Abstract
Heart failure is a pressing public health problem with no curative treatment currently available. The existing therapies provide symptomatic relief, but are unable to reverse molecular changes that occur in cardiomyocytes. The mechanisms of heart failure are complex and multiple, but mitochondrial dysfunction appears to be a critical factor in the development of this disease. Thus, it is important to focus research efforts on targeting mitochondrial dysfunction in the failing heart in order to revive the myocardium and its contractile function. This review highlights the three promising areas for the development of heart failure therapies, including mitochondrial biogenesis, mitochondrial oxidative stress and mitochondrial iron handling. Moreover, the translational potential of compounds targeting these pathways is discussed.
Keywords: Heart Failure, Mitochondria, Cardiomyocytes
Introduction
The 20th century has witnessed a dramatic improvement in patients’ survival following adverse cardiovascular events. However, heart disease still remains the number one cause of death in the industrialized world affecting over 27 million people in the United States alone. With $40 billion in annual costs, and one out of every five patients dying within one year of diagnosis (1), HF has become a major public health problem. Although significant progress has been made in the outpatient management of chronic HF, post-discharge mortality and re-hospitalization rates within 60–90 days can be as high as 15% and 30%, respectively (2).
The primary goal of treating HF patients is restoration of cardiac function. Recent studies show that heart function can be successfully recovered in patients with HF, even after structural alterations have occurred. Thus, failing myocardium is “viable but dysfunctional”, rather than irreversibly damaged, independent of the presence or absence of coronary artery disease. This finding opens up an avenue for rational design of treatments that target the cardiomyocyte itself, not the indirect pathways that suppress neurohormonal axis or induce vasodilation.
The mechanisms underlying the development of HF are multiple, complex, and are not well understood. While virtually all aspects of myocyte physiology are altered in HF, the last decade of research provided convincing evidence that mitochondrial dysfunction may be an important event in the development of hypertrophy and HF. First, genetic mutations that disrupt mitochondrial function are associated with cardiac dysfunction in mice (3) and humans (4, 5). Second, currently available therapies for HF, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor II (ATII) blockers significantly improve survival in ischemic and non-ischemic HF, and their administration also correlates with improved mitochondrial function (6, 7). Finally, it is important to note that cardiomyocytes in the failing heart remain viable, though metabolically stunned, and their function can potentially be rescued (8). Thus, therapeutic efforts should venture beyond symptomatic relief and focus on reviving the dormant myocardium by targeting the underlying molecular defects in HF. In this review we will discuss the changes that occur in the mitochondria of failing myocardium, followed by an overview of the pertinent therapeutic targets and approaches that can potentially reverse these changes and preserve cardiac health. Of note, we will refrain from discussing the changes in glucose and fatty acid utilization, as these topics have recently been reviewed in detail elsewhere (8). Instead, we will focus on mitochondrial biogenesis, production of reactive oxygen species (ROS) and maintenance of cellular iron homeostasis as promising novel therapies for HF.
I. Mitochondrial Biogenesis
Pathophysiology
One of the ways to augment energy production in the setting of increased contractile demand is to stimulate production of new mitochondria, termed mitochondrial biogenesis. Mitochondria contain about 16.5 kb of circular double-stranded DNA that encodes 13 protein components of the electron transport chain and needs to be replicated prior to the division. In addition, up to 1000 nuclear-encoded proteins must be imported into the newly formed mitochondria to make a fully functional organelle (9). Thus, generation of new mitochondria requires a coordinated transcription of mitochondrial and nuclear genomes orchestrated by peroxisome proliferator-activated receptor gamma co-activator (PGC1α) (10). PGC1α, a nuclear-encoded protein, is induced in the states of enhanced energy demand, such as increased cardiac workload, high ADP/ATP ratio, cold, exercise, and fasting (for review see (11, 12)). High PGC1α activity is associated with increased mitochondrial content, as exemplified by cardiac-specific PGC1α transgenic mice, which exhibit uncontrolled mitochondrial proliferation and increase in markers of mitochondrial biogenesis (13, 14). PGC1α stimulates mitochondrial proliferation through its interaction with several transcription factors. First, PGC1α binds to and co-activates nuclear respiratory factors 1 and 2 (NRF1/2), which in turn promote transcription of nuclear-encoded genes targeted to mitochondria (15). Second, PGC1α activates estrogen-related nuclear orphan receptors, ERRα and γ, which induce expression of genes involved in glucose and fatty acid uptake, energy production, and ATP transport (16, 17). Finally, PGC1α promotes replication of mitochondrial genome through NRF1/2-mediated induction of mitochondrial transcription factor A (Tfam) (12). Cardiac-specific deletion of NRF1 (18), ERRα (19) and Tfam (20) are all associated with decreased mitochondrial content or function, confirming their role in mitochondrial biogenesis.
Studies of rodents (21–23), dogs (24) and humans (25) suggest that disruption of mitochondrial biogenesis represents an early event in pathophysiology of HF, whose timely reversal is cardioprotective. Grossly, mitochondrial content and mtDNA copy number are significantly reduced in rodent and human failing myocardium, and downregulation of PGC1α pathway has been observed in various models of HF in mice and rats (21, 22, 26, 27) . However, the role of PGC1α in human HF remains controversial and contradictory results have also been reported (28–30). Since PGC1α is extensively regulated on the post-translational level by phosphorylation (31), acetylation (32), and protein stabilization (33), it is not clear whether PGC1α activity is reduced in the failing hearts and whether the reduction in mitochondrial number in HF in humans is due to deregulation of PGC1α signaling.
A defect in mitochondrial DNA replication was proposed as an alternative mechanism for the reduction in mitochondrial biogenesis (30, 34). Importantly, changes in mtDNA replication machinery represented a very early event detected in hypertrophied hearts that have not yet transitioned into failure (30). The actual trigger for reducing mtDNA replication in a setting of increased workload is unknown, and it would be of interest to replicate these studies in animal models and/or HF patients.
Therapeutic Strategies
Despite the controversy about the role of PGC1α in human HF, boosting mitochondrial biogenesis in failing myocardium appears to be beneficial (35). In fact, ACE inhibitor captopril was shown to increase mitochondrial content in the hearts of dogs following coronary ligation (36), suggesting that some of its beneficial effects may be due to the stimulation of mitochondrial biogenesis. While currently no drugs that specifically target mitochondrial biogenesis in HF are available, acceleration of this process through AMPK, eNOS and other pathways may represent a promising therapeutic approach (Figure 1)
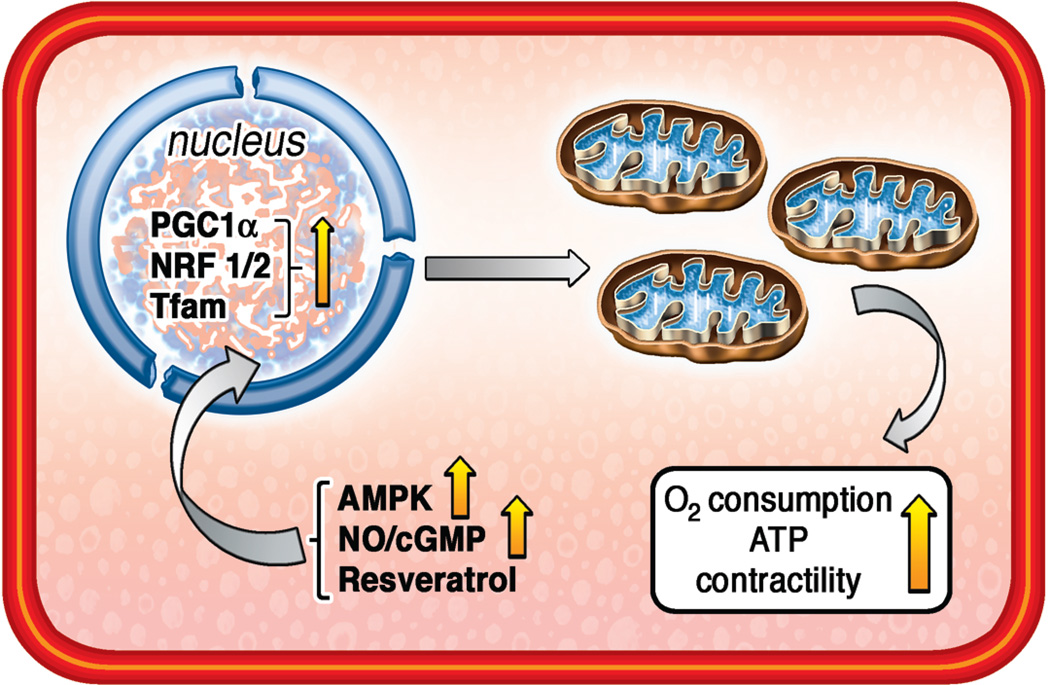
Figure 1. Mitochondrial Biogenesis.
Mitochondrial biogenesis impairment is an early event in the development of HF and reversal of this process is cardioprotective. Mitochondrial biogenesis can be enhanced therapeutically with the use of AMPK agonists, stimulants of NO/cGMP pathway (including PDE5 inhibitors), or resveratrol. All of these approaches stimulate nuclear-encoded proteins PGC1α, NRF1/2 and Tfam which, in turn, facilitate production of new mitochondria in the heart.
AMPK
AMP-activated protein kinase (AMPK) exhibits very low baseline activity in the heart, but is upregulated in response to a variety of stressors (37, 38). Importantly, activation of AMPK is thought to be the mechanism driving the increase in mitochondrial biogenesis in exercise-induced adaptive hypertrophy of the athlete’s heart (39) which, unlike the pathologic hypertrophy discussed earlier, does not lead to HF. AMPK induces PGC1α function (39, 40), activates NRF1, Tfam (41), and ERRα (28), establishing this kinase as an important modulator of mitochondrial biogenesis.
The activity of AMPK is increased in failing hearts (42), however pharmacological activation of this pathway appears to exert additional cardiac protection. For example, metformin, a commonly used anti-diabetic drug that activates AMPK signaling, reduced infarct size and preserved cardiac function in a long-term post-MI rat model in the absence of diabetes (43) and exhibited cardioprotective properties in rapid ventricular pacing canine model (44). Gundewar et al. found increased PGC1α levels and preservation of heart function in wild-type metformin-treated mice subjected to MI or I/R, but not in AMPK knockout mouse model (45). Although metformin was also shown to reduce gluconeogenesis through direct inhibition of mitochondrial complex I, the total cellular ATP content in the livers of metformin-treated rats remained unchanged, consistent with the maintenance of the cumulative mitochondrial bioenergetic capacity possibly due to increased biogenesis (46).
Accumulating evidence for cardioprotective properties of AMPK in the setting of hypertrophy and cardiac failure calls for the development of pharmacologic agonists of AMPK in the heart. Several clinically-available compounds have been shown to activate AMPK, including metformin, AICAR, thiazolidinediones and statins. Morever, ATII receptor blocker telmisartan was shown to increase phosphorylated AMPKα levels in cultured myotybes (7), suggesting that beneficial effects of this drug may partially be due to stimulation of mitochondrial biogenesis. However, the effect of current HF therapies on AMPK pathway is likely indirect via an increase in AMP/ATP ratio, inhibition of mitochondrial respiration or other cellular and systemic effects (47). Compounds targeting AMPK itself are currently in development. Of note, compound A769662 by Abbott Laboratories is a specific allosteric activator of AMPK complexes on β subunit (48). A769662 reduced infarct size in rats fed low-fat and high-fat diets (49), providing a proof of principle that a direct activation of AMPK is beneficial to the heart. Unfortunately, the compound also inhibited 26S proteasome and caused cell-cycle arrest through an AMPK-independent mechanism, limiting its clinical prospects (50). Another small molecule, PT1, appears to activate AMPKα1 and AMPKα2 isoforms by removing autoinhibition in the catalytic subunits of the kinase (51). Thus, PT1 potentially targets a vast array of AMPK complexes, though its effects on the heart remain to be studied.
Several points must be considered when developing an AMPK inhibitor agonist for the treatment of HF. First, AMPK activation may only be suitable for treatment of HF due to defined etiologies. Although AMPK activation was protective in the mouse models of MI and I/R, it failed to preserve cardiac function in rats with chronic volume overload (52). Second, AMPK is ubiquitously expressed, regulates an array of processes, and the subunit composition of AMPK heterodimeric complex differs widely between tissues (53). Thus, in order to maximize clinical benefits and minimize toxicity, specific isoforms of AMPK must be targeted. This principle is exemplified by a finding that a gain-of-function mutation in the AMPKγ2 subunit induces glycogen accumulation and progressive hypertrophic cardiomyopathy in humans (54). Studies examining expression patterns and functions of different AMPK isoforms will lay a foundation for the development of novel therapeutic agonists for the treatment of HF.
eNOS/NO/cGMP pathway
Nitric oxide (NO) is a diffusible signaling molecule released by endothelial cells through the action of endothelial nitric oxide synthase (eNOS), which enhances production of cyclic guanosine monophosphate (cGMP) and leads to smooth muscle relaxation. This enzyme is also expressed in the heart where NO and cGMP activate many potentially cardioprotective pathways (55, 56). Recent evidence suggests that eNOS/NO/cGMP pathway is an important activator of mitochondrial biogenesis (57, 58). Mice transgenic for cGMP-dependent protein kinase (cGK-TG) displayed a significant increase in mitochondrial content, size and upregulation of PGC1α, NRF1 and Tfam in skeletal muscle (59). Treatment of primary brown adipocytes with NO donor also induced mitochondrial biogenesis through cGMP and PGC1α pathways and an increase in mean mitochondrial volume density (60). Activation of the eNOS cascade also increased mitochondrial content in several established cell lines (61), while mtDNA and mitochondrial size were reduced in the heart, liver, brain, kidney and skeletal muscle of mice with global eNOS knockout (61). The mechanism by which eNOS/NO/cGMP pathway induces mitochondrial biogenesis is unknown, although increase in calcium currents and interaction with AMPK may play a role.
The eNOS/NO/cGMP pathway can be modulated pharmacologically through inhibition of phosphodiesterases (PDE), which catalyze degradation of cGMP. PDE type 5 inhibitors (PDE5Is) have been originally developed as a therapy for cardiac angina, but are currently used in treatment of erectile dysfunction and pulmonary hypertension. Importantly, PDE5I stimulate mitochondrial biogenesis through upregulation of PGC1α and subsequent increase in mtDNA content (62). Compelling evidence exists that PDE5Is can delay the progression of HF and reverse cardiac remodeling in animal models and humans. Several small clinical trials found improvement in hemodynamic and overall clinical measures in patients with congestive HF (for review see (63)), although larger samples sizes are needed to confirm the effect. A literature review from 1980–2011 found an improvement in cardiac index, ejection fraction and other markers of heart function in patients with the New York Heart Association class II or III HF treated with PDE5Is (64).
The role of PDE5I in maintenance of mitochondrial mass and function in failing hearts warrants further investigation, since the connection between eNOS/NO/cGMP pathway and mitochondrial biogenesis mostly comes from studying non-cardiac tissues and cell lines. Moreover, other PDE isoforms must be examined in relation to mitochondrial biogenesis specifically in the heart. In addition to inhibition of PDE, the NO/cGMP synthesis may be activated directly through maintenance of high eNOS activity, especially during increased cardiac workload. Hemodynamic stress is known to uncouple eNOS, leading to its loss of activity and increased generation of ROS (65). BH4 (tetrahydrobiopterin) supplementation can prevent eNOS uncoupling and was found to reduce left ventricular hypertrophy, cardiac dysfunction and fibrosis in mice with heart disease due to pressure overload (65, 66). Importantly, folic acid is known to replenish reduced BH4, and has been shown to protect the heart through increased eNOS activity. Both, folate deficiency and inhibition of BH4 synthesis were associated with reduced mitochondrial number and function (67, 68), while folate administration to rats subjected to I/R preserved cardiac function (69). Finally, the potential damaging effects of NO should be considered, as this molecule was found to inhibit mitochondrial energy production through reversible binding to cytochrome c and displacement of oxygen, which enhanced production of ROS and reactive nitrogen species (70). NO was also shown to activate mitochondrial permeability transition pore (MPTP) opening and to induce apoptotic cell death program (71).
Resveratrol
Resveratrol, a polyphenol compound responsible for the cardioprotective properties of red wine, has been recently identified as a potent stimulator of mitochondrial biogenesis (72). Resveratrol activated both eNOS (73) and AMPK (74, 75), and enhanced mitochondrial biogenesis through upregulation of PGC1α, NRFs and Tfam (76). Treatment of mice with resveratrol increased mitochondrial size and density, mtDNA content, activities of mitochondrial enzymes and oxidative capacity in skeletal muscle. Functionally, these changes were associated with improved motor function and a reduction in resting heart rate (72).
The beneficial effects of resveratrol in the heart are well documented. Two groups have independently shown that resveratrol treatment prevents cardiac dysfunction in hypertensive rats without reduction in blood pressure (75, 77), indicating the direct influence of this compound on the heart. Importantly, mitochondrial mass, biogenesis and function were preserved by resveratrol in salt-sensitive hypertensive rats (77) and rats transgenic for human renin and angiotensin genes (76). A small human clinical trial found significant improvement in diastolic heart function in 40 MI patients receiving 10 mg resveratrol daily during the three-month trial period. In addition, an improvement in endothelial function and reduction in low density lipoprotein (LDL) levels were noted in the treatment group, but mitochondrial biogenesis and function were not assessed (78). Important to note, however, is that resveratrol treatment was found to be ineffective in reversing cardiac hypertrophy induced by volume overload in rats with aortocaval shunt (79). Thus, the beneficial effects of this compound may be limited to select clinical scenarios, such as hypertensive and post-MI patients.
No significant toxicity of this compound was noted in healthy human volunteers in phase I study during four weeks of trial period (80). Unfortunately, resveratrol has a short half-life of 8–14 minutes and is extensively metabolized in the body (81). Thus, an effective human dose cannot be easily extrapolated from animal studies. Development of more potent analogs with longer half lives may help to overcome these limitations.
Other strategies
The cardioprotective effects of estrogen are well-documented in various animal models. Moreover, epidemiologic studies reveal reduced risk of cardiovascular disease in premenopausal, but not postmenopausal women compared to men. Estrogen-like compounds were shown to stimulate mitochondrial biogenesis through induction of NRF1 expression and increase in mtDNA content (82). Unfortunately, estrogen replacement therapy not only failed to reduce, but actually increased the number of cardiac events in post-menopausal women (83). The likely reasons for that are discussed by Mendelsohn et. al (84). Thus, although estrogen pathway represents a promising therapeutic target, more research is needed to understand its risks, benefits and target patient population.
The approaches discussed above are aimed at the production of new mitochondria via induction of PGC1α, NRFs and Tfam signaling. However, it is also important to note that mitochondria are dynamic organelles that constantly undergo fusion and fission. Although the significance of these processes in HF is not well understood, both fusion and fission are essential for maintenance of mitochondrial function (for review see (85, 86)). Constant exchange of mitochondrial metabolites, proteins, DNA and Ca2+ throughout the mitochondrial network by fusion/fission may protect these organelles in the setting of an insult. On the other hand, selective elimination of damaged mitochondria by the process of autophagy is also critical in cardiac physiology, as disruption of autophagy in a pressure-overloaded heart facilitated its transition into HF. Thus, mitochondrial biogenesis should be re-examined and targeted in a broader context of preserving mitochondrial number, network organization, size and quality.
II. Mitochondrial Oxidative Stress
Pathophysiology
Generation of reactive oxygen species (ROS) is significantly enhanced in the failing myocardium, as have been unequivocally shown by studies of animal models and human patients (for review see (87, 88)). The majority of ROS in the heart appear to come from uncoupling of mitochondrial electron transport chain (ETC) at the level of complexes I and III (89), although the view of mitochondria as a major source of intracellular ROS has been challenged (90). The activities of mitochondrial ETC complexes are suppressed in HF, and disruption of mitochondrial bioenergetic function was found to increase ROS and oxidative DNA damage (91), providing a possible pathophysiologic link between mitochondrial dysfunction and ROS (88, 92).
NADPH oxidase (Nox) is another important source of ROS in the heart. Five isoforms of Nox have been described, with Nox4 being the most abundant in cardiomyocytes, endothelial cells and fibroblasts. Nox4 localizes primarily to the mitochondria, does not require cytosolic subunits for its activation, and is implicated in enhanced ROS production in pressure overload and aging models of HF (93–95). Nox activity is induced by pathways that are also active in dysfunctional myocardium, including ATII stimulation, tumor necrosis factor-α (TNFα) and mechanical stretch (88). Nox activity is high in human failing hearts (96), while genetic deletion of this enzyme protects against cardiac dysfunction and remodeling in the MI mouse model (97).
The exact contribution of ROS to the development of HF is complex and remains a subject of intense debate. One likely mechanism is physical damage of cellular and mitochondrial structures, such as sarcomeric and excitation-contraction coupling proteins, which would impair the mechanical properties of the heart (98). Since the majority of ROS in HF comes from mitochondria, these organelles are the primary target of oxidative damage. MtDNA is particularly sensitive to ROS due to the lack of protective histones and less efficient DNA repair, and mutations in mtDNA-encoded genes are known to cause cardiomyopathy. Moreover, reduction of PGC1α in failing hearts can exacerbate oxidative stress and mitochondrial damage, as this protein was found to maintain mitochondrial, but not cytosolic, antioxidant defenses (99). In addition to damaging cellular components, ROS regulate several signaling cascades, including the known hypertrophic pathways such as protein kinase C, MAPK, JNK and Ras (100). Finally, ROS facilitate the remodeling of extracellular matrix by inducing matrix metalloproteases (MMPs) through direct post-translational modifications or indirectly through NFκB pathway (101). Given that ROS affect virtually all aspects of cardiomyocyte physiology, they represent an important therapeutic target for treating HF. In support of this claim, cardioprotective therapies such as ACE inhibitors and ATII receptor blockers were shown to possess antioxidant properties, although it is not known whether they target mitochondrial ROS directly or indirectly (102, 103).
Therapeutic Strategies
Several trials have assessed the efficacy of antioxidants in the treatment of HF, but the results were disappointing. Long-term supplementation with α-tocopherol (vitamin E) was actually associated with an increased risk of developing HF (104). Evidence from animal studies suggests that preferential inhibition of ROS inside the mitochondria, rather than global antioxidant treatment, may be cardioprotective. Overexpression of mitochondria-specific antioxidant peroxiredoxin-3 (Prx-3) protected the heart against failure and remodeling in the mouse model of MI (105). Similarly, overexpression of mitochondria-targeted catalase (mCAT) attenuated hypertrophy in pressure-overload (106) and hypertensive (107) mouse models. Thus, scavenging ROS within the mitochondria may protect the heart against development of HF and make it more resistant to stressful stimuli. Several approaches for targeting antioxidant compounds to the mitochondria are being explored and hold promise (Figure 2).
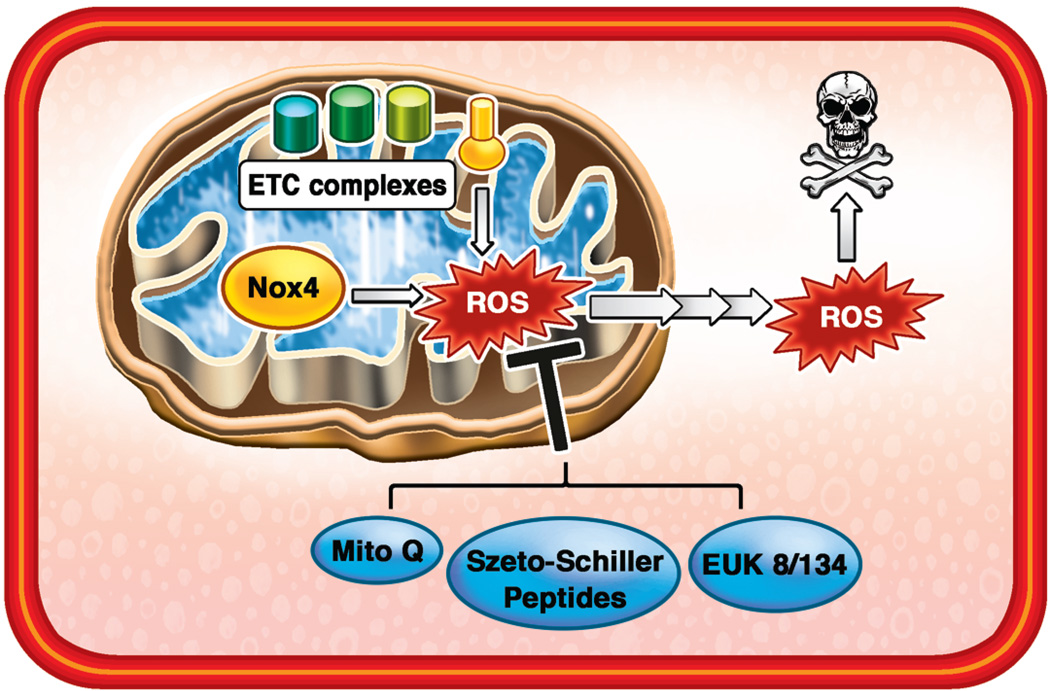
Figure 2. Targeting Mitochondrial ROS Production.
Mitochondrial ETC complexes and Nox4 enzyme generate excessive amounts of ROS in failing hearts. Moreover, mitochondria are very sensitive to oxidative stress and their function is severely impaired in HF. While non-specific antioxidants, such as vitamin E, show no benefit in HF, targeting of ROS-scavenging molecules to mitochondria is protective. Various approaches to targeting antioxidant compounds to mitochondria, including TPP conjugation (MitoQ), Szeto-Sciller peptides, and synthesis of novel MnSOD/Catalase mimetics, should be explored in the development of HF treatments.
MitoQ
The best characterized mitochondria-targeted antioxidant to date is MitoQ, a quinol ROS scavenging moiety linked to triphenylphosphonium (TPP), a lipophilic compound that easily crosses membranes and accumulates in the mitochondrial matrix as a function of membrane potential. Scavenging of ROS is achieved through oxidation of MitoQ into its quinone form, which is then recycled back into the active quinol by the action of mitochondrial complex II (108). MitoQ is bioavailable orally with no toxicity detected when administered to mice at ~20mg/kg dose. Tracer studies found the compound to be rapidly taken up into the heart, liver, brain, kidney and muscle, with highest accumulation in the heart and liver (109). Chronic administration of MitoQ had no effect on plasma glucose, insulin, free fatty acid or cholesterol levels, but was associated with significantly reduced triglycerides. Affymetrix chip analysis of the heart and liver tissue of mice receiving MitoQ revealed no significant differences in gene expression profile between the treatment and control groups (110). Thus, MitoQ is a safe, orally-available small molecule that does not significantly alter baseline physiology.
In WT mice, MitoQ does not lead to significant reduction in oxidative stress at baseline. However, administration MitoQ to rats for 2 weeks reduced oxidative stress and protected the heart against ischemia-reperfusion injury using an ex-vivo Langendorff setup. These effects were specifically due to the inhibition of ROS inside the mitochondria, as no protection was observed in control groups receiving either methylTPP which can enter the mitochondria but does not scavenge ROS, or short-chain antioxidant quinol which is impermeable to the mitochondrial membrane (111). These findings were later confirmed in a mouse model of I/R and in an established cardiac cell line (112). MitoQ also preserved cardiac function in a spontaneously hypertensive rat model of HF. However, this favorable outcome may also be attributed to the reduction in blood pressure and improvement in endothelial function observed in the MitoQ group (113). Finally, MitoQ was found protective in other models of mitochondrial oxidative stress, including cardiac damage by doxorubicin (114), liver damage by lipopolysaccharide (115), and protection of substantia nigra from 1-methyl-4-phenyl-1,2,3,6-tetrahydropryridine (MPTP) toxicity (116).
Two human trials assessed MitoQ’s efficacy in treatment of Parkinson’s disease (PROTECT) and in patients with chronic hepatitis C infection (CLEAR). While the results of the PROTECT trial were negative, it provided a wealth of data on safety of the drug administered orally for up to one year (117). On the other hand, the patients receiving 40mg and 80mg MitoQ in the CLEAR trial showed significant improvement in hepatic function (118). Importantly, no severe side effects of MitoQ regimen were reported in either of the trials.
Despite significant therapeutic potential of MitoQ and other TPP-conjugated antioxidants, there are limitations. The uptake of these compounds is governed by the mitochondrial membrane potential, which may be severely disrupted in failing hearts. Moreover, accumulation of cationic TPP in the matrix can potentially depolarize mitochondria, leading to unwanted side effects.
Szeto-Schiller Peptides
Unlike TPP conjugates, the small (<10 amino acids) Szeto-Schiller (SS) peptides selectively accumulate in the mitochondrial matrix independent of membrane potential. SS peptides are rapidly taken up by the mitochondria, with 1000–5000 fold accumulation in this organelle compared to the cytosolic compartment (119). Multiple variants of SS molecules have been synthesized to date, and the tyrosine-containing SS-02 and SS-31 peptides hold therapeutic promise due to their antioxidant properties (120). These products reduced mitochondrial ROS production in cells treated with mitochondrial complex I (121), II and III (119) inhibitors. SS compounds were also shown to be protective in vivo. Administration of SS-31 prior to ischemia and before reperfusion reduced MI size, lipid peroxidation indices, and increased ATP content in the rat heart (122). Recently, SS-31, but not the non-targeted antioxidant N-acetyl cysteine (NAC), was shown to protect the heart from cardiomyopathy due to angiotensin II administration or Gαq overexpression in mice (123), providing the first evidence for SS-31 effectiveness in a more chronic model of cardiac dysfunction.
Although peptides are typically considered poor candidates for drug development due to the issues of solubility, stability, rapid clearance and inability to cross cellular membranes, studies have revealed excellent pharmacokinetic properties of the SS peptides. These molecules are water soluble due to the 3+ net charge, and are stable in an aqueous solution at 37°C for 6 months. They can be delivered via intravenous, intraperitoneal or subcutaneous route and are rapidly distributed to highly perfused organs, including heart, kidney, lung and brain (120). Moreover, enzymatic degradation of the SS peptides is low, and they remain stable even after 2 hours of incubation in whole blood (124). Finally, the toxicity of SS compounds is low at therapeutic doses, with no side effects observed after 5 months of daily treatments of mice (125). Given these favorable pharmacokinetic properties, SS peptides appear to be good candidates for further testing in the treatment of HF. However, additional animal and human studies are required to validate these compounds as therapeutic candidates.
Manganese SOD/catalase mimetics
Superoxide dismutases (SODs) are metal-containing antioxidant enzymes that catalyze the conversion of superoxide radical to hydrogen peroxide and O2. The mitochondria-specific manganese SOD (MnSOD, or SOD2) is located in the matrix and its overexpression was shown to protect against HF (126). Several inorganic MnSOD mimetics have been synthesized and many of these compounds exert protection in conditions associated with oxidative stress (for review see (127)). Salen derivatives, such as EUK-8 and EUK-134, possess antioxidant properties of both MnSOD and catalases, and appear to be effective in the heart. While no studies have assessed the ability of these molecules to penetrate mitochondria directly, their chemical properties (small, lipophilic, water-soluble) and documented ability to reduce mitochondrial ROS and maintain activities of mitochondrial enzymes (128) support this assumption.
Both EUK-8 and EUK-134 were found to protect mitochondria and the heart against various oxidative insults. In an early study by Pucheu et al, EUK-8 protected iron-overloaded rat hearts from I/R injury, maintained left ventricular diastolic pressure, and preserved mitochondrial integrity (129). EUK-8 treatment also prevented the development of cardiomyopathy, maintained contractility and ATP content in the heart/muscle-specific MnSOD2 knockout mice, which develop HF as early as four weeks after birth. Importantly, ROS generation by isolated mitochondria was significantly reduced by EUK-8 in these mice, suggesting the ability of this molecule to offset mitochondrial oxidative stress (130). Finally, EUK-8 ameliorated pressure overload-induced cardiac dysfunction in wild type mice and mice with the deletion of mitochondrial antioxidant, apoptosis-inducing factor (AIF) (131). EUK-134, a more lipophilic derivative of EUK-8, exhibited similar protective properties in pulmonary arterial hypertension-induced HF (132) and I/R (133), and reduced apoptosis in norepinephrine-stimulated isolated adult rat ventricular myocytes (134).
New SOD/catalase mimetics are being developed and studied, thus opening up an exciting new therapeutic direction. However, most evidence for the mitoprotective and cardioprotective properties of these molecules come from genetic models of increased mitochondrial oxidative stress. The mechanism of cardiac protection conferred by EUK-8/EUK-134 and related compounds must be thoroughly investigated. In particular, cardioprotective properties of these molecules may be independent of their antioxidant effects, as EUK-8 was reported to possess significant vasodilatory properties which may increase oxygen and nutrient delivery to the heart and indirectly improve heart function (135).
Other Strategies
As our understanding of the chemistry behind mitochondrial targeting is increasing, rational design of novel therapies holds promise. A number of antioxidant moieties have been conjugated to TPP and appear to confer protection as well as control the degree of antioxidation, the duration of the effect, etc (108). In addition to synthesizing new molecules, it is important to understand various signaling pathways that regulate mitochondrial antioxidant defenses. A recent report by Lu et al. found antioxidant enzyme levels to be significantly decreased in PGC1α knockout mice with pressure-overload hypertrophy (99), suggesting that enhancement of mitochondrial biogenesis and PGC1α expression by strategies discussed earlier may have a positive effect on an antioxidant profile as well.
III. Mitochondrial Iron Homeostasis
Pathophysiology
Although the role of mitochondrial iron in HF has not been explicitly studied, indirect evidence points toward potential therapeutic implications of altering mitochondrial iron homeostasis in the diseased heart. Iron is essential for maintenance of cellular viability and function through its role in oxidative phosphorylation, antioxidant enzyme activities, ribosome biogenesis, oxygen storage and delivery, and more (136). Mitochondria are the key sites of cellular iron processing where synthesis of iron-sulfur (Fe/S) clusters and heme takes place, but are also the place where ROS are generated (137). Being a reactive metal, free iron catalyzes production of highly toxic hydroxyl radicals from less reactive species such as hydrogen peroxide or superoxide anion via the Fenton reaction (138).
While many studies examined changes in systemic iron homeostasis in animals and humans with HF, very little work has been done to characterize the intrinsic defects in iron regulatory pathways of failing cardiomyocytes. In particular, mitochondrial iron regulation remains understudied. Several lines of evidence suggest that accumulation of iron in mitochondria can cause or exacerbate cardiomyopathy. The best-documented example is cardiomyopathy of Friedreich’s Ataxia (FRDA), a human genetic disease caused by GAA triplet expansion in frataxin gene. While the precise function of frataxin is unknown, it likely plays a role in regulation of mitochondrial iron homeostasis and iron-sulfur cluster synthesis. FRDA patients develop progressive cardiomyopathy characterized by extensive mitochondrial dysfunction and oxidative damage (139). Moreover, frataxin deficiency was associated with significant accumulation of iron inside the mitochondria both in patients (140) and in mouse models of the disease (141, 142), while no difference was detected in the total iron content in FRDA hearts (140). Treatment of patients with a combination of mitochondria-permeable iron chelator deferiprone and an antioxidant partially reversed the cardiac phenotype observed in FRDA (143), supporting the role of mitochondrial iron in pathophysiology of cardiac dysfunction. Another example of a cardiomyopathy resulting from preferential iron accumulation in the mitochondria has been recently published by our group. We showed that deletion of mitochondrial ATP-binding cassette transporter 8 (ABCB8) reduces iron export from this organelle and leads to mitochondrial iron overload. Mice with inducible knockout of ABCB8 in the heart displayed progressive systolic and diastolic dysfunction 8 weeks after gene deletion, which was associated with an increase in oxidative stress, severe disruption of mitochondrial architecture, and presence of mitochondrial iron aggregates (144).
Whether or not iron accumulates in the mitochondria of failing hearts due to common etiologies such as hypertension and MI has not been examined directly. One study used EPR spectroscopy to analyze the iron status of the failing hearts in the mouse model of cardiac-specific overexpression of Gαq. Significant accumulation of iron inside cardiomyocytes was noted, although the mitochondria-specific iron pool has not been examined. However, activities of mitochondrial Fe/S cluster proteins were reduced in failing hearts, and the defect in complex III activity was specifically attributed to the lack of a Fe/S cluster center (145). These findings suggest deregulation of mitochondrial iron processing in at least one model of chronic HF. Thus, the defects in mitochondrial iron handling may contribute to the development of HF through ROS-dependent mechanism and through potential disruption of Fe/S cluster biogenesis.
Therapeutic Strategies
Extensive characterization of mitochondrial iron homeostasis in various models of HF must be performed in order to target these pathways in the translational studies. Reducing mitochondrial iron may exert cardioprotection through inhibition of hydroxyl radical formation and alleviation of oxidative stress (Figure 3). The therapy must be precisely targeted to the mitochondria, as iron homeostasis is often disrupted in HF patients and global iron deficiency is common (146). Instead of chelating iron on a systemic level and exacerbating iron deficiency, the drug must remove free iron from the mitochondria and donate it to other cellular compartments. Such redistribution of iron within a cell was reported for deferiprone (DFP), an orally-available reverse siderophore iron chelator. DFP was shown to enter the cells, reduce mitochondrial free iron levels, exit cells as an iron-chelate, and transfer chelated iron to apotransferrin in the blood, thus potentially increasing systemic iron availability (147). Oral administration of DFP to FRDA patients for six months significantly improved neurological symptoms and reduced iron accumulation in cerebellar dentate nuclei, with no hematological side effects noted (148). Moreover, DFP and idebenone treatment led to a partial reversal of FRDA cardiomyopathy in human patients (143). In addition to DFP, analogs of hydrophobic iron chelator pyridoxal isonicotinoyl hydrazone (PIH), such as 2-pyridylcarboxaldehyde isonicotinoyl hydrazone (PCIH) were shown to selectively remove radioactive isotope of iron from mitochondria of rabbit reticulocytes (149) and were proposed as a potential therapy for FRDA and other diseases associated with mitochondrial iron overload (150). The effects of PCIH on systemic iron homeostasis had not yet been examined.
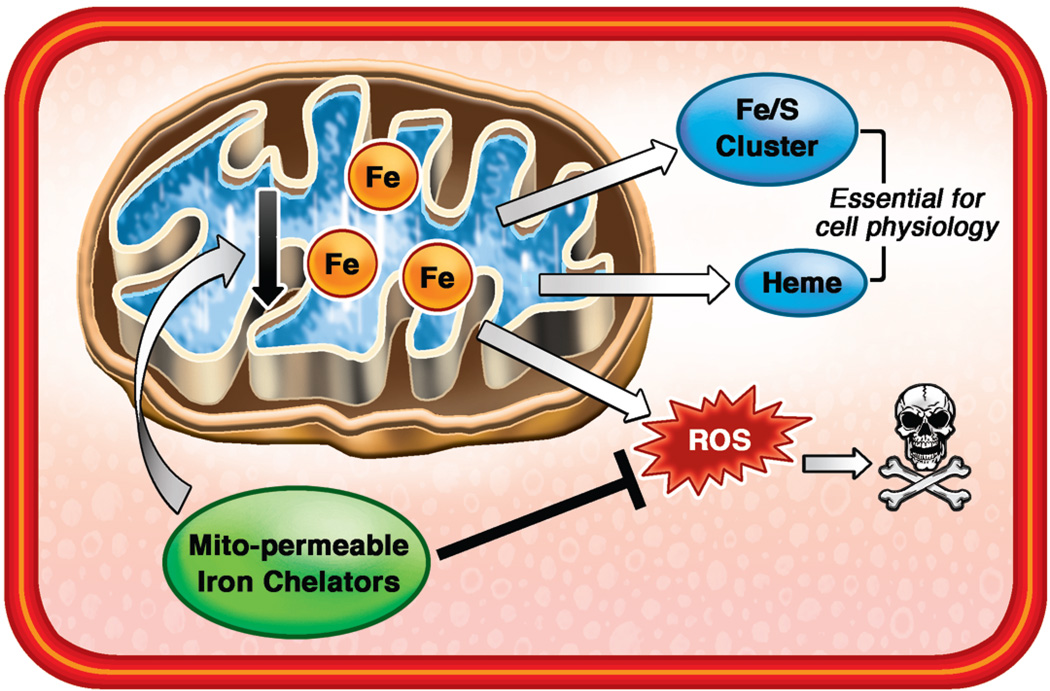
Figure 3. Mitochondrial Iron as a Promising Therapeutic Target.
Functional and structural damage to the mitochondria is a prominent feature of HF. In addition to generating ATP, mitochondria play a key role in regulation of cellular iron balance through the synthesis of heme and iron sulfur clusters. However, accumulation of iron in the mitochondria can catalyze generation of ROS and exacerbate damage. Reducing mitochondrial iron through development of mitochondria-permeable iron chelators can potentially protect the failing hearts.
Conclusions
Mitochondria are taking the center stage in our search for novel cardioprotective therapies, as their dysfunction appears early and invariably in the development of hypertrophy and HF. Maintenance of mitochondrial biogenesis against cardiac insults and reduction in mitochondrial ROS production are the two promising directions that may soon yield effective treatments. Moreover, exploration and targeting of other vital mitochondrial processes in HF, including regulation of iron homeostasis, should be actively pursued. Importantly, our basic research and translational efforts should focus on targeting the intrinsic processes of viable, but dysfunctional, cardiomyocytes.
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health K02 HL107448, R01 HL104181, and 1P01 HL108795 (to Dr. Ardehali).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Relationship to Industry:
Hossein Ardehali: Consultant, Cubist; Honorarium, Merck.
Mihai Gheorghiade: Consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer Schering Pharma AG, Cardiorentis Ltd, CorThera, Cytokinetics, CytoPherx, Inc, DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Intersection Medical, INC, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Ono Parmaceuticals USA, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT, Takeda Pharmaceuticals North America, Inc and Trevena Therapeutics; and has received signficant (> $10,000) support from Bayer Schering Pharma AG, DebioPharm S.A., Medtronic, Novartis Pharma AG, Otsuka Pharmaceuticals, Sigma Tau, Solvay Pharmaceuticals, Sticares InterACT and Takeda Pharmaceuticals North America, Inc.
REFERENCES
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Gheorghiade M, Peterson ED. Improving postdischarge outcomes in patients hospitalized for acute heart failure syndromes. Jama. 2011;305:2456–2457. doi: 10.1001/jama.2011.836. [DOI] [PubMed] [Google Scholar]
- 3.Dai DF, Chen T, Wanagat J, Laflamme M, et al. Age-dependent cardiomyopathy in mitochondrial mutator mice is attenuated by overexpression of catalase targeted to mitochondria. Aging Cell. 2010;9:536–544. doi: 10.1111/j.1474-9726.2010.00581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cizkova A, Stranecky V, Mayr JA, Tesarova M, et al. TMEM70 mutations cause isolated ATP synthase deficiency and neonatal mitochondrial encephalocardiomyopathy. Nat Genet. 2008;40:1288–1290. doi: 10.1038/ng.246. [DOI] [PubMed] [Google Scholar]
- 5.Mayr JA, Haack TB, Graf E, Zimmermann FA, et al. Lack of the mitochondrial protein acylglycerol kinase causes Sengers syndrome. Am J Hum Genet. 2012;90:314–320. doi: 10.1016/j.ajhg.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanbe A, Tanonaka K, Kobayasi R, Takeo S. Effects of long-term therapy with ACE inhibitors, captopril, enalapril and trandolapril, on myocardial energy metabolism in rats with heart failure following myocardial infarction. J Mol Cell Cardiol. 1995;27:2209–2222. doi: 10.1016/s0022-2828(95)91551-6. [DOI] [PubMed] [Google Scholar]
- 7.Feng X, Luo Z, Ma L, Ma S, et al. Angiotensin II receptor blocker telmisartan enhances running endurance of skeletal muscle through activation of the PPAR-delta/ AMPK pathway. J Cell Mol Med. 2011;15:1572–1581. doi: 10.1111/j.1582-4934.2010.01085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ardehali H, Sabbah HN, Burke MA, Sarma S, et al. Targeting myocardial substrate metabolism in heart failure: potential for new therapies. Eur J Heart Fail. 2012;14:120–129. doi: 10.1093/eurjhf/hfr173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garesse R, Vallejo CG. Animal mitochondrial biogenesis and function: a regulatory cross-talk between two genomes. Gene. 2001;263:1–16. doi: 10.1016/s0378-1119(00)00582-5. [DOI] [PubMed] [Google Scholar]
- 10.Puigserver P, Wu Z, Park CW, Graves R, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 11.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 12.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 13.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, et al. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell LK, Mansfield CM, Lehman JJ, Kovacs A, et al. Cardiac-specific induction of the transcriptional coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha promotes mitochondrial biogenesis and reversible cardiomyopathy in a developmental stage-dependent manner. Circ Res. 2004;94:525–533. doi: 10.1161/01.RES.0000117088.36577.EB. [DOI] [PubMed] [Google Scholar]
- 15.Wu Z, Puigserver P, Andersson U, Zhang C, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 16.Dufour CR, Wilson BJ, Huss JM, Kelly DP, et al. Genome-wide orchestration of cardiac functions by the orphan nuclear receptors ERRalpha and gamma. Cell Metab. 2007;5:345–356. doi: 10.1016/j.cmet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Huss JM, Torra IP, Staels B, Giguere V, et al. Estrogen-related receptor alpha directs peroxisome proliferator-activated receptor alpha signaling in the transcriptional control of energy metabolism in cardiac and skeletal muscle. Mol Cell Biol. 2004;24:9079–9091. doi: 10.1128/MCB.24.20.9079-9091.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huo L, Scarpulla RC. Mitochondrial DNA instability and peri-implantation lethality associated with targeted disruption of nuclear respiratory factor 1 in mice. Mol Cell Biol. 2001;21:644–654. doi: 10.1128/MCB.21.2.644-654.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huss JM, Imahashi K, Dufour CR, Weinheimer CJ, et al. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nat Genet. 1998;18:231–236. doi: 10.1038/ng0398-231. [DOI] [PubMed] [Google Scholar]
- 21.Garnier A, Fortin D, Delomenie C, Momken I, et al. Depressed mitochondrial transcription factors and oxidative capacity in rat failing cardiac and skeletal muscles. J Physiol. 2003;551:491–501. doi: 10.1113/jphysiol.2003.045104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Witt H, Schubert C, Jaekel J, Fliegner D, et al. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med (Berl) 2008;86:1013–1024. doi: 10.1007/s00109-008-0385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Faerber G, Barreto-Perreia F, Schoepe M, Gilsbach R, et al. Induction of heart failure by minimally invasive aortic constriction in mice: reduced peroxisome proliferator-activated receptor gamma coactivator levels and mitochondrial dysfunction. J Thorac Cardiovasc Surg. 2011;141:492–500. doi: 10.1016/j.jtcvs.2010.03.029. 500 e491. [DOI] [PubMed] [Google Scholar]
- 24.Marin-Garcia J, Goldenthal MJ, Damle S, Pi Y, et al. Regional distribution of mitochondrial dysfunction and apoptotic remodeling in pacing-induced heart failure. J Card Fail. 2009;15:700–708. doi: 10.1016/j.cardfail.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 25.Sebastiani M, Giordano C, Nediani C, Travaglini C, et al. Induction of mitochondrial biogenesis is a maladaptive mechanism in mitochondrial cardiomyopathies. J Am Coll Cardiol. 2007;50:1362–1369. doi: 10.1016/j.jacc.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 26.Sun CK, Chang LT, Sheu JJ, Wang CY, et al. Losartan preserves integrity of cardiac gap junctions and PGC-1 alpha gene expression and prevents cellular apoptosis in remote area of left ventricular myocardium following acute myocardial infarction. Int Heart J. 2007;48:533–546. doi: 10.1536/ihj.48.533. [DOI] [PubMed] [Google Scholar]
- 27.Watson PA, Reusch JE, McCune SA, Leinwand LA, et al. Restoration of CREB function is linked to completion and stabilization of adaptive cardiac hypertrophy in response to exercise. Am J Physiol Heart Circ Physiol. 2007;293:H246–H259. doi: 10.1152/ajpheart.00734.2006. [DOI] [PubMed] [Google Scholar]
- 28.Hu X, Xu X, Lu Z, Zhang P, et al. AMP activated protein kinase-alpha2 regulates expression of estrogen-related receptor-alpha, a metabolic transcription factor related to heart failure development. Hypertension. 2011;58:696–703. doi: 10.1161/HYPERTENSIONAHA.111.174128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sihag S, Cresci S, Li AY, Sucharov CC, et al. PGC-1alpha and ERRalpha target gene downregulation is a signature of the failing human heart. J Mol Cell Cardiol. 2009;46:201–212. doi: 10.1016/j.yjmcc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karamanlidis G, Bautista-Hernandez V, Fynn-Thompson F, Del Nido P, et al. Impaired mitochondrial biogenesis precedes heart failure in right ventricular hypertrophy in congenital heart disease. Circ Heart Fail. 2011;4:707–713. doi: 10.1161/CIRCHEARTFAILURE.111.961474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barger PM, Browning AC, Garner AN, Kelly DP. p38 mitogen-activated protein kinase activates peroxisome proliferator-activated receptor alpha: a potential role in the cardiac metabolic stress response. J Biol Chem. 2001;276:44495–44501. doi: 10.1074/jbc.M105945200. [DOI] [PubMed] [Google Scholar]
- 32.Rodgers JT, Lerin C, Haas W, Gygi SP, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 33.Puigserver P, Rhee J, Lin J, Wu Z, et al. Cytokine stimulation of energy expenditure through p38 MAP kinase activation of PPARgamma coactivator-1. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- 34.Karamanlidis G, Nascimben L, Couper GS, Shekar PS, et al. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Circ Res. 2010;106:1541–1548. doi: 10.1161/CIRCRESAHA.109.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeuchi M, Matsusaka H, Kang D, Matsushima S, et al. Overexpression of mitochondrial transcription factor a ameliorates mitochondrial deficiencies and cardiac failure after myocardial infarction. Circulation. 2005;112:683–690. doi: 10.1161/CIRCULATIONAHA.104.524835. [DOI] [PubMed] [Google Scholar]
- 36.Yanagishita T, Tomita M, Itoh S, Mukae S, et al. Protective effect of captopril on ischemic myocardium. Jpn Circ J. 1997;61:161–169. doi: 10.1253/jcj.61.161. [DOI] [PubMed] [Google Scholar]
- 37.Li J, Coven DL, Miller EJ, Hu X, et al. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am J Physiol Heart Circ Physiol. 2006;291:H1927–H1934. doi: 10.1152/ajpheart.00251.2006. [DOI] [PubMed] [Google Scholar]
- 38.Tian R, Musi N, D'Agostino J, Hirshman MF, et al. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 39.Gibala MJ, McGee SL, Garnham AP, Howlett KF, et al. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol. 2009;106:929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- 40.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–12022. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kukidome D, Nishikawa T, Sonoda K, Imoto K, et al. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 42.Kim M, Shen M, Ngoy S, Karamanlidis G, et al. AMPK isoform expression in the normal and failing hearts. J Mol Cell Cardiol. 2012;52:1066–1073. doi: 10.1016/j.yjmcc.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin M, van der Horst IC, van Melle JP, Qian C, et al. Metformin improves cardiac function in a nondiabetic rat model of post-MI heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H459–H468. doi: 10.1152/ajpheart.00054.2011. [DOI] [PubMed] [Google Scholar]
- 44.Sasaki H, Asanuma H, Fujita M, Takahama H, et al. Metformin prevents progression of heart failure in dogs: role of AMP-activated protein kinase. Circulation. 2009;119:2568–2577. doi: 10.1161/CIRCULATIONAHA.108.798561. [DOI] [PubMed] [Google Scholar]
- 45.Gundewar S, Calvert JW, Jha S, Toedt-Pingel I, et al. Activation of AMP-activated protein kinase by metformin improves left ventricular function and survival in heart failure. Circ Res. 2009;104:403–411. doi: 10.1161/CIRCRESAHA.108.190918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J. 2000;348 Pt 3:607–614. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou G, Myers R, Li Y, Chen Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanders MJ, Ali ZS, Hegarty BD, Heath R, et al. Defining the mechanism of activation of AMP-activated protein kinase by the small molecule A-769662, a member of the thienopyridone family. J Biol Chem. 2007;282:32539–32548. doi: 10.1074/jbc.M706543200. [DOI] [PubMed] [Google Scholar]
- 49.Song T, Lv LY, Xu J, Tian ZY, et al. Diet-induced obesity suppresses sevoflurane preconditioning against myocardial ischemia-reperfusion injury: role of AMP-activated protein kinase pathway. Exp Biol Med (Maywood) 2011;236:1427–1436. doi: 10.1258/ebm.2011.011165. [DOI] [PubMed] [Google Scholar]
- 50.Moreno D, Knecht E, Viollet B, Sanz P. A769662, a novel activator of AMP-activated protein kinase, inhibits non-proteolytic components of the 26S proteasome by an AMPK-independent mechanism. FEBS Lett. 2008;582:2650–2654. doi: 10.1016/j.febslet.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 51.Pang T, Zhang ZS, Gu M, Qiu BY, et al. Small molecule antagonizes autoinhibition and activates AMP-activated protein kinase in cells. J Biol Chem. 2008;283:16051–16060. doi: 10.1074/jbc.M710114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benes J, Kazdova L, Drahota Z, Houstek J, et al. Effect of metformin therapy on cardiac function and survival in a volume-overload model of heart failure in rats. Clin Sci (Lond) 2011;121:29–41. doi: 10.1042/CS20100527. [DOI] [PubMed] [Google Scholar]
- 53.Kim M, Tian R. Targeting AMPK for cardiac protection: opportunities and challenges. J Mol Cell Cardiol. 2011;51:548–553. doi: 10.1016/j.yjmcc.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blair E, Redwood C, Ashrafian H, Oliveira M, et al. Mutations in the gamma(2) subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet. 2001;10:1215–1220. doi: 10.1093/hmg/10.11.1215. [DOI] [PubMed] [Google Scholar]
- 55.Balligand JL, Feron O, Dessy C. eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev. 2009;89:481–534. doi: 10.1152/physrev.00042.2007. [DOI] [PubMed] [Google Scholar]
- 56.Manoury B, Montiel V, Balligand JL. Nitric oxide synthase in post-ischaemic remodelling: new pathways and mechanisms. Cardiovasc Res. 2012;94:304–315. doi: 10.1093/cvr/cvr360. [DOI] [PubMed] [Google Scholar]
- 57.Clementi E, Nisoli E. Nitric oxide and mitochondrial biogenesis: a key to long-term regulation of cellular metabolism. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:102–110. doi: 10.1016/j.cbpb.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 58.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 59.Miyashita K, Itoh H, Tsujimoto H, Tamura N, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58:2880–2892. doi: 10.2337/db09-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nisoli E, Clementi E, Tonello C, Sciorati C, et al. Effects of nitric oxide on proliferation and differentiation of rat brown adipocytes in primary cultures. Br J Pharmacol. 1998;125:888–894. doi: 10.1038/sj.bjp.0702131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nisoli E, Clementi E, Paolucci C, Cozzi V, et al. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 62.De Toni L, Strapazzon G, Gianesello L, Caretta N, et al. Effects of type 5- phosphodiesterase inhibition on energy metabolism and mitochondrial biogenesis in human adipose tissue ex vivo. J Endocrinol Invest. 2011;34:738–741. doi: 10.1007/BF03346724. [DOI] [PubMed] [Google Scholar]
- 63.Schwartz BG, Levine LA, Comstock G, Stecher VJ, et al. Cardiac uses of phosphodiesterase-5 inhibitors. J Am Coll Cardiol. 2012;59:9–15. doi: 10.1016/j.jacc.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 64.Cvelich RG, Roberts SC, Brown JN. Phosphodiesterase type 5 inhibitors as adjunctive therapy in the management of systolic heart failure. Ann Pharmacother. 2011;45:1551–1558. doi: 10.1345/aph.1Q421. [DOI] [PubMed] [Google Scholar]
- 65.Moens AL, Takimoto E, Tocchetti CG, Chakir K, et al. Reversal of cardiac hypertrophy and fibrosis from pressure overload by tetrahydrobiopterin: efficacy of recoupling nitric oxide synthase as a therapeutic strategy. Circulation. 2008;117:2626–2636. doi: 10.1161/CIRCULATIONAHA.107.737031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moens AL, Ketner EA, Takimoto E, Schmidt TS, et al. Bi-modal dose-dependent cardiac response to tetrahydrobiopterin in pressure-overload induced hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:564–569. doi: 10.1016/j.yjmcc.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chou YF, Yu CC, Huang RF. Changes in mitochondrial DNA deletion, content, and biogenesis in folate-deficient tissues of young rats depend on mitochondrial folate and oxidative DNA injuries. J Nutr. 2007;137:2036–2042. doi: 10.1093/jn/137.9.2036. [DOI] [PubMed] [Google Scholar]
- 68.Ceylan-Isik AF, Guo KK, Carlson EC, Privratsky JR, et al. Metallothionein abrogates GTP cyclohydrolase I inhibition-induced cardiac contractile and morphological defects: role of mitochondrial biogenesis. Hypertension. 2009;53:1023–1031. doi: 10.1161/HYPERTENSIONAHA.108.123422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moens AL, Champion HC, Claeys MJ, Tavazzi B, et al. High-dose folic acid pretreatment blunts cardiac dysfunction during ischemia coupled to maintenance of high-energy phosphates and reduces postreperfusion injury. Circulation. 2008;117:1810–1819. doi: 10.1161/CIRCULATIONAHA.107.725481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brown GC, Cooper CE. Nanomolar concentrations of nitric oxide reversibly inhibit synaptosomal respiration by competing with oxygen at cytochrome oxidase. FEBS Lett. 1994;356:295–298. doi: 10.1016/0014-5793(94)01290-3. [DOI] [PubMed] [Google Scholar]
- 71.Vieira HL, Belzacq AS, Haouzi D, Bernassola F, et al. The adenine nucleotide translocator: a target of nitric oxide, peroxynitrite, and 4-hydroxynonenal. Oncogene. 2001;20:4305–4316. doi: 10.1038/sj.onc.1204575. [DOI] [PubMed] [Google Scholar]
- 72.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi S, Nakashima Y. Repeated and long-term treatment with physiological concentrations of resveratrol promotes NO production in vascular endothelial cells. Br J Nutr. 2012;107:774–780. doi: 10.1017/S0007114511003588. [DOI] [PubMed] [Google Scholar]
- 74.Zang M, Xu S, Maitland-Toolan KA, Zuccollo A, et al. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor-deficient mice. Diabetes. 2006;55:2180–2191. doi: 10.2337/db05-1188. [DOI] [PubMed] [Google Scholar]
- 75.Thandapilly SJ, Wojciechowski P, Behbahani J, Louis XL, et al. Resveratrol prevents the development of pathological cardiac hypertrophy and contractile dysfunction in the SHR without lowering blood pressure. Am J Hypertens. 2010;23:192–196. doi: 10.1038/ajh.2009.228. [DOI] [PubMed] [Google Scholar]
- 76.Biala A, Tauriainen E, Siltanen A, Shi J, et al. Resveratrol induces mitochondrial biogenesis and ameliorates Ang II-induced cardiac remodeling in transgenic rats harboring human renin and angiotensinogen genes. Blood Press. 2010;19:196–205. doi: 10.3109/08037051.2010.481808. [DOI] [PubMed] [Google Scholar]
- 77.Rimbaud S, Ruiz M, Piquereau J, Mateo P, et al. Resveratrol improves survival, hemodynamics and energetics in a rat model of hypertension leading to heart failure. PLoS One. 2011;6:e26391. doi: 10.1371/journal.pone.0026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Magyar K, Halmosi R, Palfi A, Feher G, et al. Cardioprotection by resveratrol: A human clinical trial in patients with stable coronary artery disease. Clin Hemorheol Microcirc. 2012;50:179–187. doi: 10.3233/CH-2011-1424. [DOI] [PubMed] [Google Scholar]
- 79.Wojciechowski P, Juric D, Louis XL, Thandapilly SJ, et al. Resveratrol arrests and regresses the development of pressure overload- but not volume overload-induced cardiac hypertrophy in rats. J Nutr. 2010;140:962–968. doi: 10.3945/jn.109.115006. [DOI] [PubMed] [Google Scholar]
- 80.Patel KR, Scott E, Brown VA, Gescher AJ, et al. Clinical trials of resveratrol. Ann N Y Acad Sci. 2011;1215:161–169. doi: 10.1111/j.1749-6632.2010.05853.x. [DOI] [PubMed] [Google Scholar]
- 81.Wang H, Yang YJ, Qian HY, Zhang Q, et al. Resveratrol in cardiovascular disease: what is known from current research? Heart Fail Rev. 2012;17:437–448. doi: 10.1007/s10741-011-9260-4. [DOI] [PubMed] [Google Scholar]
- 82.Mattingly KA, Ivanova MM, Riggs KA, Wickramasinghe NS, et al. Estradiol stimulates transcription of nuclear respiratory factor-1 and increases mitochondrial biogenesis. Mol Endocrinol. 2008;22:609–622. doi: 10.1210/me.2007-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson GL, Limacher M, Assaf AR, Bassford T, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 84.Mendelsohn ME, Karas RH. HRT and the young at heart. N Engl J Med. 2007;356:2639–2641. doi: 10.1056/NEJMe078072. [DOI] [PubMed] [Google Scholar]
- 85.Iglewski M, Hill JA, Lavandero S, Rothermel BA. Mitochondrial fission and autophagy in the normal and diseased heart. Curr Hypertens Rep. 2010;12:418–425. doi: 10.1007/s11906-010-0147-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Palaniyandi SS, Qi X, Yogalingam G, Ferreira JC, et al. Regulation of mitochondrial processes: a target for heart failure. Drug Discov Today Dis Mech. 2010;7:e95–e102. doi: 10.1016/j.ddmec.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol. 2011;301:H2181–H2190. doi: 10.1152/ajpheart.00554.2011. [DOI] [PubMed] [Google Scholar]
- 88.Tsutsui H, Kinugawa S, Matsushima S. Oxidative stress and mitochondrial DNA damage in heart failure. Circ J. 2008;72(Suppl A):A31–A37. doi: 10.1253/circj.cj-08-0014. [DOI] [PubMed] [Google Scholar]
- 89.Ide T, Tsutsui H, Kinugawa S, Utsumi H, et al. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 90.Brown GC, Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 2012;12:1–4. doi: 10.1016/j.mito.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 91.Sung HJ, Ma W, Wang PY, Hynes J, et al. Mitochondrial respiration protects against oxygen-associated DNA damage. Nat Commun. 2010;1:5. doi: 10.1038/ncomms1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ide T, Tsutsui H, Hayashidani S, Kang D, et al. Mitochondrial DNA damage and dysfunction associated with oxidative stress in failing hearts after myocardial infarction. Circ Res. 2001;88:529–535. doi: 10.1161/01.res.88.5.529. [DOI] [PubMed] [Google Scholar]
- 93.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 94.Ago T, Kuroda J, Pain J, Fu C, et al. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kuroda J, Ago T, Matsushima S, Zhai P, et al. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci U S A. 2010;107:15565–15570. doi: 10.1073/pnas.1002178107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heymes C, Bendall JK, Ratajczak P, Cave AC, et al. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol. 2003;41:2164–2171. doi: 10.1016/s0735-1097(03)00471-6. [DOI] [PubMed] [Google Scholar]
- 97.Doerries C, Grote K, Hilfiker-Kleiner D, Luchtefeld M, et al. Critical role of the NAD(P)H oxidase subunit p47phox for left ventricular remodeling/dysfunction and survival after myocardial infarction. Circ Res. 2007;100:894–903. doi: 10.1161/01.RES.0000261657.76299.ff. [DOI] [PubMed] [Google Scholar]
- 98.Bayeva M, Ardehali H. Mitochondrial dysfunction and oxidative damage to sarcomeric proteins. Curr Hypertens Rep. 2010;12:426–432. doi: 10.1007/s11906-010-0149-8. [DOI] [PubMed] [Google Scholar]
- 99.Lu Z, Xu X, Hu X, Fassett J, et al. PGC-1 alpha regulates expression of myocardial mitochondrial antioxidants and myocardial oxidative stress after chronic systolic overload. Antioxid Redox Signal. 2010;13:1011–1022. doi: 10.1089/ars.2009.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kwon SH, Pimentel DR, Remondino A, Sawyer DB, et al. H(2)O(2) regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol. 2003;35:615–621. doi: 10.1016/s0022-2828(03)00084-1. [DOI] [PubMed] [Google Scholar]
- 101.Siwik DA, Colucci WS. Regulation of matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- 102.Yamazaki T, Tanimoto M, Gohda T, Ohara I, et al. Combination effects of enalapril and losartan on lipid peroxidation in the kidneys of KK-Ay/Ta mice. Nephron Exp Nephrol. 2009;113:e66–e76. doi: 10.1159/000228714. [DOI] [PubMed] [Google Scholar]
- 103.Goyal BR, Mehta AA. Beneficial role of spironolactone, telmisartan and their combination on isoproterenol-induced cardiac hypertrophy. Acta Cardiol. 2012;67:203–211. doi: 10.1080/ac.67.2.2154211. [DOI] [PubMed] [Google Scholar]
- 104.Marchioli R, Levantesi G, Macchia A, Marfisi RM, et al. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown) 2006;7:347–350. doi: 10.2459/01.JCM.0000223257.09062.17. [DOI] [PubMed] [Google Scholar]
- 105.Matsushima S, Ide T, Yamato M, Matsusaka H, et al. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 106.Dai DF, Hsieh EJ, Liu Y, Chen T, et al. Mitochondrial proteome remodelling in pressure overload-induced heart failure: the role of mitochondrial oxidative stress. Cardiovasc Res. 2012;93:79–88. doi: 10.1093/cvr/cvr274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dai DF, Johnson SC, Villarin JJ, Chin MT, et al. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 109.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rodriguez-Cuenca S, Cocheme HM, Logan A, Abakumova I, et al. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med. 2010;48:161–172. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 111.Adlam VJ, Harrison JC, Porteous CM, James AM, et al. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 112.Neuzil J, Widen C, Gellert N, Swettenham E, et al. Mitochondria transmit apoptosis signalling in cardiomyocyte-like cells and isolated hearts exposed to experimental ischemia-reperfusion injury. Redox Rep. 2007;12:148–162. doi: 10.1179/135100007X200227. [DOI] [PubMed] [Google Scholar]
- 113.Graham D, Huynh NN, Hamilton CA, Beattie E, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 114.Chandran K, Aggarwal D, Migrino RQ, Joseph J, et al. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J. 2009;96:1388–1398. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lowes DA, Thottakam BM, Webster NR, Murphy MP, et al. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 116.Yang L, Zhao K, Calingasan NY, Luo G, et al. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, et al. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson's disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 118.Gane EJ, Weilert F, Orr DW, Keogh GF, et al. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 119.Zhao K, Zhao GM, Wu D, Soong Y, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 120.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 121.Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- 122.Cho J, Won K, Wu D, Soong Y, et al. Potent mitochondria-targeted peptides reduce myocardial infarction in rats. Coron Artery Dis. 2007;18:215–220. doi: 10.1097/01.mca.0000236285.71683.b6. [DOI] [PubMed] [Google Scholar]
- 123.Dai DF, Chen T, Szeto H, Nieves-Cintron M, et al. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szeto HH, Lovelace JL, Fridland G, Soong Y, et al. In vivo pharmacokinetics of selective mu-opioid peptide agonists. J Pharmacol Exp Ther. 2001;298:57–61. [PubMed] [Google Scholar]
- 125.Petri S, Kiaei M, Damiano M, Hiller A, et al. Cell-permeable peptide antioxidants as a novel therapeutic approach in a mouse model of amyotrophic lateral sclerosis. J Neurochem. 2006;98:1141–1148. doi: 10.1111/j.1471-4159.2006.04018.x. [DOI] [PubMed] [Google Scholar]
- 126.Omar BA, McCord JM. Interstitial equilibration of superoxide dismutase correlates with its protective effect in the isolated rabbit heart. J Mol Cell Cardiol. 1991;23:149–159. doi: 10.1016/0022-2828(91)90102-r. [DOI] [PubMed] [Google Scholar]
- 127.Iranzo O. Manganese complexes displaying superoxide dismutase activity: a balance between different factors. Bioorg Chem. 2011;39:73–87. doi: 10.1016/j.bioorg.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 128.Melov S, Doctrow SR, Schneider JA, Haberson J, et al. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pucheu S, Boucher F, Sulpice T, Tresallet N, et al. EUK-8 a synthetic catalytic scavenger of reactive oxygen species protects isolated iron-overloaded rat heart from functional and structural damage induced by ischemia/reperfusion. Cardiovasc Drugs Ther. 1996;10:331–339. doi: 10.1007/BF02627957. [DOI] [PubMed] [Google Scholar]
- 130.Kawakami S, Matsuda A, Sunagawa T, Noda Y, et al. Antioxidant, EUK-8, prevents murine dilated cardiomyopathy. Circ J. 2009;73:2125–2134. doi: 10.1253/circj.cj-09-0204. [DOI] [PubMed] [Google Scholar]
- 131.van Empel VP, Bertrand AT, van Oort RJ, van der Nagel R, et al. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol. 2006;48:824–832. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 132.Redout EM, van der Toorn A, Zuidwijk MJ, van de Kolk CW, et al. Antioxidant treatment attenuates pulmonary arterial hypertension-induced heart failure. Am J Physiol Heart Circ Physiol. 2010;298:H1038–H1047. doi: 10.1152/ajpheart.00097.2009. [DOI] [PubMed] [Google Scholar]
- 133.Xu Y, Liu B, Zweier JL, He G. Formation of hydrogen peroxide and reduction of peroxynitrite via dismutation of superoxide at reperfusion enhances myocardial blood flow and oxygen consumption in postischemic mouse heart. J Pharmacol Exp Ther. 2008;327:402–410. doi: 10.1124/jpet.108.142372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Remondino A, Kwon SH, Communal C, Pimentel DR, et al. Beta-adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 135.Barandier C, Boucher F, Malfroy B, de Leiris J. Vasodilatory effects of a salen-manganese complex with potent oxyradical scavenger activities. J Vasc Res. 1997;34:49–57. doi: 10.1159/000159201. [DOI] [PubMed] [Google Scholar]
- 136.Anderson GJ, Vulpe CD. Mammalian iron transport. Cell Mol Life Sci. 2009;66:3241–3261. doi: 10.1007/s00018-009-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Richardson DR, Lane DJ, Becker EM, Huang ML, et al. Mitochondrial iron trafficking and the integration of iron metabolism between the mitochondrion and cytosol. Proc Natl Acad Sci U S A. 2010;107:10775–10782. doi: 10.1073/pnas.0912925107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Eaton JW, Qian M. Molecular bases of cellular iron toxicity. Free Radic Biol Med. 2002;32:833–840. doi: 10.1016/s0891-5849(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 139.Payne RM. The Heart in Friedreich's Ataxia: Basic Findings and Clinical Implications. Prog Pediatr Cardiol. 2011;31:103–109. doi: 10.1016/j.ppedcard.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Michael S, Petrocine SV, Qian J, Lamarche JB, et al. Iron and iron-responsive proteins in the cardiomyopathy of Friedreich's ataxia. Cerebellum. 2006;5:257–267. doi: 10.1080/14734220600913246. [DOI] [PubMed] [Google Scholar]
- 141.Whitnall M, Suryo Rahmanto Y, Sutak R, Xu X, et al. The MCK mouse heart model of Friedreich's ataxia: Alterations in iron-regulated proteins and cardiac hypertrophy are limited by iron chelation. Proc Natl Acad Sci U S A. 2008;105:9757–9762. doi: 10.1073/pnas.0804261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Puccio H, Simon D, Cossee M, Criqui-Filipe P, et al. Mouse models for Friedreich ataxia exhibit cardiomyopathy, sensory nerve defect and Fe-S enzyme deficiency followed by intramitochondrial iron deposits. Nat Genet. 2001;27:181–186. doi: 10.1038/84818. [DOI] [PubMed] [Google Scholar]
- 143.Velasco-Sanchez D, Aracil A, Montero R, Mas A, et al. Combined therapy with idebenone and deferiprone in patients with Friedreich's ataxia. Cerebellum. 2011;10:1–8. doi: 10.1007/s12311-010-0212-7. [DOI] [PubMed] [Google Scholar]
- 144.Ichikawa Y, Bayeva M, Ghanefar M, Potini V, et al. Disruption of ATP-binding cassette B8 in mice leads to cardiomyopathy through a decrease in mitochondrial iron export. Proc Natl Acad Sci U S A. 2012;109:4152–4157. doi: 10.1073/pnas.1119338109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Elas M, Bielanska J, Pustelny K, Plonka PM, et al. Detection of mitochondrial dysfunction by EPR technique in mouse model of dilated cardiomyopathy. Free Radic Biol Med. 2008;45:321–328. doi: 10.1016/j.freeradbiomed.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 146.van Veldhuisen DJ, Anker SD, Ponikowski P, Macdougall IC. Anemia and iron deficiency in heart failure: mechanisms and therapeutic approaches. Nat Rev Cardiol. 2011;8:485–493. doi: 10.1038/nrcardio.2011.77. [DOI] [PubMed] [Google Scholar]
- 147.Sohn YS, Breuer W, Munnich A, Cabantchik ZI. Redistribution of accumulated cell iron: a modality of chelation with therapeutic implications. Blood. 2008;111:1690–1699. doi: 10.1182/blood-2007-07-102335. [DOI] [PubMed] [Google Scholar]
- 148.Boddaert N, Le Quan Sang KH, Rotig A, Leroy-Willig A, et al. Selective iron chelation in Friedreich ataxia: biologic and clinical implications. Blood. 2007;110:401–408. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- 149.Richardson DR, Mouralian C, Ponka P, Becker E. Development of potential iron chelators for the treatment of Friedreich's ataxia: ligands that mobilize mitochondrial iron. Biochim Biophys Acta. 2001;1536:133–140. doi: 10.1016/s0925-4439(01)00041-2. [DOI] [PubMed] [Google Scholar]
- 150.Richardson DR. Friedreich's ataxia: iron chelators that target the mitochondrion as a therapeutic strategy? Expert opinion on investigational drugs. 2003;12:235–245. doi: 10.1517/13543784.12.2.235. [DOI] [PubMed] [Google Scholar]