Abstract
Objectives
To investigate the pharmacokinetics of darunavir/ritonavir and raltegravir, in HIV-infected subjects, in both plasma and at the intracellular (IC) site of action.
Methods
HIV-infected patients on antiretroviral therapy received raltegravir 400 mg twice daily for 21 days (period 1); darunavir/ritonavir 800/100 mg once daily was added for 14 days (period 2), and patients were randomized to continue raltegravir twice daily (group 1) or to switch to 800 mg once daily (group 2), then they all stopped raltegravir intake and continued darunavir/ritonavir once daily for 14 days (period 3). Drug concentrations in plasma and cells (peripheral blood mononuclear cell) were measured, and differences in geometric mean ratios (GMR) and 90% confidence intervals (CI) between period 2 versus period 3 and period 2 versus period 1 were assessed.
Results
Twenty-four patients completed the study. Group 1 GMR (90% CI) of darunavir area under the curve (AUC) with and without raltegravir was 1.24 (1.13 to 1.45) for plasma and 1.24 (1.07 to 1.73) for cells and for group 2 was 1.14 (1.07 to 1.24) and 1.03 (0.94 to 1.16). GMR (90% CI) of raltegravir AUC without and with darunavir/ritonavir (plasma and cells) for group 1 was 0.90 (0.73 to 1.44) and 1.02 (0.81 to 1.67) and for group 2 was 1.21 (1.03 to 1.77) and 1.27 (1.07 to 1.94). Geometric mean IC to plasma AUC ratios were 5.3 and 4.9 for darunavir in groups 1 and 2 when darunavir/ritonavir was given alone and 4.9 and 5.6 for raltegravir when given alone. These ratios were not altered by the coadministered drug.
Conclusions
No remarkable interactions between darunavir/ritonavir and raltegravir in plasma or cells were seen. Raltegravir IC concentrations are higher than previously reported; the difference being due to modified cell isolation procedures that reduced drug loss caused by washing.
Keywords: darunavir/ritonavir, raltegravir, intracellular pharmacokinetics, drug interactions
INTRODUCTION
The major target of most antiretroviral (ARV) agents is within cells infected with HIV, and therefore, clinical outcomes will depend on intracellular (IC) drug concentrations.1,2 Indeed, some studies have demonstrated a correlation between ARV activity and IC concentrations of nucleoside reverse transcriptase inhibitors (NRTIs) and protease inhibitors.3-7 However, determination of IC concentrations is challenging, and because standardization of IC ARV concentration measurement methods is lacking, there is considerable variability in the data reported in the literature.
IC drug concentrations are likely to be influenced by factors such as plasma protein binding, drug lipophilicity or ionization, and whether the drug is a substrate of transport proteins responsible for its cellular influx and efflux.8 Therefore, ARVs differ remarkably in terms of IC concentrations and accumulation.
Although protease inhibitor IC accumulation has been studied previously,3-6 drug interactions involving ARV agents are commonly explored in formal pharmacokinetic (PK) clinical trials investigating plasma concentrations of investigational products and IC drug interactions are rarely studied. We previously showed that coadministration of atazanavir and saquinavir/ritonavir led to an increase in both plasma and IC exposure of saquinavir but not ritonavir in HIV-infected individuals, suggesting a relationship between changes in concentrations in plasma and in cells as a result of a drug interaction.6
Darunavir is a potent protease inhibitor approved for the treatment of HIV in combination with low-dose ritonavir. Darunavir/ritonavir is administered once daily (800/100 mg) or twice daily (600/100 mg); the latter is licensed for treatment-experienced patients.9 Data on IC concentrations of darunavir are limited to a few highly treatment-experienced individuals who were administered darunavir/ritonavir 600/100 mg twice daily in combination with ritonavir, raltegravir, and etravirine. The darunavir IC to plasma ratio reported was 1.32.10
Interestingly, the same study showed that raltegravir IC concentrations were not detected.10 However, other studies have reported IC to plasma concentration ratios of 0.039, 0.07, and 0.24.11-13 Raltegravir is the first integrase inhibitor approved for the treatment of HIV infection at a dose of 400 mg twice daily. Raltegravir is not a substrate of cytochrome P450, and it is therefore characterized by a low potential for drug interactions. It is excreted as raltegravir glucuronide, and the UDP-glucuronosyltransferase 1A1 is the main enzyme responsible for the clearance of raltegravir in humans.14 However, a recent study showed that darunavir plasma trough concentrations (Ctrough) were reduced by 36% when replacing tenofovir/emtricitabine with raltegravir in HIV-infected individuals.15
A raltegravir dose of 800 mg once daily has also been studied, but it did not show the same virological response as the approved twice-daily dose,16 suggesting that concentrations at the end of the dosing interval were too low to ensure optimal viral suppression in all patients (particularly those with a high baseline viral load).
The aim of this study was to investigate the plasma and IC PK of darunavir/ritonavir and raltegravir when coadministered to HIV-infected subjects.
MATERIALS AND METHODS
Subjects
Adult male and nonpregnant nonlactating female subjects, with confirmed HIV-1 antibody–positive status, were recruited. To determine eligibility, subjects were screened to meet the following criteria: (1) age between 18 and 65 years; (2) receiving ongoing treatment with 2 NRTIs plus a non-NRTI, protease inhibitor, or integrase inhibitor, without any history of virological failure; and (3) CD4 count greater than 100 cells per cubic millimeter.
Subjects were excluded based on the presence of any active clinically significant disease or AIDS-defining illness, body mass index > 35 kg/m2, evidence of uncontrolled HIV replication (viral load > 400 copies/mL), or intake of disallowed concomitant therapies.
Approval for the study was obtained from the Riverside Research Ethics Committee, United Kingdom, and written informed consent was obtained from each study subject before study procedures were conducted.
Study Design
This was an open-label, prospective, randomized, mixed, crossover, and parallel-group PK study conducted at the Clinical Trial Unit of the St. Stephen’s AIDS Trust, Chelsea, and Westminster Hospital, London.
The study was conducted in accordance with Good Clinical Practice, the Declaration of Helsinki, and applicable regulatory requirements (EudraCT, 2008-008321-30).
Assuming a wide interindividual variability (60%) in ARV drug PK parameters, as shown by previous studies,14 data on 24 subjects completing the study provide a 90% power to show, at a 5% significance level, the presence of a significant interaction between the studied drugs.
The safety and tolerability of study medications were evaluated by questionnaires, physical examination, and laboratory parameters throughout the study, using the AIDS Clinical Trials Group Grading Scale.
After successful screening, eligible subjects were administered raltegravir 400 mg twice daily in combination with 2 NRTIs for 21 days (period 1: days 1–21). Darunavir/ritonavir 800/100 mg once daily was then added to the regimen (period 2: days 22–35), and patients were randomly assigned on a 1:1 basis to either group 1 to continue raltegravir 400 mg twice daily or group 2 to switch to raltegravir 800 mg once daily (Fig. 1). On day 36, they all stopped raltegravir intake and continued darunavir/ritonavir once daily with their background regimen for a further 14 days (period 3: days 36–49).
FIGURE 1.
Flow diagram illustrating study design. †PK profile: plasma and intracellular sampled at 1, 2, 4, 6, 8, and 12 hours (and DRV 24 hours: days 35 and 49). DRV, darunavir.
On day 21, after a standardized breakfast, all subjects had serial blood samples drawn pre dose, 1, 2, 4, 6, 8, and 12 hours post dose for determination of plasma and IC raltegravir concentrations. On day 35, plasma and cells were isolated to measure raltegravir, darunavir, and ritonavir concentrations at the time points reported above and 24 hours post dose (group 2 only for raltegravir). On day 49, darunavir and ritonavir concentrations were measured at the same time points over 24 hours.
PBMC Isolation
Blood samples were collected in two 8 mL cell preparation tubes (Becton-Dickinson Vacutainer; Oxford, United Kingdom) per time point. Tubes were gently inverted 8–10 times to mix the anticoagulant thoroughly. They were then immediately centrifuged (horizontal rotor) at room temperature (20°C) for 20 minutes (1600 relative centrifugal force). After centrifugation, the cell preparation tubes were gently inverted to suspend the peripheral blood mononuclear cells (PBMCs) in the plasma. The plasma/cells from both cell preparation tubes were transferred into a single graduated 50 mL conical tube and isotonic saline (0.9%) added to bring the total volume to 30 mL. The sample was then gently mixed by inversion, and a 40 μL aliquot was taken for initial cell counting (Digital Bio Adam Microchip Automatic Cell Counter; NanoEnTek, Inc, Seoul, Korea). The count was multiplied by 30 to calculate the total number of cells in 30 mL and recorded. A confirmatory cell count was obtained using a DNA-based method developed by Benech et al.17 The plasma/cell suspension was then centrifuged (15 minutes, 400 relative centrifugal force) to pellet the cells. The supernatant was removed taking care to avoid disruption to the cell pellet. Carefully, so as not to disrupt the cell pellet, the tube was inverted on a piece of absorbent tissue to remove as much plasma/saline as possible. The sum of this cell preparation process constituted a single cycle of washing the cells to remove the supernatant; this contrasts with the method used in other studies with differing numbers of cycles of cell washing.10-13 Ice-cold methanol (1 mL, 70%) was added to lyse and completely redissolve the pellet. This was then vortexed (3–5 minutes) and stored at −80°C. Samples were shipped on dry ice to the Liverpool Biomedical Research Centre’s Bioanalytical Facility (Good Clinical Laboratory Practice accredited) for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis.
Analytical Methods
Concentrations of raltegravir, darunavir, and ritonavir, within plasma and PBMCs, were detected and quantified by LC-MS/MS.
Plasma Extraction and Analysis
The method described by Else et al18 was adapted to incorporate the detection of raltegravir. Plasma drug concentrations were extrapolated from a validated plasma matrix standard curve with the following lower limits of quantification: raltegravir 4.7 ng/mL, darunavir 76 ng/mL, and ritonavir 13 ng/mL. Assay precision was assessed by the calculation of inter- and intra-assay variability of low quality control, medium quality control and high quality control samples and expressed in terms of a coefficient of variation (CV%). Values were raltegravir 6.9%–9.3%, darunavir 7.8%–10.6%, and ritonavir 6.7%–8.2%. The accuracy of the assay was evaluated by calculating the percent bias from 6 replicates of each quality control (QC) in relation to target QC concentrations, respectively. Accuracy (%bias) was between −10.7% and 5.7%.
Drug Quantification in PBMCs
Cell lysate samples (1 mL) were removed from the freezer and centrifuged (13,000g, 5 minutes) to remove the cell debris. Samples were then transferred into clean glass tubes and evaporated to dryness (Rotary Evaporator, Jouan RC 10-22; Scientific Laboratory Supplies, Hessle, East Yorkshire, United Kingdom). Methanol (400 μL, 70%) was added to each tube and vortexed thoroughly. Sample (100 μL) was pipetted into an autosampler vial, and internal standard quinoxaline (QX) (20 μL of 100 ng/mL) was added. The aliquots were then crimp capped, mixed carefully, and then placed on the LC-MS/MS for quantification.
Raltegravir, darunavir, ritonavir, and QX were separated on a reverse-phase Ascentis C18 column (3 mm: 100 – 2.1 mm) (Sigma Aldrich, Dorset, UK) using a stepwise gradient mobile phase [acetonitrile:water (0.1% formic acid) 5:95 and 80:20, vol/vol] at a flow rate of 400 microliters per minute over a total run time of 8 minutes. Raltegravir, darunavir, ritonavir, and QX eluted at 4.25, 4.41, 4.75, and 4.37 minutes, respectively. A TSQ Ultra triple quadrupole mass spectrometer (Thermo Scientific, Hemel Hempstead, UK) was used to perform the detection and quantification, interfaced with a heated electrospray ionization source using selective reaction monitoring and operating in positive ionization mode. All data acquisition and processing were performed using LC Quan software (Version 2.5.6; Thermo Scientific, Hemel Hempstead, United Kingdom). The intensities of the fragment ions [mass-to-charge ratios (m/z), 108.9 to 360.9 for raltegravir, 155.8 to 392.0 for darunavir, 267.9 to 295.8 for ritonavir, and 246.0 to 284.0 for QX] were measured. IC drug concentrations were extrapolated from a validated lysate matrix standard curve with the following linear ranges: raltegravir 0.45–146.1 ng/mL, darunavir 0.45–1483 ng/mL, and ritonavir 0.48–153.2 ng/mL.
Calibration curves yield drug concentrations expressed in nanograms per milliliter of the extraction solvent (methanol). The total amount of drug in PBMC pellet (in nanograms) was obtained by multiplying the drug concentration by 0.4 (ie, 0.4 mL being the volume of methanol added to the cell pellet). The final drug level in PBMCs was expressed in nanograms per milliliter using a value of 0.4 pL for the volume of a PBMC and incorporating the cell number used.
Assay precision was assessed by the calculation of inter- and intra-assay variability of low quality control, medium quality control and high quality control samples and expressed in terms of a CV%. Values were raltegravir 7.0%–9.3%, darunavir 5.1%–10.0%, and ritonavir 7.2%–9.5%. The accuracy of the assay was evaluated by calculating the percent bias from 6 replicates of each QC in relation to target QC concentrations, respectively. Accuracy (%bias) was between −2.4% and 4.2%.
PK and Statistical Analysis
Plasma and IC darunavir, ritonavir, and raltegravir maximum concentrations (Cmax) and Ctrough were derived. Area under the curves (AUCs) from 0 to 12 (for raltegravir in both groups in period 1 and in group 1 in period 2) and 0 to 24 hours for raltegravir (group 2 in period 2), darunavir, and ritonavir, were calculated using WinNonLin version 5.2 (Mountain View, CA), by noncompartmental linear-linear trapezoidal method. Interindividual variability in concentrations was assessed by calculating the CV (CV = standard deviation/mean × 100). IC accumulation was expressed as the ratio of IC drug to drug in plasma for the different PK parameters.
Within-subject changes of drug concentrations (drug alone versus drug combination) were assessed by calculating geometric means (GM), ratios (GMR), and 90% confidence intervals (CIs). The CIs were first determined using logarithms of the individual GMR values and then expressed as linear values. The changes in PK parameters were considered significant when the CI for the GMR did not cross the value of 1.
RESULTS
Demographic and Clinical Characteristics
Twenty-four HIV-infected subjects (13 in group 1 and 11 in group 2) completed all PK phases. Screening median (range) age, body mass index, and CD4 count were 37 (21–62) years, 26 (19–32) kg/m2, and 466 (249–849) cells per cubic millimeter. Of the 27 participants who were screened to participate in the study, two participants were not eligible to participate (one with positive urine screen for drugs of abuse and the other because of difficulty obtaining venous access) and 1 eligible participant withdrew before the PK assessments for personal reasons.
All were stable on ARV treatment and had a viral load <50 copies per milliliter. All subjects were males; 17 were white, 4 were of Asian origin, and 3 were black.
The study drugs were well tolerated, and no grade 3 or 4 adverse events were reported.
Concurrent ARV medications administered with the study drugs included tenofovir (n = 21), emtricitabine (n = 21), abacavir (n = 3), and lamivudine (n = 3).
Darunavir/Ritonavir PK
Darunavir and ritonavir plasma and IC PK parameters in the absence and presence of raltegravir are shown in Table 1. Steady-state plasma and IC darunavir concentrations are shown in Figure 2.
Table 1.
Plasma and IC Concentrations of Darunavir, Raltegravir, and Ritonavir and PK Parameter Comparison
| Plasma |
IC |
GM IC to Plasma Ratio |
||||
|---|---|---|---|---|---|---|
| Group 1 (n = 13) | Group 2 (n = 12) | Group 1 (n = 13) | Group 2 (n = 12) | Group 1 | Group 2 | |
| Period 1 (day 21) | ||||||
| Raltegravir | ||||||
| GM AUC, ng·h−1·mL−1 | 1944 | 1635 | 9010 | 8780 | 4.9 | 5.6 |
| GM Cmax, ng/mL | 533 | 361 | 2306 | 1871 | 4.6 | 5.5 |
| GM Ctrough, ng/mL | 23 | 28 | 101 | 115 | 5.1 | 4.6 |
| Period 2 (day 35) | ||||||
| Raltegravir | ||||||
| GM AUC, ng·h−1·mL−1 | 1759 | 1979* | 9223 | 11,183 | 5.8 | 6.0 |
| GM Cmax, ng/mL | 438 | 556* | 2173 | 2817 | 5.6 | 6.8 |
| GM Ctrough, ng/mL | 20 | 11* | 105 | 22 | 4.5 | 2.4 |
| Darunavir | ||||||
| GM AUC, ng·h−1·mL−1 | 40,064 | 50,040 | 20,2865 | 26,3148 | 5.3 | 5.6 |
| GM Cmax, ng/mL | 4235 | 5092 | 20,210 | 29,293 | 6.8 | 6.3 |
| GM Ctrough, ng/mL | 697 | 1106 | 3431 | 4555 | 4.4 | 4.3 |
| Ritonavir | ||||||
| GM AUC, ng·h−1·mL−1 | 4299 | 4359 | 33,789 | 28,683 | 10.0 | 6.9 |
| GM Cmax, ng/mL | 483 | 617 | 3883 | 3289 | 13.8 | 5.7 |
| GM Ctrough, ng/mL | 48 | 34 | 458 | 329 | 12.0 | 10.1 |
| Period 3 (day 49) | ||||||
| Darunavir | ||||||
| GM AUC, ng·h−1·mL−1 | 49,871 | 57,055 | 25,1471 | 270,194 | 5.3 | 4.9 |
| GM Cmax, ng/mL | 4106 | 5272 | 25,433 | 24,082 | 6.8 | 4.8 |
| GM Ctrough, ng/mL | 1151 | 1135 | 4984 | 4910 | 4.8 | 4.4 |
| Ritonavir | ||||||
| GM AUC, ng·h−1·mL−1 | 4568 | 4516 | 36,769 | 27,652 | 9.5 | 6.5 |
| GM Cmax, ng/mL | 528 | 527 | 4041 | 2607 | 13.5 | 5.2 |
| GM Ctrough, ng/mL | 44 | 36 | 478 | 369 | 11.7 | 10.6 |
| Period 2 versus period 1, GMR (90% CI) | ||||||
| Raltegravir | ||||||
| AUC | 0.90 (0.73 to 1.44) | 1.21 (1.03 to 1.77)† | 1.02 (0.81 to 1.67) | 1.27 (1.07 to 1.94)† | — | — |
| C max | 0.82 (0.62 to 1.67) | 1.27 (1.09 to 1.82)† | 0.94 (0.68 to 1.66) | 1.51 (1.18 to 2.59)† | — | — |
| C trough | 0.76 (0.69 to 1.12) | 0.42 (0.35 to 0.59)† | 0.70 (0.57 to 2.06) | 0.19 (0.12 to 0.50)† | — | — |
| Period 3 versus period 2, GMR (90% CI) | ||||||
| Darunavir | ||||||
| AUC | 1.24 (1.13 to 1.45) | 1.14 (1.07 to 1.24) | 1.24 (1.07 to 1.13) | 1.03 (0.94 to 1.16) | — | — |
| C max | 0.97 (0.88 to 1.11) | 1.04 (0.93 to 1.20) | 1.11 (0.95 to 1.94) | 0.82 (0.73 to 0.96) | — | — |
| C trough | 1.37 (1.16 to 2.11) | 1.03 (0.89 to 1.24) | 1.45 (1.22 to 2.41) | 1.08 (0.86 to 1.53) | — | — |
Raltegravir 800 mg once daily.
Raltegravir 400 mg twice daily versus 800 mg once daily.
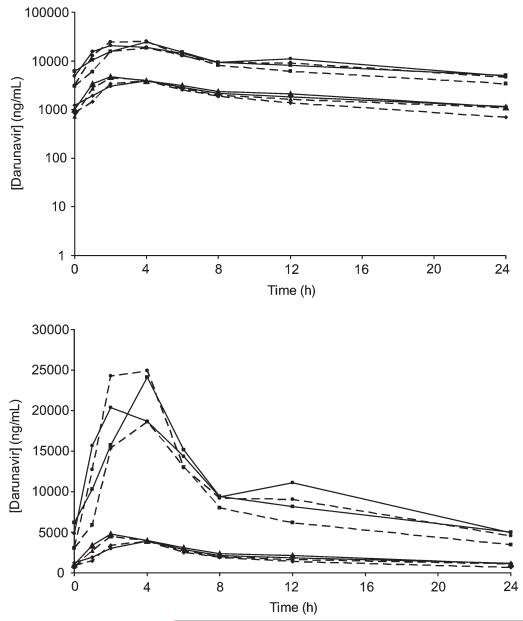
FIGURE 2.
GM plasma (diamonds, group 1; triangles, group 2) and IC (squares, group 1; circles, group 2) concentrations of darunavir in period 2 (day 35, dotted line, with raltegravir) and in period 3 (day 49, straight line, without raltegravir).
In plasma, without raltegravir, steady-state darunavir (GM, 90% CI) Cmax, Ctrough, and AUC0–24 were 4106 (3534–5339) ng/mL, 1151 (1005–1505) ng/mL, and 49,871 (44,379–60,885) ng·h−1·mL−1 for group 1 and 5272 (4755–6109) ng/mL, 1135 (1017–1339) ng/mL, and 57,055 (51,386–65,626) ng·h−1·mL−1 for group 2. With raltegravir, they were 4235 (3788–5101) ng/mL, 697 (616–877) ng/mL, and 40,064 (36,905–44,709) ng·h−1·mL−1 for group 1 and 5092 (4443–6266) ng/mL, 1106 (979–1313) ng/mL, and 50,040 (43,433–61,161) ng·h−1·mL−1 for group 2.
In cells, without raltegravir, steady-state (GM, 90% CI) Cmax, Ctrough, and AUC0–24 were 25,433 (22,050–35,538) ng/mL, 4984 (4112–8525) ng/mL, and 251,471 (217,731–338,135) ng·h−1·mL−1 for group 1 and 24,082 (22,446–26,371) ng/mL, 4910 (4259–6254) ng/mL, and 270,194 (239,620–323,007) ng·h−1·mL−1 for group 2. With raltegravir, they were 20,210 (18,168–24,324) ng/mL, 3431 (3078–4788) ng/mL, and 202,865 (181,237–243,109) ng·h−1·mL−1 for group 1 and 29,293 (25,965–35,804) ng/mL, 4555 (3914–5901) ng/mL, and 263,148 (229,991–325,739) ng·h−1·mL−1 for group 2.
Darunavir plasma AUC in both groups was slightly increased in the absence of raltegravir (24% in group 1 and 14% in group 2), and darunavir Ctrough was also higher in the absence of raltegravir in group 1 (37% increase) but not in group 2.
When comparing darunavir concentrations in the absence and presence of raltegravir in cells (Table 1), there was an increase in Ctrough (45%) in group 1 and a decrease in Cmax (18%) in group 2.
There was a marked interindividual variability in darunavir PK parameters, both in plasma (32%–87%) and in cells (32%–86%).
Raltegravir PK
Raltegravir plasma and IC PK parameters in the absence and presence of darunavir/ritonavir are shown in Table 1. Steady-state plasma and IC raltegravir concentrations when administered 400 mg twice daily and 800 mg once daily are shown in Figure 3.
FIGURE 3.
GM plasma (diamonds, group 1; triangles, group 2) and IC (squares, group 1; circles, group 2) concentrations of raltegravir in period 1 (day 21, dotted line, without darunavir) and in period 2 (day 35, straight line, with darunavir).
In plasma, without darunavir/ritonavir and at a dose of 400 mg twice daily, steady-state (GM, 90% CI) Cmax, Ctrough, and AUC0–24 were 533 (441–857) ng/mL, 23 (9–62) ng/mL, and 1944 (1686–2623) ng·h−1·mL−1 for group 1 and 361 (269–609) ng/mL, 28 (20–66) ng/mL, and 1635 (1311–2663) ng·h−1·mL−1 for group 2. With darunavir/ritonavir, they were 438 (337–999) ng/mL, 20 (17–25) ng/mL, and 1759 (1459–2937) ng·h−1·mL−1 for group 1 (400 mg twice daily) and 556 (273–1298) ng/mL, 11 (9–20) ng/mL, and 1979 (1079–4318) ng·h−1·mL−1 for group 2 (800 mg once daily) (Table 1).
In cells, without darunavir/ritonavir, steady-state (GM, 90% CI) Cmax, Ctrough, and AUC0–12 were 2307 (1892–3637) ng/mL, 101 (24–403) ng/mL, and 9010 (7852–13,519) ng·h−1·mL−1 for group 1 and 1871 (1276–4185) ng/mL, 115 (80–345) ng/mL, and 8780 (7286–16,400) ng·h−1·mL−1 for group 2. With darunavir/ritonavir, they were 2173 (1846–4299) ng/mL, 105 (51–295) ng/mL, and 9223 (7416–15,694) ng·h−1·mL−1 for group 1 (400 mg twice daily) and 2817 (2289–4153) ng/mL, 22 (16–59) ng/mL, and 11,183 (7201–23,547) ng·h−1·mL−1 for group 2 (800 mg once daily).
There was a marked interindividual variability in raltegravir PK parameters, both in plasma (53%–220%) and in cells (69%–202%), but there were no statistically significant differences in any of the PK parameters in plasma or cells when comparing raltegravir concentrations from group 1 in the absence and in the presence of darunavir/ritonavir (Table 1).
Ritonavir PK
Ritonavir PK exposure parameters were comparable between periods 2 and 3 when given in combination with and without raltegravir. In plasma, without raltegravir, steady-state ritonavir (GM, 90% CI) Cmax, Ctrough, and AUC0–24 were 528 (468–678) ng/mL, 44 (38–60) ng/mL, and 4568 (4070–5427) ng·h−1mL−1 for group 1 and 527 (427–806) ng/mL, 36 (30–54) ng/mL, and 4516 (3798–5986) ng·h−1·mL−1 for group 2. With raltegravir, they were 483 (439–564) ng/mL, 48 (5–19) ng/mL, and 4299 (3823–5176) ng·h−1·mL1 for group 1 and 617 (522–832) ng/mL, 34 (29–49) ng/mL, and 4359 (3816–5443) ng·h−1·mL−1 for group 2.
DISCUSSION
We report here the steady-state plasma and IC PK of darunavir/ritonavir and raltegravir when coadministered to HIV-infected individuals. Darunavir/ritonavir was studied at the 800/100 mg once-daily dose, whereas 2 different doses of raltegravir were investigated: 400 mg twice daily and 800 mg once daily. All study drugs were well tolerated without major adverse events being reported.
This is the first study attempting to investigate a drug interaction involving ARV belonging to different classes in plasma and within cells.
Darunavir plasma AUC was slightly higher in the absence of raltegravir in both groups 1 and 2. We do not think this change to be of any clinical significance, as it is limited and did not reflect significant changes in IC drug concentrations. Darunavir plasma Ctrough was also higher (62%) in the absence of raltegravir in group 1, but this effect was not seen in group 2 and therefore may be a consequence of the high interindividual variability in plasma and cell concentrations observed. The mechanism of a possible interaction between darunavir/ritonavir and raltegravir leading to lower concentrations of darunavir in the presence of raltegravir remains unclear. The clinical impact of this possible drug interaction needs however to be clarified by larger ongoing studies, especially in view of a recent study that showed suboptimal efficacy of ritonavir-boosted darunavir 800/100 mg once daily plus raltegravir 400 mg twice daily in treatment-naive HIV-infected individuals, with viral loads higher than 100,000 copies per milliliter, initiating ARV therapy.19 Interim results at week 24 of a 48-week study comparing darunavir/ritonavir with either tenofovir/emtricitabine or raltegravir, given to treatment-naive patients, have not confirmed this efficacy concern.20 Ritonavir plasma PK parameters were not affected by coadministration of raltegravir. Because all patients continued NRTI intake, the change in darunavir exposure cannot be explained by the absence of concomitant tenofovir administration, as previously described by a recent study comparing darunavir concentrations with tenofovir/emtricitabine to those measured with raltegravir and in the absence of NRTI.15
Raltegravir concentrations in plasma and cells were similar without or with darunavir/ritonavir in group 1. This is in contrast to a previous study that indicated a decrease of raltegravir plasma AUC and Cmax of 33% and 29%, respectively, in 10 HIV-infected individuals, 6 of whom were also on twice-daily darunavir/ritonavir.11
The measurement of IC drug concentration is complex, and methodologies lack standardization. Protease inhibitors have previously been shown to accumulate inside cells.3-6 In this study, darunavir AUC IC to plasma ratio ranged between 4.9 and 5.6 (GM). Our data show reasonable consistency with other protease inhibitor IC concentration results but are higher than previously reported for darunavir (1.32) by Ter Heine et al.10 Based on an IC to plasma ratio of between 1 and 5, it places darunavir approximately in the middle (between saquinavir that accumulates the most and indinavir the least) in the hierarchy of IC accumulation.3
Ritonavir AUC IC to plasma ratios ranged from 6.5 to 6.9 and are consistent with the value reported by Ter Heine10 of 7.72, although higher than values in some other studies.3,4 In summary, for the protease inhibitors, our limited cell washing strategy had only a small effect on the IC to plasma ratio.
In contrast, there was a very marked difference for raltegravir. Our results are considerably higher than those from 3 previously reported studies showing that either the IC concentrations of raltegravir were undetectable10 or there was a ratio of 0.07 (range 0.05–0.10),13 0.039 (range 0.007–0.177),11 or 0.24 (0.06–0.58).12
The reason for the discrepancy between our findings and previously published results is almost certainly methodological. Due to the rapid efflux of raltegravir from isolated cells, we used only a single cell wash and thereby our method clearly retained more drug associated with the cell (whereas darunavir and ritonavir were less affected). In parallel validation studies where cells were either not washed or washed 1–3 times, it was demonstrated that raltegravir “leaks” out of cells rapidly during the washing procedures.21 Furthermore, it is important to note that drug accumulation into HIV-infected cells is a dynamic process depending on drug passive diffusion, influx/efflux transmembrane transport activity, and cellular trapping. Therefore, ideally, it is the study of drug transport that may help understand the real value of IC drug concentrations.
Finally, as previously described, a wide interindividual variability has been observed for all studied drugs, especially raltegravir, both in plasma and within cells. This could be due to various reasons including drug intake with food (as the variability of raltegravir concentrations is increased in the presence of food), genetic variability of transmembrane transporters responsible for drug influx/efflux, and laboratory methodologies. However, recent population PK analysis on raltegravir in both HIV-positive and healthy individuals has demonstrated that a large proportion of the variability remains unexplained.22
In conclusion, we report darunavir/ritonavir and raltegravir plasma and IC concentrations in HIV-infected individuals during coadministration. Raltegravir IC concentrations were also assessed for the once-daily dose of 800 mg for the first time. These data increase the knowledge in IC concentration measurement and should lead to optimization of the methodologies used to assess such concentrations, as these clearly differ for each different ARV.
ACKNOWLEDGMENTS
The authors would like to thank the St. Stephen’s AIDS Trust Research Team for their hard work and the volunteers who took part in the study. Also, the authors express their thanks to the National Institute of Health Research (Department of Health), United Kingdom, and the Northwest Development Agency, United Kingdom, for infrastructural and project support.
Supported by Janssen-Cilag Ltd, United Kingdom, and St. Stephen’s AIDS Trust.
A. Jackson, D. Back, S. Khoo, B. Gazzard, and M. Boffito had received travel and research grants from and have been advisers for Tibotec, Roche, Pfizer, GlaxoSmithKline, Bristol-Myers Squibb, Merck Sharp & Dohme, Abbott, and Boehringer Ingelheim. R. Abbas is full-time employee of Janssen-Cilag Ltd, United Kingdom.
Footnotes
Some of the results of this study were presented at the 18th Conference on Retroviruses and Opportunistic Infections (CROI); February 27, to March 2, 2011; Boston, MA.
REFERENCES
- 1.Ford J, Khoo SH, Back DJ. The intracellular pharmacology of antiretroviral protease inhibitors. J Antimicrob Chemother. 2004;54:982–990. doi: 10.1093/jac/dkh487. [DOI] [PubMed] [Google Scholar]
- 2.Bazzoli C, Jullien V, Le Tiec C, et al. Intracellular pharmacokinetics of antiretroviral drugs in HIV-infected patients, and their correlation with drug action. Clin Pharmacokinet. 2010;49:17–45. doi: 10.2165/11318110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Khoo SH, Hoggard PG, Williams I, et al. Intracellular accumulation of human immunodeficiency virus protease inhibitors. Antimicrob Agents Chemother. 2002;46:3228–3235. doi: 10.1128/AAC.46.10.3228-3235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crommentuyn KM, Mulder JW, Mairuhu AT, et al. The plasma and intracellular steady-state pharmacokinetics of lopinavir/ritonavir in HIV-1-infected patients. Antivir Ther. 2004;9:779–785. [PubMed] [Google Scholar]
- 5.Breilh D, Pellegrin I, Rouzes A, et al. Virological, intracellular and plasma pharmacological parameters predicting response to lopinavir/ritonavir (KALEPHAR study) AIDS. 2004;18:1305–1310. doi: 10.1097/00002030-200406180-00009. [DOI] [PubMed] [Google Scholar]
- 6.Ford J, Boffito M, Maitland D, et al. Influence of atazanavir 200 mg on the intracellular and plasma pharmacokinetics of saquinavir and ritonavir 1600/100 mg administered once daily in HIV-infected patients. J Antimicrob Chemother. 2006;58:1009–1016. doi: 10.1093/jac/dkl379. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher CV, Kawle SP, Kakuda TN, et al. Zidovudine triphosphate and lamivudine triphosphate concentration-response relationships in HIV-infected persons. AIDS. 2000;14:2137–2144. doi: 10.1097/00002030-200009290-00010. [DOI] [PubMed] [Google Scholar]
- 8.Owen A, Khoo SH. Intracellular pharmacokinetics of antiretroviral agents. J HIV Ther. 2004;9:97–101. [PubMed] [Google Scholar]
- 9.Boffito M, Miralles D, Hill A. Pharmacokinetics, efficacy, and safety of darunavir/ritonavir 800/100 mg once-daily in treatment-naive and -experienced patients. HIV Clin Trials. 2008;9:418–427. doi: 10.1310/hct0906-418. [DOI] [PubMed] [Google Scholar]
- 10.Ter Heine R, Mulder JW, van Gorp EC, et al. Intracellular and plasma steady-state pharmacokinetics of raltegravir, darunavir, etravirine and ritonavir in heavily pre-treated HIV-infected patients. Br J Clin Pharmacol. 2010;69:475–483. doi: 10.1111/j.1365-2125.2010.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fayet Mello A, Buclin T, Franc C, et al. Cell disposition of raltegravir and newer antiretrovirals in HIV-infected patients: high inter-individual variability in raltegravir cellular penetration. J Antimicrob Chemother. 2011;66:1573–1581. doi: 10.1093/jac/dkr151. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Soon G, Goh B, et al. Time-course comparison of intracellular and plasma raltegravir after a single dose in healthy volunteers; Presented at: 50th Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA. 2011.Sep 15, [Google Scholar]
- 13.Molto J, Valle M, Back D, et al. Plasma and intracellular (peripheral blood mononuclear cells) pharmacokinetics of once-daily raltegravir (800 milligrams) in HIV-infected patients. Antimicrob Agents Chemother. 2011;55:72–75. doi: 10.1128/AAC.00789-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boffito M, Acosta E, Burger D, et al. Therapeutic drug monitoring and drug-drug interactions involving antiretroviral drugs. Antivir Ther. 2005;10:469–477. [PubMed] [Google Scholar]
- 15.Garvey L, Latch N, Erlwein OW, et al. The effects of a nucleoside-sparing antiretroviral regimen on the pharmacokinetics of ritonavir-boosted darunavir in HIV type-1-infected patients. Antivir Ther. 2010;15:213–218. doi: 10.3851/IMP1517. [DOI] [PubMed] [Google Scholar]
- 16.Eron J, Rockstroh J, Reynes J, et al. QDMRK, a Phase III study of the safety and efficacy of once daily vs twice daily RAL in combination therapy for treatment-naïve HIV-infected patients; Presented at: 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011.Mar 2, [Google Scholar]
- 17.Benech H, Theodoro F, Herbet A, et al. Peripheral blood mononuclear cell counting using a DNA-detection-based method. Anal Biochem. 2004;330:172–174. doi: 10.1016/j.ab.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Else L, Watson V, Tjia J, et al. Validation of a rapid and sensitive high-performance liquid chromatography-tandem mass spectrometry (HPLC-MS/MS) assay for the simultaneous determination of existing and new antiretroviral compounds. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1455–1465. doi: 10.1016/j.jchromb.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 19.Taiwo B, Zheng S, Gallien S, et al. Results from a single arm study of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262); Presented at: 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011.Mar 2, [Google Scholar]
- 20.Bedimo R, Drechsler H, Turner D, et al. RADAR study: raltegravir combined with boosted darunavir has similar safety and antiviral efficacy as tenofovir/emtricitabine combined with boosted darunavir in antiretroviralnaive patients; Presented at: 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. 2011. [Google Scholar]
- 21.Watson V, Liptrott N, Egan D, et al. Investigating variability in reported intracellular raltegravir concentrations: contribution of PBMC isolation methodology; Presented at: 12th International Workshop on Clinical Pharmacology of HIV Therapy; Miami, FL. 2011.Apr 14, [Google Scholar]
- 22.Arab-Alameddine M, Fayet-Mello A, Lubomirov R, et al. Population pharmacokinetic analysis and effects of raltegravir in HIV positive and healthy individuals; Presented at: 12th International Workshop on Clinical Pharmacology of HIV Therapy; Miami, FL. 2011.Apr 15, [Google Scholar]