Abstract
One of the current models of cancer proposes that oncogenes activate a DNA damage response (DDR), which would limit the growth of the tumor in its earliest stages. In this context, and in contrast to studies focused on the acute responses to a one-time genotoxic insult, understanding how cells respond to a persistent source of DNA damage might become critical for future studies in the field. We here report the discovery of a novel damage-responsive pathway, which involves p27kip1 and retinoblastoma tumour suppressors (TS), and which is only implemented after a persistent exposure to clastogens. In agreement with its late activation, we show that this pathway is critical for the maintenance –but not the initiation- of the cell cycle arrest triggered by DNA damage. Interestingly, this late response is independent of the canonical ATM- and ATR-dependent DDR, but downstream of p38 MAPK. Our results might help to reconcile the oncogene-induced DNA damage model with the clinical evidence that points to non-DDR members as the most important TSs in human cancer.
INTRODUCTION
Checkpoint responses regulate the proper transition throughout cell cycle in response to cellular stresses. For most of what we know of, even if the nature of the stress can differ significantly, the molecular circuitries that are activated share some fundamental properties. Cell cycle checkpoints are kinase-based signalling pathways that ultimately converge on the inactivation of the cyclin-CDK complexes, which are the essential engines that drive cell cycle progression. In what regards to the checkpoint responses to DNA damage, and in particular to DNA double-strand breaks (DSBs), the signalling cascade is initiated by the activation of Ataxia Telangiectasia Mutated (ATM) and ATM and Rad3-related (ATR) kinases (for a comprehensive review see (1)). In addition to the phosphorylation of targets involved in DNA repair, ATM and ATR phosphorylate Chk2 and Chk1 kinases respectively, which are essential effectors for the checkpoint function. This rapid kinase-based signalling pathway is generally known as the DNA damage response (DDR).
In addition to the rapid response that can be obtained through phosphorylation cascades, a more robust inhibition of CDK activity can also be achieved by increasing the levels of CDK-inhibitors (CKIs) (2). Two independent families of CKIs have been described in human cells. The first class includes the gene products from the INK4 locus, which specifically inhibit CDK4 and CDK6. In contrast to the INK4 family, the Cip/Kip family affects all cyclin D-, E-, and A-dependent kinase activities. This latter class of CKIs comprises three members: p21Cip1, p27Kip1 and p57Kip2; all of which contain a characteristic motif within their amino-terminal which is responsible for their CDK inhibitory capacity (3). Whereas genetic evidence suggest important tumour suppressor functions for all known CKIs, to which extent these molecules provide a checkpoint response to safeguard genomic integrity is less clear. Perhaps the only well-documented case is that of p21, where p53 mediated transcriptional control of p21 is an important regulator of the G1/S checkpoint induced by DNA damage (4, 5).
In this study, we provide a novel function of p27 in the checkpoint responses that are initiated by broken chromosomes. Our results reveal that p27 plays an important role in the response to DNA damage, subsequent to that of the DDR-p53-p21 axis, and which is only activated after a prolonged exposure to DNA breaks. Given that physiologically relevant sources of DNA damage such as oncogene-induced, eroded telomeres or aging-associated unrepairable DSBs are thought to be persistent, we believe that deciphering the molecular machinery in charge of the response to a prolonged exposure to DNA damage might emerge an important area of research in the future.
MATERIALS AND METHODS
Cell lines and chemicals
A549, IMR90, MCF7 and BJ cell lines were obtained from the ATCC. ATRflox/− cells have been described before (6). The drugs used in this study were used at the following concentrations: Dox (0.5 μM), CHX (10μg/ml), and UCN-01 (200nM).
RNA interference
Control, Rb and p27 targeting shRNA expressing lentiviruses were obtained from the TRC Mission shRNA libraries (SIGMA). An ATM shRNA expressing retroviral construct was a kind gift from Dr. Yossi Shiloh. Viral infections were performed using standard procedures and cells were selected with puromycin as a selectable marker. siRNA oligos targeting p38alpha (or control duplexes) were purchased (Dharmacon). Cells at 30% confluency were transfected with the oligos at a concentration of 50nM using DharmaFECT™ transfection reagent (Dharmacon). Analyses were performed 3 days after transfection. Results were confirmed by at least two independent siRNA duplexes.
Immunofluorescence and immunoblotting
The following primary antibodies were used in this work: γH2AX and H3S10P(Upstate biotechnology), ATM (Novus Biologicals), ATM1981P (Rockland), Chk1-S345P and Rb607P (Cell Signaling), Chk1 (Novocastra); p53, p38, p21 and p27 (Santa Cruz Biotehnology), and Rb807P (Abcam). For immunofluorescence, secondary antibodies conjugated with Alexa 488 and Alexa 594 (Molecular probes) were used. Image acquisition was done at room temperature in a Zeiss Imager Z1 fluorescence microscope with Apotome™ technology, using oil as an immersion media, an ORCA 1394 camera (Hamamatsu) and a 40X HCX PL APO-0.75 N.A. objective. All western analyses shown in this study were performed on the LICOR platform (Biosciences) which allows linearly quantitative western blot with the use of Alexa 680 and Alexa 800 conjugated secondary antibodies (Molecular Probes).
Cell cycle arrest and repair
The HR-GFP system was a kind gift from Dr. KK Khanna. G1/S checkpoint analyses were performed using standard procedures. Briefly, cells were given a 60 min pulse of BrdU (3 μg/ml) at the end of each of the treatments. Fixed cells were stained with a monoclonal anti BrdU-FITC antibody (BD-Pharmingen) and propidium iodide and analyzed by flow cytometry. For G2/M checkpoint analyses fixed cells were stained with and H3S10P antibody (Upstate) and propidium iodide and analyzed by flow cytometry.
RESULTS
A novel response to persistent DNA damage
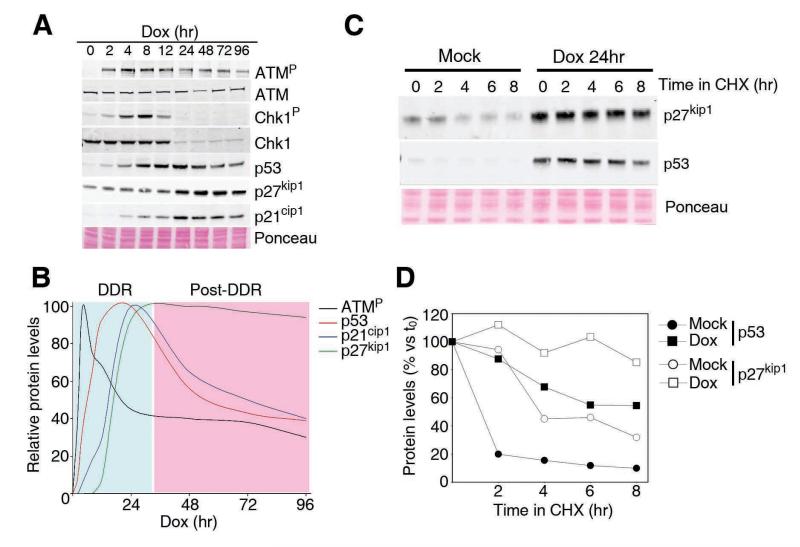
To investigate whether a prolonged exposure to DNA damage triggers a molecular signature that differs from the one activated by acute exposures, human A549 cells were cultured in the presence of Doxorubicin (Dox), a common genotoxic agent that is used in cancer chemotherapy (Fig. 1A). As expected, the addition of Dox led to a rapid and stepwise activation of ATM, Chk1, p53 and p21, which is in agreement with the modus operandi of the canonical DDR. Interestingly, the intensity of this signalling pathway seems to reach its maximum at 24hrs of exposure to Dox, after which it starts to decline even if the drug is still present (Fig. 1A,B). This is particularly notorious in the case of cell-cycle regulated genes, such a Chk1 (7), which are almost undetectable after this time due to the full implementation of cell cycle arrest. On the contrary, whereas no detectable changes were observed for the first 24 hours, the expression of p27 increased after this period and remained elevated for as long as we evaluated. This change in protein levels was not associated with p27 transcription, as tested by qRT-PCR (Supplementary Figure S1). Instead, it was the consequence of an increase of the protein stability in response to Dox (Fig. 1C,D). In contrast to p21, which is transcriptionally induced, this is the mechanism by which p27 levels are most frequently regulated (8). Thus, p27 stabilization is a secondary cellular response that is only implemented after a prolonged exposure to DNA damage, and which occurs concomitantly to the dampening of the DDR-p53-p21 response.
Figure 1. p27 stabilization in response to continuous DNA damage.
(A) Western blot data illustrating the dynamics of the different components in response to a continuous treatment with Dox. (B) Data from (A) were quantified and represented. (C) Western blot of p53 and p27 in cells treated with cicloheximide before and after exposure to Dox. (D) Representation of the data in (C).
An essential role for p27 in maintaining cell cycle arrest
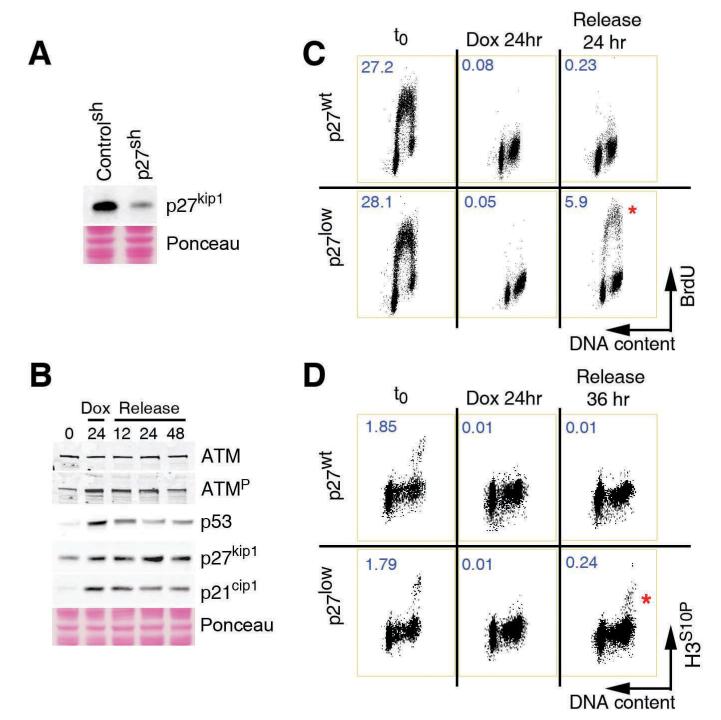
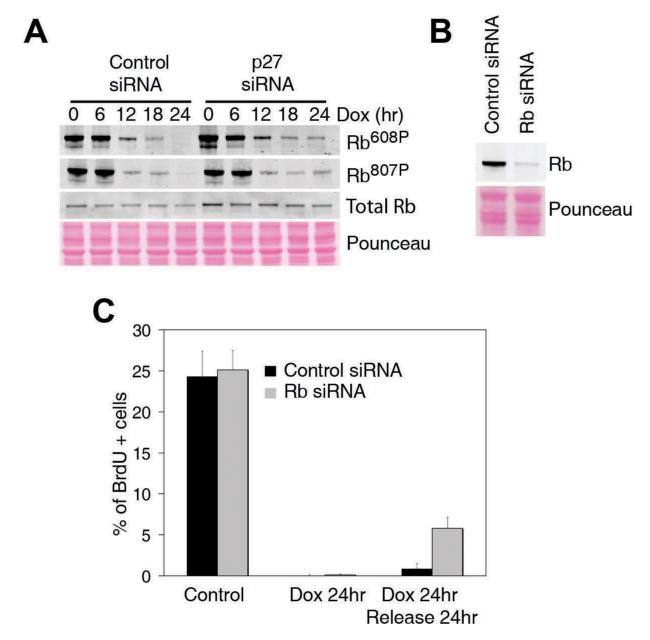
To determine the function of this late response, and because downregulation of p27 –and not complete elimination- is what is often observed in human tumours, we decided to deplete p27 by RNA interference. Stable lines of A594 human lung carcinoma cells with lentiviral-delivered shRNAs against p27 (p27low) or control shRNAs (p27wt) were generated (Fig. 2A). One initial clue about the function came from the fact that, once increased, p27 levels remained high even if the Dox was eliminated from the medium (Fig. 2B). The self-sustaining nature of this response suggested that it could be in charge of maintaining the growth-arrested state. To determine whether this was the case, cells were exposed to Dox for 24 hrs, and then released into fresh medium for another day. After the continuous exposure to Dox, both control and p27low lines had fully arrested their cell cycle, and no incorporation of BrdU was detected (Fig. 2C). However, whereas p27wt cells remained arrested even if the drug was removed from the medium, a significant fraction of p27 depleted cells showed active DNA replication at the same time (Fig. 2C). To a lesser extent, p27low cells also failed to stop at the G2 and progressed into mitosis (Fig. 2D). Importantly, these phenotypes were validated with 2 independent shRNA sequences and in several tumoral and primary human cell lines including MCF7, IMR90 and foreskin fibroblasts (data not shown). Thus, and in agreement with its late activation, p27 is dispensable for the initial cell-cycle arrest initiated by DNA damage but becomes essential for the maintenance of the arrested state once this has been implemented.
Figure 2. p27 regulates the maintenance of the cell cycle checkpoints.
(A) Western blot illustrating the extent of the depletion of p27 obtained by the infection of A549 cells with lentiviruses expressing p27 targeting -and control-shRNAs. (B) Dynamics of ATM phosphorylation, p53, p21 and p27 in cells exposed to Dox for 24hrs and subsequently released into drug-free medium. (C) Flow cytometry profiles illustrating the DNA content (propidium iodide) and S-phase index (BrdU) on p27wt and p27 low cells in the different conditions. (D) Flow cytometry profiles illustrating the DNA content (propidium iodide) and mitotic index (H3S10P) on p27wt and p27 low cells in the different conditions. Numbers indicate the percentage of BrDU positive (C) and mitotic (D) cells in each case.
A role for p27 as a guardian of the genome
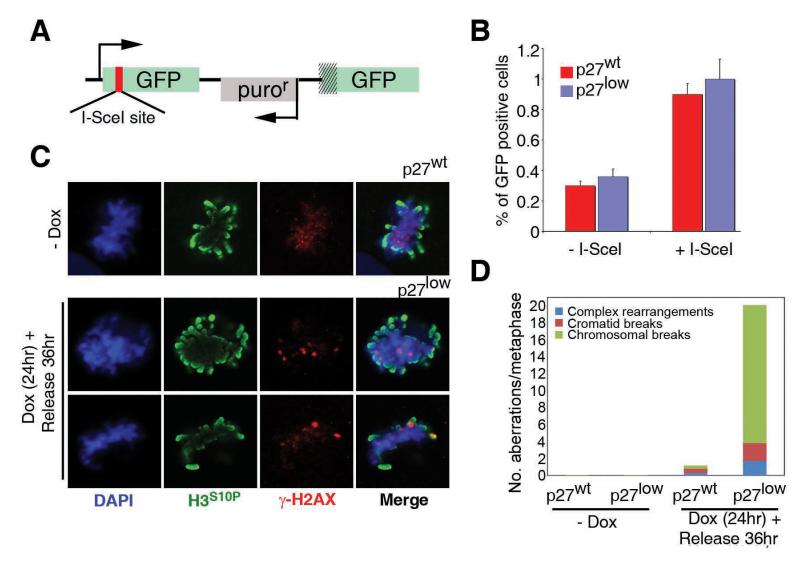
The fact that p27 depleted cells escaped the growth arrest could respond to one of two things: either these cells have a better capacity to repair and had thus already repaired all the damage; or, they did so illegitimately in the presence of chromosomal breaks. In what regards to the first option, recent data have shown that CDK activity modulates the efficiency of DNA repair, particularly by homologous recombination (HR) (9-11). To determine whether the escape from the cell cycle arrest in p27low cells was the consequence of a faster repair of DNA breaks, the efficiency of DNA repair was measured by several independent means. Neither specific assays for measuring HR or NHEJ, nor a global analysis of DNA repair based on the formation and disappearance of γH2AX foci showed significant differences between control and p27 depleted cells (Fig. 3A,B and Supplementary Figure S2).
Figure 3. Genomic Instability in p27 depleted cells.
(A) Schema of the HR-substrate used in this study. A modified GFP cDNA is expressed from a hCMV promoter. GFP is modified to include an I-SceI site with in-frame termination codons so that no full protein is expressed. The vector also has a second promoterless GFP cDNA downstream. Upon the expression of I-SceI, both GFP sequences undergo HR so that an intact GFP is generated. (B) HR frequencies on p27wt and p27low cells as measured with the system described in (A). (C) Immunofluorescence data of the localization of γH2AX and H3S10P signals on p27wt and p27low cells on the different conditions. (D) Quantification of the numbers of the different types of chromosomal abnormalities observed on Giemsa-stained metaphases. The bars indicate the average number of aberrations scored from 50 metaphases. Examples of the types of aberrations can be found on the Supplementary Figure S4.
If faster DNA repair kinetics cannot account for the earlier re-entry into the cell cycle observed in p27low cells, then this implies that cells were doing so in the presence of DNA breaks. To determine whether this was the case we first analyzed the distribution of phosphorylated histone H2AX (γH2AX) which marks sites of DSBs (12). Upon a 24hr exposure to Dox and subsequent 24hr release, p27low cells that had entered S phase did so in the presence of γH2AX foci (Supplementary Figure S3). In contrast, the few p27wt cells that were found to be replicating were consistently almost free of DDR foci. Consistently, the few control cells that reached mitosis presented an overall staining of γH2AX but no distinct foci (0/12 mitosis with γH2AX foci) (13). On the contrary, p27low cells presented mitotic figures, which, besides the disperse γH2AX staining, showed a punctuated pattern reminiscent of DSB-associated foci (20/20 cells with γH2AX foci) (Fig. 3C). Accordingly, metaphase preparations revealed a dramatic increase of genomic instability on p27 depleted cells (Fig. 3D and Supplementary Figure S4). Thus, the defective maintenance of the cell cycle arrest that is observed when p27 levels are reduced has important functional consequences, which include an accumulation of genomic instability.
A role for MAPK kinases in the damage-induced stabilization of p27
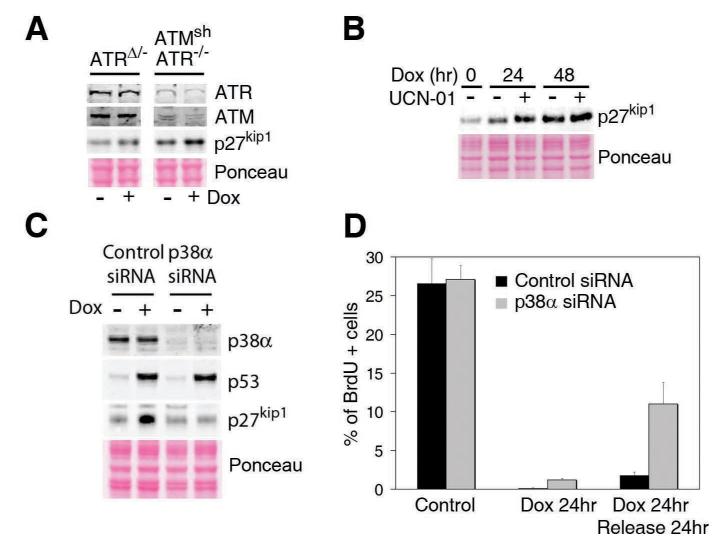
We then went on to determine the pathways responsible for the late increase in p27 levels. One unique property of p27 that is not shared by p21 or p57 is the existence of a cluster of putative target sites for the kinases of the DNA damage response (DDR) (Supplementary Figure S5). Furthermore, recent massive proteomic studies have found evidence for phosphorylation of some of these sites in vivo (14). The fact that these evolutionary conserved phosphorylation sites lie on the C-terminal part of the protein, which has been previously reported to be involved in controlling the protein’s turnover, prompted us to evaluate whether the stabilization of p27 in response to DNA damage was linked to the DDR kinases (ATM and ATR). However, even the combined depletion of ATM and ATR did not affect the increase in p27 that is observed in cells exposed to Dox (Fig. 4A). Moreover, chemical inhibition of Chk1 not only failed to abrogate the increase but even led to higher p27 levels upon exposure to Dox, which could reflect the higher endogenous breakage linked to Chk1 inhibition (Fig. 4B). As shown above, the DDR-independent nature of this pathway is consistent with the fact that p27 levels rise when the ATM/p53/p21 axis start to decline. Whereas we cannot exclude that DDR-dependent phosphorylation might regulate additional functions of the protein, our data provide genetic proof for the DDR-independent stabilization of p27 in response to DNA damage. Besides the DDR, one of the pathways for which recent evidence suggests could be directly involved in the response to chromosomal damage is that of stress kinases, and p38 MAPK in particular (15). Moreover, recent data have shown that p38 MAPK regulates p27 accumulation during contact inhibition of cell growth (16). Importantly, RNAi-mediated depletion of p38 abrogated the late increase in p27 levels initiated by Dox without affecting the activation of p53 (Fig. 4C). Of note, this p27 stabilization was not affected by scavengers of reactive oxygen species (ROS) (Supplementary Figure S6) or by RNAi mediated depletion of MK2 (data not shown). Moreover, whereas p38 depletion did not have a major impact on the implementation of the cell cycle arrest initiated by Dox, it led to a defective maintenance of the arrested state (Fig. 4D). In summary, the increase in p27 that is activated upon a continuous exposure to DNA damage is downstream of p38, but independent on the DDR.
Figure 4. p38-dependent and DDR-independent stabilisation of p27.
(A) Stabilization of p27 on cells in which ATM (by shRNA) and ATR (by genetic elimination of a conditional allele) have been eliminated. (B) Stabilization of p27 in cells exposed to UCN-01. (C) Damage induced stabilisation of p27 and p53 in cells in which p38 was eliminated by RNAi. (D) Quantification of the number of cells undergoing active replication in each condition as measured in Fig. 2C.
Two waves of CDK inhibitory activity in the response to DNA breaks
We finally tried to determine how this late increase in p27 levels could be related to the defective checkpoint maintenance observed in p27low cells. Given that p27 is a well-known CDK inhibitor, we evaluated the kinetics of CDK activity in response to the continuous treatment with Dox. As a surrogate marker of CDK activity we examined the levels of CDK-dependent phosphorylations on the retinoblastoma (Rb) TS gene product. As expected, a rapid decrease in Rb phosphorylation is initiated at about the time when the initial p53/p21 response is normally activated (Fig. 5A). Interestingly, a minimal level of CDK activity seems to persist until cells have been continuously exposed to Dox for at least 24 hrs, which is the time at which p27 levels raise (see Fig. 1). In agreement with this idea, whereas p27 depletion did not have any effect on the initial kinetics of Rb dephosphorylation, it completely abrogated the final CDK-inhibitory activity that arises at later time points (Fig. 5A). Moreover, shRNA mediated downregulation of Rb led to a defective maintenance of the cell cycle arrest, comparable to that observed in p27low cells (Fig. 5B,C). This two-step dephosphorylation of Rb is consistent with previous observations that showed a role for Rb in both the initiation and the maintenance of the growth arrest that is initiated by DNA damage (17, 18). Altogether, the data presented here demonstrate that a p38/p27/Rb-dependent pathway, independent of the canonical ATM/p53/p21 DDR plays an essential role in safeguarding genomic integrity in conditions of prolonged exposure to DNA breaks.
Figure 5. p27 dependent Rb dephosphorylation.
(A) Western blot illustrating the two-step dynamics by which Rb is dephosphorylated in response to a continuous Dox treatment. (B) Western blot illustrating the extent of the depletion of Rb obtained by the infection of A549 cells with lentiviruses expressing Rb targeting -and control- shRNAs. (C) Quantification of the number of cells undergoing active replication in each condition as measured in Fig. 2C.
DISCUSSION
Several sources of data on the functions of p27 already suggested that this CKI could have an active role in the response to DNA damage. First, and most importantly, p27 was the first haplo-insufficient TS to be ever described, and this was detected because p27+/− mice were more sensitive to tumours induced by genotoxic agents even if the tumours kept the wild type p27 allele intact (19). Of note, from the variety of tumours that were observed, lung malignancies were specific of the irradiated p27 heterozygous mice, which was one of the reasons to use lung-derived human cell lines for our study. Second, although p27 levels are often low in many human cancers, its complete loss is extremely rare. Significantly, low p27 levels correlate with a poor response to genotoxic chemotherapy (20). Third, previous works in Xenopus showed an increase in p27 levels in irradiated embryos that only occurs after the embryo has reached the mid-blastula transition, which is where the switch from apoptosis to G1/S arrest is implemented during frog development (21). Altogether, these data strongly suggested an active role of p27 in the cellular responses to DNA damage.
In what responds to mechanisms that connect p27 to DNA damage, the data are less abundant. One line of evidence revealed that p27 could limit centrosomal amplification in response to DNA damage, thus leading to genomic instability (22). A more recent report has shown that p27 is important for the initial activation of the G2/M checkpoint in response to low doses of IR, revealing that p27 could also be important at earlier timepoints (23). However, and in agreement with our observations, the authors failed to detect any changes of expression of p27 at early timepoints. Given that the initial activation of the G2/M checkpoint is directly regulated by the DDR, one intriguing possibility is that this early role could be related to the ATM phosphorylation sites that exist on the C-terminus of p27 (see Supplementary Figure S5). Nevertheless, other murine models with a deficient low-dose G2/M checkpoint and which also present strong DNA repair deficiencies have a much milder incidence of tumours when compared to p27 deficient mice (24), suggesting that additional roles of p27 must be important for tumour suppression. We believe that the role of p27 described here in maintaining the cell cycle arrest once it is implemented, could contribute to such TS activity. The dramatic accumulation of genomic instability observed in p27low cells support that p27 could indeed be a critical guardian for genomic integrity.
An issue that remains to be resolved is how p38 MAPK signalling leads to p27 stabilization. A putative role for MAP kinases in response to DNA breaks has been suggested previously. On one hand, IR exposure led to a modest increase in p38 activity, which was suggested to be dependent on TAO kinases (25). In addition, p38 dependent regulation of p21 mRNA stability has been recently shown to regulate IR-induced p21 levels independently of p53 (26). On the other hand, UV exposure is a potent activator of p38, and evidence suggests that UV damage can be converted into DNA breaks when cells replicate their DNA (27). A putative role of p38 in the response to DSBs has been recently reported, and suggested to be only relevant in the context of p53 deficiency (15). Moreover, the work showed that, in this response, p38 lies upstream of MK2 and downstream of the ATM/ATR-dependent DDR. Given that the role of p38 in stabilizing p27 occurs independently of the DDR and MK2, and that the cell lines used in this study have a proficient p53 response to DNA damage, our data further support that p38 also plays an important role in the safeguarding the genome in cells with an intact DDR-p53 axis.
In the recent model of oncogene-induced DNA damage for cancer development proposed by the laboratories of Bartek and Halazonetis, oncogenes would be a persistent source of DNA damage, which by activating the DDR will restrain tumour growth in its initial stages (28). The model implies that DDR-proteins would be important tumour suppressors in vivo. However, mutational evidence for components of the DDR is scarce, and the absence of a functional DDR is not a common observation in most cancer-derived lines used in the laboratory. Genetic evidence to validate this model is currently an important area of cancer research. Nevertheless, and based on our current data, we would want to propose that many of the common TS genes that are not commonly linked to the DDR, such as p27 or Rb, might have important roles in this second-wave response to persistent DNA damage (see model in Fig. 6). The model will then be complete, and reconcile the fact that oncogenes induce DNA damage, with the clinical evidence that points to non-DDR genes as the most important tumour suppressors.
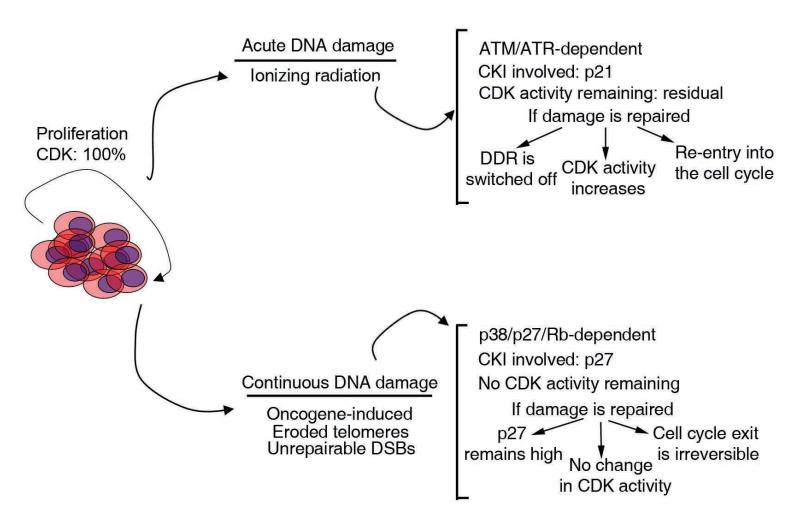
Figure 6. A two-step model for the response to DNA damage.
The figure illustrates a general overview of the model suggested by our data. In response to an acute response to DNA damage, the canonical DDR will rapidly activate a transient cell cycle arrest to allow cells for the completion of DNA repair. This checkpoint still maintains some residual CDK activity which is necessary to resume proliferation once the damage is repaired. If the damage is continuous, the DDR will eventually shut off (for instance by eliminating cell cycle regulated components such as Chk1, TopBP1...), and a second wave of CDK inhibitory activity will bring CDK activity to zero. Once all CDK activity is eliminated, the cell cycle exit is irreversible and proliferation will not restart even if the original breaks are repaired. Importantly, the model proposes that oncogene-induced DNA damage is a continuous source of replicative-damage which will ultimately induce senescence, or permanent cell cycle exit (29, 30). It is therefore interesting to point out that the components of the response to continuous DNA damage (Rb, p27..) are more commonly altered in human cancer than the DDR members (ATM, ATR, Chk1...) involved in the transient inhibition of the cell cycle.
Supplementary Material
ACKNOWLEDGMENTS
We thank I. Dolado for helpful discussions and L. Pereira for help on the ROS analysis. Work in O. F.’s laboratory is supported by grants from the Spanish Ministry of Science (RYC-2003-002731, CSD2007-00017 and BFU2005-09429), European Molecular Biology Young Investigator Programme and European Research Council (ERC StG 210520).
REFERENCES
- 1.Harper JW, Elledge SJ. The DNA damage response: ten years after. Mol Cell. 2007;28:739–45. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–12. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 3.Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14:159–69. doi: 10.1016/j.devcel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature. 1995;377:552–7. doi: 10.1038/377552a0. [DOI] [PubMed] [Google Scholar]
- 5.Deng C, Zhang P, Harper JW, Elledge SJ, Leder P. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 1995;82:675–84. doi: 10.1016/0092-8674(95)90039-x. [DOI] [PubMed] [Google Scholar]
- 6.Cortez D, Guntuku S, Qin J, Elledge SJ. ATR and ATRIP: partners in checkpoint signaling. Science. 2001;294:1713–6. doi: 10.1126/science.1065521. [DOI] [PubMed] [Google Scholar]
- 7.Carrassa L, Broggini M, Vikhanskaya F, Damia G. Characterization of the 5′flanking region of the human Chk1 gene: identification of E2F1 functional sites. Cell Cycle. 2003;2:604–9. [PubMed] [Google Scholar]
- 8.Pagano M, Tam SW, Theodoras AM, et al. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682–5. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 9.Aylon Y, Liefshitz B, Kupiec M. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. Embo J. 2004;23:4868–75. doi: 10.1038/sj.emboj.7600469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esashi F, Christ N, Gannon J, et al. CDK-dependent phosphorylation of BRCA2 as a regulatory mechanism for recombinational repair. Nature. 2005;434:598–604. doi: 10.1038/nature03404. [DOI] [PubMed] [Google Scholar]
- 11.Huertas P, Cortes-Ledesma F, Sartori AA, Aguilera A, Jackson SP. CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature. 2008;455:689–92. doi: 10.1038/nature07215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–67. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 13.Ichijima Y, Sakasai R, Okita N, Asahina K, Mizutani S, Teraoka H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem Biophys Res Commun. 2005;336:807–12. doi: 10.1016/j.bbrc.2005.08.164. [DOI] [PubMed] [Google Scholar]
- 14.Matsuoka S, Ballif BA, Smogorzewska A, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–6. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 15.Reinhardt HC, Aslanian AS, Lees JA, Yaffe MB. p53-deficient cells rely on ATM- and ATR-mediated checkpoint signaling through the p38MAPK/MK2 pathway for survival after DNA damage. Cancer Cell. 2007;11:175–89. doi: 10.1016/j.ccr.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swat A, Dolado I, Rojas JM, Nebreda AR. Cell density-dependent inhibition of epidermal growth factor receptor signaling by p38alpha mitogen-activated protein kinase via Sprouty2 downregulation. Mol Cell Biol. 2009;29:3332–43. doi: 10.1128/MCB.01955-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knudsen KE, Booth D, Naderi S, et al. RB-dependent S-phase response to DNA damage. Mol Cell Biol. 2000;20:7751–63. doi: 10.1128/mcb.20.20.7751-7763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naderi S, Hunton IC, Wang JY. Radiation dose-dependent maintenance of G(2) arrest requires retinoblastoma protein. Cell Cycle. 2002;1:193–200. [PubMed] [Google Scholar]
- 19.Fero ML, Randel E, Gurley KE, Roberts JM, Kemp CJ. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature. 1998;396:177–80. doi: 10.1038/24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito G, Pucciarelli S, Alaggio R, et al. P27kip1 expression is associated with tumor response to preoperative chemoradiotherapy in rectal cancer. Ann Surg Oncol. 2001;8:311–8. doi: 10.1007/s10434-001-0311-2. [DOI] [PubMed] [Google Scholar]
- 21.Finkielstein CV, Lewellyn AL, Maller JL. The midblastula transition in Xenopus embryos activates multiple pathways to prevent apoptosis in response to DNA damage. Proc Natl Acad Sci U S A. 2001;98:1006–11. doi: 10.1073/pnas.98.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugihara E, Kanai M, Saito S, et al. Suppression of centrosome amplification after DNA damage depends on p27 accumulation. Cancer Res. 2006;66:4020–9. doi: 10.1158/0008-5472.CAN-05-3250. [DOI] [PubMed] [Google Scholar]
- 23.Payne SR, Zhang S, Tsuchiya K, et al. p27kip1 deficiency impairs G2/M arrest in response to DNA damage, leading to an increase in genetic instability. Mol Cell Biol. 2008;28:258–68. doi: 10.1128/MCB.01536-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Celeste A, Petersen S, Romanienko PJ, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–7. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raman M, Earnest S, Zhang K, Zhao Y, Cobb MH. TAO kinases mediate activation of p38 in response to DNA damage. Embo J. 2007;26:2005–14. doi: 10.1038/sj.emboj.7601668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lafarga V, Cuadrado A, Lopez de Silanes I, Bengoechea R, Fernandez-Capetillo O, Nebreda AR. p38 Mitogen-activated protein kinase- and HuR-dependent stabilization of p21(Cip1) mRNA mediates the G(1)/S checkpoint. Mol Cell Biol. 2009;29:4341–51. doi: 10.1128/MCB.00210-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward IM, Minn K, Chen J. UV-induced ataxia-telangiectasia-mutated and Rad3-related (ATR) activation requires replication stress. J Biol Chem. 2004;279:9677–80. doi: 10.1074/jbc.C300554200. [DOI] [PubMed] [Google Scholar]
- 28.Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–5. doi: 10.1126/science.1140735. [DOI] [PubMed] [Google Scholar]
- 29.Bartkova J, Rezaei N, Liontos M, et al. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–7. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 30.Di Micco R, Fumagalli M, Cicalese A, et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature. 2006;444:638–42. doi: 10.1038/nature05327. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.