Introduction
There exists a widely held view that silymarin (a.k.a. milk thistle) promotes liver health through anti-oxidant, anti-inflammatory, anti-proliferative, and immunomodulatory effects (1). In fact, silymarin is one of the top 10 most popular natural products consumed by western society, and is the most commonly consumed botanical medicine reported in patients with chronic hepatitis C (2, 3). Presently, there is no clear evidence that any of the currently available over-the-counter preparations have efficacy in the treatment of liver disease. While there are compelling in vitro and animal data supporting the hepatoprotective effects of silymarin and inhibition of in vitro HCV infection(4), clinical data are equivocal, with some studies suggesting a protective effect of silymarin against progression of liver disease in subjects with hepatitis C (5), while other studies found no such effect (6, 7). Thus, there is clinical controversy around whether silymarin and silymarin-derived compounds protect the liver. It is the intention of this review article to summarize the current state of knowledge on whether and how silymarin (and the mixture of silymarin components known as silibinin) protects the liver and modulates HCV infection, and to make recommendations for areas of further research.
I- HISTORY
The milk thistle plant originates from the Mediterranean but is now cultivated in Asia and Europe. Silymarin first appears as Silubon in book four of the five volume treatise on medicine known as Περί ύλης ιατρικής (Peri Ylis Ialikis; PYI) or De Materia Medica (On Medical Matters). This compendium from the ancient Greek physician Pedanios Dioskurides (latinized as Pedanius Dioscorides, 20–70 CE) was written around 65CE. While silymarin is sometimes considered part of Traditional Chinese Medicine (TCM), other thistles such as Da Ji (Large Thistle) and Xiao Ji (Thistle Root) are more frequently cited in ancient Chinese medical texts such as Bencao Gangmu (Compendium of Materia Medica). Moreover, Da Ji and Xiao Ji have different chemical compositions than silymarin, which primarily consists of flavonolignans (see below). Silymarin is known as Shui Fei Ji in China, but is not cited in Shen Nong Ben Cao Jing, the earliest Material Medica from China (Jane Saxton, personal communication). Thus, silymarin is derived from ancient European medicinal practices.
Using the PubMed search term “silymarin” returns over 1750 publications, the earliest of which date back to a series of German publications from 1968 that focus on the chemical evaluation and hepatoprotective functions of silymarin (8, 9). In 1969, silymarin was shown to protect against toxic mushroom poisoning (10). In 1975, the first reference to silybin dihemisuccinate was made, as a potential antidote for mushroom poisoning (11). Today, this mixture is licensed in Germany for toxic mushroom poisoning, is undergoing a clinical trial in the USA for mushroom poisoning (NCT00915681), and has been shown to reduce HCV RNA levels in HCV-infected subjects when administered intravenously (12).
In the last 5 years alone, there have been over 700 publications on silymarin indexed on PubMed. The extract and its components display remarkable pleiotropism in biological activities, from growth inhibition of many types of cancer cells (13), to reduction of oxidative stress in multiple cell types including hepatocytes (14), macrophages (15), and neurons (16), to inhibition of many intracellular signal transduction pathways (17, 18). While a plethora of molecular mechanisms have been ascribed to silymarin and its components, no unifying mechanism of action has been forwarded.
II- CLASSIFICATION, CHEMISTRY, NOMENCLATURE, AND COMPOSITION
Classification
Silymarin is an extract from the seeds of the milk thistle plant, Silybum marianum L. Gaertn. It is a member of the Asteraceae, a large and widespread family of Angiosperms that include daisies, asters and sunflowers.
Synonyms for silymarin
The most common name for Silybum marianum is milk thistle or silymarin. However, just like the biological activities ascribed to silymarin, there exist a plethora of names including Bull thistle, cardo blanco, Cardui mariae fructus, Cardui mariae herba, Cardum marianum L., Carduus marianus L., Chardon-Marie, Emetic root, Frauendistel, Fructus Silybi mariae, fruit de chardon Marie, heal thistle, Holy thistle, Kanger, Kocakavkas, kuub, lady's thistle, Marian thistle, mariana mariana, Mariendistel, Marienkrörner, Mary thistle, mild thistle, milk ipecac, pig leaves, royal thistle, S. marianum, St. Mary's thistle, Silybi mariae fructus, snake milk, sow thistle, variegated thistle, Venus thistle, wild artichoke. An excellent resource is the link found at: http://www.naturalstandard.com/monographs/herbssupplements/milkthistle.asp
Chemistry
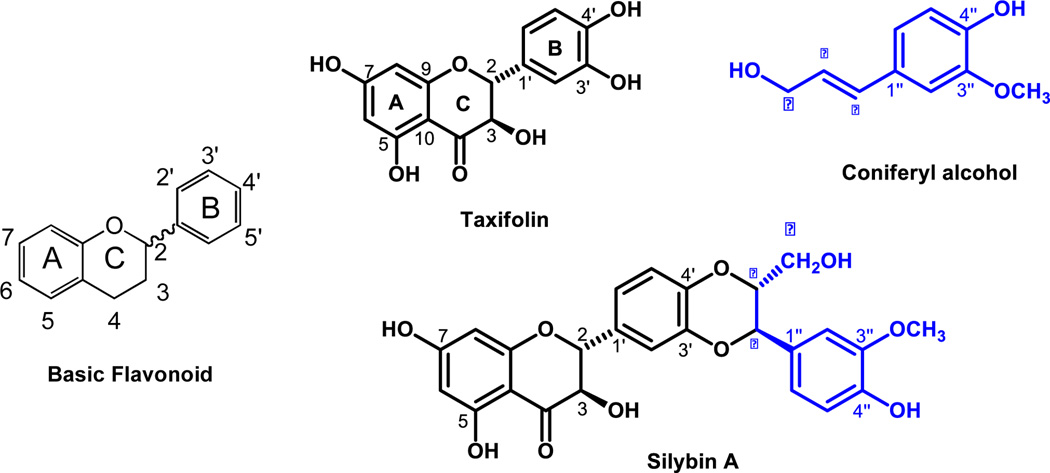
Silymarin, registered on the Chemical Abstracts Service (CAS) number 84604-20-6, is an extract from the seeds of the milk thistle plant. The major bioactive components consist of 7 flavonolignans with the same molecular weight (482) derived from the single flavonoid taxifolin (mw 326). The structure of taxifolin reveals that flavonoids are polyphenolic compounds possessing 15 carbon atoms, with two benzene rings (A and B) joined by a linear three-carbon chain (C) (Figure 1, left panel), which can be abbreviated C6-C3-C6.
Figure 1. Structural basics of silymarin.
Left panel shows the basic structure of a flavonoid. The two benzene rings are designated A and B, while the 3 carbon chain forms ring C. The wavy line that links ring C to ring B indicates the many flavonoids are actually racemic mixtures because the carbon at position 2 on ring C (C2) can be chiral provided there is a single bond between C2 and C3. If there is a double bond between C2 and C3, the flavonoid is not chiral. Right panel shows how Taxifolin (a chiral flavonoid) and coniferyl alcohol condense to form silybin A. The blue color-coding shows how conferyl alcohol is incorporated into the larger flavonolignan molecule.
As shown in Figure 1 (right panel), the condensation between taxifolin and coniferyl alcohol has two possible outcomes, thereby generating two different molecules that differ based on the position of the primary alcohol, which can either extend out of the page, or face into the page. Thus, silybin B has the alcohol protruding from the page and the benzene ring faces into the page, while silybin A has the opposite configuration. Since there are two different stereocenters that differ between silybin A and silybin B, they are called diastereoisomers.
Nomenclature and composition
Since there is inconsistency in the literature with respect to naming silymarin-derived compounds, we adopt the recommendations of Kroll and Oberlies (19):
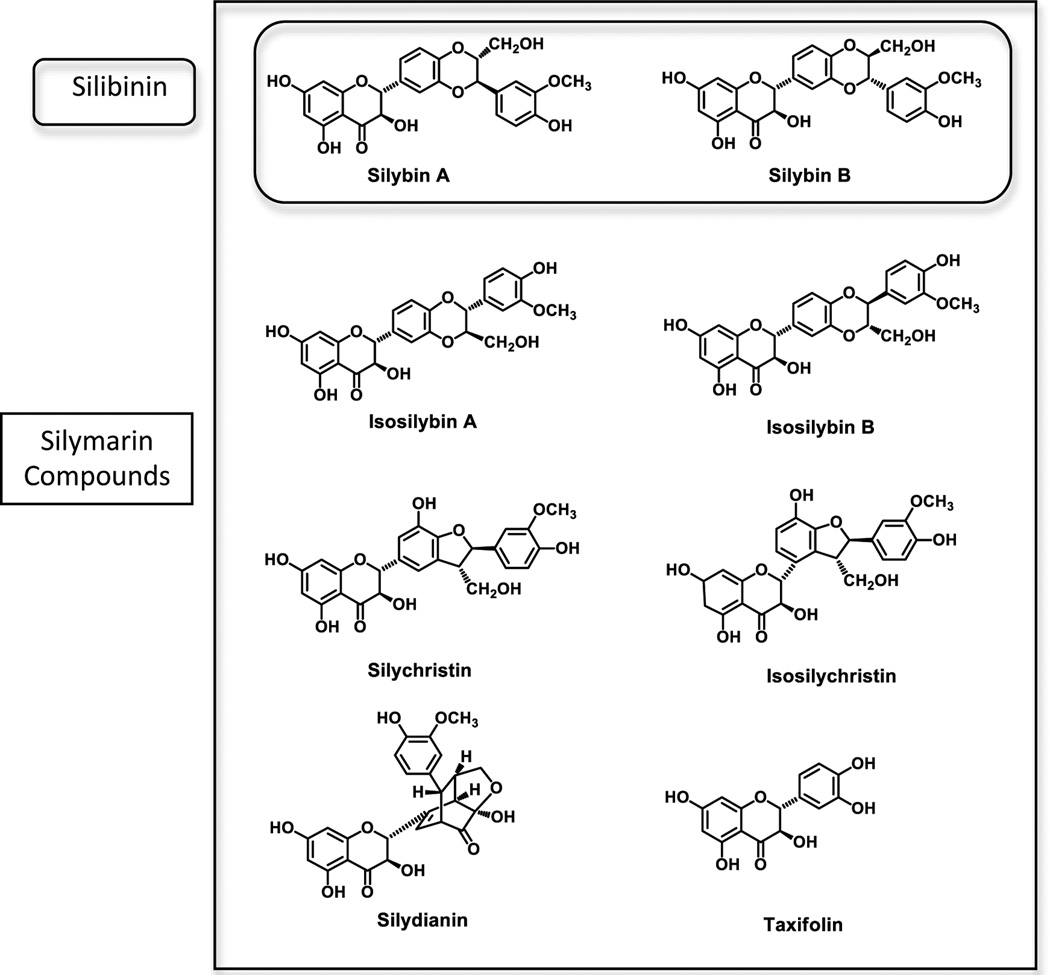
Silymarin is the entire extract containing 7 flavonolignans and taxifolin
Silibinin is a mixture of silybin A and silybin B in a 1:1 ratio
The remaining flavonolignans are: isosilybin A, isosilybin B, silychristin, isosilychristin and silydianin
The flavonoid found in silymarin is taxifolin
The structures of the 8 compounds are shown in Figure 2. In a typical preparation of silymarin, the approximate percentages of the 8 major components are silybin A (16%), silybin B (24%), isosilybin A (6%), isosilybin B (4%), silydianin (16%), silychristin (12%), isosilychristin (2%), and taxifolin (2%) (20). The product composition totals are less than 100% because a typical extract is usually labeled as 70% to 80% silymarin, with the remainder consisting of uncharacterized polyphenols and aliphatic fatty acids such as oleic and palmitic acids.
Figure 2. Structures and nomenclature of compounds found in silymarin.
Please refer to text for details.
III- PHARMACOLOGY AND TOXICOLOGY OF ORAL SILYMARIN
Pharmacokinetics of silymarin
Silymarin is fat soluble, with an oral bioavailability of 30–50%. In healthy subjects, oral dosing of silymarin results in low plasma concentrations of flavonolignans between 50–300 ng/ml because the parent compounds are rapidly metabolized to glucuronide and sulfate conjugates which are not thought to have biological activity (21–25). Plasma levels typically achieve maximum concentration after approximately 1–2 hours, with an estimated half-life of 4–6 hours (21, 24, 25). Approximately 20–40% is conjugated and excreted in the bile, 3–8% excreted in the urine, and the remainder excreted unabsorbed in the feces (26). In cirrhotic patients, the peak serum concentration and time to peak concentration are delayed, suggesting that impaired biliary excretion may be associated with reduced clearance of conjugated silibinin (26). This has also been observed for cirrhotic patients with chronic HCV, who have 3- to 5-fold higher plasma concentrations of flavonolignan conjugates as compared to healthy subjects (6, 27, 28). Silymarin may induce cytochrome P450, isoform 3A4 (29) and p-glycoprotein (30), which may alter the pharmacokinetics of oral contraceptive pills. Metabolism of other drugs may also be affected, including antihistamines, benzodiazepines, protease-inhibitors, and cholesterol-lowering agents.
In-vivo versus in-vitro exposure
One could argue that in vitro concentrations of silymarin (14) are 2–10 times higher than the concentrations achieved in human subjects (6) and therefore biological effects of silymarin should never be seen in the clinic. However, prostate cancer subjects given high doses of silipide (a formulation of silibinin with phospholipids) achieved flavonolignan plasma levels of up to 40 µg/ml (31) which are within the biologically active in-vitro dose ranges. Moreover, patients with advanced liver disease achieve 3–5 times higher plasma concentrations (28). These data indicate that with oral dosing, plasma (and presumably hepatic) levels of flavonlignans that are equivalent to in vitro doses can be achieved. However, it is clear from the discussion above that orally administered silymarin is not highly bioavailable, which likely contributes to its equivocal effects in clinical trials. Therefore, improvements in oral delivery of silymarin or development of other routes of administration should improve the bioactivity of silymarin in humans.
Safety in animal models
Perhaps the most comprehensive animal safety study on silymarin was the recent publication of the National Toxicology Program report on the toxicology and carcinogenesis of milk thistle extract in rats and mice (32). This study examined the effects of silymarin as part of rat and mouse chow for 3 month and 2 year studies. The key findings of the study were that during the 2-year feed studies, there was no evidence of carcinogenic activity of silymarin in male or female F344/N rats or B6C3F1 mice. Moreover, female rats showed decreased incidences of mammary gland neoplasms, while male mice showed decreased incidences of hepatocellular neoplasms.
Safety profile of orally administered silymarin
As an herbal remedy, silymarin is available in various forms and dosages. Therefore, the general public is undoubtedly taking a wide range of doses. Even in clinical studies, silymarin doses have varied widely, ranging from 210 mg through over 1200 mg daily, with the majority of studies using a daily dose of 420 mg (Table 1).
Table 1. Oral doses of silymarin and clinical outcomes.
| Author, year | Liver disease | N | Silymarin dose/duration | Silymarin type |
Primary Outcome Variable | P value |
|---|---|---|---|---|---|---|
| Bunout, 1992 (72) | Alcoholic liver disease | 59 | 280 mg versus placebo for up to 15 months | Legalon | No effect of silymarin on total bilirubin, alkaline phosphatases | Not reported |
| Buzzelli, 1993 (33) | Chronic viral hepatitis | 20 | 240 mg IdB1016 bid versus placebo for 7 days | Silipide | Significant reduction in mean AST, ALT, GGT, and bilirubin in treatment arm versus placebo. | <0.05 |
| El-Kamary, 2009 (73) | Acute viral hepatitis | 105 | 420 mg versus placebo for 8 weeks | Legalon | Silymarin group had quicker resolution of symptoms related to biliary retention: dark urine, jaundice and scleral icterus. | <0.05 to 0.01 |
| Federico, 2006 (57) | NAFLD and HCV with NAFLD | 85 | 376 mg silibinin-vitamin E-phospholipids vs. no treatment for 6 months. | Realsil | ALT improvement in patients with both patient groups, but improvements persisted only in NAFLD patients. | <0.01 |
| Feher, 1989 (74) | Alcoholic liver disease | 36 | 420 mg vs placebo, double-blind. 6 months. | Legalon | Serum bilirubin, AST, and ALT normalized in silymarin group. | Not reported |
| Ferenci, 1989 (75) | Cirrhosis | 170 | 420 mg vs. placebo, double blind, prospective, randomized, 2 years. | Legalon | Silymarin-treated patients had significantly longer 4-year survival rates compared to placebo. | 0.036 |
| Gordon, 2006 (46) | Chronic hepatitis C | 17 | 600-1200 mg daily versus placebo. Randomized, double-blind, placebo-controlled, crossover study. 12 weeks. | MediHerb | Mean serum ALT levels and HCV RNA titer did not change in treatment arm versus placebo. | n.s. |
| Hawke, 2010 Fried, 2012 (6, 7) | Chronic hepatitis C | 32 | 420, 840, 1680, 2100 mg, randomized, double-blind placebo controlled. 24 weeks. | Legalon | No change in ALT and HCV RNA. | n.s. |
| Huber, 2005 (49) | Chronic hepatitis C | 40 | 420mg (13 subjects), 840mg (20 subjects) 1260mg (7 subjects) silymarin per day, no control group, 12 weeks. | Legalon | ALT did not change significantly from baseline in any group and there were no differences between the treatment groups. | n.s. |
| Kiesewetter, 1977 (44) | Chronic viral hepatitis | 24 | 420 mg versus placebo daily for 365 days | Legalon | Histological improvement in 9 out of 19 on silymarin and 3 out of 17 on placebo | 0.08 |
| Lang, 1990 (76) | Alcoholic cirrhosis | 60 | 420 mg, double-blind, one month. | Legalon | Normalization of ALT, AST, and bilibrubin. | Not reported |
| Loguercio, 2012 (58) | NAFLD and NAFLD w HCV | 85 | 188mg silibinin-vitamin E-phospholipids. 12 months. | RealSil | Liver enzyme levels, hyperinsulinemia, and indexes of liver fibrosis showed an improvement in treated individuals. | <0.01 |
| Magliulo, 1978 (77) | Acute viral hepatitis | 59 | 420 mg, 5 days | Legalon | Bilirubin and GOT values decreased with silymarin. | <0.05 |
| Marcelli, 1992 (45) | Chronic hepatitis | 65 | 240 mg (120 mg bid) IdB1016 (Silipide) versus placebo for 12 weeks | Silipide | Significant reduction in mean AST and ALT in treatment arm versus placebo | <0.05 |
| Melhem, 2005 (48) | Chronic hepatitis C | 50 | 250 mg silymarin tid. No control group. Silymarin was used in conjunction with 6 other oral antioxidants and 4 intravenous antioxidants. 20 weeks. | Vital Nutrients | Normalization of serum ALT occurred in 44% of patients. Histological improvement occurred in 36% of patients. | <0.05 |
| Pares, 1998 (36) | Alcoholic cirrhosis | 200 | 450 mg vs. placebo, randomized, double-blind. 2 years. | Legalon | Silymarin did not extend survival. No effect on ALT. | n.s. |
| Salmi, 1982 (78) | Alcoholic liver disease | 97 | 420 mg vs. placebo. 4 weeks. | Legalon | Significantly greater decrease of S-SGPT (S-ALAT) and S-SGOT (S-ASAT) in the silymarin group than in controls. | <0.05 |
| Tanamly, 2004 (47) | Chronic hepatitis C | 177 | 140 mg silymarin (Legalon) tid versus multi-vitamin, double-blind. 1 year. | Legalon | No effect on HCV viremia or serum ALT in either arm. | n.s. |
n.s.=non-significant p value (>0.05).
The safety profile of silymarin in published studies has been excellent. In a review of the use of silymarin in the treatment of liver disease, the available safety data using adverse events published in nine clinical trials were summarized (26). Daily doses ranged from 240 mg (33) to 600 mg (34). As part of the Silymarin for NASH and C Hepatitis (SyNCH) phase I studies (clinicaltrials.gov, NCT00389376), subjects receiving as much as 1680 mg daily for 7 days had similar adverse effects as the placebo group (6). For 296 subjects treated in randomized, double-blinded, placebo-controlled clinical trials, no deaths or serious adverse events were attributed to silymarin. In fact, the incidence of deaths and serious adverse events was lower in the silymarin- than in the placebo-treated population. In these studies, the overall incidence of adverse events was 2.4% of 296 subjects treated in randomized trials. Of 3612 subjects treated in unblinded studies, the incidence of adverse events was 1%. Considering all published randomized trials, uncontrolled studies, and case reports, only one serious adverse event has been considered related to silymarin (diarrhea, vomiting, and collapse in a 57 year old woman) (35).
The most common symptom associated with silymarin use is a laxative effect. Other symptoms have included nausea, epigastric discomfort, arthralgia, pruritis, and urticaria, although in clinical trials, the incidence of these side effects is similar between treatment and placebo arms (36). Because silymarin has been reported to decrease bilirubin conjugation and to inhibit the cytochrome P450 enzyme system (37), clinical investigators should be aware of the potential for jaundice or drug interactions.
In summary, the various formulations of milk thistle taken as a whole have an excellent safety track record. Nevertheless, particularly at the higher dose ranges, side effects, laboratory parameters, and concomitant medications should be monitored closely.
IV- HEPATOPROTECTION WITH ORAL SILYMARIN
Hepatoprotection in animal models
There are compelling data from animal models indicating that silymarin and silymarin-derived compounds protect the liver against injury by a wide array of insults including carbon tetrachloride (38), ischemia-reperfusion (39), the toxic components of death cap mushrooms (Amanita phalloides) phalloidin (10) and alpha-amanitin (40), acetaminophen (41), alcohol (42), and the chemotherapy drug doxorubicin (43).
Hepatoprotection in clinical studies
Table 1 includes eight published studies where oral silymarin was administered to patients with chronic hepatitis C. Five of these studies included a placebo control (6, 7, 33, 44–46) while three trials included either a multivitamin control group (47) or no control group (48, 49). Results are inconsistent among the trials, with three showing ALT improvement (33, 45, 48) and five demonstrating no effect of silymarin on serum ALT (6, 44, 46, 47, 49). One small trial showed histological improvement in the absence of biochemical response (44). Thus, limited data from published studies in patients with chronic hepatitis C do not uniformly demonstrate hepatoprotection with low to high doses of silymarin. Moreover, the results from the SyNCH trial, which administered the highest oral doses of silymarin to date, were recently published (7). This study used a carefully standardized silymarin preparation, Legalon, available by prescription only, and employed a double-blind, placebo-controlled design. The patients achieved 2–2,000 ng/ml of silymarin flavonolignans and there was no significant change in serum ALT activity or RNA levels in the silymarin treatment arms during the 24-week treatment period (7).
V- ANTIVIRAL EFFECTS OF INTRAVENOUS SILIBININ
Silibinin
As described above, silymarin extract contains silibinin, which is a mixture of the flavonolignans silybin A (SA) and silybin B (SB). Silibinin has antioxidant, immunomodulatory, antiproliferative, antifibrotic, and antiviral activities (4, 14, 50) in a wide range of tissues and organs (51). Moreover, both silymarin and silibinin inhibit HCV infection in cell culture by variably blocking viral entry, viral fusion, viral RNA and protein synthesis, HCV N5SB RNA dependent RNA polymerase activity, and virus transmission (4, 14, 52–55). Despite silibinin’s promising in vitro activities and demonstrated efficacy in animal models (56), most of the clinical studies in subjects with chronic hepatitis C have administered silymarin, while silibinin has only been tested in four studies (33, 45, 57, 58).
Intravenous silibinin in HCV-infected patients
An intravenous formulation of silibinin (Silibinin-C-2’, 3-dihydrogen succinate, disodium salt), marketed as Legalon SIL®, has been tested in HCV-infected patients. At present, all published data on the use of Legalon SIL® are uncontrolled series or case reports in the following three different clinical scenarios:
1. Treatment of non-responders to pegylated interferon alpha and ribavirin
The first report on the clinical use of SIL in chronic hepatitis C demonstrated a dose-dependent decline of HCV RNA over 7 days of daily intravenous infusion in subjects who were prior non-responders to pegylated interferon alpha (PegIFN) and ribavirin (RBV) therapy. With triple SIL, PegIFN, and RBV therapy, HCV RNA further decreased and became undetectable at week 12 in 7 patients who received 15 and 20 mg/kg SIL (50%) (12). Treatment with PegIFN/RBV in responders was continued for up to further 60 weeks. A sustained virologic response was obtained in 3 patients. This seminal study showed that intravenous silibinin suppresses HCV infection in vivo in patients who failed conventional pegIFN + RBV therapy.
2. Treatment of “on-treatment” nonresponders
In a proof of concept study (59), 27 treatment-naïve patients who did not respond to PegIFN/RBV were treated with intravenous silibinin. The majority of patients had the unfavorable IL-28B T-allele (CT=22; TT=4). Patients received 20mg/kg Legalon SIL for either 14 days (n=12) or 21 days (n=15), followed by PEG/RBV re-treatment. After 7 days of Legalon SIL, 17 (62.9%) patients had undetectable HCV RNA. At the end of intravenous treatment, 23 patients (85.1 %) were HCV RNA negative. After stopping silibinin infusions, HCV RNA returned in 5 patients, and the viral rebound was associated with baseline viral loads. At the end of the PegIFN/RBV treatment, 17 patients (63%) were HCV RNA negative. During the 24 weeks of treatment-free follow up, 12 patients remained HCV RNA negative (ITT SVR rate: 45%), while 5 patients experienced virologic relapse (final update of (59)). Further analysis showed that sustained HCV RNA negativity could only be achieved if HCV RNA became undetectable during silibinin infusions. If HCV RNA persisted after Legalon SIL treatment, no patient went on to achieve SVR.
In a recent study, Biermer et al (60) reported on 20 subjects who failed various interferon-based regimens (including 4 patients receiving triple therapy with a protease inhibitor). The subjects received 1400 mg/day Legalon SIL on just two consecutive days. Complete viral suppression was induced in 13 of 20 subjects within the first week after the short-term silibinin infusion, and all but one of them remained HCV RNA-negative during the subsequent follow-up period during which PegIFN/RBV was administered. Collectively, these studies further demonstrate the clinical efficacy of SIL in patients who fail conventional IFN-based therapies.
3. Intravenous silibinin in the transplant setting
While intravenous silibinin has been used in the transplant setting in the treatment of Amanita phalloides-induced acute liver failure for over 20 years (61, 62), the mixture has only recently been evaluated in patients with hepatitis C undergoing liver transplantation. In the first case report, Legalon SIL was shown to prevent HCV reinfection of the graft (63). A second case of Legalon-SIL prevention of graft reinfection has also been reported (64). In this case report, cholestatic posttransplant hepatitis was also successfully treated by silibinin infusion. However, intravenous silibinin was unable to prevent graft reinfection in a third patient infected with a genotype 2 virus (65). Finally, Eurich et al reported 4 patients with graft reinfection with a significant decrease of HCV RNA (mean 2.8 log) after 10 days of intravenous silibinin monotherapy. One patient cleared HCV RNA under silibinin monotherapy while a second patient cleared viremia following additional PEG/RBV therapy (66). Thus, intravenous silibinin appears to have clinical utility in prevention of HCV reinfection following liver transplantation.
Safety of intravenous silibinin
Fortunately, intravenous silibinin appears to be well tolerated. Patients tend to experience a heat sensation and mild sweating during the first infusion. On the first infusion day, approximately half of the patients experience gastrointestinal symptoms including nausea, abdominal pain and diarrhea. The symptoms appear to be self-limiting and decrease on subsequent treatment days. Following Legalon SIL treatment, significant increases of bilirubin have been reported, with normalization 2 weeks after dosing. The mechanism of the increase in bilirubin is unknown, but data point to the inhibition of anion transporters OATP B1 and OATP B3 by silibinin (67, 68).
Finally, SIL has recently been shown to inhibit HCV and, to a lesser extent, HIV-1, in an HCV/HIV co-infected patient (69). Indeed, it has recently been found that SIL inhibits in vitro HIV infection of multiple cell types including PBMCs (70).
In summary, intravenous silibinin could be an alternative for patients with HCV-associated end-stage liver disease who have no further treatment options due to side effects of PegIFN/RBV therapy. Additional systematic studies that explore the length of silibinin treatment, the optimal dose, and the duration of post-silibinin PegIFN/RBV therapy should be performed.
Potential mechanisms of silibinin’s antiviral effects
Legalon SIL retains many of the same in vitro antiviral properties of the parent silibinin including inhibiting HCV fusion, replication, and production of infectious progeny virus (55). Moreover, both Legalon SIL and silibinin inhibit in vitro NS5B polymerase activity (14, 53–55). Despite clear effects of silibinin on several points of the HCV lifecycle, it is not clear which is the dominant mechanism for virus suppression. Two recent studies shed new light on how silibinin may impinge on HCV. Blaising et al (manuscript submitted) suggest that HCV enters human liver cell cultures primarily by clathrin-mediated endocytosis, and both silibinin and Legalon SIL hinder HCV entry into cells by slowing trafficking through clathrin-coated pits and vesicles. Esser-Nobis et al (manuscript submitted) selected for resistance to Legalon SIL in cell culture and isolated a mutation in the HCV nonstructural 4B (NS4B) protein conferring partial resistance to Legalon SIL treatment. These in vitro results were supported by the identification of distinct mutations affecting highly conserved amino acid residues within NS4B in a liver transplant patient who experienced viral breakthrough while on intravenous silibinin therapy. Transfer of in vivo NS4B mutations into HCV replicons conferred SIL resistance in vitro, and altered the structure of the NS4B-induced membranous web (71), the intracellular site of HCV replication. These new studies add the evolving story of how silibinin inhibits HCV infection, and also raise important questions. For example, is NS4B a direct viral target of silibinin, or does NS4B resistance to silibinin arise through accessory host cell targets? Given the plethora of ways by which silibinin can modulate cellular functions (13), it is likely that the targets of silibinin action lie within the cell, and that viral resistance is a secondary outcome of this measure. Since HCV-host interactions are required for the HCV lifecycle, silibinin (and other components of silymarin) could impact viral functions including entry, replication, and exit, by modulating host cell factors. Moreover, viral resistance to silibinin has been demonstrated and is reflected by mutations in the viral genome, but this does not prove that the viral genome or proteins are direct targets of silibinin. Therefore, the targets of silibinin could be cellular proteins or membranes/lipids. In this regard, the common link between the papers by Blaising et al. and Esser-Nobis et al. is the clear effect of silibinin on cellular processes involving membranes.
VI- ONGOING CLINICAL TRIALS WITH ORAL SILYMARIN
As listed at ClinicalTrials.org, there are multiple clinical trials on silymarin that are actively recruiting patients. At the University of Maryland, a randomized placebo-controlled trial is underway to evaluate the safety and efficacy of silymarin treatment in patients with acute viral hepatitis (NCT00755950). At the University of North Carolina at Chapel Hill, a study on the combined effects of silymarin and green tea extract in patients with chronic hepatitis C is also enrolling patients (NCT01018615). In Italy, a trial is evaluating Legalon SIL on HCV recurrence in liver transplant recipients (NCT01518933). Legalon SIL is also being evaluated in the USA for efficacy against mushroom poisoning (NCT00915681).
VII- CONCLUDING REMARKS
Even though it appears that orally administered silymarin as a monotherapy has little effect on liver enzymes and viral loads, many individuals still self-prescribe silymarin as a botanical medicine or complementary and alternative medicine practice (2). Clinicians need to be aware that their patients, who may be taking pegylated IFN alpha, ribavirin and directly acting antiviral (DAA) compounds, may also be taking silymarin. Thus, the impact of oral silymarin on current standard of treatment therapies should be considered and even investigated. Moreover, intravenously administered SIL should be further studied as a salvage therapy for previous nonresponders to IFN plus ribavirin therapy, as well as in combination with IFN, ribavirin, and DAA compounds. Further research on SIL therapy in the context of orthotopic liver transplantation is also warranted, as is continued investigation into how silibinin’s interactions with cells affect virus infection and replication.
It is clear that in vitro and in animal models, silymarin and silymarin-derived pure compounds and mixtures protect cells from injury by numerous agents, in addition to providing cytoprotection against inflammatory sequelae. The recent clinical studies of Fried and colleagues (7), while sobering, should not detract away from additional research on this interesting class of compounds. Additional randomized clinical trials are required before oral silymarin products can be endorsed as treatments for liver disease. Basic research should continue to define the mechanisms for preventing inflammatory sequelae as well as the cytoprotective mechanisms that are induced by these natural products. In doing so, the cellular targets of silymarin will be identified, which might lead to the design of more selective, potent, and orally-deliverable anti-inflammatory compounds that could prove clinically useful in liver diseases of both viral and non-viral origins.
Acknowledgements
SJP is partially supported by NIH grant R01AT006842, U19AI066328, and R56AI091840. JMP is supported by Agence Nationale de Recherche sur le SIDA et les Hépatites Virales (ANRS), Institut National de la Santé et de la Recherche Médicale (INSERM) and Fondation pour la Recherche Médicale (FRM). We thank Nicholas Oberlies (University of North Carolina at Greensboro) and Toni Kline (University of Washington) for assistance with chemical structures and for critical reading of the manuscript, Jane Saxton (Bastyr University) for research on silymarin history, Chia Wang (Virigina Mason Medical Center) and Chihiro Morishima (University of Washington) for initial compilation of silymarin clinical safety and efficacy data.
REFERENCES
- 1.NCCAM. Research Report: National Center for Complementary and Alternative Medicine. 2003. Hepatitis C and Complementary and Alternative Medicine: 2003 Update; pp. 2–20. [Google Scholar]
- 2.Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, et al. Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology. 2008;47:605–612. doi: 10.1002/hep.22044. [DOI] [PubMed] [Google Scholar]
- 3.Strader DB, Bacon BR, Lindsay KL, La Brecque DR, Morgan T, Wright EC, Allen J, et al. Use of complementary and alternative medicine in patients with liver disease. Am J Gastroenterol. 2002;97:2391–2397. doi: 10.1111/j.1572-0241.2002.05993.x. [DOI] [PubMed] [Google Scholar]
- 4.Polyak SJ, Morishima C, Shuhart MC, Wang CC, Liu Y, Lee DY. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappaB signaling, and HCV infection by standardized Silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Freedman ND, Curto TM, Morishima C, Seeff LB, Goodman ZD, Wright EC, Sinha R, Everhart JE the HALT-C Trial Group. Silymarin use and liver disease progression in the Hepatitis C Antiviral Long-Term Treatment against Cirrhosis trial. ALIMENTARY PHARMACOLOGY & THERAPEUTICS. 2010 doi: 10.1111/j.1365-2036.2010.04503.x. 2 NOV 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, et al. Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol. 2010;50:434–449. doi: 10.1177/0091270009347475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fried MW, Navarro VJ, Afdhal N, Belle SH, Wahed AS, Hawke RL, Doo E, et al. Effect of silymarin (milk thistle) on liver disease in patients with chronic hepatitis C unsuccessfully treated with interferon therapy: a randomized controlled trial. JAMA. 2012;308:274–282. doi: 10.1001/jama.2012.8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner H, Horhammer L, Munster R. On the chemistry of silymarin (silybin), the active principle of the fruits from Silybum marianum (L.) Gaertn. (Carduus marianus L.) Arzneimittelforschung. 1968;18:688–696. [PubMed] [Google Scholar]
- 9.Wagner H, Horhammer L, Seitz M. Chemical evaluation of a silymarin-containing flavonoid concentrate from Silybum marianum (L.) Gaertn. Arzneimittelforschung. 1968;18:696–698. [PubMed] [Google Scholar]
- 10.Vogel G, Temme I. Curative antagonism of phalloidin induced liver damage with silymarin as a model of an antihepatotoxic therapy. Arzneimittelforschung. 1969;19:613–615. [PubMed] [Google Scholar]
- 11.Schriewer H, Lohmann J, Rauen HM. The effect of silybin-dihemisuccinate on regulation disorders in phospholipid metabolism in acute galactosamine intoxication in the rat. Arzneimittelforschung. 1975;25:1582–1585. [PubMed] [Google Scholar]
- 12.Ferenci P, Scherzer TM, Kerschner H, Rutter K, Beinhardt S, Hofer H, Schoniger-Hekele M, et al. Silibinin is a potent antiviral agent in patients with chronic hepatitis C not responding to pegylated interferon/ribavirin therapy. Gastroenterology. 2008;135:1561–1567. doi: 10.1053/j.gastro.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 13.Ramasamy K, Agarwal R. Multitargeted therapy of cancer by silymarin. Cancer Lett. 2008;269:352–362. doi: 10.1016/j.canlet.2008.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polyak SJ, Morishima C, Lohmann V, Pal S, Lee DY, Liu Y, Graf TN, et al. Identification of hepatoprotective flavonolignans from silymarin. Proc Natl Acad Sci U S A. 2010;107:5995–5999. doi: 10.1073/pnas.0914009107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tager M, Dietzmann J, Thiel U, Hinrich Neumann K, Ansorge S. Restoration of the cellular thiol status of peritoneal macrophages from CAPD patients by the flavonoids silibinin and silymarin. Free Radic Res. 2001;34:137–151. doi: 10.1080/10715760100300131. [DOI] [PubMed] [Google Scholar]
- 16.Lu P, Mamiya T, Lu LL, Mouri A, Zou L, Nagai T, Hiramatsu M, et al. Silibinin prevents amyloid beta peptide-induced memory impairment and oxidative stress in mice. Br J Pharmacol. 2009;157:1270–1277. doi: 10.1111/j.1476-5381.2009.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deep G, Agarwal R. Antimetastatic efficacy of silibinin: molecular mechanisms and therapeutic potential against cancer. Cancer Metastasis Rev. 2010;29:447–463. doi: 10.1007/s10555-010-9237-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh RP, Agarwal R. Prostate cancer chemoprevention by silibinin: Bench to bedside. Mol Carcinog. 2006;45:436–442. doi: 10.1002/mc.20223. [DOI] [PubMed] [Google Scholar]
- 19.Kroll DJ, Shaw HS, Oberlies NH. Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther. 2007;6:110–119. doi: 10.1177/1534735407301825. [DOI] [PubMed] [Google Scholar]
- 20.Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, et al. Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005;65:4448–4457. doi: 10.1158/0008-5472.CAN-04-4662. [DOI] [PubMed] [Google Scholar]
- 21.Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos. 2008;36:65–72. doi: 10.1124/dmd.107.017566. [DOI] [PubMed] [Google Scholar]
- 22.Weyhenmeyer R, Mascher H, Birkmayer J. Study on dose-linearity of the pharmacokinetics of silibinin diastereomers using a new stereospecific assay. Int J Clin Pharmacol Ther Toxicol. 1992;30:134–138. [PubMed] [Google Scholar]
- 23.Rickling B, Hans B, Kramarczyk R, Krumbiegel G, Weyhenmeyer R. Two high-performance liquid chromatographic assays for the determination of free and total silibinin diastereomers in plasma using column switching with electrochemical detection and reversed-phase chromatography with ultraviolet detection. J Chromatogr B Biomed Appl. 1995;670:267–277. doi: 10.1016/0378-4347(95)00168-9. [DOI] [PubMed] [Google Scholar]
- 24.Gatti G, Perucca E. Plasma concentrations of free and conjugated silybin after oral intake of a silybin-phosphatidylcholine complex (silipide) in healthy volunteers. Int J Clin Pharmacol Ther. 1994;32:614–617. [PubMed] [Google Scholar]
- 25.Lorenz D, Lucker PW, Mennicke WH, Wetzelsberger N. Pharmacokinetic studies with silymarin in human serum and bile. Methods Find Exp Clin Pharmacol. 1984;6:655–661. [PubMed] [Google Scholar]
- 26.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 27.Schrieber SJ, Hawke RL, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, et al. Differences in the disposition of silymarin between patients with nonalcoholic fatty liver disease and chronic hepatitis C. Drug Metab Dispos. 2011;39:2182–2190. doi: 10.1124/dmd.111.040212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos. 2008;36:1909–1916. doi: 10.1124/dmd.107.019604. [DOI] [PubMed] [Google Scholar]
- 29.Budzinski JW, Foster BC, Vandenhoek S, Arnason JT. An in vitro evaluation of human cytochrome P450 3A4 inhibition by selected commercial herbal extracts and tinctures. Phytomedicine. 2000;7:273–282. doi: 10.1016/S0944-7113(00)80044-6. [DOI] [PubMed] [Google Scholar]
- 30.Zhou S, Lim LY, Chowbay B. Herbal modulation of P-glycoprotein. Drug Metab Rev. 2004;36:57–104. doi: 10.1081/dmr-120028427. [DOI] [PubMed] [Google Scholar]
- 31.Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, et al. A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs. 2007;25:139–146. doi: 10.1007/s10637-006-9019-2. [DOI] [PubMed] [Google Scholar]
- 32.Toxicology and carcinogenesis studies of milk thistle extract (CAS No. 84604-20-6) in F344/N rats and B6C3F1 mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 2011:1–177. [PubMed] [Google Scholar]
- 33.Buzzelli G, Moscarella S, Giusti A, Duchini A, Marena C, Lampertico M. A pilot study on the liver protective effect of silybin-phosphatidylcholine complex (IdB1016) in chronic active hepatitis. Int J Clin Pharmacol Ther Toxicol. 1993;31:456–460. [PubMed] [Google Scholar]
- 34.Velussi M, Cernigoi AM, De Monte A, Dapas F, Caffau C, Zilli M. Long-term (12 months) treatment with an anti-oxidant drug (silymarin) is effective on hyperinsulinemia, exogenous insulin need and malondialdehyde levels in cirrhotic diabetic patients. J Hepatol. 1997;26:871–879. doi: 10.1016/s0168-8278(97)80255-3. [DOI] [PubMed] [Google Scholar]
- 35.Adverse Drug Reactions Advisory Committee. An adverse reaction to the herbal medication milk thistle (Silybum marianum) Med J Aust. 1999;170:218–219. [PubMed] [Google Scholar]
- 36.Pares A, Planas R, Torres M, Caballeria J, Viver JM, Acero D, Panes J, et al. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 37.Venkataramanan R, Ramachandran V, Komoroski BJ, Zhang S, Schiff PL, Strom SC. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronosyl transferase in human hepatocyte cultures. Drug Metab Dispos. 2000;28:1270–1273. [PubMed] [Google Scholar]
- 38.Rauen HM, Schriewer H, Tegtbauer U, Lasana JE. Silymarin prevents peroxidation of lipids in carbon tetrachloride-induced liver damage. Experientia. 1973;29:1372. doi: 10.1007/BF01922826. [DOI] [PubMed] [Google Scholar]
- 39.Wu CG, Chamuleau RA, Bosch KS, Frederiks WM. Protective effect of silymarin on rat liver injury induced by ischemia. Virchows Arch B Cell Pathol Incl Mol Pathol. 1993;64:259–263. doi: 10.1007/BF02915120. [DOI] [PubMed] [Google Scholar]
- 40.Trost W, Halbach G. Anti-phalloidine and anti-alpha-amanitine action of silybin in comparison with compounds similar to structural parts of silybin. Experientia. 1978;34:1051–1052. doi: 10.1007/BF01915341. [DOI] [PubMed] [Google Scholar]
- 41.Campos R, Garrido A, Guerra R, Valenzuela A. Acetaminophen hepatotoxicity in rats is attenuated by silybin dihemisuccinate. Prog Clin Biol Res. 1988;280:375–378. [PubMed] [Google Scholar]
- 42.Valenzuela A, Lagos C, Schmidt K, Videla LA. Silymarin protection against hepatic lipid peroxidation induced by acute ethanol intoxication in the rat. Biochem Pharmacol. 1985;34:2209–2212. doi: 10.1016/0006-2952(85)90421-6. [DOI] [PubMed] [Google Scholar]
- 43.Patel N, Joseph C, Corcoran GB, Ray SD. Silymarin modulates doxorubicin-induced oxidative stress, Bcl-xL and p53 expression while preventing apoptotic and necrotic cell death in the liver. Toxicol Appl Pharmacol. 2010;245:143–152. doi: 10.1016/j.taap.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 44.Kiesewetter E, Leodolter I, Thaler H. Results of two double-blind studies on the effect of silymarine in chronic hepatitis (author's transl) Leber Magen Darm. 1977;7:318–323. [PubMed] [Google Scholar]
- 45.Marcelli R, Bizzoni P, Conte D, Lisena MO, Lampertico M, Marena C, De Marco MF, et al. Randomized controlled study of the efficacy and tolerability of a short course of IdB 1016 in the treatment of chronic persistent hepatitis. European Bulletin of Drug Research. 1992;1:131–135. [Google Scholar]
- 46.Gordon A, Hobbs DA, Bowden DS, Bailey MJ, Mitchell J, Francis AJ, Roberts SK. Effects of Silybum marianum on serum hepatitis C virus RNA, alanine aminotransferase levels and well-being in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2006;21:275–280. doi: 10.1111/j.1440-1746.2006.04138.x. [DOI] [PubMed] [Google Scholar]
- 47.Tanamly MD, Tadros F, Labeeb S, Makld H, Shehata M, Mikhail N, Abdel-Hamid M, et al. Randomised double-blinded trial evaluating silymarin for chronic hepatitis C in an Egyptian village: study description and 12-month results. Dig Liver Dis. 2004;36:752–759. doi: 10.1016/j.dld.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Melhem A, Stern M, Shibolet O, Israeli E, Ackerman Z, Pappo O, Hemed N, et al. Treatment of chronic hepatitis C virus infection via antioxidants: results of a phase I clinical trial. J Clin Gastroenterol. 2005;39:737–742. doi: 10.1097/01.mcg.0000174023.73472.29. [DOI] [PubMed] [Google Scholar]
- 49.Huber R, Futter I, Ludtke R. Oral silymarin for chronic hepatitis C - a retrospective analysis comparing three dose regimens. Eur J Med Res. 2005;10:68–70. [PubMed] [Google Scholar]
- 50.Agarwal R, Agarwal C, Ichikawa H, Singh RP, Aggarwal BB. Anticancer potential of silymarin: from bench to bed side. Anticancer Res. 2006;26:4457–4498. [PubMed] [Google Scholar]
- 51.Gazak R, Walterova D, Kren V. Silybin and silymarin--new and emerging applications in medicine. Curr Med Chem. 2007;14:315–338. doi: 10.2174/092986707779941159. [DOI] [PubMed] [Google Scholar]
- 52.Morishima C, Shuhart MC, Wang CC, Paschal DM, Apodaca MC, Liu Y, Sloan DD, et al. Silymarin inhibits in vitro T-cell proliferation and cytokine production in hepatitis C virus infection. Gastroenterology. 2010;138:671–681. doi: 10.1053/j.gastro.2009.09.021. 681 e671-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagoner J, Negash A, Kane OJ, Martinez LE, Nahmias Y, Bourne N, Owen DM, et al. Multiple effects of silymarin on the hepatitis C virus lifecycle. Hepatology. 2010;51:1912–1921. doi: 10.1002/hep.23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed-Belkacem A, Ahnou N, Barbotte L, Wychowski C, Pallier C, Brillet R, Pohl RT, et al. Silibinin and Related Compounds Are Direct Inhibitors of Hepatitis C Virus RNA-Dependent RNA Polymerase. Gastroenterology. 2010;138:1112–1122. doi: 10.1053/j.gastro.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 55.Wagoner J, Morishima C, Graf TN, Oberlies NH, Teissier E, Pecheur EI, Tavis JE, et al. Differential in vitro effects of intravenous versus oral formulations of silibinin on the HCV life cycle and inflammation. PLoS One. 2011;6:e16464. doi: 10.1371/journal.pone.0016464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saller R, Brignoli R, Melzer J, Meier R. An updated systematic review with meta-analysis for the clinical evidence of silymarin. Forsch Komplementmed. 2008;15:9–20. doi: 10.1159/000113648. [DOI] [PubMed] [Google Scholar]
- 57.Federico A, Trappoliere M, Tuccillo C, de Sio I, Di Leva A, Del Vecchio Blanco C, Loguercio C. A new silybin-vitamin E-phospholipid complex improves insulin resistance and liver damage in patients with non-alcoholic fatty liver disease: preliminary observations. Gut. 2006;55:901–902. doi: 10.1136/gut.2006.091967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Loguercio C, Andreone P, Brisc C, Brisc MC, Bugianesi E, Chiaramonte M, Cursaro C, et al. Silybin combined with phosphatidylcholine and vitamin E in patients with nonalcoholic fatty liver disease: a randomized controlled trial. Free Radic Biol Med. 2012;52:1658–1665. doi: 10.1016/j.freeradbiomed.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 59.Rutter K, Scherzer TM, Beinhardt S, Kerschner H, Stattermayer AF, Hofer H, Popow-Kraupp T, et al. Intravenous silibinin as 'rescue treatment' for on-treatment non-responders to pegylated interferon/ribavirin combination therapy. Antivir Ther. 2011;16:1327–1333. doi: 10.3851/IMP1942. [DOI] [PubMed] [Google Scholar]
- 60.Biermer M, Schlosser B, Fulop B, van Bommel F, Brodzinski A, Heyne R, Keller K, et al. High-dose silibinin rescue treatment for HCV-infected patients showing suboptimal virologic response to standard combination therapy. J Viral Hepat. 2012;19:547–553. doi: 10.1111/j.1365-2893.2011.01572.x. [DOI] [PubMed] [Google Scholar]
- 61.Klein AS, Hart J, Brems JJ, Goldstein L, Lewin K, Busuttil RW. Amanita poisoning: treatment and the role of liver transplantation. Am J Med. 1989;86:187–193. doi: 10.1016/0002-9343(89)90267-2. [DOI] [PubMed] [Google Scholar]
- 62.Pinson CW, Daya MR, Benner KG, Norton RL, Deveney KE, Ascher NL, Roberts JP, et al. Liver transplantation for severe Amanita phalloides mushroom poisoning. Am J Surg. 1990;159:493–499. doi: 10.1016/s0002-9610(05)81254-1. [DOI] [PubMed] [Google Scholar]
- 63.Neumann UP, Biermer M, Eurich D, Neuhaus P, Berg T. Successful prevention of hepatitis C virus (HCV) liver graft reinfection by silibinin mono-therapy. J Hepatol. 2010 doi: 10.1016/j.jhep.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 64.Beinhardt S, Rasoul-Rockenschaub S, Maieron A, PH S-M, Hofer H PF. Intravenous Silibinin-therapy in patients with chronic hepatitis C in the transplant setting. J Hepatology. 2012;56:S77. [Google Scholar]
- 65.Aghemo A, Bhoori S, De Nicola S, Mazzaferro V MC. Failure of Intravenous Silibinin Monotherapy to Prevent Hepatitis C Genotype 2A Liver Graft Reinfection. Hepat Mon. 2012 doi: 10.5812/hepatmon.6135. in press. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eurich D, Bahra M, Berg T, Boas-Knoop S, Biermer M, Neuhaus R, Neuhaus P, et al. Treatment of hepatitis C-virus-reinfection after liver transplant with silibinin in nonresponders to pegylated interferon-based therapy. Experimental and clinical transplantation : official journal of the Middle East Society for Organ Transplantation. 2011;9:1–6. [PubMed] [Google Scholar]
- 67.Saller R, Melzer J, Reichling J, Brignoli R, Meier R. An updated systematic review of the pharmacology of silymarin. Forsch Komplementmed. 2007;14:70–80. doi: 10.1159/000100581. [DOI] [PubMed] [Google Scholar]
- 68.Koller F. The interaction of silibinin with hepatocellular organic anion transporters. Swiss Federal Institute of Technology, Zürich; 2011. [Google Scholar]
- 69.Payer BA, Reiberger T, Rutter K, Beinhardt S, Staettermayer AF, Peck-Radosavljevic M, Ferenci P. Successful HCV eradication and inhibition of HIV replication by intravenous silibinin in an HIV-HCV coinfected patient. J Clin Virol. 2010;49:131–133. doi: 10.1016/j.jcv.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 70.McClure J, Lovelace ES, Elahi S, Maurice NJ, Wagoner J, Dragavon J, Mittler JE, et al. Silibinin Inhibits HIV-1 Infection by Reducing Cellular Activation and Proliferation. PLoS One. 2012;7:e41832. doi: 10.1371/journal.pone.0041832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Egger D, Wolk B, Gosert R, Bianchi L, Blum HE, Moradpour D, Bienz K. Expression of hepatitis C virus proteins induces distinct membrane alterations including a candidate viral replication complex. J Virol. 2002;76:5974–5984. doi: 10.1128/JVI.76.12.5974-5984.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bunout D, Hirsch S, Petermann M, de la Maza MP, Silva G, Kelly M, Ugarte G, et al. Controlled study of the effect of silymarin on alcoholic liver disease. Rev Med Chil. 1992;120:1370–1375. [PubMed] [Google Scholar]
- 73.El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, Metwally M, Mikhail N, et al. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine. 2009;16:391–400. doi: 10.1016/j.phymed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Feher J, Deak G, Muzes G, Lang I, Niederland V, Nekam K, Karteszi M. Liver-protective action of silymarin therapy in chronic alcoholic liver diseases. Orv Hetil. 1989;130:2723–2727. [PubMed] [Google Scholar]
- 75.Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, et al. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 76.Lang I, Nekam K, Deak G, Muzes G, Gonzales-Cabello R, Gergely P, Csomos G, et al. Immunomodulatory and hepatoprotective effects of in vivo treatment with free radical scavengers. Ital J Gastroenterol. 1990;22:283–287. [PubMed] [Google Scholar]
- 77.Magliulo E, Gagliardi B, Fiori GP. Results of a double blind study on the effect of silymarin in the treatment of acute viral hepatitis, carried out at two medical centres (author's transl) Med Klin. 1978;73:1060–1065. [PubMed] [Google Scholar]
- 78.Salmi HA, Sarna S. Effect of silymarin on chemical, functional, and morphological alterations of the liver. A double-blind controlled study. Scand J Gastroenterol. 1982;17:517–521. doi: 10.3109/00365528209182242. [DOI] [PubMed] [Google Scholar]