Abstract
Background and Purpose
Isoform-selective inhibitors of NOS enzymes are desirable as research tools and for potential therapeutic purposes. Vinyl-l-N-5-(1-imino-3-butenyl)-l-ornithine (l-VNIO) and Nω-propyl-l-arginine (NPA) purportedly have good selectivity for neuronal over endothelial NOS under cell-free conditions, as does N-[(3-aminomethyl)benzyl]acetamidine (1400W), which is primarily an inducible NOS inhibitor. Although used in numerous investigations in vitro and in vivo, there have been surprisingly few tests of the potency and selectivity of these compounds in cells. This study addresses this deficiency and evaluates the activity of new and potentially better pyrrolidine-based compounds.
Experimental Approach
The inhibitors were evaluated by measuring their effect on NMDA-evoked cGMP accumulation in rodent hippocampal slices, a response dependent on neuronal NOS, and ACh-evoked cGMP synthesis in aortic rings of the same animals, an endothelial NOS-dependent phenomenon.
Key Results
l-VNIO, NPA and 1400W inhibited responses in both tissues but all showed less than fivefold higher potency in the hippocampus than in the aorta, implying useless selectivity for neuronal over endothelial NOS at the tissue level. In addition, the inhibitors had a 25-fold lower potency in the hippocampus than reported previously, the IC50 values being approximately 1 μM for l-VNIO and NPA, and 150 μM for 1400W. Pyrrolidine-based inhibitors were similarly weak and nonselective.
Conclusion and Implications
The results suggest that l-VNIO, NPA and 1400W, as well as the newer pyrrolidine-type inhibitors, cannot be used as neuronal NOS inhibitors in cells without stringent verification. The identification of inhibitors with useable selectivity in cells and tissues remains an important goal.
Keywords: NOS, l-VNIO, N-propyl-l-arginine, 1400W, hippocampus, aorta, cerebellum, cGMP
Introduction
NO is an intercellular transmitter of widespread importance to mammalian physiology. NO signals are predominantly generated by NOS enzymes and are transduced by cGMP following activation of NO-targeted guanylyl cyclase (Garthwaite, 2008; Friebe and Koesling, 2009), also still known by its homogenate-based name, soluble guanylyl cyclase. Three isoforms of NOS have been identified, namely the neuronal (nNOS), endothelial (eNOS) and inducible (iNOS) subtypes. All three catalyse the mono-oxygenation of l-arginine to NO and share a common general structure (Alderton et al., 2001), although variations in their regulation and cellular distribution are thought to yield a diversity of NO signals with different, and sometimes opposing, physiological and/or pathological consequences. In the brain, for example, eNOS and nNOS may contribute to hippocampal synaptic plasticity in a temporally distinct, but complementary, manner (Hopper and Garthwaite, 2006); whereas, under ischaemic conditions, eNOS and nNOS appear to have opposing beneficial and detrimental effects respectively (Huang et al., 1994; 1996). Accordingly, isoform-selective NOS inhibitors are highly desirable, both as tools in NO research and for the potential treatment of pathologies deemed to involve increased NO concentrations (Hobbs et al., 1999; Vallance and Leiper, 2002).
Under physiological conditions, and in the absence of immune challenge, eNOS and nNOS predominate (MacMicking et al., 1997). Selective inhibitors for eNOS have yet to be found, although several apparently potent and selective nNOS inhibitors have been identified using cell-free assays. Amongst them, three commonly used compounds are vinyl-l-N-5-(1-imino-3-butenyl)-l-ornithine (l-VNIO; KI for isolated rat nNOS = 0.1 μM, bovine eNOS = 12 μM and mouse iNOS = 60 μM; Babu and Griffith, 1998b), Nω-propyl-l-arginine (NPA; KI for isolated bovine nNOS = 0.06 μM, bovine eNOS = 8.5 μM and murine iNOS = 180 μM; Zhang et al., 1997) and N-[(3-aminomethyl)benzyl]acetamidine (1400W), which is primarily used as a iNOS inhibitor but which has moderate selectivity for nNOS over eNOS (KI for human nNOS = 2 μM and human eNOS = 50 μM; Garvey et al., 1997). In tissues, nNOS-selective concentrations of l-VNIO (0.1 μM), NPA (1 μM) and 1400W (1 μM) have been defined by their ability to inhibit NMDA-induced cGMP accumulation in rodent hippocampal slices, a presumed nNOS-dependent response (Huang et al., 1993), but leave intact ACh-evoked cGMP synthesis in aortic rings (Hopper and Garthwaite, 2006), a response assumed to be eNOS-dependent (Furchgott and Zawadzki, 1980; Huang et al., 1995). The compounds have now been used to selectively inhibit nNOS in over 40 studies in vitro and in vivo (see Discussion for examples). There is, however, a surprising lack of tests of the selectivity and potency of the compounds in intact cells or tissues.
The present study was prompted by the observation (Pigott, 2012) that a form of hippocampal synaptic plasticity could be inhibited by a broad-spectrum NOS inhibitor but was sustained in eNOS knockout mice and in the presence of a concentration of l-VNIO (0.1 μM) previously found to inhibit the NMDA-evoked cGMP response in hippocampal slices (Hopper and Garthwaite, 2006). To provide a positive control for the activity of l-VNIO, tests of its potency against NMDA-stimulated cGMP accumulation in hippocampal slices were repeated. In the light of the findings with this compound, the potency and selectivity of NPA, 1400W and potentially superior, pyrrolidine-based inhibitors (called here FX-5043 and JK-5; Xue et al., 2010a) were also evaluated. In disagreement with previous results (Hopper and Garthwaite, 2006), we found that l-VNIO, NPA and 1400W are inadequately (<5-fold) selective for nNOS over eNOS in intact tissues to be of use diagnostically. FX-5043 and JK-5 were also found to be unsuitable for use as nNOS inhibitors at the tissue level.
Methods
The drug/molecular target nomenclature used in this manuscript conforms to British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2011).
Animals
All work was compliant with British Home Office regulations on laboratory animal use and welfare, and every effort to reduce the number of animals used was made. Methods and results have been reported in accordance with ARRIVE guidelines (McGrath et al., ). Experiments were conducted using a total of 18, 6- to 8-week-old, male C57Bl/6 mice and 28, 9- to 10-day-old, male Sprague–Dawley rats (both from Charles River, Kent, UK). Six male, 6- to 14-week-old, mice lacking functional eNOS (eNOS−/−) were kindly provided by Dr Adrian Hobbs (University College London, London, UK; see Huang et al., 1995) and Dr Michael Emerson (Imperial College London, London, UK; strain 002684 from Jackson Laboratory, ME, USA). Five age-, sex- and strain-matched wild-type control mice were obtained from Harlan (Cambridgeshire, UK) or Charles River. Genotyping was done using the PCR and agarose gel electrophoresis. All animals were housed in cages with soft bedding in a 12 h light/dark cycle with food and water available ad libitum.
Chemicals
ACh chloride, l-arginine hydrochloride, erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA), IBMX and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) were purchased from Sigma-Aldrich (Dorset, UK). NMDA and l-nitroarginine (l-NNA) were obtained from Tocris Cookson (Bristol, UK). N-[(3-Aminomethyl)benzyl]acetamidine dihydrochloride (1400W), 2-[(3,4-dimethoxyphenyl)methyl]-7-[(1R)-1-hydroxyethyl]-4- phenylbutyl]-5-methyl-imidazo[5,1-f][1,2,4]triazin-4(1H)-one (BAY 60-7550) and Nω-propyl-l-arginine (NPA) were from Cayman Chemical (Ann Arbor, MI) and 2-(N,N-diethylamino)-diazenolate-2-oxide diethylammonium salt (DEA/NO) was from Enzo Life Sciences (Exeter, UK). Vinyl-l-N-5-(1-imino-3-butenyl)-l-ornithine hydrochloride (l-VNIO) was purchased from Axxora or Cambridge Bioscience (Cambridge, UK). 6-{[(3R,4R)-4-(2-{[2,2-Difluoro-2-(3-fluorophenyl)ethyl]amino}ethoxy)pyrrolidin-3-yl]methyl}-4-methylpyridin-2-amine trihydrochloride (FX-5043) and 6-{[(3R,4R)-4-(2-{[2-(3-chloro-5-fluorophenyl)-2,2-difluoroethyl]amino}ethoxy)pyrrolidin-3-yl]methyl}-4-methylpyridin-2-amine trihydrochloride (JK-5) were kind gifts from Prof Richard Silverman (Northwestern University, IL). Stock solutions were made in either dimethyl sulphoxide (BAY 60–7550, FX-5043, IBMX, JK-5, l-VNIO, NPA, ODQ), equimolar HCl (l-NNA), equimolar NaOH (NMDA), 10 mM NaOH (DEA/NO) or H2O (ACh, l-arginine, EHNA, 1400W). Except in experiments in which controls for the vehicle were included; the concentrations of stock solutions were at least 100-fold higher than the final concentrations.
Tissue preparation
Animals were killed by cervical dislocation and decapitation. For experiments using mouse hippocampal slices, the brains were removed, and hippocampi were dissected out into ice-cold artificial CSF (aCSF) containing (in mM) 120 NaCl, 2.5 KCl, 1.3 MgCl2, 1 NaH2PO4, 26 NaHCO3, 10 d-glucose and 2 CaCl2, equilibrated with 95% O2/5% CO2 to pH 7.4 (at 37°C). Transverse slices (400 μm-thick) were cut from the middle of the hippocampi using a vibratome (Series 100 Sectioning System, Technical Products International, MO) and then allowed to recover for 1–2 h at room temperature on a nylon net submerged in aCSF, which was continuously bubbled with 95% O2/5% CO2.
For all other experiments, the hippocampi, cerebellum or aorta were dissected out into aCSF comprising (in mM) 120 NaCl, 2 KCl, 1.19 MgSO4, 26 NaHCO3, 1.18 KH2PO4, 11 d-glucose and 2 CaCl2 equilibrated with 95% O2/5% CO2 to pH 7.4 (at 37°C). Transverse, hippocampal or sagittal, cerebellar slices (both 400 μm thick) were cut with a tissue chopper (McIlwain, Campden Instruments, Loughborough, UK) and thoracic aortic rings (2–3 mm-thick; free from excess blood and connective tissues) using a razor blade. Tissues were then recovered for 1–2 h in flasks of aCSF held in a shaking water bath (37°C) and continuously perfused with 95% O2/5% CO2.
cGMP measurement
Methods were adapted from Hopper and Garthwaite (2006). Brain slices or aortic rings were randomly distributed to flasks of oxygenated aCSF held in a shaking water bath. For mouse hippocampal slices, the temperature was 30°C, set to match the conditions used for electrophysiology and the experiments of Hopper and Garthwaite, whereas 37°C was used for all other tissues. nNOS-dependent cGMP production was stimulated in brain slices by exposure to NMDA at a concentration and for a duration (100 μM, 2 min) shown previously to be maximal for the cGMP response in rat hippocampal slices (Hopper and Garthwaite, 2006). For experiments with aortic rings, ACh (10 μM, 1 min) was used to stimulate eNOS and the NO donor, DEA/NO (100 μM, 5 min), to directly activate the NO-targeted guanylyl cyclase. In order to improve the cGMP signal-to-noise ratio, the rings were pre-incubated with the non-selective phosphodiesterase inhibitor, IBMX and hippocampal slices with one of two inhibitors of PDE-2 (BAY 60–7550, or EHNA), the enzyme primarily responsible for cGMP hydrolysis in the hippocampus (Van Staveren et al., 2001; Van Staveren et al., 2003). PDE inhibition was not necessary to resolve the very large cGMP response observed in cerebellar slices (Garthwaite and Balazs, 1978). Concentrations of all drugs are given in the figure legends; unless otherwise stated, tissues were pre-incubated with inhibitors for 15 min.
Within 30 s of the end of stimulation, tissues were individually inactivated by submersion in 200 μL boiling buffer containing 50 mM Tris–HCl and 4 mM EDTA (pH 7.4 at room temperature). cGMP was measured by radioimmunoassay and normalized to the total tissue protein measured using a bicinchoninic acid protein assay kit (Perbio Science, Northumberland, UK). Unstimulated cGMP levels and the effect of the non-selective NOS inhibitor l-NNA (100 μM) on the stimulated cGMP response were measured in every experiment.
Chemical analysis of nNOS inhibitors
High-performance liquid chromatography-mass spectrometry was performed by Dr Matthew Gooding (University College London, London, UK) using a 6100 Series LC-Mass Spectrometer and a C-18 column (Agilent Technologies, Cheshire, UK). The mobile phase comprised a methanol to water gradient and 0.1% formic acid (which protonated the sample compounds).
Statistics
Data were obtained from tissues from at least three different animals and are reported as mean values ± SEM. Statistical significance was assessed using analysis of variance (anova) with an appropriate post hoc test, or a two-tailed t-test. Significance was concluded when P < 0.05. IC50 and Hill slope values (±SEM) were calculated from logistic fits. In some instances, successful fitting required the Hill slope to be set equal to 1 (see Table 1); adjusted R2 statistics of the fits were 0.880–0.995.
Table 1.
Potency and selectivity of alleged nNOS inhibitors
| Inhibitor | Species | Hippocampus (nNOS) | Aorta (eNOS) | IC50 ratio (aorta/hippe) | Reported KI ratio (e/nNOS)a | ||
|---|---|---|---|---|---|---|---|
| IC50 (μM) | Hill slope | IC50 (μM) | Hill slope | ||||
| l-VNIO | Mouse | 1.2 ± 0.6 | 1b | 1.9 ± 0.4d | 1b | 1.6 | 120 |
| l-VNIO | Rat | 0.27 ± 0.04 | 1.1 ± 0.1 | 0.9 ± 0.1c | 1.0 ± 0.2 | 3.3 | |
| 1400W | Rat | 154 ± 78 | 0.7 ± 0.4 | 744 ± 52c | 0.8 ± 0.1 | 4.8 | 25 |
| NPA | Rat | 1.3 ± 0.5 | 0.9 ± 0.2 | 4.3 ± 2.2 | 1b | 3.3 | 149 |
Data taken from the original description of each compound (Garvey et al., 1997; Zhang et al., 1997; Babu and Griffith, 1998b) using cell-free assays.
Value fixed to enable fitting.
Unpaired t-test, P < 0.001 compared to the IC50 measured using the same compound in rat hippocampus.
Unpaired t-test, P < 0.05 (P = 0.04) compared to the IC50 for l-VNIO in rat aorta.
hipp = hippocampus.
Results
l-VNIO, 1400W and NPA
In accordance with previous reports (East and Garthwaite, 1991; Hopper and Garthwaite, 2006), NMDA (100 μM) evoked a significant accumulation of cGMP in adult mouse hippocampal slices incubated with the PDE-2 inhibitor BAY 60–7550 (1 μM). The response was blocked by the non-selective NOS inhibitor l-NNA (100 μM), or the inhibitor of NO-targeted guanylyl cyclase ODQ (10 μM). The basal/unstimulated and NMDA-evoked cGMP levels were not significantly different between slices from eNOS−/− and matched wild-type mice (Figure 1). Considering this result, together with findings that hippocampal slices prepared from healthy animals lack iNOS (Hopper and Garthwaite, 2006), and that >90% of hippocampal, Ca2+-induced NOS activity is abolished in mice lacking the major nNOS splice variant (Huang et al., 1993), it was concluded that the NMDA-evoked cGMP response was nNOS-dependent. Unexpectedly, pretreatment of slices with l-VNIO (0.1 μM) or a second NOS inhibitor, 1400W (1 μM), each at a concentration shown previously to inhibit NMDA-evoked cGMP accumulation in adult rat hippocampal slices by 80–90% (Hopper and Garthwaite, 2006), had no effect on the magnitude of the NMDA-evoked cGMP response (Figure 2A).
Figure 1.
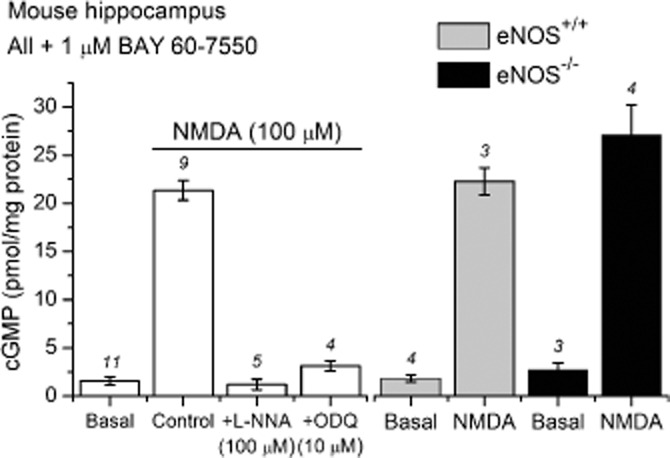
nNOS-dependent, NMDA-induced cGMP accumulation in adult mouse hippocampal slices. Slices were pre-incubated with the PDE-2 inhibitor BAY 60–7550 (1 μM) for 10 min. NMDA (100 μM, 2 min) induced a significant increase in cGMP from basal/unstimulated levels (anova with Tukey–Kramer test, P < 0.001). This increase was prevented by the non-selective NOS antagonist, l-NNA (100 μM), or the NO-targeted guanylyl cyclase inhibitor, ODQ (10 μM; anova with Tukey–Kramer test, P > 0.05 compared with basal). Basal and NMDA-induced cGMP levels did not differ significantly between slices from eNOS-deficient (eNOS−/−) and matched wild-type (eNOS+/+) mice (unpaired t-tests, eNOS−/− vs. eNOS+/+, P > 0.05). Numbers above error bars are n-values.
Figure 2.
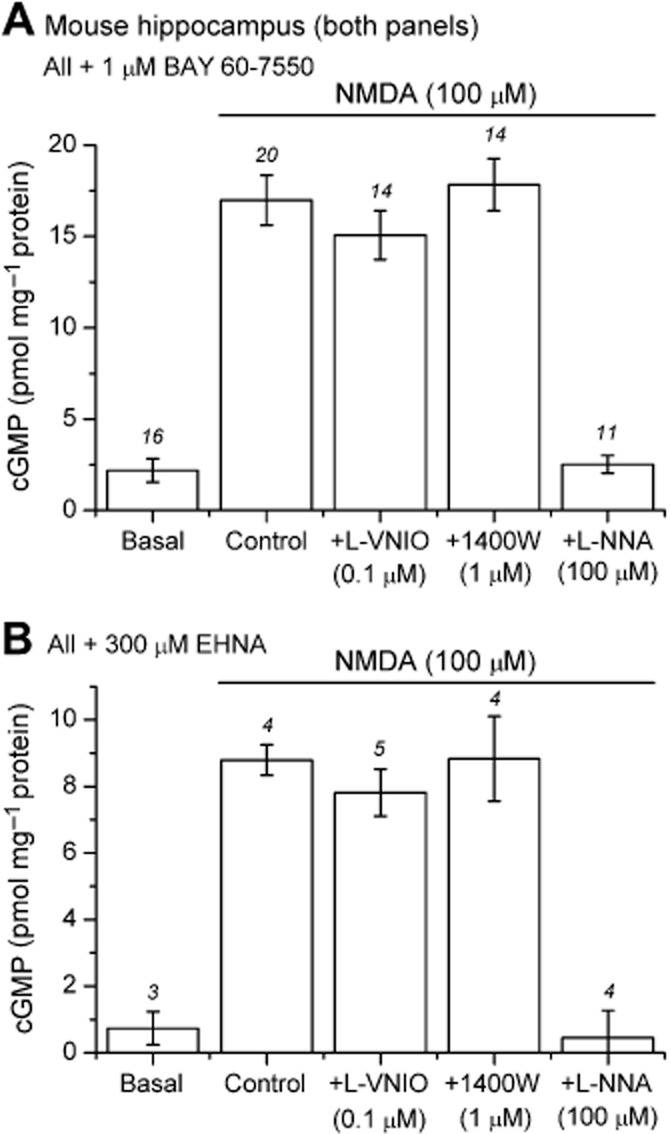
Effect of 0.1 μM l-VNIO and 1 μM 1400W on NMDA-evoked cGMP accumulation in adult mouse hippocampus. (A) Hippocampal slices were pretreated with BAY 60–7550 (1 μM) for 10 min. NMDA (100 μM, 2 min) induced a significant increase in cGMP from basal levels (P < 0.001). Concentrations of l-VNIO (0.1 μM) or 1400W (1 μM) that have been previously reported to reduce the NMDA-evoked response in hippocampal slices by 80–90% had no significant effect (P > 0.05 compared with control), although the response was abolished by l-NNA (100 μM; P > 0.05 compared with basal). The data have been compiled from two separate experiments, each using different batches of l-VNIO and 1400W. Analysed separately, neither batch of l-VNIO or 1400W had any significant effect on the NMDA-evoked cGMP response (P > 0.05 compared with control). (B) l-VNIO (0.1 μM) and 1400W (1 μM) were also without effect on the NMDA-evoked cGMP response when EHNA (300 μM, 10 min) was used in place of BAY 60–7550 (P > 0.05 compared with control). As in panel A, NMDA generated a significant accumulation of cGMP compared with basal (P < 0.001) that was blocked by l-NNA (P > 0.05 compared with basal). Statistics are anova with Tukey–Kramer test. Numbers above error bars are n-values.
The only obvious methodological difference between the present study and that of Hopper and Garthwaite (2006) was the PDE-2 inhibitor used to resolve the cGMP signal. To test whether this difference could account for the variation in l-VNIO potency, we substituted BAY 60–7550 in our experiments with the inhibitor used by Hopper and Garthwaite (EHNA at 300 μM). The NMDA-evoked and basal cGMP levels in the EHNA-pretreated slices were around half those observed in the BAY 60–7550-treated slices, presumably reflecting the superior potency of the latter inhibitor (Boess et al., 2004), but were in good agreement with those reported by Hopper and Garthwaite (2006). In contrast with the results of Hopper and Garthwaite (2006), neither l-VNIO nor 1400W had any significant effect on the cGMP response in the EHNA-pretreated slices (Figure 2B).
The difference between the results in the two studies might also have been explained were the compounds used in either study inauthentic. We obtained identical results, however, using two different batches of l-VNIO and 1400W (see Figure 2 legend). Furthermore, the molecular mass, integrity and purity of one batch of each compound were substantiated using high-performance liquid chromatography and mass spectrophotometry (Figure 3).
Figure 3.
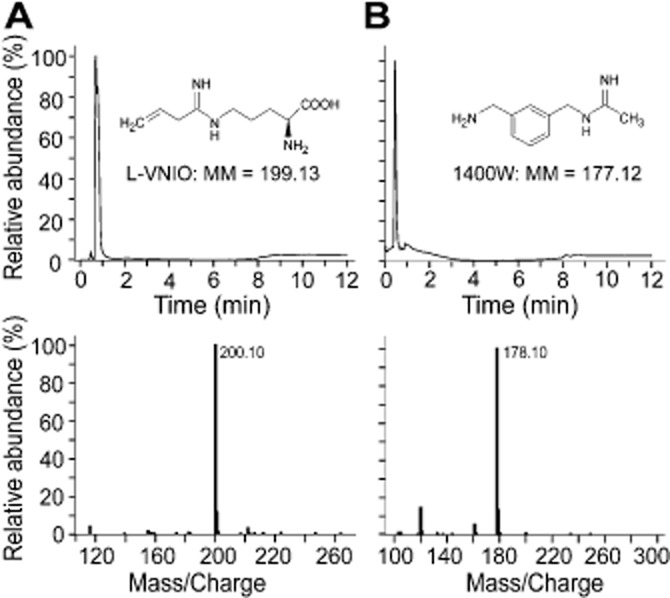
Chemical analysis of l-VNIO and 1400W. High-performance liquid chromatography of one batch of the l-VNIO (A) or 1400W (B) used in Figure 2 yielded a single major peak in each chromatogram (upper panels). Analysis of these peaks by mass spectrometry revealed that they comprised a molecular ion with a mass–charge ratio equivalent to the predicted molecular mass of the relevant compound (note that the molecular ions were protonated by formic acid in the running buffer). See insets for the chemical structures and predicted molecular mass (MM) of each compound. Relative abundance has been normalised to the maximum value recorded (100%).
To determine whether a higher concentration of l-VNIO could effect selective nNOS inhibition, we required a cell-based measure of the action of the compound on eNOS. Previously, the cGMP response to ACh in aortic rings has been used (Hopper and Garthwaite, 2006). ACh is a standard means of activating eNOS in endothelial cells (Furchgott and Zawadzki, 1980; Huang et al., 1995); but, curiously, information on the NOS isoforms involved in the cGMP response to ACh in aorta (or any other blood vessel) seems to be absent from the literature. To address this omission, we used eNOS−/− mice. In aortic rings prepared from wild-type mice and pre-incubated with the general PDE inhibitor IBMX (1 mM), ACh (10 μM) generated a significant rise in cGMP that was abolished by l-NNA (100 μM); this response was absent from aortic rings prepared from eNOS−/− mice (Figure 4). Direct stimulation of NO-targeted guanylyl cyclase using an NO donor, DEA/NO (100 μM), yielded large increases in cGMP that did not differ significantly in rings from wild-type and eNOS−/− mice, showing that the capacity of the two tissues to generate cGMP in response to NO was similar. The ACh-induced response in wild-type aortic rings was about sevenfold smaller than achievable with the NO donor, consistent with endogenous NO operating in the very low part of its concentration-response curve, probably in the picomolar range (Wood et al., 2011).
Figure 4.
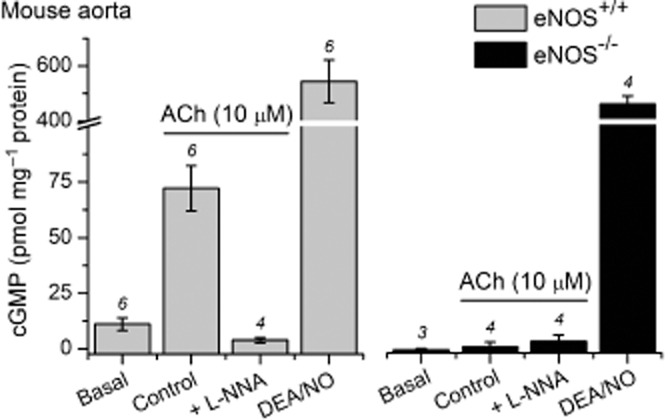
eNOS-dependent, ACh-induced cGMP accumulation in adult mouse aortic rings. Rings were pre-incubated with the non-selective PDE inhibitor IBMX (1 mM) for 20 min. In slices from wild-type (eNOS+/+) mice, ACh (10 μM, 1 min) generated a significant increase in cGMP from basal (anova with Tukey–Kramer test, P < 0.001) that was abolished by the non-selective NOS inhibitor, l-NNA (100 μM, 30 min pretreatment; P > 0.05 compared with basal). The response to ACh was absent in slices from eNOS−/− mice (anova with Tukey–Kramer test, ACh vs. basal or L-NNA: P > 0.05). The response of eNOS+/+ and eNOS−/− slices to the NO donor, DEA/NO (100 μM, 5 min), did not differ significantly (unpaired t-test, P > 0.05). Numbers above bars are n-values; note the break in the y-axis.
Having verified that the NMDA-evoked cGMP response in mouse hippocampal slices was nNOS-dependent (Figure 1), and the ACh-evoked cGMP accumulation in mouse aortic rings was eNOS-dependent (Figure 4), concentration–response curves for l-VNIO in both preparations were compiled. Using tissues from the same adult mice, it was found that the cGMP responses to NMDA in hippocampal slices and to ACh in aortic rings were inhibited by l-VNIO with very similar potencies, the IC50 values being 1–2 μM (Figure 5A and Table 1).
Figure 5.
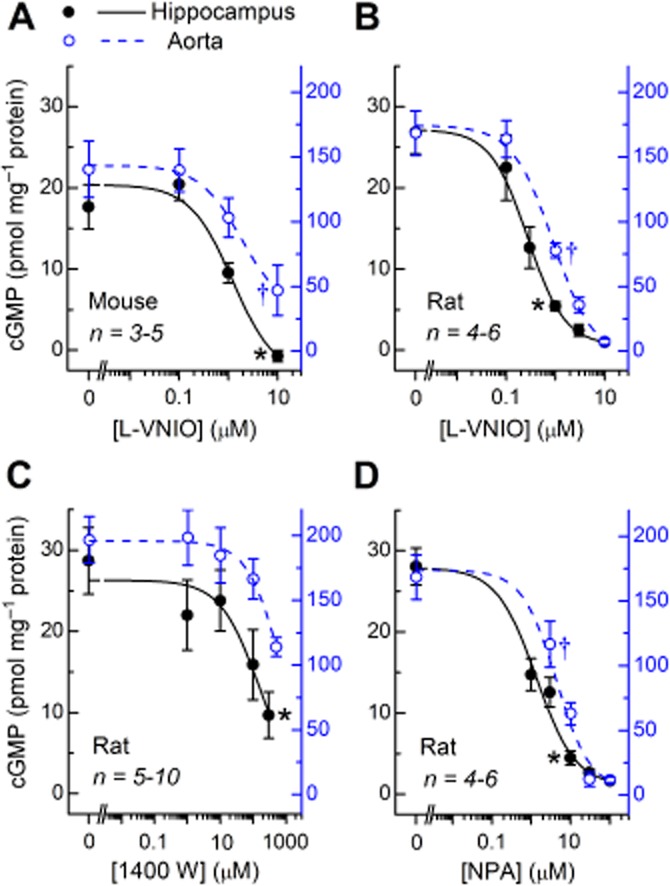
Selectivity of l-VNIO, 1400W and NPA for nNOS over eNOS. Hippocampal slices were pre-incubated with the PDE-2 inhibitor, BAY 60–7550 (1 μM for adult mouse; 10 nM for immature rat) and stimulated with NMDA (100 μM, 2 min). Aortic rings were pretreated with the non-selective phosphodiesterase inhibitor, IBMX (1 mM), and stimulated with ACh (10 μM, 1 min). l-VNIO (A–B), 1400W (C) and NPA (D; all pre-incubated for 25 min) inhibited the cGMP response to NMDA in hippocampus and to ACh in aorta in a concentration-dependent manner with similar potency (see Table 1 for IC50 and Hill slope values). In all experiments, l-NNA (100 μM; 25 min) reduced the cGMP response to NMDA or ACh by >90% (control vs. L-NNA, P < 0.001). Asterisks indicate the lowest concentration of each compound at which cGMP was not significantly different from the mean value in l-NNA-treated slices (P > 0.05). Obelisks (†) signify the lowest concentration at which l-VNIO or NPA caused a significant reduction in ACh-evoked cGMP accumulation in aortic rings (P < 0.05–0.001). Statistics are anova with Tukey–Kramer test. Within each panel, hippocampal slices and aortic rings were prepared from the same animals. Y-axes are colour-coded according to the tissue (see key in panel A).
In view of these results, tests of two other inhibitors, 1400W and NPA, that are reportedly selective for the cGMP response to NMDA in hippocampus over the response to ACh in aorta (Hopper and Garthwaite, 2006), were made. These experiments used immature rats, which provided more hippocampal slices and aortic rings than did adult mice. As in adult mice, l-VNIO was effectively non-selective in the rat tissues (Figure 5B, Table 1). Comparable non-selectivity was apparent with 1400W and NPA (Figure 5C and D, Table 1).
The results in hippocampal slices could be generalised to other nNOS-mediated responses based on testing the effect of l-VNIO on NMDA-evoked, nNOS-dependent (Morton and Bredt, 1998) cGMP accumulation in immature rat cerebellar slices. The IC50 in cerebellum was 1.3 μM (Figure 6A legend), a value fivefold higher than in hippocampus and similar to that found in aorta (0.9 μM).
Figure 6.
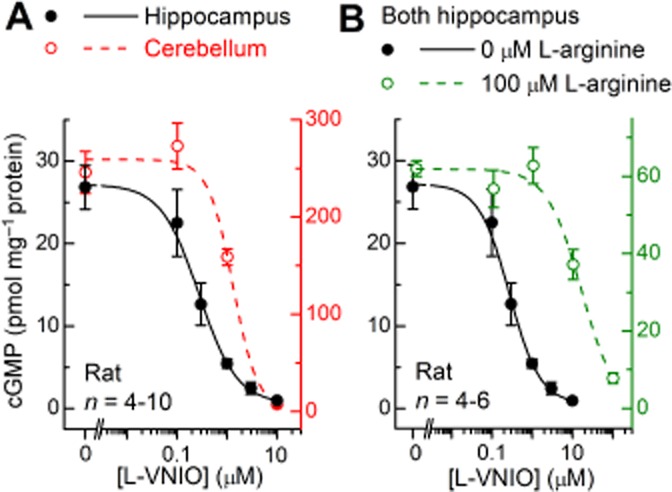
Potency of L-VNIO in cerebellum versus hippocampus and the effect of l-arginine. (A) Rat cerebellar slices were pre-incubated with l-VNIO for 25 min and stimulated with NMDA (100 μM, 2 min). As in rat hippocampal slices (data from Figure 5 re-plotted here for ease of comparison), l-VNIO inhibited the cGMP response to NMDA in the cerebellum concentration-dependently. The IC50 was 1.3 ± 0.14 μM (P < 0.001 compared with the fivefold lower value in rat hippocampal slices by unpaired t-test) and the Hill slope, 1.7 ± 0.15. (B) Rat hippocampal slices were pretreated with l-arginine (100 μM, 25 min), l-VNIO (20 min) and BAY 60–7550 (15 min). l-arginine caused an approximate 60-fold decrease in the potency of l-VNIO (IC50 value = 16 ± 6 μM; P < 0.05 compared with the IC50 in control hippocampal slices by unpaired t-test). The Hill slope was set to 1 to enable accurate fitting. The control data shown in black are from Figures 5 and 6. In panels A and B, l-NNA (100 μM; 20–25 min) reduced the cGMP response to NMDA by >90% (control vs. l-NNA, P < 0.001). Y-axes are colour-coded according to the experimental conditions.
One factor that could account for the differing potencies, and therefore selectivities, of all three compounds between the present study and that of Hopper and Garthwaite (2006) is variation in the intracellular l-arginine concentration in the brain slice preparations (the aortic data being compatible), because all three inhibitors compete with the substrate amino acid for binding to nNOS (Garvey et al., 1997; Zhang et al., 1997; Babu and Griffith, 1998b). Unfortunately, there are currently no reliable means to deplete intracellular l-arginine so the experiments examined the effect of increasing the availability of l-arginine in hippocampal slices by adding it to the incubation medium. At a concentration shown previously to be maximal for nNOS-dependent cGMP accumulation in rat cerebellar slices (100 μM; Garthwaite et al., 1989), l-arginine caused a twofold increase in the amplitude of the cGMP response to NMDA and around a 60-fold decrease in the inhibitory potency of l-VNIO (Figure 6B). These effects of l-arginine are in excellent agreement with previous studies using other NOS inhibitors (l-NNA and l-methylarginine) in immature rat cerebellar and hippocampal slices (Garthwaite et al., 1989; East and Garthwaite, 1990; East and Garthwaite, 1991).
Gem-difluorinated, pyrrolidine-based compounds
A series of pyrrolidine-based NOS inhibitors have recently been reported to have good selectivity for nNOS over eNOS in cell-free assays and improved cell permeability relative to previous compounds of this chemical class (Ji et al., 2009; Lawton et al., 2009; Xue et al., 2010a,b). Considering the above negative results with L-VNIO, NPA and 1400W, we tested the potency and selectivity of the best of these new compounds, FX-5043 (Figure 7C), which has a reported 780-fold selectivity for isolated nNOS over eNOS (KI for rat nNOS = 0.08 μM under cell-free conditions; Xue et al., 2010a), and a structurally related substance, JK-5. To circumvent the requirement for a PDE inhibitor to resolve the cGMP response, slices of immature rat cerebellum, which produces exceptionally high amplitude (Garthwaite, 1982), nNOS-dependent (Morton and Bredt, 1998), NMDA-evoked cGMP signals, were used.
Figure 7.
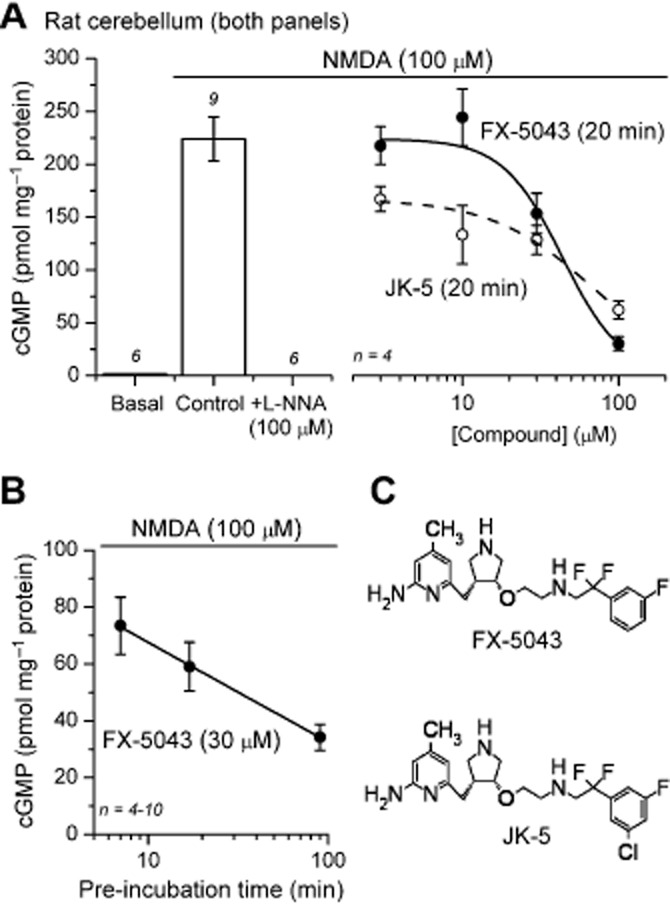
Potency of pyrrolidine-based nNOS inhibitors in intact tissues. (A) In rat cerebellar slices, the cGMP response to NMDA (100 μM, 2 min) was inhibited by FX-5043 and JK-5 (20 min pre-incubation) in a concentration-dependent manner (IC50 values both approximately 30 μM). (B) Inhibition of cGMP accumulation by 30 μM FX-5043 increased exponentially with pre-incubation time (note logarithmic x-axis). (C) Chemical structures of FX-5043 and JK-5. Numbers above error bars indicate n-values.
As expected, the NMDA-induced rise in cerebellar cGMP was blocked by l-NNA (100 μM) and inhibited by FX-5043, and JK-5 (with 20 min pre-incubation) in a concentration-dependent manner (IC50 approximately 30 μM; Figure 7A). Inhibition by FX-5043 (30 μM) increased exponentially with pre-incubation time up to 100 min (Figure 7B), consistent with a slow diffusion into the cells and implying that standard pre-incubation times were insufficient for complete penetration. With a 90 min pre-incubation, the NMDA-induced cGMP response in cerebellar slices was abolished by 100 μM FX-5043 or JK-5 (Figure 8A). The same concentrations also eliminated the cGMP response to ACh in aortic rings (Figure 8B), implying non-selectivity in intact tissues.
Figure 8.
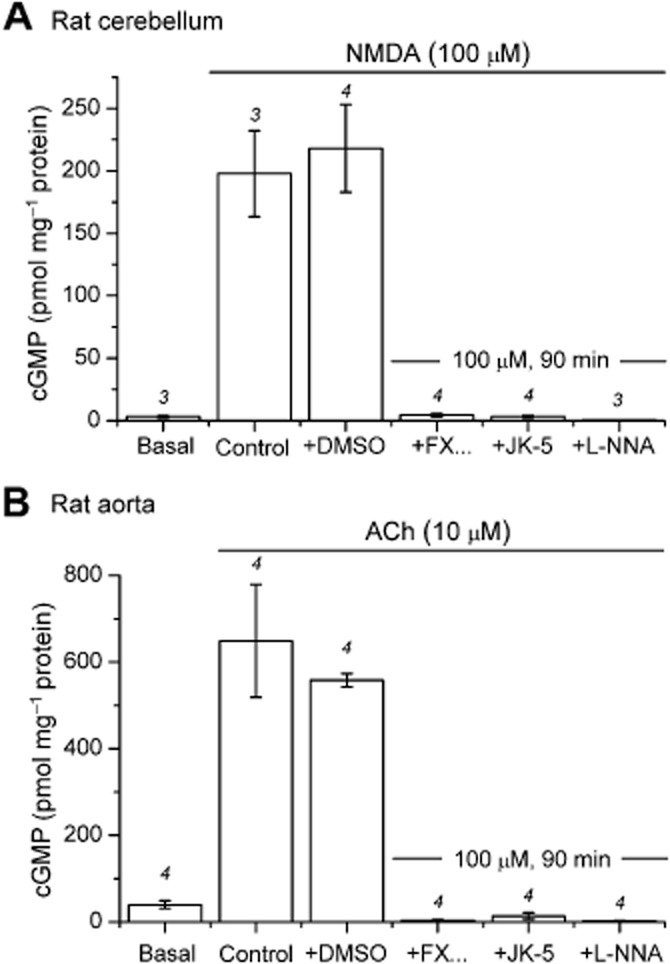
Potent concentrations of FX-5043 and JK-5 are non-selective for nNOS in intact tissues. (A) FX-5043 (abbreviated FX) or JK-5 (100 μM; 90 min pre-incubation) abolished the NMDA-evoked cGMP response (P > 0.05 compared with basal). The vehicle (dimethyl sulphoxide, DMSO) had no effect (P > 0.05 compared with control). (B) In aortic rings prepared from the same animals as used in panel A and pretreated with IBMX (1 mM, 20 min), ACh (10 μM, 1 min) initiated a significant increase in cGMP (P < 0.001). At the same concentrations as used in panel A (100 μM, 90 min), FX-5043 and JK-5 blocked the cGMP response to ACh (P > 0.05 compared with basal levels). DMSO alone had no effect (P > 0.05 compared to control). Statistics are anova with Tukey–Kramer test. Numbers above error bars indicate n-values.
Discussion
The major finding of this study is that the purported nNOS inhibitors, 1400W, NPA and l-VNIO are inadequately (<5-fold) selective for nNOS over eNOS in intact tissues to be of use diagnostically (Figure 5 and Table 1). The results challenge the marketing of the compounds as ‘potent, selective inhibitor[s] of nNOS’ (e.g. by Cayman Chemical Company), and the widespread presumption that they selectively attenuate nNOS activity in vitro and in vivo, for example in brain (e.g. Steinert et al., 2008; Chiang et al., 2009; Le Roux et al., 2009; Romberg et al., 2009; Taqatqeh et al., 2009; Cheng et al., 2011; Neitz et al., 2011), the peripheral nociceptive pathway (e.g. Lima et al., 2010; Romero et al., 2011), heart (e.g. Herring et al., 2000; Choate et al., 2001; Danson and Paterson, 2003; Lu et al., 2009), kidney (e.g. Kakoki et al., 2001; Shi et al., 2006; Foster et al., 2009; Wang and Cupples, 2009; Dautzenberg et al., 2011; Yamaleyeva et al., 2012) and gut (Mathison et al., 2004; Hatanaka et al., 2006; Fornai et al., 2007; Kato et al., 2009).
The lack of selectivity that we observed is discordant with the original characterization of all three compounds using cell-free assays (Garvey et al., 1997; Zhang et al., 1997; Babu and Griffith, 1998b: see reported selectivities in Table 1). However, other biochemical studies using isolated NOS enzymes are consistent with our findings. Notably, Boer et al. (2000) estimate the selectivity of l-VNIO, NPA and 1400W for isolated human nNOS over eNOS to be 3, 4 and 10, respectively, values that are in good agreement with our own (Table 1). Additionally, Kotthaus et al. (2008) have observed that an l-VNIO concentration sufficient to reduce recombinant rat nNOS activity by 94% (100 μM) also inhibited bovine eNOS by 45%. In contrast with the original reports (Zhang et al., 1997; Babu and Griffith, 1998b), Babu et al. (1999) have found that NPA is only around 9-fold selective for isolated nNOS over eNOS and Brzozowski et al. (2011) that l-VNIO is only 25-fold more potent for human nNOS in cell lysates than for purified eNOS. Conversely, Brzozowski et al. (2011) also report that 1400W is substantially more selective for nNOS over eNOS (332-fold) than was originally observed by Garvey et al. (1997).
It is unclear why the selectivity of the compounds varies so greatly between different cell-free assays. A comparison of the assay conditions used by Boer et al. (2000) and in the original characterisation of the inhibitors (Garvey et al., 1997; Zhang et al., 1997; Babu and Griffith, 1998b) reveals numerous methodological differences in, for example, the NOS preparation (i.e. crude lysate vs. purified protein) and species of origin, the duration of pre-incubation with the inhibitors (potentially important with time-dependent inhibitors such as l-VNIO) and the concentrations of l-arginine and cofactors, such as calmodulin.
With respect to cell-based analyses, our estimates of the selectivity (and potency) of all three compounds conflict most obviously with Hopper and Garthwaite (2006), who found that 0.1 μM l-VNIO, 1 μM 1400W and 1 μM NPA (sub-IC50 concentrations in our experiments; Figures 2 and 5; Table 1) reduced nNOS-dependent, NMDA-evoked cGMP accumulation in hippocampal slices by 80–90% but left intact the cGMP response to ACh in aortic rings, which we now confirm to be eNOS-dependent (Figure 4). It is unlikely that our results suffer from experimenter error because the results reported here using l-VNIO in rat hippocampus and aorta were obtained independently by two operators (BP and KB), several months apart. The possibility that our results were confounded by inauthentic or impure compounds is also excluded (see Results). Furthermore, our data are consistent with the results of some other cell-based studies. In particular, Overend and Martin (2007) have found a NPA IC50 of close to 10 μM both for nitrergic, endothelium-independent (presumed nNOS-dependent) and for bradykinin-induced (presumed eNOS-dependent) dilation of bovine ciliary artery (cf. 1 and 4 μM in our experiments). Also in line with our results (Figure 5C), Fang and Silverman (2009) have found that 5 μM 1400W is insufficient to attenuate the activity of recombinant nNOS in a cell-based assay.
It is likewise difficult to dismiss the results of Hopper and Garthwaite (2006) because the concentrations found to be active in their experiments are consistent with substantial functional data. For instance, Hopper and Garthwaite (2006) found that 0.1 μM l-VNIO and 1 μM 1400W significantly reduced long-term potentiation in hippocampal slices. According with the compound affecting NOS, the reduction in LTP caused by 1400W could be prevented by co-application of an NO donor. Other groups using different in vitro preparations have also reported a functionally inhibitory effect of 0.1 μM l-VNIO (e.g. Le Roux et al., 2009; Taqatqeh et al., 2009). The effects observed in these studies are coherent with NOS inhibition, since the phenomena involved were blocked by non-selective NOS inhibitors. Additionally, other concentrations of l-VNIO (0.2 and 1 μM) that were insufficient to attenuate nNOS activity under our conditions (Figure 5) have been found to inhibit NOS-dependent phenomena in vitro (e.g. Foster et al., 2009; Romberg et al., 2009).
Rather, all these functional and biochemical data point to an inherent variability in susceptibility to the nNOS inhibitors between tissues, or between studies using the same tissue. Our comparison of the potency of l-VNIO in hippocampus and cerebellum (Figure 6A) shows further variation, in this case between different brain regions. One plausible explanation lies in the intracellular l-arginine concentration. The amino acid is typically omitted from extracellular solutions used to incubate tissues and intracellular variation could result from differences in l-arginine export/loss (e.g. during tissue preparation), uptake or metabolism. The high sensitivity of the IC50 for l-VNIO in hippocampus to addition of l-arginine (Figure 6B) is consistent with this explanation. Hence, a lower intracellular l-arginine concentration in hippocampal neurones, but not in aortic endothelial cells, in the study of Hopper and Garthwaite (2006) compared with the present one could explain the discrepancies in the potency (and therefore selectivity) of the inhibitors.
Regardless of the precise explanation, our results clearly compromise the use of these compounds as selective nNOS inhibitors without rigorous controls for their activity and selectivity. Furthermore, our results complicate the interpretation of studies that have argued against (or ruled out) the involvement of nNOS in phenomena on the basis of a lack of effect of the inhibitors (e.g. Mathison et al., 2004; Fornai et al., 2007; Kato et al., 2009; Cheng et al., 2011; Neitz et al., 2011).
Unfortunately, there appear to be few, if any, alternative nNOS inhibitors available. 7-Nitroindazole is widely used as a nNOS inhibitor in vitro and in vivo (e.g. Doyle et al., 1996; Steinert et al., 2008). Nevertheless, the compound is reportedly non-selective (<5-fold) for isolated nNOS (see Alderton et al., 2001; Brzozowski et al., 2011) and has off-target effects, notably as a monoamine oxidase-B inhibitor (Castagnoli et al., 1997). S-Methyl-l-thiocitrulline (Furfine et al., 1994) is also used, albeit less frequently, to selectively inhibit nNOS in vitro and in vivo, yet studies show that the compound is <10-fold selective for isolated nNOS over eNOS (Furfine et al., 1994; Boer et al., 2000; both using human NOS isozymes). The use of other compounds with moderate or good selectivity for isolated nNOS, such as S,S′-1,4-phenylene-bis(1,2-ethanediyl)bis-isothiourea (100-fold selectivity over eNOS; tested using isolated human NOS by Boer et al., 2000), 1[2-(trifluoromethyl)phenyl]-1H-imidazole (TRIM; 38-fold selectivity over eNOS determined with homogenates of mouse cerebellum and bovine aortic endothelial cells; Handy et al., 1995) and AR-R17477 (28-fold over eNOS using human isozymes, Brzozowski et al., 2011; 100-fold for rat nNOS over human eNOS in partially purified preparations, Reif et al., 2000), is also hampered by problems such as a lack of specificity (isothioureas, see Babu and Griffith, 1998a; TRIM, Matsumura et al., 2008) and/or a lack of availability (AR-R17477).
Recently, a series of pyrrolidine-based compounds with good selectivity for nNOS in cell-free assays and moderate cell permeability have been reported (Xue et al., 2010a,b). Unfortunately, tests with two of these inhibitors indicated non-selectivity in intact tissues (Figures 7 and 8). Nonetheless, the development of nNOS inhibitors is ongoing (Xue et al., 2011; Annedi et al., 2012; Labby et al., 2012), and future tests of the selectivity of these compounds in intact tissues may reveal superior alternatives to the compounds currently available.
Acknowledgments
This work was supported by The Wellcome Trust, a Biotechnology and Biological Sciences Research Council Studentship (BP) and a Medical Research Council Studentship (KB).We are grateful to Kathryn Hampden-Smith (University College London) for assistance with radioimmunoassay, Dr Matthew Gooding (University College London) for helping with chemical analysis, Dr Adrian Hobbs (University College London) and Dr Michael Emerson (Imperial College, London) for kindly providing eNOS−/− mice, Prof Richard Silverman (Northwestern University, IL) for the gifts of FX-5043 and JK-5 and Dr Andrew Batchelor (University College London) for help with the manuscript.
Glossary
- 1400W
N-[(3-aminomethyl)benzyl]acetamidine
- aCSF
artificial CSF
- eNOS
endothelial NOS
- eNOS−/−
endothelial NOS knockout
- FX-5043
6-{[(3R,4R)-4-(2-{[2,2-difluoro-2-(3-fluorophenyl)ethyl]amino}ethoxy)pyrrolidin-3-yl]methyl}-4-methylpyridin-2-amine
- iNOS
inducible NOS
- JK-5
6-{[(3R,4R)-4-(2-{[2-(3-chloro-5-fluorophenyl)-2,2-difluoroethyl]amino}ethoxy)pyrrolidin-3-yl]methyl}-4-methylpyridin-2-amine
- l-VNIO
vinyl-l-N-5-(1-imino-3-butenyl)-l-ornithine
- nNOS
neuronal NOS
- NPA
Nω-propyl-l-arginine
Conflict of interest
The authors declare no conflict of interest.
References
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annedi SC, Ramnauth J, Maddaford SP, Renton P, Rakhit S, Mladenova G, et al. Discovery of cis-N-(1-(4-(methylamino)cyclohexyl)indolin-6-yl)thiophene-2-carboximidamide: a 1,6-disubstituted indoline derivative as a highly selective inhibitor of human neuronal nitric oxide synthase (nNOS) without any cardiovascular liabilities. J Med Chem. 2012;55:943–955. doi: 10.1021/jm201564u. [DOI] [PubMed] [Google Scholar]
- Babu BR, Griffith OW. Design of isoform-selective inhibitors of nitric oxide synthase. Curr Opin Chem Biol. 1998a;2:491–500. doi: 10.1016/s1367-5931(98)80125-7. [DOI] [PubMed] [Google Scholar]
- Babu BR, Griffith OW. N5-(1-Imino-3-butenyl)-L-ornithine. A neuronal isoform selective mechanism-based inactivator of nitric oxide synthase. J Biol Chem. 1998b;273:8882–8889. doi: 10.1074/jbc.273.15.8882. [DOI] [PubMed] [Google Scholar]
- Babu BR, Frey C, Griffith OW. L-arginine binding to nitric-oxide synthase. The role of H-bonds to the nonreactive guanidinium nitrogens. J Biol Chem. 1999;274:25218–25226. doi: 10.1074/jbc.274.36.25218. [DOI] [PubMed] [Google Scholar]
- Boer R, Ulrich WR, Klein T, Mirau B, Haas S, Baur I. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol Pharmacol. 2000;58:1026–1034. [PubMed] [Google Scholar]
- Boess FG, Hendrix M, Van der Staay FJ, Erb C, Schreiber R, Van Staveren W, et al. Inhibition of phosphodiesterase 2 increases neuronal cGMP, synaptic plasticity and memory performance. Neuropharmacology. 2004;47:1081–1092. doi: 10.1016/j.neuropharm.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Brzozowski MJ, Alcantara SL, Iravani MM, Rose S, Jenner P. The effect of nNOS inhibitors on toxin-induced cell death in dopaminergic cell lines depends on the extent of enzyme expression. Brain Res. 2011;1404:21–30. doi: 10.1016/j.brainres.2011.05.063. [DOI] [PubMed] [Google Scholar]
- Castagnoli K, Palmer S, Anderson A, Bueters T, Castagnoli N., Jr The neuronal nitric oxide synthase inhibitor 7-nitroindazole also inhibits the monoamine oxidase-B-catalyzed oxidation of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Chem Res Toxicol. 1997;10:364–368. doi: 10.1021/tx970001d. [DOI] [PubMed] [Google Scholar]
- Cheng PW, Lu PJ, Chen SR, Ho WY, Cheng WH, Hong LZ, et al. Central nicotinic acetylcholine receptor involved in Ca2+-calmodulin-endothelial nitric oxide synthase pathway modulated hypotensive effects. Br J Pharmacol. 2011;163:1203–1213. doi: 10.1111/j.1476-5381.2010.01124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang HT, Cheng WH, Lu PJ, Huang HN, Lo WC, Tseng YC, et al. Neuronal nitric oxide synthase activation is involved in insulin-mediated cardiovascular effects in the nucleus tractus solitarii of rats. Neuroscience. 2009;159:727–734. doi: 10.1016/j.neuroscience.2008.12.048. [DOI] [PubMed] [Google Scholar]
- Choate JK, Danson EJ, Morris JF, Paterson DJ. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am J Physiol Heart Circ Physiol. 2001;281:H2310–H2317. doi: 10.1152/ajpheart.2001.281.6.H2310. [DOI] [PubMed] [Google Scholar]
- Danson EJ, Paterson DJ. Enhanced neuronal nitric oxide synthase expression is central to cardiac vagal phenotype in exercise-trained mice. J Physiol. 2003;546:225–232. doi: 10.1113/jphysiol.2002.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg M, Keilhoff G, Just A. Modulation of the myogenic response in renal blood flow autoregulation by NO depends on endothelial nitric oxide synthase (eNOS), but not neuronal or inducible NOS. J Physiol. 2011;589:4731–4744. doi: 10.1113/jphysiol.2011.215897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C, Holscher C, Rowan MJ, Anwyl R. The selective neuronal NO synthase inhibitor 7-nitro-indazole blocks both long-term potentiation and depotentiation of field EPSPs in rat hippocampal CA1 in vivo. J Neurosci. 1996;16:418–424. doi: 10.1523/JNEUROSCI.16-01-00418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- East SJ, Garthwaite J. Nanomolar NG-nitroarginine inhibits NMDA-induced cyclic GMP formation in rat cerebellum. Eur J Pharmacol. 1990;184:311–313. doi: 10.1016/0014-2999(90)90623-e. [DOI] [PubMed] [Google Scholar]
- East SJ, Garthwaite J. NMDA receptor activation in rat hippocampus induces cyclic GMP formation through the L-arginine-nitric oxide pathway. Neurosci Lett. 1991;123:17–19. doi: 10.1016/0304-3940(91)90147-l. [DOI] [PubMed] [Google Scholar]
- Fang J, Silverman RB. A cellular model for screening neuronal nitric oxide synthase inhibitors. Anal Biochem. 2009;390:74–78. doi: 10.1016/j.ab.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai M, Colucci R, Antonioli L, Crema F, Buccianti P, Chiarugi M, et al. Cholecystokinin CCK2 receptors mediate the peptide's inhibitory actions on the contractile activity of human distal colon via the nitric oxide pathway. Br J Pharmacol. 2007;151:1246–1253. doi: 10.1038/sj.bjp.0707339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JM, Carmines PK, Pollock JS. PP2B-dependent NO production in the medullary thick ascending limb during diabetes. Am J Physiol Renal Physiol. 2009;297:F471–F480. doi: 10.1152/ajprenal.90760.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Koesling D. The function of NO-sensitive guanylyl cyclase: what we can learn from genetic mouse models. Nitric Oxide. 2009;21:149–156. doi: 10.1016/j.niox.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Furfine ES, Harmon MF, Paith JE, Knowles RG, Salter M, Kiff RJ, et al. Potent and selective inhibition of human nitric oxide synthases. Selective inhibition of neuronal nitric oxide synthase by S-methyl-L-thiocitrulline and S-ethyl-L-thiocitrulline. J Biol Chem. 1994;269:26677–26683. [PubMed] [Google Scholar]
- Garthwaite J. Excitatory amino acid receptors and guanosine 3′,5′-cyclic monophosphate in incubated slices of immature and adult rat cerebellum. Neuroscience. 1982;7:2491–2497. doi: 10.1016/0306-4522(82)90209-3. [DOI] [PubMed] [Google Scholar]
- Garthwaite J. Concepts of neural nitric oxide-mediated transmission. Eur J Neurosci. 2008;27:2783–2802. doi: 10.1111/j.1460-9568.2008.06285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Balazs R. Supersensitivity to the cyclic GMP response to glutamate during cerebellar maturation. Nature. 1978;275:328–329. doi: 10.1038/275328a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J, Garthwaite G, Palmer RM, Moncada S. NMDA receptor activation induces nitric oxide synthesis from arginine in rat brain slices. Eur J Pharmacol. 1989;172:413–416. doi: 10.1016/0922-4106(89)90023-0. [DOI] [PubMed] [Google Scholar]
- Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- Handy RL, Wallace P, Gaffen ZA, Whitehead KJ, Moore PK. The antinociceptive effect of 1-(2-trifluoromethylphenyl) imidazole (TRIM), a potent inhibitor of neuronal nitric oxide synthase in vitro, in the mouse. Br J Pharmacol. 1995;116:2349–2350. doi: 10.1111/j.1476-5381.1995.tb15078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatanaka Y, Hobara N, Honghua J, Akiyama S, Nawa H, Kobayashi Y, et al. Neuronal nitric-oxide synthase inhibition facilitates adrenergic neurotransmission in rat mesenteric resistance arteries. J Pharmacol Exp Ther. 2006;316:490–497. doi: 10.1124/jpet.105.094656. [DOI] [PubMed] [Google Scholar]
- Herring N, Golding S, Paterson DJ. Pre-synaptic NO-cGMP pathway modulates vagal control of heart rate in isolated adult guinea pig atria. J Mol Cell Cardiol. 2000;32:1795–1804. doi: 10.1006/jmcc.2000.1214. [DOI] [PubMed] [Google Scholar]
- Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu Rev Pharmacol Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- Hopper RA, Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci. 2006;26:11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, et al. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- Huang Z, Huang PL, Ma J, Meng W, Ayata C, Fishman MC, et al. Enlarged infarcts in endothelial nitric oxide synthase knockout mice are attenuated by nitro-L-arginine. J Cereb Blood Flow Metab. 1996;16:981–987. doi: 10.1097/00004647-199609000-00023. [DOI] [PubMed] [Google Scholar]
- Ji H, Li H, Martasek P, Roman LJ, Poulos TL, Silverman RB. Discovery of highly potent and selective inhibitors of neuronal nitric oxide synthase by fragment hopping. J Med Chem. 2009;52:779–797. doi: 10.1021/jm801220a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakoki M, Zou AP, Mattson DL. The influence of nitric oxide synthase 1 on blood flow and interstitial nitric oxide in the kidney. Am J Physiol Regul Integr Comp Physiol. 2001;281:R91–R97. doi: 10.1152/ajpregu.2001.281.1.R91. [DOI] [PubMed] [Google Scholar]
- Kato S, Ohkawa F, Ito Y, Amagase K, Takeuchi K. Role of endothelial nitric oxide synthase in aggravation of indomethacin-induced gastric damage in adjuvant arthritic rats. J Physiol Pharmacol. 2009;60:147–155. [PubMed] [Google Scholar]
- Kotthaus J, Schade D, Muschick N, Beitz E, Clement B. Structure-activity relationship of novel and known inhibitors of human dimethylarginine dimethylaminohydrolase-1: alkenyl-amidines as new leads. Bioorg Med Chem. 2008;16:10205–10209. doi: 10.1016/j.bmc.2008.10.058. [DOI] [PubMed] [Google Scholar]
- Labby KJ, Xue F, Kraus JM, Ji H, Mataka J, Li H, et al. Intramolecular hydrogen bonding: a potential strategy for more bioavailable inhibitors of neuronal nitric oxide synthase. Bioorg Med Chem. 2012;20:2435–2443. doi: 10.1016/j.bmc.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton GR, Ralay RH, Chico LK, Ji H, Xue F, Martasek P, et al. Analogues of 2-aminopyridine-based selective inhibitors of neuronal nitric oxide synthase with increased bioavailability. Bioorg Med Chem. 2009;17:2371–2380. doi: 10.1016/j.bmc.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roux N, Amar M, Moreau AW, Fossier P. Roles of nitric oxide in the homeostatic control of the excitation-inhibition balance in rat visual cortical networks. Neuroscience. 2009;163:942–951. doi: 10.1016/j.neuroscience.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Lima FO, Souza GR, Verri JWA, Parada CA, Ferreira SH, Cunha FQ, et al. Direct blockade of inflammatory hypernociception by peripheral A1 adenosine receptors: involvement of the NO/cGMP/PKG/KATP signaling pathway. Pain. 2010;151:506–515. doi: 10.1016/j.pain.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Lu XM, Zhang GX, Yu YQ, Kimura S, Nishiyama A, Matsuyoshi H, et al. The opposite roles of nNOS in cardiac ischemia-reperfusion-induced injury and in ischemia preconditioning-induced cardioprotection in mice. J Physiol Sci. 2009;59:253–262. doi: 10.1007/s12576-009-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J, Xie Q, Nathan C. Nitric oxide and macrophage function. Annu Rev Immunol. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Mathison R, Ho W, Pittman QJ, Davison JS, Sharkey KA. Effects of cannabinoid receptor-2 activation on accelerated gastrointestinal transit in lipopolysaccharide-treated rats. Br J Pharmacol. 2004;142:1247–1254. doi: 10.1038/sj.bjp.0705889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura N, Kikuchi-Utsumi K, Nakaki T. Activities of 7-nitroindazole and 1-(2-(trifluoromethylphenyl)-imidazole independent of neuronal nitric-oxide synthase inhibition. J Pharmacol Exp Ther. 2008;325:357–362. doi: 10.1124/jpet.107.135160. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton DB, Bredt DS. Norepinephrine increases cyclic cGMP levels in cerebellar cells from neuronal nitric oxide synthase knockout mice. J Neurochem. 1998;71:440–443. doi: 10.1046/j.1471-4159.1998.71010440.x. [DOI] [PubMed] [Google Scholar]
- Neitz A, Mergia E, Eysel UT, Koesling D, Mittmann T. Presynaptic nitric oxide/cGMP facilitates glutamate release via hyperpolarization-activated cyclic nucleotide-gated channels in the hippocampus. Eur J Neurosci. 2011;33:1611–1621. doi: 10.1111/j.1460-9568.2011.07654.x. [DOI] [PubMed] [Google Scholar]
- Overend J, Martin W. Differential effects of nitric oxide synthase inhibitors on endothelium-dependent and nitrergic nerve-mediated vasodilatation in the bovine ciliary artery. Br J Pharmacol. 2007;150:488–493. doi: 10.1038/sj.bjp.0707113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott B. London, UK: University College London; 2012. Nitric oxide signalling in hippocampal synaptic plasticity. PhD Thesis. [Google Scholar]
- Reif DW, McCarthy DJ, Cregan E, Macdonald JE. Discovery and development of neuronal nitric oxide synthase inhibitors. Free Radic Biol Med. 2000;28:1470–1477. doi: 10.1016/s0891-5849(00)00250-1. [DOI] [PubMed] [Google Scholar]
- Romberg C, Raffel J, Martin L, Sprengel R, Seeburg PH, Rawlins JN, et al. Induction and expression of GluA1 (GluR-A)-independent LTP in the hippocampus. Eur J Neurosci. 2009;29:1141–1152. doi: 10.1111/j.1460-9568.2009.06677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero TR, Resende LC, Duarte ID. The neuronal NO synthase participation in the peripheral antinociception mechanism induced by several analgesic drugs. Nitric Oxide. 2011;25:431–435. doi: 10.1016/j.niox.2011.08.002. [DOI] [PubMed] [Google Scholar]
- Shi Y, Wang X, Chon KH, Cupples WA. Tubuloglomerular feedback-dependent modulation of renal myogenic autoregulation by nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2006;290:R982–R991. doi: 10.1152/ajpregu.00346.2005. [DOI] [PubMed] [Google Scholar]
- Steinert JR, Kopp-Scheinpflug C, Baker C, Challiss RA, Mistry R, Haustein MD, et al. Nitric oxide is a volume transmitter regulating postsynaptic excitability at a glutamatergic synapse. Neuron. 2008;60:642–656. doi: 10.1016/j.neuron.2008.08.025. [DOI] [PubMed] [Google Scholar]
- Taqatqeh F, Mergia E, Neitz A, Eysel UT, Koesling D, Mittmann T. More than a retrograde messenger: nitric oxide needs two cGMP pathways to induce hippocampal long-term potentiation. J Neurosci. 2009;29:9344–9350. doi: 10.1523/JNEUROSCI.1902-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance P, Leiper J. Blocking NO synthesis: how, where and why? Nat Rev Drug Discov. 2002;1:939–950. doi: 10.1038/nrd960. [DOI] [PubMed] [Google Scholar]
- Van Staveren WC, Markerink-Van Ittersum M, Steinbusch HW, de Vente J. The effects of phosphodiesterase inhibition on cyclic GMP and cyclic AMP accumulation in the hippocampus of the rat. Brain Res. 2001;888:275–286. doi: 10.1016/s0006-8993(00)03081-x. [DOI] [PubMed] [Google Scholar]
- Van Staveren WC, Steinbusch HW, Markerink-Van Ittersum M, Repaske DR, Goy MF, Kotera J, et al. mRNA expression patterns of the cGMP-hydrolyzing phosphodiesterases types 2, 5, and 9 during development of the rat brain. J Comp Neurol. 2003;467:566–580. doi: 10.1002/cne.10955. [DOI] [PubMed] [Google Scholar]
- Wang X, Cupples WA. Brown Norway rats show impaired nNOS-mediated information transfer in renal autoregulation. Can J Physiol Pharmacol. 2009;87:29–36. doi: 10.1139/Y08-102. [DOI] [PubMed] [Google Scholar]
- Wood KC, Batchelor AM, Bartus K, Harris KL, Garthwaite G, Vernon J, et al. Picomolar nitric oxide signals from central neurons recorded using ultrasensitive detector cells. J Biol Chem. 2011;286:43172–43181. doi: 10.1074/jbc.M111.289777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Fang J, Lewis WW, Martasek P, Roman LJ, Silverman RB. Potent and selective neuronal nitric oxide synthase inhibitors with improved cellular permeability. Bioorg Med Chem Lett. 2010a;20:554–557. doi: 10.1016/j.bmcl.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Li H, Delker SL, Fang J, Martasek P, Roman LJ, et al. Potent, highly selective, and orally bioavailable gem-difluorinated monocationic inhibitors of neuronal nitric oxide synthase. J Am Chem Soc. 2010b;132:14229–14238. doi: 10.1021/ja106175q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue F, Fang J, Delker SL, Li H, Martasek P, Roman LJ, et al. Symmetric double-headed aminopyridines, a novel strategy for potent and membrane-permeable inhibitors of neuronal nitric oxide synthase. J Med Chem. 2011;54:2039–2048. doi: 10.1021/jm101071n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaleyeva LM, Lindsey SH, Varagic J, Zhang LL, Gallagher PE, Chen AF, et al. Amelioration of renal injury and oxidative stress by the nNOS inhibitor L-VNIO in the salt-sensitive mRen2.Lewis congenic rat. J Cardiovasc Pharmacol. 2012;59:529–538. doi: 10.1097/FJC.0b013e31824dd15b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HQ, Fast W, Marletta MA, Martasek P, Silverman RB. Potent and selective inhibition of neuronal nitric oxide synthase by Nω-propyl-L-arginine. J Med Chem. 1997;40:3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]


