Abstract
Background and Purpose
Most GABAA receptor subtypes comprise 2α, 2β and 1γ subunit, although for some isoforms, a δ replaces a γ-subunit. Extrasynaptic δ-GABAA receptors are important therapeutic targets, but there are few suitable pharmacological tools. We profiled DS2, the purported positive allosteric modulator (PAM) of δ-GABAA receptors to better understand subtype selectivity, mechanism/site of action and activity at native δ-GABAA receptors.
Experimental Approach
Subunit specificity of DS2 was determined using electrophysiological recordings of Xenopus laevis oocytes expressing human recombinant GABAA receptor isoforms. Effects of DS2 on GABA concentration–response curves were assessed to define mechanisms of action. Radioligand binding and electrophysiology utilising mutant receptors and pharmacology were used to define site of action. Using brain-slice electrophysiology, we assessed the influence of DS2 on thalamic inhibition in wild-type and δ0/0 mice.
Key Results
Actions of DS2 were primarily determined by the δ-subunit but were additionally influenced by the α, but not the β, subunit (α4/6βxδ > α1βxδ >> γ2-GABAA receptors > α4β3). For δ-GABAA receptors, DS2 enhanced maximum responses to GABA, with minimal influence on GABA potency. (iii) DS2 did not act via the orthosteric, or known modulatory sites on GABAA receptors. (iv) DS2 enhanced tonic currents of thalamocortical neurones from wild-type but not δ0/0 mice.
Conclusions and Implications
DS2 is the first PAM selective for α4/6βxδ receptors, providing a novel tool to investigate extrasynaptic δ-GABAA receptors. The effects of DS2 are mediated by an unknown site leading to GABAA receptor isoform selectivity.
Keywords: GABAA receptor, DS2, δ-subunit, α4-subunit, site of interaction, brain-slice electrophysiology
Introduction
The GABAA receptor is the major inhibitory receptor in the mammalian brain. When the neurotransmitter GABA binds to and activates the receptor, an associated conformational change of the protein permits chloride ions to pass through the receptor pore, thereby leading in most instances to neuronal hyperpolarization. GABAA receptors are pentameric proteins made from the assembly of homologous subunits of various types (α1–6, β1–3, γ1–3, δ, ε, θ and π) (Sieghart, 1995; Barnard et al., 1998; receptor nomenclature follows Alexander et al., 2011). The majority of GABAA receptors have the general stochiometry of 2α, 2β and 1γ subunit. However, a subpopulation of receptors is composed of 2α, 2β and 1δ subunit, with the δ-subunit replacing the γ-subunit. GABAA receptors incorporating the δ-subunit are primarily found in peri- or extrasynaptic locations where they are subject to activation by low ambient GABA concentrations (Belelli et al., 2009). Indeed, δ-GABAA receptors are highly sensitive to GABA and exhibit little desensitization (Nusser et al., 1998; Farrant and Nusser, 2005; Houston et al., 2009; Bright et al., 2011). GABA ‘spill-over’ from the synapse reaching a concentration in the low μM range (Mody, 2005) is believed to contribute to the tonic activation of extrasynaptic receptors. This suggestion has been supported by electrophysiological studies using brain slice preparations, where in certain neurones the GABAA receptor antagonists bicuculline, gabazine and picrotoxin, inhibit the basal current (Brickley et al., 1996; 1999; Jia et al., 2005; Chandra et al., 2006).
Three δ-GABAA receptor isoforms have been identified in vivo, namely α4β2/3δ receptors expressed in thalamic relay neurons and dentate gyrus granule cells (Porcello et al., 2003; Belelli et al., 2005; Chandra et al., 2006), α6β2/3δ receptors expressed in cerebellar granule cells (Nusser et al., 1998) and a population of α1β2/3δ receptors, proposed to occur in certain hippocampal interneurons (Sun et al., 2004; Mangan et al., 2005). Mice with a genetic deletion of the δ-subunit have provided an insight to the physiological relevance of these δ-GABAARs, knowledge that has been supplemented with a phenotypic analysis of α4 and α6 ‘knock-out’ mice, the two α-subunits primarily associated with the δ-subunit. For example, δ0/0 mice are more susceptible to seizures (Spigelman et al., 2002), have enhanced trace fear conditioning (Wiltgen et al., 2005) and have an altered response in phase 2 of the formalin-induced nociception test (Bonin et al., 2011). Further tonic currents in the thalamus and dentate gyrus of α40/0 mice (Chandra et al., 2006) and in cerebellar granule cells in α60/0 mice (Brickley et al., 2001) are significantly reduced.
From a therapeutic perspective, δ-containing receptors are emerging as a potentially important pharmacological target. Importantly, δ-containing receptors are not modulated by classical GABAA receptor modulators acting via the benzodiazepine binding site located between the γ- and α-subunits, although other known modulators of GABAA receptors such as neurosteroids, etomidate and barbiturates enhance the function of δ-GABAA receptors (Belelli et al., 2005). However, these latter modulators exhibit little selectivity for δ- over γ-containing receptors (Belelli et al., 2002; Brown et al., 2002; Wohlfarth et al., 2002; Zheleznova et al., 2008), precluding any pharmacological verification of the physiological or pathophysiological role of δ-GABAA receptors. One exception is the relatively δ-preferring agonist THIP, or gaboxadol, which has provided some insight on the role of δ-GABAA receptors in sleep regulation. For example, the effect of gaboxadol to enhance tonic inhibition in thalamocortical neurons and to produce hypnosis and ataxia were blunted in δ0/0 mice (Herd et al., 2009). Moreover, in α40/0 mice (α4 is a δ-subunit preferring partner in certain neurons), the behavioural effects of gaboxadol such as sedation, ataxia and analgesia were curtailed (Chandra et al., 2006). Based on the preclinical data on gaboxadol demonstrating a hypnotic profile in rats (Thakkar et al., 2008) and that the effects of the drug on EEG were blunted in δ0/0 mice (Winsky-Sommerer et al., 2007), it was pursued as a novel treatment for insomnia, reaching phase III clinical development (Wafford and Ebert, 2006; Roth et al., 2010). Given the literature indicating a role for δ-GABAA receptors in female stress disorders (Maguire and Mody, 2007; Smith et al., 2007), epilepsy (Mihalek et al., 1999), pain (Peng et al., 2009; Bonin et al., 2011), post-traumatic stress disorder (Wiltgen et al., 2005; Pibiri et al., 2008), schizophrenia (Marx et al., 2006), autism (Olmos-Serrano et al., 2011), major depression (Holm et al., 2010) and potentially alcoholism (Enoch, 2008; Rewal et al., 2009), the need for δ-selective tools is clear. Furthermore, it should not be overlooked that a major swathe of the literature, only partly cited above, alluding to the potential therapeutic relevance of δ-GABAA receptors has been based on studies with neurosteroids, which are not completely selective for these extrasynaptic receptors (Belelli et al., 2009). Therefore, a drug that is truly selective for δ-GABAA receptors would confirm and expand on the extant literature obtained with mostly non-selective drugs.
Few positive allosteric modulators (PAMs) selective for δ-GABAA receptors have been reported, and no selective negative allosteric modulators (NAMs) are known. The imidazopyridine DS2 is a functionally selective α4β3δ PAM, relative to its actions at α4β3γ2 and α1β3γ2 receptors, with clear effects on the tonic current of thalamic ventrobasal (VB) neurons mediated by α4β2δ receptors (Wafford et al., 2009). Recently, the triamino-benzene compound AA29504, a retigabine analogue, was described as a functionally selective α4β3δ PAM, albeit the lack of full concentration–response analysis at δ- and γ-containing GABAA receptors precludes definitive conclusions (Hoestgaard-Jensen et al., 2010). Nonetheless, AA29504 augments the effects of gaboxadol in cortical brain slices, enters the brain and is effective in some in vivo models. JM-11-43A, a dihydropyrimidinone, has also been described as a selective α4β3δ PAM, but as with AA29504, this drug appears to exhibit limited selectivity (Lewis et al., 2010).
In the current study, we have explored in detail the properties of DS2 as a δ-GABAA receptor-selective allosteric modulator, employing the Xenopus laevis oocyte expression system to conduct a comprehensive electrophysiological analysis of the actions of DS2 acting at human recombinant GABAA receptors incorporating α1–6 subunits, combined with the relevant β, γ- and δ-subunits. We demonstrate the necessity of the δ-subunit for efficacy at α4-subunit-containing receptors and analyse the influence of different β-subunits on the actions of DS2. We also provide a detailed mechanistic understanding of the effects of DS2. To determine the site of action of DS2, we have utilized both radioligand binding and electrophysiological methods employing mutant receptor constructs as necessary. Finally, we have demonstrated that the effect of DS2 at native receptors is mediated by δ-GABAA receptors by comparing its modulation of thalamic inhibition in wild-type and δ0/0 mice.
Methods
Cloning of GABAA receptor cDNAs
The cDNAs for human α1–6, β2–3 and γ2S-subunits were cloned as described previously (Mirza et al., 2008). The cDNAs for the human β1- and δ-subunit were cloned from human hippocampus poly(A+) mRNA (Clontech, Mountain View, CA) using RT-PCR. First-strand cDNA was obtained using oligo(dT) primer and Moloney murine leukaemia virus reverse transcriptase (GE Healthcare Life Sciences, UK) and full-length cDNAs were amplified using Expand HF polymerase (Roche Diagnostics, Basel, Switzerland) and gene-specific primer sets (MWG Biotech, High Point, NC). Twenty-five PCR cycles were performed using the following conditions: 94°C, 60 s; 55°C, 60 s; 72°C, 120 s, followed by 72°C, 10 min using a Robocycler (Stratagene, La Jolla, CA). The amplified product was cloned into pSwas [derived from pZErO-1 (Life Technologies, Invitrogen, Naerum, Denmark)]. Clones were sequenced bidirectionally and subcloned into the pNS3z or pNS3h vector derived from pcDNA3 (Invitrogen).
The GABAA receptor β3N265M and GABAA receptor α4T235W, Q240W subunits were constructed in a mutagenesis reaction. Briefly, uracilated β3- and α4-subunit-containing plasmids were used as templates in a mutagenesis reaction, in which mutagenic oligonucleotides and T7 DNA polymerase were used to introduce mutations. An aliquot of the mutagenesis reaction was then transformed into Escherichia coli XL1-Blue cells. Mutated plasmids were identified by the introduction or elimination of restriction sites.
The mutagenic oligonucleotides were β3N265M: GACAATGACAACCATCAtgACCCACCTTCGGGAGA and α4T235W, Q240W: ATATTCCGTGCATTATGtggGTGATctTaagTtggGTTTCATTTTGGATAAA.
All mutated constructs were verified by sequencing.
Cell culture and stable transfections
Stably transfected HEK-293 (ATCC1573) cell lines were established as described (Mirza et al., 2008) and maintained in DMEM with 10 mM HEPES and 2 mM Glutamax supplemented with 10% FBS. The cells were cultured at 37°C in a humidified atmosphere of 5% CO2 and 95% air and passaged twice a week.
[3H] muscimol, [3H]Flumazenil and [3H]Ro15-4513 binding studies
[3H]Flumazenil and [3H]Ro15-4513 binding studies were performed as described previously (Mirza et al., 2008). [3H]muscimol binding was performed using aliquots of membranes (native tissue or cell lines) resuspended in Tris–citrate buffer (50 mM, pH 7.1) and centrifuged for 10 min at 22 000× g at 4°C. The pellet was resuspended to give a protein concentration of 100–200 μg·mL−1. Binding was performed in triplicate using [3H]muscimol (25.5 Ci·mmol−1; PerkinElmer, Waltham, MA) in a final volume of 550 μL containing 50–100 μg of protein, and non-specific binding was determined in the presence of 100 μM GABA (SIGMA Aldrich, St. Louis, MO). Samples were incubated at 4°C for 40 min, and labelled membranes were harvested using rapid filtration over GF/C filters (Whatman, Maidstone, UK). The filters were washed with 2 × 5-mL Tris–citrate buffer, and the amount of radioactivity was determined by liquid scintillation counting using a Tri-Carb counter (PerkinElmer).
Preparation of cRNA
Plasmids were linearized using a unique downstream polylinker enzyme (NotI, XhoI or XbaI) for cRNA production. cRNA was prepared and capped using the mMESSAGE mMACHINE T7 Transcription kit (Ambion, Austin, TX) and was purified using the RNeasy mini kit (QIAGEN, Hilden, Germany).
Isolation of X. laevis oocytes
X. laevis (Nasco, Fort Atkinson, WIUSA) oocytes were isolated as described previously (Mirza et al., 2008). Oocytes were injected with 25 to 50 nL of cRNA mixtures of GABAAR subunits αx : βx : δ/γ2S in the ratios 1:1:2 unless otherwise stated or 1:1:5 for α1 : β2 : δ using a Pico Pump (WPI, Sarasota, FL). Oocytes were maintained at 18°C in modified Barth's solution for up to 7 days after injection.
Oocyte electrophysiology
Electrophysiological studies using X. laevis oocytes were performed using the two-electrode voltage-clamp technique (Mirza et al., 2008). Briefly, GABA was dissolved in OR2 buffer (composition, in mM: 90 NaCl, 2.5 KCl, 2.5 CaCl2, 1 MgCl2, 5 HEPES; pH adjusted to 7.4) in a concentration known to give rise to EC5-20 elicited currents for α1–3,5+β2γ2 receptors (0.5–3 μM) and EC20-50 for α4β2/3δ (0.01–1 μM) receptors. This solution was used to establish control responses and for dissolving the compounds to test in the experiment. The modulatory effects of DS2 were calculated by comparing the trace obtained in the presence of DS2 to the control trace, where a doubling of the current equals a 100% increase.
Brain slice electrophysiology
All animal care and experimental procedures complied with the UK Government Animals (Scientific Procedures) Act 1986 and were approved by the ‘University of Dundee Ethics Committee’. Studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., ). A total of 25 mice were used in the work described here. WT and δ0/0 mice were of a C57BL6 background and were bred at the University of Dundee.
Mice were killed by cervical dislocation in accordance with schedule 1 of the UK Government Animals (Scientific Procedures) Act 1986. Thalamic slices were prepared from mice of either sex (P18-24) as previously described (Belelli et al., 2005). The brain was rapidly removed and placed in oxygenated ice-cold artificial cerebrospinal fluid (aCSF) solution containing (in mM) 225 sucrose, 2.95 KCl, 1.25 NaH2PO4, 26 NaHCO3, 0.5 CaCl2, 10 MgSO4, 10 glucose, (pH of 7.4; 330–340 mOsm). The tissue was maintained in ice-cold aCSF from which horizontal 300–350 μm slices were prepared using a Vibratome (Intracel, Royston, Herts., UK). Slices were transferred to an incubation chamber containing oxygenated, extracellular solution [(ECS) in mM: 126 NaCl, 2.95 KCl, 26 NaHCO3, 1.25 NaH2PO4, 2 CaCl2, 10 glucose and 2 MgCl2 (pH 7.4; 300–310 mOsm)] for ≥1 h prior to recording. Patch pipettes (open tip resistances of 3–5 MΩ) were constructed using thick-walled borosilicate glass (Garner Glass Company, Claremont, CA) and filled with an intracellular solution that contained (in mM) 140 CsCl, 10 HEPES, 10 EGTA, 2 Mg-ATP, 1 CaCl2, 5 QX-314 (pH 7.3 with CsOH, 300–305 mOsm). Whole-cell voltage-clamp recordings were performed at 35°C from thalamic VB neurons visually identified with an Olympus BX51 (Olympus, Southall, UK) microscope equipped with DIC/IR optics as previously described (Belelli et al., 2005). Miniature inhibitory post-synaptic currents (mIPSCs) and tonic currents were recorded using an Axopatch 1D amplifier (Axon Instruments, Foster City, CA) at a holding potential of −60 mV in ECS that, contained 2 mM kynurenic acid (Sigma-Aldrich-RBI, Dorset, UK) and 0.5 μM tetrodotoxin (TTX; TCS Biologicals Ltd, Buckingham, UK) to block ionotropic glutamate receptors and sodium-dependent action potentials respectively.
Data analysis
Data were recorded onto a digital audio tape using a Biologic DTR 1200 recorder and analysed offline using the Strathclyde Electrophysiology Software, WinEDR/WinWCP (J Dempster, University of Strathclyde, UK). Individual mIPSCs (with a rise time ≤1 ms) were detected as previously described (Belelli et al., 2005). The peak amplitude, 10–90% rise time, time to decay from peak by 50% (T50), 90% (T90) and charge transfer (the area under the curve of each event) were determined for accepted events. The mIPSC frequency was determined over 10 s bins for 2 min. To calculate the time constant of mIPSC decay, a minimum of 50 accepted events were digitally averaged by alignment at the midpoint of the rising phase, and the mIPSC decay fitted by either monoexponential, or biexponential functions using the least squares method as previously described (Belelli et al., 2005). Note the average mIPSC decay was always best fit with the sum of two exponential components. Thus, a weighted decay time constant (τw) was determined as described previously. The tonic current was calculated as the difference between the holding current before (determined by averaging the current for a minimum of 200 epochs of 25 ms that did not contain phasic events) and after application of (30 μM) bicuculline methobromide. All results are reported as the arithmetic mean ± SEM. Statistical significance of mean data was assessed with paired or unpaired Student's t-test as appropriate.
Materials
DS2 was synthesized at NeuroSearch A/S, Medicinal Chemistry Department. GABA, bicuculline, etomidate, allopregnanolone (5α-pregnan-3α-ol-20-one) and pentobarbital were purchased from Sigma Aldrich (St. Louis, MO).
Results
Effect of DS2 on α4-containing GABAA receptors (α4β3δ, α4β3γ2 and α4β3): the activity of DS2 is determined by the δ-subunit
We initially determined the importance of the δ-subunit by comparing the modulatory effects of DS2 on α4β3, α4β3δ and α4β3γ2 GABAA receptors.
α4β3δ, α4β3 and α4β3γ2 receptors were expressed in X. laevis oocytes, and GABA-evoked currents were measured 5–7, 2–4 and 2–4 days after RNA injection, respectively; preliminary studies allowed us to define these optimized timing conditions (data not shown). The receptors were activated by GABA and by gaboxadol, with the latter being a more efficacious agonist than GABA on both α4β3δ (205 ± 8% relative to GABA maximal efficacy, n = 6) and α4β3 receptors (185 ± 12% relative to GABA maximal efficacy, n = 5), compared with α4β3γ2 receptors (95 ± 8% relative to GABA maximal efficacy, n = 5). Furthermore, gaboxadol had approximately 5–10× greater potency than GABA at α4β3δ (EC50 = 24 ± 1 μM, n = 6) and α4β3 (EC50 = 12 ± 1 μM, n = 6) compared with its potency at α4β3γ2 receptors (EC50 = 116 ± 34, n = 5). The relative pharmacological profile of GABA and gaboxadol at these three receptors subtypes is in agreement with earlier findings in both a mouse L(tk–) cell line (Brown et al., 2002) and Xenopus oocytes (Storustovu and Ebert, 2006).
The modulatory effect of DS2 on α4-containing receptors was critically dependent on the presence of the δ-subunit in the receptor complex. A low concentration of GABA (10 nM) activated a relatively small inward current for the three α4-containing receptor subtypes. However, only GABA-evoked currents mediated by α4β3δ receptors were enhanced by the co-application of DS2 (0.1–10 μM; Figure 1A top left panel). DS2 (10 μM) produced a maximal 10-fold increase in such GABA-evoked currents with a calculated EC50 of 5.6 ± 1.5 μM (n = 11) – Figure 1A.
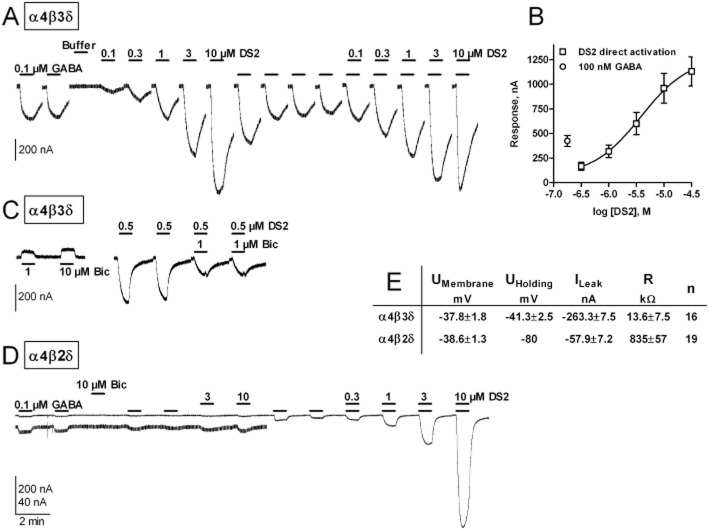
Figure 1.
Selectivity of DS2 for various human recombinant GABAA receptors determined using two-electrode voltage-clamp electrophysiology. (A) Current traces recorded from X laevis oocytes expressing human recombinant α4β3δ, α4β3 or α4β3γ2 receptors. The bars above each trace represent a time period of 1 min, where the agonist/modulator was applied. Currents were elicited using either a low GABA concentration (0.01 μM for α4β3δ and α4β3 and 10 μM for α4β3γ2) or GABA in combination with the various concentrations of DS2 listed above each bar. There is an extra 1 min wash between each of the first five traces and 2 min wash between each of the last three to allow for full receptor activation on next drug application. Note that the traces are truncated and staggered, omitting the long wash periods – 90 s at lowest concentration, and up to 4 min at the highest concentration of DS2 – to focus on key experimental data only. A new response was elicited first on return to baseline. (B) Relative modulation of 10 μM DS2 on various GABAA receptor subtypes compared with a GABA response elicited using either GABA EC5-20 for α1–5β2γ2 or EC20-50 for α1β2δ, and α4β1–3δ and α6β2δ indicated as modulation relative to agonist (Modulation rel. AG) on the y-axis. EC50 values from full concentration–response curves given at the bottom of panel B are calculated using the Hill equation. n-numbers are n = 5 for α4β1δ, n = 5 for α4β2δ, n = 11 for α4β3δ and n = 6 for α6β2δ. (C) DS2 concentration–response curves related to the GABA Imax value for each X. laevis oocyte. GABA responses elicited with no DS2 present or at a given D2 concentration for α4β2δ [using GABA EC20-50 (0.1–1 μM)] and with no DS2 present or at a given D2 concentration for α1β2γ2 [using GABA EC5-20 (0.5–3 μM)].
The very low concentration of GABA (10 nM) used in these experiments did not elicit a robust inward current from Xenopus oocytes expressing α4β3γ2 receptors. Therefore, the effect of DS2 was also investigated using 10 μM GABA to activate this receptor subtype. Under these conditions, 10 μM DS2 produced only a modest enhancement of the GABA-evoked current (Figure 1A, bottom left panel trace) being ≤20% of control compared with ≥10-fold increase at α4β3δ receptors. Thus, for α4-containing receptors, DS2 clearly discriminates between α4β3δ compared with α4β3γ2 and α4β3 receptors.
Effect of DS2 on additional GABAA receptors: DS2 is selective for extrasynaptic versus synaptic receptors
We next addressed whether DS2 modulated GABA-evoked currents at other γ2-containing GABAA receptors: namely, α1β2γ2, α2β2γ2, α3β2γ2 and α5β2γ2 receptors. With the exception of the α5β2γ2 receptor, these receptors are primarily expressed at the synapse (Fritschy and Mohler, 1995; Jacob et al., 2008). For these experiments, all γ2-containing receptors were activated with a GABA concentration that elicited a current response corresponding to EC5-20 and DS2 was co-applied with GABA in the concentration range of 100 nM to 10 μM. For these receptors, DS2 (0.1–1 μM) had no effect on the GABA-evoked current. At higher concentrations (3 and 10 μM), DS2 produced a modest but significant potentiation (range 47–139% of the submaximal GABA-evoked response) – see Figure 1B. However, these limited effects were at a low relative potency (EC50 values >10 μM for all γ2-containing receptors) compared with its EC50 (5.6 ± 1.5 μM) at α4β3δ – see summary EC50 values at bottom of Figure 1B.
Actions of DS2 are influenced by the α- but not the β-subunit
Whether the modulatory effect of DS2 with δ-GABAARs was additionally influenced by the type of α- or β-subunit present in the receptor complex was addressed using the following receptor subtypes: α4β1δ, α4β2δ, α4β3δ, α1β2δ and α6β2δ. A maximal concentration of DS2 (10 μM) produced a large enhancement (7- to 20-fold) of the GABA (1 μM) evoked current mediated by all δ-containing receptors incorporating the α4- or the α6-subunit (Figure 1B) with calculated EC50 values of 5.2 ± 1.9 (n = 5) and 7.9 ± 1.1 (n = 6) μM for α4β2δ and α6β2δ receptors respectively. By contrast, the maximal enhancement produced by DS2 of the GABA-evoked response mediated by α1β2δ receptors was modest, with a magnitude of enhancement (∼2-fold) similar to that found for DS2 modulation of γ2-containing receptors (Figure 1B). Furthermore, the EC50 value of DS2 was higher when an α1-subunit rather than an α4- or an α6-subunit was combined with the δ-subunit (see summary EC50 values at bottom of Figure 1B).
It is possible that the δ-subunit was not expressed and incorporated into the receptor complex when co-expressed with α1- and β2-subunits. However, DS2 was effective on the same batch of oocytes injected with equivalent cRNAs for α4β2δ and α6β2δ receptors. Furthermore, we found that the GABA-modulatory effect of DS2 acting at α1β2δ receptors was similar to that for oocytes injected with an increased ratio (two- or fivefold) of cRNA encoding for the δ-subunit cf. the α4- or β2-subunits (data not shown). Moreover, DS2 did not modulate the effect of GABA at α1β2 receptors (data not shown).
In addressing the influence of the β-subunit on the modulatory actions of DS2, we found no difference in the efficacy or EC50 value of DS2 at α4β1δ, α4β2δ and α4β3δ receptors (see summary EC50 values at bottom of Figure 1B).
Thus, we conclude from the above, that DS2 is selective for α4βxδ and α6β2δ receptors, exhibiting both a greater efficacy (EMAX) and a more potent modulation (EC50) for α4β1δ, α4β2δ, α4β3δ and α6β2δ receptors cf. γ2-containing receptors and the α1β2δ receptor. However, a 10 μM concentration of DS2 did enhance the function of all GABAA receptorstested, but with limited potency and efficacy.
Concentration–response curves for DS2 were also constructed at GABA Imax for the α4β2δ and α1β2γ2 receptor. The modulatory effect of DS2 on the α4β2δ receptor largely exceeds GABA Imax for this receptor in the tested concentration range. This is in contrast to the α1β2γ2 receptor where 30 μM DS2 only engenders a 30% current relative to GABA Imax (see Figure 1C).
Mechanism of action at α4β2δ and α6β2δ receptors: DS2 primarily enhances the maximum GABA response with only a modest impact on GABA potency
To investigate in more detail the mechanism of action of this δ-selective ligand, GABA concentration–response relationships were determined in the absence and presence of DS2 for α4β2δ, α6β2δ and α1β2γ2 receptors. For both δ-containing receptors DS2 (1 μM) produced only a modest leftward shift of the GABA EC50 (control α4β2δ = 1.5 ± 0.1 μM, n = 8; DS2 = 0.8 ± 0.1 μM, n = 3; control α6β2δ = 1.3 ± 0.6 μM, n = 7; DS2 = 0.5 ± 0.2 μM, n = 3), which was significant (Student's t-test on pEC50) only for the α4β2δ receptor. However, for both α4β2δ and α6β2δ receptors, DS2 produced a large increase of the apparent maximal effect of GABA (see Figure 2A and B). By contrast, a 10 times greater concentration of DS2 did not affect the GABA EC50 or the maximal effect at α1β2γ2 receptors (Figure 2C).
Figure 2.

The influence of DS2 on the GABA concentration–response relationship for human recombinant α4β2δ,α6β2δ, and α1β2γ2 GABAA receptors. GABA concentration–response relations from two-electrode voltage-clamp electrophysiology experiments performed with, or without, 1 or 10 μM DS2 for (A) α4β2δ, (B) α6β2δ and (C) α1β2γ2 receptors expressed in X. laevis oocytes. The responses are normalized to the response produced by 30 μM GABA. GABA EC50 values calculated from the curves using the Hill equation are α4β2δ [1.5 ± 0.1 μM (n = 8)], α4β2δ + 1 μM DS2 (0.8 ± 0.1 μM, n = 3), α6β2δ (1.3 ± 0.6 μM, n = 7), α6β2δ + 1 μM DS2 (0.5 ± 0.2 μM, n = 3), α1β2γ2 (1.9 ± 0.5 μM, n = 11) and α1β2γ2 + 10 μM DS2 (1.1 ± 0.3 μM, n = 11).
Direct receptor activation by DS2?
Previously, it was reported that a close analogue of DS2, namely DS1, had both GABA modulatory effects and direct activating properties (i.e. ‘GABA mimetic’) at α4β3δ receptors over the same concentration range (3–100 nM) (Wafford et al., 2009). However, in contrast to the earlier report (Wafford et al., 2009), in the absence of GABA, the application of DS2 to X. laevis oocytes expressing α4β3δ receptors induced a concentration-dependent (0.3–30 μM) inward current with a calculated EC50 of 4.9 ± 2.1 μM, n = 4, and an EMAX of ∼2- to 3-fold of that produced by 100 nM GABA as seen in Figure 3A and B.
Figure 3.
A comparison of direct receptor activation and positive allosteric modulation by DS2 on human recombinant α4β3δ and α4β2δ receptors. (A) Current traces from two-electrode voltage-clamp electrophysiology experiments addressing the apparent direct activation (traces 4–8) of α4β3δ receptors expressed in X. laevis oocytes by DS2. cf. the positive allosteric effect of DS2 (traces 13–17). Bars indicate where GABA and/or DS2 are applied for 1 min. Note that the traces are truncated and staggered, omitting the long wash periods – 90 s at lowest concentration, and up to 4 min at the highest concentration of DS2 – to focus on key experimental data only. A new response was elicited first on return to baseline. (B) Concentration–response curves for DS2 recorded at α4β3δ expressing oocytes. Direct activation by DS2 is plotted along with the response to 100 nM GABA. EC50 values using the Hill equation were determined to be 4.9 ± 2.1 μM (n = 4) for direct activation. (C) Current traces showing the blocking effect of bicuculline on α4β3δ receptors both on leak current (traces 1–2) and on the current elicited by 0.5 μM DS2 without any GABA present (traces 3–6). As in panel A above, the traces are truncated and staggered, omitting the long wash periods to focus on key experimental data only, with a new response being elicited first on return to baseline. (D) Current traces (truncated and staggered, see above) from α4β2δ receptors expressed in X. laevis oocytes exposed to GABA (traces 1–2), bicuculline (trace 3), DS2 alone (3 and 10 μM, traces 6–7) and DS2 co-applied with GABA (DS2 concentration range, 0.3–10 μM, traces 10–13). The lower traces are amplified to better illustrate the relatively small current responses. (E) Membrane potential and leak current measured from two-electrode voltage-clamp recordings from α4β3δ and α4β2δ receptors expressed in X. laevis oocytes. The membrane resistance is calculated as ((Uholding − UMembrane)/ILeak).
However, one caveat that needed to be considered before concluding that DS2 exhibits both modulatory and direct activating effects at α4β3δ receptors was that X. laevis oocytes expressing α4β3δ receptors exhibited a greater leak current than those expressing most other GABAA receptors, (e.g. γ2-containing and α4β2δ receptors). The membrane resistance of the X. laevis oocyte was calculated by dividing the difference between Uholding and UMembrane with ILeak, which revealed a significantly lower resistance of 13.6 ± 2.2 kΩ for oocytes expressing α4β3δ receptors compared with those expressing α4β2δ receptors (835 ± 57 kΩ) as listed in Figure 3E. This leak current could be due to spontaneous openings of the channels in the absence of agonist as reported for some other receptor combinations, e.g. α1βε (Maksay et al., 2003). Two experiments were performed to investigate whether DS2 activates the α4β3δ receptor directly, or whether this inward current is produced by a modulation of spontaneous channel openings. First, we demonstrated that DS2 was not able to elicit direct receptor activation in the absence of GABA when applied to X. laevis oocytes expressing α4β2δ receptors, which display little leak current (Figure 3D). Second, bicuculline, an inverse agonist at the orthosteric site (Ueno et al., 1997), was able to inhibit the leak current in X. laevis oocytes expressing α4β3δ receptors in a concentration-dependent manner, whereas it had no effect on X. laevis oocytes expressing α4β2δ receptors (compare Figure 3D and C). Moreover, direct activation of the α4β3δ receptor by DS2, in the absence of GABA, was also inhibited by bicuculline (Figure 3C). These observations suggest that α4β3δ receptors demonstrate a degree of spontaneous activity, and that DS2 can positively modulate this activity. Although β3 homomeric receptors do form spontaneously active channels when expressed in X. laevis oocytes (Wooltorton et al., 1997), these ‘receptors’ are not affected by application of DS2 (data not shown), suggesting that the presence of a δ-subunit is required.
As previous studies (Hadley and Amin, 2007) have demonstrated that spontaneous GABAAR channel opening in oocytes is more prevalent at high levels of receptor expression, we wanted to ensure that our findings above were not an artefact of the oocyte expression system. Therefore, we ran similar experiments (data not shown) to those described above with DS2 in HEK-293 cells (both a stable cell line and in transient transfections). In a HEK-293 stable cell line expressing α4β3δ receptors, we also demonstrated direct activation with DS2, which was blocked by bicuculline. Furthermore, the leak current in these HEK-293 cells stably expressing α4β3δ receptors could be blocked by picrotoxin. When we tested the effects on DS2 on HEK-293 cells engineered to transiently express α4β3δ and α4β2δ receptors, we observed direct activation when the β3, but not when the β2 subunit was present in the receptor construct. These equivalent data sets in HEK-293 and X. laevis oocyte expression systems lead us to conclude that it is likely that the presence of a β3 subunit in the receptor construct results in receptors with some constitutive/spontaneous activity, which in turn leads to an apparent ‘direct’ effect of DS2 at the α4β3δ receptor isoform.
In conclusion, these studies suggest that DS2 does not directly activate GABA α4β2δ or α4β3δ receptors but instead modulates the spontaneous current present when α4β3δ receptors are expressed in X. laevis oocytes or HEK-293 cells; a spontaneous activity that does not appear to be evident in mouse L(tk–) cells expressing α4β3δ (Wafford et al., 2009).
Interaction of DS2 with known sites on the GABAA receptor
Orthosteric site
At concentrations up to 10 μM, DS2 did not displace [3H]muscimol (2 nM) bound to the orthosteric site of α4β3δ receptors expressed in HEK-293 cells (data not shown). The lack of effect of DS2 on muscimol binding suggests that it does not directly interact with the orthosteric GABA binding site of GABAA receptors.
Benzodiazepine site
A well-characterized binding site on GABAA receptors is the benzodiazepine site located on the extracellular interface between α- and γ-subunits (Sigel, 2002; Berezhnoy et al., 2004). To ascertain if DS2 interacted with the benzodiazepine site, we determined if DS2 displaced (i) [3H]flumazenil binding to rat cortical tissue (reflecting binding predominantly to α1β2γ2, but including also α2, α3 and α5 receptor subtypes) and (ii) [3H]Ro 15–4513 binding to α4β3γ2 receptors expressed in HEK-293 cells. In rat cortex, a relatively high concentration of DS2 (30 μM) displaced 30% of specifically bound [3H]flumazenil, suggesting a possible low affinity interaction with the benzodiazepine site or, more likely, that the interaction of DS2 with the receptor causes an allosteric change in the benzodiazepine binding site leading to a change in bound [3H]flumazenil. Nonetheless, we followed up with functional electrophysiology studies and demonstrated that the modest positive modulation of α1β2γ2 receptors by DS2 was not blocked by the benzodiazepine antagonist flumazenil up to a concentration of 100 μM (data not shown). Moreover, DS2 (up to 10 μM) had no effect on the specific binding of [3H]Ro 15–4513 to recombinant α4β3γ2 receptors (data not shown). These experiments indicate that DS2 does not interact with a benzodiazepine-like site at γ-containing GABAA receptors.
There has been debate as to whether an equivalent site similar to the benzodiazepine site is present on δ-containing GABAA receptors, as Ro 15–4513 binding to δ-containing receptors has been reported (Wallner et al., 2003; Hanchar et al., 2006; Olsen et al., 2007). However, we did not obtain any specific binding of [3H]-Ro 15–4513 to recombinant α4β3δ receptors expressed in HEK-293 cells (data not shown) and therefore cannot verify the existence of such a site, a finding consistent with others (Korpi et al., 2007).
Etomidate site
Positive allosteric modulation of the GABAA receptor by the general anaesthetics etomidate and propofol is influenced by the nature of an amino acid residue located within the TM2 domain of the β-subunit (Belelli et al., 1997). Substitution of the naturally occurring asparagine 265 residue of the β2- or β3-subunit, for a methionine residue (N265M) abolishes the GABA-enhancing effects of these compounds at γ2-containing GABAA receptors (Belelli et al., 1997; Jurd et al., 2003). Both etomidate and DS2 enhanced the current induced by 100 nM GABA at α4β3δ receptors (Figure 4). In agreement with previous studies with γ2-containing receptors, we now demonstrate that the β3 N265M mutation abolished the GABA-enhancing effects of etomidate at δ-containing receptors (i.e. α4β3N265Mδ). By contrast, the GABA enhancing effects of DS2 were not influenced by this β3 N265M mutation (Figure 4). Therefore, the modulation induced by DS2 is not mediated via an interaction with this putative anaesthetic binding site.
Figure 4.

The potential interaction of DS2 with the etomidate or the neurosteroid site of GABAA receptors. The relative modulation of α4β3δ, α4β3N265Mδ or α4T235W,Q240Wβ3δ receptors expressed in Xenopus oocytes by 10 μM DS2, 10 μM etomidate or 10 μM allopregnanolone (see Results section for further details). Percentage (%) modulation was determined relative to a GABA response evoked by 0.1 μM GABA and is shown as an average ± SEM, n = 3–5.
Neurosteroid site
Previous studies have established that the introduction of two mutations in the α-subunit, namely T235W and Q240W, render GABAA receptors insensitive to positive allosteric modulation by the endogenous neurosteroid, allopregnanolone (Hosie et al., 2006). Similarly, we demonstrate here that the GABA enhancing actions of allopregnanolone (10 μM) were abolished by these mutations when incorporated in to the α4-subunit (i.e. α4T235W, Q240W β3δ). By contrast, the GABA-enhancing action of DS2 (10 μM) were not influenced by these mutations of the α4-subunit (Figure 4; right-hand bars), suggesting that DS2 does not act via the neurosteroid site.
Barbiturate site
The molecular determinants of the barbiturate site have not been identified, so an alternative approach was used to explore whether DS2 and pentobarbital interacted via a common site. First, DS2 concentration–response curves were generated in the presence of different concentrations of pentobarbital as shown for 10 μM pentobarbital in Figure 5A. We found that the DS2 EC50 decreased with higher concentrations of pentobarbital, and that clear modulation by DS2 was observed at pentobarbital concentrations up to 100 μM. Thus, pentobarbital does not block the GABA modulatory effect of DS2.
Figure 5.

The potential interaction of DS2 with the barbiturate site of GABAA receptors. (A) Current traces from DS2 concentration–response experiments performed using the co-application of GABA, pentobarbital (PB) and DS2 recorded from X. laevis oocytes expressing α4β2δ receptors. Note that the traces are truncated and staggered, omitting the long wash periods – 90 s at lowest concentration, and up to 4 min at the highest concentration of DS2 – to focus on key experimental data only. A new response was elicited first on return to baseline. Traces 1–3 show the response to 1 μM GABA applied alone, traces 4–6 show the response to 10 μM pentobarbital co-applied with 1 μM GABA and traces 7–11 show the modulatory effect of increasing concentrations of DS2 (0.3–10 μM) on responses elicited by 1 μM GABA plus 10 μM pentobarbital. (B) Current traces (truncated and staggered, see above) for a DS2 concentration–response experiment on α4β2δ receptors after 10 mM pentobarbital has been washed from the preparation (i.e. modulation by DS2 of a rebound current demonstrable after pentobarbital removal, see text in the Results section for further details).
High concentrations of pentobarbital have been reported to directly activate GABAA receptors (Muroi et al., 2009), but no direct activation with concentrations up to 10 mM pentobarbital were observed for the α4β2δ receptor. However, as seen in Figure 5B, 10 mM pentobarbital gave rise to a rebound current after removal of pentobarbital. This rebound effect was modulated by DS2 with the same potency as for the modulation of GABA (EC50 value of 5.3 μM for the modulation of the rebound effect after pentobarbital removal, compared to an EC50 value of 5.2 μM for modulation of GABA). As the EC50 value for pentobarbital-induced modulation of α4β2δ receptors is between 100 and 300 μM (data not shown), a 10 mM pentobarbital concentration would be expected to saturate the barbiturate modulatory binding site. Because, under these conditions, DS2 can still modulate the receptor, we would suggest that the DS2-induced modulation is not mediated via the same site as pentobarbital.
DS2 selectively enhances the function of the extrasynaptic δ-GABAA receptors of VB thalamocortical neurons: studies using δ0/0 mice
We have previously demonstrated that DS2 produces a concentration-dependent (1–10 μM) enhancement of the tonic current of thalamocortical VB neurons (Wafford et al., 2009). To investigate the role of native δ-GABAA receptors in this effect, we compared VB neurons derived from δ0/0 and wild-type (WT) mice. For neurons derived from WT mice, the GABAA receptor antagonist bicuculline (30 μM) produced a relatively large outward current (96 ± 8.9 pA, n = 27) (i.e. the ‘tonic’ current) (Figure 6). The outward current induced by bicuculline (30 μM) was greatly reduced (13 ± 4.7 pA, n = 9; P > 0.001; Student's unpaired t-test) for equivalent neurons derived from the δ0/0 mouse (Figure 6), confirming earlier reports that extrasynaptic δ-GABAA receptors are important contributors to the tonic current of VB neurons (Porcello et al., 2003; Belelli et al., 2005; Cope et al., 2005; Jia et al., 2005; Chandra et al., 2006). In agreement, with our previous report, the application of 10 μM DS2 to WT VB neurons induced a relatively large inward current (107 ± 9.6 pA, n = 9 – see Figure 6). The effect DS2 on the holding current (11 ± 6.6 pA, n = 5) was greatly reduced (P < 0.001 vs. WT; Student's unpaired t-test) for δ0/0 VB neurons (Figure 6). Concentrations of DS2 that greatly enhance the tonic current of VB neurons have little or no effect on the miniature inhibitory postsynaptic currents (mIPSCs) mediated by α1β2γ2 GABAA receptors located at the synapse (Wafford et al., 2009). Here, the properties of the mIPSCs recorded from δ0/0 VB neurons were identical to those of WT neurons, confirming earlier reports that δ-GABAA receptors make little contribution to such quantal synaptic events. Furthermore, in agreement with our earlier study on WT VB neurons, DS2 (10 μM) had no effect on the amplitude, kinetics or frequency of δ0/0 mIPSCs (Table 1). In conclusion, these results demonstrate DS2 enhances the tonic conductance mediated by extrasynaptic δ-GABAA receptors of thalamocortical VB neurons, with no effect on the phasic conductance mediated by synaptic GABAA receptors; that is, the δ-subunit specificity of this compound exhibited by recombinant receptors is replicated for native neuronal receptors.
Figure 6.
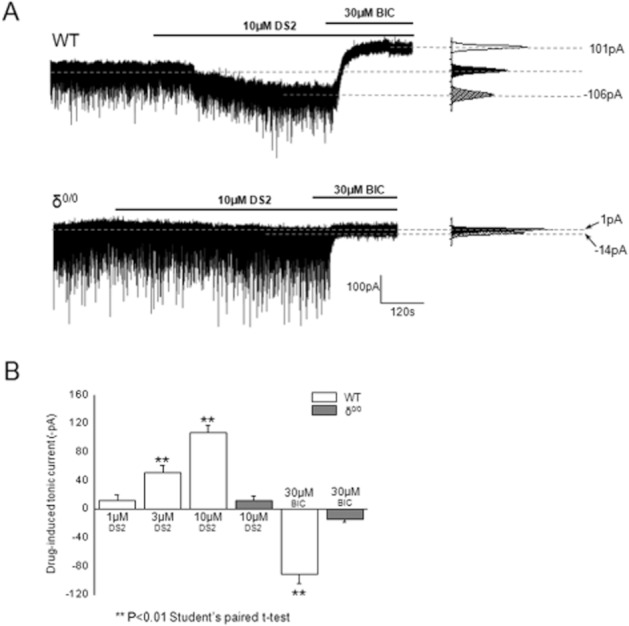
DS2 selectively interacts with extrasynaptic δ-GABAA receptors of mouse thalamic relay neurons. (A) Top trace: The tonic current (calculated as the difference between the holding current [pA] in the presence and absence of bicuculline 30 μM) recorded from a typical wild-type (WT) mouse thalamic ventrobasal neuron is greatly enhanced by DS2 (10 μM). By contrast, both the effect of DS2 (10 μM) to produce an inward current and the effect of bicuculline (30 μM) to induce an outward current are absent in a typical recording made from mouse thalamic ventrobasal neurons derived from a δ0/0 mouse. To the right of each trace is the corresponding all points histograms of the tonic current for the illustrated recordings under control conditions, in the presence of 10 μM DS2 and 30 μM bicuculline. (B) A bar graph summarizing the inward tonic current induced by 1, 3 and 10 μM DS2 for WT neurons and for 10 μM DS2 for δ0/0 neurons. The trace illustrating the effect of DS2 in WT ventrobasal neurons (panel A) and data for 1 and 3 μM DS2 (panel B) are reproduced from (Wafford et al., 2009). Note that the large outward current induced by bicuculline (30 μM) for WT neurons is absent for equivalent δ0/0 neurons. Data illustrate the mean ± SEM and were obtained from three to seven neurons (**P < 0.01, Student's paired t-test).
Table 1.
Effects of 10 μM DS2 on mIPSCs recorded from δ0/0 VB neurones
| Control (n = 5) | 10 μM DS2 (n = 5) | |
|---|---|---|
| Peak amplitude (pA) | −82 ± 5 | −85 ± 4 |
| Rise time (ms) | 0.5 ± 0.03 | 0.5 ± 0.02 |
| Area (pC) | −254 ± 26 | −285 ± 28 |
| T50 (ms) | 1.8 ± 0.1 | 1.9 ± 0.1 |
| T90 (ms) | 4.9 ± 0.2 | 5.3 ± 0.3 |
| τw (ms) | 2.6 ± 0.1 | 2.8 ± 0.1 |
| Frequency (Hz) | 17 ± 2 | 17 ± 3 |
Discussion and conclusions
A growing corpus of literature has highlighted the therapeutic potential of drugs that selectively target GABAA receptor isoforms that incorporate the δ-subunit. Specifically, targeting α4β2/3δ receptors for treating various CNS (see Introduction and references therein), as well as non-CNS (e.g. type 1 diabetes; see (Mendu et al., 2011) disorders is gaining momentum. It is now evident that δ-GABAA receptors exhibit a restricted neuronal distribution, are primarily found peri- or extrasynaptically, exhibit distinct biophysical properties compared with synaptic GABAA receptors and are not affected by clinically used benzodiazepines. Furthermore, drugs that do affect δ-GABAA receptors are relatively non-selective, exhibiting effects on their synaptic counterparts (e.g. volatile anaesthetics, barbiturates, propofol, etomidate and neurosteroids) (see Introduction and references therein).
In the current study, we have thoroughly characterized the reported α4β3δ-selective PAM DS2 first described by Wafford et al. (2009). Although our understanding of the physiological role of these receptors has advanced, given the limited availability of pharmacological tools that target δ-GABAA receptors, the following discussion compares, where applicable, the profile of DS2 to the only well-described δ-GABAA receptor selective tool gaboxadol. In addition, two other putative δ-GABAA receptor-preferring compounds have recently been described, namely the trimethylbenzylamino compound AA29504 (Hoestgaard-Jensen et al., 2010) and a dihydropyrimidinone compound JM-II-43A (Lewis et al., 2010). Therefore, where relevant data are available on these molecules, comparisons are made with our data on DS2.
GABAA receptor subtype selectivity
We have verified that DS2 is a PAM at α4β3δ receptors but have additionally demonstrated that (i) DS2 is equally effective (i.e. ∼ equal efficacy and potency) at α4βxδ and α6β2δ receptors, but not at α1β2δ receptors; (ii) DS2 clearly exhibits selectivity for α4βxδ and α6β2δ receptors c.f. GABAA receptors where a γ-subunit occurs in place of a δ-subunit, whether these receptors in vivo occur synaptically (α1β2γ, α2β2γ, α3β2γ) or extrasynaptically (α5β32γ); and (iii) the β-subunit isoform does not influence the PAM activity of DS2 when combined with α4- and δ-subunits. These studies clearly indicate that modulation of GABAA receptors by DS2 is strongly influenced by whether the δ- or the γ2-subunit is present in the receptor complex and, contrary to gaboxadol, DS2 does not affect α4β3 receptors (Shu et al., 2011). However, our data indicate further subtleties in the determinants of the DS2 selectivity profile not reported previously. First, if a γ2-subunit is present in the receptor complex, the nature of the α-subunit does not influence the limited efficacy, or potency of DS2. By contrast, when investigating δ-GABAA receptors, it is evident that DS2 modulates α4- and α6- more effectively than α1-containing receptors. These results demonstrate an interesting selectivity profile for DS2 between known in-vivo occurring populations of δ-GABAA receptors.
The best characterized δ-preferring compound described to date is the orthosteric site agonist gaboxadol. Besides the fundamental distinction that gaboxadol is an agonist, whereas DS2 is a PAM, there are also other differences between these two compounds. At γ-receptors (α1–6β1–3γ2), the maximal efficacy of gaboxadol is between 40% and 80% relative to GABA, with EC50 of ∼100 μM, indicating that relative to GABA, gaboxadol is a relatively low potency agonist of these receptors (Ebert et al., 1994). By contrast, at α6β3δ-, α4β3δ- and α4β3-receptors, gaboxadol has much higher potency (EC50 ∼10 μM for α6β3δ- and ∼50 μM for α4β3δ- and α4β3-receptors), with an apparent maximal efficacy of ∼300%, ∼200% and 164%, respectively, relative to GABA (Storustovu and Ebert, 2006). Thus, the overall selectivity profile of gaboxadol is α6β3δ > α4β3δ = α4β3 >> αxβ3γ2-receptors. By contrast, DS2 positively modulates α4/6βxδ- but not α4β3γ2-receptors, nor other γ2-receptor populations, and induces no modulation whatsoever of α4β3-receptors. That DS2 is inert at α4β3-receptors makes this drug a very useful tool to confirm the incorporation of a δ-subunit into the receptor complex; that is, the activity of DS2 is fundamentally dependent on the presence of a δ-subunit. Thus, overall, DS2 has a different selectivity profile compared with gaboxadol: α4/6βxδ > α1βxδ >> γ2-receptors > α4β3. Given that many of these receptor populations may exist in vivo (McKernan and Whiting, 1996; Pirker et al., 2000; Mortensen and Smart, 2006), the distinct selectivity profiles of DS2 and gaboxadol may lead to different effects in complex biological systems.
Although data on AA29504 (Hoestgaard-Jensen et al., 2010) and JM-II-43A (Lewis et al., 2010) are either limited, or the methodologies and techniques differ from those used here, some comparison with the selectivity profile of DS2 is possible. AA29504 was tested on α4β3δ and α1β3γ2s receptors; and, in common with DS2, this compound preferentially modulates α4β3δ over α1β3γ2S receptors (Hoestgaard-Jensen et al., 2010). However, in more detailed studies addressing the mechanism of action of AA29504, the authors showed that it decreased the EC50 for GABA acting at both α4β3δ- and α1β3γ2S-receptors. However, AA29504 only enhanced the maximal response to GABA for α4β3δ-receptors, indicating selectivity for the δ-receptor. Similarly, AA29504 increased the potency of gaboxadol acting at both α4β3δ- and α1β3γ2S-receptors. However, distinct from GABA, for α4β3δ-receptors AA29504 did not enhance but reduced the maximal response to gaboxadol. This difference in the effect of AA29504 on the maximal response to GABA and gaboxadol is probably a reflection of these two molecules being partial and full agonists at δ-GABAA receptors respectively. JM-II-43A was reported to be a δ-GABAA receptor compound on the basis of its differential influence on α1β2δ- and α1β2γ2L-receptors. However, many of the experiments were performed at 1 mM saturating GABA concentrations, making it difficult to compare the profile of this molecule with DS2. With GABA being a partial agonist at δ-GABAA receptors, the window for potentially demonstrating modulatory effects at α4β2δ is greater than for α1β2γ2L receptors where GABA induces much greater open probability (Mortensen et al., 2010). A major difference between DS2 and JM-II-43A is the influence of the α-subunit. Whereas DS2 had a preference for δ-GABAA receptors combined with the α4 or the α6 over the α1-subunit, JM-II-43A exerted a similar enhancement of δ-GABAA receptors containing an α1-, α4- or α5-subunit and a reduced effect when an α6-subunit occurred in the receptor complex. Moreover, compared with DS2 (EC50 = ∼5 μM), JM-II-43A is not very potent (EC50 value in the 100 μM range at α1β2δ-receptors).
Mechanism of action
Here we demonstrate that at δ-GABAA receptors, DS2 (i) increases the apparent maximal effect of GABA at α4βxδ and α6β2δ receptor subtypes, with only a small impact on GABA potency at δ-receptors; (ii) is a PAM of GABA acting at α4βxδ receptor subtypes, but does not directly activate such receptors, in contrast to the close analogue DS1 (Wafford et al., 2009). For α4β2δ-receptors, DS2 enhances the apparent GABA Imax by ∼10-fold.
One corollary from the mechanism of action studies is that α4β3δ-receptors expressed in oocytes caused much greater leak current than α4β2δ and all other receptors investigated in this study. This spontaneous activity could be enhanced by DS2. This property was not observed with α4β3δ-receptors expressed in mouse L(tk) cells, although spontaneous activity has been reported in oocytes injected with just β3-subunits (Wooltorton et al., 1997).
Site of action
Using radioligand binding to native tissue or recombinant receptors and functional electrophysiological studies with chimeric receptors, we demonstrated that DS2 did not (i) mediate its effects at α4βxδ-receptors via the orthosteric agonist site; (ii) bind to the ‘benzodiazepine’ site at various γ2-subunit containing receptors, and by inference any ‘benzodiazepine-like’ site suggested to be present on α4β3δ-receptors (Hanchar et al., 2006); (iii) modulate α4βxδ-receptors via an interaction with the etomidate site; (iv) modulate α4βxδ-receptors via an interaction with the neurosteroid site. Finally, DS2 was not likely to modulate α4βxδ-receptors via a site common with barbiturates based on interaction studies with pentobarbital. The conclusion that DS2 is not modulating δ-GABAA receptors via a site common with barbiturates is based on an indirect methodology compared to our studies exploring the orthosteric, benzodiazepine, etomidate and neurosteroid sites. Nonetheless, collectively, these findings suggest a unique binding site for DS2 with efficacy critically dependent on the presence of the δ-subunit in the GABAA receptor complex.
Previous studies have reported the specific binding of [3H]-Ro 15–4513 to recombinant α4/6β2/3δ receptors with low nanomolar affinity, suggesting the presence of a ‘benzodiazepine-like’ site on δ-GABAA receptors. However, in agreement with others (Korpi et al., 2007), we found no specific binding of [3H]-Ro 15–4513 to α4β3δ-receptors expressed (either transiently or stably) in HEK-293 cells. These HEK-293 cells expressing α4β3δ receptors gave clear signals to GABA in a FLIPR assay per se (data not shown) and responded to DS2, indicating the presence of the δ-subunit in the complex. Moreover, if modulation by DS2 of α4βxδ receptors was mediated through its binding to a ‘benzodiazepine-like’ site on α4βxδ receptors, then some displacement of [3H]-Ro 15–4513 binding to α4β3γ2 expressed in HEK-293 cells, or a greater degree of displacement of [3H]flumazenil binding to cortex might have been observed. Furthermore, there was no influence of flumazenil on the limited modulation of α1β2γ2-receptors by DS2 in electrophysiological studies, indicating no major interaction of DS2 with the known benzodiazepine site at this receptor isoform.
Native neuronal GABAA receptors
We previously demonstrated that DS2 enhanced a tonic current in mouse thalamocortical VB neurons (Wafford et al., 2009). Here we additionally revealed that this effect was mediated via δ-containing receptors as the modulation of the tonic current was not evident in VB neurons derived from the δ0/0 mouse. The predominant δ-GABAA receptor population in VB neurons is the α4β2δ isoform, which mediates the actions of gaboxadol in these thalamic neurons (Belelli et al., 2005; Chandra et al., 2006; Peden et al., 2008) and potentially the effects of gaboxadol on sleep (Wafford and Ebert, 2006; Winsky-Sommerer et al., 2007). As shown previously for gaboxadol, there was no influence of DS2 on mIPSCs mediated by synaptic α1β2γ2 receptors in VB neurons obtained from WT or δ0/0 mice (Belelli et al., 2005; Chandra et al., 2006; Peden et al., 2008). δ-containing receptors are not only found in the thalamus (Peng et al., 2002) – in some brain regions, the δ-subunit appears to preferentially partner with α1- and α6-subunits (e.g. hippocampal interneurons and cerebellar granule cells, respectively) (Nusser et al., 1998; Sun et al., 2004; Mangan et al., 2005), rather than α4-subunits (e.g. dentate gyrus granule cells and VB thalamocortical neurons) (Chandra et al., 2006). In this respect, it is interesting that DS2 appears to discriminate between δ-receptor populations, in that it selectively modulated δ-GABAA receptors incorporating an α4- or α6-, rather than a α1-subunit. Future studies exploring the effects of DS2 on neurons where α1β2δ and α6β2δ populations predominate would clarify whether this selectivity at recombinant receptors is observed in native preparations expressing these subtypes.
In vivo properties
Although not reported in this paper, we have attempted to ascertain the utility of DS2 as an in vivo tool compound. However, in an initial pharmacokinetic study in both mice and rats, we found that whereas plasma exposure after a 10 mg·kg−1 p.o. dose of DS2 was very high in both rats (Cmax ∼30 μM) and mice (Cmax ∼15 μM), in both species, DS2 had very poor brain penetration (brain/plasma ∼0.1). Further assessment in the rat at a higher dose (30 mg·kg−1, p.o.), as well as being delivered via other routes of administration (s.c. and i.p.), did not result in any significant improvement in brain-penetrant properties. This poor brain/plasma profile was confirmed by a lack of effect of DS2 at doses up to 100 mg·kg−1 p.o. in animal models of gross behaviour (Irwin screen, locomotor activity, rotarod), anxiety (e.g. zero maze and stress-induced hyperthermia), psychosis (MK-801 hyperactivity, PPI) and pain (formalin). Although it is plausible that these models might be unresponsive to a compound with the GABAA receptor selectivity profile of DS2 and that more appropriate models should be considered [e.g. (Maguire and Mody, 2007; Smith et al., 2007), given the poor brain/plasma ratio achieved with DS2 in rodents and the clear in vivo effects described for gaboxadol in similar models (Wafford and Ebert, 2006; – see Introduction), this explanation is unlikely. Clearly, this does not preclude DS2 being administered directly into brain ventricles or discrete brain regions in future studies. However, potentially of more interest, given the excellent plasma exposure of DS2 in mice and rats, is to consider assessing DS2 in animal models of disorders with a peripheral/non-CNS basis (Mendu et al., 2011).
In contrast to the poor CNS bioavailability of DS2, AA29504 (Hoestgaard-Jensen et al., 2010) over a range of doses has been reported to have an excellent brain/plasma ratio of ∼1.5 in rats with absolute brain concentrations reaching 1.6 μM at a dose of 4 mg·kg−1 after s.c. administration. At a dose of 4 mg·kg−1, some in vivo effects were reported in rodents: anxiolysis, impairment of rotarod performance and a synergistic interaction with ethanol leading to impaired rotarod performance. Although these data are of interest, as noted above a 1 μM concentration (brain level in rat after 4 mg·kg−1 = 1.6 μM) modulates both IPSCs and tonic inhibition (in the presence of gaboxadol) in cortical slices. Furthermore, since AA29504 is an analogue of the neuronal Kv7 channel opener retigabine, care should be exercised in associating its actions exclusively with GABAA receptors. Indeed, the authors indicate that in the rat amygdaloid kindling model of partial seizures the effects seen with AA29504 at 10 mg·kg−1 are likely to be linked to activation of Kv7 channels. Moreover, the effects described at the 4 mg·kg−1 dose in the anxiety, rotarod and ethanol interaction tests are consistent with the profile of retigabine (Blackburn-Munro et al., 2005; Korsgaard et al., 2005). Recently, Damgaard et al. (2011) have shown that AA29504 reverses recognition memory deficits in an animal model of schizophrenia, albeit retigabine is also effective in schizophrenia models (Sotty et al., 2009).
In conclusion, we have conducted an in-depth characterization of DS2 demonstrating its unique selectivity for a subset of δ-receptors amongst a range of human GABAA receptors, verified that this selectivity is maintained when assessing native (rodent) GABAA receptors, shown that the predominant mechanism of action of DS2 was to enhance the maximal GABA response at δ-GABAA receptors, and determined that DS2 was unlikely to mediate its effects via currently known sites on GABAA receptors. DS2 was distinguished from gaboxadol in having a different selectivity profile, in addition to being a PAM rather than an agonist. Although not a convenient tool after systemic administration for in vivo studies of CNS disorders, direct brain administration and assessment of DS2 in animal models of peripheral disorders is feasible. This in-depth characterization of DS2 increases our understanding of effects being mediated by this and other δ-subunit selective drugs and suggests that the effects of DS2 are mediated by an as yet uncharacterized site on δ-GABAA receptors.
Acknowledgments
We would like to acknowledge Carsten Jessen for the synthesis of DS2 and Elsebeth Østergaard Nielsen for help with the binding experiments. Work was supported by an AJ Clark Studentship (JJL & DB), MRC Grant G1000008 (DB & JJL) and Tenovus Scotland (DB).
Glossary
- DS1
delta selective compound 1
- DS2
delta selective compound 2
- ECx
effective concentration giving the activation degree of X
- GABAAR
GABAA receptor
- NAM
negative allosteric modulator
- PAM
positive allosteric modulator
- VB neurons
ventrobasal thalamic neurons
Conflict of interest
None.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br. J. Pharmacol. (5th edn) 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type A receptor is influenced by a single amino acid. Proc Natl Acad Sci U S A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–661. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezhnoy D, Nyfeler Y, Gonthier A, Schwob H, Goeldner M, Sigel E. On the benzodiazepine binding pocket in GABAA receptors. J Biol Chem. 2004;279:3160–3168. doi: 10.1074/jbc.M311371200. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005;11:1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin RP, Labrakakis C, Eng DG, Whissell PD, De Koninck Y, Orser BA. Pharmacological enhancement of delta-subunit-containing GABA(A) receptors that generate a tonic inhibitory conductance in spinal neurons attenuates acute nociception in mice. Pain. 2011;152:1317–1326. doi: 10.1016/j.pain.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol. 1996;497(Pt 3):753–759. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Cull-Candy SG, Farrant M. Single-channel properties of synaptic and extrasynaptic GABAA receptors suggest differential targeting of receptor subtypes. J Neurosci. 1999;19:2960–2973. doi: 10.1523/JNEUROSCI.19-08-02960.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKenzie G, Hosie AM, et al. Profound desensitization by ambient GABA limits activation of delta-containing GABAA receptors during spillover. J Neurosci. 2011;31:753–763. doi: 10.1523/JNEUROSCI.2996-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, et al. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope DW, Hughes SW, Crunelli V. GABAA receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damgaard T, Plath N, Neill JC, Hansen SL. Extrasynaptic GABAA receptor activation reverses recognition memory deficits in an animal model of schizophrenia. Psychopharmacology (Berl) 2011;214:403–413. doi: 10.1007/s00213-010-2039-9. [DOI] [PubMed] [Google Scholar]
- Ebert B, Wafford KA, Whiting PJ, Krogsgaard-Larsen P, Kemp JA. Molecular pharmacology of gamma-aminobutyric acid type A receptor agonists and partial agonists in oocytes injected with different alpha, beta, and gamma receptor subunit combinations. Mol Pharmacol. 1994;46:957–963. [PubMed] [Google Scholar]
- Enoch MA. The role of GABA(A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Hadley SH, Amin J. Rat alpha6beta2delta GABAA receptors exhibit two distinct and separable agonist affinities. J Physiol. 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, et al. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci U S A. 2006;103:8546–8551. doi: 10.1073/pnas.0509903103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herd MB, Foister N, Chandra D, Peden DR, Homanics GE, Brown VJ, et al. Inhibition of thalamic excitability by 4,5,6,7-tetrahydroisoxazolo[4,5-c]pyridine-3-ol: a selective role for delta-GABA(A) receptors. Eur J Neurosci. 2009;29:1177–1187. doi: 10.1111/j.1460-9568.2009.06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoestgaard-Jensen K, Dalby NO, Wolinsky TD, Murphey C, Jones KA, Rottlander M, et al. Pharmacological characterization of a novel positive modulator at alpha 4 beta 3 delta-containing extrasynaptic GABA(A) receptors. Neuropharmacology. 2010;58:702–711. doi: 10.1016/j.neuropharm.2009.12.023. [DOI] [PubMed] [Google Scholar]
- Holm MM, Nieto-Gonzalez JL, Vardya I, Henningsen K, Jayatissa MN, Wiborg O, et al. Hippocampal GABAergic dysfunction in a rat chronic mild stress model of depression. Hippocampus. 2010;21:422–433. doi: 10.1002/hipo.20758. [DOI] [PubMed] [Google Scholar]
- Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- Houston CM, Bright DP, Sivilotti LG, Beato M, Smart TG. Intracellular chloride ions regulate the time course of GABA-mediated inhibitory synaptic transmission. J Neurosci. 2009;29:10416–10423. doi: 10.1523/JNEUROSCI.1670-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob TC, Moss SJ, Jurd R. GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition. Nat Rev Neurosci. 2008;9:331–343. doi: 10.1038/nrn2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, et al. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Debus F, Linden AM, Malecot C, Leppa E, Vekovischeva O, et al. Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol. 2007;41:163–176. doi: 10.1016/j.alcohol.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strobaek D, Mirza NR. Anxiolytic effects of Maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther. 2005;314:282–292. doi: 10.1124/jpet.105.083923. [DOI] [PubMed] [Google Scholar]
- Lewis RW, Mabry J, Polisar JG, Eagen KP, Ganem B, Hess GP. Dihydropyrimidinone positive modulation of delta-subunit-containing gamma-aminobutyric acid type A receptors, including an epilepsy-linked mutant variant. Biochemistry. 2010;49:4841–4851. doi: 10.1021/bi100119t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKernan RM, Whiting PJ. Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–2162. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksay G, Thompson SA, Wafford KA. The pharmacology of spontaneously open alpha 1 beta 3 epsilon GABA A receptor-ionophores. Neuropharmacology. 2003;44:994–1002. doi: 10.1016/s0028-3908(03)00116-3. [DOI] [PubMed] [Google Scholar]
- Mangan PS, Sun C, Carpenter M, Goodkin HP, Sieghart W, Kapur J. Cultured hippocampal pyramidal neurons express two kinds of GABAA receptors. Mol Pharmacol. 2005;67:775–788. doi: 10.1124/mol.104.007385. [DOI] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, et al. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006;31:1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendu SK, Akesson L, Jin Z, Edlund A, Cilio C, Lernmark A, et al. Increased GABA(A) channel subunits expression in CD8(+) but not in CD4(+) T cells in BB rats developing diabetes compared to their congenic littermates. Mol Immunol. 2011;48:399–407. doi: 10.1016/j.molimm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, et al. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci U S A. 1999;96:12905–12910. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza NR, Larsen JS, Mathiasen C, Jacobsen TA, Munro G, Erichsen HK, et al. NS11394 [3′-[5-(1-hydroxy-1-methyl-ethyl)-benzoimidazol-1-yl]-biphenyl-2-carbonitr ile], a unique subtype-selective GABAA receptor positive allosteric modulator: in vitro actions, pharmacokinetic properties and in vivo anxiolytic efficacy. J Pharmacol Exp Ther. 2008;327:954–968. doi: 10.1124/jpet.108.138859. [DOI] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Smart TG. Extrasynaptic alphabeta subunit GABAA receptors on rat hippocampal pyramidal neurons. J Physiol. 2006;577:841–856. doi: 10.1113/jphysiol.2006.117952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol. 2010;588:1251–1268. doi: 10.1113/jphysiol.2009.182444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muroi Y, Theusch CM, Czajkowski C, Jackson MB. Distinct structural changes in the GABAA receptor elicited by pentobarbital and GABA. Biophys J. 2009;96:499–509. doi: 10.1016/j.bpj.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olmos-Serrano JL, Corbin JG, Burns MP. The GABA(A) receptor agonist THIP ameliorates specific behavioral deficits in the mouse model of fragile X syndrome. Dev Neurosci. 2011;33:395–403. doi: 10.1159/000332884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the ‘one glass of wine’ receptors. Alcohol. 2007;41:201–209. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden DR, Petitjean CM, Herd MB, Durakoglugil MS, Rosahl TW, Wafford K, et al. Developmental maturation of synaptic and extrasynaptic GABAA receptors in mouse thalamic ventrobasal neurones. J Physiol. 2008;586:965–987. doi: 10.1113/jphysiol.2007.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng HY, Chen GD, Lee SD, Lai CY, Chiu CH, Cheng CL, et al. Neuroactive steroids inhibit spinal reflex potentiation by selectively enhancing specific spinal GABA(A) receptor subtypes. Pain. 2009;143:12–20. doi: 10.1016/j.pain.2008.12.023. [DOI] [PubMed] [Google Scholar]
- Peng Z, Hauer B, Mihalek RM, Homanics GE, Sieghart W, Olsen RW, et al. GABA(A) receptor changes in delta subunit-deficient mice: altered expression of alpha4 and gamma2 subunits in the forebrain. J Comp Neurol. 2002;446:179–197. doi: 10.1002/cne.10210. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Porcello DM, Huntsman MM, Mihalek RM, Homanics GE, Huguenard JR. Intact synaptic GABAergic inhibition and altered neurosteroid modulation of thalamic relay neurons in mice lacking delta subunit. J Neurophysiol. 2003;89:1378–1386. doi: 10.1152/jn.00899.2002. [DOI] [PubMed] [Google Scholar]
- Rewal M, Jurd R, Gill TM, He DY, Ron D, Janak PH. Alpha4-containing GABAA receptors in the nucleus accumbens mediate moderate intake of alcohol. J Neurosci. 2009;29:543–549. doi: 10.1523/JNEUROSCI.3199-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth T, Lines C, Vandormael K, Ceesay P, Anderson D, Snavely D. Effect of gaboxadol on patient-reported measures of sleep and waking function in patients with Primary Insomnia: results from two randomized, controlled, 3-month studies. J Clin Sleep Med. 2010;6:30–39. [PMC free article] [PubMed] [Google Scholar]
- Shu HJ, Bracamontes J, Taylor A, Wu K, McCollum M, Akk G, et al. Characteristics of alpha4/delta-containing concatemeric GABA(A) receptors expressed in Xenopus oocytes. Br J Pharmacol. 2011;165:2228–2243. doi: 10.1111/j.1476-5381.2011.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Curr Top Med Chem. 2002;2:833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotty F, Damgaard T, Montezinho LP, Mork A, Olsen CK, Bundgaard C, et al. Antipsychotic-like effect of retigabine [N-(2-Amino-4-(fluorobenzylamino)-phenyl)carbamic acid ester], a KCNQ potassium channel opener, via modulation of mesolimbic dopaminergic neurotransmission. J Pharmacol Exp Ther. 2009;328:951–962. doi: 10.1124/jpet.108.146944. [DOI] [PubMed] [Google Scholar]
- Spigelman I, Li Z, Banerjee PK, Mihalek RM, Homanics GE, Olsen RW. Behavior and physiology of mice lacking the GABAA-receptor delta subunit. Epilepsia. 2002;43(Suppl. 5):3–8. doi: 10.1046/j.1528-1157.43.s.5.8.x. [DOI] [PubMed] [Google Scholar]
- Storustovu SI, Ebert B. Pharmacological characterization of agonists at delta-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J Pharmacol Exp Ther. 2006;316:1351–1359. doi: 10.1124/jpet.105.092403. [DOI] [PubMed] [Google Scholar]
- Sun C, Sieghart W, Kapur J. Distribution of alpha1, alpha4, gamma2, and delta subunits of GABAA receptors in hippocampal granule cells. Brain Res. 2004;1029:207–216. doi: 10.1016/j.brainres.2004.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Winston S, McCarley RW. Effect of microdialysis perfusion of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridine-3-ol in the perifornical hypothalamus on sleep-wakefulness: role of delta-subunit containing extrasynaptic GABAA receptors. Neuroscience. 2008;153:551–555. doi: 10.1016/j.neuroscience.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Bracamontes J, Zorumski C, Weiss DS, Steinbach JH. Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor. J Neurosci. 1997;17:625–634. doi: 10.1523/JNEUROSCI.17-02-00625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Ebert B. Gaboxadol-a new awakening in sleep. Curr Opin Pharmacol. 2006;6:30–36. doi: 10.1016/j.coph.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wafford KA, van Niel MB, Ma QP, Horridge E, Herd MB, Peden DR, et al. Novel compounds selectively enhance delta subunit containing GABAA receptors and increase tonic currents in thalamus. Neuropharmacology. 2009;56:182–189. doi: 10.1016/j.neuropharm.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiltgen BJ, Sanders MJ, Ferguson C, Homanics GE, Fanselow MS. Trace fear conditioning is enhanced in mice lacking the delta subunit of the GABAA receptor. Learn Mem. 2005;12:327–333. doi: 10.1101/lm.89705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winsky-Sommerer R, Vyazovskiy VV, Homanics GE, Tobler I. The EEG effects of THIP (Gaboxadol) on sleep and waking are mediated by the GABA(A)delta-subunit-containing receptors. Eur J Neurosci. 2007;25:1893–1899. doi: 10.1111/j.1460-9568.2007.05455.x. [DOI] [PubMed] [Google Scholar]
- Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooltorton JR, Moss SJ, Smart TG. Pharmacological and physiological characterization of murine homomeric beta3 GABA(A) receptors. Eur J Neurosci. 1997;9:2225–2235. doi: 10.1111/j.1460-9568.1997.tb01641.x. [DOI] [PubMed] [Google Scholar]
- Zheleznova N, Sedelnikova A, Weiss DS. alpha1beta2delta, a silent GABAA receptor: recruitment by tracazolate and neurosteroids. Br J Pharmacol. 2008;153:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]