Abstract
Background and Purpose
Airway inflammation in cystic fibrosis (CF) patients is characterized by accumulations of neutrophils in the airway and T cells in bronchial tissue, with activation of platelets in the circulation. CF patients are routinely treated with systemic or inhaled tobramycin for airway infection with Pseudomonas aeruginosa. Clinical trials have indicated an anti-inflammatory effect of tobramycin beyond its bactericidal activity. Here, we investigate the anti-inflammatory properties of tobramycin in vitro and consider if these relate to the ability of tobramycin to bind copper, which is elevated in blood and sputum in CF.
Experimental Approach
A copper–tobramycin complex was synthesized. The effect of tobramycin and copper–tobramycin on neutrophil activation and migration of T cells and neutrophils across human lung microvascular endothelial cells in response to thrombin-activated platelets were investigated in vitro. Tobramycin uptake was detected by immunocytochemistry. Intracellular reactive oxygen species were detected using the fluorescent indicator, 2′,7′-dichlorofluorescein diacetate (DCFDA). Neutrophil superoxide, hydrogen peroxide and neutrophil elastase activity were measured using specific substrates. Copper was measured using atomic absorption spectroscopy.
Key Results
Tobramycin and copper–tobramycin were taken up by endothelial cells via a heparan sulphate-dependent mechanism and significantly inhibited T-cell and neutrophil transendothelial migration respectively. Copper–tobramycin has intracellular and extracellular superoxide dismutase-like activity. Neutrophil elastase inhibition by α1-antitrypsin is enhanced in the presence of copper–tobramycin. Tobramycin and copper–tobramycin are equally effective anti-pseudomonal antibiotics.
Conclusions and Implications
Anti-inflammatory effects of tobramycin in vivo may relate to the spontaneous formation of a copper–tobramycin complex, implying that copper–tobramycin may be more effective therapy.
Keywords: aminoglycoside antibiotic, anti-inflammatory therapy, copper chelation, neutrophil, T-cell, platelet, endothelium, reactive oxygen species (ROS), neutrophil elastase, IL-8
Introduction
Tobramycin is a widely prescribed antibiotic for the treatment of infection with Gram-negative bacteria and in particular for the treatment of chronic pulmonary infection with Pseudomonas aeruginosa, the most frequently described opportunistic pathogen in patients with cystic fibrosis (CF) (Ratjen et al., 2009). In the CF airway, the genetic defect in the cystic fibrosis transmembrane conductance regulator (CFTR) gene, which codes for an epithelial chloride ion channel, promotes the formation of a niche favouring colonization with P. aeruginosa. Adaptation of P. aeruginosa to this niche renders eradication of this organism by host defences or antibiotics difficult, if not impossible, unless infection is treated early (Ratjen et al., 2009; Doring, 2010).
Tobramycin may be administered intravenously, especially during acute exacerbations, or via the inhaled route. Inhalation achieves high local concentrations in the airways, which are required to overcome the inhibitory effect of sputum binding on bioactivity, while avoiding systemic toxicity (Geller et al., 2002). In addition to anti-pseudomonal efficacy, intermittent administration of inhaled tobramycin was reported to improve pulmonary function and decrease risk of hospitalization (Ramsey et al., 1999). This study also hinted at the possibility that inhaled tobramycin may have an anti-inflammatory effect, beyond that mediated by its antibacterial activity (Ramsey et al., 1999). In this context, tobramycin was previously reported to protect lung epithelial cells against oxidant-induced injury (Cantin and Woods, 1993) and more recently has been reported to inhibit mucin production by human lung epithelial cells (Nakamura et al., 2011).
Copper is an essential trace metal that plays an important physiological role in the redox chemistry of a number of enzymes (Tapeiro et al., 2003), but which becomes toxic when present in excess and therefore requires tight homeostatic regulation (Camakaris et al., 1999). Pro-inflammatory roles for copper have been described, including activation of NF-κB in vivo (Persichini et al., 2006), induction of RANTES (Spisni et al., 2009) and IL-8 (Bar-Or et al., 2003) synthesis and angiogenesis (Hu, 1998). Copper levels are increased in the circulation (Percival et al., 1999) and in sputum (Gray et al., 2010) in patients with CF, and other inflammatory conditions. However, the aminoglycosides, by virtue of multiple vicinal amino and hydroxyl groups, bind copper (II) ions at physiological pH into stable 1:1 chelates (Kozlowski et al., 2005; Gokhale et al., 2007). Many drugs, when complexed with copper, demonstrate superoxide dismutase (SOD)-like activity, and it has been suggested that complexes contemporaneously formed with endogenous copper in vivo are the real active forms of some of the most common anti-inflammatory drugs (reviewed in Milanino and Buchner, 2006). Thus, we hypothesized that in the presence of copper, tobramycin spontaneously forms a copper–tobramycin (CuT) complex with antioxidant and anti-inflammatory properties.
A hyperinflammatory state exists in the CF airway that exceeds that expected considering the level of infection (reviewed in Elizur et al., 2008). Pulmonary inflammation in CF patients is characterized by IL-8-mediated neutrophil accumulation in the airway lumen (Elizur et al., 2008), accumulation of activated T cells in bronchial tissue (Hubeau et al., 2001) and activation of platelets in the circulation (O'Sullivan and Michelson, 2006). In addition, the endothelium, the initial barrier to the inflammatory response, is activated in CF (Solic et al., 2005). We therefore tested our hypothesis using in vitro models of neutrophil and T-cell activation and migration across monolayers of TNF-α-activated endothelial cells in response to thrombin-activated platelets. We found that endothelial cells accumulate tobramycin and a CuT complex, which has SOD-like activity, and demonstrate anti-inflammatory properties of both tobramycin and CuT, and previously unrecognized roles for platelets in neutrophil and T-cell recruitment.
Methods
Ethical approval for the isolation of T cells, neutrophils and platelets from the blood of normal healthy volunteers was obtained from the University of Portsmouth Ethical Committee.
Isolation and activation of normal human T cells
Whole blood from normal healthy volunteers was diluted 1:2 and layered on Lymphoprep (Axis-Shield, Dundee, UK), followed by centrifugation for 30 min at 438× g at 20°C. PBMC from the plasma/Lymphoprep interface were resuspended in RPMI/10%FCS supplemented with 2 mM l-glutamine, 1 mM sodium pyruvate, 100 units·mL−1 penicillin, 100 μg·mL−1 streptomycin and 250 ng·mL−1 amphotericin B and depleted of monocytes by adherence overnight on plastic. Non-adherent T cells were recovered and activated with PHA (1 μg·mL−1) for 2 days, followed by IL-2 (200 U·mL−1) for 3 days at 37°C and 5% CO2, (adapted from Loetscher et al., 1996).
Platelet isolation
For co-culture with T cells, platelets were prepared from the plasma layer from above, by centrifugation at 2500× g for 15 min, washing twice in 10 mM EDTA and resuspending in RPMI/10%FCS.
For co-culture with neutrophils, platelets were isolated from 18 mL EDTA-anti-coagulated normal venous blood. Platelet-rich plasma was prepared by adding 0.1 volume of 0.15 M NaCl plus 77 mM EDTA, pH 7.4 to whole blood and centrifuging at 200× g for 15 min at 20°C and platelets were pelleted at 2500× g for 15 min at room temperature. The pellet was washed first with PBS (–Ca/Mg) containing 10 mM EDTA and second with PBS (–Ca/Mg) without EDTA. Cells were finally resuspended in appropriate buffer, counted using trypan blue and haemocytometer and diluted to the required cell number.
Platelet activation with thrombin for cytokine release
Isolated platelets were resuspended in PBS (+Ca/Mg), diluted to 2 × 108 cells·mL−1 and thrombin (Sigma Aldrich Inc., Poole, Dorset, UK) added to obtain 2 U·mL−1 final concentration in 0.5 mL of platelet suspension. Platelets were incubated for 30 min at 37°C with or without thrombin stimulation. The material was then centrifuged at 2500× g for 15 min at 4°C. The supernatant was removed, and the cell pellet was lysed in 0.5 mL of 1% (v/v) Triton X-100 containing double-strength protease inhibitor (Roche, Welwyn Garden City, Hertfordshire, UK). The material was stored at −80°C before ELISA analysis.
Neutrophil isolation
Neutrophils were isolated from EDTA-anti-coagulated venous blood from healthy donors (adapted from Petreccia et al., 1987). Red blood cells (RBCs) were removed by dextran sedimentation, by mixing blood in a 2:1 ratio with 6% (v/v) Dextran 70 (Baxter Healthcare Ltd, Thetford, UK) for 45 min. Leukocyte-rich plasma was underlayered with an equal volume of Lymphoprep and centrifuged at 450× g for 30 min at room temperature. The upper layers were discarded and RBCs remaining in the granulocyte pellet were subjected to hypotonic lysis. Cells were finally resuspended in appropriate buffer, counted using trypan blue and diluted to the required density. Cell viability was assessed by trypan blue exclusion to be >99% with purity >98%. Contaminating cells were mostly eosinophils and rarely monocytes.
Endothelial cell culture
Human lung microvascular endothelial cells (HLMVEC) (Lonza Ltd, Slough, Berkshire, UK) were maintained in full culture medium (Lonza) consisting of EBM-2MV basal medium supplemented with 5% FBS, 0.04% hydrocortisone, 0.4% hFGF, 0.1% VEGF, 0.1% IGF-1, 0.1% ascorbic acid, 0.1% hEGF and 0.1% GA-100 (gentamicin and amphotericin). The growth medium was changed 1 day after seeding and then every other day. The cell cultures were maintained at 37°C in 5% CO2 and 95% air. The cells were subcultured when they were 60–90% confluent using trypsin/EDTA, which was neutralized with an equal volume of warmed Trypsin Neutralising Solution (Lonza). Cells were used in subsequent experiments at up to passage 10.
T-cell transendothelial migration
HLMVEC were seeded (60 000 cells in 300 μL EGM-2MV) into 3 μm pore size, uncoated, polyethylene terephthalate culture inserts (BD Biosciences, Oxford, UK) in 24-well companion plates with 800 μL medium in the lower well. Cells were grown for 14 days, until confluent, changing the medium every other day. RANTES synthesis was induced with IFN-γ (100 U·mL−1) and TNF-α (10 ng·mL−1) for 24 h. Copper chelators were added to upper and lower wells for a further 24 h where indicated. A neutralizing RANTES antibody (10 μg·mL−1) (Peprotech EC, London, UK) was added to upper and lower wells 30 min before the start of T-cell transendothelial migration.
Activated T cells (2 × 106 cells·mL−1, 300 μL) were added to the upper wells, and platelets (1 × 108 cells·mL−1) activated with 1 U·mL−1 thrombin added to the upper or lower wells as indicated, in RPMI/10% FCS containing 2 mM l-glutamine and 25 mM HEPES (adapted from Kawai et al., 1999). Transwells were incubated for 5 h at 37°C and 5% CO2, and cells in the lower compartment counted following addition of 10 mM EDTA to dislodge adherent migrated cells.
Neutrophil transendothelial migration
Primary HLMVEC were cultured in monolayers on polyethylene terephthalate (PET) 3 μM pore size uncoated Transwells cell culture inserts, 70 mm diameter (Marathon Labs Supplies, London, UK) until confluent over 14 days. HLMVEC were pretreated with tobramycin and CuT (0.01–0.5 mM) 30 min before stimulation with 10 ng·mL−1 TNF-α, in the presence of the drugs, for 16 h. The experiment was carried out in RPMI/2.5% FCS containing 25 mM HEPES and 2 mM l-glutamine (23.11 ± 1.98 μg·L−1 copper). Platelets (1 × 108 cells·mL−1) were added (800 μL) to the basal compartment of Transwells and activated with 2 U·mL−1 thrombin for 30 min. When IL-8 was the chemoattractant, human recombinant IL-8 (Peprotech EC) was added at 6.25 × 10−8 M to wells underneath unactivated HLMVEC for 30 min. Neutrophils (2 × 106 cells·mL−1, 250 μL, in a final volume of 300 μL) were applied to the apical compartment of Transwells. Following 3 h incubation at 37°C and 5% CO2, the plate was placed on ice for 10 min; 10 mM EDTA final concentration was added to the bottom wells for 15 min to allow neutrophils to detach from the lower surface of the membrane. The apical supernatants were collected and cleared by centrifugation at 900× g for 10 min at 4°C. Neutrophils were harvested from the medium in the lower wells by centrifugation at 450× g for 10 min at 4°C. The supernatant was stored at −80°C for further analysis. The cell pellets were resuspended in 100 μL PBS (–Ca/Mg) and counted using trypan blue in a haemocytometer.
Immunohistochemical staining of HLMVEC for tobramycin and CuT
The cells were subcultured (0.7 × 105 cells·mL−1, 250 μL per well) into eight-well chamber slides (Thermo Scientific, Waltham, MA) coated with 0.1 mg·mL−1 collagen IV (Sigma Aldrich). At confluency, some cell cultures were pretreated with 5 U·mL−1 heparitinase II from Flavobacterium heparinum (Sigma Aldrich) for 30 min, followed by co-incubation with tobramycin or CuT (0.01–0.5 mM) for 3.5 h at 37°C and 5% CO2. The experiments were carried out in full culture medium. The cells were fixed using 500 μL per well 4% (w/v) PFA in PBS (–Ca/Mg) for 30 min at room temperature, permeabilized with 500 μL per well ice-cold methanol (100%) for 5 min at −20°C and blocked with 500 μL per well 1% (w/v) BSA in PBS (–Ca/Mg) at 4°C for 16 h to block non-specific binding sites. Sheep anti-tobramycin antibody (Randox Laboratories Ltd, Crumlin, Co. Antrim, UK) at 1:584 dilution was added to cultures for 1 h at room temperature, followed by three washing steps with 500 μL per well 0.02% Tween-20 in PBS (–Ca/Mg) and a 1 h incubation with donkey anti-sheep Alexa Fluor 594 antibody (Molecular Probes, Invitrogen Ltd, Paisley, UK) diluted 1:400. The slide was mounted in FluorPreserve™ Reagent (Calbiochem, Nottingham, Nottinghamshire, UK) before analysis on a Zeiss LSM 710 confocal microscope (excitation, 591; emission, 618).
Fluorescence detection of intracellular ROS
HLMVEC were cultured in collagen IV-coated Microtek eight-well chamber slide at 0.7 × 105 cells·mL−1 (250 μL per well) and grown to confluency for at least 2 days. Cell layers were treated with tobramycin and CuT (0.01, 0.1, 0.5 mM) for 3 h, 10 μM DCFDA (Sigma Aldrich) for 30 min, followed by 10 min incubation with fresh medium pre-warmed to 37°C. HLMVEC were then activated with TNF-α (10 ng·mL−1) for 15 min at 37°C and 5% CO2. The experiment was carried out in full culture medium supplemented with tobramycin or CuT. The cells underwent fixation, permeabilization and the blocking step described above for the tobramycin uptake experiment. The slide was finally mounted in FluorPreserve™ Reagent and viewed on a Zeiss LSM 710 Confocal Microscope (green fluorescent stain excitation, 488 nm, emission, 540 nm).
Opsonisation of zymosan
The preparation (adapted from Petreccia et al., 1987) was made fresh daily. Serum was prepared from normal venous blood collected into Vacuette clot activator tubes (Greiner-BioOne Ltd, Stonehouse, Gloucestershire, UK), allowed to clot for 10 min at room temperature and centrifuged at 1500× g for 10 min. Zymosan A from Saccharomyces cerevisae (Sigma Aldrich) was prepared by washing twice in deionized water and then opsonising in fresh human serum at 5 mg·mL−1 for 60 min at 37°C with gentle shaking. The reaction was stopped by placing on ice. Opsonized zymosan (OPZ) was washed twice with ice-cold water and centrifuged at 1500× g for 10 min at 4°C. A final resuspension was made in HBSS (+Ca/Mg) containing 20 mM HEPES, pH 7.4 at 10 mg·mL−1.
Superoxide assay
Superoxide ion release from neutrophils was measured by the reduction of cytochrome C (adapted from Petreccia et al., 1987). The experiment was carried out in HBSS (+Ca/Mg) containing 20 mM HEPES, pH 7.4 in triplicates. Neutrophils (1 × 107 cells·mL−1, 200 μL) were incubated with tobramycin, CuT (0.001–0.5 mM) or bovine erythrocyte SOD (Sigma Aldrich) (3.125–100 U·mL−1) in the presence of cytochrome C (40 μM) and with OPZ activation (1 mg·mL−1) at 37°C for 15 min with gentle shaking in a final volume of 250 μL. There was no pre-incubation with drugs, and the reaction was started by the addition of cells. The reactions were stopped by placing on ice, and the samples were centrifuged at 1100× g for 5 min at 4°C. The supernatant was transferred into a 96-well plate, and absorbance was measured at 550 nm.
Hydrogen peroxide assay
Hydrogen peroxide release from neutrophils was detected using the Amplex Red Neuraminidase (Sialidase) Assay (Molecular Probes, Invitrogen Ltd) according to the manufacturer's instructions, but in the absence of fetuin and galactose oxidase. Hydrogen peroxide consumption by neutrophil myeloperoxidase and catalase was inhibited with 1 mM sodium azide (Sigma Aldrich). The experiment was carried out in HBSS (+Ca/Mg) containing 20 mM HEPES, pH 7.4 in duplicate. Neutrophils (1 × 107 cells·mL−1, 100 μL) were incubated with tobramycin, CuT (0.01–0.5 mM) or SOD (100 U·mL−1) upon OPZ activation (1 mg·mL−1) at 37°C for 15 min with gentle shaking. There was no pre-incubation with drugs, and the reaction was started by the addition of cells. The reactions were stopped on ice, and the samples were cleared by centrifugation at 1100× g for 5 min at 4°C for measurement of hydrogen peroxide concentration, calculated from a hydrogen peroxide standard curve in the range 0–100 μM H2O2.
Neutrophil elastase activity
Neutrophil elastase (NE) activity was measured using the specific NE substrate, N-methoxysuccinyl-ala-ala-pro-val-p-nitroanilide (Sigma Aldrich Inc.). The experiment was carried out in HBSS (+Ca/Mg) containing 20 mM HEPES, pH 7.4 in duplicates. Neutrophils (1 × 107 cells·mL−1, 200 μL) were pre-incubated with tobramycin or CuT (0.01–0.5 mM) in the absence or presence of α1-antitrypsin (α1-AT, 2 mg·mL−1) for 15 min in a final volume of 250 μL, followed by OPZ activation (1 mg·mL−1) for 60 min at 37°C with gentle shaking. The reactions were stopped by placing on ice, and the samples were centrifuged at 1100× g for 5 min at 4°C. The cell pellets were lysed in an equal volume of 1% (v/v) Triton X-100 in water. Supernatants and cell pellets were pre-warmed for 1 min at 37°C with gentle shaking followed by addition of 0.555 mM NE substrate and the absorbance at 405 nm was read over 30 min.
Synthesis of a CuT complex
A 1:1 CuT complex was synthesized as described for copper–kanamycin (Sreedhara et al., 2000) and precipitated with 40% ethanol from which it was dried under vacuum.
Superoxide dismutase (SOD) assay
SOD activity was measured by inhibition of nitro blue tetrazolium reduction, which was induced using NADH plus phenazine methosulphate as a non-enzymic superoxide generator (Ewing and Janero, 1995).
Catalase activity assay
The catalase activity of tobramycin and copper tobramycin (0.01 to 0.5 mM) was measured using the Amplex Red Assay (Molecular Probes), as described earlier. Additionally, the catalase activity of tobramycin and copper tobramycin (0.5 mM) was measured as the rate of decomposition of hydrogen peroxide to water and molecular oxygen at 240 nm in quartz cuvettes on a Uvikon 923 double beam UV/VIS spectrophotometer, with a catalase standard in the concentration range 1–10 U·mL−1.
UV-VIS spectrophotometry
Stock solutions of 50 mM tobramycin, copper sulphate and CuT were prepared in deionized water. Measurements were carried out using 0.5 cm light-path quartz cuvettes at 37°C. Wavelength scanning was performed on a Uvikon 923 double beam UV/VIS spectrophotometer.
Quantitation of cytokines by ELISA
RANTES, IFN-γ, IL-8, NAP-2 and TNF-α were quantified by elisa using elisa development kits from Peprotech according to the manufacturer's instructions.
Cell viability assays
T-cell LDH activity release was assayed using the TOX-7 assay kit (Sigma Aldrich).
TACS™ annexin V-biotin apoptosis detection kit (Trevigen, from AMS Biotechnology Ltd, Abingdon, UK) with biotin-FITC conjugate (Becton Dickson Biosciences, Oxford) was used to assess cytotoxicity of tested drugs on PMN. The assay was performed on a FACS Calibur Immunocytometry System. HLMVEC viability was assessed using a Caspase-Glo® 3/7 Assay or CytoToxGlo Cytotoxicity Assay (Promega, Southampton, UK) on cells grown in collagen IV-coated 96-well plates.
Copper analysis
Cells were digested for copper analysis using a H2O2/HNO3 digestion system (Alcock, 1987), and total copper was analysed by graphite furnace-atomic absorption spectroscopy (GF-AAS).
Statistical analysis
All statistical analyses were performed using GraphPad Prism 4. The results were expressed as mean ± SEM. Student's two-tailed paired or unpaired t-test and one-way ANOVA with Dunnett's multiple comparison post hoc test were performed as required. A P-value less than 0.05 was considered statistically significant.
Results
Platelet-derived RANTES induces T-cell transendothelial migration
Platelets are a rich source of both RANTES (Kameyoshi et al., 1992) and copper (Schmitt, 1997), and we previously reported that a copper–hydrogen peroxide redox system induces oligomerization of RANTES (MacGregor et al., 2011) which is essential for in vivo activity (Proudfoot et al., 2002). We therefore established a model of platelet-derived RANTES-induced T-cell migration across a confluent monolayer of HLMVECs in which we tested the effect of a RANTES-neutralizing antibody and copper chelators.
Responsiveness of normal primary T cells towards RANTES was induced by activation in vitro with PHA, followed by IL-2, which is essential for CC chemokine receptor expression and RANTES responsiveness (Loetscher et al., 1996). A non-significant increase in spontaneous T-cell migration across a confluent layer of HLMVECs was observed following activation of HLMVECs with IFN-γ plus TNF-α (Figure 1A). However, thrombin-activated platelets added to the lower well induced a significant (P < 0.01) increase in T-cell migration that was significantly (P < 0.01) inhibited by anti-RANTES antibody in the cultures. Conversely, when thrombin-activated platelets were added to the upper well spontaneous T-cell migration was arrested, suggesting platelet aggregation.
Figure 1.
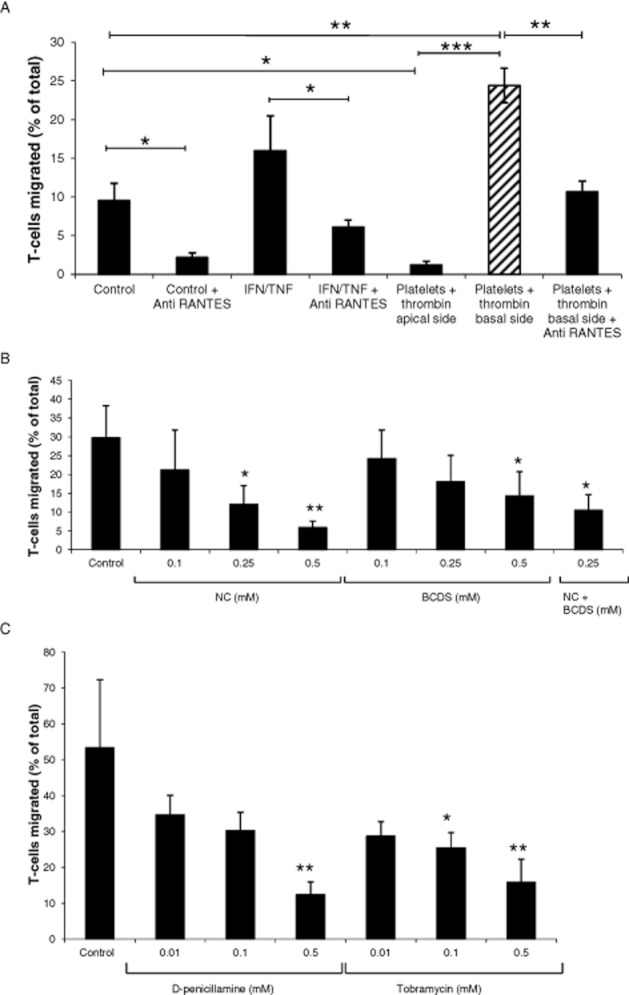
RANTES-induced transendothelial migration of T cells. (A) Spontaneous migration was stimulated when HLMVECs were pre-activated with IFN-γ plus TNF-α for 24 h, or when thrombin (1 U·mL−1)-activated platelets (108 mL−1) were added basally 30 min before the assay (hatched bar in panel A, control conditions in panels B and C) and inhibited by a RANTES-neutralizing antibody (10 μg·mL−1). Results are presented as mean ± SEM, n = 3, * P < 0.05, ** P < 0.01 and *** P < 0.001. (B) The copper chelators, neocuproine (NC) and bathocuproine disodium salt (BCDS), added to both the apical and basal wells 24 h before the assay inhibited T-cell migration. Results are presented as mean ± SEM, n = 3. (C) d-penicillamine and tobramycin added to both the apical and basal wells 24 h before the assay inhibited T-cell migration. Results are presented as mean ± SEM, n = 5.
It was reported that RANTES binds preferentially to activated endothelial cells (Baltus et al., 2008). Since IFN-γ and TNF-α were not added exogenously to the platelet/HLMVEC/T-cell co-cultures, we measured IFN-γ and TNF-α in apical supernatants from experiments in which T-cell migration was induced by thrombin-activated platelets in the basal compartment (Figure 1A, hatched bar). The amount of IFN-γ (3.6 ± 0.3 ng·mL−1) produced in the cultures was higher than that added exogenously (100 U·mL−1 or 5 pg·mL−1), and TNF-α (2.4 ± 1.1 ng·mL−1) was of the same order as TNF-α added exogenously (10 ng·mL−1), indicating activation of HLMVECs by endogenously produced cytokines in our model.
Copper chelators inhibit T-cell transendothelial migration
Exposure of HLMVECs to the copper chelators neocuproine (NC) and bathocuproine disodium salt (BCDS) for 24 h prior to transendothelial migration assays induced significant concentration-dependent inhibition of platelet-induced T-cell migration (Figure 1B), although their effects were not additive. d-penicillamine and tobramycin also chelate copper (Jezowska-Bojczuk et al., 1998; Brewer, 2008) and similarly induced significant concentration-dependent inhibition of platelet-induced T-cell migration (Figure 1C). Penicillamine and tobramycin (0.5 mM) significantly (P < 0.01) inhibited T-cell migration by 67.1 ± 3.4 % and 66.5 ± 5.8 % respectively. The inhibitory effects of the copper chelators were not due to cytotoxicity since lactate dehydrogenase activity in culture supernatants was not increased under any condition. Also, the copper chelators had no effect on the concentration of RANTES in supernatants, as measured by ELISA, and no significant effect on the multimeric form of RANTES, which was detected by Western blotting predominantly as a 32 kDa tetramer both in apical and basal supernatants and in cell lysates under all conditions (data not shown).
A CuT complex has SOD-like activity
Previous reports indicated free radical scavenging activity of copper complexed to a number of ligands (reviewed in Milanino and Buchner, 2006), and an inhibitory effect of extracellular SOD (SOD3) on T-cell migration in animal models in vivo (Laurila et al., 2009). We therefore considered the possibility that inhibition of T-cell transendothelial migration was mediated by tobramycin binding copper in situ to generate a complex with SOD-like activity. To test this idea, we synthesized a CuT (CuT) complex. The UV-VIS spectrum showed an absorption maximum at 250 nm for the complex (Figure 2A) with a molar extinction coefficient of 1229·M−1·cm−1. The blue complex formed rapidly and spontaneously when tobramycin was titrated against a fixed concentration of CuSO4 in water (Figure 2B). It was stable in powder form at room temperature and in solution in water at 4°C over many weeks. A dissociation constant of 0.048 mM was calculated for the CuT complex.
Figure 2.
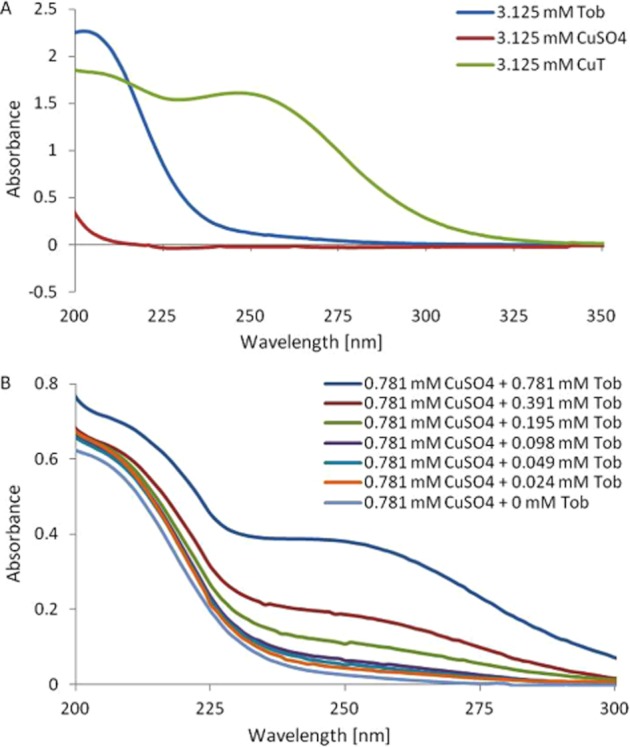
UV spectrum of tobramycin binding copper at 37°C. (A) UV spectrum of tobramycin, copper sulphate and CuT complex (3.125 mM). (B) UV spectrum of tobramycin (0–0.781 mM) titrated against a constant concentration of copper sulphate (0.781 mM).
In a cell-free assay, copper-tobramycin was found to have concentration-dependent SOD-like activity with IC50 200 nM (Figure 3), while tobramycin had no SOD-like activity. The copper II ion is reported to the most potent SOD known and in the NBT reduction assay, copper sulphate inhibited NBT reduction by superoxide over the same concentration range as copper tobramycin, presumably via dismutation of superoxide in this enzyme-free assay. Although free copper II ions dissociated from the complex cannot be completely excluded as a contributory factor to the observed SOD-like activity of copper-tobramycin, preliminary experiments showing differential inhibition by EDTA of the superoxide scavenging activity of copper sulphate and CuT, particularly at higher concentrations, indicate that free copper II ions are not essential for the effect of the complex.
Figure 3.
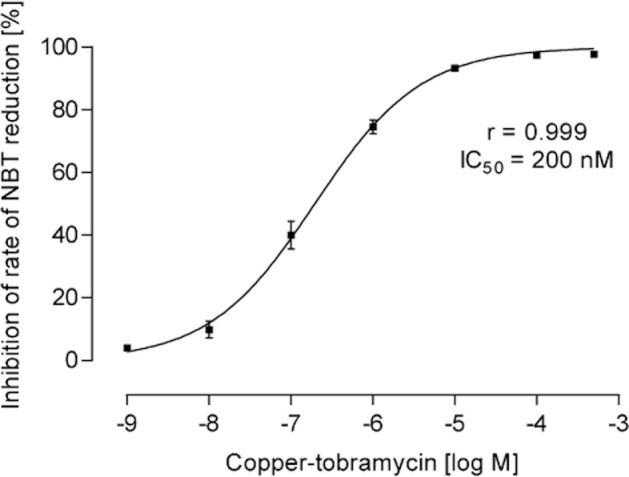
Superoxide dismutative activity of copper-tobramycin measured as inhibition of the rate of nitro blue tetrazolium reduction. Copper-tobramycin potently inhibited NBT reduction with an IC50 of 200 nM. Tobramycin had no inhibitory effect at concentrations up to 500 μM.
In cell-free assays, no catalase activity was detected for tobramycin or CuT.
Antibiotic activity
Using Mueller-Hinton agar-gel diffusion plates with 7 mm diameter wells and P. aeruginosa (ATCC® 15442) as the target microorganism, the antimicrobial susceptibility to tobramycin and CuT in the concentration range 10–100 μM was not significantly different and zones of inhibition of bacterial growth over 72 h were the same (data not shown).
Tobramycin and CuT uptake by HLMVECs
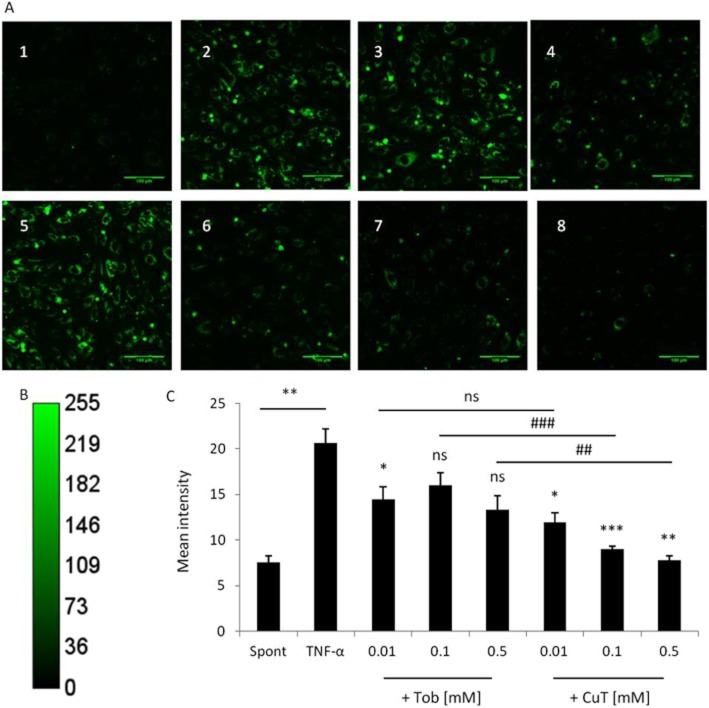
The uptake of tobramycin and CuT by HLMVECs in culture was investigated using immunohistochemistry and semi-quantitative analysis of confocal images (Figure 4). Over 3.5 h, the uptake of both tobramycin (Figure 4A, panels 2–4) and CuT (Figure 4A, panels 6–7) were concentration-dependent. Confocal analysis of the intracellular distribution using 1 μM z-stacks revealed that the antibiotics were mostly cytoplasmic and perinuclear. Previous studies indicated that cationic aminoglycosides such as tobramycin interact with anionic cell surface molecules (Cantin and Woods, 1993). In addition, guanidinylated aminoglycosides were taken up by CHO cells in a heparan sulphate-dependent manner (Elson-Schwab et al., 2007). We therefore tested the effect of pretreatment with heparitinase II (5 U·mL−1) for 4 h, which significantly (P < 0.01), but not completely, inhibited uptake of 0.5 mM tobramycin (Figure 4A, panels 4 and 5) and 0.5 mM CuT (Figure 4A, panels 8 and 9), as assessed by semiquantitative analysis of fluorescence intensity. Tobramycin and copper tobramycin were not cytotoxic, at any concentration, to HLMVEC under the conditions used in these experiments.
Figure 4.
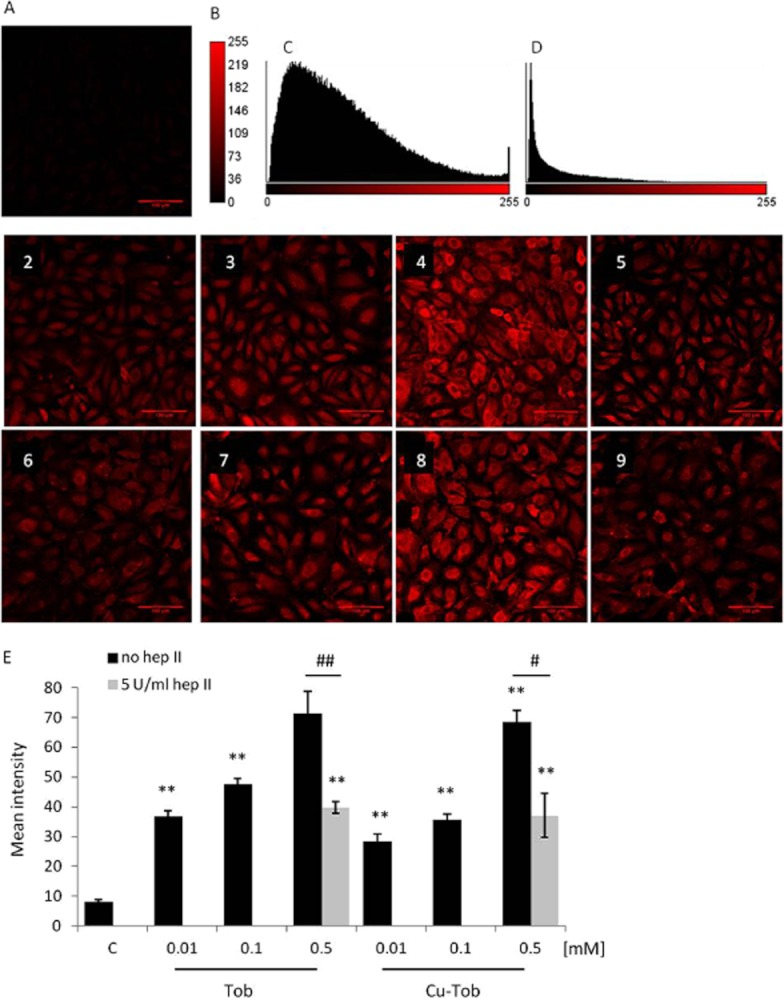
Immunohistochemical analysis of tobramycin and CuT uptake by HLMVEC and the effect of pretreatment with heparitinase II. (A) Confocal laser scanning microscopy (CLSM) images of untreated HLMVEC (1) and HLMVEC treated with increasing concentrations of tobramycin at 0.01 mM (2), 0.1 mM (3), 0.5 mM (4) before and after (5) heparitinase II treatment and copper-tobramycin at 0.01 mM (6), 0.1 mM (7), 0.5 mM (8) before and after (9) heparitinase II treatment. HLMVEC were pretreated with heparitinase II (5 U mL−1) for 30 min, followed by treatment with tobramycin or copper–tobramycin (0.01–0.5 mM) for 3.5 h at 37°C and under 5% CO2. Scale bar 100 μm. (B) Calibration bar of total fluorescence intensity. (C) Fluorescence intensity histogram of Figure 4, panel 4. (D) Fluorescence intensity histogram of Figure 4, panel 5. (E) The fluorescence intensity for each condition was analysed as the mean of at least three fields of view. The data expressed as mean ± SEM were analysed using one-way anova and Dunnett's post hoc test versus the negative control and *P < 0.05, **P < 0.01, ***P < 0.001. Uptake following heparitinase treatment was compared with uptake of 0.5 mM tobramycin and CuT by untreated cells using a Student's two-tailed unpaired t-test, with #P < 0.05, ##P < 0.01. Representative images from a single experiment, typical of at least three independent experiments, are shown.
The intracellular antioxidant activity of tobramycin and CuT
In view of the SOD-like activity of CuT in a cell-free system and its uptake by HLMVECs we tested the intracellular antioxidant activity of both tobramycin and CuT in TNF-activated HLMVECs using the fluorescent indicator DCFDA. DCFDA reacts with multiple reactive oxygen and nitrogen species (Freitas et al., 2009), and the green fluorescence seen 15 min after the addition of TNF-α (10 ng·mL−1) (Figure 5A, panel 5), but not in unstimulated cells (Figure 5A, panel 1) is likely to reflect the generation of both. However, both tobramycin and CuT had significant intracellular antioxidant activity (Figure 5C), although CuT (0.1 and 0.5 mM) was significantly more effective than tobramycin.
Figure 5.
The effect of tobramycin and CuT on TNF-α-activated intracellular ROS generation in HLMVEC. (A) CLSM images of untreated HLMVEC (1), cells treated with 10 ng·mL−1 TNF-α alone (5), and with 10 ng·mL−1 TNF-α following treatment for 3 h with 0.01 mM Tob (2), 0.1 mM Tob (3), 0.5 mM Tob (4), 0.01 mM Cu-Tob (6), 0.1 mM Cu-Tob (7), 0.5 mM Cu-Tob (8). Scale bar 100 μm. (B) A calibration of total fluorescent intensity. (C) Fluorescent intensity for each condition analysed as the mean of at least four fields of view. The data expressed as mean ± SEM was analysed using one-way anova and Dunnett's post hoc test versus the TNF-α positive control, where * P < 0.05, **P < 0.01, ***P < 0.001. Differences between the effects of tobramycin and CuT were analysed using Student's two-tailed unpaired t-test with ##P < 0.01 and ###P < 0.001. Representative confocal images from a single experiment, representative of at least four independent experiments.
The extracellular SOD-like activity of tobramycin and CuT
We could find no evidence, using immunocytochemistry, for the uptake of tobramycin and CuT by normal neutrophils in suspension. However, normal neutrophils undergo a respiratory burst following C3b and Fc receptor activation and uptake of opsonized zymosan (Petreccia et al., 1987), with extracellular release of superoxide anions. We therefore tested the extracellular SOD-like activity of tobramycin and CuT, and compared them with the effect of bovine erythrocyte SOD, an exogenous enzyme that does not penetrate the cell membrane. OPZ induced a strong respiratory burst and production of superoxide ions, detected as reduction of cytochrome C at 550 nm (Figure 6A). Tobramycin had no significant effect on superoxide detection, while CuT potently and significantly reduced the levels of detectable superoxide ions, as did SOD. Combined with the results shown in Figure 3, the data indicate that copper-tobramycin has extracellular SOD-like activity, although we cannot rule out a contribution of free copper II ions dissociated from the complex.
Figure 6.
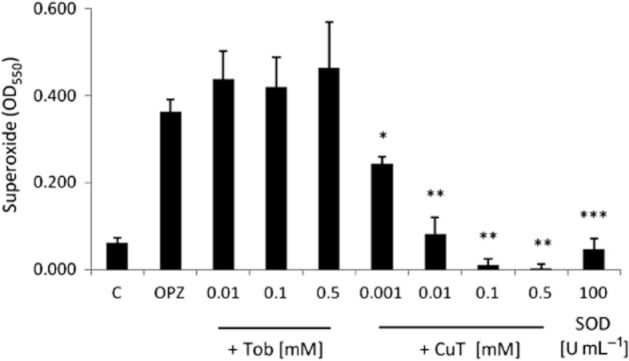
The effect of tobramycin, CuT and SOD on superoxide release from OPZ-activated neutrophils. Neutrophils at 107 mL−1 suspended in HBSS with Ca/Mg + 20 mM HEPES, pH 7.4 were treated with OPZ alone and OPZ in the presence of a range of tobramycin, CuT and SOD concentrations added at the same time as freshly prepared OPZ (1 mg·mL−1) for 15 min at 37°C with gentle shaking. Superoxide ions were measured by the reduction of cytochrome C (n = 3, independent experiments) at 550 nm. C is the spontaneous generation of O2− and OPZ is the generation of O2− by OPZ alone. The effect of the drugs was compared with OPZ alone. Data are represented as mean ± SEM. * P < 0.05, **P < 0.01, ***P < 0.001.
Since endogenous SOD dismutates superoxide ions to hydrogen peroxide, we compared the effect of the drugs on the levels of hydrogen peroxide detected following OPZ-activation of neutrophils. Detection of hydrogen peroxide required the presence of sodium azide to inhibit consumption of hydrogen peroxide by neutrophil-derived myeloperoxidase (MPO) and catalase (Nakagawara et al., 1981). Under these conditions, OPZ stimulated the production of 57.9 ± 5.8 μM hydrogen peroxide. Neither tobramycin or CuT, even at concentrations that significantly reduced the levels of detectable superoxide anions (0.001 and 0.01 M), or bovine erythrocyte SOD (100 U·mL−1) had a significant effect on the hydrogen peroxide concentrations detected. It appears that the hydrogen peroxide concentration is unaffected by the extracellular SOD-like activity of CuT or SOD per se, as previously reported for SOD (Liochev and Fridovich, 2007).
Indirect inhibitory effects of CuT on neutrophil elastase activity
Following activation, released neutrophil elastase (NE) is largely bound to the heparan sulphate and chondroitin sulphate GAGs of the cell surface (Campbell and Owen, 2007). Since tobramycin and its copper complex were found to utilize heparan sulphate for uptake by HLMVECs (Figure 4), we therefore considered the possibility that tobramycin might compete with and displace NE from the surface of OPZ-activated neutrophils. NE activity was measured in cell supernatants (Figure 7A) and in cell lysates (Figure 7B). As expected, little NE activity was detected in the supernatants following activation with OPZ, with most (96.24 ± 1.53 %) being measured in cell lysates. Also as expected, NE activity was not significantly inhibited by α1-antitrypsin (2 mg·mL−1) in the untreated cell lysates. CuT (0.01–0.5 mM), but not tobramycin, significantly decreased elastase activity in cell pellets, while increasing elastase activity in cell supernatants (concentration curve not shown). In the presence of 0.5 mM CuT, NE activity was significantly increased in cell supernatants and was significantly inhibited by α1-antitrypsin, both in cell supernatants (Figure 7A) and cell lysates (Figure 7B). Tobramycin was less effective and induced inhibition of NE activity by α1-antitrypsin only in supernatants (Figure 7A). Copper sulphate (0.01–0.5 mM) had no effect at any concentration on the activity of neutrophil elastase in cell pellets. In the serum-free conditions used in these assays, CuT (but not tobramycin) at 0.1 and 0.5 mM induced neutrophil apoptosis (59.51 ± 2.54 % and 98.96 ± 0.15% respectively). However, apoptosis does not induce degranulation and, in addition, the effect appeared to be specific for NE since copper tobramycin did not induce an increase in neutrophil IL-8 release (data not shown).
Figure 7.
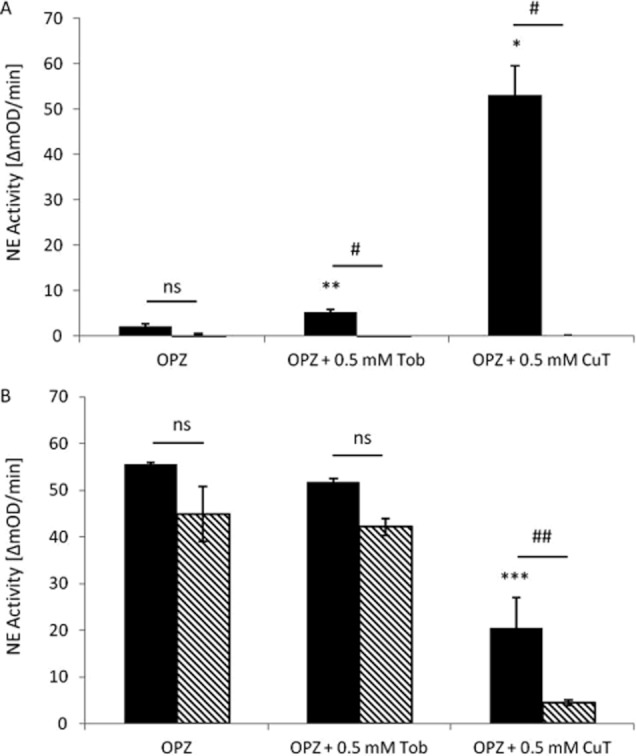
The effect of tobramycin and CuT on NE activity and inhibition by α1-AT. Neutrophils at 107 mL−1 suspended in HBSS with Ca/Mg + 20 mM HEPES, pH 7.4 were pretreated without or with tobramycin and CuT (0.5 mM), as indicated, for 15 min and activated with freshly prepared OPZ (1 mg·mL−1) at 37°C with gentle shaking for 1 h. NE activity was measured using N-methoxysuccinyl-ala-ala-pro-val-p-nitroanilide in supernatants (A) and whole cell lysates (B). The optical density (OD) was monitored at 550 nm in the absence and presence α1-AT (2 mg·mL−1). The data are expressed as mean ± SEM and analysed using two-tailed, paired t-test, all assays were carried out in duplicate in three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 for comparison of the effect of the treatments with OPZ alone (OPZ) and #P < 0.05, ##P < 0.01 for the comparison between neutrophil elastase activity in the absence and presence of α1-AT.
Release of chemokines by thrombin-stimulated platelets
Platelets activated with 2 U·mL−1 thrombin for 30 min released IL-8 (4.81 ± 0.46 pg·mL−1, n = 7), RANTES (20.85 ± 4.55 ng·mL−1, n = 4) and NAP-2 (18.97 ± 3.13 μg·mL−1, n = 3), which in all cases was significantly higher than the spontaneous release from unstimulated cells.
Platelets stimulate neutrophil transendothelial migration
Platelets activated by thrombin stimulate the migration of neutrophils over unstimulated and TNF-α-stimulated HLMVEC monolayers (Figure 8), and the response on activated HLMVEC was not significantly different to the response to an optimum concentration (6.25 × 10−8 M) of IL-8 added to the basal compartment of inactivated HLMVECs. TNF-α induced IL-8 release from endothelial cells, and the mean concentration of IL-8 in the basal compartment was 2.38 × 10−10 M (n = 3), which was not increased in the presence of activated platelets.
Figure 8.
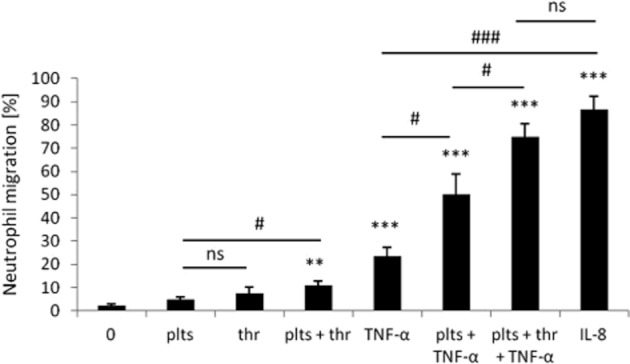
The effect of activated platelets on neutrophil transendothelial migration. Transendothelial migration of neutrophils in response to subendothelial platelets (plts) with and without thrombin (thr) activation compared with the response to IL-8 (6.25 × 10−8 M) alone over 3 h. Neutrophil (2 × 106 cells mL−1) migration through unstimulated and TNF-α (10 ng·mL−1)-stimulated HLMVEC was measured (n ≥ 3). Data are expressed as mean ± SEM and analysed using two-tailed unpaired t-test, **P < 0.01, ***P < 0.001 compared with spontaneous migration (0) and #P < 0.05, ###P < 0.001 for comparison between conditions.
Pre-treatment of Transwells for 30 min with anti-IL8 or anti-NAP-2 antibodies significantly (P < 0.01) inhibited neutrophil transendothelial migration by 17.2 ± 2.1% (n = 4) and 30.0 ± 5.3% (n = 5), respectively, whereas the PAF antagonist WEB2086 at 10 μM had no effect.
Of the antioxidants tested (Table 1 ), pretreatment of endothelial cells for 30 min with SOD plus catalase together, or MnTBAP [MnTBAP (Mn (III) tetrakis (4-benzoic acid) porphyrin chloride] (Sigma Aldrich), which has combined intracellular SOD/catalase activity, significantly (P < 0.05) reduced transendothelial migration of PMN. However, SOD and catalase tested separately did not affect transendothelial migration of PMN. Interestingly, following a 16 h pretreatment of endothelial cells, a significant inhibition of neutrophil transendothelial migration was observed for MnTBAP (37.4 ± 5.7%, n = 3), tobramycin at the low concentration of 0.01 mM (20.3 ± 3.6%, n = 5) and CuT at 0.5 mM (33.8 ± 10.4%, n = 5).
Table 1.
The effect of antioxidants on neutrophil TEM
| Inhibitor | Drug concentration | Inhibition of control [%] | n | Significance | Inhibition of control [%] | n | Significance |
|---|---|---|---|---|---|---|---|
| 30 min pretreatment | 16 h pretreatment | ||||||
| SOD | 2000 U·mL−1 | −10.8 ± 16.5 | 3 | ns | 14.6 ± 2.6 | 2 | ns |
| Catalase | 250 U·mL−1 | 15.3 ± 10.2 | 4 | ns | 32.2 ± 3.4 | 3 | P < 0.05 |
| SOD + Catalase | 2000 U·mL−1, 250 U·mL−1 | 25.7 ± 11.0 | 4 | P < 0.05 | |||
| MnTBAP | 50 μM | 43.5 ± 15.3 | 3 | P < 0.05 | 37.4 ± 5.7 | 3 | P < 0.05 |
| Tob | 0.01 mM | 19.4 ± 32.6 | 3 | ns | 20.3 ± 3.6 | 5 | P < 0.01 |
| Tob | 0.1 mM | 18.9 ± 8.4 | 3 | ns | 12.3 ± 5.2 | 5 | P < 0.05 |
| Tob | 0.5 mM | −8.8 ± 20.6 | 3 | ns | 10.1 ± 2.8 | 5 | ns |
| CuT | 0.01 mM | −5.0 ± 13.8 | 4 | ns | 3.2 ± 7.9 | 5 | ns |
| CuT | 0.1 mM | 11.6 ± 9.3 | 4 | ns | 16.3 ± 6.8 | 5 | ns |
| CuT | 0.5 mM | 19.1 ± 7.6 | 4 | ns | 33.8 ± 10.4 | 5 | P < 0.05 |
The treatment was compared with neutrophil (2 × 106 cells·mL−1) migration through TNF-α (10 ng mL−1)-stimulated HLMVEC towards thrombin (2 U·mL−1)-activated platelets (1 × 108 cells·mL−1). Inhibitors were added to upper and lower wells for 30 min or 16 h prior to TEM for 3 h. The data are expressed as mean ± SEM for at least three independent experiments. Data were analysed using two-tailed, paired t-test and one-way ANOVA with Dunnett's multiple comparison test, as appropriate.
Copper sulphate (0.01–0.5 mM) had no effect on neutrophil transendothelial migration at any concentration and we found no effect of any inhibitor on the oligomerization of IL-8, which was detected on Western blots as a tetramer in cells and supernatants under every condition tested (results not shown).
Under the assay conditions used for neutrophil transendothelial migration, namely RPMI supplemented with 2.5% FCS, tobramycin and CuT were not cytotoxic at any concentration to HLMVEC or neutrophils over 3.5 h.
Copper concentrations in platelets, neutrophils and HLMVECs
Because we considered that tobramycin might have an effect through binding copper contemporaneously in the assay, we investigated potential cellular sources of copper. Using GF-AAS, we measured 23.37 ± 1.11 ng copper per 106 HLMVEC and 14.33 ± 2.49 ng copper per 109 platelets, whereas copper was undetectable in neutrophils using this method. Of the media that was used in transendothelial migration assays, EGM contained 38.48 ± 1.01 μg·L−1and RPMI/2.5% FCS contained 23.11 ± 1.98 μg·L−1 copper.
Discussion
In summary, our data demonstrate a previously unrecognized role for sub-endothelial platelets in directing the recruitment of activated T cells and neutrophils across the human lung microvasculature, and anti-inflammatory effects of tobramycin and a CuT complex with SOD-like activity.
Platelets release a number of T-cell activating chemokines including RANTES, MCP-3, MIP-1α and TARC (Sallusto et al., 1998; Pitchford, 2007; von Hundelhausen, 2007) and here we demonstrate a role for platelet-derived RANTES in directing T-cell transendothelial migration. Normal peripheral T cells were activated with IL-2 to respond to RANTES through both CCR3 and CCR5 receptors, although the target cell for recruitment across the endothelium in our model is likely to be the subset of CCR5-expressing cells (Kawai et al., 1999). This is supported by the observation that CCR5 is expressed by Th1 cells (Sallusto et al., 1998), which are also the likely source of IFN-γ in our model (Romagni, 1994). Our model of platelet-dependent T-cell recruitment did not require exogenously added cytokines for endothelial activation, since T-cell derived cytokines were present in sufficient concentration. However, platelet-dependent neutrophil recruitment required exogenously added TNF-α for endothelial activation. In view of the concentrations of chemokines released by thrombin-activated platelets, neutrophil migration appeared to be dependent on platelet-derived NAP-2, while IL-8 was derived from TNF-activated endothelium. Clearly, other unidentified neutrophil chemoattractants were responsible for the part of the response that was not inhibited by antibodies to NAP-2 or IL-8.
T-cell transendothelial migration in response to thrombin-activated platelets was significantly inhibited by the copper chelators used in this study, including tobramycin. The copper chelators had no effect on the concentration of RANTES in cells or supernatants, or on its multimeric form, which was predominantly a tetramer under all conditions. However, many drugs that bind copper form complexes with SOD-like activity (Milanino and Buchner, 2006). Lymphocyte transendothelial migration was previously reported to be dependent on VCAM-1-dependent activation of endothelial cell NADPH oxidase, the generation of superoxide anions and low concentrations of hydrogen peroxide (Cook-Mills, 2006) and was inhibited in vitro and in vivo by the antioxidants bilirubin and SOD3 (Cook-Mills, 2006; Laurila et al., 2009). Our data indicate that tobramycin chelates copper present in the cells and/or media to generate a SOD-like complex, with the potential to limit the expression of a wide range of adhesion molecules and inflammatory cytokines that are regulated by the NF-κB pathway (Milanino and Buchner, 2006; Laurila et al., 2009) and limit T-cell migration (Cook-Mills, 2006; Milanino and Buchner, 2006). Similarly, copper–penicillamine has SOD-like activity (Lengfelder et al., 1979) and anti-inflammatory properties of copper–penicillamine have also previously been reported (Milanino and Buchner, 2006).
In support of the notion that a CuT complex formed contemporaneously in our assays of transendothelial migration, we found that tobramycin rapidly and spontaneously forms a stable complex with copper. In addition, the CuT complex, but not tobramycin per se, is a scavenger of superoxide ions in a cell-free and enzyme-free system. The SOD activity of many different types of copper complex has been reported (Weder et al., 2002). However, the measured IC50 (200 nM) for CuT indicates that it is more potent than most, with the exception of SOD itself (40 nM). Based on the copper content of the medium used in our assays of T-cell transendothelial migration, we estimate that CuT may have been present at up to 1.5 μM, sufficient to have antioxidant effects that potentially were responsible for the inhibition of T-cell migration.
Endothelial cells were able to take up both tobramycin and CuT via a heparan-sulphate-dependent mechanism, and we demonstrated intracellular antioxidant activity for the CuT complex as well as for higher concentrations of tobramycin in endothelial cells. Copper is taken up by cells from all the major forms of copper-binding proteins in plasma (Tapeiro et al., 2003) and was measured in HLMVEC in culture. This again suggests that tobramycin binds copper present in the media and/or within endothelial cells to form a complex with SOD-like activity. The DCFDA sensor used to detect oxidative stress in endothelial cells does not distinguish reactive oxygen and nitrogen species (Freitas et al., 2009), but since hydrogen peroxide and reactive oxygen nitrogen species depend initially on superoxide formation, it appears that the SOD-like activity of CuT decreases levels of these species while generating highly diffusible hydrogen peroxide, resulting in the observed overall decrease in intracellular ROS. Thus, CuT represents a novel low molecular weight SOD mimetic (reviewed in Muscoli et al., 2003).
In addition to the intracellular antioxidant properties of CuT in endothelial cells, this complex had potent extracellular effects on the neutrophil respiratory burst. CuT had no detectable catalase activity, supporting the evidence that hydrogen peroxide concentrations were unaffected by CuT. Together, these observations confirm that CuT has both intracellular and extracellular SOD-like antioxidant properties.
A role for superoxide anions in neutrophil chemotaxis (Hattori et al., 2010) and leukocyte extravasation (Boueiz and Hassoun, 2009) has been reported, and SOD3 is reported to inhibit neutrophil infiltration and lung injury in animal models (Muscoli et al., 2003). However, we found that although tobramycin significantly inhibited neutrophil migration at low concentrations, it was more effective as an inhibitor of T-cell migration. This may be because neutrophils do not use the VCAM-1 pathway of cell adhesion and endothelial activation previously reported (Laurila et al., 2009), but utilize the ICAM-1-dependent pathway in which no role for endothelial NADPH oxidase has been described (reviewed in Muller, 2011). We speculate that the lack of effect of tobramycin at the highest concentration reflects competition for endothelial cell uptake between low levels of the contemporaneously formed CuT complex and excess free, uncomplexed, tobramycin for heparan sulphate binding sites.
In contrast, CuT had a concentration-dependent inhibitory effect on neutrophil migration that was significant at the highest concentration tested. Although the inhibitory effect was similar to that of the combined intracellular SOD/catalase activity of MnTBAP, the mechanism of action may depend only on superoxide scavenging because CuT has no catalase activity associated with it. In addition to its anti-oxidant properties, we suggest that the inhibitory effect of CuT on neutrophil transmigration may also relate to its ability to displace neutrophil elastase from heparan sulphate on the neutrophil cell surface where it is normally bound and protected from inhibition by high molecular weight naturally occurring inhibitors following cell activation and degranulation (Campbell and Owen, 2007). We showed that, once displaced from the cell surface by CuT, neutrophil elastase is sensitive to inhibition by α1-antitrypsin, which during transendothelial migration could be derived either from activated neutrophils (Paakko et al., 1996) or from FCS added in the assay.
In our model of transendothelial migration, endothelial cells were grown on Transwell inserts for 14 days, in which time they lay down a basement membrane (Huber and Weiss, 1989). Although the role of neutrophil elastase in neutrophil transendothelial migration is controversial (Huber and Weiss, 1989; Rowe and Weiss, 2008), and both proteolytic and non-proteolytic pathways are proposed (Rowe and Weiss, 2008), a decrease in neutrophil cell surface elastase was recently shown to inhibit neutrophil migration in vivo and in vitro (Mizuguchi et al., 2009).
Overall, our data point to tobramycin being a pro-drug having anti-inflammatory effects as the CuT complex, which inhibits leukocyte migration via effects on oxidative and proteolytic pathways. These pathways interact (Witko-Sarsat and Descamps-Latscha, 1994); and, for example, CuT may protect α1-antitrypsin from oxidative inactivation, further limiting elastase activity. The copper levels in normal human plasma are approximately 1 μg·mL−1 (15 μM), of which 30% (5 μM) is in an exchangeable pool bound to albumin and transcuprein (Tapeiro et al., 2003). Serum levels of tobramycin are reported to be 5–15 μM via parenteral administration (Ratjen et al., 2009) and approximately 2 μM following inhalation of 300 mg tobramycin twice daily (Ramsey et al., 1999; Geller et al., 2002). These values indicate the potential for CuT formation in vivo following tobramycin therapy with significant antioxidant activity at these concentrations and thus the anti-inflammatory effects of inhaled tobramycin that were previously reported (Ramsey et al., 1999).
The oxidant/antioxidant and proteinase/anti-proteinase balance is important in the pathophysiology of CF lung disease. There are three mammalian SOD enzymes, SOD1 (Cu/Zn SOD), SOD2 (Mn SOD) and SOD3 (EC-SOD), which has Cu and Zn at the catalytic centre (Muscoli et al., 2003). SOD1 and SOD2 are intracellular enzymes, while SOD3 is an extracellular enzyme found bound to the tissue matrix and is present at highest concentration in the lungs (Muscoli et al., 2003). In CF patients, a deficiency in lung antioxidant defences, especially SOD3, is associated with increased oxidative stress contributing to epithelial cell apoptosis and the decline in lung function, pointing to new therapeutic pathways to reduce oxidative stress (Rottner et al., 2009; 2011). Many copper complexes of non-steroidal anti-inflammatory drugs that are non-cytotoxic, and have SOD-like activity and superior anti-inflammatory activity in animal models to the parent compound, are in development and used in veterinary practise (Weder et al., 2002). It remains to be seen whether CuT has an appropriate pharmacological and pharmacokinetic profile for further therapeutic development.
The models we developed to investigate the effects of tobramycin and CuT on leukocyte migration are relevant to CF. Intrinsic platelet activation and a pro-inflammatory function for platelets have been reported in CF (O'Sullivan and Michelson, 2006). In part, this may be related to dysfunctional platelet CFTR associated with reduced production of the anti-inflammatory mediator lipoxin A4 and delayed resolution of inflammation (Mattoscio et al., 2010). The factors that direct the initial recruitment of platelets into lung tissue are relatively unknown, although platelet recruitment into lung tissue in allergic asthma is mediated by allergen via allergen-specific IgE bound to FcεR1 on platelets (Pitchford et al., 2008) and platelet migration to bacterial peptides in animal models has been reported (Pitchford, 2007).
In summary, tobramycin is widely used as an intravenous and inhaled therapy to treat lung infection with P. aeruginosa. CF patients receiving intermittent inhaled tobramycin over 11 months maintained continued improvement in lung function despite smaller reductions in bacterial density, leading the authors to suggest that inhaled tobramycin may have anti-inflammatory effects in addition to its bactericidal activity (Ramsey et al., 1999). Inhaled tobramycin achieves high concentrations (∼2 mM) in CF sputum (Geller et al., 2002) that may bind with elevated sputum copper (0.31 μM) (Gray et al., 2010), to generate low levels of an antioxidant complex in the airways. Additionally, intravenous and inhaled tobramycin may achieve concentrations in the circulation that have some of the anti-inflammatory effects described here. We propose that these additional effects of tobramycin relate to its ability to chelate copper and the associated SOD-like activity of the CuT complex.
Glossary
- BCDS
bathocuproine disodium salt
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CHO
Chinese hamster ovary
- CuT
copper–tobramycin
- DCFDA
2′,7′-dichlorofluorescein diacetate
- GF-AAS
graphite furnace atomic absorption spectroscopy
- HLMVEC
human lung microvascular endothelial cells
- NC
neocuproine
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TEM
transendothelial migration
- Tob
tobramycin
Conflict of interest
None.
References
- Alcock NW. A hydrogen peroxide digestion system for tissue trace metal analysis. Biol Trace Elem Res. 1987;13:363–370. doi: 10.1007/BF02796647. [DOI] [PubMed] [Google Scholar]
- Baltus T, von Hundelshausen P, Mause SF, Buhre W, Rossaint R, Weber C. Differential and additive effects of platelet-derived chemokines on monocyte arrest on inflamed endothelium under flow conditions. J Leuk Biol. 2008;78:435–441. doi: 10.1189/jlb.0305141. [DOI] [PubMed] [Google Scholar]
- Bar-Or D, Thomas GW, Yuki RL, Rael LT, Shimonkevitz RP, Curtis CG, et al. Copper stimulates the synthesis and release of interleukin-8 in human endothelial cells: a possible early role in systemic inflammatory responses. Shock. 2003;20:154–158. doi: 10.1097/01.shk.0000068318.49350.3a. [DOI] [PubMed] [Google Scholar]
- Boueiz A, Hassoun PM. Regulation of endothelial barrier function by reactive oxygen and nitrogen species. Microvasc Res. 2009;77:26–34. doi: 10.1016/j.mvr.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Brewer GJ. The risks of free copper in the body and the development of useful anticopper drugs. Curr Opin Clin Nutr Metab Care. 2008;11:727–732. doi: 10.1097/MCO.0b013e328314b678. [DOI] [PubMed] [Google Scholar]
- Camakaris J, Voskoboinik I, Mercer JF. Molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun. 1999;261:225–232. doi: 10.1006/bbrc.1999.1073. [DOI] [PubMed] [Google Scholar]
- Campbell EJ, Owen CA. The sulfate groups of chondrotin sulfate and heparan sulfate-containing proteoglycans in neutrophil plasma membranes are novel binding sites for human leukocyte elastase and cathepsin G. J Biol Chem. 2007;282:14645–14654. doi: 10.1074/jbc.M608346200. [DOI] [PubMed] [Google Scholar]
- Cantin A, Woods DE. Protection by antibiotics against myeloperoxidase-dependent cytotoxicity to lung epithelial cells in vitro. J Clin Invest. 1993;91:38–45. doi: 10.1172/JCI116196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook-Mills JM. Hydrogen peroxide activation of endothelial cell-associated MMPs during VCAM-1-dependent leukocyte migration. Cell Mol Biol (Noisy-Le-Grand) 2006;52:8–16. [PMC free article] [PubMed] [Google Scholar]
- Doring G. Prevention of Pseudomonas aeruginosa infection in cystic fibrosis patients. Int J Med Microbiol. 2010;300:573–577. doi: 10.1016/j.ijmm.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Elizur A, Cannon C, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- Elson-Schwab L, Garner OB, Schuksz M. Crawford BE, Esko JD, Tor Y. Guanindinylated neomycin delivers large, bioactive cargo into cells through a heparan sulfate-dependent pathway. J Biol Chem. 2007;282:13585–13591. doi: 10.1074/jbc.M700463200. [DOI] [PubMed] [Google Scholar]
- Ewing JF, Janero DR. Microplate superoxide dismutase assay employing a nonenzymatic superoxide generator. Anal Biochem. 1995;232:243–248. doi: 10.1006/abio.1995.0014. [DOI] [PubMed] [Google Scholar]
- Freitas M, Lima JLFC, Fernandes E. Optical probes for detection and quantification of neutrophils oxidative burst. A review. Anal Chim Acta. 2009;649:8–23. doi: 10.1016/j.aca.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Geller DE, Pitlick WH, Nardella PA, Tracewell WG, Ramsey BW. Pharmacokinetics and bioavailability of aerosolised tobramycin in cystic fibrosis. Chest. 2002;122:219–226. doi: 10.1378/chest.122.1.219. [DOI] [PubMed] [Google Scholar]
- Gokhale N, Patwardhan A, Cowan JA. Metalloaminoglycosides: chemistry and biological relevance. In: Arya DP, editor. Aminoglycoside Antibiotics: From Chemical Biology to Drug Discovery. Hoboken, NJ: John Wiley and Sons; 2007. pp. 235–254. [Google Scholar]
- Gray RD, Duncan A, Noble D, Imrie M, O'Reilly DSJ, Innes JA, et al. Sputum trace metals are biomarkers of inflammatory and suppurative lung disease. Chest. 2010;137:635–641. doi: 10.1378/chest.09-1047. [DOI] [PubMed] [Google Scholar]
- Hattori H, Subramanian KK, Sakai J, Jia Y, Li Y, Porter TF, et al. Small-molecule screen identifies reactive oxygen species as key regulators of neutrophil chemotaxis. Proc Natl Acad Sci U S A. 2010;107:3546–3551. doi: 10.1073/pnas.0914351107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G-F. Copper stimulates proliferation of human endothelial cells under culture. J Cell Biochem. 1998;69:326–335. doi: 10.1002/(sici)1097-4644(19980601)69:3<326::aid-jcb10>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Hubeau C, Lorenzato M, Couetil JP, Hubert D, Dusser D, Puchelle E, et al. Quantitative anaysis of inflammatory cells infiltrating the cystic fibrosis airway mucosa. Clin Exp Immunol. 2001;124:69–76. doi: 10.1046/j.1365-2249.2001.01456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber AR, Weiss SJ. Disruption of the subendothelial basement membrane during neutrophil diapedesis in an in vitro construct of a blood vessel wall. J Clin Invest. 1989;83:1122–1136. doi: 10.1172/JCI113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Hundelhausen CW. Platelets as immune cells bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- Jezowska-Bojczuk M, Karaczyn A, Kozlowski H. Copper (II) binding to tobramycin: potentiometric and spectroscopic studies. Carbohydr Res. 1998;313:265–269. doi: 10.1016/s0008-6215(98)00288-2. [DOI] [PubMed] [Google Scholar]
- Kameyoshi Y, Dorschner A, Mallet AI, Christophers E, Schroder J-M. Cytokine RANTES released by thrombin-stimulated platelets is a potent attractant for human eosinophils. J Exp Med. 1992;176:587–592. doi: 10.1084/jem.176.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Seki M, Hiromatsu K, Eastcott JW, Watts GFM, Sugai M, et al. Selective diapedesis of Th1 cells induced by endothelial cell RANTES. J Immunol. 1999;163:3269–3278. [PubMed] [Google Scholar]
- Kozlowski H, Kowalik-Jankowska T, Jezowska-Bojczuk M. Chemical and biological aspects of Cu2+ interactions with peptides and aminoglycosides. Coord Chem Rev. 2005;249:2323–2334. [Google Scholar]
- Laurila JP, Laatikainen LE, Castellone MD, Laukkanen MO. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS ONE. 2009;4:e5786. doi: 10.1371/journal.pone.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengfelder E, Fuchs C, Younes M, Weser U. Functional aspects of the superoxide dismutative action of Cu-penicillamine. Biochim Biophys Acta. 1979;567:492–502. doi: 10.1016/0005-2744(79)90135-9. [DOI] [PubMed] [Google Scholar]
- Liochev SI, Fridovich I. The effects of superoxide dismutase on H2O2 formation. Free Rad Biol Med. 2007;42:1465–1469. doi: 10.1016/j.freeradbiomed.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Loetscher P, Seitz M, Baggiolini M, Moser B. Interleukin-2 regulates CC chemokine receptor expression and chemotactic responsiveness in T lymphocytes. J Exp Med. 1996;184:569–577. doi: 10.1084/jem.184.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor HJ, Kato Y, Marshall LJ, Nevell TG, Shute JK. A copper-hydrogen peroxide redox system induces dityrosine cross-links and chemokine oligomerisation. Cytokine. 2011;56:669–675. doi: 10.1016/j.cyto.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Mattoscio D, Evangelista V, De Cristofaro R, Recchiuti A, Pandolfi A, Di Silvetsre S, et al. Cystic fibrosis transmembrane conductance regulator (CFTR) expression in human platelets: impact on mediators and mechanisms of the inflammatory response. FASEB J. 2010;24:3970–3980. doi: 10.1096/fj.10-159921. [DOI] [PubMed] [Google Scholar]
- Milanino R, Buchner V. Copper: role of the ‘endogenous’ and ‘exogenous’ metal on the development and control of inflammatory processes. Rev Environ Health. 2006;21:153–214. doi: 10.1515/reveh.2006.21.3.153. [DOI] [PubMed] [Google Scholar]
- Mizuguchi S, Stephen J, Bihari R, Markovic N, Suehiro S, Capretta A, et al. CORM-3-derived CO modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase. Am J Physiol Heart Circ Physiol. 2009;297:H920–H929. doi: 10.1152/ajpheart.00305.2009. [DOI] [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annu Rev Pathol Mech Dis. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscoli C, Cuzzocrea S, Riley DP, Zweier JL, Thiemermann C, Wang Z-Q SD. On the selectivity of superoxide dismutase mimetics and its importance in pharmacological studies. Br J Pharmacol. 2003;140:445–460. doi: 10.1038/sj.bjp.0705430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawara A, Nathan CF, Cohn ZA. Hydrogen peroxide metabolism in human monocytes during differentiation in vitro. J Clin Invest. 1981;68:1243–1252. doi: 10.1172/JCI110370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Yanagihara K, Araki N, Yamada K, Morinaga Y, Izumikawa K, et al. High-dose tobramycin inhibits lipopolysaccharide-induced MUC5AC production in human lung epithelial cells. Eur J Pharmacol. 2011;659:67–71. doi: 10.1016/j.ejphar.2011.03.002. [DOI] [PubMed] [Google Scholar]
- O'Sullivan BP, Michelson AD. The inflammatory role of platelets in cystic fibrosis. Am J Respir Crit Care Med. 2006;173:483–490. doi: 10.1164/rccm.200508-1243PP. [DOI] [PubMed] [Google Scholar]
- Paakko P, Kirby M, do Bois RM, Gillissen A, Ferrans VJ, Crystal RG. Activated neutrophils secrete stored α1-antitrypsin. Am J Respir Crit Care Med. 1996;154:1829–1833. doi: 10.1164/ajrccm.154.6.8970377. [DOI] [PubMed] [Google Scholar]
- Percival SS, Kauwell GPA, Bowser E, Wagner M. Altered copper status in adult men with cystic fibrosis. J Am Coll Nutr. 1999;18:614–619. doi: 10.1080/07315724.1999.10718896. [DOI] [PubMed] [Google Scholar]
- Persichini T, Percario Z, Mazzon E, Colasanti M, Cuzzocrea S, Musci G. Copper activates the NF-κB pathway in vivo. Antioxid Redox Signal. 2006;8:1897–1904. doi: 10.1089/ars.2006.8.1897. [DOI] [PubMed] [Google Scholar]
- Petreccia DC, Nauseef WM, Clark RA. Respiratory burst of normal human eosinophils. J Leuk Biol. 1987;41:283–288. doi: 10.1002/jlb.41.4.283. [DOI] [PubMed] [Google Scholar]
- Pitchford SC. Novel uses for anti-platelet agents as anti-inflammatory drugs. Br J Pharmacol. 2007;152:987–1002. doi: 10.1038/sj.bjp.0707364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchford SC, Momi S, Baglioni S, Casali L, Giannini S, Rossi R, et al. Allergen induces the migration of platelets to lung tissue in allergic asthma. Am J Respir Crit Care Med. 2008;177:604–612. doi: 10.1164/rccm.200702-214OC. [DOI] [PubMed] [Google Scholar]
- Proudfoot AEI, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2002;100:1885–1890. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey BW, Pepe MS, Quan JM, Otto KL, Montgomery AB, Williams-Warren J, et al. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N Engl J Med. 1999;340:23–30. doi: 10.1056/NEJM199901073400104. [DOI] [PubMed] [Google Scholar]
- Ratjen F, Brockhaus F, Angyalosi G. Aminoglycoside therapy against Pseudomonas aeruginosa in cystic fibrosis: a review. J Cyst Fibros. 2009;8:361–369. doi: 10.1016/j.jcf.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Romagni S. Lymphokine production by human T cells in disease states. Annu Rev Immunol. 1994;12:227–257. doi: 10.1146/annurev.iy.12.040194.001303. [DOI] [PubMed] [Google Scholar]
- Rottner M, Freyssinet J-M, Martinez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res. 2009;10:23. doi: 10.1186/1465-9921-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner M, Tual-Chalot S, Mostefai HA, Andriantsitohaina R, Freyssinet J-M, Martinez MC. Increased oxidative stress induces apoptosis in human cystic fibrosis cells. PLoS ONE. 2011;6:e24880. doi: 10.1371/journal.pone.0024880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;18:568–574. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- Schmitt Y. Copper and zinc determination in plasma and corpuscular components of peripheral blood of patients with preterminal and terminal renal failure. J Trace Elements Med Biol. 1997;11:210–214. doi: 10.1016/S0946-672X(97)80015-6. [DOI] [PubMed] [Google Scholar]
- Solic N, Wilson J, Wilson SJ, Shute JK. Endothelial activation and increased heparan sulphate expression in cystic fibrosis. Am J Respir Crit Care Med. 2005;172:892–898. doi: 10.1164/rccm.200409-1207OC. [DOI] [PubMed] [Google Scholar]
- Spisni E, Valerii MC, Manerba M, Strillacci A, Polazzi E, Mattia T, et al. Effect of copper on extracellular levels of ket pro-inflammatory molecules in hypothalamic GN11 and primary neurons. Neurotoxicology. 2009;30:605–612. doi: 10.1016/j.neuro.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Sreedhara A, Freed JD, Cowan JA. Efficient inorganic deoxyribonucleases. Greater than 50-million-fold rate enhancement in enzyme-like DNA cleavage. J Am Chem Soc. 2000;122:8814–8824. [Google Scholar]
- Tapeiro H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper Biomed Pharmacother. 2003;57:386–398. doi: 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder JE, Dillon CT, Hambley TW, Kennedy BJ, Lay PA, Biffin JR, et al. Copper complexes of non-steroidal anti-inflammatory drugs: an opportunity yet to be realized. Coord Chem Rev. 2002;232:95–126. [Google Scholar]
- Witko-Sarsat V, Descamps-Latscha B. Neutrophil derived oxidants and proteinases as immunomodulatory mediators in inflammation. Mediators Inflamm. 1994;3:257–273. doi: 10.1155/S0962935194000360. [DOI] [PMC free article] [PubMed] [Google Scholar]