Abstract
Peroxisome proliferator activated receptors (PPARs) are ligand-activated transcription factors expressed in trophoblasts, which regulate both cell differentiation and proliferation. In recent years, evidence has linked PPARs to playing an integral role in pregnancy; specifically, PPAR-β and PPAR-γ have been shown to play an integral role in placentation, with PPAR-γ additionally serving to regulate trophoblast differentiation. Recent evidence has shown that PPAR-γ expression is altered in many complications of pregnancy such as intrauterine growth restriction (IUGR), preterm birth, pre-clampsia and gestational diabetes. Thus, at present, accumulating evidence from the literature suggests both a pivotal role for PPAR-γ in the progression of a healthy pregnancy and the possibility that PPAR-γ may act as a therapeutic target in complicated pregnancies. This review aims to provide a succinct and comprehensive assessment of the role of PPAR-γ in normal pregnancy and pregnancy complications, and finally its potential as a therapeutic target in the treatment and/or prevention of adverse pregnancy outcomes.
Keywords: peroxisome proliferator activated receptor gamma, pregnancy, pre-eclampsia, trophoblasts, placenta
Introduction
Peroxisome proliferator activated receptors (PPARs) are ligand-activated transcription factors that regulate the expression of a number of genes involved in cell differentiation and proliferation (Mueller et al., 1998), energy homeostasis, fatty acid catabolism and adipogenesis (Kastner et al., 1994; Mangelsdorf et al., 1995; Escher and Wahli, 2000; Lazennec et al., 2000; Willson et al., 2000). In terms of the PPAR subtype, PPAR-γ, it has been suggested that this receptor has an integral role in the progression of pregnancy; and although much of the data relating to this proposed role has been obtained from experimental animal studies, as PPARs are highly conserved across species (>80% of amino acid homology; Guan et al., 1997; Kersten et al., 2000), these findings may be relevant to human pregnancy and complications of human pregnancy. PPARs possess a variety of beneficial functions and as a consequence have been suggested as therapeutic targets in a number of pathophysiological conditions, including polycystic ovarian syndrome (Azziz et al., 2001), type II diabetes, myocardial infarction (Cao et al., 2007), multiple sclerosis, endometriosis (Feinstein et al., 2002; Aytan et al., 2007) and more recently Alzheimer's and Parkinson's disease (Mandrekar-Colucci and Landreth, 2011; Sadeghian et al., 2012). The role of PPAR-γ in pregnancy (in particular trophoblast development) (Parast et al., 2009) and complications of pregnancy such as intrauterine growth restriction (IUGR; Challis et al., 2009), pre-clampsia (McCarthy et al., 2011a) and gestational diabetes (Heude et al., 2011) has been extensively studied over recent years. This review aims to provide a succinct summary of the role of PPAR-γ in normal pregnancy and pregnancy complications.
PPARs
The PPAR nuclear receptor family is comprised of three isotypes: α, β and γ, all of which are encoded for by distinct single copy genes located on human chromosomes 22 (α), 6 (β) and 3 (γ) (Greene et al., 1995; Yoshikawa et al., 1996). Throughout the body, all three isotypes differ in their tissue distribution and function. PPAR-α is predominantly expressed in both the liver and brown fat and plays a role in lipid homeostasis via the up-regulation of the expression of enzymes involved in fatty acid oxidation/catabolism (Issemann and Green, 1990; Lee et al., 1995). PPAR-β (also called δ and NUC1) is the least understood of the three human PPAR isotypes. PPAR-β has been implicated in lipid metabolism, cell survival, wound healing, embryonic implantation and development of the central nervous system (Berger et al., 2005). PPAR-β also appears to have an essential role to play in placentation in addition to that of PPAR-γ. Elevated PPAR-β expression has been detected at implantation sites and in decidual cells in the rat uterus (Lim and Dey, 2000). The absence of PPAR-β in a PPAR-β knockout (PPAR-β–/–) murine model resulted in severe placental defects including abnormally loose connections between the placenta and the decidua, a smaller than normal placental labyrinth, and the occurrence of severe maternal haemorrhages into the labyrinth zone (Barak et al., 2002). As a consequence of these malformed placentas, PPAR-β–/– mice are associated with a high degree of embryonic death, and any surviving pups are characterized by severe fetal growth restriction.
PPAR-γ has four isoforms, γ1, γ2, γ3 and γ4, and is involved in the control of adipocyte differentiation, lipid storage (adipogenesis) and macrophage differentiation. PPAR-γ1 is expressed throughout the body, including endothelial cells (Satoh et al., 1999; Diep and Schiffrin, 2001), vascular smooth muscle cells (Law et al., 2000), cardiomyocytes (Park et al., 1997) and macrophages, including the foam cells of atherosclerotic lesions (Fajas et al., 1997; Ricote et al., 1998; Spiegelman, 1998; Tontonoz et al., 1998; Desvergne and Wahli, 1999). In contrast, PPAR-γ2 expression is more conservative, and this PPAR-γ subtype is found in adipocytes and the liver (Rangwala and Lazar, 2004) and regulates the action of insulin on skeletal muscle and adipose tissue (Willson et al., 2001; Berger et al., 2005; Sharma and Staels, 2007).
The mechanism of action of the PPARs is described in detail elsewhere (McCarthy et al., 2011b). In brief, PPAR is composed of five modular domains with domain E acting as the ligand binding domain. Domain E mediates ligand-dependent transcriptional activation (Berger and Moller, 2002), which induces conformational changes in these receptors, leading to recruitment of cofactor proteins/co-activators and the subsequent heterodimerization of these receptors with the retinoid X receptor (RXR) (Kliewer et al., 1992a,b). Following activation, PPARs may regulate gene expression in one of two ways, either via transactivation or transrepression. During transactivation, the co-activators interact with nuclear receptors in a ligand-dependent way and influence the set of genes transcribed. In contrast, PPAR-γ mediated transrepression suppresses gene transcription by negatively interfering with other signal-transduction pathways, such as the NF-κB signalling pathway, in a DNA binding independent manner (Chinetti et al., 2000; Rosen and Spiegelman, 2001; Yki-Jarvinen, 2004). The action of PPAR-γ does not always involve direct DNA binding to regulate gene transcription. The conformational changes that occur on ligand binding allow further interaction with co-activators containing histone acetyl-transferase or co-repressor release. These co-activators include steroid receptor co-activator 1 and PPAR-γ co-activator 1, through which PPAR-γ can then indirectly exert its effects (Lehrke and Lazar, 2005).
PPAR-γ in healthy pregnancy
In mice, the role of PPAR-γ in the regulation of trophoblast activity appears to be essential as early as gestational day 10, at which time the nutritional support of the developing embryo switches from the primary yolk sac to the placenta. In the rat, low levels of expression of PPAR-γ are detected at day 11 (equivalent to mouse gestational day 9), with maximal levels detected at day 13 and subsequent down-regulation of the nuclear receptor by day 15 (Asami-Miyagishi et al., 2004). The vital role of PPAR-γ in embryonic development appears to be restricted to the placenta. In humans, circulating activators of PPAR-γ (fatty acids and lipid metabolites) are elevated in normal pregnancy (approximately twofold), suggesting that PPAR-γ may act as a regulator of maternal metabolism and immune function in normal pregnancy (Waite et al., 2000). Furthermore, humans with heterozygous PPAR-γ mutations exhibit partial lipodystrophy, severe insulin resistance, hypertension and steatohepatitis, all of which are classic hallmarks of pre-clampsia, a pregnancy-specific condition characterized by maternal hypertension and proteinuria (Barroso et al., 1999; Agarwal and Garg, 2002; Hegele et al., 2002; Savage et al., 2003).
Within the human placenta, PPAR-γ is expressed predominantly in trophoblasts and is required not only for trophoblast differentiation but also for trophoblast maturation to establish maternal fetal transport (Schaiff et al., 2000; Rodie et al., 2005). During the first trimester, PPAR-γ protein is expressed primarily in villous cytotrophoblasts and invading extravillous trophoblasts as early as 7 weeks of human gestation (Wang et al., 2004). In the second trimester, PPAR-γ expression is detected in columns of the anchoring villi and cytotrophoblasts (Waite et al., 2000). In contrast, PPAR-γ is not detected in non-invasive, differentiated syncytiotrophoblasts in either of the first or second trimesters (Waite et al., 2000; Capparuccia et al., 2002; Rodie et al., 2005). Furthermore, for PPAR-γ expression in the third trimester, human placenta is localized to villous syncytiotrophoblasts, as well as villous and extravillous cytotrophoblasts (Schaiff et al., 2000; Tarrade et al., 2001; Wang et al., 2004).
The exact role PPAR-γ plays in both trophoblast invasion and differentiation has yet to be elucidated with apparently paradoxical functions being described in both rodent and human placentas. Much of the data related to understanding PPAR-γ in humans has resulted from in vitro experiments. In terms of rodents, PPAR-γ activation appears to be essential for placental development and specifically trophoblast invasion and differentiation (Barak et al., 1999; Asami-Miyagishi et al., 2004). In contrast, the exact role of PPAR-γ in the human placenta remains controversial as studies have demonstrated that PPAR-γ activation both enhances and inhibits differentiation of cytotrophoblasts (Schaiff et al., 2000; 2006). Moreover, while PPAR-γ activation has been shown to induce the differentiation of trophoblasts into syncytiotrophoblasts, activation of this receptor has also been shown to hamper cytotrophoblast invasion while PPAR-γ antagonists have been shown to enhance this process (Tarrade et al., 2001; Handschuh et al., 2006). Although PPAR-γ appears essential for placentation, some studies have demonstrated that PPAR-γ ligands reduce the production of VEGF, which is essential for placental vascularization (Peeters et al., 2005). Furthermore, recent evidence has shown that overactivation of PPAR-γ at the maternal–fetal interface impaired implantation and placentation and therefore embryonic development (Fournier et al., 2011).
Data has demonstrated that homozygous PPAR-γ-deficient mice embryos die due to placental dysfunction (Kubota et al., 1999). In particular, it appears that ablation of the PPAR-γ gene induces a disruption in both the terminal differentiation of the trophoblast and placental vascularization, leading to severe myocardial thinning and death. Furthermore, these PPAR-γ-null placentas develop a malformed labyrinth zone, with no permeation of fetal blood vessels and dilation and rupture of maternal blood sinuses. In addition, labyrinthine trophoblasts fail to properly differentiate and spongiotrophoblasts abnormally phagocytose maternal red blood cells (Barak et al., 1999). The myocardial thinning that occurs in PPAR-γ-null embryos can be circumvented by the use of tetraploid chimeras. This rescue was only partial with 2 of 5 mutants rescued by 12.5 days of gestation; 1 of 10 by 16.5 days and 1 of 6 at birth (Barak et al., 1999). A recent study has subsequently shown that an epiblastic-specific deletion of PPAR-γ in the placenta was sufficient to rescue the embryonic lethality of PPAR-γ–/– zygotes (Nadra et al., 2010). This demonstrates that the likely lethality associated with the PPAR-γ–/– embryos, occurs as a result of a placental defect.
In PPAR-γ-null embryos, which were rescued to term, there was no visible white adipose tissue and the embryos possessed a fatty liver emphasising the importance of PPAR-γ in adipogenesis (Barak et al., 1999). This was further emphasized by in vitro work demonstrating that embryonic stem cells lacking PPAR-γ were unable to differentiate into adipocytes (Rosen et al., 1999). Several co-activators of PPAR-γ including PBP/DRIP205/TRAP220 and RAP250/PRIP, may also be necessary for placental development. Mice with mutations in these proteins exhibit placental phenotypes that resemble aspects of PPAR-γ-null mice (Zhu et al., 2000; 2003; Antonson et al., 2003). More recently, the mucin gene (Muc1) has emerged as a target gene for PPAR-γ in trophoblast development in mice and also as a measure of trophoblast downstream activity of PPAR-γ (Shalom-Barak et al., 2004). In the placenta, Muc1 protein is localized on the labyrinthine trophoblast around maternal blood sinuses. More importantly, Muc1 appears to be the only direct target for PPAR-γ, and it appears to be independent of lipid and energy metabolism pathways.
PPAR-γ and pregnancy-specific diseases
PPAR-γ plays a predominant role in normal vascular function (Beyer et al., 2008) and in the differentiation of labyrinthine trophoblast lineages (Schaiff et al., 2000), which along with the fetal endothelium forms the vascular exchange interface with maternal blood essential for the progression of a healthy pregnancy (Parast et al., 2009). More recently, accumulating evidence has suggested that aberrations in PPAR-γ expression and/or function contribute at least in part to a variety of pathophysiological states associated with complicated pregnancies (summarized in Table 1.). In particular, with regard to preterm birth, recent evidence has suggested that this may be linked to a genetic susceptibility factor. In a study conducted by Meirhaeghe et al. (2007) in a cohort of patients in Northern Ireland, it was found that there may be a link between PPAR-γ and susceptibility to preterm birth (Meirhaeghe et al., 2007). The PPAR-γ polymorphism, Pro12Ala, could potentially influence the duration of gestation and in addition birth weight, implicating an important role for PPAR-γ in genetic susceptibility to preterm birth. Furthermore, the above polymorphism could act as a potential biomarker for preterm birth and be possibly used in the early detection of the condition.
Table 1.
Complicated pregnancy-related conditions and associated PPAR-γ effects
| Condition | Associated effect on PPAR-γ | Species | Reference |
|---|---|---|---|
| Pre-eclampsia | ↑ DNA binding activity of PPAR –γ | Rat | Crews et al., 2000 |
| Pre-eclampsia | ↓ circulating activators of PPAR-γ and ↓ placental expression of PPAR-γ | Human | Waite et al., 2005 |
| Pre-eclampsia | ↓ placental expression of PPAR-γ | Rat | Mattace Raso et al., 2008 |
| Pre-eclampsia | Pharmacological antagonism of PPAR-y | Rat | McCarthy et al., 2011a |
| Intrauterine growth restriction | ↓ fetal lung expression of PPAR-γ | Rat | Joss-Moore et al., 2010 |
| Restricted embryonic development | Over-activation of PPAR-γ | Human | Fournier et al., 2011 |
| Embryonic lethality | Homozygous deletion of PPAR-γ gene | Mouse | Barak et al., 1999; Kubota et al., 1999 |
| Embryonic lethality | Mutations of PPAR-γ cofactors (PBP/DRIP205/TRAP220 and RAP250/PRIP) | Mouse | Antonson et al., 2003; Zhu et al., 2000; Zhu et al., 2003 |
| Preterm birth | Presence of PPAR-γ polymorphism (Pro12Ala) | Human | Meirhaeghe et al., 2007 |
| Gestational diabetes mellitus | Presence of PPAR-γ polymorphisms (Pro12Ala and C 1431 T) | Human | Heude et al., 2011 |
Pre-eclampsia
A reduction in the placental expression of PPAR-γ activators has been demonstrated in some women who develop severe pre-clampsia (Waite et al., 2005), and significantly higher PPAR-γ DNA binding activity has been demonstrated in placentas from women with both IUGR and pre-eclampsia (Crews et al., 2000). In spontaneously hypertensive rats, there is reduced protein expression of placental PPAR-γ (Mattace Raso et al., 2008). Waite et al. (2005) demonstrated that serum extracts from pregnant women contained PPAR-γ activators, and women with severe early-onset pre-clampsia had significantly reduced circulating PPAR-γ activators compared with serum extracts from healthy pregnant women, suggesting that reduced PPAR-γ activity may contribute to pre-clampsia (Waite et al., 2005). In contrast, Holdsworth-Carson et al. (2010) examined placental expression of PPAR-γ and demonstrated that placentas from women with pre-clampsia did not demonstrate any differences in mRNA or protein expression of PPAR-γ compared with healthy controls (Holdsworth-Carson et al., 2010).
In our own work with rodents, we initially investigated the role of PPAR-γ in the progression of rodent pregnancy by administering a PPAR-γ-specific antagonist, T0070907 (from gestational days 11–15) to healthy pregnant rats (McCarthy et al., 2011a). Antagonism of PPAR-γ during pregnancy appeared to adversely affect these rats as they were characterized by hypertension, proteinuria, endothelial dysfunction, fetal growth restriction, platelet hyperaggregability, disturbances in the angiogenic balance (VEGF/soluble fms like tyrosine kinase 1; sFlt-1) and a placental labyrinthine trophoblast that exhibited adaptive angiogenesis, increased cellular proliferation and was less differentiated than those from healthy pregnant rats (McCarthy et al., 2011a). Taken together, these findings suggest a pivotal role for PPAR-γ in the progression of a healthy pregnancy and, in addition, a possible role for this receptor in the pathogenesis of pre-clampsia. To investigate the latter, we carried out a separate study in which we administered a PPAR-γ agonist, rosiglitazone, to pregnant rats that had undergone chronic surgical reduction of uteroplacental perfusion to produce a preeclamptic-like state [reduced uterine perfusion pressure (RUPP) rat model of pre-clampsia] (McCarthy et al., 2011b). RUPP rats were characterized by hypertension, endothelial dysfunction and elevated microalbumin creatinine ratios, all classical hallmarks of pre-clampsia; and rosiglitazone administration ameliorated all of these symptoms, suggesting activation of PPAR-γ as a possible therapeutic intervention for pre-clampsia. Furthermore, the majority of beneficial effects observed following rosiglitazone administration were abrogated in the presence of a heme oxygenase-1 (HO-1) inhibitor, which suggests at least part of the mechanistic pathway involved in the protective effects mediated by PPAR-γ in this animal model of pre-clampsia (McCarthy et al., 2011b).
In relation to the mechanism(s) involved in PPAR-γ-mediated effects in pregnancy, NO may act as one of several possible downstream mediators. While pregnancy is associated with an increase in NO levels, studies have yielded conflicting results as to whether nitrite/nitrate (NOx; metabolites of NO) levels are increased (Smarason et al., 1997; Shaamash et al., 2000), decreased (Var et al., 2003; Aydin et al., 2004) or unchanged (Davidge et al., 1996; Daniel et al., 1998; Conrad et al., 1999) relative to normotensive pregnancies in pre-clampsia. In addition, asymmetric dimethylarginine (ADMA) levels (an endogenous inhibitor of NOS) are increased in pre-clampsia compared with normotensive pregnancies (Fickling et al., 1993; Savvidou et al., 2003). ADMA produces superoxide (SO) rather than NO, augmenting oxidative stress and preventing production of NO from l-arginine, which may account for any observed reductions in NO levels. Activation of PPAR-γ has been shown to both restore NO bioavailability (Sorrentino et al., 2007) and increase NO release from human aortic endothelial cells (Polikandriotis et al., 2005) in addition to decreasing ADMA levels.
Pre-eclampsia is characterized by increased platelet aggregation/activation, which may be due to several NO depletion-related mechanisms. Under physiological conditions, NO inhibits platelet aggregation (Loscalzo, 2001); thus, in pathophysiological situations characterized by reduced NO bioavailability, this inhibitory effect would be attenuated considerably. Furthermore, increasing concentrations of ADMA augments platelet aggregation, most likely as a consequence of reduced NO production (Brunini et al., 2006), and peroxynitrite (albeit at high concentrations) has been shown to activate platelets, thus increasing platelet aggregation (Brown et al., 1998). Activated platelets (which express PPAR-γ) act as a major source of reactive oxygen species (ROS) in several pathological conditions (myocardial ischaemia, diabetes and atherosclerosis) and therefore may contribute considerably to the oxidative stress associated with pre-clampsia.
Gestational diabetes mellitus
Gestational diabetes mellitus has been a disease of increasing prevalence in recent years. This disease is characterized by impaired glucose tolerance during pregnancy, affecting 2–8% of all pregnancies (Arck et al., 2010), a figure that has risen by 50% in the last 20 years (Ferrara, 2007). Along with other complications, gestational diabetes mellitus can result in the development of type II diabetes mellitus in both mother and child. PPAR-γ is a major regulator of both glucose and lipid metabolism through its involvement in the regulation of adipogenesis and intracellular insulin signalling processes that ultimately controls glucose homeostasis (Tontonoz et al., 1994). In gestational diabetes, PPAR-γ expression is often dysregulated, leading to changes in insulin sensitivity profiles (Arck et al., 2010). Furthermore, a recent study has suggested that the PPAR-γ variants, Pro12Ala and C1431T, and their haplotypes may play a role in the susceptibility of developing gestational diabetes (Heude et al., 2011), further demonstrating their potential as biomarkers for disease and possible therapeutic targets for translational medicine.
IUGR
IUGR is a disease characterized by poor fetal growth during pregnancy, resulting in low birth weight and often preterm delivery (Lawn et al., 2005). Additionally, IUGR is often associated with altered lung development in both humans and rat models (Joss-Moore et al., 2010). IUGR has a similar placental pathology to pre-clampsia, but there is no exaggerated dyslipidaemia or hypertension (Rodie et al., 2005). PPAR-γ is thought to have a role in fetal lung development via its direct regulation of chromatin-modifying enzymes such as Setd8. In a study carried out by Joss-Moore et al. (2010), it was shown that lung PPAR-γ expression was reduced by IUGR in both male and female neonatal rats, and this reduction was associated with a decrease in Setd8. This could suggest that down-regulation of PPAR-γ may significantly contribute to abnormal fetal lung development associated with IUGR.
PPAR-γ as a therapeutic target
PPAR-γ activation has been shown to play a key role in metabolic processes related to cell differentiation, proliferation and lipid metabolism; and, as a consequence, substantial emphasis has been placed on elucidating the mechanisms involved in its activation due to the potential for its use as a pharmacological target for the treatment of pathophysiological metabolic syndromes such as type II diabetes, atherosclerosis and obesity. Thiazolidinediones such as rosiglitazone activate PPAR-γ in order to improve insulin sensitivity profile; however, the thiazolidinediones currently on the market are first-generation, nonspecific agonists of PPAR-γ, resulting in the deleterious side effects (Kung and Henry, 2012) that have caused their limited usage and in some cases have been withdrawn from the market (Mutalik, 2011). While there has yet to be an assessment of the risks associated with thiazolidinedione administration during pregnancy and a safety profile remains to be established (Arck et al., 2010), there is hope on the horizon in terms of modulators of PPAR-γ activity. The new development of highly targeted selective PPAR-γ modulators (SPPARγMs) and dual PPAR-γ/PPAR-α agonists (Kung and Henry, 2012) has led to the re-emergence of PPAR-γ as a potential therapeutic target.
Anti-inflammatory effects of PPAR-γ
A beneficial role for PPAR-γ in conditions associated with an inflammatory response, such as IUGR, preterm labour and spontaneous abortion (Challis et al., 2009), has been suggested on the basis of its numerous anti-inflammatory effects (Marx et al., 2000; Pasceri et al., 2000; Verrier et al., 2004; Sasaki et al., 2005). PPAR-γ activation has been shown to prevent microparticle (plasma membrane vesicles with pro-coagulant and pro-inflammatory properties) induced vascular hypo-reactivity through the regulation of pro-inflammatory proteins. In particular, ligand-induced activation of PPAR-γ attenuated the microparticle-mediated up-regulation of NF-κB transcription, expression and activation, which ultimately prevented increases in both interleukin (IL)-6 and IL-8 (Tesse et al., 2008). In both monocytes and macrophages, PPAR-γ activation has been shown to reduce the production of cytokines including TNF-α (Hofmann et al., 1994), IL-1β and IL-6 via the inhibition of pro-inflammatory transcription factors such as AP-1, STAT and NF-κB (see Figure 1; Jiang et al. 1998; Ricote et al. 1998; Barak et al. 2002; Matsumoto et al. 2008). Furthermore, PPAR-γ activation suppresses the expression of pro-inflammatory adhesion molecules including intercellular adhesion molecule-1 (ICAM-1), vascular adhesion molecule-1 (VCAM-1) and E-selectin (Wang et al., 2002), which would reduce the increase in adherence of monocytes to both endothelial and polymorphonuclear cells (Imamoto et al., 2004). Zhang et al. (2012) demonstrated that PPAR-γ and PPAR-α act as regulators of IFNγ and IL-17α production by human T cells in a sex-specific way. In mice where androgen levels were elevated, PPAR-α levels were increased, and levels of PPAR-γ were decreased. In some pregnancies, androgen levels are raised, such as in the patients suffering from polycystic ovary syndrome, which can be a risk factor in the development of pre-clampsia (Roos et al., 2011). Similarly, platelets express PPAR-γ and activation of this receptor can inhibit the pro-inflammatory effects of platelets, inhibiting both platelet aggregation and the release of both thromboxane A2 (TXA2) and adenosine triphosphate from platelets reducing the generation of further mediators associated with the syndrome of pre-clampsia (Akbiyik et al., 2004). This could additionally serve as a therapeutic target for autoimmune disease and could serve a further cardioprotective and vasculoprotective role in the prevention of clot formation for patients at risk of recurrent stroke.
Figure 1.
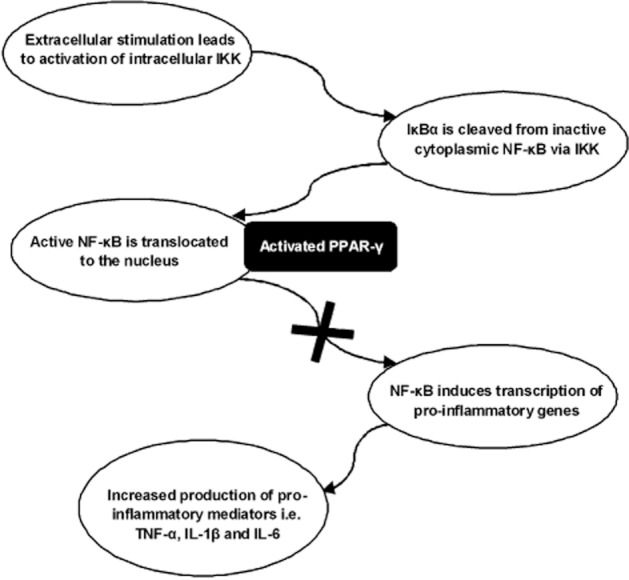
Anti-inflammatory effects of PPAR-γ via NF-κB transrepression (adapted from Li and Yang, 2011). In the transcriptional control of inflammation, pro-inflammatory cytokines act on membrane bound receptors to induce the activation of the enzyme IκB kinase (IKK). IKK then phosphorylates the IκBα protein bound to the inactive cytosolic NF-κB, leading to the dissociation of the two dimers and activation of NF-κB. The activated NF-κB is then translocated to the nucleus where it induces the transcription of a number of pro-inflammatory genes, leading to an increase in cytokine (TNF-α, IL-1β & IL-6 etc.) production. Following the binding of a ligand to PPAR-γ, the latter prevents the recruitment of transcriptionally active NF-κB, leading to inhibition of inflammatory gene expression and a resultant anti-inflammatory effect.
As pre-clampsia is thought to have an underlying autoimmune component (Xia and Kellems, 2009; Biggar et al., 2010), targeting PPAR-γ pharmacologically could serve to ameliorate the autoimmune response and potentially act to prevent the inception of the disease in genetically susceptible individuals. Additionally, this could also serve as a future pharmacological target for severe cases of autoimmune disease such as pemphigus vulgaris, rheumatoid arthritis and Crohn's disease.
In the vasculature, PPAR-γ activation leads to inhibition of endothelial inflammation via the suppression of key pro-inflammatory gene expression that is NF-κB. PPAR-γ is expressed by both endothelial cells where it appears to play an important role in the regulation of diet induced hypertension (Nicol et al., 2005) and vascular smooth muscle cells where PPAR-γ activation inhibits cell proliferation and migration while promoting apoptosis. In addition, PPAR-γ activation inhibits NO overproduction, IL-6 and TNF-α expression, and suppresses COX-2 and iNOS induction via the suppression of NF-κB and activator protein-1 activation (Inoue et al., 2000; Maggi et al., 2000).
Vasculoprotective effects of PPAR-γ
Endothelial dysfunction plays a vital role in the pathophysiology of many conditions, including hypertension, stroke, atherosclerosis, hypercholesterolaemia and diabetes (Cai and Harrison, 2000; Kobayashi et al., 2000; 2004; 2005; Landmesser et al., 2004; Matsumoto et al., 2007). In several of these pathophysiological cardiovascular conditions, activation of PPAR-γ restores vascular structure and corrects endothelial dysfunction (Walcher and Marx, 2004; Schiffrin, 2005; Li and Palinski, 2006; Cao et al., 2007). In particular, PPAR-γ agonists improve endothelium dependent vasodilatation and also reduce the elevated blood pressure associated with a number of hypertensive conditions (Kaufman et al., 1995; Caballero et al., 2003; Forst et al., 2005), including pre-eclampsia (McCarthy et al., 2011b). PPAR-γ mediates its vasculoprotective effects via several mechanisms, including the suppression of the pro-inflammatory cytokine, TNF-α, in type II diabetics (Pistrosch et al., 2004; Horio et al., 2005; Sourij et al., 2006) and the attenuation of endothelin-1 (ET-1) levels in an animal model of diabetes (Kanie et al., 2003). In terms of the latter pathway, chronic administration of a PPAR-γ agonist to streptozotocin diabetic rats normalized both mRNA for prepro-ET-1 and the plasma concentrations of ET-1 via the inhibition of the AP-1 pathway (Delerive et al., 1999a,b; Satoh et al., 1999).
In addition to their endothelial-stabilizing effects, PPAR-γ agonists may also restore the balance of angiogenic factors in pre-eclampsia, as this condition is characterized by a significant increase in the anti-angiogenic molecule, sFlt-1 (Ahmad and Ahmed, 2004). PPAR-γ agonists have been shown to up-regulate HO-1 expression an anti-oxidant enzyme that negatively regulates the production of the anti-angiogenic mediator, sFlt-1 (Cudmore et al., 2007; Kronke et al., 2007). Moreover PPAR-γ agonists serve to increase the production of the pro-angiogenic hormone, VEGF, in macrophages, vascular smooth muscle cells and endothelial cells (Jozkowicz et al., 2000; Yamakawa et al., 2000; Inoue et al., 2001a,b; Negro et al., 2005; Suwaki et al., 2007).
As mentioned previously, activation of PPAR-γ has been shown to restore NO bioavailability via the inhibition of SO production by NADPH oxidase in endothelial progenitor cells from patients with type II diabetes (Sorrentino et al., 2007). Furthermore, PPAR-γ ligands increase NO release from porcine and human aortic endothelial cells (Martens et al., 2002; Polikandriotis et al., 2005) and decrease ADMA levels, all of which improves NO bioavailability (Cho et al., 2004; Polikandriotis et al., 2005; Goya et al., 2006; Wang et al., 2006). These findings provide further evidence that PPAR-γ agonists have the potential both to induce direct modification of endothelial cell function and to modulate the production of NO, a critical mediator in the maintenance of normal vascular physiology and in the pathogenesis of pre-clampsia.
Effect of PPAR-γ activation on fetal development and preterm birth
In the case of IUGR, PPAR-γ may equally serve as a potential drug target as in the case of pre-clampsia. As mentioned earlier, Joss-Moore et al. (2010) demonstrated that abnormal lung development in IUGR may be linked to a decrease in PPAR-γ expression. Moreover, the adverse effects associated with this decrease in PPAR-γ expression were ameliorated in neonatal IUGR rat offspring by the administration of maternal docosahexanoic acid (DHA), an endogenous PPAR-γ activator. This suggests a potential supplementary role for maternal DHA, dietary DHA and/or synthetic DHA in the treatment of IUGR. Furthermore, this could also indicate another therapeutic avenue for future PPAR-γ-targeted treatments.
PPAR-γ may also prove to be effective as a drug target for patients at risk for preterm birth. It has been suggested that the aforementioned PPAR-γ polymorphism Pro12Ala may represent a genetic susceptibility factor to low birth weight and preterm labour (Meirhaeghe et al., 2007). In targeting Pro12Ala with an exact PPAR-γ antagonist for this polymorphism, this could serve to reduce the risk of preterm birth in genetically susceptible individuals. Similarly, in the case of gestational diabetes, by targeting the Pro12Ala and C1431T variants of PPAR-γ in this manner, PPAR-γ could be exploited to serve a protective role for genetically susceptible individuals to gestational diabetes.
Conclusion
As discussed above, the applications of PPAR-γ as a pharmacological target are numerous, serving a wide range of functions, not just in the aetiology and treatment of pre-clampsia but also across a range of other cardiovascular, neurological, autoimmune and metastatic diseases. As of yet, there have been no pharmacological developments in PPAR-γ-targeted drug treatments for pre-clampsia, however, as a future drug target for this disease, PPAR-γ holds promise. In recent years, there has been increased interest in this area of research and new novel PPAR-γ agonists are under development to replace the ‘dying’ drug class that is the glitazones. The majority of these are aimed at the improvement of insulin sensitivity and metabolic profile (Cho et al., 2011; Chaudhary et al., 2012; Rikimaru et al., 2012); however, the aims of this research area could similarly be applied to the development of a drug geared towards the prevention and treatment of pre-clampsia and other adverse pregnancy outcomes, such as in the treatment of neonatal hyperoxia-induced lung injury for which rosiglitazone may serve a protective role (Cai and Xu, 2012). As yet, it is unknown whether PPAR-γ agonists are toxic to the embryo and/or possess teratogenic effects; thus, caution must be exercised in the extrapolation of any data from experimental models to the clinical situation. While previous studies have demonstrated cardioprotective effects of rosiglitazone in an experimental model of myocardial ischaemia/reperfusion injury (Yue et al., 2005; Molavi et al., 2006; Gonon et al., 2007), concern has been expressed regarding the safety profile of this glitazone as reports linking it with a major risk of death from cardiovascular causes have also emerged (Home et al., 2007; Krall, 2007; Nissen and Wolski, 2007; Dluhy and McMahon, 2008). Therefore, it is essential that appropriate teratogenicity studies in animals be carried out as part of any investigation into the therapeutic potential of PPAR-γ agonists in complicated pregnancies. Finally, PPAR-γ is rapidly becoming an exciting new area for pharmacological research, its potential for physiological benefit encompassing a vast range of pathological disease states, but it is its potential for the treatment of pre-clampsia and other adverse pregnancy conditions that is perhaps the most intriguing. As there is no current treatment for pre-clampsia other than delivery of the baby, which can pose significant risks for the developing fetus depending on the severity of the disease and period when diagnosed, this makes PPAR-γ's potential for therapeutic benefit all the more poignant. Thus far, there has been little investigation into the use of PPAR-γ as a possible pharmacological target for the treatment and prevention of pre-clampsia. In the face of mounting evidence supporting a role for PPAR-γ in the pathogenesis of pre-clampsia, it seems pertinent to explore this avenue of prospective treatment for expectant mothers everywhere.
Glossary
- ET-1
endothelin-1
- HO-1
heme oxygenase-1
- ICAM-1
intercellular adhesion molecule-1
- IKK
IκB kinase
- RXR
retinoid X receptor
- SO
superoxide
- TXA2
thromboxane A2
- VCAM-1
vascular adhesion molecule-1
Conflicts of interest
None.
References
- Agarwal AK, Garg A. A novel heterozygous mutation in peroxisome proliferator-activated receptor-gamma gene in a patient with familial partial lipodystrophy. J Clin Endocrinol Metab. 2002;87:408–411. doi: 10.1210/jcem.87.1.8290. [DOI] [PubMed] [Google Scholar]
- Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ Res. 2004;95:884–891. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- Akbiyik F, Ray DM, Gettings KF, Blumberg N, Francis CW, Phipps RP. Human bone marrow megakaryocytes and platelets express PPARgamma, and PPARgamma agonists blunt platelet release of CD40 ligand and thromboxanes. Blood. 2004;104:1361–1368. doi: 10.1182/blood-2004-03-0926. [DOI] [PubMed] [Google Scholar]
- Antonson P, Schuster GU, Wang L, Rozell B, Holter E, Flodby P, et al. Inactivation of the nuclear receptor coactivator RAP250 in mice results in placental vascular dysfunction. Mol Cell Biol. 2003;23:1260–1268. doi: 10.1128/MCB.23.4.1260-1268.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arck P, Toth B, Pestka A, Jeschke U. Nuclear receptors of the peroxisome proliferator-activated receptor (PPAR) family in gestational diabetes: from animal models to clinical trials. Biol Reprod. 2010;83:168–176. doi: 10.1095/biolreprod.110.083550. [DOI] [PubMed] [Google Scholar]
- Asami-Miyagishi R, Iseki S, Usui M, Uchida K, Kubo H, Morita I. Expression and function of PPARgamma in rat placental development. Biochem Biophys Res Commun. 2004;315:497–501. doi: 10.1016/j.bbrc.2004.01.074. [DOI] [PubMed] [Google Scholar]
- Aydin S, Benian A, Madazli R, Uludag S, Uzun H, Kaya S. Plasma malondialdehyde, superoxide dismutase, sE-selectin, fibronectin, endothelin-1 and nitric oxide levels in women with preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2004;113:21–25. doi: 10.1016/S0301-2115(03)00368-3. [DOI] [PubMed] [Google Scholar]
- Aytan H, Caliskan AC, Demirturk F, Aytan P, Koseoglu DR. Peroxisome proliferator-activated receptor-gamma agonist rosiglitazone reduces the size of experimental endometriosis in the rat model. Aust N Z J Obstet Gynaecol. 2007;47:321–325. doi: 10.1111/j.1479-828X.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Azziz R, Ehrmann D, Legro RS, Whitcomb RW, Hanley R, Fereshetian AG, et al. Troglitazone improves ovulation and hirsutism in the polycystic ovary syndrome: a multicenter, double blind, placebo-controlled trial. J Clin Endocrinol Metab. 2001;86:1626–1632. doi: 10.1210/jcem.86.4.7375. [DOI] [PubMed] [Google Scholar]
- Barak Y, Nelson MC, Ong ES, Jones YZ, Ruiz-Lozano P, Chien KR, et al. PPAR gamma is required for placental, cardiac, and adipose tissue development. Mol Cell. 1999;4:585–595. doi: 10.1016/s1097-2765(00)80209-9. [DOI] [PubMed] [Google Scholar]
- Barak Y, Liao D, He W, Ong ES, Nelson MC, Olefsky JM, et al. Effects of peroxisome proliferator-activated receptor delta on placentation, adiposity, and colorectal cancer. Proc Natl Acad Sci USA. 2002;99:303–308. doi: 10.1073/pnas.012610299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE. The mechanisms of action of PPARs. Annu Rev Med. 2002;53:409–435. doi: 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends Pharmacol Sci. 2005;26:244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Beyer AM, Baumbach GL, Halabi CM, Modrick ML, Lynch CM, Gerhold TD, et al. Interference with PPARgamma signaling causes cerebral vascular dysfunction, hypertrophy, and remodeling. Hypertension. 2008;51:867–871. doi: 10.1161/HYPERTENSIONAHA.107.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar RJ, Poulsen G, Ng J, Melbye M, Boyd HA. HLA antigen sharing between mother and fetus as a risk factor for eclampsia and preeclampsia. Hum Immunol. 2010;71:263–267. doi: 10.1016/j.humimm.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Brown AS, Moro MA, Masse JM, Cramer EM, Radomski M, Darley-Usmar V. Nitric oxide-dependent and independent effects on human platelets treated with peroxynitrite. Cardiovasc Res. 1998;40:380–388. doi: 10.1016/s0008-6363(98)00182-5. [DOI] [PubMed] [Google Scholar]
- Brunini TM, Roberts NB, Yaqoob MM, Ellory JC, Mann GE, Mendes Ribeiro AC. Activation of L-arginine transport in undialysed chronic renal failure and continuous ambulatory peritoneal dialysis patients. Clin Exp Pharmacol Physiol. 2006;33:114–118. doi: 10.1111/j.1440-1681.2006.04333.x. [DOI] [PubMed] [Google Scholar]
- Caballero AE, Saouaf R, Lim SC, Hamdy O, Abou-Elenin K, O'Connor C, et al. The effects of troglitazone, an insulin-sensitizing agent, on the endothelial function in early and late type 2 diabetes: a placebo-controlled randomized clinical trial. Metabolism. 2003;52:173–180. doi: 10.1053/meta.2003.50023. [DOI] [PubMed] [Google Scholar]
- Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- Cai Q, Xu MY. Protective effect of rosiglitazone against hyperoxia-induced lung injury in neonatal rats. Zhongguo Dang Dai Er Ke Za Zhi. 2012;14:301–305. [PubMed] [Google Scholar]
- Cao Z, Ye P, Long C, Chen K, Li X, Wang H. Effect of pioglitazone, a peroxisome proliferator-activated receptor gamma agonist, on ischemia-reperfusion injury in rats. Pharmacology. 2007;79:184–192. doi: 10.1159/000100870. [DOI] [PubMed] [Google Scholar]
- Capparuccia L, Marzioni D, Giordano A, Fazioli F, De Nictolis M, Busso N, et al. PPARgamma expression in normal human placenta, hydatidiform mole and choriocarcinoma. Mol Hum Reprod. 2002;8:574–579. doi: 10.1093/molehr/8.6.574. [DOI] [PubMed] [Google Scholar]
- Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, III, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- Chaudhary S, Dube A, Kothari V, Sachan N, Upasani CD. NS-1: a novel partial peroxisome proliferator-activated receptor γ agonist to improve insulin sensitivity and metabolic profile. Eur J Pharmacol. 2012;684:154–160. doi: 10.1016/j.ejphar.2012.03.033. [DOI] [PubMed] [Google Scholar]
- Chinetti G, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res. 2000;49:497–505. doi: 10.1007/s000110050622. [DOI] [PubMed] [Google Scholar]
- Cho DH, Choi YJ, Jo SA, Jo I. Nitric oxide production and regulation of endothelial nitric-oxide synthase phosphorylation by prolonged treatment with troglitazone: evidence for involvement of peroxisome proliferator-activated receptor (PPAR) gamma-dependent and PPARgamma-independent signaling pathways. J Biol Chem. 2004;279:2499–2506. doi: 10.1074/jbc.M309451200. [DOI] [PubMed] [Google Scholar]
- Cho MC, Lee DH, Kim EJ, Lee JY, Kang JW, Song JH, et al. Novel PPARγ partial agonists with weak activity and no cytotoxicity; identified by a simple PPARγ ligand screening system. Mol Cell Biochem. 2011;358:75–83. doi: 10.1007/s11010-011-0923-1. [DOI] [PubMed] [Google Scholar]
- Conrad KP, Kerchner LJ, Mosher MD. Plasma and 24-h NO(x) and cGMP during normal pregnancy and preeclampsia in women on a reduced NO(x) diet. Am J Physiol. 1999;277:F48–F57. doi: 10.1152/ajprenal.1999.277.1.F48. [DOI] [PubMed] [Google Scholar]
- Crews JK, Herrington JN, Granger JP, Khalil RA. Decreased endothelium-dependent vascular relaxation during reduction of uterine perfusion pressure in pregnant rat. Hypertension. 2000;35:367–372. doi: 10.1161/01.hyp.35.1.367. [DOI] [PubMed] [Google Scholar]
- Cudmore M, Ahmad S, Al-Ani B, Fujisawa T, Coxall H, Chudasama K, et al. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- Daniel Y, Kupferminc MJ, Baram A, Jaffa AJ, Wolman I, Shenhav M, et al. Plasma soluble endothelial selectin is elevated in women with pre-eclampsia. Hum Reprod. 1998;13:3537–3541. doi: 10.1093/humrep/13.12.3537. [DOI] [PubMed] [Google Scholar]
- Davidge ST, Stranko CP, Roberts JM. Urine but not plasma nitric oxide metabolites are decreased in women with preeclampsia. Am J Obstet Gynecol. 1996;174:1008–1013. doi: 10.1016/s0002-9378(96)70341-1. [DOI] [PubMed] [Google Scholar]
- Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, et al. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999a;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, et al. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999b;85:394–402. doi: 10.1161/01.res.85.5.394. [DOI] [PubMed] [Google Scholar]
- Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- Diep QN, Schiffrin EL. Increased expression of peroxisome proliferator-activated receptor-alpha and -gamma in blood vessels of spontaneously hypertensive rats. Hypertension. 2001;38:249–254. doi: 10.1161/01.hyp.38.2.249. [DOI] [PubMed] [Google Scholar]
- Dluhy RG, McMahon GT. Intensive glycemic control in the ACCORD and ADVANCE trials. N Engl J Med. 2008;358:2630–2633. doi: 10.1056/NEJMe0804182. [DOI] [PubMed] [Google Scholar]
- Escher P, Wahli W. Peroxisome proliferator-activated receptors: insight into multiple cellular functions. Mutat Res. 2000;448:121–138. doi: 10.1016/s0027-5107(99)00231-6. [DOI] [PubMed] [Google Scholar]
- Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre AM, Saladin R, et al. The organization, promoter analysis, and expression of the human PPARgamma gene. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- Feinstein DL, Galea E, Gavrilyuk V, Brosnan CF, Whitacre CC, Dumitrescu-Ozimek L, et al. Peroxisome proliferator-activated receptor-gamma agonists prevent experimental autoimmune encephalomyelitis. Ann Neurol. 2002;51:694–702. doi: 10.1002/ana.10206. [DOI] [PubMed] [Google Scholar]
- Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care. 2007;30:S141–S146. doi: 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- Fickling SA, Williams D, Vallance P, Nussey SS, Whitley GS. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and pre-eclampsia. Lancet. 1993;342:242–243. doi: 10.1016/0140-6736(93)92335-q. [DOI] [PubMed] [Google Scholar]
- Forst T, Lubben G, Hohberg C, Kann P, Sachara C, Gottschall V, et al. Influence of glucose control and improvement of insulin resistance on microvascular blood flow and endothelial function in patients with diabetes mellitus type 2. Microcirculation. 2005;12:543–550. doi: 10.1080/10739680500253402. [DOI] [PubMed] [Google Scholar]
- Fournier T, Guibourdenche J, Handschuh K, Tsatsaris V, Rauwel B, Davrinche C, et al. PPARγ and human trophoblast differentiation. J Reprod Immunol. 2011;90:41–49. doi: 10.1016/j.jri.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Gonon AT, Bulhak A, Labruto F, Sjoquist PO, Pernow J. Cardioprotection mediated by rosiglitazone, a peroxisome proliferator-activated receptor gamma ligand, in relation to nitric oxide. Basic Res Cardiol. 2007;102:80–89. doi: 10.1007/s00395-006-0613-4. [DOI] [PubMed] [Google Scholar]
- Goya K, Sumitani S, Otsuki M, Xu X, Yamamoto H, Kurebayashi S, et al. The thiazolidinedione drug troglitazone up-regulates nitric oxide synthase expression in vascular endothelial cells. J Diabetes Complications. 2006;20:336–342. doi: 10.1016/j.jdiacomp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Greene ME, Blumberg B, McBride OW, Yi HF, Kronquist K, Kwan K, et al. Isolation of the human peroxisome proliferator activated receptor gamma cDNA: expression in hematopoietic cells and chromosomal mapping. Gene Expr. 1995;4:281–299. [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Zhang Y, Davis L, Breyer MD. Expression of peroxisome proliferator-activated receptors in urinary tract of rabbits and humans. Am J Physiol. 1997;273:F1013–F1022. doi: 10.1152/ajprenal.1997.273.6.F1013. [DOI] [PubMed] [Google Scholar]
- Handschuh K, Guibourdenche J, Guesnon M, Laurendeau I, Evain-Brion D, Fournier T. Modulation of PAPP-A expression by PPARgamma in human first trimester trophoblast. Placenta. 2006;27:S127–S134. doi: 10.1016/j.placenta.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51:3586–3590. doi: 10.2337/diabetes.51.12.3586. [DOI] [PubMed] [Google Scholar]
- Heude B, Pelloux V, Forhan A, Bedel JF, Lacorte JM, Clément K, et al. Association of the Pro12Ala and C1431T variants of PPARgamma and their haplotypes with susceptibility to gestational diabetes. J Clin Endocrinol Metab. 2011;96:E1656–E1660. doi: 10.1210/jc.2011-0381. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Lorenz K, Braithwaite SS, Colca JR, Palazuk BJ, Hotamisligil GS, et al. Altered gene expression for tumor necrosis factor-alpha and its receptors during drug and dietary modulation of insulin resistance. Endocrinology. 1994;134:264–270. doi: 10.1210/endo.134.1.8275942. [DOI] [PubMed] [Google Scholar]
- Holdsworth-Carson SJ, Lim R, Mitton A, Whitehead C, Rice GE, Permezel M, et al. Peroxisome proliferator-activated receptors are altered in pathologies of the human placenta: gestational diabetes mellitus, intrauterine growth restriction and preeclampsia. Placenta. 2010;31:222–229. doi: 10.1016/j.placenta.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, et al. Rosiglitazone evaluated for cardiovascular outcomes – an interim analysis. N Engl J Med. 2007;357:28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- Horio T, Suzuki M, Takamisawa I, Suzuki K, Hiuge A, Yoshimasa Y, et al. Pioglitazone-induced insulin sensitization improves vascular endothelial function in nondiabetic patients with essential hypertension. Am J Hypertens. 2005;18:1626–1630. doi: 10.1016/j.amjhyper.2005.05.035. [DOI] [PubMed] [Google Scholar]
- Imamoto E, Yoshida N, Uchiyama K, Kuroda M, Kokura S, Ichikawa H, et al. Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 2004;20:37–47. doi: 10.1002/biof.5520200104. [DOI] [PubMed] [Google Scholar]
- Inoue H, Tanabe T, Umesono K. Feedback control of cyclooxygenase-2 expression through PPARgamma. J Biol Chem. 2000;275:28028–28032. doi: 10.1074/jbc.M001387200. [DOI] [PubMed] [Google Scholar]
- Inoue I, Goto S, Matsunaga T, Nakajima T, Awata T, Hokari S, et al. The ligands/activators for peroxisome proliferator-activated receptor alpha (PPARalpha) and PPARgamma increase Cu2+,Zn2+-superoxide dismutase and decrease p22phox message expressions in primary endothelial cells. Metabolism. 2001a;50:3–11. doi: 10.1053/meta.2001.19415. [DOI] [PubMed] [Google Scholar]
- Inoue M, Itoh H, Tanaka T, Chun TH, Doi K, Fukunaga Y, et al. Oxidized LDL regulates vascular endothelial growth factor expression in human macrophages and endothelial cells through activation of peroxisome proliferator-activated receptor-gamma. Arterioscler Thromb Vasc Biol. 2001b;21:560–566. doi: 10.1161/01.atv.21.4.560. [DOI] [PubMed] [Google Scholar]
- Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–650. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- Jiang C, Ting AT, Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Joss-Moore LA, Wang Y, Baack ML, Yao J, Norris AW, Yu X, et al. IUGR decreases PPARγ and SETD8 Expression in neonatal rat lung and these effects are ameliorated by maternal DHA supplementation. Early Hum Dev. 2010;86:785–791. doi: 10.1016/j.earlhumdev.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozkowicz A, Dulak J, Piatkowska E, Placha W, Dembinska-Kiec A. Ligands of peroxisome proliferator-activated receptor-gamma increase the generation of vascular endothelial growth factor in vascular smooth muscle cells and in macrophages. Acta Biochim Pol. 2000;47:1147–1157. [PubMed] [Google Scholar]
- Kanie N, Matsumoto T, Kobayashi T, Kamata K. Relationship between peroxisome proliferator-activated receptors (PPAR alpha and PPAR gamma) and endothelium-dependent relaxation in streptozotocin-induced diabetic rats. Br J Pharmacol. 2003;140:23–32. doi: 10.1038/sj.bjp.0705414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, et al. Genetic analysis of RXR alpha developmental function: convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Kaufman LN, Peterson MM, DeGrange LM. Pioglitazone attenuates diet-induced hypertension in rats. Metabolism. 1995;44:1105–1109. doi: 10.1016/0026-0495(95)90000-4. [DOI] [PubMed] [Google Scholar]
- Kersten S, Desvergne B, Wahli W. Roles of PPARs in health and disease. Nature. 2000;405:421–424. doi: 10.1038/35013000. [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Mangelsdorf DJ, Evans RM. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992a;355:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992b;358:771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Matsumoto T, Kamata K. Mechanisms underlying the chronic pravastatin treatment-induced improvement in the impaired endothelium-dependent aortic relaxation seen in streptozotocin-induced diabetic rats. Br J Pharmacol. 2000;131:231–238. doi: 10.1038/sj.bjp.0703572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi T, Taguchi K, Yasuhiro T, Matsumoto T, Kamata K. Impairment of PI3-K/Akt pathway underlies attenuated endothelial function in aorta of type 2 diabetic mouse model. Hypertension. 2004;44:956–962. doi: 10.1161/01.HYP.0000147559.10261.a7. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Matsumoto T, Kamata K. The PI3-K/Akt pathway: roles related to alterations in vasomotor responses in diabetic models. J Smooth Muscle Res. 2005;41:283–302. doi: 10.1540/jsmr.41.283. [DOI] [PubMed] [Google Scholar]
- Krall RL. Cardiovascular safety of rosiglitazone. Lancet. 2007;369:1995–1996. doi: 10.1016/S0140-6736(07)60824-1. [DOI] [PubMed] [Google Scholar]
- Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, et al. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- Kubota N, Terauchi Y, Miki H, Tamemoto H, Yamauchi T, Komeda K, et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Mol Cell. 1999;4:597–609. doi: 10.1016/s1097-2765(00)80210-5. [DOI] [PubMed] [Google Scholar]
- Kung J, Henry RR. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11:565–579. doi: 10.1517/14740338.2012.691963. [DOI] [PubMed] [Google Scholar]
- Landmesser U, Hornig B, Drexler H. Endothelial function: a critical determinant in atherosclerosis? Circulation. 2004;109:II27–II33. doi: 10.1161/01.CIR.0000129501.88485.1f. [DOI] [PubMed] [Google Scholar]
- Law RE, Goetze S, Xi XP, Jackson S, Kawano Y, Demer L, et al. Expression and function of PPARgamma in rat and human vascular smooth muscle cells. Circulation. 2000;101:1311–1318. doi: 10.1161/01.cir.101.11.1311. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Cousens S, Zupan J Lancet Neonatal Survial Steering Team. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- Lazennec G, Canaple L, Saugy D, Wahli W. Activation of peroxisome proliferator-activated receptors (PPARs) by their ligands and protein kinase A activators. Mol Endocrinol. 2000;14:1962–1975. doi: 10.1210/mend.14.12.0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, et al. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Li AC, Palinski W. Peroxisome proliferator-activated receptors: how their effects on macrophages can lead to the development of a new drug therapy against atherosclerosis. Annu Rev Pharmacol Toxicol. 2006;46:1–39. doi: 10.1146/annurev.pharmtox.46.120604.141247. [DOI] [PubMed] [Google Scholar]
- Li MD, Yang X. A retrospective on nuclear receptor regulation of inflammation: lessons from GR and PPARs. PPAR Res. 2011;742785:1–9. doi: 10.1155/2011/742785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Dey SK. PPAR delta functions as a prostacyclin receptor in blastocyst implantation. Trends Endocrinol Metab. 2000;11:137–142. doi: 10.1016/s1043-2760(00)00243-5. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC, et al. Evidence implicating peroxisome proliferator-activated receptor-γ in the pathogenesis of preeclampsia. Hypertension. 2011a;58:882–887. doi: 10.1161/HYPERTENSIONAHA.111.179440. [DOI] [PubMed] [Google Scholar]
- McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator-activated receptor-γ as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011b;58:280–286. doi: 10.1161/HYPERTENSIONAHA.111.172627. [DOI] [PubMed] [Google Scholar]
- Maggi LB, Jr, Sadeghi H, Weigand C, Scarim AL, Heitmeier MR, Corbett JA. Anti-inflammatory actions of 15-deoxy-delta 12,14-prostaglandin J2 and troglitazone: evidence for heat shock-dependent and -independent inhibition of cytokine-induced inducible nitric oxide synthase expression. Diabetes. 2000;49:346–355. doi: 10.2337/diabetes.49.3.346. [DOI] [PubMed] [Google Scholar]
- Mandrekar-Colucci S, Landreth GE. Nuclear receptors as therapeutic targets for Alzheimer's disease. Expert Opin Ther Targets. 2011;15:1085–1097. doi: 10.1517/14728222.2011.594043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens FM, Visseren FL, Lemay J, de Koning EJ, Rabelink TJ. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002;62:1463–1480. doi: 10.2165/00003495-200262100-00004. [DOI] [PubMed] [Google Scholar]
- Marx N, Mach F, Sauty A, Leung JH, Sarafi MN, Ransohoff RM, et al. Peroxisome proliferator-activated receptor-gamma activators inhibit IFN-gamma-induced expression of the T cell-active CXC chemokines IP-10, Mig, and I-TAC in human endothelial cells. J Immunol. 2000;164:6503–6508. doi: 10.4049/jimmunol.164.12.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Noguchi E, Kobayashi T, Kamata K. Mechanisms underlying the chronic pioglitazone treatment-induced improvement in the impaired endothelium-dependent relaxation seen in aortas from diabetic rats. Free Radic Biol Med. 2007;42:993–1007. doi: 10.1016/j.freeradbiomed.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Kobayashi T, Kamata K. Relationships among ET-1, PPARgamma, oxidative stress and endothelial dysfunction in diabetic animals. J Smooth Muscle Res. 2008;44:41–55. doi: 10.1540/jsmr.44.41. [DOI] [PubMed] [Google Scholar]
- Mattace Raso G, Bianco G, Esposito E, Iacono A, Meli R, Autore G. Evaluation of placental protein modifications in normotensive and spontaneously hypertensive rats. Placenta. 2008;29:429–435. doi: 10.1016/j.placenta.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Meirhaeghe A, Boreham CA, Murray LJ, Richard F, Davey Smith G, Young IS, et al. A possible role for the PPARG Pro12Ala polymorphism in preterm birth. Diabetes. 2007;56:494–498. doi: 10.2337/db06-0915. [DOI] [PubMed] [Google Scholar]
- Molavi B, Chen J, Mehta JL. Cardioprotective effects of rosiglitazone are associated with selective overexpression of type 2 angiotensin receptors and inhibition of p42/44 MAPK. Am J Physiol Heart Circ Physiol. 2006;291:H687–H689. doi: 10.1152/ajpheart.00926.2005. [DOI] [PubMed] [Google Scholar]
- Mueller E, Sarraf P, Tontonoz P, Evans RM, Martin KJ, Zhang M, et al. Terminal differentiation of human breast cancer through PPAR gamma. Mol Cell. 1998;1:465–470. doi: 10.1016/s1097-2765(00)80047-7. [DOI] [PubMed] [Google Scholar]
- Mutalik M. The story of glitazones. Int J Cur Biomed Phar Res. 2011;1:141–147. [Google Scholar]
- Nadra K, Quignodon L, Sardella C, Joye E, Mucciolo A, Chrast R, et al. PPARgamma in placental angiogenesis. Endocrinology. 2010;151:4969–4981. doi: 10.1210/en.2010-0131. [DOI] [PubMed] [Google Scholar]
- Negro R, Mangieri T, Dazzi D, Pezzarossa A, Hassan H. Rosiglitazone effects on blood pressure and metabolic parameters in nondipper diabetic patients. Diabetes Res Clin Pract. 2005;70:20–25. doi: 10.1016/j.diabres.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Nicol CJ, Adachi M, Akiyama TE, Gonzalez FJ. PPARgamma in endothelial cells influences high fat diet-induced hypertension. Am J Hypertens. 2005;18:549–556. doi: 10.1016/j.amjhyper.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Nissen SE, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Parast MM, Yu H, Ciric A, Salata MW, Davis V, Milstone DS. PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. Plos ONE. 2009;4:e8055. doi: 10.1371/journal.pone.0008055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KS, Ciaraldi TP, Abrams-Carter L, Mudaliar S, Nikoulina SE, Henry RR. PPAR-gamma gene expression is elevated in skeletal muscle of obese and type II diabetic subjects. Diabetes. 1997;46:1230–1234. doi: 10.2337/diab.46.7.1230. [DOI] [PubMed] [Google Scholar]
- Pasceri V, Wu HD, Willerson JT, Yeh ET. Modulation of vascular inflammation in vitro and in vivo by peroxisome proliferator-activated receptor-gamma activators. Circulation. 2000;101:235–238. doi: 10.1161/01.cir.101.3.235. [DOI] [PubMed] [Google Scholar]
- Peeters LL, Vigne JL, Tee MK, Zhao D, Waite LL, Taylor RN. PPARgamma represses VEGF expression in human endometrial cells: implications for uterine angiogenesis. Angiogenesis. 2005;8:373–379. doi: 10.1007/s10456-005-9027-4. [DOI] [PubMed] [Google Scholar]
- Pistrosch F, Passauer J, Fischer S, Fuecker K, Hanefeld M, Gross P. In type 2 diabetes, rosiglitazone therapy for insulin resistance ameliorates endothelial dysfunction independent of glucose control. Diabetes Care. 2004;27:484–490. doi: 10.2337/diacare.27.2.484. [DOI] [PubMed] [Google Scholar]
- Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–1816. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. 2004;25:331–336. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- Rikimaru K, Wakabayashi T, Abe H, Imoto H, Maekawa T, Ujikawa O, et al. A new class of non-thiazolidinedione, non-carboxylic-acid-based highly selective peroxisome proliferator-activated receptor (PPAR) γ agonists: design and synthesis of benzylpyrazole acylsulfonamides. Bioorg Med Chem. 2012;20:714–733. doi: 10.1016/j.bmc.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Rodie VA, Young A, Jordan F, Sattar N, Greer IA, Freeman DJ. Human placental peroxisome proliferator-activated receptor delta and gamma expression in healthy pregnancy and in preeclampsia and intrauterine growth restriction. J Soc Gynecol Investig. 2005;12:320–329. doi: 10.1016/j.jsgi.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Roos N, Kieler H, Sahlin L, Ekman-Ordeberg G, Falconer H, Stephansson O. Risk of adverse pregnancy outcomes in women with polycystic ovary syndrome: population based cohort study. BMJ. 2011;343:d6309. doi: 10.1136/bmj.d6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. J Biol Chem. 2001;276:37731–37734. doi: 10.1074/jbc.R100034200. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, et al. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4:611–617. doi: 10.1016/s1097-2765(00)80211-7. [DOI] [PubMed] [Google Scholar]
- Sadeghian M, Marinova-Mutafchieva L, Broom L, Davis JB, Virley D, Medhurst AD, et al. Full and partial peroxisome proliferation-activated receptor-gamma agonists, but not delta agonist, rescue of dopaminergic neurons in the 6-OHDA Parkinsonian model is associated with inhibition of microglial activation and MMP expression. J Neuroimmunol. 2012;246:69–77. doi: 10.1016/j.jneuroim.2012.03.010. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Jordan P, Welbourne T, Minagar A, Joh T, Itoh M, et al. Troglitazone, a PPAR-gamma activator prevents endothelial cell adhesion molecule expression and lymphocyte adhesion mediated by TNF-alpha. BMC Physiol. 2005;5:1–12. doi: 10.1186/1472-6793-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Tsukamoto K, Hashimoto Y, Hashimoto N, Togo M, Hara M, et al. Thiazolidinediones suppress endothelin-1 secretion from bovine vascular endothelial cells: a new possible role of PPARgamma on vascular endothelial function. Biochem Biophys Res Commun. 1999;254:757–763. doi: 10.1006/bbrc.1998.0126. [DOI] [PubMed] [Google Scholar]
- Savage DB, Tan GD, Acerini CL, Jebb SA, Agostini M, Gurnell M, et al. Human metabolic syndrome resulting from dominant-negative mutations in the nuclear receptor peroxisome proliferator-activated receptor-gamma. Diabetes. 2003;52:910–917. doi: 10.2337/diabetes.52.4.910. [DOI] [PubMed] [Google Scholar]
- Savvidou MD, Hingorani AD, Tsikas D, Frolich JC, Vallance P, Nicolaides KH. Endothelial dysfunction and raised plasma concentrations of asymmetric dimethylarginine in pregnant women who subsequently develop pre-eclampsia. Lancet. 2003;361:1511–1517. doi: 10.1016/S0140-6736(03)13177-7. [DOI] [PubMed] [Google Scholar]
- Schaiff WT, Carlson MG, Smith SD, Levy R, Nelson DM, Sadovsky Y. Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. J Clin Endocrinol Metab. 2000;85:3874–3881. doi: 10.1210/jcem.85.10.6885. [DOI] [PubMed] [Google Scholar]
- Schaiff WT, Barak Y, Sadovsky Y. The pleiotropic function of PPAR gamma in the placenta. Mol Cell Endocrinol. 2006;249:10–15. doi: 10.1016/j.mce.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Peroxisome proliferator-activated receptors and cardiovascular remodeling. Am J Physiol Heart Circ Physiol. 2005;288:H1037–H1043. doi: 10.1152/ajpheart.00677.2004. [DOI] [PubMed] [Google Scholar]
- Shaamash AH, Elsnosy ED, Makhlouf AM, Zakhari MM, Ibrahim OA, EL-dien HM. Maternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsia. Int J Gynaecol Obstet. 2000;68:207–214. doi: 10.1016/s0020-7292(99)00213-1. [DOI] [PubMed] [Google Scholar]
- Shalom-Barak T, Nicholas JM, Wang Y, Zhang X, Ong ES, Young TH, et al. Peroxisome proliferator-activated receptor gamma controls Muc1 transcription in trophoblasts. Mol Cell Biol. 2004;24:10661–10669. doi: 10.1128/MCB.24.24.10661-10669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AM, Staels B. Review: peroxisome proliferator-activated receptor gamma and adipose tissue – understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386–395. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- Smarason AK, Allman KG, Young D, Redman CW. Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with pre-eclampsia. Br J Obstet Gynaecol. 1997;104:538–543. doi: 10.1111/j.1471-0528.1997.tb11528.x. [DOI] [PubMed] [Google Scholar]
- Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- Sourij H, Zweiker R, Wascher TC. Effects of pioglitazone on endothelial function, insulin sensitivity, and glucose control in subjects with coronary artery disease and new-onset type 2 diabetes. Diabetes Care. 2006;29:1039–1045. doi: 10.2337/diacare.2951039. [DOI] [PubMed] [Google Scholar]
- Spiegelman BM. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- Suwaki N, Masuyama H, Masumoto A, Takamoto N, Hiramatsu Y. Expression and potential role of peroxisome proliferator-activated receptor gamma in the placenta of diabetic pregnancy. Placenta. 2007;28:315–323. doi: 10.1016/j.placenta.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Tarrade A, Schoonjans K, Pavan L, Auwerx J, Rochette-Egly C, Evain-Brion D, et al. PPARgamma/RXRalpha heterodimers control human trophoblast invasion. J Clin Endocrinol Metab. 2001;86:5017–5024. doi: 10.1210/jcem.86.10.7924. [DOI] [PubMed] [Google Scholar]
- Tesse A, Al-Massarani G, Wangensteen R, Reitenbach S, Martinez MC, Andriantsitohaina R. Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents microparticle-induced vascular hyporeactivity through the regulation of proinflammatory proteins. J Pharmacol Exp Ther. 2008;324:539–547. doi: 10.1124/jpet.107.130278. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Hu E, Spiegelman BM. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM. PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 1998;93:241–252. doi: 10.1016/s0092-8674(00)81575-5. [DOI] [PubMed] [Google Scholar]
- Var A, Yildirim Y, Onur E, Kuscu NK, Uyanik BS, Goktalay K, et al. Endothelial dysfunction in preeclampsia. Increased homocysteine and decreased nitric oxide levels. Gynecol Obstet Invest. 2003;56:221–224. doi: 10.1159/000074824. [DOI] [PubMed] [Google Scholar]
- Verrier E, Wang L, Wadham C, Albanese N, Hahn C, Gamble JR, et al. PPARgamma agonists ameliorate endothelial cell activation via inhibition of diacylglycerol-protein kinase C signaling pathway: role of diacylglycerol kinase. Circ Res. 2004;94:1515–1522. doi: 10.1161/01.RES.0000130527.92537.06. [DOI] [PubMed] [Google Scholar]
- Waite LL, Person EC, Zhou Y, Lim KH, Scanlan TS, Taylor RN. Placental peroxisome proliferator-activated receptor-gamma is up-regulated by pregnancy serum. J Clin Endocrinol Metab. 2000;85:3808–3814. doi: 10.1210/jcem.85.10.6847. [DOI] [PubMed] [Google Scholar]
- Waite LL, Louie RE, Taylor RN. Circulating activators of peroxisome proliferator-activated receptors are reduced in preeclamptic pregnancy. J Clin Endocrinol Metab. 2005;90:620–626. doi: 10.1210/jc.2004-0849. [DOI] [PubMed] [Google Scholar]
- Walcher D, Marx N. Insulin resistance and cardiovascular disease: the role of PPARgamma activators beyond their anti-diabetic action. Diab Vasc Dis Res. 2004;1:76–81. doi: 10.3132/dvdr.2004.011. [DOI] [PubMed] [Google Scholar]
- Wang LH, Yang XY, Zhang X, Huang J, Hou J, Li J, et al. Transcriptional inactivation of STAT3 by PPARgamma suppresses IL-6-responsive multiple myeloma cells. Immunity. 2004;20:205–218. doi: 10.1016/s1074-7613(04)00030-5. [DOI] [PubMed] [Google Scholar]
- Wang N, Verna L, Chen NG, Chen J, Li H, Forman BM, et al. Constitutive activation of peroxisome proliferator-activated receptor-gamma suppresses pro-inflammatory adhesion molecules in human vascular endothelial cells. J Biol Chem. 2002;277:34176–34181. doi: 10.1074/jbc.M203436200. [DOI] [PubMed] [Google Scholar]
- Wang TD, Chen WJ, Cheng WC, Lin JW, Chen MF, Lee YT. Relation of improvement in endothelium-dependent flow-mediated vasodilation after rosiglitazone to changes in asymmetric dimethylarginine, endothelin-1, and C-reactive protein in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2006;98:1057–1062. doi: 10.1016/j.amjcard.2006.05.027. [DOI] [PubMed] [Google Scholar]
- Willson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- Willson TM, Lambert MH, Kliewer SA. Peroxisome proliferator-activated receptor gamma and metabolic disease. Annu Rev Biochem. 2001;70:341–367. doi: 10.1146/annurev.biochem.70.1.341. [DOI] [PubMed] [Google Scholar]
- Xia Y, Kellems RE. Is preeclampsia an autoimmune disease? Clin Immunol. 2009;133:1–12. doi: 10.1016/j.clim.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa K, Hosoi M, Koyama H, Tanaka S, Fukumoto S, Morii H, et al. Peroxisome proliferator-activated receptor-gamma agonists increase vascular endothelial growth factor expression in human vascular smooth muscle cells. Biochem Biophys Res Commun. 2000;271:571–574. doi: 10.1006/bbrc.2000.2665. [DOI] [PubMed] [Google Scholar]
- Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004;351:1106–1118. doi: 10.1056/NEJMra041001. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Brkanac Z, Dupont BR, Xing GQ, Leach RJ, Detera-Wadleigh SD. Assignment of the human nuclear hormone receptor, NUC1 (PPARD), to chromosome 6p21.1-p21.2. Genomics. 1996;35:637–638. doi: 10.1006/geno.1996.0417. [DOI] [PubMed] [Google Scholar]
- Yue TL, Bao W, Gu JL, Cui J, Tao L, Ma XL, et al. Rosiglitazone treatment in Zucker diabetic Fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes. 2005;54:554–562. doi: 10.2337/diabetes.54.2.554. [DOI] [PubMed] [Google Scholar]
- Zhang MA, Rego D, Moshkova M, Kebir H, Chruscinski A, Nguyen H, et al. Peroxisome proliferator-activated receptor (PPAR)α and -γ regulate IFNγ and IL-17A production by human T cells in a sex-specific way. Proc Natl Acad Sci USA. 2012;109:9505–9510. doi: 10.1073/pnas.1118458109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Qi C, Jia Y, Nye JS, Rao MS, Reddy JK. Deletion of PBP/PPARBP, the gene for nuclear receptor coactivator peroxisome proliferator-activated receptor-binding protein, results in embryonic lethality. J Biol Chem. 2000;275:14779–14782. doi: 10.1074/jbc.C000121200. [DOI] [PubMed] [Google Scholar]
- Zhu YJ, Crawford SE, Stellmach V, Dwivedi RS, Rao MS, Gonzalez FJ, et al. Coactivator PRIP, the peroxisome proliferator-activated receptor-interacting protein, is a modulator of placental, cardiac, hepatic, and embryonic development. J Biol Chem. 2003;278:1986–1990. doi: 10.1074/jbc.C200634200. [DOI] [PubMed] [Google Scholar]


