Summary
OBJECTIVES
To describe specific causes of the high rates of stillbirth, neonatal death and early child childhood death in Zambia.
METHODS
We conducted a household-based survey in rural Zambia. Socio-demographic and delivery characteristics were recorded, alongside a maternal HIV test. Verbal autopsy questionnaires were administered to elicit mortality-related information and independently reviewed by three experienced paediatricians who assigned a cause and contributing factor to death. For this secondary analysis, deaths were categorized into: stillbirths (foetal death ≥28 weeks of gestation), neonatal deaths (≤28 days) and early childhood deaths (>28 days to <2 years).
RESULTS
Among 1679 households, information was collected on 148 deaths: 34% stillbirths, 26% neonatal and 40% early childhood deaths. Leading identifiable causes of stillbirth were intrauterine infection (26%) and birth asphyxia (18%). Of 32 neonatal deaths, 38 (84%) occurred within the first week of life, primarily because of infections (37%) and prematurity (34%). The majority of early childhood deaths were caused by suspected bacterial infections (82%). HIV prevalence was significantly higher in mothers who reported an early childhood death (44%) than mothers who did not (17%; P < 0.01). Factors significantly associated with mortality were lower socio-economic status (P < 0.01), inadequate water or sanitation facilities (P < 0.01), home delivery (P = 0.04) and absence of a trained delivery attendant (P < 0.01).
CONCLUSION
We provide community-level data about the causes of death among children under 2 years of age. Infectious etiologies for mortality ranked highest. At a public health level, such information may have an important role in guiding prevention and treatment strategies to address perinatal and early childhood mortality.
Keywords: autopsy, stillbirth, infant, Zambia, Africa, cause of death
Introduction
Perinatal and early child mortality rates in Zambia are among the highest in the world (Hyder et al. 2003; Madise et al. 2003; Stanton et al. 2006). Similar to many other resource-constrained settings however, causes of death are generally unavailable (Van Eijk et al. 2008). Numerous factors conspire to make their ascertainment a challenge. In rural areas, for example, clinics are often understaffed and have inadequate systems for data collection or disease monitoring. Causes of death may be recorded without an autopsy, laboratory data or clinical examination. Additionally up to half of pregnant women deliver at home for reasons that include logistical difficulties in accessing clinical care, transportation costs and a lack of adequate health education (Stekelenburg et al. 2004, Central Statistical Office et al. 2009). As a result, many delivery and infant outcomes go unreported (Van Dijk et al. 2009).
In such settings, verbal autopsies (VAs) may prove valuable in understanding the possible causes of child mortality (Anker et al. 1999; Nongkynrih et al. 2003; Kanungo 2005). With this method, trained interviewers administer a detailed questionnaire to relatives of the deceased; where possible, supplemental information such as medical records may be gathered. These data are then reviewed and analysed by medical personnel – often using data-derived algorithms – to determine cause and contributing factors associated with death (Quigley et al. 1996). VAs can reasonably identify major causes of child and neonatal death compared to physician-assigned cause-of-death data, with sensitivity of 60–83% and specificity of 76–85% (Kalter et al. 1990; Snow et al. 1992; Nykanen et al. 1995; Thatte et al. 2009). Comparisons of VAs and hospital records show a lower diagnostic accuracy of 64% for stillbirths (Edmond et al. 2008). However, in settings with poor diagnostic capacity and low accessibility to healthcare, VAs can still play an important role in policy decisions by providing targeted information about paediatric morbidity and mortality (Mobley et al. 1996; Quigley et al. 1999).
Method
We sought to determine the causes of stillbirth (foetal death of ≥28 weeks gestation), neonatal death (birth to ≤28 days) and early childhood mortality (>28 days to <2 years) across four communities in rural Zambia. We analysed data from a community survey designed to determine the public health impact of offering routine antiretroviral therapy (ART) for perinatal HIV transmission on under-2 HIV-free survival. The four selected communities were in Kafue district, roughly 50 km outside the capital city of Lusaka. Two rounds of the community survey are planned; the results reported here represent baseline community data obtained prior to programme implementation.
The sampling for the survey was established according to government-demarcated catchments areas for the community’s primary public health facility. Zones in each catchment area – defined by the facility’s Neighborhood Health Committees for community outreach activities – were randomly selected into a specific sequence and a central point was identified for each. At this starting point, a ‘spin the bottle’ approach was used to determine initial direction (Giganti et al. 2010). Every third house was selected and eligibility assessed. A household was deemed eligible if a child under 2 years of age was currently living within its confines or if the family reported the death of a child under 2 years of age within the past 2 years. If the household was eligible, written consent was obtained from the child’s mother for a 165-question survey, performed to better understand the demographic and socioeconomic characteristics of the household and the mother’s medical history. Alongside this questionnaire (itself modelled after the Zambian Demographic and Health Survey), separate written consent was obtained for blood specimen collection from mothers and eligible children (Stringer et al. 2008; Central Statistical Office et al. 2009). The maternal samples were later tested for HIV antibodies and, in those who were seropositive the exposed infants were also tested using PCR. In households where the newborn child had died within the past 2 years, survey staff administered a comprehensive VA questionnaire. Surveys were administered in the local language, adapted from previously developed age-specific VA tools (World Health Organization 2007). Sampling continued in a clockwise fashion until a zone had been canvassed. At that point, team members went to the next listed zone and started the process anew. Zones were sampled until 387 eligible households in each community were surveyed. If the target was reached midway through a zone, sampling for the entire zone was completed before activities were halted.
Completed VA questionnaires were independently reviewed by three paediatricians (CBM, MMM and MBG), all with substantial clinical experience in Zambia. Each provided a cause of death (i.e. underlying disease or action responsible for death) and up to two contributing factors to death (i.e. medical condition or circumstance thought to have significantly contributed to death). The group then convened to discuss cases and review individual decisions. Agreement between two of the three reviewers was required for assignment of a final cause of death or contributing factor. If no agreement could be reached or if the reviewers agreed that cause of death could not be determined, the cause of death was listed as ‘unknown’. Reviewers were blinded to the mother’s HIV test results and to the assessments of the other reviewers. In our analysis, we collated causes of and contributing factors for death, and further stratified them according to timing (stillbirth, neonatal death or early child death) and maternal HIV status.
The stillbirth rate was calculated as the number of events per 1000 deliveries. Fresh stillbirth was defined as stillbirths occurring in the intrapartum period which are generally normal in appearance, while a macerated stillbirth was defined as one with skin not intact or macerated, implying a death >12 h before delivery (Chi et al. 2007). Owing to the design and eligibility criteria of this one-time household survey, not all living children included in the analysis had reached 28 days (for neonates), or 2 years (for early childhood). For this reason, it was not possible to present neonatal and early childhood mortality according to standard metrics (i.e. per 1000 live births). We instead described these rates by deaths per 100 person-months.
Using Chi-Squared tests and Wilcoxon Rank Sum Tests, household socio-demographic, antenatal and delivery characteristics were compared between houses with at least one recorded death in the last 2 years among children under 2 years of age and houses with no deaths. All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC, USA). This study was approved by ethical review committees at the University of Zambia (Lusaka, Zambia) and the University of Alabama at Birmingham (Birmingham, AL, USA).
Results
We approached 3612 households between November 2008 and May 2009. Of these, 1716 (48%) were not eligible and 217 (6%) refused to participate. Of the remaining 1679 households, 1541 (92%) reported at least one live child under the age of 2 at time of survey, while 138 (8%) households reported at least one child who had died in the last 2 years under the age of 2. A total of 148 child deaths were recorded in 138 households: 50 (34%) were stillbirths, 38 (26%) were neonatal deaths and 60 (40%) were early childhood deaths. Overall, 42 (28%) were born to mothers who tested HIV positive. Four (3%) had a mother who had died. The rate of stillbirths was 27 per 1000 deliveries (95% CI: 20, 36), and the rate of neonatal deaths was 2.4 per 100 person-months (95%CI: 1.7, 3.2). The rate of early childhood deaths among children with greater than 28 days of follow-up was 0.33 per 100 person-months (95%CI: 0.25, 0.42).
We aggregated the causes of death and then stratified our population according to timing of death and maternal HIV status (Table 1). The contributing factors to death, as assigned by our reviewers, were likewise categorized according to age group (Figure 1a, b, c). Among the 148 total deaths, 33 (22%) had no contributing factors assigned by reviewers, 77 (52%) had one and 38 (26%) had two.
Table 1.
Causes of stillbirth, neonatal death and early childhood death in Kafue, Zambia from households surveyed between November 2008 and May 2009 (N = 148)
| Total | HIV-exposed | No HIV exposure | HIV exposure unknown | |
|---|---|---|---|---|
| Stillbirth (foetal death ≥28 weeks gestation, n = 50) | ||||
| Intrauterine infection (macerated SB, n = 7) | 13 | 4 | 7 | 2 |
| Birth asphyxia/placental insufficiency | 9 | 0 | 9 | 0 |
| Cord compromise/prolapse | 6 | 0 | 6 | 0 |
| Maternal malaria (macerated SB, n = 1) | 4 | 1 | 3 | 0 |
| Anencephaly | 1 | 0 | 1 | 0 |
| Induced abortion | 1 | 0 | 1 | 0 |
| Pre-eclampsia | 1 | 0 | 1 | 0 |
| Unknown (macerated SB, n = 6) | 15 | 6 | 8 | 1 |
| Neonatal (≤28 days, n = 38) | ||||
| Infection (n = 14) | ||||
| Pneumonia | 6 | 2 | 4 | 0 |
| Sepsis | 5 | 1 | 3 | 1 |
| Meningitis | 2 | 0 | 2 | 0 |
| Congenital infection | 1 | 0 | 1 | 0 |
| Prematurity (n = 13) | ||||
| Respiratory distress syndrome prematurity | 3 | 1 | 2 | 0 |
| Other prematurity | 10 | 1 | 8 | 1 |
| Birth asphyxia/placental insufficiency | 6 | 1 | 5 | 0 |
| Congenital genitourinary abnormality | 1 | 0 | 1 | 0 |
| Exsanguination | 1 | 1 | 0 | 0 |
| Unknown | 3 | 0 | 3 | 0 |
| Early Childhood (>28 days to <2 years, n = 60) | ||||
| Infection (n = 49) | ||||
| Gastroenteritis/enteritis | 15 | 4 | 8 | 3 |
| Pneumonia | 15 | 8 | 6 | 1 |
| Meningitis | 8 | 1 | 7 | 0 |
| Malaria | 5 | 2 | 3 | 0 |
| Cerebral malaria | 4 | 2 | 1 | 1 |
| Sepsis | 1 | 0 | 1 | 0 |
| Other CNS infection | 1 | 0 | 1 | 0 |
| Malnutrition | 5 | 5 | 0 | 0 |
| Congenital liver disease | 1 | 0 | 1 | 0 |
| Burns | 1 | 0 | 1 | 0 |
| Prematurity | 1 | 1 | 0 | 0 |
| Necrotizing enterocolitis (NEC) | 1 | 1 | 0 | 0 |
| Renal failure | 1 | 0 | 1 | 0 |
| Unknown | 1 | 0 | 1 | 0 |
Figure 1.
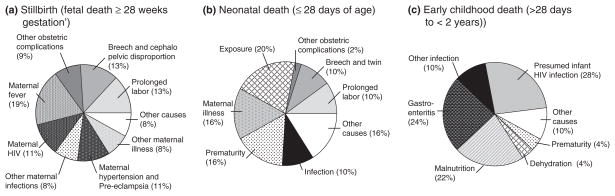
Contributing factors to stillbirth, neonatal death and early childhood death in Kafue, Zambia from households surveyed between November 2008 and May 2009 (N = 148).
In a high proportion of stillbirth cases (30%), reviewers were unable to assign a cause because of insufficient reported detail (Table 1). Among those with a listed cause of death, the most common diagnoses were infections (e.g. chorioamnionitis; 26%), birth asphyxia (18%) and other obstetric complications (12%). Foetal death was suspected to have occurred during the late antenatal or intrapartum period in the majority (36 of 50; 72%) of cases; accordingly, these were described as fresh stillbirths. Among the 14 stillbirths categorized as macerated, causes included intrauterine infection and maternal malaria. There was no significant difference in HIV positivity when we compared postpartum women with a reported stillbirth and those without (23.9% vs. 17.7%, P = 0.28). Reviewers did not assign any contributing factors to 13 of 50 (26%) stillbirths. In the remaining 37, contributing factors included maternal illnesses (57%) and obstetric complications (35%) such as breech presentation, twin gestation and prolonged labour (Figure 1a). Other contributing factors included maternal ingestion of traditional medicines to induce abortion and home delivery (8%).
The leading causes of neonatal death were infection (37%), prematurity (34%) and birth asphyxia (16%; Table 1). Most infections were classified as bacterial, with pneumonia, sepsis and meningitis mostly described. The majority (32 of 38) of neonatal deaths occurred within the first week, and over half (20 of 38) died within 24 h of birth. Rare causes of death included congenital anomalies (n = 1) and exsanguination from the umbilical stump (n = 1). Again, the proportions of mothers with HIV were no different between those with and without a reported neonatal death (19.4% vs. 17.7%, P = 0.79). Reviewers did not assign a contributing factor to 7 of 38 (18%) neonatal deaths. Major contributors to the remaining 31 neonatal deaths were obstetric complications (38%) and associated maternal illness (16%) such as fever, syphilis and assault (Figure 1b).
The majority (49 of 60) of early childhood deaths appeared to result from bacterial infection, including pneumonia (n = 15), gastroenteritis (n = 15) and meningitis (n = 8; Table 1). The maternal HIV prevalence was significantly higher in those mothers reporting an early childhood death (43.6%) compared with those not reporting one (17.2%; P < 0.01). Additionally all five children who died from malnutrition had HIV-infected mothers. One 2-month-old child was reportedly found dead in her cot with blood around the mouth and nose; because of insufficient detail, our review committee listed the cause of death as unknown. Severe burns from a house fire caused the death of another child. Reviewers did not assign a contributing factor to 13 of 60 (22%) early childhood deaths. The primarily contributors to the remaining 47 deaths were reported HIV child infection (28%) and gastroenteritis (24%; Figure 1c). Malnutrition as a contributing factor was not related to HIV exposure of the child.
We compared household, antenatal and delivery characteristics among those with and without a reported death in the past 2 years (Tables 2 and 3). In a univariate analysis, households with lower socio-economic status, described by the availability of electricity and various household commodities, were more likely to have had a recent child death (P < 0.01). Households without running water or a flush toilet were also associated with increased child death (P < 0.01). Additionally child death was associated with mothers who delivered at home (P = 0.04) and/or did not have a trained attendant present at delivery (P < 0.01). Maternal age, marital status and education level of mother were not significantly different between the two groups.
Table 2.
Comparison of household characteristics among women with and without a reported stillbirth, neonatal death or early childhood death in Kafue, Zambia surveyed between November 2008 and May 2009 (N = 1679)
| Household Characteristics | Households with no deaths* (N = 1541) (%) | Households with a death (n = 138) (%) | P-value |
|---|---|---|---|
| Water Supply to Household | |||
| Piped water into house | 226 (14.7) | 6 (4.3) | <0.01 |
| Piped water outside | 371 (24.2) | 26 (18.8) | |
| Public tap/borehole/protected well | 594 (38.7) | 73 (52.9) | |
| Unprotected well/surface water/other | 343 (22.4) | 33 (23.9) | |
| Water Treatment | |||
| Boil/All bleach chlorine | 897 (58.5) | 74 (53.6) | 0.26 |
| Nothing | 636 (41.5) | 64 (46.4) | |
| Main Material of Floor | |||
| Natural floor | 598 (39.0) | 71 (51.4) | <0.01 |
| Finished Floor | 936 (61.0) | 67 (48.6) | |
| Toilet Facilities in household | |||
| Flush Toilet | 268 (17.5) | 8 (5.9) | <0.01 |
| Pit latrine | 1075 (70.2) | 111 (81.6) | |
| No facility | 188 (12.3) | 17 (12.5) | |
| Mosquito net in household for child | 1142 (74.5) | 95 (68.8) | 0.15 |
| Electricity in household | 511 (33.3) | 25 (18.1) | <0.01 |
| Television in household | 618 (40.3) | 34 (24.6) | <0.01 |
| Cell phone in household | 944 (61.5) | 67 (48.6) | <0.01 |
| Refrigerator in household | 313 (20.4) | 11 (8.0) | <0.01 |
| Car or motorbike in household | 65 (4.2) | 3 (2.2) | 0.24 |
| Bicycle in household | 673 (43.9) | 49 (35.5) | 0.06 |
| Usually enough food for household | 703 (45.9) | 56 (40.6) | 0.23 |
Households reporting a child under 2 years of age but with no reported stillbirth, neonatal death or early childhood death in the previous 2 years.
Table 3.
Comparison of maternal and delivery characteristics among women with and without a reported stillbirth, neonatal death, or early childhood death in Kafue, Zambia surveyed between November 2008 to May 2009 (N = 1679)
| Maternal and Delivery Characteristics | Mothers with no deaths (N = 1585)* (%) | Mothers with a death (n = 138) (%) | P-value |
|---|---|---|---|
| Maternal Age | 26.0 (22.0, 31.0) | 26.0 (21.5, 31.0) | 0.93 |
| Married or living with partner | 1299 (87.1) | 121 (91.7) | 0.13 |
| Education Level of Mother | |||
| Primary or did not attend school | 739 (48.9) | 77 (57.9) | 0.06 |
| Secondary | 769 (50.9) | 56 (42.1) | |
| Higher | 2 (0.1) | 0 (0.0) | |
| Women who sought antenatal care | 1,431 (95.3) | 104 (86.7) | <0.01 |
| Syphilis test performed at antenatal visit | 1,232 (82.2) | 88 (73.9) | 0.03 |
| Positive syphilis test | 38 (3.1) | 8 (9.1) | <0.01 |
| HIV test performed at antenatal visit | 1,305 (86.9) | 93 (78.2) | <0.01 |
| Positive HIV test | 120 (9.2) | 13 (14.1) | 0.16 |
| Place of delivery | |||
| Home | 646 (41.0) | 72 (52.2) | 0.04 |
| Government hospital | 396 (25.1) | 36 (26.1) | |
| Government health clinic/post | 441 (28.0) | 26 (18.8) | |
| Private or mission hospital | 16 (1.0) | 0 (0.0) | |
| Mission health clinic/post | 77 (4.9) | 4 (2.9) | |
| Attendant at Delivery | |||
| Doctor | 62 (3.9) | 8 (5.8) | <0.01 |
| Nurse/Midwife | 871 (55.3) | 59 (43.1) | |
| Traditional Birth Attendant | 198 (12.6) | 10 (7.3) | |
| No trained attendant | 445 (28.2) | 60 (43.8) | |
Mothers reporting a child under 2 years of age but with no reported stillbirth, neonatal death or early childhood death in the previous 2 years
Discussion
In this report, we describe causes of mortality during the perinatal, neonatal and early childhood periods in rural Zambia. Our VA methodology allowed reasonable approximations about the causes of death; however, precise diagnoses were often difficult to ascertain. Infection was a very common cause of death across all age strata and, although not an outcome of this analysis, reviewers commented on the preventable nature of many illnesses. Our analysis suggests that improved water and sanitation facilities, increased access to trained birth attendants and early entry into paediatric HIV care may be reasonable interventions to reduce mortality in this setting.
The high prevalence of infectious etiologies, related to death in all age groups of this study, is consistent with other reports from southern Africa and from other resource-limited settings (Mobley et al. 1996; Hinderaker et al. 2003; Nongkynrih et al. 2003; Chi et al. 2007). Like other studies, neonates appeared at highest risk from infection in the first week of life (Lawn et al. 2006; Lawoyin et al. 2010). Empiric antibiotic treatment for preterm premature rupture of membranes in low-income countries has been proposed to decrease infection-related mortality by as much as 8% (Cousens et al. 2010). Such strategies could be deployed here to tackle the high mortality rate from infection.
At nearly 18%, maternal HIV prevalence in this population corresponds to previous literature in pregnant women from this area (Stringer et al. 2008). Despite our small sample size, HIV exposure was high and observed in a large proportion of early childhood deaths attributed to malnutrition and to infectious causes such as pneumonia and gastroenteritis. Infant HIV testing at 6 weeks and early initiation of antiretroviral treatment for HIV-exposed children is known to reduce early child mortality by 76% (Violari et al. 2008). The Ministry of Health has adopted this approach as part of its most recent guidelines for paediatric HIV treatment. Successfully implemented, this strategy could lead to more rapid immune reconstitution and improved infant and early childhood survival.
Home delivery – either alone or in the presence of unskilled attendants – was reported by our participants at a frequency similar to other studies in rural Zambia (Van Den Boogaard et al. 2008). Prior research has shown links between home delivery and poor birth outcomes (Lawoyin et al. 2010), because of obstetric complications and infection. Although we are unable to show causality using our study design, the medical literature strongly suggests that better access to institutional delivery may result in better survival (World Health Organization 1994). Where financial cost and/or cultural norms prevent the regular use of facility-based delivery services, the training of community health workers to recognize danger signs in labour and to perform basic neonatal resuscitation may significantly reduce asphyxia-related deaths and intrapartum (i.e. fresh) stillbirths (Deorari et al. 2001; Carlo et al. 2010). Outreach and educational efforts focused on clean home delivery, hygienic cord care and pneumonia case management could also minimize neonatal infection and reduce mortality (Darmstadt et al. 2005). Engagement of traditional healers and traditional birth attendants may also be important, particularly in the establishment of referral systems for complicated pregnancies and deliveries (Banda et al. 2007; Van Den Boogaard et al. 2008).
Our study’s VA methodology, whereby physicians assigned causes of death using information collected from a standardized instrument, has been validated in other trials (Nykanen et al. 1995; Quigley et al. 1999; Baiden et al. 2007). Nevertheless, we recognize several limitations to the approach. Because VAs rely on patient-reported information – sometimes long after the event – this methodology risks misclassification around timing and cause of death, particularly when medical records are not available for validation (Quigley et al. 1996; Thatte et al. 2009). Suboptimal ascertainment of death was a general concern. This was most apparent in one of our four target communities, where a religious group with specific beliefs opposed to health care strongly discouraged its members from participating. Finally, this sub-study was not designed to identify predictors for stillbirth, neonatal mortality and early childhood mortality, and the small sample size restricted our ability to conduct multivariable analyses. As a result, it is possible that our findings may be subject either to type I (i.e. report of an association that does not truly exist) or type II (i.e. failure to detect a true association) errors.
In this rural Zambian setting, where routine autopsy or mortality data are not readily available, we provide important information about the causes of stillbirth, neonatal mortality and early childhood mortality. Despite limitations around its precision, VA-based surveys have a clear role in public health programmes seeking to target broad areas for health improvement. Investments in the diagnosis and treatment of infectious diseases and the development of novel community-based strategies could have notable impacts on public health outcomes. Surveillance around the causes of childhood mortality should continue longitudinally, using the best-available data to guide policy decisions.
Acknowledgments
The authors acknowledge the Zambian Ministry of Health for consistent and high-level support of operations research in the context of PMTCT. Investigator salary was provided by the National Institutes of Health (R24-TW007988, D43-TW001035, K01-TW06670; P30-AI027767), and a Clinical Scientist Development Award from the Doris Duke Charitable Foundation (2007061).
References
- Anker M, Black R, Coldham C, Kalter H, Quigley M, Ross D. A standard verbal autopsy method for investigating causes of death in infants and children. 1999. [Google Scholar]
- Baiden F, Bawah A, Biai S, et al. Setting international standards for verbal autopsy. Bulletin of the World Health Organization. 2007;85:570–571. doi: 10.2471/BLT.07.043745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banda Y, Chapman V, Goldenberg RL, et al. Use of traditional medicine among pregnant women in Lusaka, Zambia. Journal of Alternative and Complementary Medicine. 2007;13:123–127. doi: 10.1089/acm.2006.6225. [DOI] [PubMed] [Google Scholar]
- Carlo WA, Goudar SS, Jehan I, et al. Newborn-care training and perinatal mortality in developing countries. New England Journal of Medicine. 2010;362:614–623. doi: 10.1056/NEJMsa0806033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Central Statistical Office, Ministry of Health, Tropical Diseases Research Centre, University of Zambia & Inc. M. I. Zambia demographic and health survey 2007. 2009. [Google Scholar]
- Chi BH, Wang L, Read JS, et al. Predictors of stillbirth in sub-saharan Africa. Obstetrics and Gynecology. 2007;110:989–997. doi: 10.1097/01.AOG.0000281667.35113.a5. [DOI] [PubMed] [Google Scholar]
- Cousens S, Blencowe H, Gravett M, Lawn JE. Antibiotics for pre-term pre-labour rupture of membranes: prevention of neonatal deaths due to complications of pre-term birth and infection. International Journal of Epidemiology. 2010;39(Suppl 1):i134–i143. doi: 10.1093/ije/dyq030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darmstadt GL, Bhutta ZA, Cousens S, Adam T, Walker N, De Bernis L. Evidence-based, cost-effective interventions: how many newborn babies can we save? Lancet. 2005;365:977–988. doi: 10.1016/S0140-6736(05)71088-6. [DOI] [PubMed] [Google Scholar]
- Deorari AK, Paul VK, Singh M, Vidyasagar D. Impact of education and training on neonatal resuscitation practices in 14 teaching hospitals in India. Annals of Tropical Paediatrics. 2001;21:29–33. [PubMed] [Google Scholar]
- Edmond KM, Quigley MA, Zandoh C, et al. Diagnostic accuracy of verbal autopsies in ascertaining the causes of stillbirths and neonatal deaths in rural Ghana. Paediatric and Perinatal Epidemiology. 2008;22:417–429. doi: 10.1111/j.1365-3016.2008.00962.x. [DOI] [PubMed] [Google Scholar]
- Giganti MJ, Levy JW, Banda Y, et al. Methods and baseline results of a repeated cross-sectional survey to assess the public health impact of antiretroviral therapy in Lusaka, Zambia. American Journal of Tropical Medicine and Hygiene. 2010;82:971–977. doi: 10.4269/ajtmh.2010.09-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderaker SG, Olsen BE, Bergsjo PB, et al. Avoidable stillbirths and neonatal deaths in rural Tanzania. British Journal of Obstetrics and Gynaecology. 2003;110:616–623. [PubMed] [Google Scholar]
- Hyder AA, Wali SA, Mcguckin J. The burden of disease from neonatal mortality: a review of South Asia and Sub-Saharan Africa. British Journal of Obstetrics and Gynaecology. 2003;110:894–901. [PubMed] [Google Scholar]
- Kalter HD, Gray RH, Black RE, Gultiano SA. Validation of postmortem interviews to ascertain selected causes of death in children. International Journal of Epidemiology. 1990;19:380–386. doi: 10.1093/ije/19.2.380. [DOI] [PubMed] [Google Scholar]
- Kanungo S. Bulletin of the World Health. World Health Organization; Geneva, Switzerland: 2005. WHO technical consultation on verbal autopsy tools. [Google Scholar]
- Lawn JE, Wilczynska-Ketende K, Cousens SN. Estimating the causes of 4 million neonatal deaths in the year 2000. International Journal of Epidemiology. 2006;35:706–718. doi: 10.1093/ije/dyl043. [DOI] [PubMed] [Google Scholar]
- Lawoyin TO, Onadeko MO, Asekun-Olarimoye EO. Neonatal mortality and perinatal risk factors in rural south-western Nigeria: a community-based prospective study. West African Journal of Medicine. 2010;29:19–23. doi: 10.4314/wajm.v29i1.56183. [DOI] [PubMed] [Google Scholar]
- Madise NJ, Banda EM, Benaya KW. Infant mortality in Zambia: socioeconomic and demographic correlates. Social Biology. 2003;50:148–166. doi: 10.1080/19485565.2003.9989069. [DOI] [PubMed] [Google Scholar]
- Mobley CC, Boerma JT, Titus S, Lohrke B, Shangula K, Black RE. Validation study of a verbal autopsy method for causes of childhood mortality in Namibia. Journal of Tropical Pediatrics. 1996;42:365–369. doi: 10.1093/tropej/42.6.365. [DOI] [PubMed] [Google Scholar]
- Nongkynrih B, Anand K, Kapoor SK. Use of verbal autopsy by health workers in under-five children. Indian Pediatrics. 2003;40:766–771. [PubMed] [Google Scholar]
- Nykanen M, Tamaona W, Cullinan T, Van Oosterzee V, Ashorn P. Verbal autopsy as a technique to establish causes of infant and child mortality. East African Medical Journal. 1995;72:731–734. [PubMed] [Google Scholar]
- Quigley MA, Armstrong Schellenberg JR, Snow RW. Algorithms for verbal autopsies: a validation study in Kenyan children. Bulletin of the World Health Organization. 1996;74:147–154. [PMC free article] [PubMed] [Google Scholar]
- Quigley MA, Chandramohan D, Rodrigues LC. Diagnostic accuracy of physician review, expert algorithms and data-derived algorithms in adult verbal autopsies. International Journal of Epidemiology. 1999;28:1081–1087. doi: 10.1093/ije/28.6.1081. [DOI] [PubMed] [Google Scholar]
- Snow RW, Armstrong JR, Forster D, et al. Childhood deaths in Africa: uses and limitations of verbal autopsies. Lancet. 1992;340:351–355. doi: 10.1016/0140-6736(92)91414-4. [DOI] [PubMed] [Google Scholar]
- Stanton C, Lawn JE, Rahman H, Wilczynska-Ketende K, Hill K. Stillbirth rates: delivering estimates in 190 countries. Lancet. 2006;367:1487–1494. doi: 10.1016/S0140-6736(06)68586-3. [DOI] [PubMed] [Google Scholar]
- Stekelenburg J, Kyanamina S, Mukelabai M, Wolffers I, Van Roosmalen J. Waiting too long: low use of maternal health services in Kalabo, Zambia. Tropical Medicine and International Health. 2004;9:390–398. doi: 10.1111/j.1365-3156.2004.01202.x. [DOI] [PubMed] [Google Scholar]
- Stringer EM, Chintu NT, Levy JW, et al. Declining HIV prevalence among young pregnant women in Lusaka, Zambia. Bulletin of the World Health Organization. 2008;86:697–702. doi: 10.2471/BLT.07.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatte N, Kalter HD, Baqui AH, Williams EM, Darmstadt GL. Ascertaining causes of neonatal deaths using verbal autopsy: current methods and challenges. Journal of Perinatology. 2009;29:187–194. doi: 10.1038/jp.2008.138. [DOI] [PubMed] [Google Scholar]
- Van Den Boogaard J, Arntzen B, Chilwana J, Liyungu M, Mantingh A, Stekelenburg J. Skilled or traditional birth attendant? Choices of communities in Lukulu District, rural Zambia. World Health and Population. 2008;10:34–43. doi: 10.12927/whp.2008.19736. [DOI] [PubMed] [Google Scholar]
- Van Dijk JH, Sutcliffe CG, Munsanje B, Hamangaba F, Thuma PE, Moss WJ. Barriers to the care of HIV-infected children in rural Zambia: a cross-sectional analysis. BMC Infectious Diseases. 2009;9:169. doi: 10.1186/1471-2334-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eijk AM, Adazu K, Ofware P, Vulule J, Hamel M, Slutsker L. Causes of deaths using verbal autopsy among adolescents and adults in rural western Kenya. Tropical Medicine and International Health. 2008;13:1314–1324. doi: 10.1111/j.1365-3156.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. New England Journal of Medicine. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Safe Mother. 1994/07/01. World Health Organisation; Geneva: 1994. The mother-baby package: WHO’s guide to saving women’s and infants’ lives. [PubMed] [Google Scholar]
- World Health Organization. Verbal Autopsy Standards: Ascertaining and Attributing Cause of Death. World Health Organisation; Geneva: 2007. [Google Scholar]


