Abstract
Objectives
To investigate the critical period for overweight development during early childhood by examining growth trajectory and related sex differences.
Methods
Using piecewise-linear mixed models and logistic regression, we examined the effect of growth trajectory at different periods on overweight at age 4–5 by sex among 136,971 regularly followed children (mean: 12.2 times) during 2000–05 in south China.
Results
The high-BMI group (> top tertile of BMI Z score at age of 4–5 years) faster growth rates of BMI, BMI Z score, weight and height than the low-BMI group in the first 3 months of life. Boys were more likely to be overweight (OR = 2.0, 95% CI: 1.5–2.7) than girls; the male high-BMI group had higher growth rates during the first 3 months than girls with high-BMI, independently of environmental factors. Those fast grown (in the upper tertile of growth rates in BMI and BMI Z score) in periods 0–3 months had relatively higher odds ratio of at risk of overweight at age of 4–5years than those in other periods.
Conclusions
Overweight risk develops during the first 3 months of life. Boys have an earlier peak in growth than girls, which may help explain why overweight is more prevalent in boys in China.
Keywords: Child, obesity, sex, rapid weight gain, China
INTRODUCTION
Childhood obesity may track into adulthood and increase the risk of cardiovascular disease in later life.1 A number of studies have examined the risk factors that contribute to childhood obesity. Rapid weight gain during infancy has been considered as an important risk factor for childhood obesity as well as a key factor to be addressed in early obesity prevention in western countries.2 Although a normal range of weight gain during childhood is a biological phenomenon and necessary to physical growth, rapid growth can lead to metabolic-related disorders such as obesity, hypertension and diabetes mellitus.3
The prevalence of childhood overweight and obesity has increased rapidly in China during the past two decades, mainly a result of the recent socio-economic transition.4–6 Data from nationwide surveys show that the prevalence of overweight in Beijing and Shanghai increased from below 4% in 1985 to approximately 10% in 2000.7 Considering the direction of current economic development, it is likely that more Chinese children may face rapid growth due to excessive dietary intakes and lack of physical activity, both of which are influenced by parenting styles and economic factors.8
Sex differences in obesity prevalence have been observed among children worldwide.9–11 In China, boys have a much higher prevalence than girls, and the sex-difference starts early in life.12 Previous research has examined diet, physical activity and socio-economic status as possible reasons for the sex difference,13 but did not pay attention to the difference in rapid postnatal growth rates between boys and girls, which may be a result of different feeding patterns. Moreover, previous studies on child growth were mainly based on cross-sectional data or longitudinal data collected from predominantly Western populations, with irregular follow-ups at selected periods. Consequently, the results from these studies have presented different periods as the critical windows for child growth and suggested various risk levels based on their limited data sets.
Our data, collected in China, used a regularly measured childhood growth trajectory combined with a large sample size, which together allowed for investigation of the critical period of overweight development longitudinally from birth, thus helping to advance understanding about Asian children. Our study aimed to: 1) investigate the growth trajectory and critical period for overweight development, and 2) study the sex- difference in this relationship.
METHODS AND MATERIALS
Study design and subjects
The data were collected as part of a large population-based health surveillance system in four provinces in China. The Jiaxing cohort was initiated by the Perinatal Health Care Surveillance System (PHCSS) and the Child Health Care Surveillance System (CHCSS) in China in 1993, which was for the intervention program of folic acid supplementation on pregnant women to prevent neural tube defects 14. This program was supported by the Chinese Ministry of Health under collaboration between the US CDC and the National Center for Maternal and Infant Health (NCMIH) at Peking University Health Science Center (PUHSC). It collected various information about demographics (education level, occupation, etc.), prenatal care, labor and delivery (gestational age, birth characteristics, etc.) from booklets given to and filled out by obstetricians and primary physicians as well as the children’s physical growth after birth. The 2000–2005 data provided information on 138,616 babies who were from Jiaxing, a middle-income county (include both urban and rural residents) in Southeast China. Our study analyzed the growth trajectory of 136,971 of the subjects from birth to age five, excluding twins, children with congenital disorders of sex development, and extreme anthropometric measures (Z scores for weight, height and BMI; weight for age Z score < -6 or > 5, height for age Z score < -6 or > 6, BMI for age Z score < -5 or > 5 based on the 2006 WHO Growth Standards)15.
This study was approved by the Institutional Review Boards of the PUHSC and the Johns Hopkins University Bloomberg School of Public Health.
Key measurements
Anthropometric assessments
Children’s weight and height were measured by trained primary physicians in local clinics following a standardized protocol and related national recommendations at birth, once every 3 months in first year after birth, every six months from ages 2 to 3, and then annually up to six year (in total, 11 times). Although effort was made to adhere to these protocol times, there was some variation in the time of actual assessments; the average number of times of measurement was 12.2 and ranged from 3 to 24 times. Additional health-related information (breast feeding, residential area, etc.) was collected using survey questionnaires. Regarding data quality, our validation study showed high agreement for anthropometric measurements made by NCMIH staff and by local health workers in 2004 (mean difference level: height = −0.04 cm [p = 0.92], weight = 0.11 kg [p = 0.83]).
BMI and BMI Z-scores
BMI was calculated as weight (kg) divided by squared length/height (m2). Overweight at 4–5 years was defined as BMI-for-age Z score ≥ 2, according to the 2006 WHO Growth Standard.16 Rapid growth was defined as an increase weight-for-age Z score >0.67 standard deviation (SD) during the first 2 years, which represents the upward crossing of major percentile lines of a usual growth reference17. Due to the relatively small number of children with available data at the age of 5 years (n = 604), the child’s final measurement after the age of 4 was used for defining overweight status.
Covariates
Birth weight and gestational age were adjusted as covariates, because of the high risks of rapid growth and overweight due to low birth weight and being pre-term. In addition, as indicators of environmental factors, residence (urban vs. rural), exclusive breast feeding before 6 months (yes vs. no), maternal education (≥ high school vs. less) and occupation (unemployed, housework or temporary work vs. routine jobs) were included as covariates.
Statistical analysis
General characteristics of longitudinal growth and related environmental factors were compared across groups using chi-square test and ANOVA. The BMI and BMI Z score growth curves by overweight status at 4–5 years of age were presented using locally weighted scatter plot smoothing curve. The overweight risk at 4–5 years of age associated with rapid growth was estimated using logistic regression.
To define the effect of growth trajectory on the risk of overweight, we selected 11,839 subjects having outcome measurements at age 4–5 and at least two times records previously. For estimating period-specific growth rate, we used random intercept mixed models with piece-wise specification of the 9 age periods (every 3 months in the first year after birth, every 6 months in years 2–3, and 1 time in the 4th year, in conformity with the previous literature on the time windows for rapid weight gain18, 19) as independent variables to capture the periodic growth slope specifically, since BMI curves went up and down during the earliest ages and the slope of early growth could be extremely sensitive to the time intervals. We treated age periods as continuous and substrated values by the starting point of each period to present the association between time and growth as several separated linear regression slopes. The primary model without covariates was as follows:
Yij = β0i (ageij -0) + β1i (ageij -3) + β2i (ageij -6) + β3i (ageij -9) + β4i (ageij -12) + β5i (ageij -18) + β6i (ageij -24) + β7i (ageij -30) + β8i (ageij -36) + β9i (ageij -48) + εij
Yij was the continuous BMI, BMI Z score, height and weight; the term (ageij –Ck) with age interval Ck ∈ {0, 3, 6, 9, 12, 18, 24, 30, 36, 48} meant that child i at measurement j had (ageij –Ck) if Ck-1 < ageij ≤ Ck; otherwise (ageij –Ck) was equal to 0 during the other 8 age periods.
To compare the slopes between children with BMI Z scores at the top tertile and the others, we allowed the interaction terms of rapid growth status and the 9 age periods mentioned above; the differences in periodic growth rates between boys and girls in the upper-BMI group were also compared using a similar approach.
Next, we fit logistic regression models in examining which period had the biggest effect on at risk of overweight (defined by BMI for age Z score > 1 at 4–5 years according to the 2006 WHO Growth Standard) using the following model (without covariates):
E (Yi |Xi) = 1/ [1 + exp (−Z)] + εij
E (Yi |Xi) was the High-BMI risk at 4–5 ages; the variable Z was defined as Z = β1 (high growth rate in period 0–3 months) + β2 (those in period 3–6 months) + β3 (those in period 6–9 months) + β4 (those in period 9–12 months) + β5 (those in period 12–18 months) + β6 (those in period 18–24 months) + β7 (those in period 24–30 months) + β8 (those in period 30–36 months) + β9 (those in period 36–48 months).
Individual growth rates in different periods were calculated using mixed models (see above); and then high growth rate was assigned the value 1, if someone’s growth rate was in the top tertile; otherwise, assigned 0.
Statistical significance for the regression coefficients and the interaction terms were set at p <0.05. All analysis was conducted using SAS 9.2 (SAS Institute, Cary, NC).
RESULTS
Characteristics of study subjects
BMI was highest among toddlers (1–2 years old, p <0.01) (Table 1). Low-birth-weight was 2.3% and preterm birth was 3.3%. Over 30% of the children were rapid growers. The overall prevalence of overweight at 4–5 years was 2.1%, and rapid growers had a much higher prevalence than non-rapid growers (3.9% vs. 1.3%, p < 0.001). After adjustment for covariates (breast feeding, residential area, maternal education and occupation, birth weight and gestational age), rapid growers had a 7 times higher risk of being overweight at 4–5 years of age than the others (OR=7.7, 95%CI: 5.3–11.1).
Table 1.
Characteristics of the study subjects in China*
| Age | Birth | <1 year | 1–2 year | 2–3 year | 3–4 year | 4–6year | p value |
|---|---|---|---|---|---|---|---|
| Total visits | 133,954 | 663,452 | 167,125 | 113,415 | 38,739 | 12,726 | - |
| Number of subjects | 133,954 | 127,944 | 93,678 | 64,781 | 33,478 | 11,839 | - |
| Male | 64,630 (48.3) | 61,727 (48.3) | 48,446 (51.7) | 31,339 (48.4) | 17,175 (51.3) | 5,657 (47.8) | < 0.01 |
| Urban residence | 18,127 (13.5) | 17,411 (13.9) | 12,920 (14.1) | 8,696 (13.8) | 4,612 (14.2) | 2,311 (20.0) | < 0.01 |
| Highly educated mother† | 28,363 (23.0) | 27,501 (23.6) | 19,124 (22.7) | 12,006 (21.1) | 5,956 (20.9) | 1,707 (19.6) | < 0.01 |
| Preterm | 4,053 (3.3) | 3,972 (3.4) | 2,840 (3.4) | 1,932 (3.4) | 966 (3.4) | 285 (3.3) | 0.59 |
| Routinely working mother‡ | 25,720 (20.8) | 24,867 (21.3) | 17,927 (21.2) | 11,762 (20.7) | 6,137 (21.5) | 1,840 (21.1) | < 0.01 |
| Breast fed§ | 105,643 (84.6) | 107,544 (84.2) | 79,243 (84.8) | 55,916 (86.5) | 28,975 (86.8) | 10,216 (87.2) | < 0.01 |
| BMI mean level | 13.3 (1.4) | 14.3 (2.0) | 17.4 (1.4) | 16.3 (1.3) | 15.8 (1.2) | 15.4 (1.3) | < 0.01 |
| Underweight (BMI Z∫<-2) | 7,662 (5.7) | 10,939 (8.6) | 337 (0.4) | 298 (0.5) | 265 (0.8) | 133 (1.1) | < 0.01 |
| Overweight (BMI Z≥ 2) | 3,361 (2.5) | 2,196 (1.7) | 6,325 (6.8) | 2,858 (4.4) | 1,008 (3.0) | 239 (2.0) | < 0.01 |
| Obesity (BMI Z≥ 3) | 375 (0.3) | 322 (0.3) | 795 (0.9) | 367 (0.6) | 145 (0.4) | 47 (0.4) | < 0.01 |
Data were presented by mean (SD: standard deviation) or frequency (%) from Chi-square test and ANOVA.
Highly educated was defined by maternal education more than high school.
Routine work included all work except for house work and temporary work.
Breast fed was defined by whole breast feeding before first 6 month.
BMI-Z was calculated based on the 2006 WHO Growth Standard.
Comparison of growth trajectories in overweight vs. non-overweight children
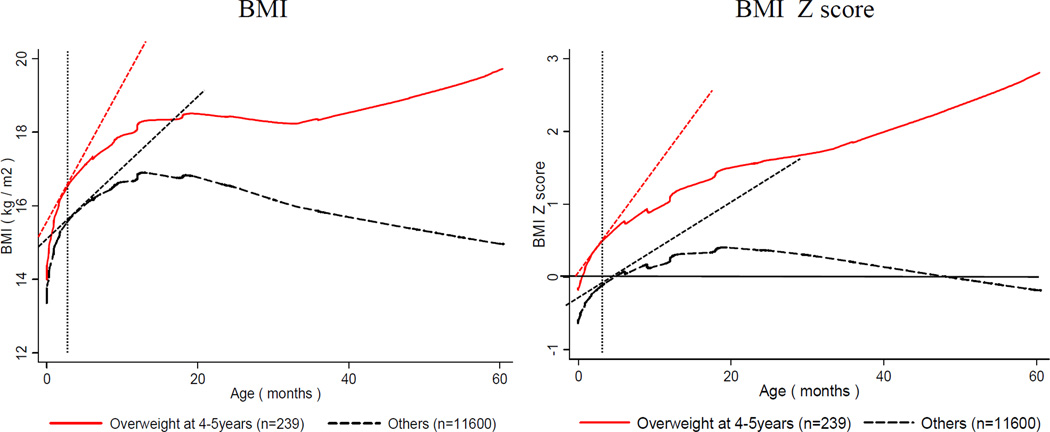
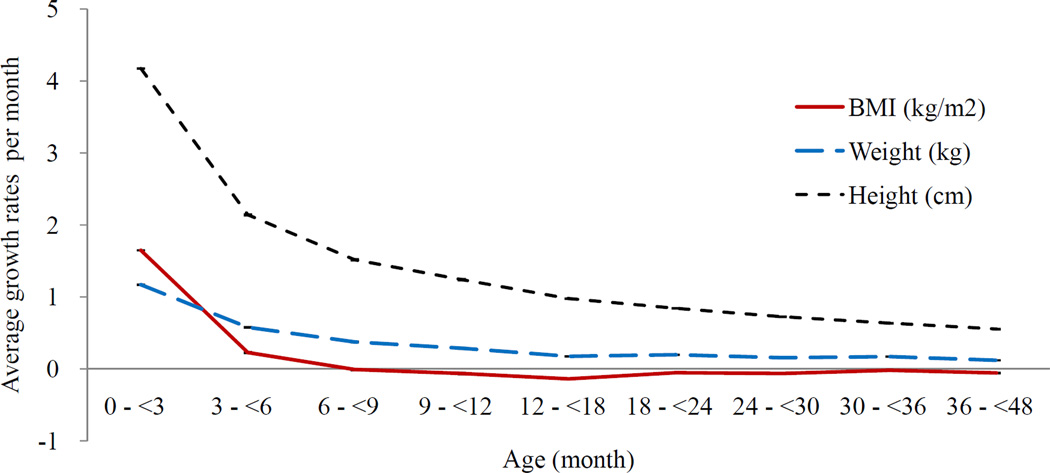
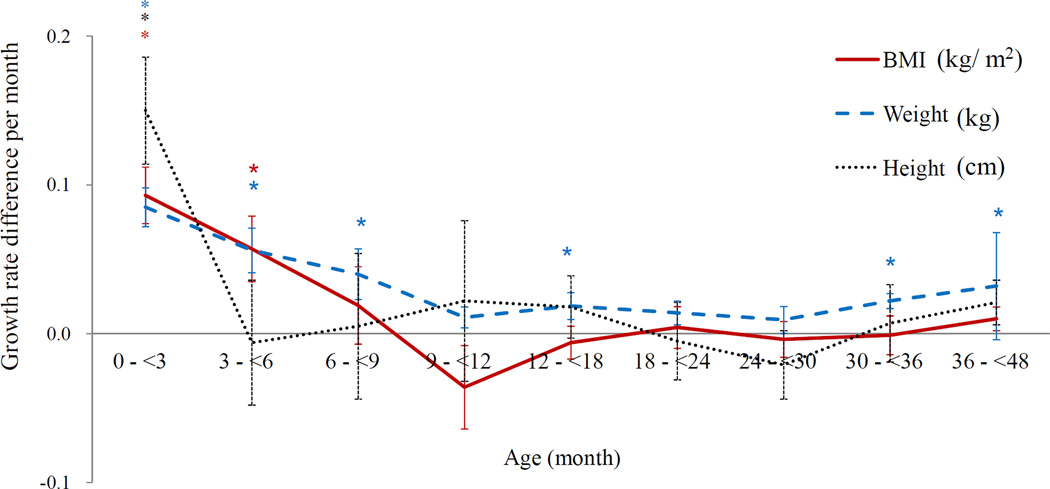
The growth curves of BMI and BMI Z scores of overweight children at 4–5 years old diverged from those of non-overweight children from the early months (Figure 1). Based on the top tertile BMI Z score at 4–5 years, the children were divided into two groups: high and low BMI. Figure 2 shows overall growth rate in 9 periods (n = 8,490) (Figure 2a) and the different growth rates of these two groups (Figure 2b). The BMI, BMI Z score, weight and height growth rates were drastically higher in the first 3 months than afterwards (growth rate of BMI β [SE] = 1.65 [0.00]; BMI Z score = 0.06 [0.01]; weight = 1.17 [0.01]; height = 4.17 [0.02]). The high-BMI group (n = 2,133) grew significantly faster in BMI, weight and height during the first 3 months. The pattern in the slope differences was similar when children were grouped by at the overweight risk at age 4–5 (BMI Z score ≥1) the difference of β [SE] = 0.08 [0.03], p=0.002).
Figure 1. Comparison of BMI and BMI Z score from birth to 5 years old between children being overweight vs. non-overweight at 4–5 years in China*†‡.
*BMI for age Z score was calculated based on the 2006 WHO Growth Standard.
†Overweight (n = 239): BMI Z score ≥ 2 SD at 4–5 years; others (n = 11,600): non- overweight.
‡The BMI and BMI Z score growth curves by overweight at 4–5 years were presented using locally weighted scatter plot smoothing curve.
The 4 tangent lines on each figure showed how the growth rates in figure 2 were drawn from these growth curves.
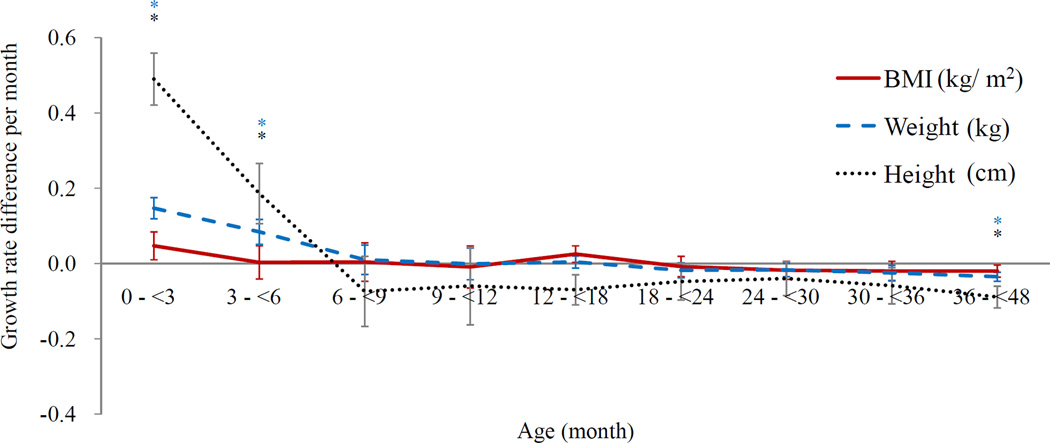
Figure 2. Growth rates per month for BMI, weight and height development during the first 4 years.
a. Average growth rates †
b. The differences in growth rates by BMI status at 4–5 years*†‡
c. The differences in growth rates by sex*†§
Each beta coefficient for periodic growth rate and their standard error bars were presented.
*Difference of growth rates between groups, p < 0.05.
†Piecewise- linear mixed model with random intercept was fitted with adjustment for breast feeding, residential area, gestational age, birth weight, maternal education and occupation (n=8,490).
‡BMI status was defined by top tertile of BMI for age Z score at 4–5 years; differences by BMI status (n=8,490) = Growth rate of high BMI group - growth rate of low BMI group.
§Differences by sex in the high BMI group (n = 2,133) = Growth rates of boys - growth rates of girls in the high BMI group.
Sex-difference in risk of overweight by having rapid growth vs. not
More were overweight than girls (OR = 2.0, 95% CI: 1.5–2.7). Boys grew faster in BMI, weight and height than girls during the first 3 months in the BMI upper group (the difference of weight = 0.15 [0.03], p < 0.01; height = 0.49 [0.07], p < 0.01) (Figure 2c). Maternal education level, employment status and family residence were not different between boys and girls, however, more girls received exclusive breast feeding during their first 6 months of life than boys (87.9% vs. 86.2%, p=0.01).
Breast feeding: After adjustment for sex, birth weight, gestational age, residential area, and maternal education and occupation, breast feeding was protective against rapid growth (OR = 0.82, 95% CI: 0.69– 0.97), but not against overweight (OR = 1.08, 95% CI: 0.67– 1.75). To find the possible influence of boys being less exclusively breast-fed on the sex difference of rapid growth risk, stratified analyses were done by exclusively breast-fed. Higher rapid growth risk among boys as compared to girls was not different between those in the exclusively breast-fed group and those in the other group (exclusive breast fed group: OR = 1.44, 95% CI: 1.28– 1.62; non- exclusive breast fed group: OR = 1.56, 95% CI: 1.1– 1.9, p of OR difference = 0.76).
Period difference in risk of overweight by growth rate
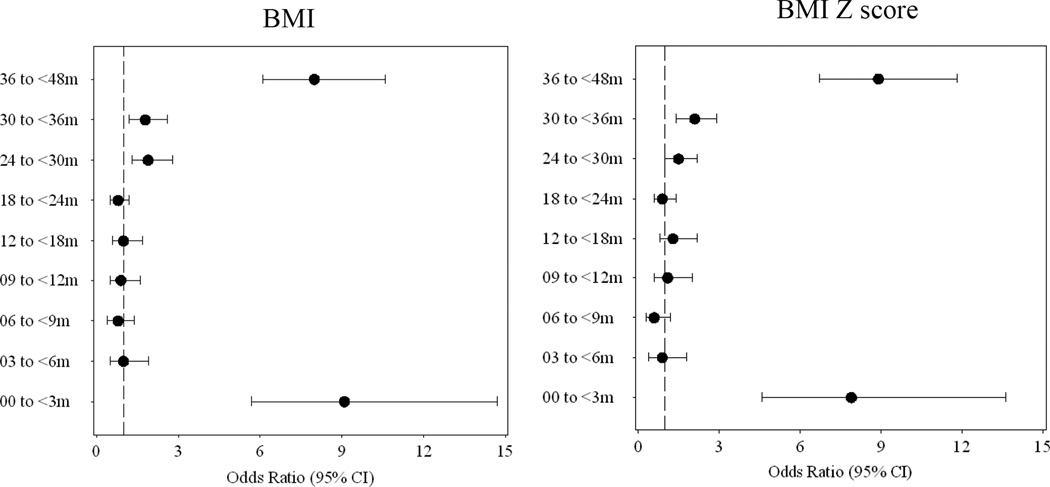
Growth rate had time-dependent effects on overweight development. OR of at risk of overweight (BMI Z score ≥ 1) by having fast growth rates of BMI (in the highest tertile) was the biggest in the first 3 months of life (OR = 9.1, 95% CI: 5.7–14.7; n = 11,839) (Figure 3). Similar trends were observed by using BMI Z score growth rates (OR of at risk of overweight in period 0–3 months =7.9, 95% CI: 4.6– 13.6]; in period 36–48 months [OR = 8.9, 95% CI: 6.7– 11.8]).Our sensitivity analysis confirmed similar results in those who had >=6 times follow-up information of physical growth in their first 3 months of life (OR = 6.7, 95% CI: 4.1– 10.9; n = 10,861) and in those who have more than 7 times (OR = 7.7, 95% CI: 4.5– 13.2; n = 9,297).
Figure 3. The odds ratio of at risk of overweight at 4–5 years with high growth rates of BMI and BMI Z score in different periods*†‡.
*At risk of overweight was defined by BMI for age Z score > 1 at 4–5 years according to the 2006 WHO Growth Standard.
†Individual growth rates of BMI and BMI Z score in different periods were calculated by previous mixed model and then high growth rates was assigned the value 1, if someone’s growth rate was in the highest tertile of growth rate values in the entire population; otherwise it was assigned the value 0.
‡Logistic regression was used to present odds ratio and 95%CI of at risk of overweight with high growth rates of BMI and BMI Z score in different periods after adjusted for breast feeding, residential area, gestational age, birth weight maternal education and occupation.
DISCUSSION
Our results based on longitudinal data collected from 136,971 children in Southeast China suggest that overweight develops early in life. The childhood growth curves showed that those being overweight at age 4–5 had consistently higher BMI levels than their counterparts from the time of infancy; their growth rates diverged from the others during first 3 months, with steeper slopes. Chinese boys, who were at higher risk for overweight than girls, also showed higher growth rates than girls at the same age period; their higher risk seemed to be independent of environmental factors such as birth weight, gestational age, breast feeding, residential area, and maternal education and occupation. In particular, fast growth during the period of first 0–3 months of life predicted higher risk of high-BMI at age 4–5 than those in other periods.
Previous studies have shown that the subsequent rapid growth after intrauterine growth retardation may be a cause of metabolic problems.3 In addition, recent studies on children with appropriate gestational age20 or full-term births21, 22 discussed the risk of high infancy weight gain and subsequent higher BMI and body fat percentage during childhood. Our data indicate that rapid growth can be an independent factor for obesity and metabolic disease development.
Based on measures taken regularly (about 11 times) from 136,971 children, we tested the periodic growth rates of BMI from birth to 4–5 years; and found those that experienced a higher growth rate during their first 3 months of life were more likely to be overweight at age 4–5. This was consistent with results from the GINI and LISA birth cohorts in German, period-specific models.23 However, the German study did not test when the overweight group became faster growers than the others. They compared the probability of being overweight at different time periods (birth to < 3months, 3 to < 6 months, 6 to < 12 months, 12 to < 24 months, and 24 to < 72 months). They concluded that the monthly velocity for overweight accelerated through the 12th month and then diminished afterward.
In addition, our data showed that within the same period, those who had a higher BMI level at age 4–5 grew significantly faster than their counterparts. This result may explain why the rapid growers in previous and our studies had a significantly higher risk of later overweight than others. Early infancy is considered to be a critical period for body fat mass accumulation, which persistently affects body fat mass in older ages; newborns, in particular, attain a peak of adiposity with a fat mass before a period of about 4–6 months.24
In previous studies, differences in study design have resulted in the use of various definitions of “rapid growth” in terms of amount of weight gain and periods of time. As a result, the reported effects on overweight risks are inconsistent. One multi-cohort study and one New York state study in the U.S. used weight gain in the first a few months (100 g/month) and presented similar results; children with increasing weight gain by 4 months or by 6 months were more likely to be overweight at age 4 (OR = 1.4 [1.3– 1.6])25 or at age 7 (OR = 1.4 [1.3– 1.4]).21 However using “more than 0.67 SD in weight Z score change” as the rapid growth definition, Japanese rapid growers during the first 3–4 months of life had a much higher overweight risk at 3 years of age (OR = 6.77 [2.18– 21.01]).26 In the U.K., groups with rapid growth during the first 2 years showed significantly higher BMI (difference: 0.79 SD score at 2 year, 0.63 SD score at 5 year) and body fat percentage (difference: 1.4%) than their counterparts at 5 years old.27 Ethnic differences may contribute to the relationships between rapid growth and overweight risk, as well.
Other studies have compared the effects of weight gain in different periods on later overweight risk. Swedish children in Stockholm showed a stronger linear relationship between weight gain by 3 to 6 years (β = 1.5 [0.9– 2.1]) and BMI at 17 years than those with a linear relationship by the first 6 months of life (β = 0.9 [0.5– 1.3]).17 On the other hand, Brazilian children showed relatively higher overweight risk at 15 years of age in those who had been rapid growers by 2 year (OR = 1.6 [1.1– 2.4]) and than in those who had been rapid growers during ages 2 to 4 (OR = 1.9 [1.3– 2.8]).28 But another Brazilian study suggested the upepr quartile weight gain group during years 1 to 4 had the most significant p-value for group difference on BMI mean levels at year 9, compared to the other p-values in different periods.29 Although a recent systematic review of 18 studies on infant size or growth within the first two years of life and subsequent obesity summarized the odds ratios of obesity among rapid growers ranging from 1.17 to 5.7030, it was still difficult to determine the critical period of overweight development, since the studies had selective follow-up periods and were thus only able to cover some specific time ranges rather than an overall growth trajectory.
We also found more Chinese boys were overweight than girls. The sex-difference was consistent with previous studies, which are mainly among school students.4, 12 In our study, boys had a greater growth rate of BMI than girls during their first 3 months of life. This sex difference was independent of breast feeding and other covariates, although rapid growth in boys and girls was negatively affected by breast feeding.
It is still not clear why Chinese boys have rapid growth and subsequent overweight than girls, independent of the related environmental factors. More studies are needed to understand what contributes to the ethnic differences in sex-dependent risks of obesity across countries11. Some other studies, such as those done in the Netherlands and Romania out of a total of 18 E.U. countries9 and in Indonesia compared to China and Vietnam10, showed a higher obesity rate in girls than in boys. Further studies are needed to examine possible ethnic and cultural effects to help understand these various sex-differences in obesity rates across populations.
Our study has a few limitations. First, no data on dietary intakes were collected after infancy, except for breast feeding. Second, we could not suggest whether fat or lean mass made the rapid weight gain during early postnatal age as such measures were not collected. Despite lack of such information, regular follow-up data and our mixed model allow us to cover the most of critical periods in growth until age 5 for studying the association between growth rates and overweight development. The large sample size made subgroup analysis possible by high-BMI status and sex.
Along with economic development in China, more food has been accessible and caloric intake has been greater than ever before.31 We may suspect that without guidelines, parents in these new social conditions may feed their young children with more foods to make grow faster and thus may also put them at risk of developing obesity6. As a result, their risk for chronic diseases later in life may increase faster than the past. The increase in non-communicable chronic diseases such as type 2 diabetes and cardiovascular disease and some types of cancer has become an alarming public health threat in China.5 Prevention of such chronic diseases needs to start earlier in life.
CONCLUSION
Based on data collected from a large population-based cohort in China, our study suggests that overweight risk develops in the first three months after birth and persists afterwards. Chinese boys have a faster growth rate than girls, even after adjusted for selected environmental factors, which helps explain in part why obesity is more prevalent in boys in China.
What’s already known about this subject
Rapid postnatal growth during infancy has been considered as an important risk factor for childhood obesity.
However, it was difficult to determine the critical period for overweight development due to the limitation of previous study designs and ethnic diversity.
What this study adds
Our analysis of longitudinal data collected from 136,971 children in Southeast China suggests that overweight develops early in life.
Those who grew fast during ages of 0–3 months had a higher risk of having high BMI than those in other periods.
Faster growth during the first 3 months in boys could partly explain their higher risk of overweight at 4–5 years of age than girls in China.
Acknowledgements
All authors have contributed to the study including writing the paper and had approved the submitted and published versions. The present analysis was supported in part by the Johns Hopkins Bloomberg School of Public Health Faculty Innovation Award, the NIH/NIDDK (P60 DK0079637), the NIH/NICHD and the NIH/OBSSR (1 U54 HD070725-01).
Abbreviations
- BMI
body mass index
- OR
odds ratio
- CI
confidence interval
Footnotes
Conflicts of interest statement
None declared.
References
- 1.Barker DJ. The developmental origins of chronic adult disease. Acta Paediatr Suppl. 2004;93(446):26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 2.Ong KK. Size at birth, postnatal growth and risk of obesity. Horm Res. 2006;65(Suppl 3):65–69. doi: 10.1159/000091508. [DOI] [PubMed] [Google Scholar]
- 3.Nobili V, Alisi A, Panera N, Agostoni C. Low birth weight and catch-up-growth associated with metabolic syndrome: a ten year systematic review. Pediatr Endocrinol Rev. 2008;6(2):241–247. [PubMed] [Google Scholar]
- 4.Chen CM. Overview of obesity in Mainland China. Obes Rev. 2008;9 Suppl 1:14–21. doi: 10.1111/j.1467-789X.2007.00433.x. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond) 2007;31(1):177–188. doi: 10.1038/sj.ijo.0803354. [DOI] [PubMed] [Google Scholar]
- 6.Cui Z, Huxley R, Wu Y, Dibley MJ. Temporal trends in overweight and obesity of children and adolescents from nine Provinces in China from 1991-2006. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2010;5(5):365–374. doi: 10.3109/17477166.2010.490262. [DOI] [PubMed] [Google Scholar]
- 7.Ji CY. The prevalence of childhood overweight/obesity and the epidemic changes in 1985-2000 for Chinese school-age children and adolescents. Obes Rev. 2008;9(Suppl 1):78–81. doi: 10.1111/j.1467-789X.2007.00443.x. [DOI] [PubMed] [Google Scholar]
- 8.Kumanyika SK. Environmental influences on childhood obesity: ethnic and cultural influences in context. Physiol Behav. 2008;94(1):61–70. doi: 10.1016/j.physbeh.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 9.Cattaneo A, Monasta L, Stamatakis E, et al. Overweight and obesity in infants and pre-school children in the European Union: a review of existing data. Obes Rev. 2010;11(5):389–398. doi: 10.1111/j.1467-789X.2009.00639.x. [DOI] [PubMed] [Google Scholar]
- 10.Tuan NT, Nicklas TA. Age, sex and ethnic differences in the prevalence of underweight and overweight, defined by using the CDC and IOTF cut points in Asian children. Eur J Clin Nutr. 2009;63(11):1305–1312. doi: 10.1038/ejcn.2009.90. [DOI] [PubMed] [Google Scholar]
- 11.Olds T, Maher C, Zumin S, et al. Evidence that the prevalence of childhood overweight is plateauing: data from nine countries. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2011;6(5-6):342–360. doi: 10.3109/17477166.2011.605895. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Schouten EG, Hu X, Cui Z, Luan D, Ma G. Obesity prevalence and time trend among youngsters in China, 1982-2002. Asia Pac J Clin Nutr. 2008;17(1):131–137. [PubMed] [Google Scholar]
- 13.Isganaitis E, Levitsky LL. Preventing childhood obesity: can we do it? Curr Opin Endocrinol Diabetes Obes. 2008;15(1):1–8. doi: 10.1097/MED.0b013e3282f44a07. [DOI] [PubMed] [Google Scholar]
- 14.Li S, Moore CA, Li Z, et al. A population-based birth defects surveillance system in the People's Republic of China. Paediatr Perinat Epidemiol. 2003;17(3):287–293. doi: 10.1046/j.1365-3016.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Child Growth Standards SAS igrowup package. p4. http://www.who.int/entity/childgrowth/software/readme_sas.pdf.
- 16.WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. Geneva: World Health Organization; 2006. [Google Scholar]
- 17.Ekelund U, Ong K, Linne Y, et al. Upward weight percentile crossing in infancy and early childhood independently predicts fat mass in young adults: the Stockholm Weight Development Study (SWEDES) Am J Clin Nutr. 2006;83(2):324–330. doi: 10.1093/ajcn/83.2.324. [DOI] [PubMed] [Google Scholar]
- 18.Toschke AM, Grote V, Koletzko B, von Kries R. Identifying children at high risk for overweight at school entry by weight gain during the first 2 years. Arch Pediatr Adolesc Med. 2004;158(5):449–452. doi: 10.1001/archpedi.158.5.449. [DOI] [PubMed] [Google Scholar]
- 19.Dietz WH. Periods of risk in childhood for the development of adult obesity--what do we need to learn? J Nutr. 1997;127(9):1884S–1886S. doi: 10.1093/jn/127.9.1884S. [DOI] [PubMed] [Google Scholar]
- 20.Karaolis-Danckert N, Buyken AE, Bolzenius K, Perim de Faria C, Lentze MJ, Kroke A. Rapid growth among term children whose birth weight was appropriate for gestational age has a longer lasting effect on body fat percentage than on body mass index. Am J Clin Nutr. 2006;84(6):1449–1455. doi: 10.1093/ajcn/84.6.1449. [DOI] [PubMed] [Google Scholar]
- 21.Stettler N, Zemel BS, Kumanyika S, Stallings VA. Infant weight gain and childhood overweight status in a multicenter, cohort study. Pediatrics. 2002;109(2):194–199. doi: 10.1542/peds.109.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Stettler N, Kumanyika SK, Katz SH, Zemel BS, Stallings VA. Rapid weight gain during infancy and obesity in young adulthood in a cohort of African Americans. Am J Clin Nutr. 2003;77(6):1374–1378. doi: 10.1093/ajcn/77.6.1374. [DOI] [PubMed] [Google Scholar]
- 23.Rzehak P, Sausenthaler S, Koletzko S, et al. Period-specific growth, overweight and modification by breastfeeding in the GINI and LISA birth cohorts up to age 6 years. Eur J Epidemiol. 2009;24(8):449–467. doi: 10.1007/s10654-009-9356-5. [DOI] [PubMed] [Google Scholar]
- 24.Adair LS. Child and adolescent obesity: epidemiology and developmental perspectives. Physiol Behav. 2008;94(1):8–16. doi: 10.1016/j.physbeh.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Dennison BA, Edmunds LS, Stratton HH, Pruzek RM. Rapid infant weight gain predicts childhood overweight. Obesity (Silver Spring) 2006;14(3):491–499. doi: 10.1038/oby.2006.64. [DOI] [PubMed] [Google Scholar]
- 26.Akaboshi I, Haraguchi Y, Mizumoto Y, Kitano A, Kan H. Taller stature after postnatal rapid weight gain in early infancy predicts overweight status at age 3. Acta Paediatr. 2008;97(10):1460–1464. doi: 10.1111/j.1651-2227.2008.00932.x. [DOI] [PubMed] [Google Scholar]
- 27.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monteiro PO, Victora CG, Barros FC, Monteiro LM. Birth size, early childhood growth, and adolescent obesity in a Brazilian birth cohort. Int J Obes Relat Metab Disord. 2003;27(10):1274–1282. doi: 10.1038/sj.ijo.0802409. [DOI] [PubMed] [Google Scholar]
- 29.Wells JC, Hallal PC, Wright A, Singhal A, Victora CG. Fetal, infant and childhood growth: relationships with body composition in Brazilian boys aged 9 years. Int J Obes (Lond) 2005;29(10):1192–1198. doi: 10.1038/sj.ijo.0803054. [DOI] [PubMed] [Google Scholar]
- 30.Baird J, Fisher D, Lucas P, Kleijnen J, Roberts H, Law C. Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ. 2005;331(7522):929. doi: 10.1136/bmj.38586.411273.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Seo DC, Kolbe L, Middlestadt S, Zhao W. Trends in overweight among school children and adolescents in seven Chinese Provinces, from 1991-2004. International journal of pediatric obesity : IJPO : an official journal of the International Association for the Study of Obesity. 2010;5(5):375–382. doi: 10.3109/17477161003592592. [DOI] [PubMed] [Google Scholar]