Abstract
The continuous two bottle choice test is the most common measure of alcohol consumption but there is remarkably little information about the development of tolerance or dependence with this procedure. We showed that C57BL/6JxFVB/NJ and FVB/NJxC57BL/6J F1 hybrid mice demonstrate greater preference for and consumption of alcohol than either parental strain. In order to test the ability of this genetic model of high alcohol consumption to produce neuroadaptation, we examined development of alcohol tolerance and dependence after chronic self-administration using a continuous access two-bottle choice paradigm. Ethanol-experienced mice stably consumed about 16–18 g/kg/day of ethanol. Ethanol-induced withdrawal severity was assessed (after 59 days of drinking) by scoring handling-induced convulsions; withdrawal severity was minimal and did not differ between ethanol-experienced and -naïve mice. After 71 days of drinking, the rate of ethanol clearance was similar for ethanol-experienced and -naïve mice. After 77 days of drinking, ethanol-induced loss of righting reflex (LORR) was tested daily for 5 days. Ethanol-experienced mice had a shorter duration of LORR. For both ethanol-experienced and -naïve mice, blood ethanol concentrations taken at gain of righting reflex were greater on day 5 than on day 1, indicative of tolerance. After 98 days of drinking, ethanol-induced hypothermia was assessed daily for 3 days. Both ethanol-experienced and –naïve mice developed rapid and chronic tolerance to ethanol-induced hypothermia, with significant group differences on the first day of testing. In summary, chronic, high levels of alcohol consumption in F1 hybrid mice produced rapid and chronic tolerance to both the sedative/hypnotic and hypothermic effects of ethanol; additionally, a small degree of metabolic tolerance developed. The development of tolerance supports the validity of using this model of high alcohol consumption in genetic studies of alcoholism.
Keywords: Alcohol, ethanol, tolerance, hypothermia, sedation, ethanol clearance
1. Introduction
Alcohol abuse is a prerequisite for development of alcoholism and is associated with a high cost to health and quality of life. In 2004, the US Department of Health estimated that 22.5 million Americans have experienced substance abuse or dependence (Chou and Narasimhan, 2005). According to the Diagnostic and Statistical Manual-IV, dependence on alcohol is accompanied by signs of abuse, compulsive drinking behavior, tolerance, and withdrawal, all of which promote alcohol intake and increase the damaging effects of alcohol over time. Development of alcohol tolerance requires that more alcohol is needed to achieve an effect; therefore the effect of a given dose of ethanol decreases as tolerance develops. Three categories of ethanol tolerance have been described according to the sequential order in which they appear. Acute-functional tolerance occurs when an initial response to ethanol is reduced within a single drinking session in a metabolism-independent manner (Mellanby, 1919). Acute functional tolerance occurs within a time frame lasting minutes to hours. Rapid tolerance occurs 8 to 24 hours after a single ethanol administration (Crabbe et al., 1979; Rustay and Crabbe, 2004). Chronic tolerance occurs after repeated ethanol administration and can last for days, months, or years. Chronic tolerance can be environment-dependent or -independent. Characterization of rapid and chronic tolerance has been described for repeated trials of ethanol-induced hypothermia and loss of righting reflex (LORR; Crabbe, 1989; Crabbe, 1994; Crowell et al., 1981; Le et al., 1979; Melchoir and Tabakoff, 1981; Silvers et al., 2003). Human and animal studies indicate that some aspects of tolerance are genetically determined; thus tolerance has an innate component (Browman et al., 2000; Crabbe 1994; Crabbe et al., 1994; Carcia-Andrade et al., 1997; Phillips et al., 1996; Schuckit and Gold, 1988; Schuckit et al., 2004).
Alcohol dependence is also characterized by the presence of withdrawal symptoms (physical and psychological) after drinking ceases, which results from physical dependence on alcohol. Withdrawal from alcohol in humans is characterized by central nervous system hyperexcitability, seizures, autonomic dysregulation, anxiety, restlessness, nausea, sleeplessness, and depression. Nearly all studies of ethanol withdrawal in mice focus on one or two behaviors – seizure severity and anxiety-like behavior. In rodent models, dependence on ethanol is frequently induced either by offering an ethanol-containing liquid diet as the sole source of nutrition or by ethanol vapor inhalation. Once dependence is induced, withdrawal seizure severity is measured using handling-induced convulsion (HIC) scores (Goldstein and Pan, 1971). Increased HIC scores can be seen after a single injection of ethanol using a modification of the HIC index, allowing the detection of withdrawal severity with more sensitivity (Crabbe et al., 1991). This behavioral index ranges from facial grimace to tonic-clonic convulsions. The severity of withdrawal depends on genetic background, as well as dose of ethanol and duration of exposure (Crabbe et al., 1991; Goldstein and Pan, 1971; Metten et al., 1998). For a more detailed review of alcohol tolerance and dependence, several excellent reviews are available (Harris and Buck, 1990; Kalant et al., 1971; Kalant, 1998; Koob and Bloom, 1988; Phillips et al., 1994).
The use of rodent models to model human disease has been a powerful tool in the advancement of understanding disease and improving treatments. There are several rodent models in place to study alcohol-related phenotypes. We found that C57BL/6JxFVB/NJ (B6xFVB) and FVB/NJxC57BL/6J (FVBxB6) F1 hybrid mice self-administer unusually large amounts of alcohol during two-bottle preference tests (females consume 20–35 g/kg/day, males 7–25 g/kg/day, depending on concentration) (Blednov et al., 2005). The distinction between the hybrids is discerned by the order of the listed cross, where the first strain listed is the dam and the second strain of the cross is the sire. This new genetic model has significant advantages when compared to existing inbred strains, including evidence of drinking to intoxication (Blednov et al., 2005). Using this mouse model of high alcohol consumption, we studied tolerance and withdrawal following chronic alcohol consumption. To our knowledge, this is the first study to evaluate the acute withdrawal, metabolic, sedative/hypnotic, and hypothermic responses to ethanol after chronic self-administration using a continuous access two-bottle choice paradigm. Two sets of chronic ethanol drinking experiments were carried out using the two-bottle choice paradigm. The first set of experiments consisted of chronic ethanol drinking followed by testing for physical dependence (ethanol-induced acute withdrawal), rapid and chronic hypothermic tolerance, and rapid sedative/hypnotic tolerance. The second set of experiments consisted of chronic ethanol drinking followed by testing for metabolic tolerance (ethanol clearance) and rapid and chronic sedative/hypnotic tolerance. We found that chronic, high levels of alcohol consumption in F1 hybrid mice produced rapid and chronic tolerance to both the sedative/hypnotic and hypothermic effects of ethanol; additionally, a small degree of metabolic tolerance developed. The development of tolerance supports the validity of using this model of high alcohol consumption in genetic studies of alcoholism.
2. Methods
2.1 Animals
Studies were conducted using intercross F1 hybrid female mice derived from C57BL/6J and FVB/NJ mice (B6xFVB F1 and FVBxB6 F1). C57BL/6J and FVB/NJ breeders were purchased from The Jackson Laboratory (Bar Harbor, ME) and mated at 7–8 weeks. Mice were housed individually in standard cages. Behavioral testing did not begin until the mice were at least 70 days old. Ethanol-naïve and ethanol-experienced groups were closely matched for age, and littermates were split between the two groups. Except for one occasion in the first set of experiments, all mice were tested only once. Ethanol-naïve and -experienced mice were tested for acute ethanol withdrawal and retested approximately one month later for ethanol-induced loss of righting reflex. Experiments were conducted in the isolated behavioral testing rooms in the animal facility to avoid external distractions. The University of Texas facility is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experiments were approved by the Institutional Animal Care and Use Committee and adhered to NIH Guidelines.
2.2 Drugs
Ethanol was obtained from Aaper (Shelbyville, KT) and was dissolved in 0.9% saline and injected intraperitoneally (i.p.) in a volume of 0.2 ml per 10 g of body weight.
2.3 Continuous Access Two-Bottle Choice
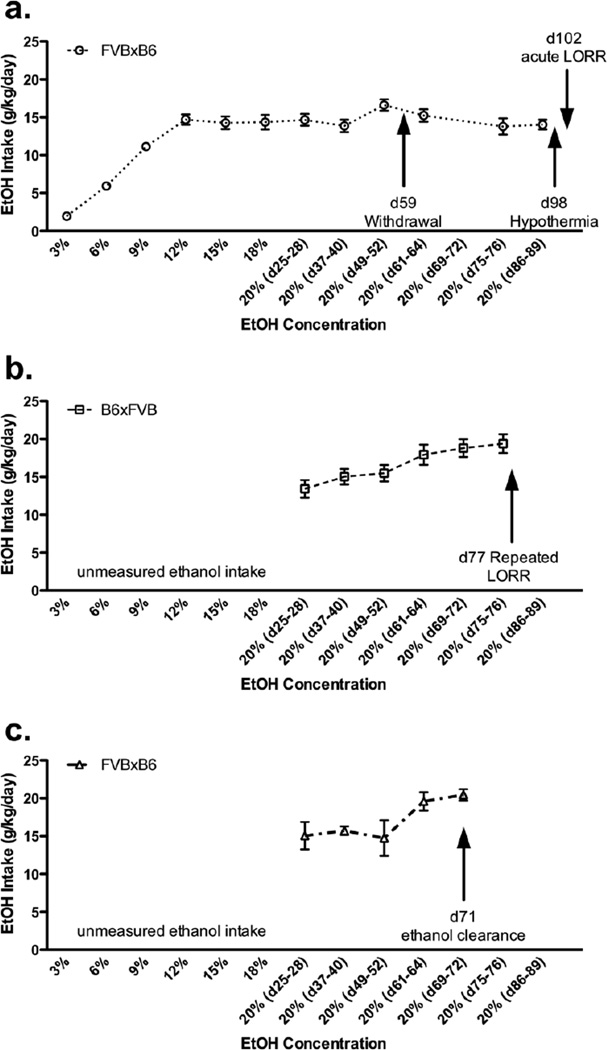
For ethanol-naïve groups, water was available ad libitum throughout the study. For ethanol-experienced groups, water and an ethanol solution were presented in identical bottles and continuously available in the two-bottle choice paradigm. Bottle positions were changed daily for both ethanol-experienced and -naïve groups. Mice were weighed every 8 days throughout the experiment. Initially, the mice were offered water and 3% ethanol (v/v in tap water) for 4 days, and then escalating concentrations (3% increases up to 18%, then 20% ethanol thereafter) were offered versus water for 4 days each. Initially, fluid consumption was measured daily, found to be stable and therefore, only measured every other day (beginning eight days following introduction of the 20% ethanol solution). FVBxB6 female mice were used for the first set of experiments (Figure 1a, ethanol-experienced group, n=23 total). B6xFVB female mice were used for the repeated LORR experiment in the second set of experiments (Figrue 1b, ethanol-experienced group, n=9). FVBxB6 female mice were used for the clearance experiment in the second set of experiments (Figure 1c, ethanol-experienced group, n=4). Figures 1b and c represent a second set of experiments carried out at the same time. Refer to Figure 1 for details on the timing of behavioral testing and the genotypes used for each test. Furthermore, ethanol, but not water, was removed several hours before behavioral testing. Behavioral testing occurred during mid-day (during lights on and presumably lower levels of ethanol were consumed in the prior hours). Mice were acclimated to the behavioral rooms one hour prior to loss of righting reflex and ethanol clearance testing. Whereas, hypothermia and handling-induced convulsion testing was measured from home cages.
Figure 1. B6xFVB and FVBxB6 F1 hybrid females consume stable, large amounts of ethanol from a high ethanol concentration solution.
Voluntary ethanol (EtOH) consumption during continuous access two-bottle choice presented as g/kg/day (mean ± SEM). d = day, for example d25–28 = days 25–28. a. FVBxB6 (open circles) female mice were used for the first set of experiments (ethanol-experienced group, n=23). b. B6xFVB (open squares) female mice were used for the repeated LORR experiment (ethanol-experienced group, n=9). c. FVBxB6 (open triangles) female mice were used for the ethanol clearance experiment (ethanol-experienced group, n=4). Figures 1b and c represent a second set of experiments carried out at the same time.
2.4 Ethanol-induced acute withdrawal
After 59 days of two-bottle choice, ethanol-experienced (n=11) and ethanol-naïve (n=10) mice were scored for handling-induced convulsion (HIC) severity 30 min before ethanol administration (3.8 g/kg). The HIC score was tested every hour until the HIC level reached baseline. Each mouse was picked up gently by the tail and, if necessary, gently rotated 180°, and the HIC was scored as follows: 5, tonic-clonic convulsion when lifted; 4, tonic convulsion when lifted; 3, tonic-clonic convulsion after a gentle spin; 2, no convulsion when lifted, but tonic convulsion elicited by a gentle spin; 1, facial grimace only after a gentle spin; 0, no convulsion after a gentle spin (18).
2.5 Ethanol Clearance
After 71 days of two-bottle choice, ethanol-experienced (n=4) and ethanol-naïve (n=4) mice received ethanol (4 g/kg), and rates of ethanol clearance were determined using a spectrophotometric enzyme assay. Blood samples (50 uL) were taken from the retro-orbital sinus (at 30, 60, 120, 180, and 240 min post-injection), added to 2 mL 3% perchloric acid, and centrifuged for 10 min at 1000 × g. Resulting supernatants were used to determine blood ethanol concentration using an ethanol dehydrogenase enzyme assay (Lundquist, 1959).
2.6 Loss of Righting Reflex
There were two separate ethanol-induced loss of righting reflex tests: acute (one trial) and chronic (five trials). The acute test was carried out during the first set of experiments after 102 days of two-bottle choice, where latency to LORR and duration of LORR were measured after ethanol was administered at a dose of 3.6 g/kg (n=6–7/group). The chronic test was carried out during the second set of experiments. After 77 days of two-bottle choice, ethanol was administered at a dose of 3.6 g/kg for five consecutive days (n=8–9/group). Mice were injected with ethanol and when they became ataxic, they were placed in the supine position in V-shaped plastic troughs until they were able to right themselves three times within 60 seconds. Peri-orbital blood samples were taken at gain of righting reflex on day 1 and day 5, and blood ethanol levels were determined as described above. Mice that failed to lose their righting reflex (misplaced injections) or had a latency or duration greater than two standard deviations from the group mean were excluded from data analysis.
2.7 Ethanol-Induced Hypothermia
After 98 days of two-bottle choice, ethanol-experienced (n=6) and ethanol-naïve (n=5) mice received 2.6 g/kg ethanol daily for 3 consecutive days. Baseline temperatures were taken just before the injection of ethanol. Following injections, mice were returned to individual home cages, and temperatures were again taken at 30, 60, 90, 120, 180, 240, 300, 360, and 420 min after injection. For all temperature measurements, a 0.5-mm probe connected to a temperature monitor was inserted into the rectum of each mouse to measure core body temperature (readings were taken 5 seconds after probe insertion).
2.8 Statistical Procedures
Data are reported as the mean ± S.E.M. value. Statistics were performed using Statistica version 6 (StatSoft, Tulsa, OK) and GraphPad Prism version 4.00 (GraphPad Software, San Diego, CA). Two-way ANOVAs were carried out for ethanol clearance and LORR data to evaluate differences between groups (ethanol-experienced and -naïve) and trials. Student’s t-tests were used to evaluate differences between groups for LORR and acute ethanol-induced withdrawal data. For ethanol-induced hypothermia data, a three-way ANOVA was carried out to evaluate differences between groups (ethanol-experienced and -naïve), trials, and time points within a trial. Bonferroni’s post-hoc and repeated measures (or paired comparisons) were used when appropriate.
3. Results
3.1 Continuous Access Two-Bottle Choice
Ethanol consumption increased as the ethanol concentration offered increased and when 20% ethanol was available, ethanol consumption remained high and stable for the remainder of the experiment (Figure 1). Collectively, B6xFVB and FVBxB6 females consumed an average of 15.3 ± 0.7 g/kg/day ethanol from a 20% ethanol solution.
3.2 Ethanol-induced acute withdrawal
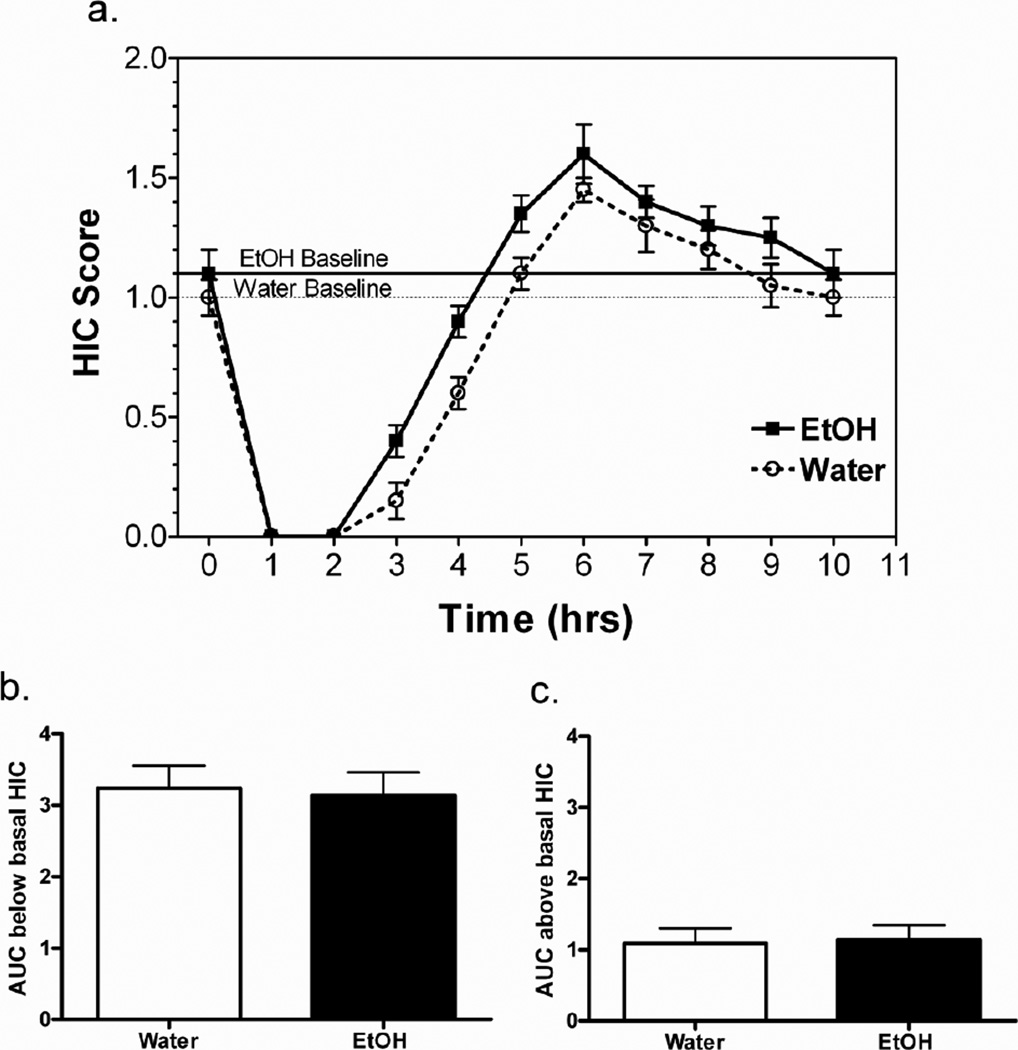
The severity of ethanol-induced acute withdrawal was assessed by monitoring HIC scores following 59 days of voluntary consumption of ethanol and water or water only. A single 3.8 g/kg ethanol dose suppressed basal HIC scores for 4.5 to 5 hours, followed by an increase in HIC scores that returned to baseline 5 hours later (Figure 2a). The area under the curve (AUC) was determined for each subject for the negative area (area below baseline-representing the seizure suppressive effects of ethanol) and the positive area (area above baseline-representing ethanol withdrawal). Ethanol-naïve and -experienced mice had similar negative (Figure 2b) and positive (Figure 2c) AUC (p > 0.05 - negative AUC; p > 0.05 - positive AUC).
Figure 2. Chronic voluntary ethanol consumption does not alter acute ethanol withdrawal severity.
a. Handling induced convulsion (HIC) scores after a single injection of ethanol (3.8 g/kg). Dashed horizontal line denotes baseline HIC level for ethanol-naïve mice, and solid horizontal line denotes baseline HIC level for ethanol-experienced mice. b. Area under HIC curve (AUC) and below the basal HIC level does not differ with chronic ethanol consumption. c. Area under HIC curve (AUC) and above the basal HIC level does not differ with chronic ethanol consumption. FVBxB6 females, n=10–11/group. Data shown are mean ± SEM.
3.3 Ethanol Clearance
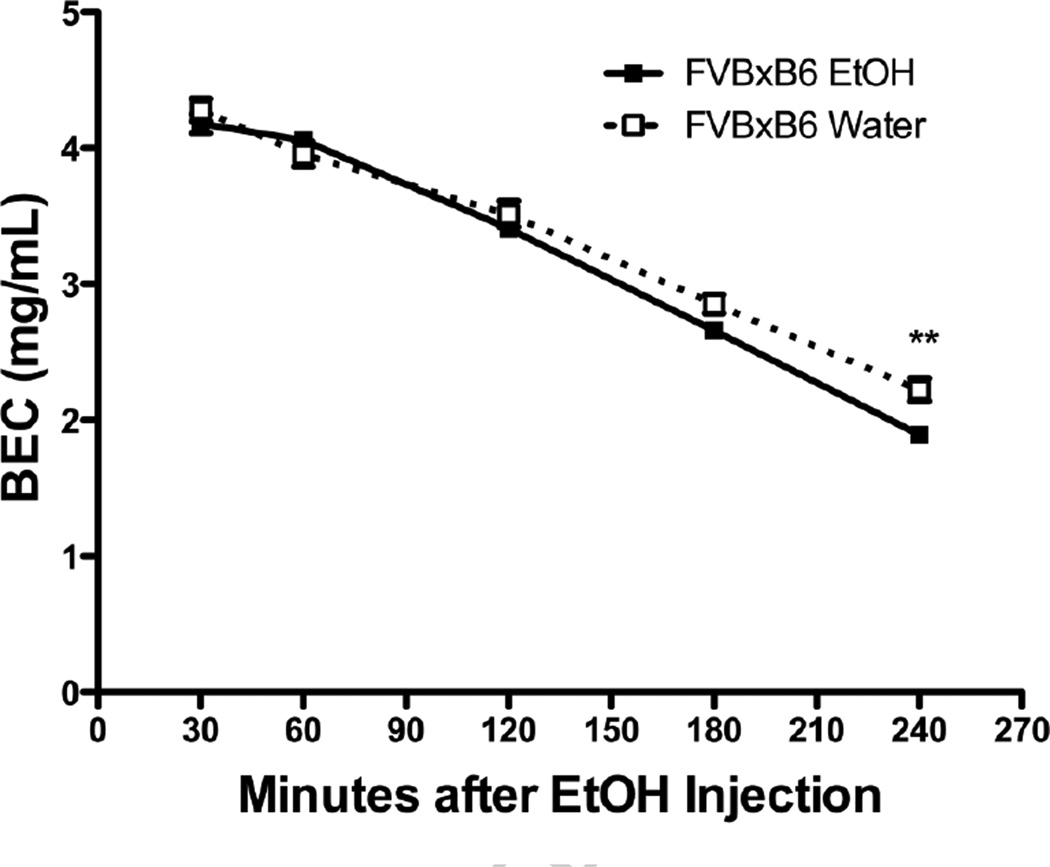
Chronic ethanol consumption can lead to increased ethanol metabolism (metabolic tolerance), so we determined blood ethanol concentrations for both ethanol-naïve and -experienced mice (after 71 days of voluntary consumption) at 30, 60, 120, 180, and 240 minutes after a single injection of 4.0 g/kg ethanol (Figure 3). A two-way ANOVA revealed a significant group × time interaction (F(4,24) = 11.35; p < 0.0001) and a main effect of time (F(4, 24) = 1457; p < 0.0001); however, there was no main effect of group (F(1,24) = 2.16; p > 0.05). Bonferroni post-hoc analysis revealed a significant difference between groups at the 240 minute time point (p < 0.01). Ethanol-experienced mice had a 15 ± 3% increase in ethanol metabolism as compared with ethanol-naïve mice. The volume of distribution was calculated for each mouse as the initial dose (determined from y-intercepts of individual slopes) divided by body weight. The volume of distribution did not differ between ethanol-naïve (21.99 ± 0.61 mL) and -experienced groups (22.12 ± 0.62 mL).
Figure 3. Ethanol clearance after chronic voluntary ethanol consumption.
BEC (blood ethanol concentration in mg/mL) after a single injection of 4.0 g/kg ethanol in ethanol-experienced compared to -naïve mice. FVBxB6 females, n=4/group. Data shown are mean ± SEM. ** = p < 0.01
3.4 Loss of Righting Reflex
There were two separate ethanol-induced loss of righting reflex tests: acute (one trial) and chronic (five trials). The acute test was carried out during the first set of experiments after 102 days of voluntary ethanol (or water) intake, where latency to LORR and duration of LORR were measured after ethanol was administered at a dose of 3.6 g/kg (n=6–7/group). Latency to LORR did not differ over time within or between groups (ethanol-experienced group = 1.55 ± 0.04 min; ethanol-naïve group = 1.41 ± 0.13 min). However, the duration of LORR was much shorter in ethanol-experienced (37 ± 3 min) compared with ethanol-naïve mice (59 ± 3 min) (p < 0.001).
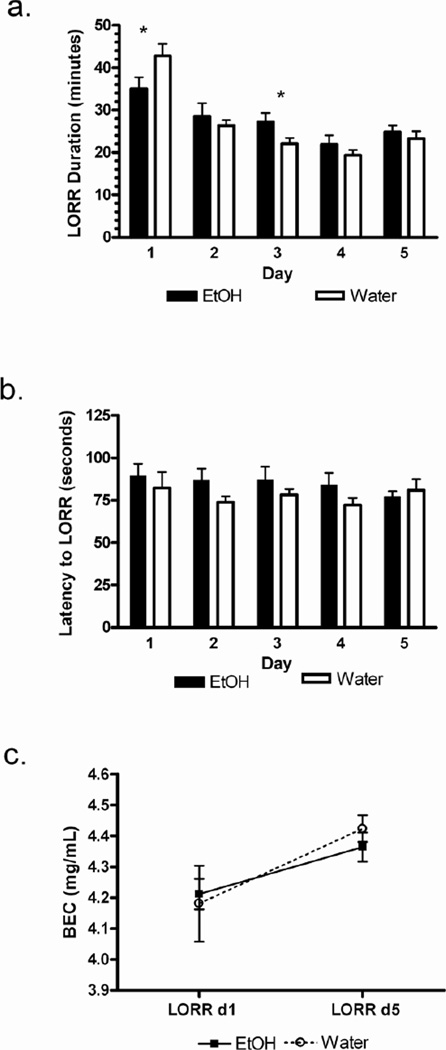
The chronic test was carried out during the second set of experiments. After 77 days of voluntary ethanol (or water) intake, 3.6 g/kg ethanol was administered for five consecutive days (n=6–9/group). We wanted to measure and compare the slopes derived from linear regression of the data (ethanol experienced vs naïve). We hypothesized ethanol-naïve mice would have a steeper (negative) slope than ethanol-experienced mice. However, we found no evidence to support the linearity of tolerance development in this behavioral measure. Thus, we used more conventional statisticss to analyze the results. A two-way ANOVA of the data which revealed a group × day interaction statistic of F(4,57)=2.40; p < 0.06. On the first day of testing, the duration of LORR was shorter in ethanol-experienced mice as compared with ethanol-naïve mice (p < 0.05) (Figure 4a). However, ethanol-experienced mice exhibited increased LORR duration on Day 3. By the fifth day of testing, both groups demonstrated a reduction in duration of LORR as compared with the first day of testing (ethanol-naïve group, p < 0.001; ethanol-experienced group, p < 0.05). Latency to LORR did not differ over time within or between groups (Figure 4b). Differences in ethanol-induced LORR can be due to differences in metabolism; thus, we determined blood ethanol concentrations at gain of righting reflex on day 1 and day 5 of testing. Blood ethanol concentrations did not differ between groups on either day (Figure 4c). However, both groups had a higher blood ethanol level at gain of righting reflex on day 5 compared with day 1 (ethanol-naïve group, p < 0.001; ethanol-experienced group, p < 0.01).
Figure 4. Chronic ethanol consumption reduced duration of loss of righting reflex.
a. Shorter duration of loss of righting reflex (LORR) on day 1 for ethanol-experienced compared to ethanol-naïve mice after injection of 3.6 g/kg ethanol. b. Latency to lose righting reflex (seconds) does not differ between ethanol-experienced and -naïve mice. c. Blood ethanol concentration (BEC) at gain of righting reflex were not significantly different on days 1 and 5. B6xFVB females, n=6–7/group. Data shown are mean ± SEM.
3.5 Ethanol-Induced Hypothermia
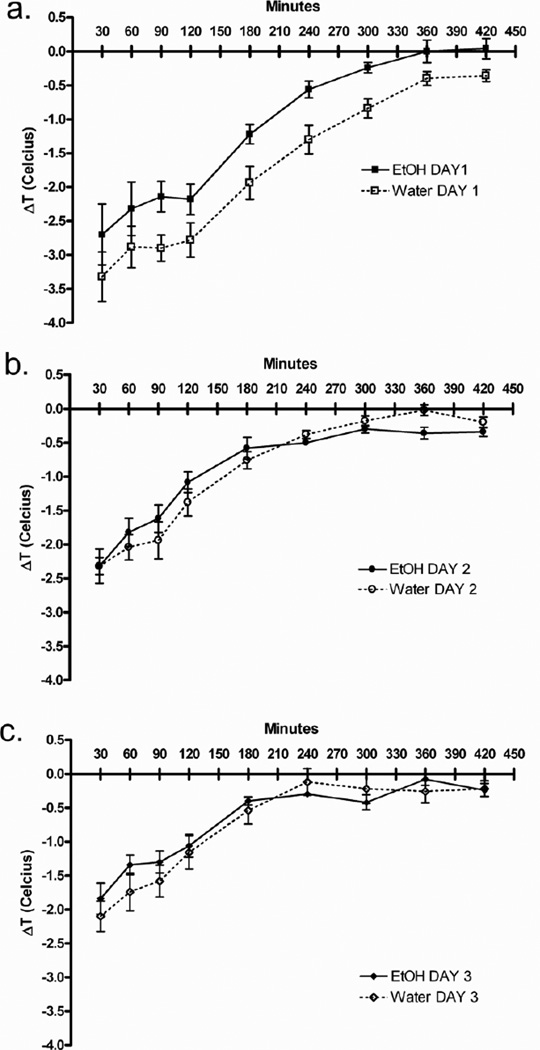
After 98 days of the voluntary ethanol (or water) intake, ethanol (2.6 g/kg) was administered i.p. for 3 consecutive days. Baseline temperatures were taken just prior to the injection of ethanol. Following injections, mice were returned to individual home cages, and temperatures were again taken at 30, 60, 90, 120, 180, 240, 300, 360, and 420 min after injection. For each trial, mean changes from baseline are reported for each group (Figures 5a,b,c). Three-way ANOVA revealed three interesting significant interactions: trial × time point (F(16, 128) = 9.88; p < 0.0001), time point × group (F(8, 64) = 2.25; p < 0.05), and trial × group (F(2, 16) = 2.87; p < 0.05). Ethanol-naïve mice developed more severe ethanol-induced hypothermia than ethanol-experienced mice on day 1(day 1: main effect of group – F(1,8) = 5.23; p = 0.05); however, both groups experienced less severe ethanol-induced hypothermia over repeated trials.
Figure 5. Chronic ethanol consumption results in tolerance to ethanol-induced hypothermia.
a. The first day of 2.6 g/kg ethanol injections revealed the greatest difference in hypothermia between ethano-naïve and –experiences mice. b. Hypothermia after the second day of 2.6 g/kg ethanol injection. c. Hypothermia after the third day of 2.6 g/kg ethanol injection. FVBxB6 females n=5–6/group. Data shown are mean ± SEM.
4. Discussion
Tolerance to the distinct effects of ethanol develops differentially and varies in extent and persistence. There is evidence that tolerance develops more to the aversive effects than to the rewarding effects. For example, initial exposure to ethanol is associated with sedation and conditioned taste aversion; there is evidence that with continued use, these effects dissipate while the activating or pleasurable effects persist (Lopez et al., 2012; Tabakoff and Kiianmaa, 1982; Stewart et al., 1991). Additionally, there are genetic components to alcohol tolerance, and much research has been devoted to study selected rodent lines of high and low alcohol responders (Crabbe, 1989; Crabbe and Phillips, 1993; Phillips et al., 1989, Radcliffe et al., 2005). However, most studies of alcohol tolerance have focused on acute tolerance produced by a single injection or chronic tolerance produced by a few daily injections (Hu et al., 2008; Wu et al., 2001). One of the few studies of tolerance following chronic two bottle choice drinking found that three weeks of consumption by C57BL/6 mice was sufficient to produce tolerance to the discriminative stimulus effects of ethanol (Middaugh et al., 2003). B6xFVB and FVBxB6 F1 hybrid mice are known to stably consume high levels of ethanol under several different schedules (Blednov et al., 2005). For reference, reported ethanol intake in these hybrids is variable, but still high, in all published studies. Blednov et al. (2005) reported 20–35 g/kg/day ethanol intake, Blednov et al. (2010) reported 18–30 g/kg/day ethanol intake, Ozburn et al. (2010) reported 12–18 g/kg/day ethanol intake, and Ozburn et al. (2012 - BMC Neuroscience, in press) paper reports 18–20 g/kg/day. Here, we report ethanol intakes of 16–18 g/kg/day. Measurement of consumption was as similar as possible to the earlier studies but the consumption is somewhat lower in the present study for reasons that are not immediately apparent. The goal of this study was to determine if chronic self-administration of ethanol led to the development of tolerance and/or dependence, which would further validate the use of B6xFVB and FVBxB6 hybrids as a genetic model of high alcohol consumption. B6xFVB and FVBxB6 F1 hybrid female mice consumed stable, high levels of ethanol (16–18 g/kg/day) from a high ethanol (20%) solution for anywhere between 59 and 102 days, depending on the particular test. We found that tolerance to the sedative/hypnotic effects of ethanol developed and were accompanied by metabolic tolerance. Further, tolerance to the hypothermic effects of ethanol developed; however, we did not detect the development of dependence.
It has been shown that repeated ethanol injections produce tolerance to the sedative/hypnotic and hypothermic effects of ethanol (Crabbe, 1994; Crowell et al., 1981; Le et al., 1979; Melchior and Tabakoff, 1981). We propose that the shorter duration of LORR seen in mice that consumed ethanol for 77 or 102 days due to metabolic and chronic tolerance. After 77 days, ethanol-induced LORR was tested daily for 5 days. On LORR day 1, ethanol-experienced mice had a shorter duration of LORR than ethanol-naïve mice. From day 1 to day 2, ethanol-experienced and -naïve mice developed rapid tolerance. By day 5, all mice developed chronic tolerance to the daily injections, consistent with earlier studies (Tabakoff et al., 1980). Both groups had a higher blood ethanol level at gain of righting reflex on day 5 as compared with day 1. It is important to note that there was no difference in the blood ethanol concentrations of ethanol-experienced and -naïve mice at gain of righting reflex on day 1, indicating metabolic tolerance is responsible for the difference in LORR duration. We cannot rule out chronic tolerance, or decreased brain sensitivity, to ethanol, since the difference in LORR day 1 BEC at awakening is very small (i.e. a small difference in BEC, so small it is hard to measure, may provide a clear difference in LORR duration).
Ethanol-experienced mice developed tolerance to ethanol-induced hypothermia when measured after 98 days of drinking. We propose that the reduced effect of ethanol-induced hypothermia in these mice is a model of chronic tolerance. Given these measurements were taken using a rectal probe, a potentially stressful event, it is possible that the changes in body temperature are changed stress responses based on a history of chronic voluntary ethanol drinking. Future studies could benefit from the use of telemetry devices to measure body temperature remotely, thus reducing handling stress that may arise from a temperature probe. After 98 days, ethanol-induced hypothermia was tested daily for 3 days. From day 1 to day 2, ethanol-experienced and -naïve mice developed rapid tolerance. By day 3, all mice developed chronic tolerance to the daily injections. There is a commonality in the results for the repeated LORR and hypothermia experiments. Ethanol-experienced mice exhibited an initial tolerance that is attenuated in comparison to the chronic tolerance that develops by the end of the repeated trials. The initial tolerance seen in ethanol-experienced mice is the result of the chronic, high level of ethanol drinking, which may, at least in part, be due to small changes in ethanol clearance rates.
After 59 days of continuous access ethanol drinking, acute withdrawal severity was assessed by scoring HIC after ethanol. Withdrawal severity was minimal and did not differ between ethanol-experienced and ethanol-naïve mice. B6xFVB demonstrated high ethanol preference and low acute ethanol withdrawal severity, further supporting findings by Metten et al. (1998) demonstrating a negative correlation between ethanol preference and acute ethanol withdrawal severity. Dependence and tolerance are frequently induced either by offering an ethanol containing liquid diet or ethanol vapor inhalation, making it difficult to directly compare our two-bottle choice paradigm to others (33, 34). We know of only one study demonstrating signs of dependence in mice after continuous access ethanol consumption. Phillips et al. (1994) reported that ethanol consumption (~ 10 g/kg/day for 16 days) resulted in significant withdrawal symptoms as measured by HIC scores. The severity of withdrawal positively correlated with sweetened ethanol consumption for the strains tested (C57BL/6, DBA/2J, and 19 BxD RI strains derived from an F2 cross of C57BL/6 and DBA/2J progenitors) (21). A critical observation (and future direction) is that a more sensitive measure of dependence, such as withdrawal induced anxiety, might reveal signs of dependence missed by HIC scores. Kliethermes et al. (2004) reported that mice made ethanol dependent by vapor inhalation showed increased anxiety-like behaviors in the elevated zero maze and light/dark box. Similar withdrawal signs have been demonstrated in the P rat, a rat line selected for high alcohol consumption (Waller et al., 1982).
Metabolic tolerance is associated with liver enzymes activated after chronic drinking which increase ethanol degradation, thereby reducing the duration of ethanol's effects (Lieber, 1988; Lieber, 1991; Tabakoff et al., 1986). As a consequence of chronic, high alcohol consumption, we found evidence for the development of metabolic tolerance. Data that support this finding are the group × time interaction for ethanol clearance and the decreased duration of LORR unaccompanied by difference in BEC at gain of righting relfex). Studies of chronic tolerance using five days of alcohol injections or five cycles of ethanol vapor inhalation did not find any metabolic tolerance, although they did detect functional tolerance (Tabakoff et al., 1980; Lopez et al., 2012). Additionally, we attempted to determine whether acute functional tolerance (fixed speed rotorod) developed, but ethanol-experienced mice were unable to successfully perform this task. Spontaneous locomotor activity in B6xFVB is quite high; thus, it is not surprising that these mice jumped off the rotorod (Ozburn, unpublished). Perhaps functional tolerance could be measured successfully using a balance beam task as described in Crabbe et al., 2009.
5. Conclusions
As a consequence of chronic, high levels of ethanol consumption, B6xFVB and FVBxB6 mice developed rapid and chronic tolerance to the sedative/hypnotic and hypothermic effects of ethanol, which may be due, at least in part, to the development of metabolic tolerance. The development of tolerance in these hybrid mice supports their role as a powerful genetic model for the study of alcoholism. The high blood alcohol levels and the tolerance seen in this model are distinct from other mouse models, making it a valuable genetic model for future studies on alcoholism.
Highlights.
-
-
We use valuable genetic mouse model of high ethanol intake
-
-
Chronic, high ethanol intake results in the development of tolerance
-
-
Tolerance is a distinction from other genetic mouse models.
6. Acknowledgments
We would like to thank Marni Martinez, Danielle Walker, and Jennifer Stokes for their assistance in animal husbandry and data collection and Dr. Jody Mayfield for assistance with manuscript editing. This research was supported by the Integrative Neuroscience Initiative on Alcoholism Consortium Grant AA13520 and National Institute on Alcohol Abuse and Alcoholism Grants AA06399-S and AA16424.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
The authors have no conflict of interest to declare.
References
- Anji A, Kumari M. Supplementing the liquid alcohol diet with chow enhances alcohol intake in C57 BL/6 mice. Drug Alcohol Depend. 2008;97:86–93. doi: 10.1016/j.drugalcdep.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blednov YA, Metten P, Finn DA, Rhodes JS, Bergeson SE, Harris RA, Crabbe JC. Hybrid C57BL/6J x FVB/NJ mice drink more alcohol than do C57BL/6J mice. Alcohol Clin Exp Res. 2005;29:1949–1958. doi: 10.1097/01.alc.0000187605.91468.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browman KE, Rustay NR, Nikolaidis N, Crawshaw L, Crabbe JC. Sensitivity and tolerance to ethanol in mouse lines selected for ethanol-induced hypothermia. Pharmacol Biochem Behav. 2000;67:821–829. doi: 10.1016/s0091-3057(00)00427-5. [DOI] [PubMed] [Google Scholar]
- Chou IH, Narasimhan K. Neurobiology of addiction. Nat Neurosci. 2005;8:1427. [Google Scholar]
- Crabbe JC, Rigter H, Uijlen J, Strijbos C. Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther. 1979;208:128–133. [PubMed] [Google Scholar]
- Crabbe JC. Genetic animal models in the study of alcoholism. Alcohol Clin Exp Res. 1989;13:120–127. doi: 10.1111/j.1530-0277.1989.tb00296.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Merrill CD, Belknap JK. Effects of convulsants on handling-induced convulsions in mice selected for ethanol withdrawal severity. Brain Res. 1991;550:1–6. doi: 10.1016/0006-8993(91)90397-e. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ. Selective breeding for alcohol withdrawal severity. Behav Genet. 1993;23:171–177. doi: 10.1007/BF01067422. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Tolerance to ethanol hypothermia in HOT and COLD mice. Alcohol Clin Exp Res. 1994;18:42–46. doi: 10.1111/j.1530-0277.1994.tb00878.x. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Mitchell SR, Crawshaw LI. Quantitative trait loci mapping of genes that influence the sensitivity and tolerance to ethanol-induced hypothermia in BXD recombinant inbred mice. J Pharmacol Exp Ther. 1994;269:184–192. [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell CR, Hinson RE, Siegel S. The role of conditional drug responses in tolerance to the hypothermic effects of ethanol. Psychopharmacology. 1981;73:51–54. doi: 10.1007/BF00431101. [DOI] [PubMed] [Google Scholar]
- Garcia-Andrade C, Wall TL, Ehlers CL. The firewater myth and response to alcohol in Mission Indians. Am J Psychiatry. 1997;154:983–988. doi: 10.1176/ajp.154.7.983. [DOI] [PubMed] [Google Scholar]
- Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- Goldstein DB. Relationship of alcohol dose to intensity of withdrawal signs in mice. J Pharmacol Exp Ther. 1972;180:203–215. [PubMed] [Google Scholar]
- Harris RA, Buck KJ. The processes of alcohol tolerance and dependence - Special Focus: Alcohol and the Brain. Alcohol Health & Research World. 1990;13(1) [Google Scholar]
- Homanics GE, Le NQ, Kist F, Mihalek R, Hart AR, Quinlan JJ. Ethanol tolerance and withdrawal responses in GABA(A) receptor alpha 6 subunit null allele mice and in inbred C57BL/6J and strain 129/SvJ mice. Alcohol Clin Exp Res. 1998;22:259–265. [PubMed] [Google Scholar]
- Hu W, Saba L, Kechris K, Bhave SV, Hoffman PL, Tabakoff B. Genomic insights into acute alcohol tolerance. J Pharmacol Exp Ther. 2008;326:792–800. doi: 10.1124/jpet.108.137521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to, and dependence on, some non-opiate psychotropic drugs. Pharmacol Rev. 1971;23:135–191. [PubMed] [Google Scholar]
- Kalant H. Research on tolerance: what can we learn from history? Alcohol Clin Exp Res. 1998;22:67–76. doi: 10.1111/j.1530-0277.1998.tb03618.x. [DOI] [PubMed] [Google Scholar]
- Kliethermes CL, Cronise K, Crabbe JC. Anxiety-like behavior in mice in two apparatuses during withdrawal from chronic ethanol vapor inhalation. Alcohol Clin Exp Res. 2004;28:1012–1019. doi: 10.1097/01.alc.0000131976.40428.8f. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242:715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Lê AD, Poulos CX, Cappell H. Conditioned tolerance to the hypothermic effect of ethyl alcohol. Science. 1979;206:1109–1110. doi: 10.1126/science.493999. [DOI] [PubMed] [Google Scholar]
- Lieber CS. The microsomal ethanol oxidizing system: Its role in ethanol and xenobiotic metabolism. Biochemical Society Transactions. 1988;16:232–239. doi: 10.1042/bst0160232. [DOI] [PubMed] [Google Scholar]
- Lieber CS. Metabolism of ethanol and associated hepatotoxicity. Drug and Alcohol Review. 1991;10:175–202. doi: 10.1080/09595239100185231. [DOI] [PubMed] [Google Scholar]
- Lopez MF, Griffin WC, 3rd, Melendez RI, Becker HC. Repeated Cycles of Chronic Intermittent Ethanol Exposure Leads to the Development of Tolerance to Aversive Effects of Ethanol in C57BL/6J Mice. Alcohol Clin Exp Res. 2012 Feb 6; doi: 10.1111/j.1530-0277.2011.01717.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist F. The determination of ethyl alcohol in blood and tissue. Methods Biochem Anal. 1959;7:217–251. [Google Scholar]
- Mellanby E. Alcohol: Its absorption into and disappearance from the blood under different conditions. Med Res Committee Special Rep (Lond) 1919;31:1–48. [Google Scholar]
- Melchior CL, Tabakoff B. Modification of environmentally cued tolerance to ethanol in mice. J Pharmacol Exp Ther. 1981;219:175–180. [PubMed] [Google Scholar]
- Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- Middaugh LD, Szumlinski KK, Van Patten Y, Marlowe AL, Kalivas PW. Chronic ethanol consumption by C57BL/6 mice promotes tolerance to its interoceptive cues and increases extracellular dopamine, an effect blocked by naltrexone. Alcohol Clin Exp Res. 2003;27:1892–1900. doi: 10.1097/01.ALC.0000099264.36220.48. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Feller DJ, Crabbe JC. Selected mouse lines, alcohol and behavior. Experientia. 1989;45:805–827. doi: 10.1007/BF01954056. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Crabbe JC, Metten P, Belknap JK. Localization of genes affecting alcohol drinking in mice. Alcohol Clin Exp Res. 1994;18:931–941. doi: 10.1111/j.1530-0277.1994.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Phillips TJ, Lessov CN, Harland RD, Mitchell SR. Evaluation of potential genetic associations between ethanol tolerance and sensitization in BXD/Ty recombinant inbred mice. J Pharmacol Exp Ther. 1996;277:613–623. [PubMed] [Google Scholar]
- Radcliffe RA, Floyd KL, Drahnak JA, Deitrich RA. Genetic dissociation between ethanol sensitivity and rapid tolerance in mouse and rat strains selectively bred for differential ethanol sensitivity. Alcohol Clin Exp Res. 2005;29:1580–1589. doi: 10.1097/01.alc.0000179208.05882.1f. [DOI] [PubMed] [Google Scholar]
- Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol's incoordinating effects in mice: inbred strains and artificial selection. Behav Genet. 2004;34:441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Gold EO. A simultaneous evaluation of multiple markers of ethanol/placebo challenges in sons of alcoholics and controls. Archives of General Psychiatry. 1988;45:211–216. doi: 10.1001/archpsyc.1988.01800270019002. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J. Findings across subgroups regarding the level of response to alcohol as a risk factor for alcohol use disorders: a college population of women and Latinos. Alcohol Clin Exp Res. 2004;10:1499–1508. doi: 10.1097/01.alc.0000141814.80716.32. [DOI] [PubMed] [Google Scholar]
- Silvers JM, Tokunaga S, Mittleman G, Matthews DB. Chronic intermittent injections of high-dose ethanol during adolescence produce metabolic, hypnotic, and cognitive tolerance in rats. Alcohol Clin Exp Res. 2003;27:1606–1612. doi: 10.1097/01.ALC.0000090141.66526.22. [DOI] [PubMed] [Google Scholar]
- Stewart RB, McBride WJ, Lumeng L, Li TK, Murphy JM. Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacology. 1991;105:530–534. doi: 10.1007/BF02244375. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Ritzmann RF, Raju TS, Deitrich RA. Characterization of acute and chronic tolerance in mice selected for inherent differences in sensitivity to ethanol. Alcohol Clin Exp Res. 1980;4:70–73. doi: 10.1111/j.1530-0277.1980.tb04794.x. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Kiianmaa K. Does tolerance develop to the activating, as well as the depressant, effects of ethanol? Pharmacology Biochemistry & Behavior. 1982;17:1073–1076. doi: 10.1016/0091-3057(82)90496-8. [DOI] [PubMed] [Google Scholar]
- Tabakoff B, Cornell N, Hoffman PL. Alcohol tolerance. Annals of Emergency Medicine. 1986;15:1005–1012. doi: 10.1016/s0196-0644(86)80119-6. [DOI] [PubMed] [Google Scholar]
- Wu PH, Tabakoff B, Szabó G, Hoffman PL. Chronic ethanol exposure results in increased acute functional tolerance in selected lines of HAFT and LAFT mice. Psychopharmacology (Berl) 2001;155:405–412. doi: 10.1007/s002130100722. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacol Biochem Behav. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]