Abstract
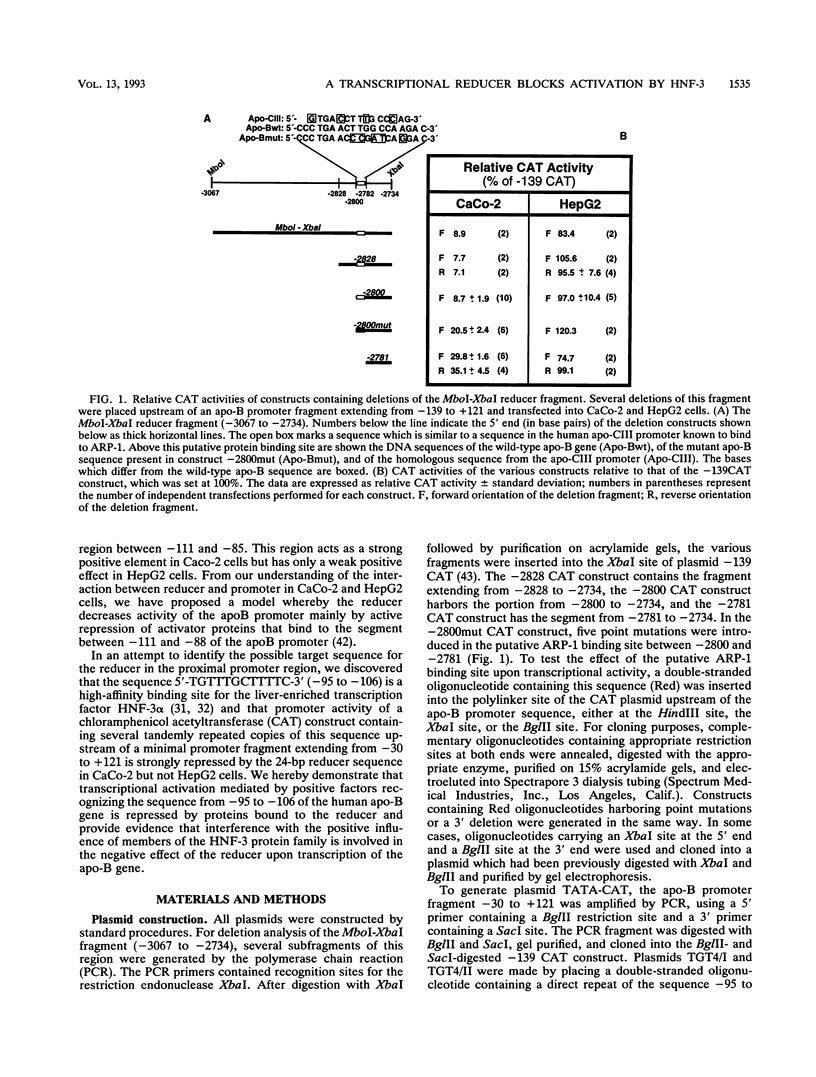
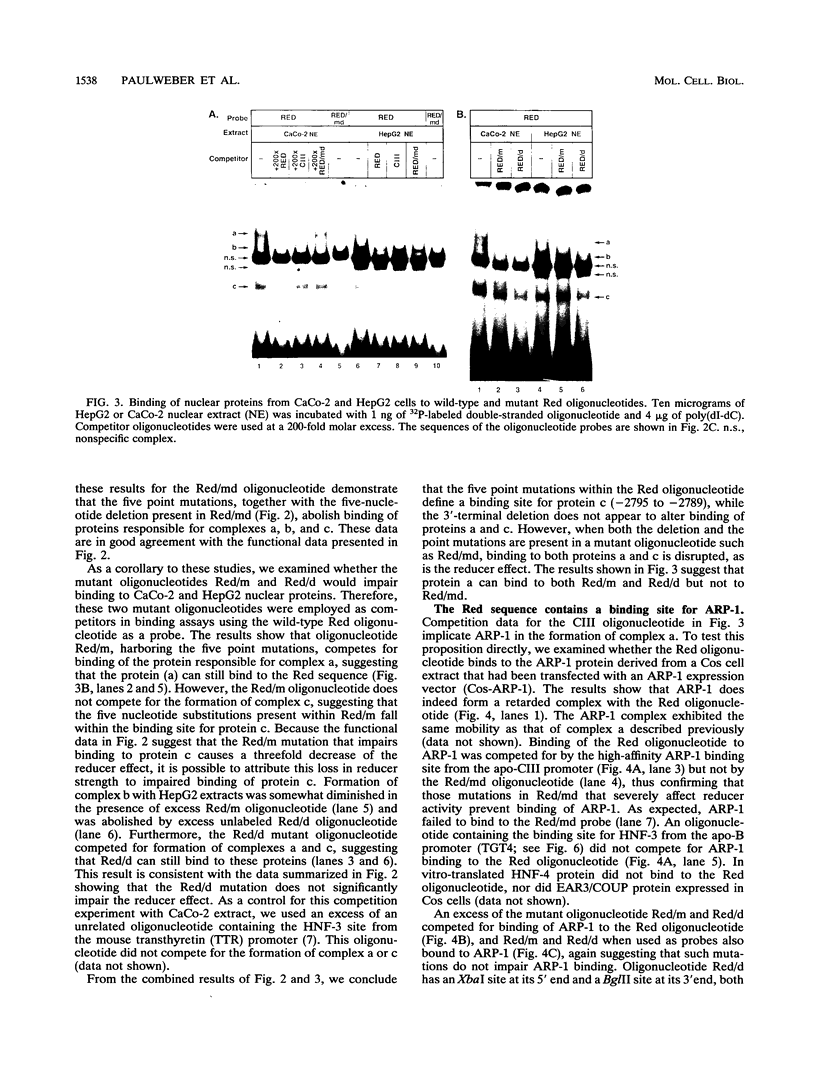
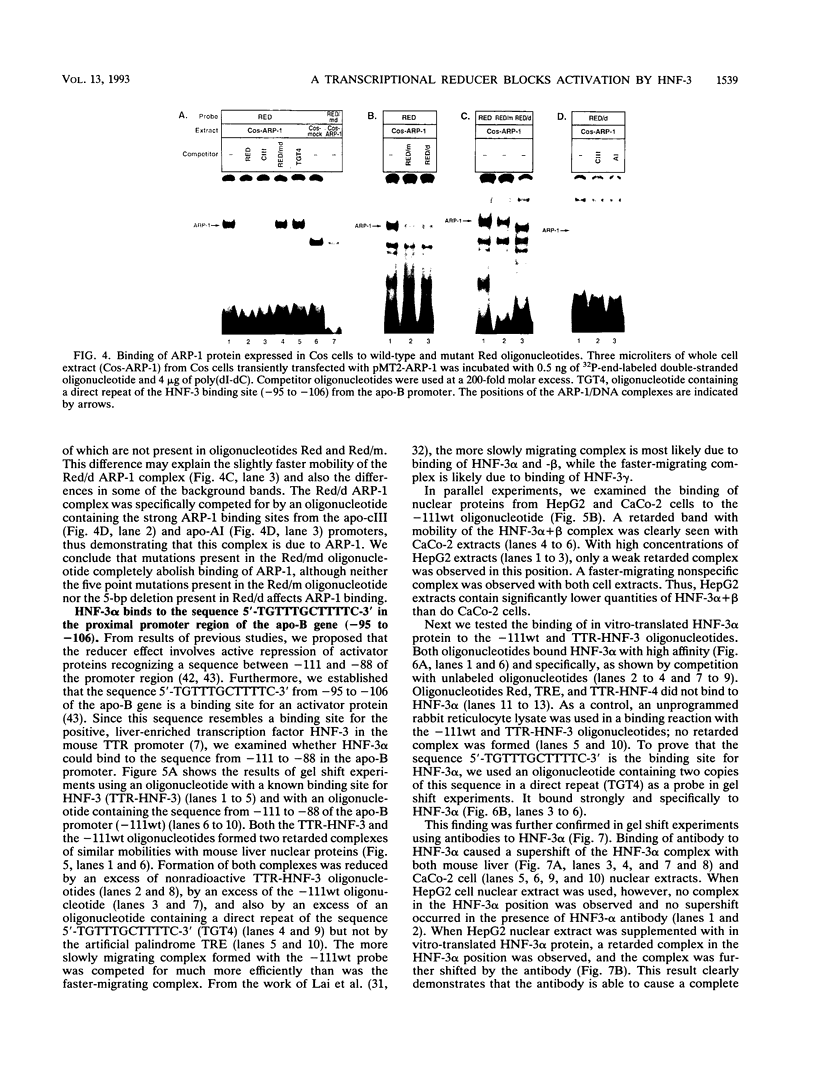
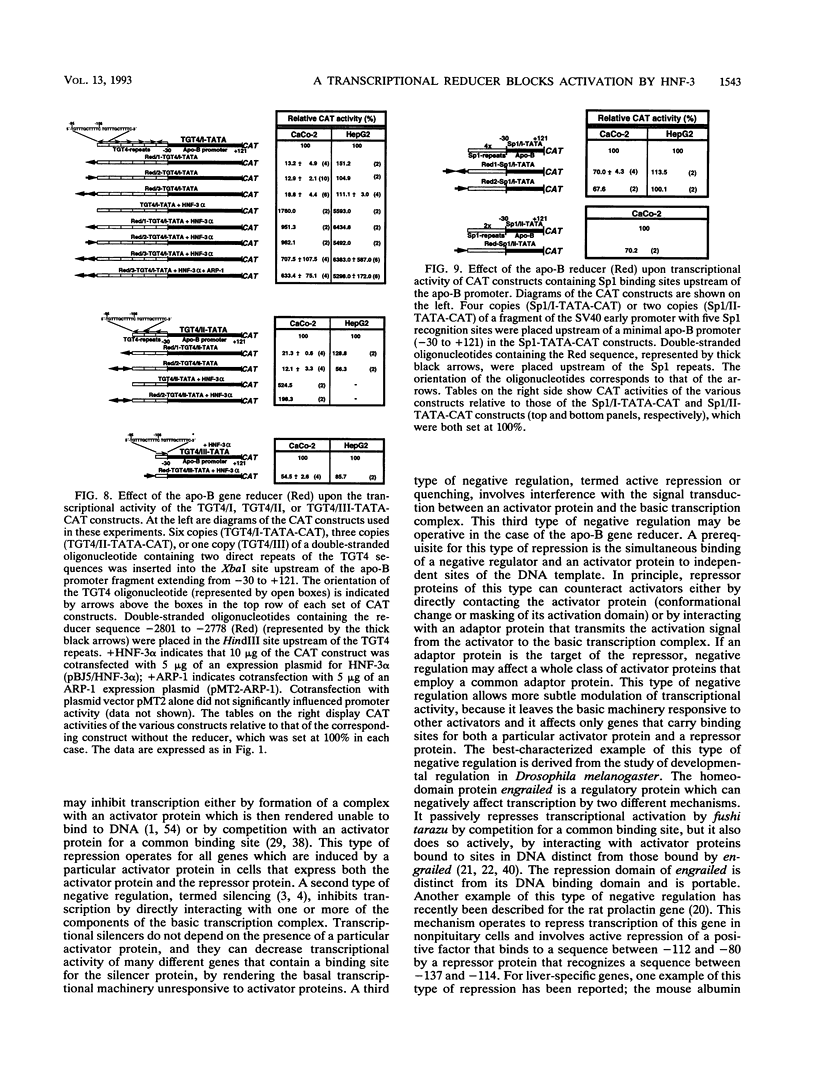
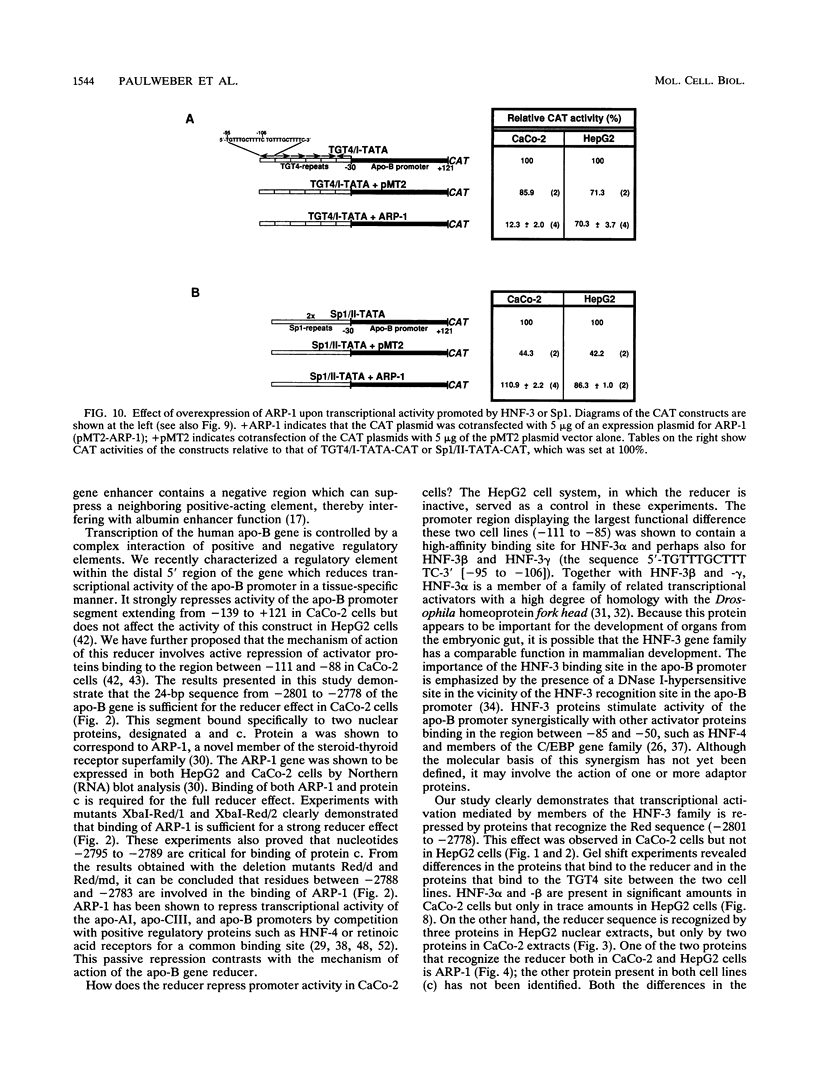
Previously, we showed that when a DNA fragment extending from -3067 to -2734 of the human apolipoprotein B (apo-B) gene is inserted immediately upstream of an apo-B promoter segment (-139 to +121), transcription from this promoter is reduced by about 10-fold in cultured colon carcinoma cells (CaCo-2) but not in cultured hepatoma cells (HepG2). We postulated that this reducer operates by a mechanism involving active repression of a transcriptional activator that binds to the segment from -111 to -88 of the apo-B promoter (B. Paulweber and B. Levy-Wilson, J. Biol. Chem. 266:24161-24168 1991). In the current study, the reducer element has been localized to a 24-bp sequence from -2801 to -2778 of the apo-B gene that contains a binding site for the negative regulatory protein ARP-1. Furthermore, we have demonstrated that the transcription factor hepatocyte nuclear factor 3 alpha (HNF-3 alpha) binds to the sequence 5'-TGTTTGCTTTTC-3' from -95 to -106 of the apo-B promoter, to stimulate transcription. Transcriptional activation by HNF-3 is repressed when the reducer sequence is inserted immediately upstream of the HNF-3 binding site, suggesting a mechanism by which the reducer-bound protein blocks the activation promoted by HNF-3. Data from cotransfection experiments in which ARP-1 is overexpressed in the absence of its binding site suggest that ARP-1 interacts either directly or via a mediator protein with proteins recognizing the HNF-3 site and that this interaction is sufficient to repress transcriptional activation by HNF-3. Because transcriptional activation by Sp1 is not affected by the reducer, it is unlikely that the reducer interacts directly with basic components of the transcriptional machinery.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auwerx J., Sassone-Corsi P. IP-1: a dominant inhibitor of Fos/Jun whose activity is modulated by phosphorylation. Cell. 1991 Mar 8;64(5):983–993. doi: 10.1016/0092-8674(91)90322-p. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Köhne A. C., Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992 Mar;11(3):1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baniahmad A., Steiner C., Köhne A. C., Renkawitz R. Modular structure of a chicken lysozyme silencer: involvement of an unusual thyroid hormone receptor binding site. Cell. 1990 May 4;61(3):505–514. doi: 10.1016/0092-8674(90)90532-j. [DOI] [PubMed] [Google Scholar]
- Brand A. H., Breeden L., Abraham J., Sternglanz R., Nasmyth K. Characterization of a "silencer" in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell. 1985 May;41(1):41–48. doi: 10.1016/0092-8674(85)90059-5. [DOI] [PubMed] [Google Scholar]
- Brooks A. R., Blackhart B. D., Haubold K., Levy-Wilson B. Characterization of tissue-specific enhancer elements in the second intron of the human apolipoprotein B gene. J Biol Chem. 1991 Apr 25;266(12):7848–7859. [PubMed] [Google Scholar]
- Brooks A. R., Levy-Wilson B. Hepatocyte nuclear factor 1 and C/EBP are essential for the activity of the human apolipoprotein B gene second-intron enhancer. Mol Cell Biol. 1992 Mar;12(3):1134–1148. doi: 10.1128/mcb.12.3.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das H. K., Leff T., Breslow J. L. Cell type-specific expression of the human apoB gene is controlled by two cis-acting regulatory regions. J Biol Chem. 1988 Aug 15;263(23):11452–11458. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynlacht B. D., Hoey T., Tjian R. Isolation of coactivators associated with the TATA-binding protein that mediate transcriptional activation. Cell. 1991 Aug 9;66(3):563–576. doi: 10.1016/0092-8674(81)90019-2. [DOI] [PubMed] [Google Scholar]
- Flanagan P. M., Kelleher R. J., 3rd, Sayre M. H., Tschochner H., Kornberg R. D. A mediator required for activation of RNA polymerase II transcription in vitro. Nature. 1991 Apr 4;350(6317):436–438. doi: 10.1038/350436a0. [DOI] [PubMed] [Google Scholar]
- Goodbourn S. Negative regulation of transcriptional initiation in eukaryotes. Biochim Biophys Acta. 1990 Jun 1;1032(1):53–77. doi: 10.1016/0304-419x(90)90012-p. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Herbst R. S., Boczko E. M., Darnell J. E., Jr, Babiss L. E. The mouse albumin enhancer contains a negative regulatory element that interacts with a novel DNA-binding protein. Mol Cell Biol. 1990 Aug;10(8):3896–3905. doi: 10.1128/mcb.10.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson M. E. Negative regulation of eukaryotic transcription. J Cell Sci. 1991 Sep;100(Pt 1):1–7. doi: 10.1242/jcs.100.1.1. [DOI] [PubMed] [Google Scholar]
- Jackson R. L., Morrisett J. D., Gotto A. M., Jr Lipoprotein structure and metabolism. Physiol Rev. 1976 Apr;56(2):259–316. doi: 10.1152/physrev.1976.56.2.259. [DOI] [PubMed] [Google Scholar]
- Jackson S. M., Keech C. A., Williamson D. J., Gutierrez-Hartmann A. Interaction of basal positive and negative transcription elements controls repression of the proximal rat prolactin promoter in nonpituitary cells. Mol Cell Biol. 1992 Jun;12(6):2708–2719. doi: 10.1128/mcb.12.6.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., O'Farrell P. H. Activation and repression of transcription by homoeodomain-containing proteins that bind a common site. Nature. 1988 Dec 22;336(6201):744–749. doi: 10.1038/336744a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaynes J. B., O'Farrell P. H. Active repression of transcription by the engrailed homeodomain protein. EMBO J. 1991 Jun;10(6):1427–1433. doi: 10.1002/j.1460-2075.1991.tb07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Kardassis D., Hadzopoulou-Cladaras M., Ramji D. P., Cortese R., Zannis V. I., Cladaras C. Characterization of the promoter elements required for hepatic and intestinal transcription of the human apoB gene: definition of the DNA-binding site of a tissue-specific transcriptional factor. Mol Cell Biol. 1990 Jun;10(6):2653–2659. doi: 10.1128/mcb.10.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardassis D., Zannis V. I., Cladaras C. Organization of the regulatory elements and nuclear activities participating in the transcription of the human apolipoprotein B gene. J Biol Chem. 1992 Feb 5;267(4):2622–2632. [PubMed] [Google Scholar]
- Kelleher R. J., 3rd, Flanagan P. M., Kornberg R. D. A novel mediator between activator proteins and the RNA polymerase II transcription apparatus. Cell. 1990 Jun 29;61(7):1209–1215. doi: 10.1016/0092-8674(90)90685-8. [DOI] [PubMed] [Google Scholar]
- Knott T. J., Rall S. C., Jr, Innerarity T. L., Jacobson S. F., Urdea M. S., Levy-Wilson B., Powell L. M., Pease R. J., Eddy R., Nakai H. Human apolipoprotein B: structure of carboxyl-terminal domains, sites of gene expression, and chromosomal localization. Science. 1985 Oct 4;230(4721):37–43. doi: 10.1126/science.2994225. [DOI] [PubMed] [Google Scholar]
- Ladias J. A., Hadzopoulou-Cladaras M., Kardassis D., Cardot P., Cheng J., Zannis V., Cladaras C. Transcriptional regulation of human apolipoprotein genes ApoB, ApoCIII, and ApoAII by members of the steroid hormone receptor superfamily HNF-4, ARP-1, EAR-2, and EAR-3. J Biol Chem. 1992 Aug 5;267(22):15849–15860. [PubMed] [Google Scholar]
- Ladias J. A., Karathanasis S. K. Regulation of the apolipoprotein AI gene by ARP-1, a novel member of the steroid receptor superfamily. Science. 1991 Feb 1;251(4993):561–565. doi: 10.1126/science.1899293. [DOI] [PubMed] [Google Scholar]
- Lai E., Prezioso V. R., Smith E., Litvin O., Costa R. H., Darnell J. E., Jr HNF-3A, a hepatocyte-enriched transcription factor of novel structure is regulated transcriptionally. Genes Dev. 1990 Aug;4(8):1427–1436. doi: 10.1101/gad.4.8.1427. [DOI] [PubMed] [Google Scholar]
- Lai E., Prezioso V. R., Tao W. F., Chen W. S., Darnell J. E., Jr Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev. 1991 Mar;5(3):416–427. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Levy-Wilson B., Fortier C., Blackhart B. D., McCarthy B. J. DNase I- and micrococcal nuclease-hypersensitive sites in the human apolipoprotein B gene are tissue specific. Mol Cell Biol. 1988 Jan;8(1):71–80. doi: 10.1128/mcb.8.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Wilson B., Paulweber B., Nagy B. P., Ludwig E. H., Brooks A. R. Nuclease-hypersensitive sites define a region with enhancer activity in the third intron of the human apolipoprotein B gene. J Biol Chem. 1992 Sep 15;267(26):18735–18743. [PubMed] [Google Scholar]
- Meisterernst M., Roy A. L., Lieu H. M., Roeder R. G. Activation of class II gene transcription by regulatory factors is potentiated by a novel activity. Cell. 1991 Sep 6;66(5):981–993. doi: 10.1016/0092-8674(91)90443-3. [DOI] [PubMed] [Google Scholar]
- Metzger S., Leff T., Breslow J. L. Nuclear factors AF-1 and C/EBP bind to the human ApoB gene promoter and modulate its transcriptional activity in hepatic cells. J Biol Chem. 1990 Jun 15;265(17):9978–9983. [PubMed] [Google Scholar]
- Mietus-Snyder M., Sladek F. M., Ginsburg G. S., Kuo C. F., Ladias J. A., Darnell J. E., Jr, Karathanasis S. K. Antagonism between apolipoprotein AI regulatory protein 1, Ear3/COUP-TF, and hepatocyte nuclear factor 4 modulates apolipoprotein CIII gene expression in liver and intestinal cells. Mol Cell Biol. 1992 Apr;12(4):1708–1718. doi: 10.1128/mcb.12.4.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Ohkuma Y., Horikoshi M., Roeder R. G., Desplan C. Binding site-dependent direct activation and repression of in vitro transcription by Drosophila homeodomain proteins. Cell. 1990 May 4;61(3):475–484. doi: 10.1016/0092-8674(90)90529-n. [DOI] [PubMed] [Google Scholar]
- Paulweber B., Brooks A. R., Nagy B. P., Levy-Wilson B. Identification of a negative regulatory region 5' of the human apolipoprotein B promoter. J Biol Chem. 1991 Nov 15;266(32):21956–21961. [PubMed] [Google Scholar]
- Paulweber B., Levy-Wilson B. The mechanisms by which a human apolipoprotein B gene enhancer and reducer interact with the promoter are different in cultured cells of hepatic and intestinal origin. J Biol Chem. 1991 Dec 15;266(35):24161–24168. [PubMed] [Google Scholar]
- Paulweber B., Onasch M. A., Nagy B. P., Levy-Wilson B. Similarities and differences in the function of regulatory elements at the 5' end of the human apolipoprotein B gene in cultured hepatoma (HepG2) and colon carcinoma (CaCo-2) cells. J Biol Chem. 1991 Dec 15;266(35):24149–24160. [PubMed] [Google Scholar]
- Ptashne M., Gann A. A. Activators and targets. Nature. 1990 Jul 26;346(6282):329–331. doi: 10.1038/346329a0. [DOI] [PubMed] [Google Scholar]
- Pugh B. F., Tjian R. Diverse transcriptional functions of the multisubunit eukaryotic TFIID complex. J Biol Chem. 1992 Jan 15;267(2):679–682. [PubMed] [Google Scholar]
- Renkawitz R. Transcriptional repression in eukaryotes. Trends Genet. 1990 Jun;6(6):192–197. doi: 10.1016/0168-9525(90)90176-7. [DOI] [PubMed] [Google Scholar]
- Roeder R. G. The complexities of eukaryotic transcription initiation: regulation of preinitiation complex assembly. Trends Biochem Sci. 1991 Nov;16(11):402–408. doi: 10.1016/0968-0004(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Rottman J. N., Widom R. L., Nadal-Ginard B., Mahdavi V., Karathanasis S. K. A retinoic acid-responsive element in the apolipoprotein AI gene distinguishes between two different retinoic acid response pathways. Mol Cell Biol. 1991 Jul;11(7):3814–3820. doi: 10.1128/mcb.11.7.3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sladek F. M., Zhong W. M., Lai E., Darnell J. E., Jr Liver-enriched transcription factor HNF-4 is a novel member of the steroid hormone receptor superfamily. Genes Dev. 1990 Dec;4(12B):2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- Sniderman A., Shapiro S., Marpole D., Skinner B., Teng B., Kwiterovich P. O., Jr Association of coronary atherosclerosis with hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc Natl Acad Sci U S A. 1980 Jan;77(1):604–608. doi: 10.1073/pnas.77.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. H., Tsai S. Y., Cook R. G., Beattie W. G., Tsai M. J., O'Malley B. W. COUP transcription factor is a member of the steroid receptor superfamily. Nature. 1989 Jul 13;340(6229):163–166. doi: 10.1038/340163a0. [DOI] [PubMed] [Google Scholar]
- Widom R. L., Rhee M., Karathanasis S. K. Repression by ARP-1 sensitizes apolipoprotein AI gene responsiveness to RXR alpha and retinoic acid. Mol Cell Biol. 1992 Aug;12(8):3380–3389. doi: 10.1128/mcb.12.8.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G. Recent progress in understanding apolipoprotein B. Circulation. 1990 Nov;82(5):1574–1594. doi: 10.1161/01.cir.82.5.1574. [DOI] [PubMed] [Google Scholar]
- Zabel U., Baeuerle P. A. Purified human I kappa B can rapidly dissociate the complex of the NF-kappa B transcription factor with its cognate DNA. Cell. 1990 Apr 20;61(2):255–265. doi: 10.1016/0092-8674(90)90806-p. [DOI] [PubMed] [Google Scholar]
- Zawel L., Reinberg D. Advances in RNA polymerase II transcription. Curr Opin Cell Biol. 1992 Jun;4(3):488–495. doi: 10.1016/0955-0674(92)90016-6. [DOI] [PubMed] [Google Scholar]
- Zuo P., Stanojević D., Colgan J., Han K., Levine M., Manley J. L. Activation and repression of transcription by the gap proteins hunchback and Krüppel in cultured Drosophila cells. Genes Dev. 1991 Feb;5(2):254–264. doi: 10.1101/gad.5.2.254. [DOI] [PubMed] [Google Scholar]