Abstract
Objectives
The objective of this study was to develop a simultaneous population pharmacokinetic (PK) model to describe atazanavir/ritonavir (ATV/RTV) PK (300/100 mg) and to assess the effect of RTV dose reduction on ATV PK. Simulations of ATV concentration-time profiles were performed at doses of ATV/RTV 300/50 mg, 200/50 mg, and 200/100 mg once daily.
Methods
A total of 288 ATV and 312 RTV plasma concentrations from 30 patients were included to build a population PK model using the stochastic approximation expectation maximization algorithm implemented in MONOLIX 3.2 software.
Results
A one-compartment model with first-order absorption and lag-time best described the data for both drugs in the final simultaneous model. A maximum-effect model in which RTV inhibited the elimination of ATV was used to describe the relationship between RTV concentrations and ATV clearance (CL/F). An RTV concentration of 0.22 mg/L was associated with 50% maximum inhibition of ATV CL/F. The population prediction of ATV CL/F in the absence of RTV was 16.6 L/h (relative standard error, 7.0%), and the apparent volume of distribution and absorption rate constant were 106 L (relative standard error, 8%) and 0.87 per hour (fixed), respectively. Simulated average ATV trough concentrations at ATV/RTV 300/50 mg, 200/50 mg, and 200/100 mg once daily were 45%, 63%, and 33% lower, respectively, than that of the standard dose.
Conclusion
Although simulated median ATV trough concentrations after dose reductions were still more than the ATV minimum effective concentration (2.9-, 1.9-, and 3.6-fold for ATV/RTV 300/50 mg, 200/50 mg, and 200/100 mg, respectively); our modeling predicted a high proportion of individuals with subtherapeutic trough concentrations on the 200/50 mg dose. This suggests that 300/50 mg and 200/100 mg dosing are preferred candidate regimens in future clinical studies.
Keywords: population PK, nonlinear mixed effect, atazanavir, ritonavir, dose reduction
INTRODUCTION
Atazanavir (ATV) is an HIV protease inhibitor (PI) administered once daily at a dose of 300 mg with 100 mg of ritonavir (RTV) to “boost” its plasma concentrations.1 ATV can be used without boosting at 400 mg once daily.2,3 Although 400 mg once daily is not licensed in Europe,4 in the United States, it is licensed for the treatment of naive patients who cannot tolerate RTV.5
ATV is mainly metabolized by cytochrome P450 3A4 (CYP3A4), which is present in the intestine and liver. There is marked interindividual variability in CYP3A4 expression and function that is not explained by current knowledge of single nucleotide polymorphisms in the CYP3A4 gene. ATV is also a substrate for ABCB1 (P-glycoprotein), and this transporter may influence both intestinal absorption and excretion into bile.6,7 ATV plasma exposure correlates with virological response, and a trough concentration (Ctrough) of 0.150 mg/L has been proposed as the minimum effective concentration (MEC).8,9 An upper limit more than 0.85 mg/L has been associated with a risk of unconjugated hyperbilirubinaemia10 because of uridine diphosphate glucuronyltransferase 1A1 (UGT1A1) inhibition.
In general, RTV boosting of PIs increases systemic exposure and decreases interpatient variability in pharmacokinetics (PKs). This effect is mainly because of the inhibitory effect of RTV on CYP3A4 and P-glycoprotein.11 Coadministration of RTV increases the ATV area under the curve (AUC) 3.4-fold and the Cmax 1.9-fold, but the Cmin is raised 11.9-fold: thus the main effect of RTV on ATV PK is inhibition of ATV clearance in the liver.12
RTV is not well tolerated and has been shown to elevate lipid levels even at doses of 100–200 mg daily.13 In a study of healthy volunteers, higher RTV exposure was correlated with increases in lipids.13 Recently, a PK trial of 13 healthy individuals (majority white),14 showed a Ctrough slightly lower, but significantly more than the suggested MEC, when boosted with 50 mg or 100 mg of RTV (0.59 vs 0.79 mg/L, mean), and the lower RTV dose was associated with a reduced impact on lipid profiles. There are clear advantages to lower doses of RTV, including improved tolerability.
In this analysis, we used data from HIV-positive patients who were included in a previous population PK study,15 where RTV AUC was used as a covariate. We developed separate models for ATV and RTV, and then integrated these models to describe the interaction between ATV and RTV in a simultaneous population PK model using a sigmoid function. This method allowed assessment of the effect of RTV dose reduction on ATV PK. Simulations of ATV concentration–time profiles were performed at doses of ATV/RTV 300/50 mg, 200/50 mg, and 200/100 mg once daily.
METHODS
Patients
The PK model was developed using data from previously reported studies15-17 in HIV-positive patients receiving a regimen containing ATV/RTV 300/100 mg once daily. All individuals were recruited and assessed at one UK study centre (St Stephen’s Centre, Chelsea and Westminster Foundation Trust, London, UK); the study was approved by the local Research Ethics Committee, and patients provided written informed consent.
A total of 288 ATV and 312 RTV plasma concentrations from 30 patients were available; 27 were men, 5 were receiving tenofovir (TDF) 300 mg once daily and the median (range) age and body weight were 43 (22–62) years and 75.5 (46–110) kg, respectively. Blood samples were collected from each individual on a single occasion. The majority of the patients were white (5 Black-Africans and 3 Hispanics). The patients had median HIV viral load of 61 copies/mL (,50–72) at baseline. The median number of samples per patient was 11. Plasma was isolated (1000 g; 10 min; 48°C) within 2 hours of collection and stored (−80°C) until analyzed. ATV and RTV were quantified by a fully validated high-pressure liquid chromatography–tandem mass spectrometry method. Details of assay performance have been reported previously.18
Population PK Modelling
A nonlinear mixed-effect model was used for the population analysis in which the ATV and RTV concentration profiles were described simultaneously, assuming a direct relationship between RTV concentration and ATV clearance:
where CL/FATV is the ATV clearance, CL0/FATV is the ATV clearance in the absence of RTV, and I(t) is the inhibition of ATV by RTV, which can be modeled as a linear or a sigmoid function. A sigmoid function best described the data:
where IMAX is the maximum inhibitory effect of RTV on CL/FATV, and IC50 is the CRTV producing 50% of the IMAX.
The model-building process was in 3 stages: (1) a separate model was developed for ATV, (2) a separate model was developed for RTV, and finally, (3) a combined model was developed, incorporating the influence of RTV concentration on ATV clearance, simultaneously.
Population PK analysis was performed using MONO-LIX nonlinear mixed effects modeling software (version 3.2, http://wfn.software.monolix.org).19 The stochastic approximation expectation maximization (SAEM) algorithm combined with the Markov chain Monte Carlo simulation procedure with no approximations was used to estimate the maximum likelihood of the model. In all the model-building stages, the strategy was as follows. One- and 2-compartment models with first- or zero-- order absorption without and with lag time were fitted to the data using the SAEM algorithm. Proportional, constant, and combined proportional and constant error models were evaluated to describe residual variability. Interindividual random effects were described by an exponential model: θi = θ × exp (ηi), where θi is the PK parameter of the ith individual, θ is the average population value, ηi is the interpatient random effect assumed to have a mean of zero and variance ω2.
The likelihood ratio test, including the log-likelihood, the Akaike information criterion, the Bayesian information criterion, visual inspection of goodness-of-fit plots, and precision of parameter estimates were used to test different hypotheses regarding the selection of the final model, including covariate selection for the separate models for ATV and RTV. The effect of each patient covariate was systematically tested via generalized additive modeling on the basic model.
The following covariates were explored: gender, ethnicity, body weight, age, and TDF coadministration. For continuous variables (eg, body weight), plots of covariates versus individual predicted PK parameters were performed to determine possible relationships. Continuous variables were introduced into the model by linear functions. For dichotomous variables, here defined as X (such as male/female sex), the following equation was applied, using CL/F as an example:
where CL/F is the typical value of the ATV clearance in the population for a male (denoted by X = 0, and thus equal to θ1), and θ2 is the relative difference in the CL/F for a female (X = 1).
To assess the stability and performance of the model, a visual predictive check20 was carried out. A thousand data sets were simulated using the parameter estimates defined by the final model. From the simulated data, 90% prediction intervals (P5–P95) were constructed, and observed data from the original data set were plotted together; the agreement between simulations and observations was assessed visually; the percentage of observed data outside the 90% prediction interval from the simulated data was calculated to assess the model. At least 90% of data points within the prediction interval (5% above and below) was indicative of an adequate model. The standard errors of all parameters were also calculated using a stochastic approximation of the Fisher information matrix.
Simulation of Dose Reductions
One thousand simulations of the original data set were performed by using the fixed and random effects of the final model at ATV/RTV doses of 300/50 mg, 200/50 mg, and 200/100 mg once daily; trough concentrations were compared.
RESULTS
ATV and RTV PK Models
Separate models for ATV and RTV were developed. A 1-compartment model with first-order absorption and a combined proportional–constant error model best described the data for both drugs. A 1-compartment model with zero-order absorption or a 2-compartment model did not improve the fit. The incorporation of an absorption lag time also improved the fit. Interindividual variability was included for the apparent oral clearance (CL/F), apparent volume of distribution (V/F), absorption rate constant (ka), and lag time. For RTV covariance in the random effects structure between CL/F and V/F was found to improve the model fit.
None of the demographic covariates or comedication (TDF) showed a significant improvement of the model fit and therefore, they were not retained in the final model. Parameter estimates for the separate final models are summarized in Table 1.
TABLE 1. Parameter Estimates of the Separate PK Models for ATV and RTV.
| Parameter | Mean Estimate | RSE (%) |
|---|---|---|
| ATV | ||
| CL/F (L/h) | 7.17 | 9 |
| V/F (L) | 91.2 | 11 |
| ka (per hour) | 1.81 | 22 |
| Lag-time (h) | 0.87 | 13 |
| IIV CL/F (%) | 70.1 | 13 |
| IIV V/F (%) | 75.5 | 14 |
| IIV ka (%) | 88.7 | 28 |
| IIV Lag-time (%) | 76.8 | 17 |
| Residual error | ||
| Proportional (%) | 44.8 | 6 |
| Additive (mg/L) | 0.0611 | 37 |
| RTV | ||
| CL/F (L/h) | 13.2 | 10 |
| V/F (L) | 81 | 10 |
| ka (per hour) | 0.898 | 12 |
| Lag-time (h) | 1.05 | 9 |
| IIV CL/F (%) | 72.8 | 14 |
| IIV V/F (%) | 73.3 | 15 |
| IIV ka (%) | 69.1 | 30 |
| IIV Lag-time (%) | 67.2 | 15 |
| Correlation (V, CL) | 0.873 | 6 |
| Residual error | ||
| Proportional (%) | 51.6 | 5 |
| Additive (mg/L) | 0.00115 | 65 |
CL/F, apparent oral clearance; IIV, interindividual variability; RSE (%), relative standard error; RSE defined as: (SEestimate/estimate) × 100, where SE is standard error; V/F, apparent volume of distribution; ka, absorption rate constant.
Simultaneous Combined ATV-RTV Model
A total of 600 plasma concentrations (288 ATV and 312 RTV) were combined and analyzed, simultaneously. A 1-compartment model with first-order absorption best described both drugs. The residual variability was best described by a proportional structure for ATV and RTV. The final estimates of the separate ATV and RTV models were used as initial parameter estimates for the combined model. The initial value for the CL/FATV without RTV was set to a value previously published.21 Estimation of all parameters simultaneously produced numerical instability. To attain a successful minimization of the model, ka for ATV and RTV were fixed to the final estimates of the separated models. The inhibition of ATV CL/F by RTV concentrations was described by a maximum-effect model. The maximum inhibitory effect of RTV on ATV CL/F was estimated to be 0.988. The estimated concentration of RTV associated with half-maximal inhibition of ATV CL/F (IC50) was 0.221 mg/L. Final estimates for fixed and random effects for ATV and RTV are summarized in Table 2, and diagnostic plots are shown in Figures 1A, 2A, respectively.
TABLE 2. Parameter Estimates of the Combined PK Models for ATV and RTV.
| Parameter | Mean Estimate | RSE % |
|---|---|---|
| CL/FRTV (L/h) | 13.2 | 12 |
| CL/FATV (L/h) | 16.6 | 7 |
| V/FRTV (L) | 124 | 11 |
| V/FATV (L) | 106 | 7 |
| Lag TRTV (h) | 1.05 | 1 |
| Lag TATV (h) | 0.87 | 2 |
| kaRTV (per hour) fixed | 0.898 | — |
| kaATV (per hour) fixed | 1.81 | — |
| Imax | 0.988 | 1 |
| IC50 | 0.221 | 13 |
| Correlation (CLRTV, VRTV) | 0.75 | 13 |
| IIV CL/FRTV (%) | 77 | 14 |
| IIV V/FRTV (%) | 73 | 16 |
| IIV V/FATV (%) | 53 | 23 |
| Residual error | ||
| ProportionalRTV (%) | 73 | 5 |
| ProportionalATV (%) | 63 | 18 |
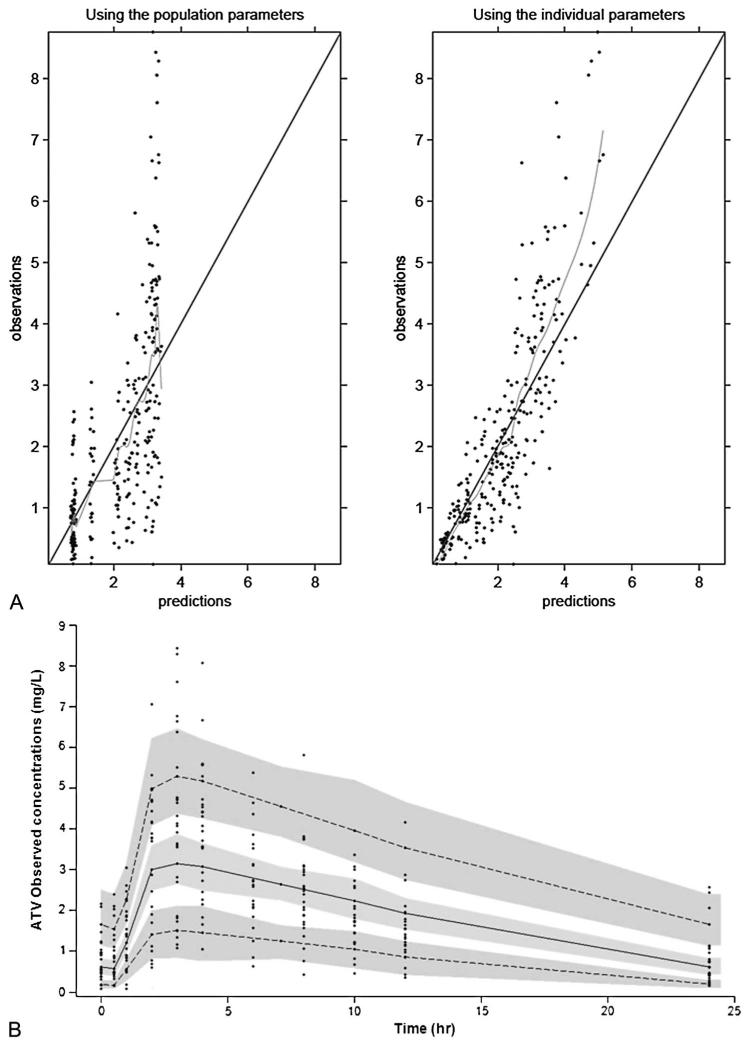
FIGURE 1.
A, Goodness of fit plots for the final PK model illustrating population and individual predictions of ATV versus observed concentrations (milligrams per liter). The continuous line shows the line of unity. B, The figure shows the 90% prediction intervals determined from simulated data of boosted ATV 300 mg once daily. The gray areas are the 90% confidence intervals for the median, 5th percentile, and the 90th percentile of the simulated data. Observed concentrations are displayed as circles.
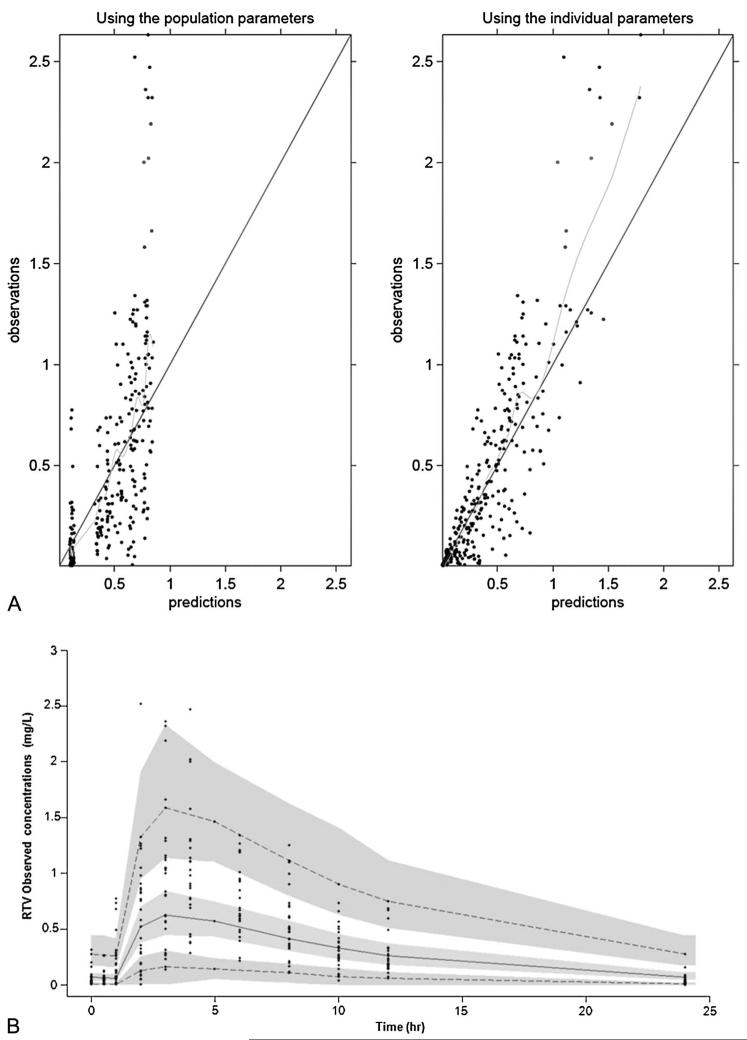
FIGURE 2.
A, Goodness of fit plots for the final PK model illustrating population and individual predictions of RTV versus observed concentrations (milligram per liter). The continuous line shows the line of unity. B, The figure shows 90% prediction intervals determined from simulated data of RTV 100 mg once daily. The gray areas are the 90% confidence intervals for the median, 5th percentile, and the 90th percentile of the simulated data. Observed concentrations are displayed as circles.
Internal Model Validation
A 90% prediction interval was generated from 1000 simulations for ATV/RTV 300/100 mg once daily. Observed data from patients used in the model-building process were superimposed onto the prediction interval. Of 288 plasma ATV concentrations, 4.3% were more than P95 and 4.5% were less than P5; of 312 RTV concentrations, 5.1% were more than P95 and 3.8% were less than P5. These results suggested that, overall, the final combined model performed adequately for both drugs (Figs. 1B, 2B).
Simulations of the Dose Reduction Strategy
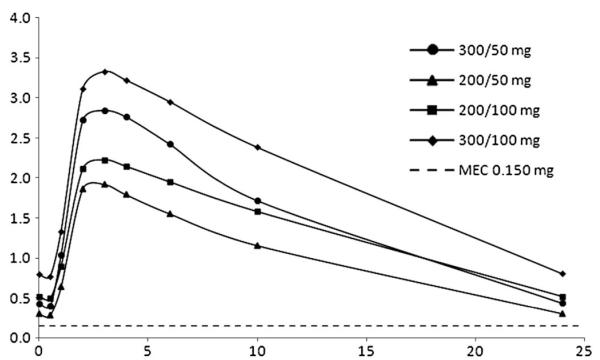
To evaluate the clinical impact of the ATV/RTV dose reduction, simulated concentration–time courses of ATV/RTV (at steady state) were carried out at doses of ATV/RTV 300/50 mg, 200/50 mg, and 200/100 mg once daily. Overall, the mean (SD) predicted ATV trough concentration was 0.437 (0.366) mg/L in patients receiving ATV/RTV 300/50 mg daily, 0.303 (0.278) mg/L in patients receiving ATV/RTV 200/50 mg daily, and 0.520 (0.448) mg/L for a dose of 200/100 mg ATV/RTV. The proportion of a thousand simulated individuals with potentially subtherapeutic (ie, less than proposed MEC) ATV trough concentration were 17.8% with a dose of 300/50 mg, 33.9% with a dose of 200/50 mg, and 15.3% with a dose of 200/100 mg (Fig. 3). At the licensed dose of 300/100 mg, the simulated mean and proportion of individuals less than MEC were 0.80 mg/L (0.676) and 6%, respectively.
FIGURE 3.
ATV mean plasma concentration predictions determined from 1000 simulations at 3 different doses.
DISCUSSION
ATV is a PI with a low pill burden, favorable lipid profile, and PK, which allows once-daily dosing.22 Conversely, RTV is associated with a number of dose-related adverse effects. Diarrhea has been shown to be the most common adverse event, followed by nausea, elevations in cholesterol, triglycerides and liver enzymes, gastrointestinal symptoms and circumoral parasthaesia.12,23 The lipid elevations and gastrointestinal adverse events experienced during treatment with RTV boosted ATV and also with other PIs may be partly caused by RTV. The reduction of RTV dose could minimize the risk of developing adverse events and also lower medication costs. In the present study, we evaluated the impact of RTV dose reduction on ATV plasma concentrations, developing and evaluating a model to describe the ATV PK as a function of the RTV plasma concentration. A direct-effect model with a maximum-effect function best described the relationship between RTV and the inhibition of the ATV CL/F. This type of model was used previously to describe the PK of lopinovir/ritonavir.24-27 This approach, with the estimation of the inhibition effect of RTV, has not been reported for boosted ATV.
A 1-compartment model with first-order absorption and elimination with the presence of lag time described the PK of both ATV and RTV separately, which was consistent with previous studies.10,15,24,25,27,28 Zhang et al26 described a 2-compartment model for RTV, using a transit absorption model. We tried to incorporate the transit absorption, however, the model had problems converging, so was not included in the final combined model. Population estimates for ATV CL/F were similar, and as with previous analyses, the interindividual variability (IIV) of parameters was relatively wide, particularly ka (78%). However, previous studies reported IIV in excess of 100%.10,28 This could be partially attributed to few samples being taken in the absorption phase. ATV absorption can be affected by food intake and it is highly dependent on gut pH, which is also variable between subjects. Among the different demographic covariates tested, there was no apparent effect of age, gender, ethnicity, or body weight on ATV PK parameters, which confirmed previous studies.10,21,28 No significant differences were observed with concomitant use of TDF, which has been shown in some29,30 but not all studies10,28 to lower ATV concentrations.29,30 However, our analysis is underpowered to evaluate this interaction as only 5 of the 30 individuals were receiving TDF.
Population estimates for RTV were difficult to compare with other PK models because there are no data describing RTV PK in the presence of ATV. None of the covariates tested showed a significant effect on any of the PK parameters. The goodness-of-fit diagnostic plots and visual predictive check for the separate models showed adequate predictions. RTV structural model of RTV is similar to previous reported studies.24,25
The interaction between ATV and RTV was described by a combined model using a direct concentration-dependent relationship. This strategy allowed us to evaluate the effect of RTV on ATV plasma concentrations estimating the RTV concentration necessary for producing half the inhibition of ATV CL/F (IC50) and simulate different dosing scenarios. The estimation of ka was difficult, thus, to have a successful-minimization, the ka of the drugs were fixed using the estimates from the separate models, which provided stability. RTV dose reduction could affect the absorption because RTV is an inhibitor of gut CYP3A and P-glycoprotein. However, the study by Estevaz et al14 showed an ATV Cmax and Tmax not significantly different between 300/100 mg and 300/50 mg, which indicates that the effect of reducing the RTV dose (from 100 mg to 50 mg) is not affecting the ATV ka. Thus, the fixing of ka could be considered an acceptable approximation.
The estimate of ATV CL/F without RTV was 16.6 L/h, which was similar to the value reported in a previous population PK study of unboosted ATV (18.4 L/h).21 The addition of IIV on ATV CL/F contributed to the instability of the model. This might be because of over parameterization of the model in combination of a relatively small number of subjects, thus IIV on ATV CL/F was not included in the final model.
It is important to note that the present model assumes only the inhibition of ATV CL/F by RTV and does not take into consideration any influence that ATV may have on RTV concentrations. Although RTV is given in combination with PIs to boost concentrations, other PIs may also impact RTV PK, as it is not only an inhibitor of CYP3A4 but also a substrate. Previous data showed that RTV plasma concentrations increase in the presence of ATV by approximately 70% compared with those of RTV administered alone.31 Although this or inhibition of transporter-mediated efflux transport were not taken into consideration, the model for RTV still provided a reasonable fit to the data. The main focus of this study was not to model the influence of ATV on RTV PK but to describe the changes in ATV concentrations and to evaluate the effect of dose reduction on ATV plasma trough concentrations. This was successfully accomplished. We previously reported an association between homozygosity for the T allele for PXR63396 and higher unboosted ATV clearance.21 This polymorphism may have an effect on the present study, which may also explain the relatively high IIV of some parameters. However, the boosting action of RTV is probably limiting the effect of PXR63396 on ATV clearance.
The recent study by Estevez et al of ATV/RTV PK in 13 healthy volunteers14 showed that a dose of ATV/RTV of 300/100 mg and 300/50 mg once daily generated mean ATV trough concentrations (0.79 and 0.59 mg/L, respectively) similar to the trough concentrations reported in our study (0.80 and 0.44 mg/L, respectively). Additionally, in the present study, a dose reduction to 200/50 mg was considered and the model prediction of the mean ATV trough concentration was more than the recommended MEC. However, some of the simulated individuals in the 90% prediction interval were below the MEC. The reduction to 200/100 mg once daily showed a Ctrough similar to the dose 300/50 mg. However, the main difference was in predicted mean maximum concentration and the AUC, which were higher for 300/50 mg (2.85 vs 2.19 mg/L and 36.6 vs 32.3 mg·h/L, respectively). A study32 in Thai HIV-1–infected adults showed that a lower dose of 200/100 mg once daily has proven to be effective and well tolerated. Currently, a clinical study is in progress to assess the efficacy and safety of the 200/100 mg regime in a Thai population.33 Hill et al12,34 have published a number of analyses showing the potential benefit of reducing the dose of RTV for several PIs (eg, reduction of cost and toxicity) and have suggested the development of a 50-mg RTV tablet, which could also have useful paediatric applications.
In conclusion, a population model to simultaneously describe the PK of ATV and RTV was developed and validated in HIV-infected individuals. The simulated median ATV trough concentrations after dose reductions were lower than those achieved with the licensed dose but were still more than the recommended ATV MEC (2.9-, 1.9-, and 3.6-fold for ATV/RTV 300/50 mg, 200/50 mg, and 200/100 mg, respectively). Simulated data for the 300/50 mg regimen were consistent with clinical data. However, a high proportion of individuals on 200/50 mg (33.9%) had simulated Ctrough less than the MEC. This figure was lower and comparable between 300/50 and 200/100 mg, suggesting that these 2 doses would be better candidate regimens for clinical evaluation. Given that PK differences between 200/100 and 300/50 mg are anticipated to be relatively minor, the choice may be determined by the likelihood of known gastrointestinal intolerance and lipid elevation with higher dose of RTV on the one hand and lack of a 50-mg tablet formulation of RTV on the other. This modeling approach aids our understanding of the interaction between ATV and RTV and informs the design of dose reduction strategies, particularly in relation to RTV.
Acknowledgments
Supported by Wellcome Trust Grant 083851/Z/07/Z.
D. Back and S. Khoo have received research grants and travel bursaries from Merck, Bristol-Myers Squibb, GlaxoSmithKline Abbott, ViiV, Boehringer Ingelheim, and Jassen.
Footnotes
Presented at the 13th International Workshop on Clinical Pharmacology of HIV, June 5–8, 2012, Venice, Italy.
All other authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bristol-Myers Squibb Pharmaceuticals Ltd. Reyatazw US. [Accessed August 9, 2012]; Available at: http://packageinserts.bms.com/pi/pi_reyataz.pdf.
- 2.Malan DR, Krantz E, David N, et al. Efficacy and safety of atazanavir, with or without ritonavir, as part of once-daily highly active antiretroviral therapy regimens in antiretroviral-naive patients. J Acquir Immune Defic Syndr. 2008;47:161–167. doi: 10.1097/QAI.0b013e31815ace6a. [DOI] [PubMed] [Google Scholar]
- 3.Molto J, Santos JR, Valle M, et al. Monitoring atazanavir concentrations with boosted or unboosted regimens in HIV-infected patients in routine clinical practice. Ther Drug Monit. 2007;29:648–651. doi: 10.1097/FTD.0b013e31815704c1. [DOI] [PubMed] [Google Scholar]
- 4.European Medicines Agency [Accessed August 9, 2012];Human medicines, Reyataz, product information. Available at: http://www.ema.europa.eu/docs/en_GB/document_ library/EPAR_-_Product_Information/human/000494/WC500056380.pdf.
- 5.US Food and Drug Administration. CDER. Reyataz [Accessed August 9, 2012]; Available at: http://www.fda.gov/Safety/MedWatch/SafetyInformation/ucm211884.htm.
- 6.Choo EF, Leake B, Wandel C, et al. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28:655–660. [PubMed] [Google Scholar]
- 7.Kim RB, Fromm MF, Wandel C, et al. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J Clin Invest. 1998;101:289–294. doi: 10.1172/JCI1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.la Porte CJL, Back D, Blaschke T, et al. Updated guideline to perform therapeutic drug monitoring for antiretroviral agents. Rev Antiviral Ther. 2006;3:4–14. [Google Scholar]
- 9. [Accessed August 9, 2012];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Available at: http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 10.Colombo S, Buclin T, Cavassini M, et al. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50:3801–3808. doi: 10.1128/AAC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perloff ES, Duan SX, Skolnik PR, et al. Atazanavir: effects on P-glycoprotein transport and CYP3A metabolism in vitro. Drug Metab Dispos. 2005;33:764–770. doi: 10.1124/dmd.104.002931. [DOI] [PubMed] [Google Scholar]
- 12.Hill A, van der Lugt J, Sawyer W, et al. How much ritonavir is needed to boost protease inhibitors? Systematic review of 17 dose-ranging pharmacokinetic trials. AIDS. 2009;23:2237–2245. doi: 10.1097/QAD.0b013e328332c3a5. [DOI] [PubMed] [Google Scholar]
- 13.Shafran SD, Mashinter LD, Roberts SE. The effect of low-dose ritonavir monotherapy on fasting serum lipid concentrations. HIV Med. 2005;6:421–425. doi: 10.1111/j.1468-1293.2005.00328.x. [DOI] [PubMed] [Google Scholar]
- 14.Estevez JA, Molto J, Tuneu L, et al. Ritonavir boosting dose reduction from 100 to 50 mg does not change the atazanavir steady-state exposure in healthy volunteers. J Antimicrob Chemother. 2012;67:2013–2019. doi: 10.1093/jac/dks152. [DOI] [PubMed] [Google Scholar]
- 15.Dickinson L, Boffito M, Back D, et al. Population pharmacokinetics of ritonavir-boosted atazanavir in HIV-infected patients and healthy volunteers. J Antimicrob Chemother. 2009;63:1233–1243. doi: 10.1093/jac/dkp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boffito M, Kurowski M, Kruse G, et al. Atazanavir enhances saquinavir hard-gel concentrations in a ritonavir-boosted once-daily regimen. AIDS. 2004;18:1291–1297. doi: 10.1097/00002030-200406180-00007. [DOI] [PubMed] [Google Scholar]
- 17.Waters LJ, Moyle G, Bonora S, et al. Abacavir plasma pharmacokinetics in the absence and presence of atazanavir/ritonavir or lopinavir/ritonavir and vice versa in HIV-infected patients. Antivir Ther. 2007;12:825–830. [PubMed] [Google Scholar]
- 18.Dickinson L, Robinson L, Tjia J, et al. Simultaneous determination of HIV protease inhibitors amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir and saquinavir in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;829:82–90. doi: 10.1016/j.jchromb.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 19.Kuhn E, Lavielle M. Maximum likelihood estimation in nonlinear mixed effects models. Comput Stat Data Analysis. 2005;49:1020–1038. [Google Scholar]
- 20.Holford N. The visual predictive check: superiority to standard diagnostic (Rorschach) plots; Presented at: 14th Population Approach Group in Europe; Pamplona, Spain. 2005.Jun 16-17, [Google Scholar]
- 21.Schipani A, Siccardi M, D’Avolio A, et al. Population pharmacokinetic modeling of the association between 63396C->T pregnane X receptor polymorphism and unboosted atazanavir clearance. Antimicrob Agents Chemother. 2010;54:5242–5250. doi: 10.1128/AAC.00781-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldsmith DR, Perry CM. Atazanavir. Drugs. 2003;63:1679–1693. doi: 10.2165/00003495-200363160-00003. discussion 1694–1675. [DOI] [PubMed] [Google Scholar]
- 23.Markowitz M, Saag M, Powderly WG, et al. A preliminary study of ritonavir, an inhibitor of HIV-1 protease, to treat HIV-1 infection. N Engl J Med. 1995;333:1534–1539. doi: 10.1056/NEJM199512073332204. [DOI] [PubMed] [Google Scholar]
- 24.Molto J, Barbanoj MJ, Miranda C, et al. Simultaneous population pharmacokinetic model for lopinavir and ritonavir in HIV-infected adults. Clin Pharmacokinet. 2008;47:681–692. doi: 10.2165/00003088-200847100-00005. [DOI] [PubMed] [Google Scholar]
- 25.Dickinson L, Boffito M, Back D, et al. Sequential population pharmacokinetic modeling of lopinavir and ritonavir in healthy volunteers and assessment of different dosing strategies. Antimicrob Agents Chemother. 2011;55:2775–2782. doi: 10.1128/AAC.00887-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang C, McIlleron H, Ren Y, et al. Population pharmacokinetics of lopinavir and ritonavir in combination with rifampicin-based antitubercular treatment in HIV-infected children. Antivir Ther. 2012;17:25–33. doi: 10.3851/IMP1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schipani A, Egan D, Dickinson L, et al. Estimation of the effect of SLCO1B1 polymorphisms on lopinavir plasma concentration in HIV-infected adults. Antivir Ther. 2012;17:861–868. doi: 10.3851/IMP2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Solas C, Gagnieu MC, Ravaux I, et al. Population pharmacokinetics of atazanavir in human immunodeficiency virus-infected patients. Ther Drug Monit. 2008;30:670–673. doi: 10.1097/FTD.0b013e3181897bff. [DOI] [PubMed] [Google Scholar]
- 29.Dailly E, Tribut O, Tattevin P, et al. Influence of tenofovir, nevirapine and efavirenz on ritonavir-boosted atazanavir pharmacokinetics in HIV-infected patients. Eur J Clin Pharmacol. 2006;62:523–526. doi: 10.1007/s00228-006-0122-2. [DOI] [PubMed] [Google Scholar]
- 30.Taburet AM, Piketty C, Chazallon C, et al. Interactions between atazanavir-ritonavir and tenofovir in heavily pretreated human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 2004;48:2091–2096. doi: 10.1128/AAC.48.6.2091-2096.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seminari E, Guffanti M, Villani P, et al. Steady-state pharmacokinetics of atazanavir given alone or in combination with saquinavir hard-gel capsules or amprenavir in HIV-1-infected patients. Eur J Clin Pharmacol. 2005;61:545–549. doi: 10.1007/s00228-005-0966-x. [DOI] [PubMed] [Google Scholar]
- 32.Avihingsanon A, van der Lugt J, Kerr SJ, et al. A low dose of ritonavir-boosted atazanavir provides adequate pharmacokinetic parameters in HIV-1-infected Thai adults. Clin Pharmacol Ther. 2009;85:402–408. doi: 10.1038/clpt.2008.244. [DOI] [PubMed] [Google Scholar]
- 33.National Institutes of Health (U.S.) [Accessed August 9, 2012];Low dose atazanavir/r versus standard dose atazanavir/r (LASA) Available at: http://clinicaltrials.gov/ct2/show/NCT01159223.
- 34.Hill A, Khoo S, Boffito M, Back D. Should we switch to a 50-mg boosting dose of ritonavir for selected protease inhibitors? J Acquir Immune Defic Syndr. 2011;58:E137–E138. doi: 10.1097/QAI.0b013e318237ccae. [DOI] [PubMed] [Google Scholar]