adhesion); 7) type of AAdAs if complications were noted after use; 8) the attitude toward the idea that AAdAs prevent postoperative bowel obstruction; 9) the location of application (directly below the skin wound, gastrectomy site, others). AAdAs brands included in the questionnaire were those currently on the market in Korea. More detailed information about AAdA types are shown in Table 2. For questions 3) to 5), we classified each answer according to the type of surgical procedures, namely, (1) open radical gastrectomy with standard lymph node dissection for gastric cancer; (2) laparoscopic radical gastrectomy with standard lymph node dissection for gastric cancer; and (3) surgery for postoperative bowel obstruction, including both open and laparoscopic procedures. In addition, the number of respondents for each type of question was different.
Table 1.
Questionnaire survey on antiadhesive agents
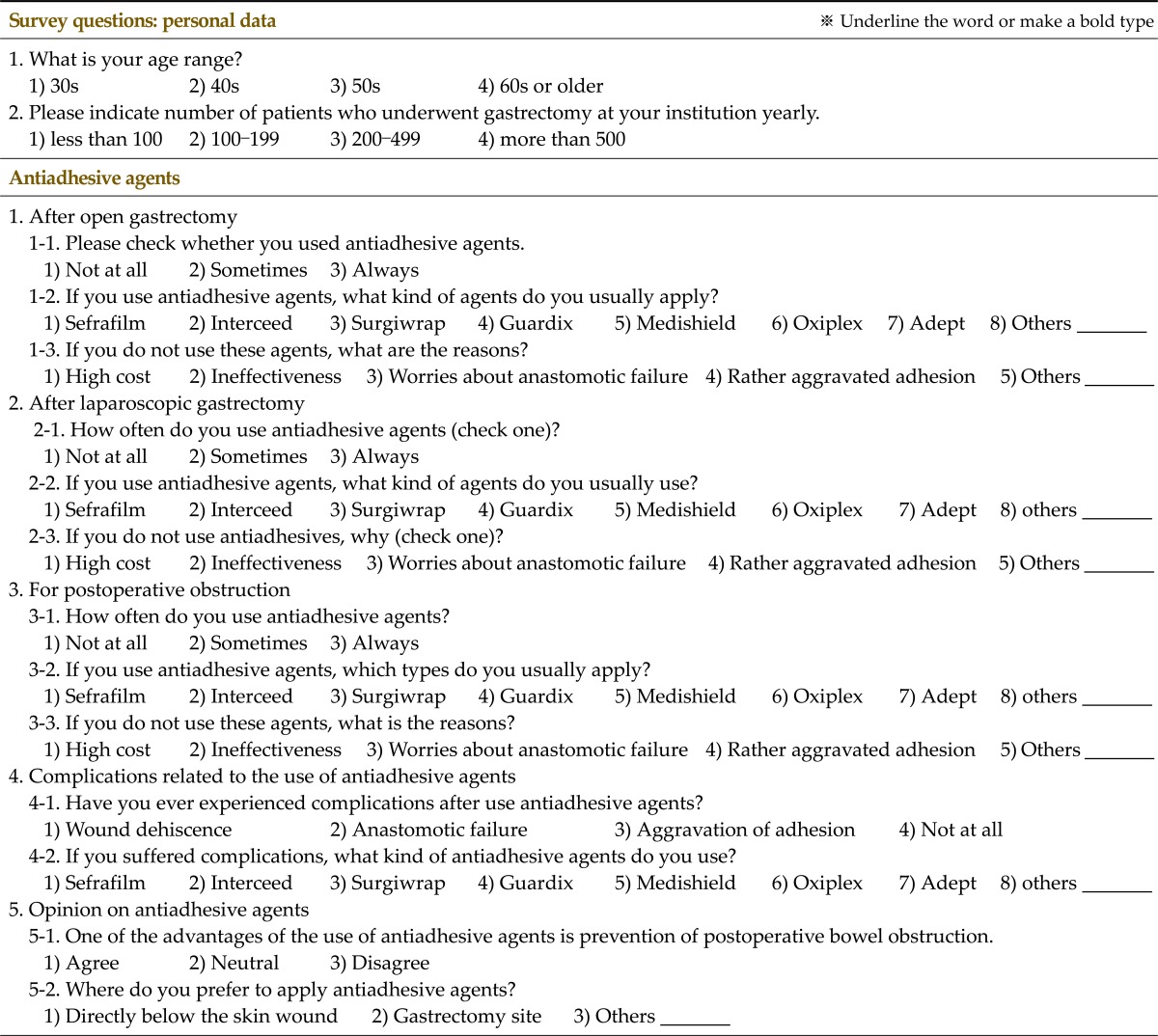
Table 2.
Informations of antiadhesive agents came into the market in Korea
We divided into the respondents into the following 3 groups depending on the frequency of AAdAs application: (1) frequent use group - these respondents almost always used AAdAs; (2) occasional use group - these respondents occasionally used AAdAs; and (3) no use group - these respondents never used AAdAs.
Statistical analyses were performed using PASW ver. 18.0 (IBM Co., Armonk, NY, USA). The significance of differences was determined using the χ2 test. Statistical tests were two-sided. P ≤ 0.05 was considered statistically significant.
RESULTS
Responses were obtained from 63 of 300 (21.0%) members at 35 institutions (36.8%, 35/95). Twenty (31.7%) of the respondents were in their 30s, 24 (38.1%) were in their 40s, 18 (28.6%) were in their 50s, and 1 (2.0%) was in his 60s. The respondents' hospitals were grouped according to the number of patients who underwent gastrectomy for gastric cancer each year at the hospital: 17 (27.0%), more than 500 operations; 27 (42.9%), 200 to 500 operations; 15 (23.8%), 100 to 200 operations; 4 (6.3%), less than 100 operations. The response rates of each grouped hospital according to annual number of gastrectomies were: 44.7% (17/38), more than 500 operations, 8 hospitals; 50% (27/54), 200 to 500 operations, 12 hospitals; 24.2% (15/62), 100 to 200 operations, 11 hospitals; 44.4% (4/9), less than 100 operations, 4 hospitals.
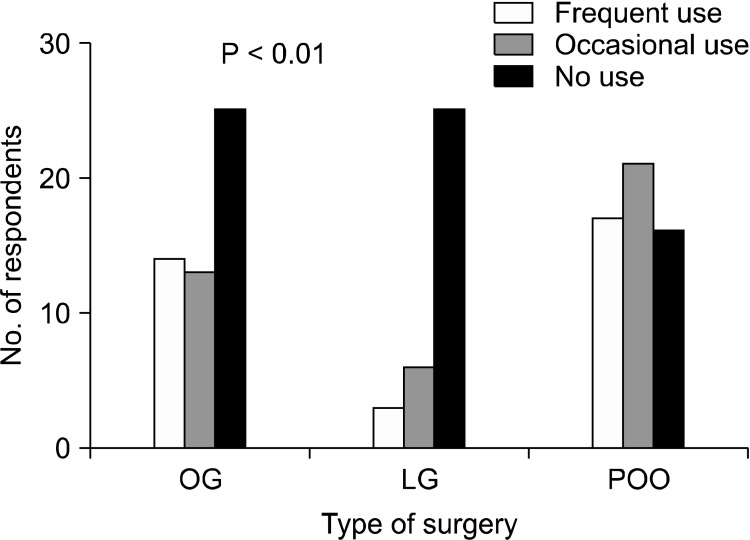
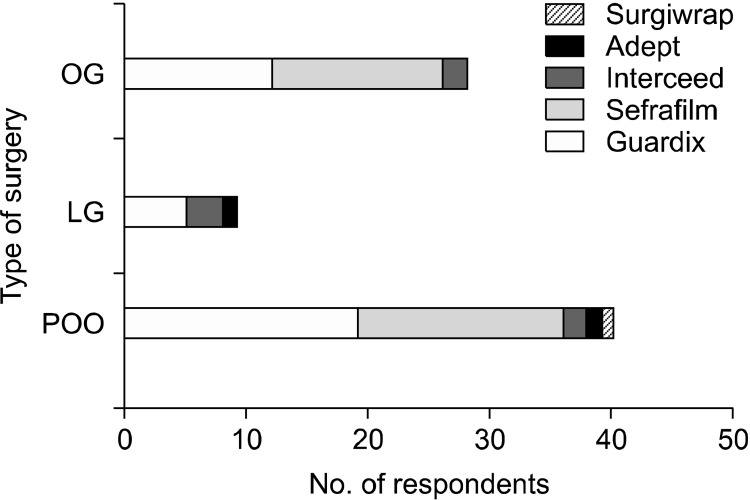
The use and type of AAdAs are shown in Figs. 1, 2. Rates of frequent use group were 26.9% (14/52), 5.9% (3/51), and 31.5% (17/54), respectively (P = 0.005), for open gastrectomy, laparoscopic gastrectomy, and surgery for postoperative bowel obstruction. The rates for these procedures in the use group, including the occasional use group, were 51.9% (27/52), 19.6% (10/51), and 70.4% (38/54) respectively (P < 0.001). The AAdAs used mainly were Sefrafilm and Guardix for open procedures, and Guardix and Interceed for laparoscopic surgery.
Fig. 1.
Whether to use of antiadhesive agents. OG, open gastrectomy; LG, laparoscopic gastrectomy; POO, surgery for postoperative bowel obstruction.
Fig. 2.
Type of applying antiadhesive agents. OG, open gastrectomy; LG, laparoscopic gastrectomy; POO, surgery for postoperative bowel obstruction.
The findings for each procedure are as follows.
Open gastrectomy for gastric cancer
Nearly half of the respondents (48.1%, 25/52) did not use AAdAs. Fourteen surgeons (26.9%) applied them usually, and 13 surgeons used AAdAs (25.0%) occasionally. Among those using AAdAs, 51.9% (14/27) usually applied Sefrafilm, while the others generally used Guardix (44.4%, 12/27) and Interceed (4.0%, 1/27). The main reasons for nonuse were as follows: ineffectiveness (52.0%, 13/25), high cost (40.0%, 10/25), worries about anastomotic failure (4.0%, 1/25), rather aggravated adhesion (4.0%, 1/25), and others (no interest, etc.).
Laparoscopic gastrectomy for gastric cancer
More than three quarters of respondents (78.4%, 40/51) did not apply AAdAs. Three surgeons (5.9%) applied AAdAs usually, and 6 surgeons (11.8%) applied AAdAs occasionally. Among the surgeons using AAdAs, more than half usually applied Guardix (55.6%, 5/9), and the others generally used Interceed (33.3%, 3/9) and Adept (11.1%, 1/9). The reasons for nonuse were as follows: ineffectiveness (50.0%, 20/40), high cost (22.5%, 9/40), worries about anastomotic failure (2.5%, 1/40), rather aggravated adhesion (2.4%, 1/42), and others (difficulty of application, no interest, etc.).
Surgery for postoperative bowel obstruction
More respondents used AAdAs under these circumstances. Thirty-one percent of respondents (17/54) always used AAdAs, 21 surgeons (38.9%) occasionally used them, and 16 surgeons (29.6%) did not use AAdAs at all. Among those using AAdAs, 47.5% of respondents (19/40) usually applied Guardix, others generally used Sefrafilm (42.5%, 17/40), Interceed (5.0%, 2/40), Surgiwrap (2.5%, 1/40), and Adept (2.5%, 1/40). The reasons for nonuse were as follows: ineffectiveness (62.5%, 10/16) and high cost (37.5%, 6/16).
Complication related to use of AAdAs
Ninety percent of respondents (36/40) observed no complications associated with application of AAdAs. Four surgeons (10%) reported postapplication morbidity (deterioration of adhesion, 3; anastomotic failure, 1). The AAdAs used in cases with complications were Sefrafilm (50%, 2/4) and Guardix (50%, 2/4).
Opinion on antiadhesive agents
Only 17.3% (9/52) of respondents expressed a positive attitude toward AAdAs, and 9.6% (5/52) of the respondents expressed a negative attitude. A clear majority of respondents (73.1%, 38/52) had neutral opinions. AAdAs were applied directly below the main wound (73.7%, 28/38), gastrectomy site (23.7%, 9/38), and to other locations (2.6%, 1/38; nearby adhesive band).
DISCUSSION
Gastrectomy for gastric cancer treatment is associated with a high risk of bowel obstruction (incidence, 11.7-8.5%) [8,9]. In one study conducted in a high-volume institute in Korea, intestinal obstruction was the most frequent complication requiring reoperation after gastrectomy for gastric cancer [14]. Furthermore, previous clinical and experimental studies have shown the relationship between peritoneal adhesion and intraperitoneal recurrence [15,16]. Peritoneal recurrence is among the most common patterns of recurrence (29-40%) in gastric cancer [17,18]. Peritoneal healing causes damage to the peritoneum and adhesion formation, which may promote intraperitoneal growth of tumor cells [15]. Prevention of peritoneal adhesion during gastric cancer surgery is an unavoidable issue for gastric cancer surgeons. However, there is little evidence available for guidance in choosing AAdAs, except for the classic surgical strategies for adhesion prevention.
Therefore, we conducted a nationwide survey assessing the utilization of AAdAs among Korean gastric cancer surgeons.
The strategies for adhesion prevention or reduction include the classical surgical principles to reduce surgical trauma and use of AAdAs. AAdAs can be classified into 4 categories: solid barrier, fluid or gel barrier, cellular strategies, and pharmaceuticals [7]. In our, study, we focused on barrier membrane and gel-type AAdAs, which are used primarily in clinical settings in Korea.
Our results indicate that AAdAs were commonly used in 26.9%, 5.9%, and 31.5% of all open gastrectomy, laparoscopic gastrectomy, and surgery for postoperative bowel obstruction procedures, respectively. By including the data from the occasional use group, the rates of use increased to 51.9%, 17.7%, and 70.4% respectively. Considering the surgical and economic significance of adhesion-related complications, these usage rates can be considered to be quite low. In addition, only 17.3% of the respondents reacted positively on being enquired about the effectiveness of AAdAs. The main factors influencing the rates of AAdAs usage were the efficacy of AAdAs and their high price. In addition, some respondents expressed concerns over anastomotic failure, aggravation of adhesions, and difficulty in laparoscopic application of AAdAs, while another small group of respondents believed that postoperative adhesion may have positive physiologic effects.
The preferred AAdAs among Korean gastric cancer surgeons were Seprafilm and Guardix for open gastrectomy, Guardix and Interceed for laparoscopic gastrectomy, and Guardix and Seprafilm for surgery for postoperative bowel obstruction.
Seprafilm is a solid sheet of sodium hyaluronate and sodium carboxymethylcellulose, which is a transparent and resorbable membrane capable of mechanically separating 2 opposite tissue areas over a 7-day period of peritoneal reformation. In many randomized, controlled human trials, Seprafilm was shown to reduce the incidence, severity, and extent of abdominal adhesion [7,19-22]. Furthermore, Seprafilm has the unique distinction of being approved by the US Food and Drug Administration (FDA) as an AAdA for patients undergoing abdominal or pelvic laparotomy [19]. However, there is debate over its tendency to induce inflammatory reaction, the anastomotic instability associated with its use, and its limited laparoscopic applicability [7,23]. Two clinical trials reported the results obtained with Seprafilm after gastrectomy in gastric cancer operation. In one randomized controlled, trial comprising 150 gastric cancer patients, the use of Seprafilm did not significantly reduce the incidence of small bowel obstruction [10]. However, another retrospective study of 282 patients statistically proved the effectiveness of Seprafilm in reducing the incidence of adhesive obstruction after distal gastrectomy [11].
Interceed is a fabric barrier of oxidized regenerated cellulose that typically undergoes biodegradation within 1 to 2 weeks. A number of well-designed studies in humans have indicated the efficacy of Interceed in preventing adhesions [7,24]. One review of 15 randomized, controlled trials in humans showed that Interceed has superior effectiveness to Seprafilm in pelvic surgery [25]. However, Interceed suffers from a number of limitations related to difficulty in handling, susceptibility to infection, ineffectiveness in a blood infiltration environment, mobility in the presence of excess peritoneal fluid, and laparoscopic application. Interceed was approved by the US FDA as an AAdA in only open gynecologic pelvic surgery after meticulous hemostasis is completed [6,7].
Adept, a 4% icodextrin solution, has been approved as a fluid barrier by the US FDA for only laparoscopic gynecological surgery. It shows antiadhesive effects by separating the damaged tissues and allowing prolonged "hydroflotation" of the peritoneal cavity for 3 to 4 days after the operation [7,26]. Although many clinical and experimental studies have validated the antiadhesive effect of Adept, it has clear limitations and is contraindicated for patients with infection or allergy to cornstarch as well as in operations including laparotomy incision, bowel resection, or appendectomy [7].
Guardix is a solution of carboxymethylcellulose and hyaluronic acid, the same ingredients that are used to create Seprafilm, and has been recently developed and found to significantly reduce postoperative adhesions [12,27]. It is cost-effective, since it is developed in South Korea, and offers several clinical advantages such as ease of application in multifocal trauma and suitability in laparoscopic procedures. Although there are several reports describing the clinical efficacy of Guardix, its biggest limitation is the absence of a randomized, controlled trial in humans that definitively validates its use in preventing abdominal adhesions in abdominal surgery. Moreover, it could not gain approval by the US FDA as an AAdA for any abdomino-pelvic surgery, excluding rhinologic surgery. However, it was approved by European Conformity Certification (CE marking) and Korean Food & Drug Administration (KFDA) as an AAdAs for intraabdominal surgery [28,29].
The AAdAs in current surgical use represent solid, fluid, and gel barriers. The effects of AAdAs on prevention of peritoneal adhesion are supported and proved by many evidence-based studies. However, these AAdAs have several limitations and problems involving difficulty in application, susceptibility to infection, laparoscopic application, anastomotic failure, promotion of inflammatory reaction and adhesions, and ineffectiveness in cases showing blood infiltration and intra-abdominal fluid retention. Surgery for gastric cancer is inevitably accompanied with intestinal anastomosis and the risk of bleeding, intraperitoneal fluid collection, and infection. Therefore, we should carefully consider the use of AAdAs introduced. During operation for postoperative bowel obstruction without intestinal resection and anastomosis, application of AAdAs is worth considering. The major AAdAs are Seprafilm, Interceed, Guardix in conventional open surgery and Guardix and Adept in laparoscopic procedures.
This survey-based study had several limitations. The 21% low response rate, small-sized sample, and a questionnaire analysis that was restricted to gastric cancer surgeons would reflect a selection bias. Postoperative adhesions are common problems through all fields of abdominal and pelvic surgery and are associated with major morbidity, mortality, and financial burdens. In a retrospective analysis of 144 cases of small-bowel obstruction from adhesions in the US, the main causative procedures were appendectomy, colorectal resection, and gynecologic procedures. These are responsible for about 60% of all abdomino-pelvic surgeries [30]. Despite these drawbacks, we can also ascertain some advantages. Our survey recruited faculty members of expert groups for gastric cancer surgery. The rate of response from hospitals with more than 100 cases of patients who underwent gastrectomy for gastric cancer was 93.7% (59/63). Moreover, this study was conducted without any financial support.
In conclusion, despite the positive and encouraging implications of numerous clinical and experimental trials for prevention of postoperative adhesion, application rates of AAdAs are still low. Surgeons cite low reliability and high cost to performance ratio in elective gastric cancer surgery as the reasons for this phenomenon. However, the use rate in operations for intestinal obstruction was rather high in comparison to that in gastric cancer operations. We anticipate the emergence of new and promising antiadhesive strategies far beyond the limitations of current products.
Footnotes
This paper was presented orally at the 63rd Annual Congress of the Korean Surgical Society, November 2011.
No potential conflict of interest relevant to this article was reported.
References
- 1.Diamond MP, Freeman ML. Clinical implications of postsurgical adhesions. Hum Reprod Update. 2001;7:567–576. doi: 10.1093/humupd/7.6.567. [DOI] [PubMed] [Google Scholar]
- 2.Liakakos T, Thomakos N, Fine PM, Dervenis C, Young RL. Peritoneal adhesions: etiology, pathophysiology, and clinical significance. Recent advances in prevention and management. Dig Surg. 2001;18:260–273. doi: 10.1159/000050149. [DOI] [PubMed] [Google Scholar]
- 3.Ellis H, Moran BJ, Thompson JN, Parker MC, Wilson MS, Menzies D, et al. Adhesion-related hospital readmissions after abdominal and pelvic surgery: a retrospective cohort study. Lancet. 1999;353:1476–1480. doi: 10.1016/S0140-6736(98)09337-4. [DOI] [PubMed] [Google Scholar]
- 4.Ray NF, Denton WG, Thamer M, Henderson SC, Perry S. Abdominal adhesiolysis: inpatient care and expenditures in the United States in 1994. J Am Coll Surg. 1998;186:1–9. doi: 10.1016/s1072-7515(97)00127-0. [DOI] [PubMed] [Google Scholar]
- 5.Wilson MS. Practicalities and costs of adhesions. Colorectal Dis. 2007;9(Suppl 2):60–65. doi: 10.1111/j.1463-1318.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 6.Johns A. Evidence-based prevention of post-operative adhesions. Hum Reprod Update. 2001;7:577–579. doi: 10.1093/humupd/7.6.577. [DOI] [PubMed] [Google Scholar]
- 7.Ward BC, Panitch A. Abdominal adhesions: current and novel therapies. J Surg Res. 2011;165:91–111. doi: 10.1016/j.jss.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Inaba T, Okinaga K, Fukushima R, Iinuma H, Ogihara T, Ogawa F, et al. Prospective randomized study of two laparotomy incisions for gastrectomy: midline incision versus transverse incision. Gastric Cancer. 2004;7:167–171. doi: 10.1007/s10120-004-0291-6. [DOI] [PubMed] [Google Scholar]
- 9.Korenaga D, Yasuda M, Takesue F, Honda M, Inutsuka S, Nagahama S, et al. Factors influencing the development of small intestinal obstruction following total gastrectomy for gastric cancer: the impact of reconstructive route in the Roux-en-Y procedure. Hepatogastroenterology. 2001;48:1389–1392. [PubMed] [Google Scholar]
- 10.Hayashi S, Takayama T, Masuda H, Kochi M, Ishii Y, Matsuda M, et al. Bioresorbable membrane to reduce postoperative small bowel obstruction in patients with gastric cancer: a randomized clinical trial. Ann Surg. 2008;247:766–770. doi: 10.1097/SLA.0b013e3181656d4e. [DOI] [PubMed] [Google Scholar]
- 11.Kawamura H, Yokota R, Yokota K, Watarai H, Tsunoda Y, Yamagami H, et al. A sodium hyaluronate carboxymethylcellulose bioresorbable membrane prevents postoperative small-bowel adhesive obstruction after distal gastrectomy. Surg Today. 2010;40:223–227. doi: 10.1007/s00595-008-4059-1. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Cha SJ, Kim BG, Choi YS, Kwon GY, Kang H, et al. An assessment of the effects of a hyaluronan-based solution on reduction of postsurgical adhesion formation in rats: a comparative study of hyaluronan-based solution and two film barriers. J Surg Res. 2011;168:49–55. doi: 10.1016/j.jss.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Ahn HS, Lee HJ, Yoo MW, Jeong SH, Han TS, Kim WH, et al. Efficacy of an injectable thermosensitive gel on postoperative adhesion in rat model. J Korean Surg Soc. 2010;79:239–245. [Google Scholar]
- 14.Oh SJ, Choi WB, Song J, Hyung WJ, Choi SH, Noh SH, et al. Complications requiring reoperation after gastrectomy for gastric cancer: 17 years experience in a single institute. J Gastrointest Surg. 2009;13:239–245. doi: 10.1007/s11605-008-0716-3. [DOI] [PubMed] [Google Scholar]
- 15.Van den Tol PM, van Rossen EE, van Eijck CH, Bonthuis F, Marquet RL, Jeekel H. Reduction of peritoneal trauma by using nonsurgical gauze leads to less implantation metastasis of spilled tumor cells. Ann Surg. 1998;227:242–248. doi: 10.1097/00000658-199802000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adams M, Lee TH, Busch MP, Heitman J, Marshall GJ, Gjerset GF, et al. The Transfusion Safety Study Group. Rapid freezing of whole blood or buffy coat samples for polymerase chain reaction and cell culture analysis: application to detection of human immunodeficiency virus in blood donor and recipient repositories. Transfusion. 1993;33:504–508. doi: 10.1046/j.1537-2995.1993.33693296814.x. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz RE, Zagala-Nevarez K. Recurrence patterns after radical gastrectomy for gastric cancer: prognostic factors and implications for postoperative adjuvant therapy. Ann Surg Oncol. 2002;9:394–400. doi: 10.1007/BF02573875. [DOI] [PubMed] [Google Scholar]
- 18.D'Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kling J. Genzyme's Seprafilm gets FDA marketing nod. Nat Biotechnol. 1996;14:572. doi: 10.1038/nbt0596-572a. [DOI] [PubMed] [Google Scholar]
- 20.Diamond MP Seprafilm Adhesion Study Group. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Fertil Steril. 1996;66:904–910. [PubMed] [Google Scholar]
- 21.Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Mater Res. 2002;63:10–14. doi: 10.1002/jbm.10040. [DOI] [PubMed] [Google Scholar]
- 22.Salum M, Wexner SD, Nogueras JJ, Weiss E, Koruda M, Behrens K, et al. Does sodium hyaluronate- and carboxymethylcellulose-based bioresorbable membrane (Seprafilm) decrease operative time for loop ileostomy closure? Tech Coloproctol. 2006;10:187–190. doi: 10.1007/s10151-006-0278-x. [DOI] [PubMed] [Google Scholar]
- 23.Erturk S, Yuceyar S, Temiz M, Ekci B, Sakoglu N, Balci H, et al. Effects of hyaluronic acid-carboxymethylcellulose antiadhesion barrier on ischemic colonic anastomosis: an experimental study. Dis Colon Rectum. 2003;46:529–534. doi: 10.1007/s10350-004-6594-1. [DOI] [PubMed] [Google Scholar]
- 24.Nordic Adhesion Prevention Study Group. The efficacy of Interceed(TC7)* for prevention of reformation of postoperative adhesions on ovaries, fallopian tubes, and fimbriae in microsurgical operations for fertility: a multicenter study. Fertil Steril. 1995;63:709–714. [PubMed] [Google Scholar]
- 25.Farquhar C, Vandekerckhove P, Watson A, Vail A, Wiseman D. Barrier agents for preventing adhesions after surgery for subfertility. Cochrane Database Syst Rev. 2000;(2):CD000475. doi: 10.1002/14651858.CD000475. [DOI] [PubMed] [Google Scholar]
- 26.Verco SJ, Peers EM, Brown CB, Rodgers KE, Roda N, diZerega G. Development of a novel glucose polymer solution (icodextrin) for adhesion prevention: pre-clinical studies. Hum Reprod. 2000;15:1764–1772. doi: 10.1093/humrep/15.8.1764. [DOI] [PubMed] [Google Scholar]
- 27.Kim JH, Lee JH, Yoon JH, Chang JH, Bae JH, Kim KS. Antiadhesive effect of the mixed solution of sodium hyaluronate and sodium carboxymethylcellulose after endoscopic sinus surgery. Am J Rhinol. 2007;21:95–99. doi: 10.2500/ajr.2007.21.2911. [DOI] [PubMed] [Google Scholar]
- 28.EVPU Slovak testing centre SKTC 101. List of issued certificated [Internet] EVPU; c2012. [cited 2012 Aug 22]. Available from: http://www.evpu.sk/sktc/certificates/index.php?language=english. [Google Scholar]
- 29.Korea Food & Drug Administration [Internet] Cheongwon: Korea Food & Drug Administration; c2013. [cited 2012 Aug 22]. Available from: http://www.kfda.go.kr/index.jsp. [Google Scholar]
- 30.Cox MR, Gunn IF, Eastman MC, Hunt RF, Heinz AW. The operative aetiology and types of adhesions causing small bowel obstruction. Aust N Z J Surg. 1993;63:848–852. doi: 10.1111/j.1445-2197.1993.tb00358.x. [DOI] [PubMed] [Google Scholar]