Abstract
The repair of abdominal aortic aneurysm (AAA) in patients with functioning renal transplant is critical because it is important to avoid ischemic and reperfusion injury to the transplanted kidney. Endovascular aneurysm repair (EVAR) avoids aortic cross clamping and can prevent renal graft ischemia. Here we report the endovascular management and outcome of AAA in two renal transplant patients using a bifurcated aortic stent graft. One patient underwent EVAR using a small amount of contrast (30 mL) due to decreased renal function resulting from chronic rejection. Another patient had EVAR performed with iliac conduit because of the heavily calcified, stenotic lesion of external iliac artery. EVAR in patients with a renal transplant is a feasible option without impairing renal arterial flow.
Keywords: Abdominal aortic aneurysm, Kidney transplantation, Endovascular procedures
INTRODUCTION
The presence of an abdominal aortic aneurysm (AAA) in a patient with a functioning renal transplant poses a significant surgical challenge because it is important to avoid ischemic and reperfusion injury to the transplanted kidney. Conventional open repair of AAA in these patients requires ischemia-protecting procedures for transplanted kidney such as a temporary axillo-femoral bypass, aortoiliac shunt, aorto-femoral shunt, atrio-femoral bypass, femorofemoral bypass with extracorporeal circulation, topical cooling, and cold perfusion of the graft [1,2].
Endovascular AAA repair (EVAR) avoids aortic cross clamping and can be an attractive option due to little or no impairment of renal arterial flow. Here we report EVAR in two patients with renal transplant.
CASE REPORT
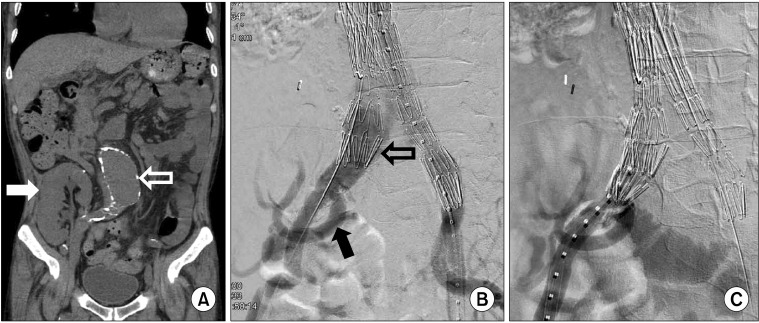
The first patient was a 60-year-old man presenting with an asymptomatic, 6.0 cm expanding infrarenal AAA (Fig. 1A). He underwent a renal transplantation 21 years prior to his current presentation due to renal failure secondary to diabetes and hypertension. The renal artery of the transplanted kidney arose from the right internal iliac artery. His renal function was marginal due to chronic rejection with creatinine and blood urea nitrogen (BUN) values of around 4.0 mg/dL and 50 mg/dL, respectively. His AAA was repaired with a Zenith (Cook Medical Inc., Bloomington, IN, USA) bifurcated stent graft. A 30 mm × 117 mm Zenith main body device was placed through the left common femoral artery with "preclose technique" using 7F Proglide (Abbott Vascular, Redwood City, CA, USA). An 18 mm × 71 mm limb extender was used as the ipsilateral extension. A 24 mm × 71 mm was used as contralateral extension. A type Ib endoleak arising from the right iliac artery was noted on the completion angiogram (Fig. 1B). Another 24 mm × 54 mm limb extender was placed to overcome this type Ib endoleak. There was no endoleak with final angiogram (Fig. 1C). There was no change in renal perfusion after EVAR according to the completion angiogram. A total of 30 mL of Visipaque (iodixanol) was used for this patient. The total fluoroscopic time was 90 minutes. The patient's renal function was slightly decreased after EVAR (creatinine and BUN of 4.1-4.4 mg/dL and 53-58 mg/dL, respectively). The hemodialysis was not needed after EVAR. The aggravation of chronic rejection led to hemodialysis 4 months after EVAR. Over the following 12 months, no types of endoleak were detected as evaluated by serial colorized duplex scan.
Fig. 1.
Patient I: coronal view of preoperative computed tomography (CT) scan and endovascular procedure. (A) Preoperative, nonenhanced CT scan showed the calcified abdominal aortic aneurysm (open arrow) and transplanted kidney in right lower abdomen (white arrow). (B) The type Ib endoleak (open arrow) was seen at the right distal landing zone after placement of stent-graft. The renal artery of transplanted kidney arose from the right internal iliac artery (black arrow). (C) Final angiogram showed the disappearance of endoleak and well perfused transplanted kidney.
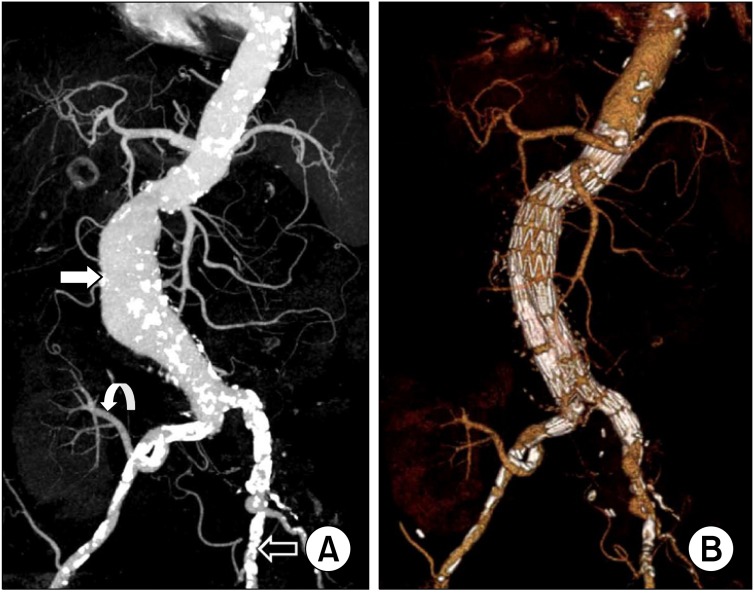
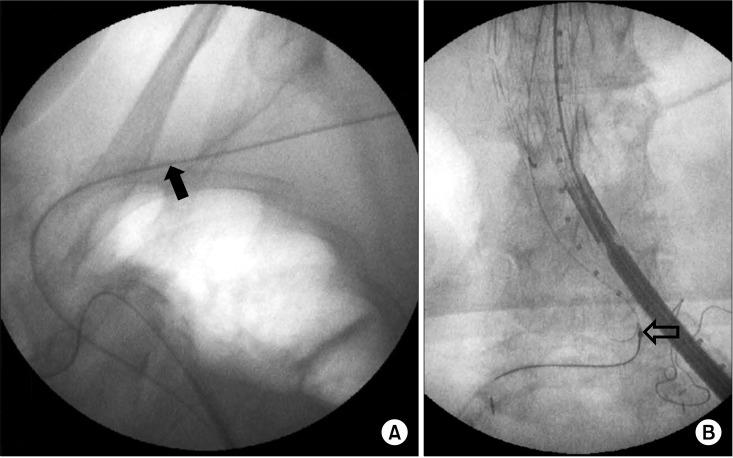
The second patient was a 67-year-old woman presenting with asymptomatic 6.5 cm-sized infrarenal AAA. Nineteen years ago, she underwent a renal transplantation for renal failure secondary to diabetes and hypertension. The transplanted kidney was well functioning after transplantation. At 2 months prior to admission, she underwent ureteroneocystostomy due to recurrent vesicoureteral reflux. Preoperative computed tomographyangiography (CTA) revealed a heavily calcified, stenotic lesion in the left external iliac artery. Fig. 2 shows the maximal intensity projection view of the preoperative CTA and the volume rendering view of the postoperative CTA. Iliac conduit was a possible option to overcome this stenotic lesion. After the creation of the iliac conduit on the left iliac bifurcation using 10 mm Dacron graft (Fig. 3A), her AAA was repaired with an Zenith (Cook Medical Inc.) bifurcated stent graft. A 28 × 117 mm Zenith main body device was placed through the iliac conduit (Fig. 3B). A 14 mm × 71 mm limb extender was used as the ipsilateral extension. Two limb extenders of 14 mm × 71 mm and 12 mm × 54 mm were used as contralateral extension. The guidewire was not passed during the cannulation of contralateral gate due to sharp angulation and heavy calcium on the right common iliac artery (CIA). The guidewire could be passed after snaring the wire, which was inserted though left brachial artery (Fig. 4). The completion angiogram showed a well-perfused kidney. There was no endoleak. A total of 120 mL of Visipaque (iodixanol) was used for this patient. The total fluoroscopic time was 95 minutes. Over the following 20 months, no endoleak was detected as evaluated by serial CT scan.
Fig. 2.
Patient II: preoperative and postoperative computed tomography angiography (CTA). (A) The maximal intensity projection view of preoperative CTA showed the abdominal aortic aneurysm (white arrow), the renal artery of functioning transplanted kidney originated right internal iliac artery (curved arrow), and heavily calcified left external iliac artery (open arrow). (B) The volume rendering view of postoperative CTA showed no endoleak and well perfused transplanted kidney.
Fig. 3.
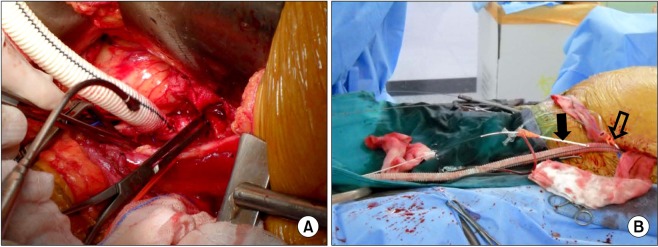
Iliac conduit. (A) The iliac conduit was created on left iliac bifurcation using 10 mm Dacrongraft. (B) There are two introducer sheaths, distal one (black arrow) for angiography and proximal one (open arrow) for the delivery of a main stent-graft.
Fig. 4.
Brachial approach and snaring of the guidewire. (A) There is the angiographic catheter passed through left brachial artery (black arrow). (B) The successful snaring was done for the cannulation of contralateral gate (open arrow).
DISCUSSION
Several case series have shown EVAR is a promising alternative to open surgical treatment [3-6]. We have described endovascular aortic aneurysm repair in 2 patients with transplant kidney and multiple comorbid medical conditions. Conventional open repair can be done safely. Lacombe reported the open repair of aortoiliac aneurysm in 18 patients with kidney transplantation without mortality [7]. During the open repair, however, aortic cross clamping was needed. The arterial occlusion time between aortic clamping and revascularization of the transplant ranged from 25 to 50 minutes (mean, 38 minutes). The maximum increase of blood creatinine value occurred on the third postoperative day even in patients with normal transplant function. Most patients with renal transplantation have heavily calcified aortas and iliac arteries. This feature might be a possible factor for longer clamping time. Longer aortic clamping time led to more decreased renal function.
Comparisons between patients treated with open surgery versus EVAR were performed in terms of renal function [8]. In the EVAR group, the pre- and postoperative creatinine (Cr) was 1.69 and 1.73 mg/dL, respectively, (P = 0.412) without any protective kidney allograft perfusion measures. In the open group, the pre- and postoperative Cr was 1.45 and 1.37 mg/dL, respectively (P = 0.055). In 16%, no adjuvant allograft perfusion was provided. In the remainder, temporary axillofemoral bypasses were used most often. Most outcomes were favorable (57%). No differences in Cr between EVAR and open techniques (P = 0.13) were seen. They concluded that EVAR without special allograft protection measures seems to be equally effective as open surgery with or without adjuvant kidney transplant perfusion. In our series, we performed EVAR without any allograft protection maneuver. The first patient showed decreased renal function 4 months after EVAR. This was related to the aggravation of chronic rejection confirmed by the nephrologist. The second patient showed unchanged renal function.
On preoperative planning for EVAR, special concern is where to put the main body of the device. There are both advantages and disadvantages to stent-graft insertion through the transplant side [6]. Scarring from previous transplant surgery often fixes the artery, prevents straightening of tortuous vessels, and thus may impede insertion of the delivery system. Also, it is crucial that the stent-graft length determinations be very accurate in order to avoid exclusion of the origin of the transplant artery by inadvertent coverage with stent-graft material. Alternatively, insertion of the delivery system through the contralateral iliac system may be easier and safer. In our case, we inserted the main body through the contralateral side. Another concern is the cannulation of contralateral gate of the bifurcated device due to heavy calcium and acute angulation of iliac arteries. If it is difficult to cannulate the contralateral gate, a brachial approach and snaring the wire is a possible option as in our case. The preparation and draping of left arm is needed when difficulty of contralateral cannulation is expected. Another important concern is the possibility of embolization of the transplanted kidney or damage to the iliac artery [9]. Even though all endovascular procedures carry the risks of embolization, special care is needed in the region of the transplant artery origin to prevent dissection during insertion of the guidewire or device. If there is much mural thrombi in the iliac artery or aneurysmal sac, more gentle manipulation should be done. In our opinion, EVAR is indicated only in patients with suitable anatomy of aneurysm for endovascular repair. If the CIA shows circumferential calcium with severe stenosis, extensive mural thrombus, or short working length for endovascular procedure, we recommend conventional open repair.
Iliac access conduits are valuable tools to facilitate endovascular arterial access in clinical situations such as the presence of small and/or diseased (calcified) femoral and external iliac arteries and the need for introduction of a large-bore sheath or catheter ≥size 22F [10]. Standard surgical techniques are used to achieve retroperitoneal exposure of the CIA via a relatively short oblique incision in the lower quadrant of the abdomen. A left-side approach is preferred. A 10-mm diameter Dacron graft is the best conduit as it provides enough luminal space for introduction of all delivery systems. The anastomosis is sewn end-to-side between the graft and the CIA using a running suture technique. In the second patient, the left external iliac artery showed a heavily calcified, stenotic lesion with a diameter less than 5 mm. We overcame this lesion with the creation of a left iliac conduit using 10 mm Dacron graft. Another option is the internal endoconduit, which creates a controlled rupture of the iliac artery and facilitates remote femoral artery access. The variety of available stent-grafts can be used in an off-label fashion to create an endoconduit.
In summary, EVAR in patients with a renal transplant is a feasible option without impairing renal arterial flow.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Karkos CD, McMahon G, Fishwick G, Lambert K, Bagga A, McCarthy MJ. Endovascular abdominal aortic aneurysm repair in the presence of a kidney transplant: therapeutic considerations. Cardiovasc Intervent Radiol. 2006;29:284–288. doi: 10.1007/s00270-005-0043-y. [DOI] [PubMed] [Google Scholar]
- 2.Kim HK, Ryuk JP, Choi HH, Kwon SH, Huh S. Abdominal aortic aneurysm repair in patient with a renal allograft: a case report. J Korean Med Sci. 2009;24:166–169. doi: 10.3346/jkms.2009.24.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Machado R, Almeida P, Loureiro L, Almeida R. Renal transplantation after endovascular aortic aneurysm repair: case report and literature review. Transplant Proc. 2011;43:150–152. doi: 10.1016/j.transproceed.2010.12.038. [DOI] [PubMed] [Google Scholar]
- 4.Hseino H, McGrath F, Hickey D, Hill AD, Moneley D. Endovascular abdominal aortic aneurysm repair in kidney transplant recipients: case series. Surgeon. 2011;9:115–117. doi: 10.1016/j.surge.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Kaskarelis IS, Koukoulaki M, Lappas I, Karkatzia F, Dimopoulos N, Filias V, et al. Successful endovascular repair of ruptured abdominal aortic aneurysm in a renal transplant recipient. Cardiovasc Intervent Radiol. 2006;29:279–283. doi: 10.1007/s00270-004-9205-6. [DOI] [PubMed] [Google Scholar]
- 6.Sawhney R, Chuter TA, Wall SD, Reilly LM, Kerlan RK, Canto CJ, et al. Aortic stent-grafts in patients with renal transplants. J Endovasc Ther. 2000;7:286–291. doi: 10.1177/152660280000700405. [DOI] [PubMed] [Google Scholar]
- 7.Lacombe M. Surgical treatment of aortoiliac aneurysms in renal transplant patients. J Vasc Surg. 2008;48:291–295. doi: 10.1016/j.jvs.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Leon LR, Jr, Glazer ES, Hughes JD, Bui TD, Psalms SB, Goshima KR. Aortoiliac aneurysm repair in kidney transplant recipients. Vasc Endovascular Surg. 2009;43:30–45. doi: 10.1177/1538574408322654. [DOI] [PubMed] [Google Scholar]
- 9.Roach DM, Thompson MM, Patrick GM, Fitridge RA. Aortic aneurysm repair with a functioning renal transplant: therapeutic options. ANZ J Surg. 2004;74:65–67. doi: 10.1046/j.1445-1433.2003.02889.x. [DOI] [PubMed] [Google Scholar]
- 10.Criado FJ. Iliac arterial conduits for endovascular access: technical considerations. J Endovasc Ther. 2007;14:347–351. doi: 10.1583/06-2059.1. [DOI] [PubMed] [Google Scholar]