Abstract
Background and Purpose
Recent studies demonstrated that the sympathetic nervous system regulates bone metabolism via β2-adrenoceptors. Although α-adrenoceptors are also expressed in osteogenic cells, their functions in bone metabolism have been less studied. We previously demonstrated that noradrenaline suppressed potassium currents via α1B-adrenoceptors in the human osteoblast SaM-1 cell line. The aim of this study was to investigate the signal transduction pathway and the physiological role of noradrenaline in human osteoblasts in more detail.
Experimental Approach
To investigate signal transduction through α1B-adrenoceptors, we used whole-cell patch clamp recording and Ca fluorescence imaging. Potassium channels regulate membrane potential and cell proliferation activity in non-excitable cells, so we evaluated cell proliferation activity by BrdU incorporation and WST assay.
Key Results
In SaM-1 cells, bath-applied noradrenaline elevated intracellular Ca2+ concentration and this effect was abolished by both chloroethylclonidine, an α1B-adrenoceptor antagonist, and U73122, a PLC inhibitor. However, the inhibitory effect of noradrenaline on whole-cell current was unaffected by U73122. In contrast, in cells pretreated with either Pertussis toxin, a Gi/o-protein-coupled receptor inhibitor, or gallein, a Gβγ-protein inhibitor, the inhibitory effect of noradrenaline on whole-cell current was significantly suppressed. Noradrenaline-induced enhancement of cell proliferation was inhibited by CsCl, a non-selective potassium channel blocker, gallein and H89, a PKA inhibitor, but not by U73122.
Conclusions and Implications
Noradrenaline facilitated cell proliferation by regulation of potassium currents in human osteoblasts via Gi/o-protein-coupled α1B-adrenoceptors, not via coupling to Gq-proteins.
Keywords: sympathetic nervous system, osteoblast, potassium channels, cell proliferation, heterotrimeric G-protein
Introduction
Bones are continuously resorbed by osteoclasts and formed by osteoblasts, and bone mass is maintained by the balance between their functions. In recent years, many studies have demonstrated that the sympathetic nervous system is involved in bone metabolism (Cherruau et al., 1999; Elefteriou et al., 2005; Togari and Arai, 2008; He et al., 2011). Bone loss can be induced by continuously high sympathetic tone and this is reversed by β-adrenoceptor blockade (Bonnet et al., 2008; Sato et al., 2010). Previous studies, including ours, showed that mRNAs of both α- and β-adrenoceptors were expressed in human osteoblasts (Togari et al., 1997; Togari, 2002; Huang et al., 2009). Although many studies have suggested that up-regulation of osteoclastogenesis and osteoclastic activity via β-adrenoceptors enhanced bone resorption (Arai et al., 2003; Elefteriou et al., 2005; Kondo and Togari, 2011), the physiological role of α-adrenoceptors in bone metabolism has been less well studied. Adrenaline stimulated cell proliferation and differentiation via α1-adrenoceptors in MC3T3-E1 osteoblast-like cells (Suzuki et al., 1998; 2001). The expression levels of osteoprotegerin and receptor activator of NF-κB ligand (RANKL) were increased by α- and β-adrenoceptor agonists (Takeuchi et al., 2001; Nishiura and Abe, 2007; Huang et al., 2009; receptor nomenclature follows Alexander et al., (2011).
In general, GPCRs exert their functions via the phosphoinositide–phospholipase C (PI–PLC) pathway or the cAMP/PKA pathway, but recent studies suggested that ion channels also play important roles downstream of the signal transduction pathway of several GPCRs, including adrenoceptors (Inoue et al., 2001; Marx et al., 2002; Kim et al., 2005; O-uchi et al., 2008). We previously demonstrated that noradrenaline suppressed Cs-sensitive and tetraethylammonium-insensitive (TEA-insensitive) potassium channels via α1B-adrenoceptors in human osteoblasts, SaM-1 cells, by using whole-cell patch clamp recordings (Kodama and Togari, 2010). Several types of potassium channel had been found to regulate membrane potential and cell proliferation (Pardo et al., 2005; Liebau et al., 2006; Henney et al., 2009; Jang et al., 2011). In osteoblasts, blockade of large conductance Ca-activated potassium (BK) channels, ERG channels and TREK-1 channels promoted cell proliferation (Hughes et al., 2006; Hernandez et al., 2007), while a high dose of TEA, a potassium channel inhibitor, impaired cell proliferation (Hernandez et al., 2007; Henney et al., 2009). The physiological roles of the inhibitory effects of noradrenaline on potassium channels in human osteoblasts have not yet been defined.
In the present study, we undertook more detailed investigation of the signal transduction pathway in terms of the effect of noradrenaline and whether it is involved in cell proliferation in human osteoblasts, SaM-1 cells. Pharmacological analysis suggested that both Gq- and Gi/o-protein-coupled α1B-adrenoceptors were expressed in SaM-1 cells, and the latter was involved in the inhibitory effect of noradrenaline on potassium channels. Additionally, Gi/o-coupled α1-adrenoceptors, not those coupled to Gq-proteins, participated in noradrenaline-induced enhancement of cell proliferation. These results suggested that noradrenaline regulates cell proliferation via Gi/o-protein-coupled α1B-adrenoceptors by regulating potassium channel activity in human osteoblasts.
Methods
Cell culture
The human osteoblasts used in this study, SaM-1 cells, were provided by Dr Koshihara, who prepared them with informed consent from an explant of ulnar periosteum tissue from a 20-year-old male patient who underwent curative surgery (Koshihara et al., 1987). These cells have a mitotic lifespan of 34 population doubling levels (PDLs), and we used them at PDL of 22–24 for our experiments. We confirmed the cells are capable of calcifying at this level (Komoto et al., 2012). The cells were cultured in α-modified minimum essential medium (α-MEM; Invitrogen, Carlsbad, CA) containing 10% FBS (Moregate Biotech, Bulimba, Australia) and 60 μg·mL−1 kanamycin at 37°C in 95% humidified air containing 5% CO2. The growth media were renewed every 2 days. For optical measurements of intracellular calcium concentration ([Ca2+]i) and whole-cell patch clamp recording, they were seeded on a cover slip, 1–2 days before the experiments.
Optical measurements of [Ca2+]i
SaM-1 cells were loaded with fluo-3AM (2.5 μM) for 30 min and washed three times with extracellular solution, which contained 124 mM NaCl, 3 mM KCl, 1 mM MgCl2, 2 mM CaCl2, 14 mM d-glucose and 10 mM HEPES (pH adjusted to 7.4 with NaOH), just before use. Then, the glass coverslip was transferred to a superfusion chamber on the stage of a confocal laser scanning microscope (LSM710, Carl Zeiss, Hallbergmoos, Germany). Cells were superfused with extracellular solution at a rate of 2 mL·min−1. The fluorescence was recorded every 2 s at room temperature at an excitation wavelength of 488 nm, and the data were analysed using ZEN 2009 software (Carl Zeiss). Stock solutions of drugs were prepared and diluted 1000-fold into extracellular solution just before use, and they were applied by bath perfusion.
Whole-cell patch clamp recording
SaM-1 cells were seeded on a glass coverslip. After 1–2 days of culture, the glass coverslip was transferred to a superfusion chamber on the stage of an inverted microscope (Axiovert200, Carl Zeiss). Cells were superfused with extracellular solution at 1–1.5 mL·min−1. Whole-cell voltage clamping was performed using patch electrodes (3–5 MΩ) filled with internal solution containing 140 mM KCl, 2 mM MgCl2, 10 mM HEPES, 0.1 mM EGTA, 3 mM Na2ATP and 0.3 mM Na3GTP (pH adjusted to 7.4 with KOH) and Axopatch200B patch clamp amplifier (Axon Instruments, Sunnyvale, CA). In the experiments to investigate the involvement of [Ca2+]i, we used internal solution containing 130 mM KCl, 2 mM MgCl2, 10 mM HEPES, 5 mM EGTA, 3 mM Na2ATP and 0.3 mM Na3GTP (pH adjusted to 7.4 with KOH).
The holding potential was −60 mV, and voltage–-current relationships were recorded using voltage steps or ramps. In the voltage step protocol, 10 mV voltage steps from −100 to +40 mV from a holding potential of −60 mV for 500 ms were applied. In the voltage ramp protocol, 1 s voltage ramp from −100 to +40 mV following 150 ms voltage steps of −100 mV was applied every 10 s. The signals were low-pass filtered at 10 kHz and digitized at 20 kHz for analysis with pClamp9.2 software (Molecular Devices, Silicon Valley, CA). Stock solutions of drugs were prepared and diluted 1000-fold into extracellular solution just before use, and they were applied by bath perfusion.
Cell proliferation assay
5-Bromo-2′-deoxyuridine (BrdU) incorporation
Cell proliferation activity was assessed by BrdU incorporation using Cell Proliferation elisa, BrdU kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions. Cells were seeded in a 96-well plate at a density of 10 000 cells·per well in 100 μL of culture medium. After 1 day, culture media were replaced with α-MEM containing 2.5% FBS and kanamycin, and then the cells were treated with noradrenaline for 20 h at 37°C. BrdU labelling solution (100 μM) was added at 10 μL·per well, and the cells were incubated for an additional 4 h at 37°C. After this, the labelling media were removed, the cells were fixed and DNA was denatured with FixDenat solution. The cells were incubated with peroxidase-conjugated anti-BrdU antibody for 1.5 h at room temperature. The cells were then washed three times with PBS, followed by the addition of substrate solution (tetraethyl-benzidine) at 100 μL·per well. After 15 min incubation, 1 M H2SO4 was added at 25 μL·per well to stop the peroxidase reaction, and then absorbance of wells was measured at 450 nm using Multiskan FC (Thermo Fisher Scientific, Waltham, MA).
Water-soluble tetrazolium (WST) assay
Cell number was assessed as dehydrogenase activity by using Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan). In this assay, WST-8 is reduced to water-soluble formazan outside the cells. Cells were seeded in a 96-well plate at a density of 10 000 cells·per well in 100 μL of culture medium. After 1 day, culture media were replaced with α-MEM containing 2.5% FBS and kanamycin, and then the cells were treated with noradrenaline for 22.5 h at 37°C. CCK-8 solution was added at 10 μL·per well, and the cells were incubated for an additional 1.5 h at 37°C. Then absorbance of wells was measured at 450 nm using Multiskan FC (Thermo Fisher Scientific).
To confirm that the experimental conditions were adequate, the data from non-drug-treated cultures were also recorded in each experiment.
Statistical analysis
All data are expressed as mean ± SEM. In the voltage step protocol, the changes of whole-cell currents in a stable state were evaluated using the paired t-test. In the voltage ramp protocol, the effects of the drugs on whole-cell currents were evaluated by comparing average currents (six traces for 1 min) taken during the peak response to each drug with those before drug application. Cell proliferation activities were evaluated by comparing with the data from a control group. The two-tailed t-test combined with Bonferroni's correction following one-way anova was used for multiple comparisons. Differences with P-values <0.05 were considered significant.
Materials
l-Noradrenaline; chloroethylclonidine (CEC), an α1B-adrenoceptor-selective antagonist; U73122, a PLC inhibitor; H89, a PKA inhibitor; clonidine, prazosin and propranolol were purchased from Sigma Aldrich (St. Louis, MO, USA). Pertussis toxin (PTX) was purchased from Merck KGaA (Darmstadt, Germany). CsCl was purchased from Nacalai Tesque (Kyoto, Japan). A Gβγ -protein inhibitor, gallein, was purchased from Tocris Biosciences (Bristol, UK). A calcium fluorophore, fluo-3AM, was purchased from Dojindo. U73122, gallein and H89 were dissolved in dimethyl sulfoxide. All other chemicals used were of reagent grade.
Results
Involvement of the PI–PLC pathway in the effects of noradrenaline
In Ca2+ fluorescence imaging, elevation of fluo-3AM fluorescence intensity was induced by bath application of 1 μM noradrenaline in 50.4 ± 6.4% of cells examined and the responses were reproducible on the same cells with repeated application in SaM-1 cells (13 individual experiments; Figure 1A). In the cells pretreated with 100 μM CEC for 45 min at 37°C, bath-applied noradrenaline had no effects on fluorescence (five individual experiments, data not shown). Additionally, the effect of noradrenaline on [Ca2+]i was eliminated by pretreatment with the PLC inhibitor U73122 for 10 min (five individual experiments; Figure 1B). On the other hand, in whole-cell patch clamp recording, the currents induced by voltage steps were significantly reduced and reversal potential was shifted rightwards by 1 μM bath-applied noradrenaline, as shown in our previous study (n = 6; Figure 1C; Kodama and Togari, 2010). The ratios between the current amplitude at the beginning (50 ms) of the pulse and that at the end of pulse (500 ms) were 95.1 ± 2.5% and 90.1 ± 4.4% in the absence and presence of noradrenaline respectively. There was no apparent effect of noradrenaline on the current kinetics. Similarly, in the voltage ramp protocol, whole-cell current was reduced, at 40 mV (n = 7; Figure 1D). These inhibitory effects of noradrenaline on whole-cell current were shown in almost all cells tested (48 of 50 independent experiments, including preliminary data). Bath-applied U73122 slightly suppressed whole-cell current (n = 5), and treatment with noradrenaline following U73122 exhibited a similar extent of inhibition as in the control cells (n = 5; Figure 1D, E). Additionally, the inhibitory effect of noradrenaline was unaffected by using the internal solution containing 5 mM EGTA (n = 5; Figure 1D).
Figure 1.
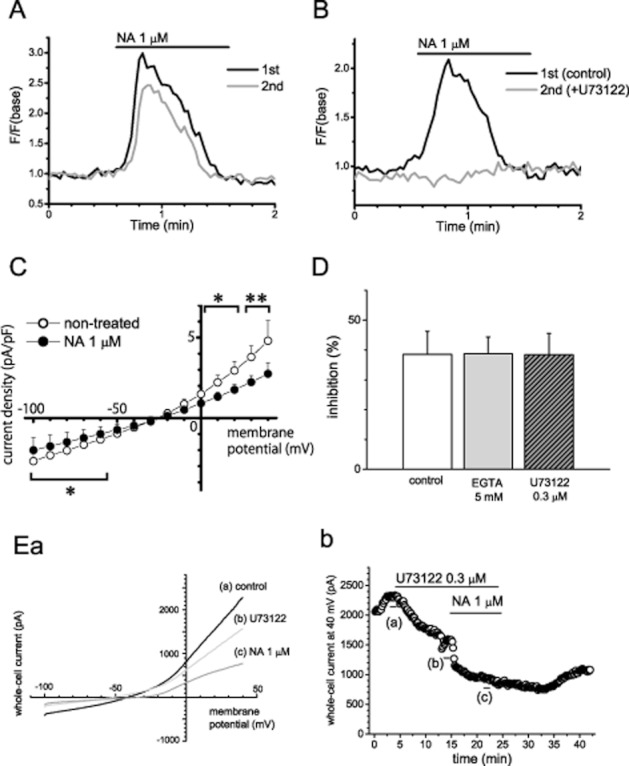
Involvement of the Gq/PI–PLC pathway in the inhibitory effect of noradrenaline (NA) on whole-cell current in human osteoblasts. (A and B) Representative traces of [Ca2+]i elevation induced by repeated application of noradrenaline in SaM-1 cells. (A) The representative recordings from cells that responded to noradrenaline (B) Representative responses to noradrenaline in the absence (the first application) and presence (the second application) of U73122, an inhibitor of PLC. (C) The averaged voltage–current density relationship recorded by voltage step from −100 to +40 mV. (D) Summary of the influence of the presence of EGTA, a Ca chelator, at 5 mM in the pipette solution and pretreatment with U73122, on the effects of noradrenaline (control, n = 7; EGTA, U73122, n = 5). (E) Representative averaged traces of six consecutive whole-cell currents during a −100 to +40 mV voltage ramp (a) and time course of whole-cell currents at +40 mV (b). The bars indicate the time points when the averaged traces were recorded. Values are shown as the mean ± SEM. *P < 0.05, **P < 0.01 compared with non-treated control. Each figure is representative data of five independent experiments in panels A, B and E.
Involvement of Gi/o- and Gβγ-protein in the effects of noradrenaline on whole-cell current
We next examined whether Gi/o-protein is involved in the inhibitory effect of noradrenaline on whole-cell current. In the cells pretreated with Gi/o-coupled receptor inhibitor, PTX, at 150 ng·mL−1 for 24 h, noradrenaline-induced suppression of whole-cell current was significantly attenuated (n = 11, 5, respectively; Figure 2A, D). Treatment with PTX inhibits not only the effect via Giα-protein but also that via Gβγ-protein (Katz et al., 1992). Then we examined the effect of Gβγ-protein inhibitor, gallein and PKA inhibitor, H89. Pretreatment with gallein at 10 μM for 30 min just before the experiment significantly reduced the inhibitory effect of noradrenaline (n = 5; Figure 2B, D). Meanwhile, bath application of H89 at 2.5 μM slightly suppressed whole-cell current (n = 5), and the inhibitory effect of noradrenaline following H89 application tended to be attenuated (n = 5; Figure 2C, D).
Figure 2.
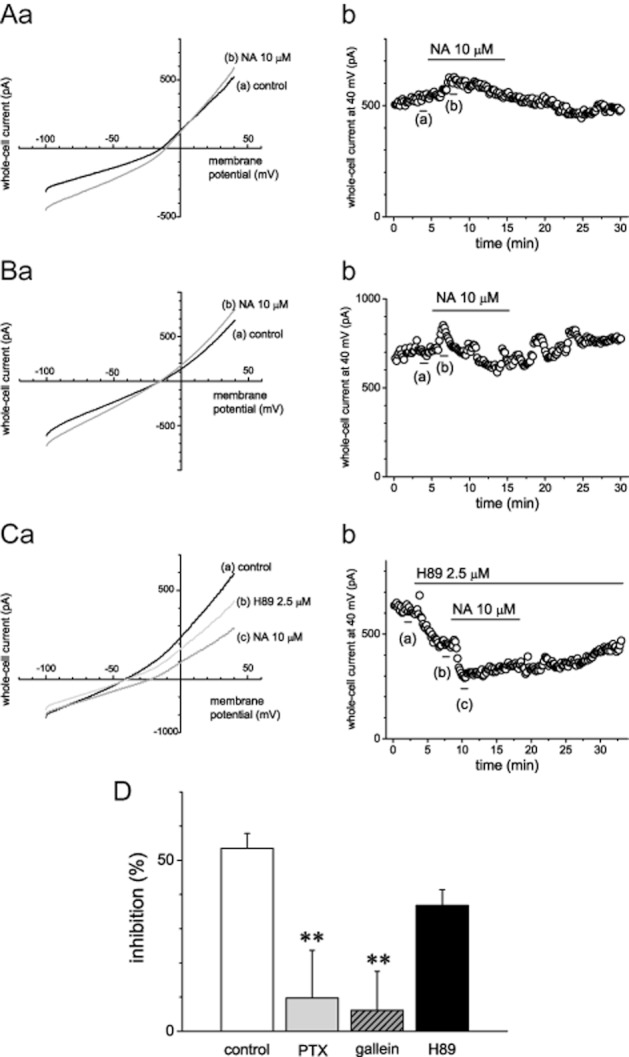
Involvement of Gi/o- and Gβγ-proteins in the inhibitory effects of noradrenaline (NA) on whole-cell current. (A–C) Representative averaged traces of six consecutive whole-cell currents during a −100 to +40 mV voltage ramp (a) and time course of whole-cell currents recorded at +40 mV (b) in the cells pretreated with Pertussis toxin (PTX), a Gi/o-protein-coupled receptor inhibitor (A), gallein, a Gβγ-protein inhibitor (B), or H89, a PKA inhibitor (C). The bars indicate the time points when the averaged traces were recorded. (D) Summary results of the pretreatment with PTX, gallein or H89 on the effects of noradrenaline (control, n = 11; PTX, gallein, H89, n = 5). Values are shown as the mean ± SEM. **P < 0.01 compared with control. Each figure shows representative data of five independent experiments in panels A, B and C.
In our previous study, α2B-adrenoceptors were expressed at a low level in SaM-1 cells (Togari et al., 1997; Togari, 2002). In general, α2-adrenoceptors are known to be coupled with Gi/o-protein. We then examined the effect of the α2-adrenoceptor agonist, clonidine, on whole-cell current. Bath application of clonidine had no obvious effect on the voltage–current relationship (n = 5; Figure 3).
Figure 3.
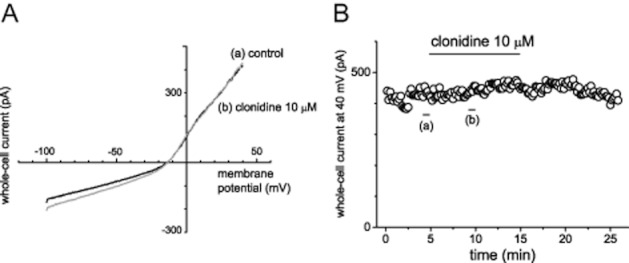
The effects of clonidine, an α2-adrenoceptor agonist, on whole-cell current in human osteoblasts. (A and B) Representative averaged traces of six consecutive whole-cell currents during a −100 to +40 mV voltage ramp (A) and time course of whole-cell currents recorded at +40 mV (B). These are representative data from five independent experiments. The bars indicate the time points when the averaged traces were recorded.
Effects of adrenoceptor ligands on proliferation in SaM-1 cells
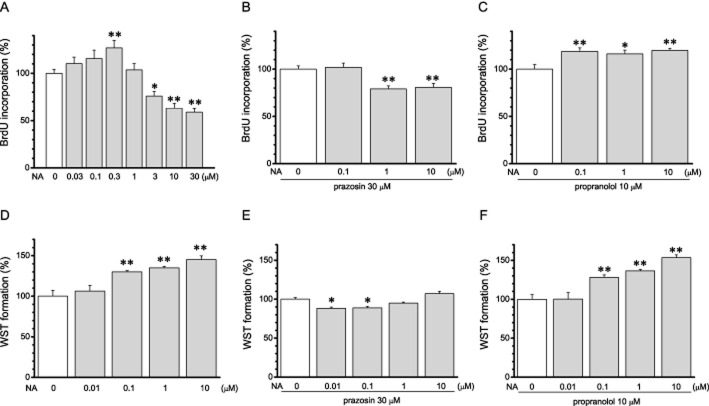
Cell proliferation activity was evaluated as DNA synthesis by BrdU test, and live cell number was evaluated as dehydrogenase activity by WST assay. In SaM-1 cells, treatment with noradrenaline for 24 h increased BrdU incorporation at a lower dose (0.03 − 0.3 μM,) and suppressed it at higher doses (1 μM − 30 μM; n = 8; Figure 4A). On the other hand, noradrenaline only increased formazan formation in WST assays, over the whole concentration range (n = 8; Figure 4D). In the presence of prazosin, an α1-adrenoceptor antagonist, at 30 μM, noradrenaline induced only decreased effects in both the BrdU test and the WST assay (n = 8; Figure 4B, E). In contrast, in the presence of a β-adrenoceptor antagonist, propranolol, at 10 μM, noradrenaline showed only positive effects in both assays (n = 8; Figure 4C, F). These results suggested that cell proliferation was facilitated via α1-adrenoceptors and inhibited via β-adrenoceptors.
Figure 4.
The effects of noradrenaline (NA) on cell proliferation of human osteoblasts through α1- and β-adrenoceptors. (A–C) Effects of noradrenaline on DNA synthesis measured by BrdU incorporation. (A) Dose–response relationship of the effect of noradrenaline (n = 8). (B, C) Effects of noradrenaline in the presence of the α1-blocker prazosin at 30 μM (B) and β-blocker propranolol at 10 μM (C) (n = 8). (D–F) Effects of noradrenaline on water-soluble tetrazolium salt (formazan) formation by dehydrogenase in live cells were assessed by WST assay. (D) Dose–response relationship of the effect of noradrenaline (n = 8). (E, F) Effects of noradrenaline in the presence of prazosin at 30 μM (E) and propranolol at 10 μM (F) (n = 8). Values are shown as the mean ± SEM. *P < 0.05, **P < 0.01 compared with control. Each figure is representative data from three to five independent experiments.
Signal pathways involved in the effects of noradrenaline on cell proliferation
Previously, we demonstrated that Cs-sensitive potassium channels were involved in the inhibitory effects of noradrenaline on whole-cell current (Kodama and Togari, 2010). Here, we have examined whether CsCl affected the increasing effects of noradrenaline via α1-adrenoceptors. In the presence of 1 mM CsCl and 10 μM propranolol, noradrenaline had no effect on BrdU incorporation and formazan formation (n = 8; Figure 5).
Figure 5.
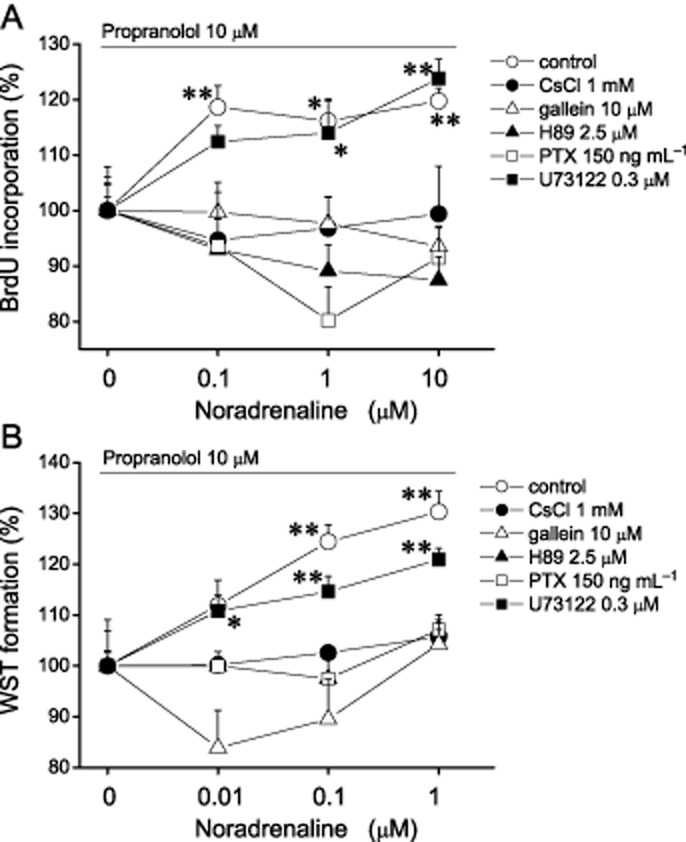
Involvement of noradrenaline-induced potassium channel inhibition on its effect on cell proliferation. The effects of noradrenaline (NA) via α1-adrenoceptor on cell proliferation were evaluated as DNA synthesis by BrdU test (A) and as dehydrogenase activity by WST assay (B) in the presence of propranolol and CsCl, gallein, H89 or U73122 (BrdU test: control, CsCl; n = 8, gallein, H89, U73122; n = 14; WST assay: n = 8). Open square indicates the data from cells pretreated with PTX for 24 h before noradrenaline application (n = 8). Values are shown as the mean ± SEM. *P < 0.05, **P < 0.01 compared with each non-NA-treated group. Each figure shows representative data from three independent experiments.
Next, to study whether signal pathways were involved in the positive effects on cell proliferation of noradrenaline, we evaluated BrdU incorporation and formazan formation in the presence of propranolol and gallein, H89, PTX or U73122. The positive effects of noradrenaline in both assays were eliminated by pretreatment with PTX for 24 h (n = 8; Figure 5). Likewise, in the presence of gallein or H89, noradrenaline had no effect in both assays (n = 8; Figure 5). In contrast, in the cells treated with U73122, the positive effect via α1-adrenoceptors was shown in both assays (n = 8; Figure 5). These results suggested that regulation of potassium current via Gi/o-coupled α1-adrenoceptors was involved in the positive effects on cell proliferation.
Discussion
Recent studies have demonstrated that a single subtype of receptor can be associated with different types of heterotrimeric G-proteins (Wenzel-Seifert and Seifert, 2000; Gazi et al., 2003; Cordeaux et al., 2004). It is also suggested that coupling with a particular type of G-protein can be regulated by the location, phosphorylation and expression of GPCRs (Davies et al., 1999; Xiang et al., 2002; Hasseldine et al., 2003). The results presented in this study suggest that α1B-adrenoceptors can be coupled to both Gq-protein and Gi/o-protein in human osteoblasts. We previously reported that mRNAs of α1B-, α2B- and β2-adrenoceptors were expressed in SaM-1 cells (Togari et al., 1997; Togari, 2002). Bath application of noradrenaline induced [Ca2+]i elevation, and the effect was inhibited by the α1B-adrenoceptor-selective inhibitor, CEC, and the PLC inhibitor, U73122 (Figure 1A, B). These results indicated that noradrenaline activated the PI–PLC pathway via Gq-protein-coupled α1B-adrenoceptors in SaM-1 cells. Meanwhile, noradrenaline-induced suppression of whole-cell current, which was inhibited by pretreatment with CEC in our previous study (Kodama and Togari, 2010), was affected by neither addition of Ca2+ chelator to internal solution nor treatment with U73122 (Figure 1D, E). In contrast, the inhibitory effect of noradrenaline on whole-cell current was significantly attenuated by pretreatment with PTX (Figure 2A). However, the α2-adrenoceptor agonist, clonidine, had no effect on the whole-cell current (Figure 3). These results indicated that noradrenaline inhibited whole-cell current via Gi/o-protein-coupled α1B-adrenoceptors. Although noradrenaline-induced [Ca2+]i elevations were detected in about half of SaM-1 cells, the inhibitory effect of noradrenaline on whole-cell current appeared in almost all cells tested. This suggested the possibility that α1B-adrenoceptors were mainly, but not all, coupled to Gi/o-proteins in human osteoblasts.
The effects of Gi/o-protein-coupled GPCRs are exerted not only through the Giα subunit but also through Gβγ subunits. Many studies reported that G-protein-gated inward rectifying potassium channels (GIRK channels) are regulated by Gβγ subunits (Filippov et al., 2004; Lüscher and Slesinger, 2010). In addition, recent studies reported that Gβγ subunits could interact with several proteins, for example, L-type calcium channels, connexin 43 and GPCR kinase (O-uchi et al., 2008; Sato et al., 2009; Casey et al., 2010). In this study, the inhibitory effect of noradrenaline on whole-cell current was significantly attenuated by pretreatment with the Gβγ-protein inhibitor, gallein (Figure 2B). The inhibitory effect of noradrenaline also tended to be attenuated following application of a PKA inhibitor, H89 (Figure 2C). In SaM-1 cells, the reversal potential of whole-cell current was considered to be less negative. This suggested the possibility that not only potassium channels but also chloride channels, contributed to the whole-cell current. However, noradrenaline-induced suppression was eliminated in the presence of CsCl, a non-selective potassium channel blocker, in our previous study (Kodama and Togari, 2010). Therefore, these results suggested that the potassium channels were inhibited by Gβγ-proteins and were partly regulated by PKA. Although the GIRK channels are a major target of Gβγ-proteins, the whole-cell current suppressed by noradrenaline did not show inward rectification. Zhou et al., (2008) demonstrated that BK channels were inhibited by Gβγ-protein and we previously reported that BK channels were expressed in SaM-1 cells (Hirukawa et al., 2008). However, BK channels are inhibited by TEA and activated by [Ca2+]i elevation (Henney et al., 2009; Lee and Cui, 2010). The inhibition of the current by noradrenaline was seen at cell potentials below −60 mV, and further inhibition was seen over 0 mV (Figure 1C). Additionally, there was no apparent effect of noradrenaline on the current kinetics. These results suggested that the channels inhibited by noradrenaline were non-inactivated, outward rectifying and open at the resting potential. Recent studies demonstrated that the two-pore domain K channel family is responsible for non-inactivated, leak or background, potassium current (Patel and Honoré, 2001)and TREK-1, a mechanosensitive member of the two-pore domain K channels, was expressed and regulated cell proliferation in human osteoblasts (Hughes et al., 2006). The current through TREK-1 is outwardly rectifying and is insensitive to classical potassium channel blockers, including TEA (Patel and Honoré, 2001). However, TREK-1 activity was inhibited by Gs-protein-coupled receptors and stimulated by Gi/o-protein-coupled receptors (Mathie, 2007). Rivard et al. (2009) reported that overexpression of α1B-adrenoceptors decreased outwards potassium current and several types of potassium channel were involved in its effects in heart. Further investigations are needed to identify the potassium channels suppressed by noradrenaline in SaM-1 cells.
A number of studies have demonstrated that membrane potential and cell proliferation capacity were regulated by activities of potassium channels in several types of cells including osteoblasts (Pardo et al., 2005; Liebau et al., 2006; Henney et al., 2009; Jang et al., 2011). Adequate inhibition of potassium channels is thought to cause membrane depolarization and activate voltage-dependent calcium channels, leading to cell proliferation (Burg et al., 2008). Additionally, activation of potassium channels plays an important role at an early stage of apoptosis (Burg et al., 2008; Valencia-Cruz et al., 2009). In the present study, noradrenaline increased DNA synthesis at a low dose and decreased it at a high dose (Figure 4A). In WST assays, noradrenaline also increased formazan formation in a dose-dependent manner (Figure 4D). While the results of the BrdU tests indicated DNA synthesis during 20–24 h after treatment with noradrenaline, those from the WST assays indicated cumulative effects on cell proliferation. These differences might account for the divergent results of the BrdU test and the WST assay, at high concentrations of noradrenaline. In the BrdU test, the positive and negative effects of noradrenaline were inhibited by prazosin and propranolol respectively (Figure 4B, C). Additionally, in the WST assay, positive effects of noradrenaline were inhibited by prazosin but not by propranolol (Figure 4E, F). These results suggested that cell proliferation in human osteoblasts is bi-directionally regulated by noradrenaline via α1- and β-adrenoceptors. This is consistent with a previous study in human osteoblasts (Huang et al., 2009). A number of studies have demonstrated involvement of β2-adrenoceptors in bone metabolism. In general, the β2-adrenoceptor is coupled with Gs-protein and is thought to activate the cAMP/PKA pathway and Fu et al., (2005) demonstrated that β2-adrenoceptors regulated osteoblast proliferation via the cAMP/PKA/CREB signalling pathway in vivo.
As observed with whole-cell current, the positive effects of noradrenaline via α1-adrenoceptors, shown in BrdU tests and WST assays, was inhibited in the presence of CsCl (Figure 5). This suggested that noradrenaline enhanced cell proliferation by blocking Cs-sensitive potassium channels. Additionally, the positive effects of noradrenaline via α1-adrenoceptors on cell proliferation in both assays were suppressed by inhibition of Gi/o-protein-coupled receptors by PTX, Gβγ-protein by gallein or the cAMP/PKA pathway by H89, but not by inhibition of the PI–PLC pathway by U73122 (Figure 5). These results suggested the possibility that noradrenaline-induced suppressions of potassium channels via Gβγ-protein and the cAMP–PKA pathway were involved in its effect on cell proliferation mediated by α1B-adrenoceptors. Suzuki et al. (1998; 2001) reported that adrenaline stimulated cell proliferation and alkaline phosphatase activity and increased type III Pi transporter expression via α1-adrenoceptors, and these effects were inhibited by PTX in MC3T3-E1 osteoblast-like cells. Activation of α1-adrenoceptors induced the expression of RANKL, and this effect was inhibited by a PKC inhibitor (Nishiura and Abe, 2007). Therefore, Gq- and Gi/o-coupled α1-adrenoceptors are thought to play different roles in osteoblasts.
Both experimental and clinical studies have demonstrated that β-adrenoceptor blockade is effective against osteoporosis accompanied by a highly active sympathetic nervous system (Pasco et al., 2004; Bonnet et al., 2007; 2008; Sato et al., 2010; Yang et al., 2011). There is also a report that α1-adrenoceptor blockade increased the risk of hip/femur fracture (Souverein et al., 2003). Phenylephrine, an α1-adrenoceptor agonist, promotes osteogenesis in bone fracture healing (McDonald et al., 2011). All these reports suggest the possibility that activation of α1-adrenoceptors could lead to a treatment for osteoporosis. The positive effects via α1B-adrenoceptors on osteoblast proliferation might contribute to bone fracture healing and treatment of osteoporosis. However, neither knockout nor overexpression of α1B-adrenoceptors has been reported to result in obvious skeletal phenotypes (Battaglia et al., 2003; Rivard et al., 2009; Docherty, 2010). Detailed analysis of bone metabolism in a pathophysiological state showing high sympathetic tone, for example, in leptin-injected mice, ovariectomized mice or spontaneous hypertensive rats, is required to investigate the physiological role of α1B-adrenoceptor (Elefteriou et al., 2005; Sato et al., 2010).
In conclusion, the present study suggested that noradrenaline suppressed potassium channels via Gi/o-coupled α1B-adrenoceptors and that the Gβγ-proteins play an important role in this effect. Additionally, this effect was involved in the noradrenaline-induced increase in proliferation of human osteoblasts, SaM-1 cells. These results will aid understanding of the mechanism behind the regulation of bone metabolism by the sympathetic nervous system.
Acknowledgments
This work was partly supported by a grant-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 21890276 to D.K.) and by a Grant-in-Aid from Strategic Research AGU-Platform Formation (2008-2012).
Glossary
- α-MEM
α-modified minimum essential medium
- BK channel
large-conductance calcium-activated potassium channel
- BrdU
5-bromo-2′-deoxyuridine
- CCK-8
cell counting kit-8
- CEC
chloroethylclonidine
- CREB
cAMP response element binding protein
- ERG channel
ether-a-go-go-related gene channel
- GIRK
G-protein-gated inward rectifying potassium channel
- MC3T3-E1
mouse calvaria-derived osteoblastic cells
- PDL
population doubling level
- PI–PLC
phosphoinositide-PLC
- PTX
Pertussis toxin
- RANKL
receptor activator of NF-κB ligand
- SaM-1
human periosteum-derived osteoblastic cells
- TEA
tetraethylammonium
- TREK-1
TWIK-related potassium channel 1
- WST
water-soluble tetrazolium
Conflicts of interest
All authors have no conflicts of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai M, Nagasawa T, Koshihara Y, Yamamoto S, Togari A. Effects of beta-adrenergic agonists on bone-resorbing activity in human osteoclast-like cells. Biochim Biophys Acta. 2003;1640:137–142. doi: 10.1016/s0167-4889(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Battaglia G, Fornai F, Busceti CL, Lembo G, Nicoletti F, De Blasi A. Alpha-1B adrenergic receptor knockout mice are protected against methamphetamine toxicity. J Neurochem. 2003;86:413–421. doi: 10.1046/j.1471-4159.2003.01867.x. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Gadois C, McCloskey E, Lemineur G, Lespessailles E, Courteix D, et al. Protective effect of beta blockers in postmenopausal women: influence on fractures, bone density, micro and macroarchitecture. Bone. 2007;40:1209–1216. doi: 10.1016/j.bone.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Bonnet N, Benhamou CL, Malaval L, Goncalves C, Vico L, Eder V, et al. Low dose beta-blocker prevents ovariectomy-induced bone loss in rats without affecting heart functions. J Cell Physiol. 2008;217:819–827. doi: 10.1002/jcp.21564. [DOI] [PubMed] [Google Scholar]
- Burg ED, Remillard CV, Yuan JX. Potassium channels in the regulation of pulmonary artery smooth muscle cell proliferation and apoptosis: pharmacotherapeutic implications. Br J Pharmacol. 2008;153(Suppl. 1):S99–S111. doi: 10.1038/sj.bjp.0707635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, et al. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res. 2010;107:532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherruau M, Facchinetti P, Baroukh B, Saffar JL. Chemical sympathectomy impairs bone resorption in rats: a role for the sympathetic system on bone metabolism. Bone. 1999;25:545–551. doi: 10.1016/s8756-3282(99)00211-2. [DOI] [PubMed] [Google Scholar]
- Cordeaux Y, Ijzerman AP, Hill SJ. Coupling of the human A1 adenosine receptor to different heterotrimeric G proteins: evidence for agonist-specific G protein activation. Br J Pharmacol. 2004;143:705–714. doi: 10.1038/sj.bjp.0705925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MG, Huynh TT, Hagen PO. Functional characterization of alpha1-adrenergic receptors in experimental vein grafts. J Surg Res. 1999;84:40–45. doi: 10.1006/jsre.1999.5600. [DOI] [PubMed] [Google Scholar]
- Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010;67:405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature. 2005;434:514–520. doi: 10.1038/nature03398. [DOI] [PubMed] [Google Scholar]
- Filippov AK, Fernández-Fernández JM, Marsh SJ, Simon J, Barnard EA, Brown DA. Activation and inhibition of neuronal G protein-gated inwardly rectifying K(+) channels by P2Y nucleotide receptors. Mol Pharmacol. 2004;66:468–477. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. The molecular clock mediates leptin-regulated bone formation. Cell. 2005;122:803–815. doi: 10.1016/j.cell.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Gazi L, Nickolls SA, Strange PG. Functional coupling of the human dopamine D2 receptor with G alpha i1, G alpha i2, G alpha i3 and G alpha o G proteins: evidence for agonist regulation of G protein selectivity. Br J Pharmacol. 2003;138:775–786. doi: 10.1038/sj.bjp.0705116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasseldine AR, Harper EA, Black JW. Cardiac-specific overexpression of human beta2 adrenoceptors in mice exposes coupling to both Gs and Gi proteins. Br J Pharmacol. 2003;138:1358–1366. doi: 10.1038/sj.bjp.0705191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JY, Jiang LS, Dai LY. The roles of the sympathetic nervous system in osteoporotic diseases: a review of experimental and clinical studies. Ageing Res Rev. 2011;10:253–263. doi: 10.1016/j.arr.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Henney NC, Li B, Elford C, Reviriego P, Campbell AK, Wann KT, et al. A large-conductance (BK) potassium channel subtype affects both growth and mineralization of human osteoblasts. Am J Physiol Cell Physiol. 2009;297:C1397–C1408. doi: 10.1152/ajpcell.00311.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L, Park KH, Cai SQ, Qin L, Partridge N, Sesti F. The antiproliferative role of ERG K+ channels in rat osteoblastic cells. Cell Biochem Biophys. 2007;47:199–208. doi: 10.1007/s12013-007-0006-9. [DOI] [PubMed] [Google Scholar]
- Hirukawa K, Muraki K, Ohya S, Imaizumi Y, Togari A. Electrophysiological properties of a novel Ca(2+)-activated K(+) channel expressed in human osteoblasts. Calcif Tissue Int. 2008;83:222–229. doi: 10.1007/s00223-008-9167-9. [DOI] [PubMed] [Google Scholar]
- Huang HH, Brennan TC, Muir MM, Mason RS. Functional alpha1- and beta2-adrenergic receptors in human osteoblasts. J Cell Physiol. 2009;220:267–275. doi: 10.1002/jcp.21761. [DOI] [PubMed] [Google Scholar]
- Hughes S, Magnay J, Foreman M, Publicover SJ, Dobson JP, El Haj AJ. Expression of the mechanosensitive 2PK+ channel TREK-1 in human osteoblasts. J Cell Physiol. 2006;206:738–748. doi: 10.1002/jcp.20536. [DOI] [PubMed] [Google Scholar]
- Inoue R, Okada T, Onoue H, Hara Y, Shimizu S, Naitoh S, et al. The transient receptor potential protein homologue TRP6 is the essential component of vascular alpha(1)-adrenoceptor-activated Ca(2+)-permeable cation channel. Circ Res. 2001;88:325–332. doi: 10.1161/01.res.88.3.325. [DOI] [PubMed] [Google Scholar]
- Jang SS, Park J, Hur SW, Hong YH, Hur J, Chae JH, et al. Endothelial progenitor cells functionally express inward rectifier potassium channels. Am J Physiol Cell Physiol. 2011;301:C150–C161. doi: 10.1152/ajpcell.00002.2010. [DOI] [PubMed] [Google Scholar]
- Katz A, Wu D, Simon MI. Subunits beta gamma of heterotrimeric G protein activate beta 2 isoform of phospholipase C. Nature. 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- Kim Y, Park MK, Uhm DY, Shin J, Chung S. Modulation of delayed rectifier potassium channels by alpha1-adrenergic activation via protein kinase C zeta and p62 in PC12 cells. Neurosci Lett. 2005;387:43–48. doi: 10.1016/j.neulet.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Kodama D, Togari A. Modulation of potassium channels via the α1B-adrenergic receptor in human osteoblasts. Neurosci Lett. 2010;485:102–106. doi: 10.1016/j.neulet.2010.08.073. [DOI] [PubMed] [Google Scholar]
- Komoto S, Kondo H, Fukuta O, Togari A. Comparison of β-adrenergic and glucocorticoid signaling on clock gene and osteoblast-related gene expressions in human osteoblast. Chronobiol Int. 2012;29:66–74. doi: 10.3109/07420528.2011.636496. [DOI] [PubMed] [Google Scholar]
- Kondo H, Togari A. Continuous treatment with a low-dose β-agonist reduces bone mass by increasing bone resorption without suppressing bone formation. Calcif Tissue Int. 2011;88:23–32. doi: 10.1007/s00223-010-9421-9. [DOI] [PubMed] [Google Scholar]
- Koshihara Y, Kawamura M, Oda H, Higaki S. In vitro calcification in human osteoblastic cell line derived from periosteum. Biochem Biophys Res Commun. 1987;145:651–657. doi: 10.1016/0006-291x(87)91014-x. [DOI] [PubMed] [Google Scholar]
- Lee US, Cui J. BK channel activation: structural and functional insights. Trends Neurosci. 2010;33:415–423. doi: 10.1016/j.tins.2010.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebau S, Pröpper C, Böckers T, Lehmann-Horn F, Storch A, Grissmer S, et al. Selective blockage of Kv1.3 and Kv3.1 channels increases neural progenitor cell proliferation. J Neurochem. 2006;99:426–437. doi: 10.1111/j.1471-4159.2006.03967.x. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Slesinger PA. Emerging roles for G protein-gated inwardly rectifying potassium (GIRK) channels in health and disease. Nat Rev Neurosci. 2010;11:301–315. doi: 10.1038/nrn2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SJ, Dooley PC, McDonald AC, Djouma E, Schuijers JA, Ward AR, et al. α(1) adrenergic receptor agonist, phenylephrine, actively contracts early rat rib fracture callus ex vivo. J Orthop Res. 2011;29:740–745. doi: 10.1002/jor.21302. [DOI] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, et al. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–499. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- Mathie A. Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol. 2007;578:377–385. doi: 10.1113/jphysiol.2006.121582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiura T, Abe K. Alpha1-adrenergic receptor stimulation induces the expression of receptor activator of nuclear factor kappaB ligand gene via protein kinase C and extracellular signal-regulated kinase pathways in MC3T3-E1 osteoblast-like cells. Arch Oral Biol. 2007;52:778–785. doi: 10.1016/j.archoralbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- O-Uchi J, Sasaki H, Morimoto S, Kusakari Y, Shinji H, Obata T, et al. Interaction of alpha1-adrenoceptor subtypes with different G proteins induces opposite effects on cardiac L-type Ca2+ channel. Circ Res. 2008;102:1378–1388. doi: 10.1161/CIRCRESAHA.107.167734. [DOI] [PubMed] [Google Scholar]
- Pardo LA, Contreras-Jurado C, Zientkowska M, Alves F, Stühmer W. Role of voltage-gated potassium channels in cancer. J Membr Biol. 2005;205:115–124. doi: 10.1007/s00232-005-0776-1. [DOI] [PubMed] [Google Scholar]
- Pasco JA, Henry MJ, Sanders KM, Kotowicz MA, Seeman E, Nicholson GC. Beta-adrenergic blockers reduce the risk of fracture partly by increasing bone mineral density: Geelong Osteoporosis Study. J Bone Miner Res. 2004;19:19–24. doi: 10.1359/JBMR.0301214. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Rivard K, Trépanier-Boulay V, Rindt H, Fiset C. Electrical remodeling in a transgenic mouse model of alpha1B-adrenergic receptor overexpression. Am J Physiol Heart Circ Physiol. 2009;296:H704–H718. doi: 10.1152/ajpheart.00337.2008. [DOI] [PubMed] [Google Scholar]
- Sato M, Jiao Q, Honda T, Kurotani R, Toyota E, Okumura S, et al. Activator of G protein signaling 8 (AGS8) is required for hypoxia-induced apoptosis of cardiomyocytes: role of G betagamma and connexin 43 (CX43) J Biol Chem. 2009;284:31431–31440. doi: 10.1074/jbc.M109.014068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Arai M, Goto S, Togari A. Effects of propranolol on bone metabolism in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2010;334:99–105. doi: 10.1124/jpet.110.167643. [DOI] [PubMed] [Google Scholar]
- Souverein PC, Van Staa TP, Egberts AC, De la Rosette JJ, Cooper C, Leufkens HG. Use of alpha-blockers and the risk of hip/femur fractures. J Intern Med. 2003;254:548–554. doi: 10.1111/j.1365-2796.2003.01227.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Palmer G, Bonjour JP, Caverzasio J. Catecholamines stimulate the proliferation and alkaline phosphatase activity of MC3T3-E1 osteoblast-like cells. Bone. 1998;23:197–203. doi: 10.1016/s8756-3282(98)00099-4. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Palmer G, Bonjour JP, Caverzasio J. Stimulation of sodium-dependent inorganic phosphate transport by activation of Gi/o-protein-coupled receptors by epinephrine in MC3T3-E1 osteoblast-like cells. Bone. 2001;28:589–594. doi: 10.1016/s8756-3282(01)00459-8. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Tsuboi T, Arai M, Togari A. Adrenergic stimulation of osteoclastogenesis mediated by expression of osteoclast differentiation factor in MC3T3-E1 osteoblast-like cells. Biochem Pharmacol. 2001;61:579–586. doi: 10.1016/s0006-2952(00)00591-8. [DOI] [PubMed] [Google Scholar]
- Togari A. Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech. 2002;58:77–84. doi: 10.1002/jemt.10121. [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M. Pharmacological topics of bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci. 2008;106:542–546. doi: 10.1254/jphs.fm0070227. [DOI] [PubMed] [Google Scholar]
- Togari A, Arai M, Mizutani S, Mizutani S, Koshihara Y, Nagatsu T. Expression of mRNAs for neuropeptide receptors and beta-adrenergic receptors in human osteoblasts and human osteogenic sarcoma cells. Neurosci Lett. 1997;233:125–128. doi: 10.1016/s0304-3940(97)00649-6. [DOI] [PubMed] [Google Scholar]
- Valencia-Cruz G, Shabala L, Delgado-Enciso I, Shabala S, Bonales-Alatorre E, Pottosin II, et al. Kbg and Kv1.3 channels mediate potassium efflux in the early phase of apoptosis in Jurkat T lymphocytes. Am J Physiol Cell Physiol. 2009;297:C1544–C1533. doi: 10.1152/ajpcell.00064.2009. [DOI] [PubMed] [Google Scholar]
- Wenzel-Seifert K, Seifert R. Molecular analysis of beta(2)-adrenoceptor coupling to G(s)-, G(i)-, and G(q)-proteins. Mol Pharmacol. 2000;58:954–966. doi: 10.1124/mol.58.5.954. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Rybin VO, Steinberg SF, Kobilka B. Caveolar localization dictates physiologic signaling of beta 2-adrenoceptors in neonatal cardiac myocytes. J Biol Chem. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- Yang S, Nguyen ND, Center JR, Jr, Eisman JA, Nguyen TV. Association between beta-blocker use and fracture risk: the Dubbo Osteoporosis Epidemiology Study. Bone. 2011;48:451–455. doi: 10.1016/j.bone.2010.10.170. [DOI] [PubMed] [Google Scholar]
- Zhou XB, Wulfsen I, Lutz S, Utku E, Sausbier U, Ruth P, et al. M2 muscarinic receptors induce airway smooth muscle activation via a dual, Gbetagamma-mediated inhibition of large conductance Ca2+-activated K+ channel activity. J Biol Chem. 2008;283:21036–21044. doi: 10.1074/jbc.M800447200. [DOI] [PMC free article] [PubMed] [Google Scholar]