Abstract
Very low doses of ionizing radiation, 5 to 100 mGy, can induce adaptive responses characterized by elevation in cell survival and reduction in micronuclei formation. Utilizing these end points, RKO human colon carcinoma and transformed mouse embryo fibroblasts (MEF), wild-type or knockout cells missing TNF receptors 1 and 2 (TNFR1−R2−), and C57BL/6 and TNFR1−R2− knockout mice, we demonstrate that intact TNF signaling is required for induction of elevated manganese superoxide dismutase (SOD2) activity (P < 0.001) and the subsequent expression of these SOD2-mediated adaptive responses when cells are challenged at a later time with 2 Gy. In contrast, amifostine’s free thiol form WR1065 can directly activate NF-κB giving rise to elevated SOD2 activity 24 h later and induce an adaptive response in both MEF wild-type and TNF signaling defective TNFR1−R2− cells. Transfection of cells with SOD2 siRNA completely abolishes both the elevation in SOD2 activity and expression of the adaptive responses. These results were confirmed in vivo using a micronucleus assay in splenocytes derived from C57BL/6 and TNFR1−R2− knockout mice that were exposed to 100 mGy or 400 mg/kg amifostine 24 h prior to exposure to a 2 Gy whole-body dose. A dose of 100 mGy also conferred enhanced protection to C57BL/6 mice exposed 24 h later to 100 mg/kg of N-Ethyl-N-nitrosourea (ENU). While very low radiation doses require an intact TNF signaling process to induce a SOD2-mediated adaptive response, amifostine can induce a similar adaptive response in both TNF receptor competent and knockout cells, respectively.
INTRODUCTION
The adaptive response, defined as the ability of cells or organisms exposed to a very low dose of ionizing radiation in the range of 0.1–100 mGy to confer enhanced resistance to a subsequent exposure to a much larger dose of radiation, was initially observed in the context of elevating the inherent resistance of human lymphocytes against the toxicity of high doses of ionizing radiation and chemical mutagens (1). This phenomenon has recently garnered extensive interest given the expanding use of computerized axial tomography (CAT) as an essential and pervasive tool for rapid and highly sensitive analysis of both diagnostic- and therapeutic-associated medical issues (2, 3). The very low-dose radiation-induced adaptive response is not a universal phenomenon with responses differing or lacking in different cell types or even the same cell type in different individuals. These variable results suggest that the underlying mechanism(s) leading to adaptive responses are modifiable and may be present or absent in the same cell type as a function of extra- and intracellular signaling pathways and their temporal responses to the initial very low-dose exposures. An important pathway that has been found to be implicated in this adaptive response is controlled by tumor necrosis factor (TNF) signaling (4–6). Also implicated in this process are nuclear factor κB (NF-κB) activation and subsequent manganese superoxide dismutase (SOD2) gene expression and enzymatic activity (7–10). Each of these components of the molecular pathway by virtue of the robustness of their respective response and temporal association can modify the initiation, subsequent duration and magnitude of an adaptive response. Utilizing a human colon carcinoma cell line and two transformed mouse embryo fibroblast (MEF) cell systems along with two mouse models, we have evaluated and characterized two different adaptive responses potentially capable of affecting survival and genomic instability outcomes in both normal and malignant cells after their subsequent exposure to 2 Gy radiation or to the alkylating agent N-ethyl-N-nitrosourea (ENU).
MATERIALS AND METHODS
Cells and Culture Conditions
A subclone of RKO colorectal carcinoma cells designated RKO36 was used in selected experiments (10). Mouse embryo fibroblasts were isolated from either 14- to 16-day-old pregnant female C57BL/6 WT or TNFR1−R2− C57BL/6 knockout mice. Mice were euthanized, and the uterus was removed and placed in a culture dish containing sterile PBS. Organs, tail, limbs and head were removed for genotyping. Carcasses were placed in PBS with 0.25% trypsin and finely minced with scissors. Minced tissues were incubated for 15 min at 37°C and pipetted to dissociate the tissue. This process was repeated 2–3 times after which supernatants were collected and centrifuged. Cells were re-suspended in culture medium containing (1:1) DMEM:F12 (Invitrogen, Carlsbad, CA), 10% fetal bovine serum (Atlantic Biologicals, Lawrenceville, GA) and 100 units/ml penicillin and 100 mg/ml streptomycin and were plated in 60 mm dishes at a density of 106 cells/ dish. Cell cultures were maintained at 37°C in a humidified environment containing 5% CO2.
All MEF used in this study were derived from both wild-type and TNFR1−R2− knockout mice and then transformed with c-myc and H-Ras. Generation of transformed MEF cell lines was as follows: the pLXSN vector for co-expression of c-myc and H-RasVal12 with an internal ribosomal entry site (a gift from A. Gudkov) was co-transfected with packaging plasmids in 293T cells using ProFection Mammalian Transfection calcium phosphate system (Promega, Madison, WI). Culture supernatants containing infectious retrovirus were harvested 48 h post-transfection. Virus-containing supernatants were pooled and filtered through a 0.45-μm membrane and exponentially growing MEFs were transfected. RKO and MEF cells were grown in Dulbecco’s modified Eagle’s medium. In all experiments cells were grown to confluence and then re-fed with fresh medium and maintained for an additional 3 days. Cultures were again re-fed with fresh medium 1 day prior to each experiment.
Animal Models
Female C57BL/6 mice 6–8 weeks of age were purchased from Harlan Laboratories (Indianapolis, IN). Breeding pairs of TNF receptor 1/2 deficient mice (p55 and p75 deficient) B6:129S-Tnfrsflatm1 Imx and Tnfrsflbtm1 Imx/J were purchased from The Jackson Laboratory (Bar Harbor, ME). The care and treatment of experimental animals was in accordance with Institutional Guidelines and adherence to the NIH Guide for the Care and Use of Laboratory Animals.
Irradiation Conditions
RKO36 cells and MEF wild-type and TNFR1−R2− cells that were nontransfected or transfected with SOD2 siRNA as well as C57BL/6 wild-type and TNFR1−R2− knockout mice were irradiated at room temperature using a Philips X-ray generator operating at 250 kVp and 15 mA at a dose rate of 0.368 Gy/min to deliver a dose of 100 mGy or 1.65 Gy/min to deliver a dose of 2 Gy. For doses ranging from 5–100 mGy, the X-ray source was run at 10 mA and a dose rate of 0.0441 Gy/min.
Drug Treatment
Amifostine and WR1065 (2-[(aminopropyl)amino]ethanethiol), the active form of amifostine were supplied by the Drug Synthesis and Chemistry Branch, Division of Cancer Treatment, National Cancer Institute. For in vivo studies, amifostine was dissolved in phosphate-buffered saline (PBS; Invitrogen Life Technologies) and administered to C57BL/6 wild-type and TNFR1−R2− knockout mice intraperitoneally (i.p.) as a single dose of 400 mg/kg. For in vitro studies WR1065 is weighed and immediately before use dissolved in PBS at a working concentration of 1 M and then sterilized by passing through a 0.2-μm syringe filter. Cells were exposed to a final concentration of 40 μM. At this concentration the drug is rapidly taken up and is undetectable in the growth medium (11).
N-Ethyl-N-nitrosourea (ENU) was obtained from Sigma-Aldrich and was diluted in citrate phosphate buffer and filter sterilized just prior to use. ENU was administered i.p. to mice weighing 18 g in a 0.1 ml volume to give a final dose of 100 mg/kg. Approximately 3 h after ENU administration, animals were sacrificed and their spleens removed for determination of micronuclei formation.
Cell Survival Assay
RKO36, MEF wild-type and TNFR1−R2− knockout cells were irradiated with 5, 20, 50 or 100 mGy X rays, or treated with WR1065 24 h prior to irradiation with 2 Gy. Unirradiated cells and cells exposed only to WR1065 served as controls. Immediately after exposure to 2 Gy, cells were counted, diluted to specific cell concentrations, and known numbers were seeded into 60 mm dishes to yield from 25–100 colonies after 10–14 days of growth.
Micronucleus Assay
To assess the effects of various treatments on chromosomal integrity as it relates to the adaptive response, the frequencies of micronuclei formation using a standard cytokinesis block assay was used as described in detail elsewhere (12). For cultured RKO and MEF cells, 106 sham- or drug/radiation-treated cells were grown in T-25 culture flasks in the presence of 1.5 μg/ml cytochalasin B and incubated for 48 h at 37°C. At this concentration cytochalasin B was not toxic to either cell type. For spleen cells, spleens were aseptically removed and teased using a 22 g syringe and 3 ml of serum-free RPMI 1640 media (Invitrogen Life Technologies). Cells were passed through a #200 steel screen mesh, centrifuged at 1,000 rpm for 5 min, resulting pellet was disrupted into a single cell suspension and then layered onto room-temperature histopaque (Ficoll, Sigma Aldrich, St. Louis, MO) and centrifuged at 200g for 35 min at room temperature. The buffy coat was removed and washed with PBS three times after which the cells were placed in complete RPMI 1640 medium containing 10% fetal bovine serum (Atlanta Biologicals, Atlanta, GA), 5 mg/ml Concanavalin-A (Sigma-Aldrich), 10,000 U/ml interleukin-2 (Sigma-Aldrich), 0.05 M β-mercaptoethanol (Sigma-Aldrich) and 1% penicillin/streptomycin (Invitrogen Life Technologies). Cells in complete medium were then grown for 48 h to allow for stimulation into cell cycle. Afterward cells were removed, centrifuged at 1,000 rpm for 5 min at 4°C and then exposed to 1 μg/ml cytochalasin B (Sigma-Aldrich) and fixed following the procedure used for cultured cells.
All cells were harvested and fixed with cold methanol:acetic acid (3:1) and stored at −20°C. Slides were prepared and stained with acridine orange (0.01% in PBS) and examined under a fluorescence microscope (60×, dual-band filter). At least 1,000 binucleated cells with well-defined cytoplasm were scored for the presence of micronuclei. The frequency of micronucleus formation was calculated as the ratio of the number of binucleate cells containing micronuclei relative to the total number of binucleate cells scored.
SOD2 siRNA Transfection
The method of SOD2 siRNA transfection is described in detail elsewhere according to both the manufacturer’s instructions in the Lipofectamine Kit and previously published work (13). Briefly, MEF cells were grown to confluence and were transfected with 100 nM, final concentration, SOD2 or negative control (NC) short interfering RNA (siRNA, Applied Biosystems/Ambion, Foster City, CA). The RNA sequence was 5′-AAGGAACAACAGGCCTTATTC-3′. Lipofectamine 2000 reagent (Invitrogen Life Technologies) was used for transfection with siRNA oligomer in serum free medium. The siRNA-Lipofectamine 2000 complexes were added to culture dishes and were gently mixed to allow for a uniform distribution. Cells were incubated with transfection complexes for 24 h under normal growth conditions. Cells were then washed with PBS at 37°C and fresh growth medium was added.
SOD2 Enzyme Activity Assay
SOD2 activity was measured using the Superoxide Dismutase Assay Kit from Trevigen (Gaithersburg, MD) following the manufacturer’s instructions, and as described in detail elsewhere (8, 13). MEF cells were grown to confluence and harvested by scraping on ice and lysed with 350 μl lysis solution. Protein concentrations were determined following the standard Bradford assay and adjusted to 5 μg/μl with lysis buffer. The spectrophotometric SOD activity assay is a competitive inhibition assay, where the SOD enzyme inhibits the reaction of NBT with superoxide to form blue formazan, using the catalytic conversion of xanthine to uric acid by xanthine oxidase as a source of superoxide. Activity was measured using 50–500 μg of total cellular protein in the presence of 5 mM sodium cyanide (Sigma-Aldrich) to inhibit CuZnSOD (SOD1) activity. The percentage inhibition was calculated and plotted as a function of protein concentration for each treatment condition. The highest maximum percentage inhibition for each group of sample curves to be compared was determined and used to calculate the amount of protein that inhibited NBT reduction by 50% of this maximum value. SOD2 activities in U/mg protein were then calculated.
Statistical Analysis
Means and standard errors were calculated for all data points from three independent experiments. Pairwise comparisons of SOD2 activity, micronuclei frequency and survival between each of the experimental conditions were performed using Student’s two-tailed t test.
RESULTS
Very Low-Dose Range Induction of the Adaptive Response
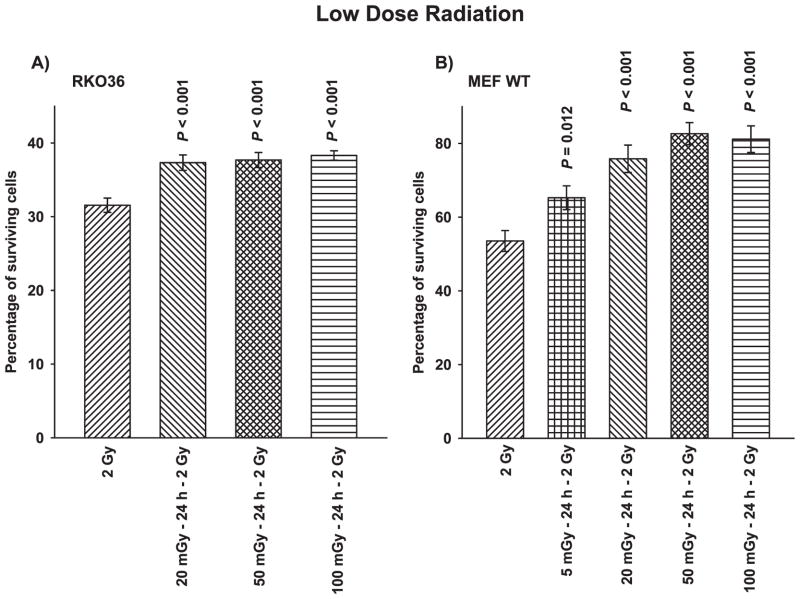
Figure 1A and B present demonstrations of the effectiveness of very low radiation doses that can induce adaptive responses in both human (RKO36 colon carcinoma cells) and mouse embryo fibroblast (MEF) cells. When cells are challenged with a 2 Gy dose of radiation 24 h after exposure to radiation doses ranging from 5–100 mGy, cell survival was found to be significantly enhanced in the range from 5–20%.
FIG. 1.
The effects of very low doses of ionizing radiation on the induction of an adaptive response following an exposure to a 2 Gy challenge dose as evidenced by an elevation in cell survival compared to cells irradiated with only 2 Gy. Panel A: The adaptive response in RKO36 human colon carcinoma cells. Panel B: The adaptive response in wild-type transformed mouse embryo fibroblasts (MEF WT). P values above the bar compare treatment groups with the corresponding 2 Gy only control. Each experiment was performed two times and error bars represent the standard error of the mean (SEM).
Treatment Effects on SOD2 Activity
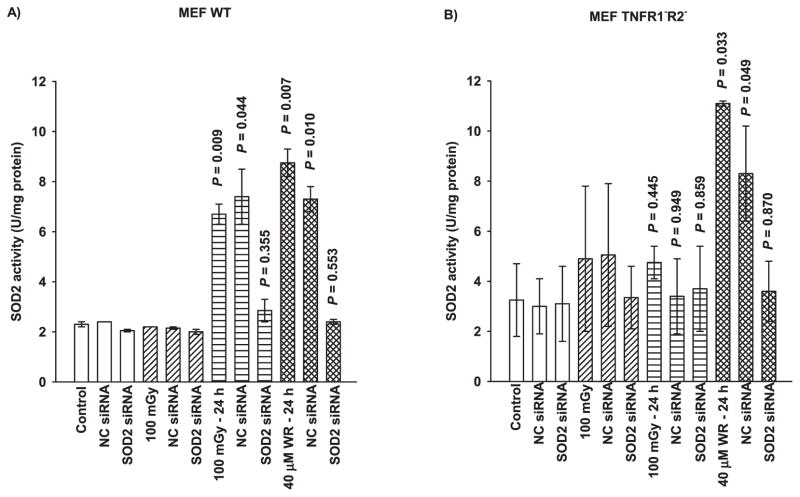
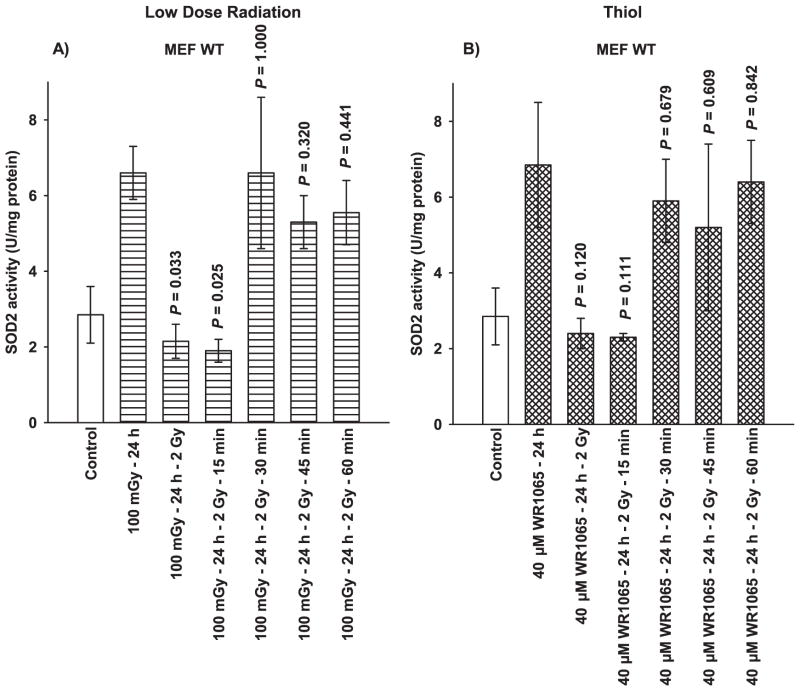
SOD2 is known to play an important role in the adaptive response (7–10, 13). Figure 2 shows data that describe the inductive effects of 100 mGy very low-dose radiation and the free thiol metabolite of amifostine, WR1065, on SOD2 activity as a function of TNF signaling and SOD2 siRNA transfection. Exposure to 100 mGy irradiation or 40 μM WR1065 induces a robust elevation in SOD2 activity in MEF WT 24 h after exposure and transfection of cells and to with SOD2 siRNA inhibits this increase to background levels (Fig. 2A). The negative control (NC) siRNA containing scrambled sequences of bases was also used in transfection studies as a transfection control. It had no significant effect on SOD2 activity measured after either very low-dose irradiation or thiol exposure in both cell systems (Fig. 2). MEF TNFR1−R2− cells deficient in both receptors required for TNF signaling exhibited no elevation in SOD2 activity 24 h after irradiation with 100 mGy (Fig. 2B). However, WR1065 treatment induced a robust elevation in SOD2 activity in these cells consistent with the magnitude of elevation seen in MEF WT cells. Transfection with SOD2 siRNA resulted in a diminution of this elevated SOD2 activity in MEF TNFR1−R2− to background levels.
FIG. 2.
The effects of 100 mGy irradiation and 40 μM WR1065 (WR) on the subsequent elevation of SOD2 activity 24 h later in (panel A) wild-type and (panel B) TNFR1−R2− MEF as a function of NC siRNA and SOD2 siRNA transfection. SOD2 activity was measured in untreated control cells and was measured in 100 mGy and WR1065-exposed cells either immediately following or 24 h later. NC siRNA represents negative control siRNA. Each experiment was repeated at least two times and error bars represent the SEM. P values comparing cells exposed to 100 mGy or 40 μM WR1065 to their respective untreated controls are presented.
Very Low-Dose Radiation-Induced Adaptive Response
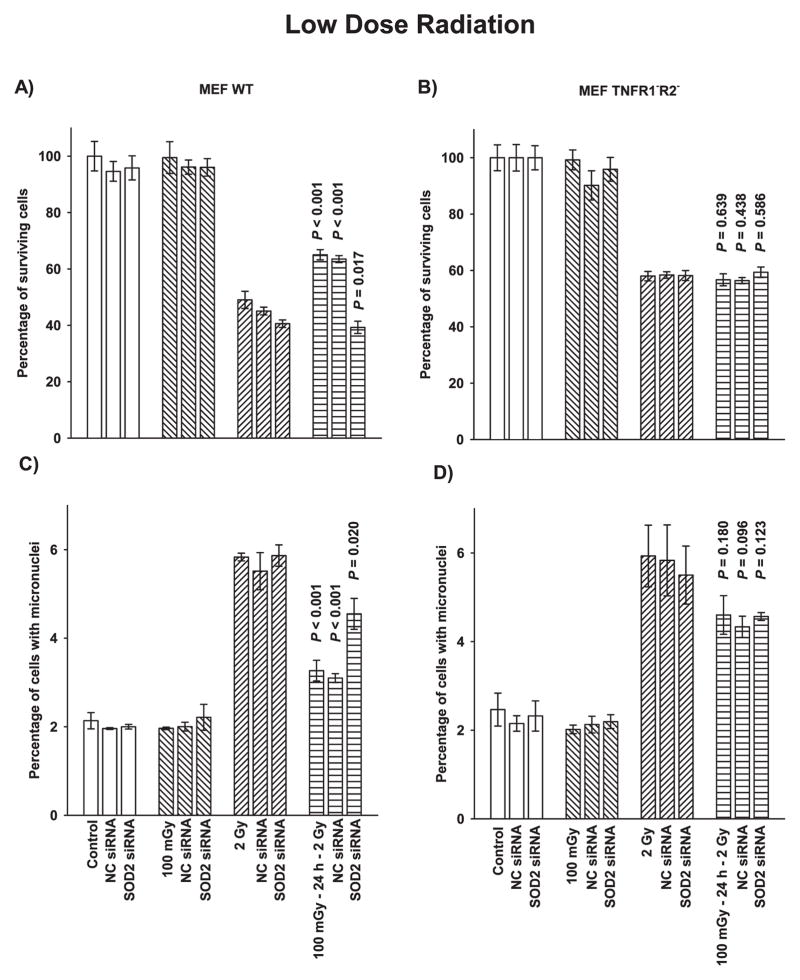
The effects of very low-dose irradiation on adaptive responses was measured using two end points, cell survival and micronuclei formation, as a function of TNF signaling capability and transfection with SOD2 siRNA. As described in Fig. 3A and C, MEF wild-type irradiated with 100 mGy exhibited a robust adaptive response 24 h later when challenged with a 2 Gy dose as characterized by a highly significant increase in survival (P < 0.001) and a reduction in micronuclei formation (P < 0.001), respectively. Transfection of MEF wild-type cells with SOD2 siRNA significantly inhibited the adaptive response demonstrating the importance of elevated SOD2 enzymatic activity to the demonstration of this adaptive response regardless of the two end points measured.
FIG. 3.
The effects of 100 mGy exposure and SOD2 siRNA transfection on the adaptive response end points of cell survival as determined by colony forming ability and micronuclei formation. Panels A and B describe the survival response of MEF wild-type (WT) cells and TNFR1−R2−knockout cells, respectively. Panels C and D describe micronuclei formation as an end point in MEF WT and TNFR1−R2− cells, respectively. Experiments were repeated at least three times and error bars represent the SEM. P values were obtained for comparisons between cells exposed to 100 mGy without or with transfection with NC siRNA or SOD2 siRNA and cells exposed to 2 Gy only. Adaptive responses for cell survival and micronuclei formation were found to be highly significant only in MEF WT cells.
MEF TNFR1−R2− cells did not exhibit an adaptive response for either end point when exposed to 100 mGy followed 24 h later by a 2 Gy challenge dose (Fig. 3B and D). Transfection of these cells with SOD2 siRNA likewise had no effect on either of these end points.
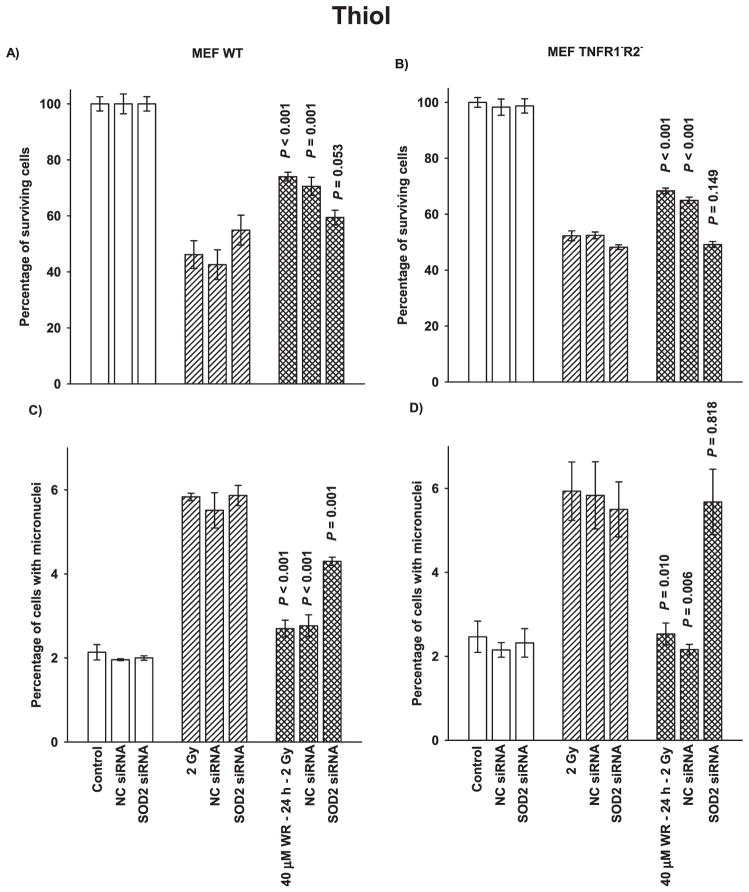
WR1065 Thiol-Induced Adaptive Response
Both MEF wild-type and TNFR1−R2− cells exhibit adaptive responses when they are exposed to WR1065 24 h prior to being given a 2 Gy challenge dose of radiation (Fig. 4). Cell survival for both cell systems was significantly elevated after thiol exposure (Fig. 4A and B) as was reduction in micronuclei formation (Fig. 4C and D) compared to cells irradiated with 2 Gy, but not previously exposed to WR1065. Transfection of both cell systems with SOD2 siRNA resulted in either a complete inhibition of the adaptive responses (Fig. 4B and D) or a significant reduction in the magnitude of the responses (Fig. 4A and C) indicating that elevations in SOD2 enzymatic activity induced by WR1065 is independent of the TNF signaling pathway and that it is an important factor in the adaptive response.
FIG. 4.
The effects of 40 μM WR1065 (WR) exposure and SOD2 siRNA transfection on the adaptive response end points of cell survival as determined by colony forming ability and micronuclei formation. Panels A and B show the survival response of MEF wild-type and TNFR1−R2− cells, respectively. Panels C and D show micronuclei formation as an end point in MEF wild-type and TNFR1−R2− cells, respectively. Experiments were repeated at least three times and error bars represent the SEM. P values were obtained for comparisons between cells exposed to 40 μM WR1065 without or with transfection with NC- and SOD2 siRNA and cells exposed to 2 Gy only. Adaptive responses for cell survival and micronuclei formation were found to be highly significant for both MEF wild-type and TNFR1−R2− cells.
Effect of High-Dose Irradiation on SOD2 Activity
SOD2 activity was significantly elevated over background levels 24 h following either exposure to 100 mGy or 40 μM WR1065 (Fig. 2). Figure 5 describes the effects of a 2 Gy dose on SOD2 activity in MEF wild-type as a function of time after radiation exposure. Immediately following radiation exposure and for at least 15 min thereafter, SOD2 activity levels fell to approximately unirradiated control levels and then raised to pre-2 Gy irradiation levels by 30 min. The results demonstrated that SOD2 activity can be affected quickly following oxidative stress caused by an exposure to 2 Gy.
FIG. 5.
Effects of a 2 Gy radiation exposure as a function of time on SOD2 activity levels induced by a 100 mGy or 40 uM WR1065 exposure 24 h earlier. Activity levels are contrasted to unirradiated control cells. Times in minutes represent times post 2 Gy irradiation. Each experiment was repeated at least twice and error bars represent the SEM.
Demonstration of an Adaptive Response in Animal Systems
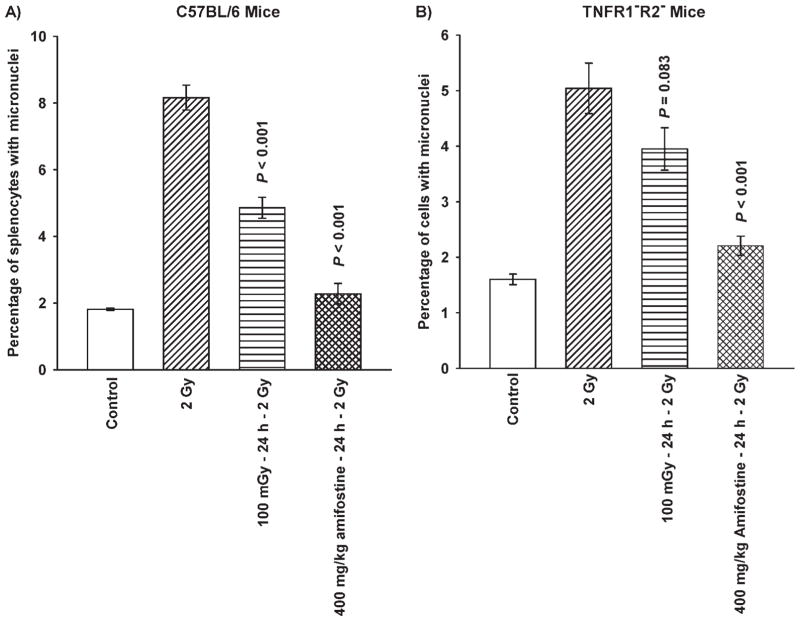
Micronuclei formation was chosen as the end point for the characterization of the adaptive response in our mouse models due to its ease of use and the demonstration of its effectiveness under in vitro conditions as being a meaningful end point. Figure 6 describes the relative effects of very low-dose irradiation and amifostine on inducing adaptive responses in wild-type C57BL/6 and TNFR1−R2− knockout mice. C57BL/6 mice exhibited significant adaptive responses (P < 0.001) after either very low-dose radiation or thiol drug treatment 24 h prior to challenge with 2 Gy. Under in vivo conditions amifostine was observed to be a more potent inducer of this protective response (Fig. 6A). Amifostine works independently of TNF signaling in activating NF-κB and subsequent SOD2 gene expression. This may, in part, account for this more robust response. Consistent with in vitro results using MEF TNFR1−R2− knockout cells, very low-dose irradiation failed to induce a significant adaptive response (P = 0.083) as robust as amifostine (P < 0.001) (Fig. 6B).
FIG. 6.
Effects of 100 mGy whole-body irradiation and 400 mg/kg amifostine i.p. treatment, each administered 24 h prior to a whole-body challenge dose of 2 Gy, on micronuclei formation in splenocytes from C57BL/6 wild-type (panel A) and corresponding TNFR1−R2− knockout mice (panel B). Each experiment was repeated twice and error bars represent the SEM. P values were obtained by comparing 100 mGy and 400 mg/kg amifostine pre-treatments with their corresponding 2 Gy only radiation groups.
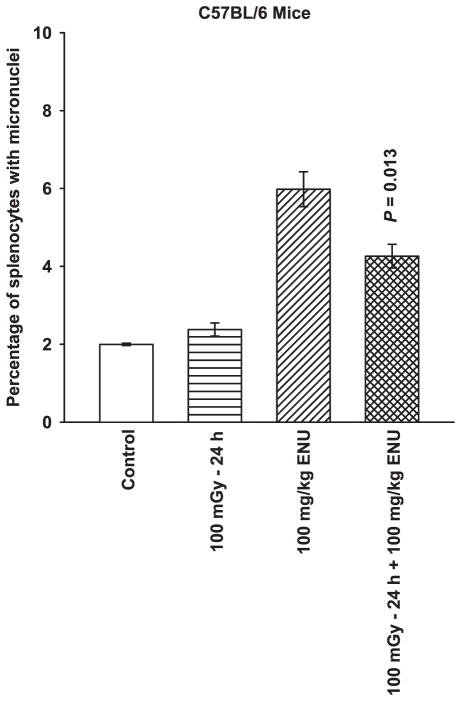
To determine whether the adaptive response could be extended to other deleterious agents other than ionizing radiation, C57BL/6 mice were exposed to the alkylating agent ENU, 100 mg/kg, 24 h after their exposure to 100 mGy (Fig. 7). ENU induced micronuclei formation was significantly reduced (P = 0.013) in splenocytes derived from wild-type mice previously exposed to 100 mGy compared to nonirradiated mice.
FIG. 7.
The effects of 100 mGy whole-body irradiation, 100 mg/kg ENU treatment, and 100 mGy irradiation followed 24 h later with exposure to 100 mg/kg ENU on micronuclei formation in splenocytes in C57BL/6 mice. The experiment was repeated twice and error bars represent the SEM. P value was obtained by comparing the percentage of micronuclei formation in the 100 mGy 24 h +100 mg/kg ENU group to the ENU only group.
DISCUSSION
The very low-dose radiation-induced adaptive response has been investigated over the years as a phenomenon of general interest to the radiation research community. With the expanded development and implementation of CAT in both diagnostic and therapeutic applications as well as the use of thiol containing agents such as amifostine during cancer therapy, the potential effects of inducible adaptive responses in the context of factors capable of mitigating therapeutic outcomes requires more study. Recently we reported that murine BFS fibrosarcoma cells defective in both TNFR1 and R2 could not be induced by a 100 mGy exposure to exhibit an adaptive response to a subsequent 2 Gy challenge dose (13). Cells defective in only TNFR1 were able to exhibit a very low dose initiated adaptive response while a thiol containing drug like amifostine could induce this response in cells regardless of their TNFR status. As described in Fig. 1, the very low-dose-induced adaptive response can be induced by a range of doses in both human and animal tumor models. The underlying mechanism of action that is proposed to describe this phenomenon is the ability of very low radiation doses to initiate a TNF signaling pathway (4–6) that can activate NF-κB. Exposure of cells to thiols can bypass TNF signaling and directly activate NF-κB (7, 14, 15). Activation of NF-κB then can lead to induction of SOD2 gene expression (16). The subsequent kinetics of SOD2 protein increase has been characterized in a number of human and rodent cell lines. Consistent with each experimental system tested in our laboratory, the level of SOD2 activity begins to increase at 4 h, peaks at about 24 h after exposure to TNF or thiols and then decays back to background levels in about 48 h (8–10, 13). The effects of very low-dose radiation and thiol exposure on the induction of adaptive processes using both wild-type and engineered TNFR1−R2− mouse embryo fibroblasts are in agreement with those observed with similarly modified BFS fibrosarcoma wild-type and TNFR1−R2− knockout cells (13). WR1065, the free thiol form of amifostine, because of its ability to directly activate NF-κB and subsequent SOD2 gene expression (7–9, 13–15) was used as a positive control to specifically affect and thus identify the importance of elevated SOD2 activity in this adaptive response. Data presented in Fig. 2A and B demonstrate that very low-dose irradiation induces a robust elevation of SOD2 activity 24 h later in wild-type cells only. In contrast, WR1065 can induce a similar response 24 h later in both wild-type and TNFR1−R2− cells. Transfection of these cells with SOD2 siRNA significantly inhibited the induction of SOD2 activity. These results served as the basis for interpreting the adaptive response end points of elevated survival and reduced micronuclei formation as a function of the various treatment regimens.
Previous studies describing the kinetics of SOD2 changes in both protein level and activity after exposure to 100 mGy, TNF-α, or thiols indicate that maximum SOD2 activity occurs approximately 24 h later in both murine and human cells in culture (8–10, 13). Data in Fig. 3 shows adaptive responses, reflected by enhanced cell survival and reduced micronuclei formation in TNF wild-type, but absent in TNFR1−2− knockout cells at 24 h are consistent with this kinetic pattern of changes in SOD2 activity. The importance of elevated SOD2 activity in these adaptive responses is also supported by the profound inhibitory effectiveness of transfection with SOD2 siRNA and the lack of an effect exhibited after transfection with NC siRNA.
NF-κB activation is known to play an important role in SOD2 gene expression. It is activated through TNF signaling (4–6) and direct exposure to thiol-containing drugs such as N-acetyl-cysteine, oltipraz, mesna, captopril, and WR1065 (7, 14–16). Thiols activate NF-κB through the reduction of disulfide bonds in cysteine residues in its p50 and p65 subunits (7, 17), and its activation affects an intronic binding element in the SOD2 gene that has been identified as being essential for the rapid activation of the gene to stress-inducing agents such as ionizing radiation and TNF-α (16). The data in Fig. 4 support this model of TNF independent, thiol mediated activation of NF-κB and subsequent elevation of SOD2 activity as evidenced by the adaptive responses observed in both the wild-type and TNFR1−R2− knockout MEFs. Transfection with SOD2 siRNA also prevented thiol-induced adaptive responses in both cell types (Fig. 4). Regardless of the inducing agent, the expression of these adaptive responses is directly correlated to elevated levels of SOD2 activity at the time of exposure to high doses of radiation. This activity is significantly but transiently reduced as a result of oxidative challenge as demonstrated in Fig. 5. The rapid inactivation of SOD2 has been attributed to a process of biological protein nitration induced by oxidative damage (18). This is a very dynamic process in which nitration, denitration, and renitration can occur within minutes within the mitochondria following oxidative stress. Mitochondria can completely eliminate 3-nitrotyrosyl adducts during 20 min of hypoxia-anoxia induced stress (19).
The inverse correlation between effects on cell survival and micronuclei formation as end points for describing the extent of the adaptive responses induced by very low-dose irradiation allow for the use of micronuclei formation as a relatively easily implemented biomarker to assess the presence of an adaptive response in human and animal models. As described in Fig. 6 and consistent with the in vitro data, a radiation dose of 100 mGy significantly induced an adaptive response in C57BL/6 wild-type mice, but not in the TNFR1−R2− knockout mice. Amifostine, consistent with in vitro data, was equally effective in reducing the frequency of micronuclei formation in both mouse models. The ability of very low-dose irradiation to induce an adaptive response was also demonstrated for C57BL/6 mice exposed to the alkylating agent ENU (Fig. 7) demonstrating the usefulness of the micronuclei end point for assessing the expression of adaptive responses under in vivo conditions where cell survival assays are not easily applicable.
SOD2 activity has been reported to be elevated in a transplantable murine tumor 24 h after administration of 400 mg/kg of amifostine to the host animals resulting in an increased resistance to ionizing radiation (20). Using a mouse sarcoma in vitro model, daily or every other day administration with WR1065 was found to induce an elevated SOD2 activity level that was accompanied by an enhanced resistance to radiation-induced cell killing, and this effect could be maintained for at least 14 days (21). The potential clinical relevance of these observations is that thiol containing drugs and very low-dose ionizing radiation, consistent with doses in the range delivered by CAT, are capable of inducing adaptive responses in tumor cells. The timing of CAT procedures in relationship to administration of therapeutic treatments can become an important parameter to minimize these potential adverse adaptive responses due to elevated SOD2 activity. Depending on the timing and frequency of very low radiation doses delivered as a result of therapy coupled imaging procedures, increases in cell survival as low as 5–10% after each imaging procedure through a multi-treatment course of radiotherapy could impact overall anticipated tumor response.
Acknowledgments
We acknowledge the excellent technical assistance of Mr. Ken Baker in the scoring of micronuclei data and Mr. Michael Becket for the isolation and development of the transformed wild-type and TNFR1−R2− MEFs. Both are members of the Department of Radiation and Cellular Oncology, The University of Chicago, Chicago, IL. This work was supported in part by the DOE Low Dose Program/Project Grant DE-SC0001271 awarded to Drs. Grdina, Li, Woloschak. Dr. Grdina was also supported by NIH NCI R01-CA132998 and Dr. Weichselbaum by NIH NCI R01-CA111423.
Footnotes
Conflict of interest disclosure: Dr. David J. Grdina is a paid consultant to Pinnacle Biologics. Drs. Grdina and Murley are minority equity partners in Pinnacle Oncology LLC regarding the potential novel uses of amifostine. Dr. Weichselbaum is a consultant to Reflexion, a radiotherapy company.
References
- 1.Wolff S, Afzal V, Wiencke JK, Olivieri G, Michaeli A. Human lymphocytes exposed to low doses of ionizing radiations become refractory to high doses of radiation as well as to chemical mutagens that induce double strand breaks in DNA. Int J Radiat Biol Relat Stud Phys Chem Med. 1988;53:39–47. doi: 10.1080/09553008814550401. [DOI] [PubMed] [Google Scholar]
- 2.Bonner WM. Low-dose radiation: thresholds, bystander effects, and adaptive responses. PNAS USA. 2003;100:4973–75. doi: 10.1073/pnas.1031538100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phan N, De Lisio M, Parise G, Boreham DR. Biological effects and adaptive response from single and repeated computed tomography scans in reticulocytes and bone marrow of C57BL/6 mice. Radiat Res. 2012;177:164–75. doi: 10.1667/rr2532.1. [DOI] [PubMed] [Google Scholar]
- 4.Neta R. Modulation of radiation damage by cytokines. Stem Cells. 1997;15(Suppl 2):87–94. doi: 10.1002/stem.5530150713. [DOI] [PubMed] [Google Scholar]
- 5.Neta R. Modulation with cytokines of radiation injury: Suggested mechanisms of action. Environ Health Perspect. 1997;105(Suppl 6):1463–65. doi: 10.1289/ehp.97105s61463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh VK, Yadav VS. Role of cytokines and growth factors in radioprotection. Exp Mol Pathol. 2005;78:156–69. doi: 10.1016/j.yexmp.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Murley JS, Kataoka Y, Hallahan DE, Roberts JC, Grdina DJ. Activation of NFκB and MnSOD gene expression by free radical scavengers in human microvascular endothelial cells. Free Radic Biol Med. 2001;30:1426–39. doi: 10.1016/s0891-5849(01)00554-8. [DOI] [PubMed] [Google Scholar]
- 8.Murley JS, Kataoka Y, Weydert CJ, Oberley LW, Grdina DJ. Delayed radioprotection by nuclear transcription factor kappaB-mediated induction of manganese superoxide dismutase in human microvascular endothelial cells after exposure to the free radical scavenger WR1065. Free Radic Biol Med. 2006;40:1004–16. doi: 10.1016/j.freeradbiomed.2005.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murley JS, Kataoka Y, Baker KL, Diamond AM, Morgan WF, Grdina DJ. Manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by the free thiol form of amifostine and tumor necrosis factor alpha. Radiat Res. 2007;167:465–74. doi: 10.1667/RR0758.1. [DOI] [PubMed] [Google Scholar]
- 10.Murley JS, Kataoka Y, Miller RC, Li JJ, Woloschak G, Grdina DJ. SOD2-mediated effects induced by WR1065 and low dose ionizing radiation on micronucleus formation in RKO human colon carcinoma cells. Radiat Res. 2011;175:57–65. doi: 10.1667/RR2349.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grdina DJ, Shigematsu N, Dale P, Newton GL, Aguilera JA, Fahey RC. Thiol and disulfide metabolites of the radiation protector and potential chemopreventive agent WR2721 are linked to both its anticytotoxic and antimutagenic mechanisms of action. Carcinogenesis. 1995;16:767–74. doi: 10.1093/carcin/16.4.767. [DOI] [PubMed] [Google Scholar]
- 12.Fenech M. Cytokinesis-block micronucleus cytome assay. Nat Protoc. 2007;2:1084–104. doi: 10.1038/nprot.2007.77. [DOI] [PubMed] [Google Scholar]
- 13.Murley JS, Baker KL, Miller RC, Darga TE, Weichselbaum RR, Grdina DJ. SOD2-mediated adaptive responses induced by low-dose ionizing radiation via TNF signaling and amifostine. Free Radic Biol Med. 2011;51:1918–25. doi: 10.1016/j.freeradbiomed.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das KC, Lewis-Molock Y, White CW. Activation of NFκB and elevation of MnSOD gene expression by thiol reducing agents in lung adenocarcinoma (A549) cells. Am J Physiol. 1995;269:L588–602. doi: 10.1152/ajplung.1995.269.5.L588. [DOI] [PubMed] [Google Scholar]
- 15.Antras-Ferry J, Maheo K, Chevanne M, Dubos MP, Morel F, Guillouzo A, et al. Oltipraz stimulates the transcription of the manganese superoxide dismutase gene in rat hepatocytes. Carcinogenesis. 1997;18:2113–17. doi: 10.1093/carcin/18.11.2113. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y, Devalara JA, Yeh CC, Majima H, Kasarskis EJ, St Clair DK. An intronic NF-κB element is essential for induction of human manganese superoxide dismutase gene by tumor necrosis factor-α and interleukin 1β. DNA Cell Biol. 1999;18:709–22. doi: 10.1089/104454999314999. [DOI] [PubMed] [Google Scholar]
- 17.Matthews JR, Wakasugi N, Virelizier J, Yodoi J, Hay RT. Thioredoxin regulates the DNA binding activity of NFκB by reducing of a disulphide bond involving cysteine 62. Nucleic Acids Res. 1992;20:3821–30. doi: 10.1093/nar/20.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aulak KS, Koeck T, Crabb JW, Stuehr DJ. Dynamics of protein nitration in cells and mitochondria. Am J Physiol Heart Circ Physiol. 2004;286:H30–8. doi: 10.1152/ajpheart.00743.2003. [DOI] [PubMed] [Google Scholar]
- 19.Koeck T, Fu X, Hazen SL, Crabb JW, Stuehr DJ, Aulak KS. Rapid and selective oxygen-regulated protein tyrosine denitration and nitration in mitochondria. J Biol Chem. 2004;279:27257–62. doi: 10.1074/jbc.M401586200. [DOI] [PubMed] [Google Scholar]
- 20.Grdina DJ, Murley JS, Kataoka Y, Baker KL, Kunnavakkam R, Coleman MC, et al. Amifostine induces antioxidant enzymatic activities in normal tissues and a transplantable tumor that can affect radiation response. Int J Radiat Oncol Biol Phys. 2009;73:886–96. doi: 10.1016/j.ijrobp.2008.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murley JS, Nantajit D, Baker KL, Kataoka Y, Li JJ, Grdina DJ. Maintenance of manganese superoxide dismutase (SOD2)-mediated delayed radioprotection induced by repeated administration of the free thiol form of amifostine. Radiat Res. 2008;169:495–505. doi: 10.1667/RR1194.1. [DOI] [PMC free article] [PubMed] [Google Scholar]